Abstract
A comprehensive evaluation of the risk of serious infections in biologic therapies for psoriasis is lacking. We performed a systematic review and meta-analysis of randomized controlled trials (RCTs) and prospective cohort studies reporting serious infections in people taking any licensed biologic therapy for psoriasis compared with those taking placebo, nonbiologic therapy, or other biologic therapies. The quality of the studies was assessed using Grading of Recommendations Assessment, Development and Evaluation criteria. No significant heterogeneity was detected in data from 32 RCTs (n = 13,359 participants) and one cohort study (n = 4,993 participants). In adults, low- to very-low-quality RCT data showed no significant difference between any biologic therapy and placebo at weeks 12–16 (overall pooled Peto odds ratio = 0.71, 95% confidence interval = 0.36–1.41) and weeks 20–30 (odds ratio = 2.27, 95% confidence interval = 0.45–11.49). No significant differences were found in any of the other comparisons in underpowered RCT data. Prospective cohort study data of low quality suggests that only adalimumab (adjusted hazard ratio [adjHR] = 2.52, 95% confidence interval = 1.47–4.32) was associated with a significantly higher risk of serious infection compared with retinoid and/or phototherapy in adults. No association between biologic therapies and serious infections in patients with psoriasis who were eligible for RCTs was detected. Further observational studies are needed to inform the uncertainty around this risk in the real world.
Abbreviations: adjHR, adjusted hazard ratio; CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development and Evaluation criteria; OR, odds ratio; RCT, randomized controlled trial
Introduction
Three classes of biologic therapies are used for the treatment of psoriasis: the tumor necrosis factor inhibitors infliximab, etanercept, and adalimumab; ustekinumab, an IL-12/IL-23 antagonist; and secukinumab, an IL-17 antagonist. Pharmacovigilance registries indicate that over 27,000 patients are receiving a biologic therapy for the treatment of psoriasis worldwide (Garcia-Doval et al., 2013, Iskandar et al., 2015, Kalb et al., 2015). These treatments inhibit cytokine pathways of critical importance in the immune system. TNF-α is integral for the immune response against intracellular infections and formation of granulomas (Rychly and DiPiro, 2005), whereas IL-12 and IL-23 regulate cell-mediated immunity through IFN-γ induction (Watford et al., 2004), and IL-23 is involved in T-helper 17 cell differentiation and secretion of IL-17, which is important for defense against fungal infections (Puel et al., 2010). The risk of serious infections leading to morbidity and/or mortality in association with biologic therapies for psoriasis is therefore a legitimate concern for patients, clinicians, and health care providers, but there is significant uncertainty surrounding the extent of this risk and whether this risk is different between biologic therapy classes.
There is limited evidence regarding the risk of serious infections conferred by biologic therapies in patients with psoriasis. Individual randomized controlled trials (RCTs) are inadequately powered to study rare events, and long-term extensions to these trials often lack a comparator arm. One systematic review of RCTs with meta-analysis reported no increased risk of serious infection with the short-term use of tumor necrosis factor inhibitors in patients with psoriasis and psoriatic arthritis compared with placebo (odds ratio [OR] = 0.70, 95% confidence interval [CI] = 0.40–1.21) (Dommasch et al., 2011). However, in addition to trials involving participants with psoriasis predominantly, this review also included trials involving only participants with psoriatic arthritis, limiting its generalizability to patients with only cutaneous manifestations of psoriasis. This review was not able to take into account more recent clinical trials involving ustekinumab and secukinumab.
An updated review of the evidence is needed to investigate the risk of serious infection for the new biologic therapies and to include more recent studies involving the tumor necrosis factor inhibitors, thereby offering more power to evaluate the rare event of serious infection, to focus on a homogenous study population of patients with predominately cutaneous disease, and to inform development work for the updating of the British Association of Dermatologists national guideline for the use of biologic therapies in patients with psoriasis. We aimed to review RCTs and prospective cohort studies to assess the risk of serious infection of the currently licensed biologic therapies for the treatment of psoriasis, both against placebo, against nonbiologic systemic therapies, and where possible compared with each other, based on data currently available.
Results
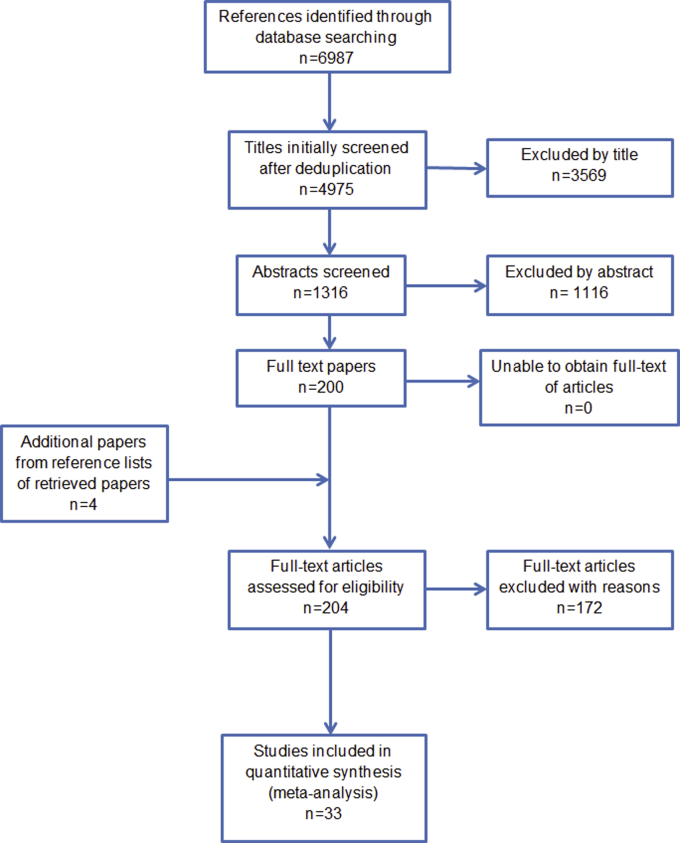
The systematic literature search yielded 6,987 results. Overall, 204 articles were assessed for eligibility in full text; data were extracted from the 30 RCT articles (reporting 32 studies) and one cohort study that met the inclusion criteria (Figure 1, and Supplementary Table S1 online). The reasons for exclusion of studies are given in Supplementary Table S2 online. A total of 48 articles were excluded because of study design, and 28 were excluded because of lack of reporting of serious infection outcome. A total of 13,359 participants from RCT studies and 4,993 participants in one cohort study were included.
Figure 1.
Flow diagram showing the identification of literature in the PRISMA statement format.
No statistical evidence of heterogeneity was detected for any of the comparisons, and therefore no subgroups, including dosing regimens, were investigated separately.
Thirteen RCTs included past serious infection as an exclusion criterion in the methods report of the article. Two RCTs assessed patients under 18 years of age. All of the included studies investigated participants with plaque-type psoriasis. Seventeen studies reported inclusion of participants who had previously received biologic therapy; the proportion ranged from 9.3% to 60% in a treatment arm within the RCT studies, whereas the cohort study reported that 87.4% of participants were biologic experienced.
The total number of serious infections reported across all RCTs was low (n = 54). Eight studies did not report any serious infections in either study arm.
Risk of bias
Overall, using the risk of bias assessment checklist from the National Institute for Health and Care Excellence and the National Clinical Guideline Centre, the risk of bias varied among the individual studies, ranging from low to very high (see Supplementary Table S3 online). Regarding selection bias and performance bias, 26 of 31 (83.9%) studies had a low risk of selection bias, and 26 of 31 (83.9%) had a low risk of performance bias. A total of 27 studies (87.1%) did not clearly report blinding of the investigators to important confounding or prognostic factors. The intervention was open label in one study (Gordon et al., 2015). All studies reviewed were financially sponsored by the pharmaceutical industry, and all studies had industry involvement in both the analysis of the data and the writing of the manuscript. There was a low risk of attrition bias in most individual studies. The percentage of patients discontinuing allocated treatment at the time of the primary outcome overall was significantly higher in the placebo group (median 8.1%) compared with the treatment group (median = 4.5%, P < 0.01, Mann-Whitney test). However, it is unclear whether these participants continued under follow-up for adverse events after discontinuation of therapy.
Three studies defined the outcome of serious infection (Bachelez et al., 2015, Kalb et al., 2015, Reich et al., 2005), with two of these studies clearly defining this in the results section (Bachelez et al., 2015, Reich et al., 2005) (see Supplementary Table S4 online). There was heterogeneity in the nomenclature of the outcome, with 22 studies defining it as serious infection, whereas other studies used various terms such as serious infection event, individual severe adverse event, infectious severe adverse event, serious infectious adverse event, and hospitalization due to infection (see Supplementary Table S1).
For outcomes that were able to be assessed by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria, the overall quality of evidence was found to be either low or very low. This was due to either very serious imprecision and/or serious risk of bias.
Regarding publication bias, a funnel plot did not show any significant asymmetry for the studies examining biologic therapies versus placebo at 3 or 4 months, and the number of studies was too low for the other outcomes to be evaluated for publication bias in this way.
Sensitivity meta-analyses using Mantel-Haenszel methods for both fixed- and random-effects models did not influence the conclusions of any comparisons.
Evidence from RCTs: risk of serious infection with biologic therapies compared with placebo in adults
At 12–16 weeks
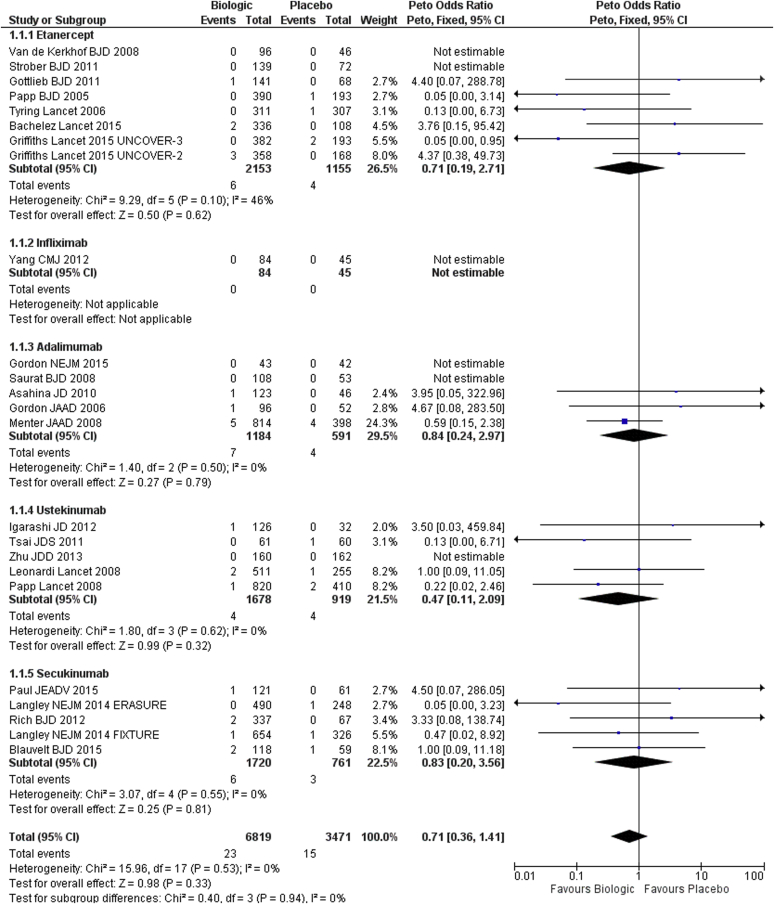
There were 24 placebo-controlled trials across the different biologic therapies reporting a serious infection event rate of 0.4% in the placebo arm and 0.3% in the biologics arm at 12–16 weeks (Figure 2). No significant heterogeneity was found across the different biologic therapies (I2 = 46% for etanercept, I2 = 0% for all other biologic therapies and overall).
Figure 2.
Forest plot for dose-independent comparison between biologic therapies and placebo. Serious infection at week 12–16 for adults (randomized controlled trials). CI, confidence interval.
The pooled Peto OR for individual biologic therapies did not show any significant differences compared with placebo. Although most earlier studies, especially those of etanercept and adalimumab (6/8 etanercept studies and 4/5 adalimumab studies), were conducted in US or European sites only; later studies varied between inclusion of sites worldwide (4/5 secukinumab studies) and predominantly Asian populations (3/5 ustekinumab studies).
The pooled Peto OR for all biologic therapies versus placebo was 0.71 (95% CI = 0.36–1.41, I2 = 0%), showing no significant difference in the risk of serious infection (Figure 2).
Only one small study of 129 patients was eligible for comparison of infliximab with placebo, with the outcome measured at 10 weeks and with very-low-quality evidence (Yang et al., 2012).
The quality of the evidence across the studies ranged from low for studies involving etanercept (Bachelez et al., 2015, Gottlieb et al., 2011, Griffiths et al., 2015, Papp et al., 2005, Strober et al., 2011, Tyring et al., 2006, van de Kerkhof et al., 2008) and secukinumab (Blauvelt et al., 2015, Langley et al., 2014, Paul et al., 2015, Rich et al., 2013) to very low for studies involving infliximab (Yang et al., 2012), adalimumab (Asahina et al., 2010, Gordon et al., 2015, Gordon et al., 2006, Menter et al., 2008, Saurat et al., 2008), and ustekinumab (Igarashi et al., 2012, Leonardi et al., 2008, Papp et al., 2008, Tsai et al., 2011, Zhu et al., 2013) (Supplementary Table S5 online).
At 20–30 weeks
There were four placebo-controlled trials reporting a serious infection event rate of 0.4% in the placebo arm and 0.9% in the biologics arm between 20 and 30 weeks. One study evaluated etanercept (Gottlieb et al., 2003), two studies evaluated infliximab (Gottlieb et al., 2004, Reich et al., 2005), and one study evaluated ustekinumab (Krueger et al., 2007). The pooled Peto OR for all four studies was 2.27 (95% CI = 0.45–11.49, I2 = 0%), and none of the individual ORs were statistically significant, with a low quality of evidence overall (see Supplementary Figure S1 and Supplementary Table S6 online).
Evidence from RCTs: risk of serious infection with biologic therapies compared with nonbiologic therapy and between biologic therapies in adults
Biologic therapy versus nonbiologic therapy (methotrexate) at 12–16 weeks
Only one study evaluated a biologic therapy (adalimumab) (n = 108) versus methotrexate (n = 110) (Saurat et al., 2008). No serious infection events were reported in any of the treatment arms (Supplementary Table S7 online).
Between biologic therapies at 12–16 weeks and at 1 year
The comparisons eligible for inclusion were ustekinumab versus etanercept (Griffiths et al., 2010) (OR = 2.19, 95% CI = 0.36–13.31, low-quality evidence) and secukinumab versus etanercept at week 12 (Langley et al., 2014) (OR = 4.47, 95% CI = 0.07–286.66, low-quality evidence), secukinumab versus ustekinumab (Thaci et al., 2015) at week 16 (OR = 0.52, 95% CI = 0.05–4.97, very-low-quality evidence), and secukinumab versus etanercept at 1 year (Langley et al., 2014) (OR = 1.00, 95% CI = 0.30–3.34, low-quality evidence) (Supplementary Tables S8 and S9 online).
Evidence from RCTs: risk of serious infection with biologic therapy compared with nonbiologic therapy and between biologic therapies in children
At 12–16 weeks
One study assessed patients with psoriasis from 4–17 years of age receiving etanercept (Paller et al., 2008), and another study assessed patients from 12–17 years of age receiving ustekinumab (Landells et al., 2015). The studies evaluated a total of 321 patients, and did not report any serious infections in either the treatment or the placebo arm (Supplementary Table S10 online).
Evidence from prospective cohort studies: risk of serious infection with biologic therapies compared with nonbiologic therapies in adults
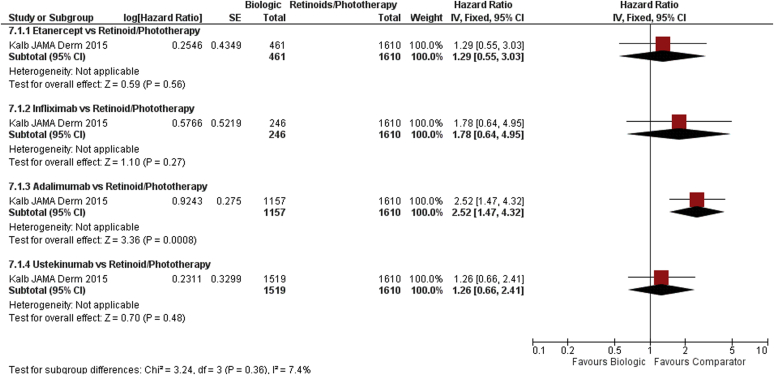
One prospective observational registry published adjusted comparisons for risk of serious infection between biologic therapies and nonbiologic systematic therapies (Kalb et al., 2015). This study reported adjHRs in a Cox proportional hazard model for time-to-event data, taking into account differences in the population including key potential confounders such as age, sex, ethnicity, body mass index, Physician’s Global Assessment score at baseline, and history of significant infection. For the incident population (population starting a biologic therapy while registered), adalimumab was shown to have an increased risk of serious infection compared with a population who received systemic retinoids and/or psoralen plus UVA/UVB (adjHR = 2.52, 95% CI = 1.47–4.34), with a low quality of evidence (Figure 3). Ustekinumab (adjHR 1.26, 95% CI = 0.66–2.42), infliximab (adjHR = 1.78, 95% CI = 0.64–4.98), and etanercept (adjHR = 1.29, 95% CI = 0.55–3.01) did not show any significant difference of serious infection risk compared with the same population (very-low-quality evidence) (Supplementary Table S11 online).
Figure 3.
Forest plot for dose-independent comparison between biologic therapies and retinoid therapy/phototherapy. Serious infection in adults (cohort studies). CI, confidence interval; IV, inverse variance; SE, standard error.
Discussion
This systematic review and meta-analysis provides an up-to-date synthesis of the published evidence regarding the risk of serious infection for biologic therapies in the treatment of psoriasis, and to our knowledge represents the only review of the risk of serious infection of ustekinumab and secukinumab to date.
None of the biologic therapies showed a significantly higher risk of serious infection compared with placebo in RCTs. In a worst-case scenario, taking the upper 95% CI for the pooled Peto OR from RCT data, it is unlikely that the odds of having an episode of serious infection in the first 3–4 months of treatment with biologic therapies overall are higher than 1.4 times the background odds (i.e., 2 more cases of serious infection per 1,000 patients treated).
The cohort study reported here showed a different result, reporting that adalimumab had a significantly higher risk of serious infection at 2.5 times the risk of retinoids and/or phototherapy. Several factors may explain the difference in the results between the RCT meta-analysis and the cohort study data. The population at risk in the clinical trials was different from the real-world population—for example, 13 RCTs reported the exclusion of patients who have had a serious infection. This may be important, because a history of “significant infections” before registry entry was an independent predictor of serious infections (adjHR = 1.67; 95% CI = 1.28–2.18) in the multivariate analysis performed by Kalb et al. (2015). The external validity of safety outcome results from clinical trials is limited by the varied ineligibility criteria (Garcia-Doval et al., 2012), with one study estimating that approximately 30% of patients in a real-world clinic are ineligible for enrolment in RCTs.
The cohort study used a time-to-event analysis. We were unable to perform this meta-analysis for the RCT data and adjust for person-year of follow-up because we did not have the individual patient data available.
There is varied nomenclature describing the outcome of serious infection, with poor reporting of the definition in the method section of trial reports. In addition, observational studies may have different methods of classifying the serious infection outcome; for example, the cohort study reviewed here included any infections associated with a life-threatening condition or another medically important condition (Kalb et al., 2015) (see Supplementary Table S4). This approach may capture more events than definitions used by clinical trials, which equate severe infectious adverse events with those requiring hospitalization directly. In addition, it is often unclear whether RCTs pursued an active or passive monitoring strategy, and combined with a lack of publically available individual patient data there is a possibility of underreporting and, therefore, misclassification of serious infections in RCTs.
Most of the trials limited the placebo-controlled period to 3–4 months’ duration. In a rheumatoid arthritis population, the risk of serious infections was increased in the first 6 months of treatment with tumor necrosis factor inhibitor therapy (Galloway et al., 2011). Thus, analysis at 4 months or less has the potential to miss a significant effect of biologic therapies on serious infection risk 5–6 months after initiation of therapy. In comparison, the average follow-up period in the cohort study was between 1 and 2 years.
These differences in study design between RCTs and observational studies likely account for the reason that RCTs reported fewer serious infection events in total (n = 54) compared with the one cohort study reported here (n = 323).
The limited number of head-to-head trials, or trials comparing biologic therapies with a traditional systemic therapy, restricted our ability to analyze the comparative risk of biologic therapies. The imprecision of the meta-analyzed estimates (Figure 2 and see Supplementary Figures S1–S3 online) showed that the study is underpowered to make a confident estimate of the true risk of serious infection for biologic therapies based on RCT data. Similarly, there were only two published trials for patients under the age of 18 years, and they did not report any serious infection events in either arm.
By comparison, a recent systematic review of serious infection risk in patients with rheumatoid arthritis receiving biologic therapies analyzed more than 3 times the number of participants compared with the current review, reporting 10 times the number of serious infection events in total (Singh et al., 2015). However, there are differences between this rheumatoid arthritis population and psoriasis populations, including age, sex, and disease pathogenesis. In addition, RCTs in patients with rheumatoid arthritis often allow co-therapy with other oral immune suppressants (which is not allowed in psoriasis RCTs), and patients with psoriasis are less likely to be affected by mobility issues or be treated with concomitant intra-articular or enteral corticosteroid therapy. Thus, rheumatoid arthritis patients have a higher baseline infection risk, and this may partially account for the lower event rates seen in psoriasis RCTs.
The serious infection safety profile from the meta-analysis of RCT data here is in keeping with the findings of two previous systematic reviews and meta-analyses (Dommasch et al., 2011, Nast et al., 2015), one of which investigated adverse events rather than serious infection specifically (Nast et al., 2015). These studies likely share the same limitations as the current study in terms of number of events and sample size, duration of treatment, and exclusion criteria listed above.
Observational data are also likely to be limited by factors such as residual confounding. Different thresholds for hospitalization of patients among the 301 centers from 16 different countries (e.g., between predominantly privately funded vs. publicly funded health systems) may lead to outcome misclassification in patients from the cohort study (Kimball et al., 2014). It is unclear whether this misclassification would be differential, because the relative prevalence for each prescribed therapy is not given for each country. However, it is probable that this type of misclassification would affect multinational, multicenter RCTs to the same extent.
The selection of a close comparator cohort is important, but some baseline differences between the two cohorts were not provided in the study by Kalb et al. (2015) (e.g., psoriasis area and severity index, number of previous treatments). The main analysis used a prevalent population, that is, inclusive of patients who had started a biologic therapy before registration. This presents a problem of left truncation: those who have had the event of interest before registration are not captured in the analysis, introducing selection bias favoring the interventions. This is especially pertinent given the probability that the risk of serious infection may follow a time-dependent trajectory of a higher risk in the first 6 months. The use of the adjHRs for the incident population in this review may help take this into account (Yoshida et al., 2015).
Data were particularly scarce for the assessment of the risk of serious infections for infliximab. Three eligible trials were examined, and the incident population cohort reported by Kalb et al. (2015) for infliximab included 246 patients and 324 patient-years only.
Conclusions
In summary, no increased short-term risk of serious infection was identified in adults with psoriasis who were eligible for enrolment in the RCTs. No differential serious infection risk between the biologic therapies was detected where head-to-head RCT data were available. Data from a real-world population, however, suggest that adalimumab is associated with a higher risk of serious infections compared with acitretin and/or phototherapy in adults.
The results from the RCTs should be interpreted with caution given limitations, which include the lack of long-term data, the differences among the characteristics of the study population compared with the target population of patients in real-world settings, and the unclear reporting of outcome measures of serious infections in RCTs.
Other limitations include a low event rate, the lack of data informing the serious infection risk of infliximab, and the lack of comparative data either between biologic therapies or against traditional systemic therapies. Future research priority should focus on standardization of the nomenclature, definition, and reporting of the outcome for serious infection in RCTs. Strict adherence of the extension of the CONSORT statement to report harms in clinical trials will help make the definition, ascertainment, and statistical evaluation of adverse events more transparent (Ioannidis et al., 2004). Future RCTs should conduct head-to-head comparisons between biologic therapies above and beyond 6 months. More RCTs should be conducted in children from different populations. Individual patient data and trial protocols from the RCT should be shared after publication for replication of results and to facilitate time-to-event meta-analysis (Taichman et al., 2016).
Analysis of further adequately powered registry data with adequate adjustment for potential confounding should be performed to clarify the risk of serious infection of biologic therapies against a suitable comparator cohort. This may inform the current uncertainty about the risk of serious infection when biologic therapies are used in patients with psoriasis. Clinicians should encourage their patients to enroll in prospective pharmacovigilance registries and should remain vigilant for serious infections in patients receiving biologic therapies for psoriasis.
Materials and Methods
The current systematic review and meta-analysis was conducted in accordance with the PRISMA statement. The review protocol was registered on the PROSPERO International prospective register of systematic reviews (2015:CRD42015017538).
Predefined search strategy and selection criteria
An a priori protocol was established as follows. The patient population included all people with psoriasis who were being treated primarily for their skin disease. Children (up to 12 years) and young people (12–18 years), people with different psoriasis phenotypes, and people receiving a second biologic were considered in different strata if data were available. Factors such as biologic dosing regimen, methotrexate use, disease severity, skin type and ethnicity, psoriatic arthritis, and body mass index were considered for subgroup analysis if heterogeneity of the results was present. Published studies, including RCTs, systematic reviews, or prospective cohort studies, were considered for inclusion if the intervention consisted of one or more of the following: adalimumab, etanercept, infliximab, ustekinumab, and secukinumab. The comparison arm could consist of any of the listed biologic therapies or of placebo or nonbiologic systemic interventions. Only prospective studies were considered. For observational studies, only studies that presented adjusted estimates (e.g., adjHRs) were eligible. The outcome was the occurrence of a serious infection episode at time points reported at the end of the placebo- or comparator-controlled period; serious infection was defined by the investigator.
Studies were excluded if there were fewer than 50 participants or if there were fewer than 25 participants in each intervention arm. Studies involving indirect populations were excluded, with populations involving a treated proportion for psoriatic arthritis of greater than 50% considered indirect.
The systematic literature search was conducted in the PubMed, Medline, Embase, and Cochrane databases from inception up to September 29, 2015, with the results de-duplicated, titles reviewed, and irrelevant studies excluded (LE). The search terms and strategy are presented in the Supplementary Materials, section S1, online. All studies reported in a language other than English were excluded.
Titles and abstracts of studies were screened in a two-step process, initially by two assessors (ZY and ZJL), with any disagreement reviewed by a third assessor (CS). The full-text articles were obtained, read, and rechecked against the protocol, with those that did not meet it excluded (LE). Systematic reviews and meta-analyses were screened for additional studies (LE). The RCTs and identified cohort studies were distributed among the coauthors for detailed appraisal and extraction of data using a standardized data extraction tool. For the studies that did not report serious infection as a main outcome, the relevant pharmaceutical company and/or the lead author for the published study was contacted. Data were provided for the following referenced studies in this way: Griffiths et al., 2015, Langley et al., 2014; and Thaci et al., 2015. The data extraction and appraisal were then repeated by one assessor for all eligible articles (ZY). The methodological quality of each study, including the risk of bias of individual studies, was assessed using checklists from National Institute for Health and Care Excellence (United Kingdom) and the National Clinical Guideline Centre, and the quality of the evidence for outcomes across studies was assessed by the GRADE criteria.
Data analysis and quality assessment of evidence
The meta-analysis was performed using Peto’s method with a fixed effects model to give an estimate for the pooled OR from the individual studies (Review Manager 5.3, The Nordic Cochrane Centre, The Cochrane Collaboration, 2014, Copenhagen), because it has been shown that this method gives the least-biased results and is preferable when the events are rare (Bradburn et al., 2007). Under Peto’s method, studies were dropped from the pooled analysis if they reported no events in either arm. In situations where in one cell there were zero cases, 0.5 was added to all four cells of a 2×2 table. A sensitivity analysis was performed using the Mantel-Haenszel risk ratios to add robustness to the results.
Heterogeneity was assessed using the I2 test. Selection bias, lack of blinding, attrition bias, and measurement and outcome reporting bias were assessed using the National Institute for Health and Care Excellence checklists for individual studies. The GRADE assessment profile table was generated using software from the Cochrane Collaboration (GRADEpro 3.6, The Nordic Cochrane Centre, The Cochrane Collaboration, 2014, Copenhagen). The quality of the evidence for each comparison across studies was classed as very low, low, moderate, and high based on an assessment of the risk of bias, inconsistency, indirectness of evidence, imprecision, and publication or reporting bias. Based on the GRADE criteria, evidence for observational studies is downgraded to start with a low quality. This can be graded upward if there are no reasons for downgrading because of the described assessment and if the magnitude of the treatment effect is large, there is evidence of a dose-response relationship, and if all plausible confounders would have ordinarily decreased the magnitude of an apparent treatment effect.
ORCID
Zenas Z. N. Yiu: http://orcid.org/0000-0002-1831-074X
Conflict of Interest
ADB consults and lectures for Abbvie, Amgen, Novartis, Pfizer, Celgene, Janssen, and Boehringer Ingelheim. RM has served on advisory boards and/or has been a speaker for AbbVie, Janssen, Pfizer, and Novartis. DMA has received grant funding from the National Institute for Health Research (NIHR) and grant funding from Abbvie and has served on advisory boards for Pfizer and GlaxoSmithKline. CEMG has received honoraria and/or research grants from Abbvie, Actelion, Amgen, Celgene, Lilly, GlaxoSmithKline-Stiefel, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, and Sandoz. RBW has acted as a consultant and/or speaker and/or has received research grants for Abbvie, Amgen, Celgene, Boehringer, Eli Lilly, Medac, Pfizer, Leo, Novartis, Xenoport, and Janssen. ZZNY, LSE, ZJL, MFMM, EJS, CMO, RP, VV, and CHS report no conflict of interest.
Acknowledgments
ZZNY is funded by a National Institute for Health Research (NIHR) Doctoral Research Fellowship (ref no: DRF-2015-08-089). The research was supported by the NIHR Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the author and not necessarily those of the National Health Service, the NIHR, or the Department of Health. CEMG is an NIHR Senior Investigator.
This systematic review and meta-analysis was supported by the British Association of Dermatologists to inform the next update to the guidelines for biologic interventions for psoriasis. The authors would like to acknowledge the funding made available by the British Association of Dermatologists for the consultancy work provided by the National Clinical Guideline Centre. The authors are grateful to Dr. Victoria R. Cornelius for her review of the manuscript and her helpful comments.
This paper has been registered with the PROSPERO database at http://www.crd.york.ac.uk/PROSPERO/ (PROSPERO number 2015:CRD42015017538).
accepted manuscript published online 13 April 2016; corrected proof published online 14 June 2016
Footnotes
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at http://dx.doi.org/10.1016/j.jid.2016.03.035.
Contributor Information
Catherine H. Smith, Email: catherine.smith@kcl.ac.uk.
Richard B. Warren, Email: richard.warren@manchester.ac.uk.
Supplementary Material
References
- Asahina A., Nakagawa H., Etoh T., Ohtsuki M., Adalimumab M.S.G. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a Phase II/III randomized controlled study. J Dermatol. 2010;37:299–310. doi: 10.1111/j.1346-8138.2009.00748.x. [DOI] [PubMed] [Google Scholar]
- Bachelez H., van de Kerkhof P.C., Strohal R., Kubanov A., Valenzuela F., Lee J.H. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet. 2015;386:552–561. doi: 10.1016/S0140-6736(14)62113-9. [DOI] [PubMed] [Google Scholar]
- Blauvelt A., Prinz J.C., Gottlieb A.B., Kingo K., Sofen H., Ruer-Mulard M. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE) Br J Dermatol. 2015;172:484–493. doi: 10.1111/bjd.13348. [DOI] [PubMed] [Google Scholar]
- Bradburn M.J., Deeks J.J., Berlin J.A., Russell Localio A. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med. 2007;26:53–77. doi: 10.1002/sim.2528. [DOI] [PubMed] [Google Scholar]
- Dommasch E.D., Abuabara K., Shin D.B., Nguyen J., Troxel A.B., Gelfand J.M. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035–1050. doi: 10.1016/j.jaad.2010.09.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway J.B., Hyrich K.L., Mercer L.K., Dixon W.G., Fu B., Ustianowski A.P. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology (Oxford) 2011;50:124–131. doi: 10.1093/rheumatology/keq242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Doval I., Carretero G., Vanaclocha F., Ferrandiz C., Dauden E., Sanchez-Carazo J.L. Risk of serious adverse events associated with biologic and nonbiologic psoriasis systemic therapy: patients ineligible vs eligible for randomized controlled trials. Arch Dermatol. 2012;148:463–470. doi: 10.1001/archdermatol.2011.2768. [DOI] [PubMed] [Google Scholar]
- Garcia-Doval I., Rustenbach S.J., Stern R., Dam T.N., Cohen A.D., Baker C. Systemic psoriasis therapy shows high between-country variation: a sign of unwarranted variation? Cross-sectional analysis of baseline data from the PSONET registries. Br J Dermatol. 2013;169:710–714. doi: 10.1111/bjd.12344. [DOI] [PubMed] [Google Scholar]
- Gordon K.B., Duffin K.C., Bissonnette R., Prinz J.C., Wasfi Y., Li S. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136–144. doi: 10.1056/NEJMoa1501646. [DOI] [PubMed] [Google Scholar]
- Gordon K.B., Langley R.G., Leonardi C., Toth D., Menter M.A., Kang S. Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label extension study. J Am Acad Dermatol. 2006;55:598–606. doi: 10.1016/j.jaad.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Gottlieb A.B., Evans R., Li S., Dooley L.T., Guzzo C.A., Baker D. Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2004;51:534–542. doi: 10.1016/j.jaad.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Gottlieb A.B., Leonardi C., Kerdel F., Mehlis S., Olds M., Williams D.A. Efficacy and safety of briakinumab vs. etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. 2011;165:652–660. doi: 10.1111/j.1365-2133.2011.10418.x. [DOI] [PubMed] [Google Scholar]
- Gottlieb A.B., Matheson R.T., Lowe N., Krueger G.G., Kang S., Goffe B.S. A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol. 2003;139:1627–1632. doi: 10.1001/archderm.139.12.1627. [DOI] [PubMed] [Google Scholar]
- Griffiths C.E., Reich K., Lebwohl M., van de Kerkhof P., Paul C., Menter A. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541–551. doi: 10.1016/S0140-6736(15)60125-8. [DOI] [PubMed] [Google Scholar]
- Griffiths C.E., Strober B.E., van de Kerkhof P., Ho V., Fidelus-Gort R., Yeilding N. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118–128. doi: 10.1056/NEJMoa0810652. [DOI] [PubMed] [Google Scholar]
- Igarashi A., Kato T., Kato M., Song M., Nakagawa H. Japanese Ustekinumab Study G. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39:242–252. doi: 10.1111/j.1346-8138.2011.01347.x. [DOI] [PubMed] [Google Scholar]
- Ioannidis J.P., Evans S.J., Gotzsche P.C., O'Neill R.T., Altman D.G., Schulz K. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med. 2004;141:781–788. doi: 10.7326/0003-4819-141-10-200411160-00009. [DOI] [PubMed] [Google Scholar]
- Iskandar I.Y., Ashcroft D.M., Warren R.B., Yiu Z.Z., McElhone K., Lunt M. Demographics and disease characteristics of patients with psoriasis enrolled in the British Association of Dermatologists Biologic Interventions Register. Br J Dermatol. 2015;173:510–518. doi: 10.1111/bjd.13908. [DOI] [PubMed] [Google Scholar]
- Kalb R.E., Fiorentino D.F., Lebwohl M.G., Toole J., Poulin Y., Cohen A.D. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR) JAMA Dermatol. 2015;151:961–969. doi: 10.1001/jamadermatol.2015.0718. [DOI] [PubMed] [Google Scholar]
- Kimball A.B., Leonardi C., Stahle M., Gulliver W., Chevrier M., Fakharzadeh S. Demography, baseline disease characteristics and treatment history of patients with psoriasis enrolled in a multicentre, prospective, disease-based registry (PSOLAR) Br J Dermatol. 2014;171:137–147. doi: 10.1111/bjd.13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger G.G., Langley R.G., Leonardi C., Yeilding N., Guzzo C., Wang Y. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580–592. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- Landells I., Marano C., Hsu M.C., Li S., Zhu Y. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J Am Acad Dermatol. 2015;73:594–603. doi: 10.1016/j.jaad.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Langley R.G., Elewski B.E., Lebwohl M., Reich K., Griffiths C.E., Papp K. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371:326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- Leonardi C.L., Kimball A.B., Papp K.A., Yeilding N., Guzzo C., Wang Y. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- Menter A., Tyring S.K., Gordon K., Kimball A.B., Leonardi C.L., Langley R.G. Adalimumab therapy for moderate to severe psoriasis: A randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58:106–115. doi: 10.1016/j.jaad.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Nast A., Jacobs A., Rosumeck S., Werner R.N. Efficacy and safety of systemic long-term treatments for moderate-to-severe psoriasis: a systematic review and meta-analysis. J Invest Dermatol. 2015;135:2641–2648. doi: 10.1038/jid.2015.206. [DOI] [PubMed] [Google Scholar]
- Paller A.S., Siegfried E.C., Langley R.G., Gottlieb A.B., Pariser D. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358:241–251. doi: 10.1056/NEJMoa066886. [DOI] [PubMed] [Google Scholar]
- Papp K.A., Langley R.G., Lebwohl M., Krueger G.G., Szapary P., Yeilding N. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371:1675–1684. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- Papp K.A., Tyring S., Lahfa M., Prinz J., Griffiths C.E., Nakanishi A.M. A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol. 2005;152:1304–1312. doi: 10.1111/j.1365-2133.2005.06688.x. [DOI] [PubMed] [Google Scholar]
- Paul C., Lacour J.P., Tedremets L., Kreutzer K., Jazayeri S., Adams S. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE) J Eur Acad Dermatol Venereol. 2015;29:1082–1090. doi: 10.1111/jdv.12751. [DOI] [PubMed] [Google Scholar]
- Puel A., Picard C., Cypowyj S., Lilic D., Abel L., Casanova J.L. Inborn errors of mucocutaneous immunity to Candida albicans in humans: a role for IL-17 cytokines? Curr Opin Immunol. 2010;22:467–474. doi: 10.1016/j.coi.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich K., Nestle F.O., Papp K., Ortonne J.P., Evans R., Guzzo C. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366:1367–1374. doi: 10.1016/S0140-6736(05)67566-6. [DOI] [PubMed] [Google Scholar]
- Rich P., Sigurgeirsson B., Thaci D., Ortonne J.P., Paul C., Schopf R.E. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013;168:402–411. doi: 10.1111/bjd.12112. [DOI] [PubMed] [Google Scholar]
- Rychly D.J., DiPiro J.T. Infections associated with tumor necrosis factor-alpha antagonists. Pharmacotherapy. 2005;25:1181–1192. doi: 10.1592/phco.2005.25.9.1181. [DOI] [PubMed] [Google Scholar]
- Saurat J.H., Stingl G., Dubertret L., Papp K., Langley R.G., Ortonne J.P. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION) Br J Dermatol. 2008;158:558–566. doi: 10.1111/j.1365-2133.2007.08315.x. [DOI] [PubMed] [Google Scholar]
- Singh J.A., Cameron C., Noorbaloochi S., Cullis T., Tucker M., Christensen R. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Lancet. 2015;386:258–265. doi: 10.1016/S0140-6736(14)61704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober B.E., Crowley J.J., Yamauchi P.S., Olds M., Williams D.A. Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. 2011;165:661–668. doi: 10.1111/j.1365-2133.2011.10419.x. [DOI] [PubMed] [Google Scholar]
- Taichman D.B., Backus J., Baethge C., Bauchner H., de Leeuw P.W., Drazen J.M. Sharing clinical trial data—a proposal from the International Committee of Medical Journal Editors. N Engl J Med. 2016;374:384–386. doi: 10.1056/NEJMe1515172. [DOI] [PubMed] [Google Scholar]
- Thaci D., Blauvelt A., Reich K., Tsai T.F., Vanaclocha F., Kingo K. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400–409. doi: 10.1016/j.jaad.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Tsai T.F., Ho J.C., Song M., Szapary P., Guzzo C., Shen Y.K. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL) J Dermatol Sci. 2011;63:154–163. doi: 10.1016/j.jdermsci.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Tyring S., Gottlieb A., Papp K., Gordon K., Leonardi C., Wang A. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- van de Kerkhof P.C., Segaert S., Lahfa M., Luger T.A., Karolyi Z., Kaszuba A. Once weekly administration of etanercept 50 mg is efficacious and well tolerated in patients with moderate-to-severe plaque psoriasis: a randomized controlled trial with open-label extension. Br J Dermatol. 2008;159:1177–1185. doi: 10.1111/j.1365-2133.2008.08771.x. [DOI] [PubMed] [Google Scholar]
- Watford W.T., Hissong B.D., Bream J.H., Kanno Y., Muul L., O'Shea J.J. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev. 2004;202:139–156. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- Yang H.Z., Wang K., Jin H.Z., Gao T.W., Xiao S.X., Xu J.H. Infliximab monotherapy for Chinese patients with moderate to severe plaque psoriasis: a randomized, double-blind, placebo-controlled multicenter trial. Chin Med J (Engl) 2012;125:1845–1851. [PubMed] [Google Scholar]
- Yoshida K., Solomon D.H., Kim S.C. Active-comparator design and new-user design in observational studies. Nat Rev Rheumatol. 2015;11:437–441. doi: 10.1038/nrrheum.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Zheng M., Song M., Shen Y.K., Chan D., Szapary P.O. Efficacy and safety of ustekinumab in Chinese patients with moderate to severe plaque-type psoriasis: results from a phase 3 clinical trial (LOTUS) J Drugs Dermatol. 2013;12:166–174. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.