SUMMARY
The emergence of invasive fungal wound infections (IFIs) in combat casualties led to development of a combat trauma-specific IFI case definition and classification. Prospective data were collected from 1133 US military personnel injured in Afghanistan (June 2009–August 2011). The IFI rates ranged from 0·2% to 11·7% among ward and intensive care unit admissions, respectively (6·8% overall). Seventy-seven IFI cases were classified as proven/probable (n = 54) and possible/unclassifiable (n = 23) and compared in a case-case analysis. There was no difference in clinical characteristics between the proven/probable and possible/unclassifiable cases. Possible IFI cases had shorter time to diagnosis (P = 0·02) and initiation of antifungal therapy (P = 0·05) and fewer operative visits (P = 0·002) compared to proven/probable cases, but clinical outcomes were similar between the groups. Although the trauma-related IFI classification scheme did not provide prognostic information, it is an effective tool for clinical and epidemiological surveillance and research.
Key words: Epidemiology, fungi – infections due to, surveillance
INTRODUCTION
Invasive fungal infections (IFIs), such as mucormycosis or aspergillosis, generally develop in immunocompromised patients with the potential of resulting in substantial morbidity, and also considerable mortality (rates range as high as 80% and may exceed 90% with dissemination) [1–4]. Less is known about locally invasive IFIs in immunocompetent individuals who sustain traumatic injuries as the available data are limited to a small number of case reports or series [5–13]. Even fewer studies are available related to this emerging trauma-related disease in wounded military personnel [14–17].
In early 2011, an IFI case investigation among combat-injured US military personnel medically evacuated from Afghanistan (June 2009–December 2010) to Landstuhl Regional Medical Center (LRMC), which is located in Germany and provides trauma care prior to transfer to the USA, was conducted and reported an IFI rate of 3·5% in the fourth quarter of 2010 among trauma admissions [17]. Commonly cited IFI case definitions (proven, probable, possible) from the European Organization for Research and Treatment of Cancer/IFI Cooperative Group and the National Institute of Allergy and Infectious Disease Mycoses Study Group (EORTC/MSG) Consensus Group [18] were reviewed for the case investigation. However, the EORTC/MSG IFI case definitions and diagnostic criteria were targeted for immunocompromised patients without traumatic injuries; thus, the case definitions were revised to reflect traumatic injury as the underlying risk factor for IFI. The result was a combat trauma-specific IFI classification scheme [17]. This scheme was used for the initial case investigation [17]; however, due to the small sample size (37 patients including only four probable cases), the previous report focused on a description of the clinical presentation and management of combat-related IFI and only a limited comparison of the various classes of IFI was briefly discussed. In order to better assess the utility of the modified IFI case definition and classification, we reviewed data from an expanded case series of medically evacuated US combat casualties diagnosed with IFIs and conducted a case-case analysis [histopathology confirmed proven/probable (P/P) vs. possible/unclassifiable (P/U) cases] with the objective of determining if there were any clinically significant differences in presentation or outcomes between the different IFI classes.
METHODS
Study population
Prospective data were collected from US military personnel with combat-related injuries (Fig. 1; 1 June 2009–31 August 2011), medically evacuated from Afghanistan (Operation Enduring Freedom) to LRMC in Germany, then transferred to one of three US tertiary-care military treatment facilities (MTFs): Walter Reed Army Medical Center, Washington DC, National Naval Medical Center, Bethesda, MD, and San Antonio Military Medical Center, San Antonio, TX, as described previously [19]. Patient demographics, clinical and trauma history, injury patterns, surgical management, and treatment data were obtained through the Department of Defense Trauma Registry (formerly the Joint Theater Trauma Registry) [20]. Clinical outcome variables for evaluation of the classification scheme included total days hospitalization, intensive care unit (ICU) length of stay, time to IFI diagnosis, time to administration of first antifungal agent, and mortality rate.
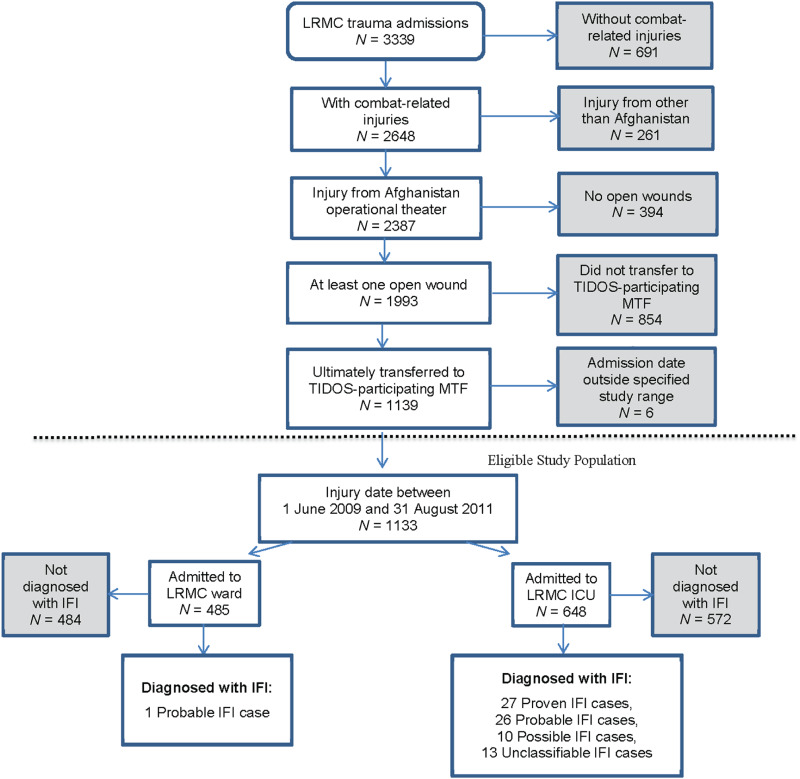
Fig. 1.
[colour online]. Flow diagram showing the eligible study population and resulting invasive fungal infection (IFI) cases from the total number of Landstuhl Regional Medical Center (LRMC) trauma admissions. Factors triggering review of data for IFI diagnosis were wound culture with fungal growth, pathology with fungal elements, or administration of ⩾2 days of antifungal agents. Confirmation of IFI diagnosis required recurrent wound tissue necrosis following two or more surgical debridements in addition to the presence of tissue invasion with fungal hyphae angioinvasion (proven classification), histopathological fungal elements (probable classification), and/or fungal growth on culture (possible classification). Unclassifiable IFIs are cases with fungal culture evidence, but histopathology was not sent for evaluation; therefore, they are not able to be specified to a specific classification on the basis of the available evidence. ICU, Intensive care unit; MTF, military treatment facility; TIDOS, Trauma Infectious Disease Outcomes Study.
IFI case identification, definitions, and investigation
Cases were identified and classified for analysis after reviewing the Trauma Infectious Disease Outcomes Study database [19] for positive fungal wound cultures, histology, and antifungal therapy during the period of interest. Case data from the infectious disease and trauma surgery services were also evaluated. The identified IFI cases include the 37 cases detailed in the original case series [17] as well as 40 additional cases. This study was approved by the Infectious Disease Institutional (Ethical) Review Board of the Uniformed Services University of the Health Sciences, Bethesda, Maryland.
The diagnostic criteria required a traumatic wound with recurrent tissue necrosis following at least two surgical debridements, with either evidence of tissue invasive mould infection on histopathology or mould growth from tissue. A proven IFI case was confirmed by angioinvasive fungal elements on histopathology, whereas a probable IFI case had fungal elements identified on histopathology without angioinvasion (all histopathology specimens were reviewed by two surgical pathologists). While fungal cultures were performed for all suspected cases, classification as proven or probable was independent of culture results. A possible IFI described all cases in which wound tissue grew mould; however, histopathology was negative for fungal elements. Unclassifiable IFI cases had wound tissue which grew mould, meeting the definition of a possible case, but histopathology was not sent for evaluation; therefore, these cases could not be specifically classified.
Statistical analysis
P/P cases were compared to the P/U cases in a case-case analysis. Descriptive data from the negative histopathology possible cases (no evidence of fungal elements on histology) and absent histopathology unclassifiable cases (tissue specimens not sent for histopathological analysis) are also presented in tabular format for discussion purposes. χ2 and Fisher's exact tests were used to test the difference between the categorical variables by IFI classification. Non-parametric tests (Wilcoxon Rank Sum and t tests) were used to compare the overall distribution of continuous variables between the groups. Statistical analysis was conducted using SAS v. 9.3 (SAS Institute Inc., USA) and R v. 2.13.2 (R Project for Statistical Computing, Austria). Significance was defined as P<0·05.
RESULTS
Demographic information and injury patterns
From 1133 eligible combat casualties (Fig. 1), 77 patients met the IFI case definition leading to an IFI incidence rate of 6·8% with 27 (35%) categorized as proven, 27 (35%) as probable, and 23 (30%) as P/U. Among patients admitted to the ICU at LRMC, the IFI rate was 11·7%, while it was 0·2% for patients admitted to the ward at LRMC. Nearly all (99%) of the 77 IFI patients were injured in Helmand or Kandahar provinces of southern Afghanistan (Table 1). All cases were men with a median age of 23 years for the P/P group and 24 years for the P/U group. Blast injury (>90%) sustained while dismounted (>78%) was the primary mode of injury. Among the 77 IFI cases, 65% sustained isolated lower extremity amputations, 1·3% had isolated upper extremity amputations, and 13% experienced both upper and lower extremity amputations.
Table 1.
Demographic characteristics and injury circumstances of combat-injured US service members (2009–2011) by invasive fungal infection case classification
| Characteristics | Proven (N = 27) | Probable (N = 27) | Proven/probable (N = 54) | Possible/unclassifiable (N = 23) | Possible: negative histopathology* (N = 10) | Unclassifiable: no histopathology† (N = 13) |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, years, median (IQR) | 22·6 (21·6–28·7) | 22·4 (21·7–26·6) | 22·6 (21·7–26·9) | 24·2 (21·9–25·4) | 24·3 (23·4–25·1) | 22·7 (20·7–26·5) |
| Enlisted, n (%) | 27 (100) | 24 (88·9) | 51 (94·4) | 21 (91·3) | 9 (90·0) | 12 (92·3) |
| Marine, n (%) | 20 (74·1) | 23 (85·2) | 43 (79·6) | 15 (65·2) | 9 (90·0) | 6 (46·2) |
| Army, n (%) | 6 (22·2) | 2 (7·4) | 8 (14·8) | 5 (21·7) | 0 | 5 (38·5) |
| Injury circumstances/severity, n (%) | ||||||
| Blast injury | 27 (100) | 26 (96·3) | 53 (98·1) | 21 (91·3) | 9 (90·0) | 12 (92·3) |
| Injured on foot patrol | 26 (96·3) | 25 (92·6) | 51 (94·4) | 18 (78·3) | 9 (90·0) | 9 (69·2) |
| Amputations, n (%) | ||||||
| Lower extremity | 21 (77·8) | 15 (55·6) | 36 (66·7) | 14 (60·9) | 8 (80·0) | 6 (46·2) |
| Upper extremity | 1 (3·7) | 0 | 1 (1·9) | 0 | 0 | 0 |
| Both upper/lower extremities | 2 (7·4) | 5 (18·5) | 7 (13·0) | 3 (13·0) | 1 (10) | 2 (15·4) |
IQR, Interquartile range.
Tissue specimens sent for histopathological analysis and no fungal elements were reported.
Tissue specimens were not sent for histopathological analysis.
Clinical characteristics
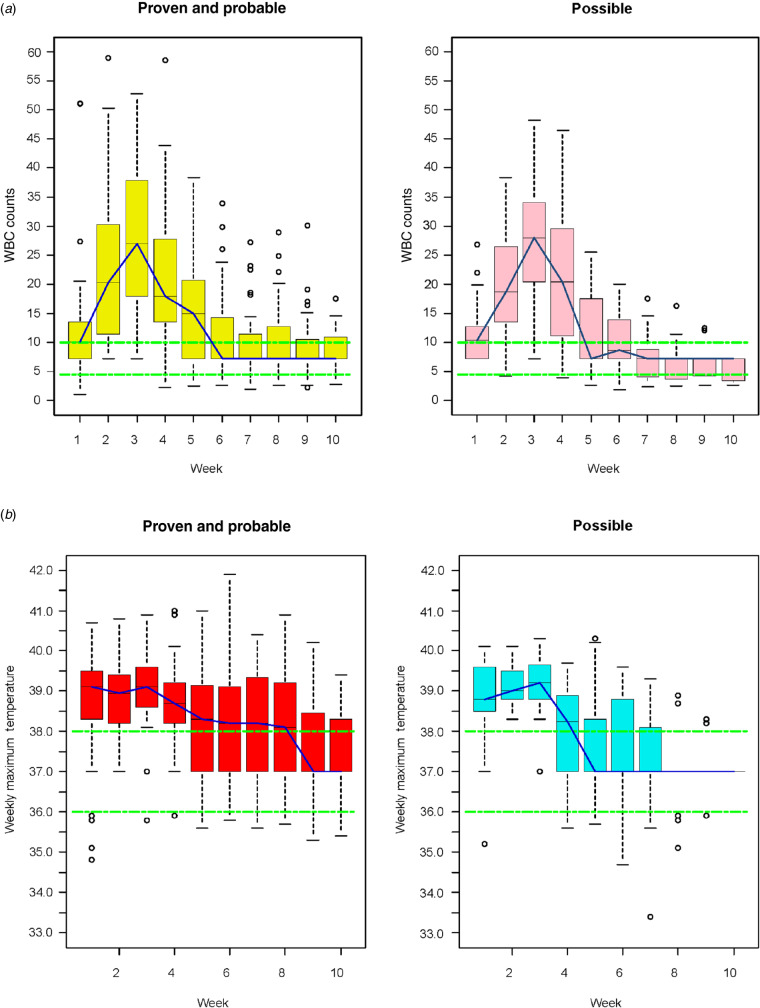
Shock indices recorded on presentation to the first combat support hospital (i.e. heart rate, systolic blood pressure, blood gas) and clinical characteristics at both LRMC and US MTFs were not statistically different between the P/P and P/U groups (Table 2). Large volume blood product transfusions were required for all IFI cases during the first 24 h post-injury and there were no statistical differences between the groups. Injury severity was high in both the P/P and P/U cases. There were no statistical differences among the median admission Sequential Organ Failure Assessment scores determined at LRMC or the US MTFs for the P/U group compared to P/P cases (Table 2). Excluding the unclassifiable cases from the possible group did not reveal any differences in the above clinical characteristics between the P/P cases and the possible cases (data not shown). Moreover, the P/U cases' maximal weekly temperature and white blood cell counts were not statistically different from the P/P group (Fig. 2).
Table 2.
Median (IQR) clinical characteristics by invasive fungal infection case classification among US military personnel injured in combat (2009–2011)
| Clinical parameters | Proven (N = 27) | Probable (N = 27) | Proven/probable (N = 54) | Possible/unclassifiable (N = 23) | Possible: negative histopathology (N = 10)* | Unclassifiable: no histopathology (N = 13)† | P value |
|---|---|---|---|---|---|---|---|
| In-theatre shock indices (combat support hospital) | |||||||
| Heart rate | 134 (118–152) | 114 (97–136) | 127 (101–140) | 119 (107–132) | 121 (104–135) | 119 (109–127) | 0·54 |
| Systolic blood pressure | 100 (80–120) | 99 (75–132) | 100 (80–128) | 97 (65–117) | 96 (65–132) | 97 (81–112) | 0·63 |
| Blood gas – base deficit | 11 (14 to 8) | 6 (10 to 3) | 9 (13 to 4) | 7 (9 to 4) | 6 (11 to 2) | 7 (9 to 5) | 0·40 |
| Blood gas – pH | 7·2 (7·0–7·3) | 7·3 (7·2–7·3) | 7·3 (7·1–7·3) | 7·2 (7·1–7·3) | 7·3 (7·2–7·3) | 7·2 (7·1–7·3) | 0·66 |
| Blood product requirements in-theatre | |||||||
| PRBC | 31 (26–42) | 30 (15–44) | 31 (18–42) | 22 (13–33) | 33 (22–37) | 18 (13–24) | 0·17 |
| Plasma‡ | 31 (26–40) | 24 (15–42) | 30 (18–40) | 22 (13–36) | 30 (20–36) | 18 (12–31) | 0·11 |
| LRMC | |||||||
| White blood cell total (109 cells/l) | 7·5 (5·0–10·0) | 8·1 (6·3–9·6) | 7·9 (5·6–9·8) | 7·6 (6·1–9·3) | 7·1 (5·4–10·9) | 7·9 (7·1–8·4) | 0·89 |
| Blood urea nitrogen (mg/dl) | 14 (9–21) | 12 (8–15) | 13 (8–18) | 12 (10–19) | 16 (12–29) | 10 (6–13) | 0·93 |
| Aspartate aminotransferase (U/l) | 133 (93–210) | 101 (69–218) | 116 (75–217) | 136 (104–234) | 178 (128–301) | 122 (103–142) | 0·28 |
| Alanine aminotransferase (U/l) | 43 (36–67) | 38 (28–63) | 40 (30–65) | 50 (36–75) | 51 (42–82) | 46 (30–63) | 0·31 |
| US MTF | |||||||
| White blood cell total (109 cells/l) | 11·4 (7·8–20·1) | 10·9 (8·8–13·0) | 11·1 (8·4–13·5) | 11·3 (9·4–14·9) | 12·6 (9·5–17·9) | 11·1 (9·4–12·1) | 0·80 |
| Blood urea nitrogen (mg/dl) | 15 (10–23) | 15 (12–18) | 15 (11–20) | 12 (9–18) | 16 (11–40) | 11 (9–15) | 0·27 |
| Aspartate aminotransferase (U/l) | 78 (60–122) | 55 (45–110) | 72 (50–115) | 97 (68–126) | 106 (73–125) | 89 (66–126) | 0·46 |
| Alanine aminotransferase (U/l) | 45 (32–64) | 38 (26–53) | 43 (28–63) | 44 (36–54) | 41 (35–52) | 44 (40–59) | 0·93 |
| LRMC Injury severity score | 21 (18–28) | 22 (18–25) | 21 (17–26) | 21 (15–26) | 24 (21–28) | 18 (14–21) | 0·41 |
| LRMC SOFA score | 8 (5–11) | 8 (3–9) | 8 (4–9) | 7 (4–11) | 9 (5–12) | 6 (4–8) | 0·88 |
| US MTF SOFA score | 6 (4–8) | 5 (0–7) | 5 (2–8) | 2 (1–8) | 4 (1–12) | 2 (1–7) | 0·49 |
IQR, Interquartile range; LRMC, Landstuhl Regional Medical Center; MTF, military treatment facility; PRBC, packed red blood cells plus whole blood; SOFA, Sequential Organ Failure Assessment.
P value represents comparison between proven/probable and possible/unclassifiable groups.
Tissue specimens sent for histopathological analysis and no fungal elements were reported.
Tissue specimens were not sent for histopathological analysis.
Plasma is fresh frozen plasma plus whole blood.
Fig. 2.
[colour online]. Boxplots of (a) maximum white blood cell (WBC, 109 cells/l) counts and (b) maximum weekly temperatures (°C) among US military personnel injured in combat (2009–2011). Weekly data combined from Landstuhl Regional Medical Center and US military treatment facilities.
IFI mycology
According to the IFI classification scheme, 100% of P/U cases had cultures with fungal growth compared to 76% of P/P cases (Table 3). Mucorales, Aspergillus, and Fusarium were the predominant moulds isolated from wound cultures. Although the fungal growth distribution profiles were slightly different between the classification groups, the dissimilarities were not statistically significant.
Table 3.
Wound culture results by cases, n (%), following combat-related injuries, 2009–2011
| Proven (N = 27) | Probable (N = 27) | Proven/ probable (N = 54) | Possible/unclassifiable (N = 23) | Possible: negative histopathology (N = 10)* | Unclassifiable: no histopathology (N = 13)† | P value | |
|---|---|---|---|---|---|---|---|
| Cases with positive wound cultures | 22 (81·5) | 19 (70·4) | 41 (75·9) | 23 (100) | 10 (100) | 13 (100) | 0·01 |
| Cases with wound cultures growing‡ | |||||||
| Mucorales | 14 (51·9) | 7 (25·9) | 21 (38·9) | 5 (21·7) | 1 (10·0) | 4 (30·8) | 0·19 |
| Aspergillus | 8 (29·6) | 5 (18·5) | 13 (24·1) | 11 (47·8) | 5 (50·0) | 6 (46·2) | 0·07 |
| Fusarium | 8 (29·6) | 5 (18·5) | 13 (24·1) | 4 (17·4) | 2 (20·0) | 2 (15·4) | 0·77 |
| Other non-Mucorales organisms§ | 11 (40·7) | 11 (40·7) | 22 (40·7) | 9 (39·1) | 4 (40·0) | 5 (38·5) | 1·0 |
| Mucorales and non-Mucorales organisms | 11 (40·7) | 3 (11·1) | 14 (25·9) | 2 (8·7) | 1 (10·0) | 1 (7·7) | 0·13 |
P value represents comparison between proven/probable and possible/unclassifiable groups.
Tissue specimens sent for histopathological analysis and no fungal elements were reported.
Tissue specimens were not sent for histopathological analysis.
Wound cultures commonly grew more than one organism; therefore, the column data total more than the number of cases with positive wound cultures.
Growth of non-Mucorales organisms other than Aspergillus and Fusarium.
IFI management
Overall, 16% of the 77 IFI cases did not receive any antifungal treatment (Tables 4, 5), of which the P/U group contributed a larger proportion (26%) compared to the P/P cases (11%), but the difference was not statistically significant (Table 4). Among the 12 IFI cases that did not receive antifungal treatment (Table 5), 58% experienced lower extremity amputations with a median injury severity score (ISS) of 23, which was comparable to the overall classification groups. Of those who did receive antifungal therapy, there was no difference between P/P and P/U cases in time to IFI diagnosis or initiation of therapy. However, when excluding the unclassifiable cases there was a significantly shorter duration between injury and IFI diagnosis (P = 0·02) and between injury and initiation of antifungal therapy (P = 0·05) for possible cases compared to P/P cases.
Table 4.
Invasive fungal infection (IFI) management and clinical outcomes among military personnel injured in combat (2009–2011)
| Proven (N = 27) | Probable (N = 27) | Proven/probable (N = 54) | Possible/unclassifiable (N = 23) | Possible: negative histopathology (N = 10)† | Unclassifiable: no histopathology (N = 13)‡ | P value | |
|---|---|---|---|---|---|---|---|
| IFI antifungal treatment regimen, n (%) | |||||||
| No treatment | 0 | 6 (22·2) | 6 (11 1) | 6 (26·1) | 2 (20·0) | 4 (30·8) | 0·17 |
| Single agent | 1 (3·7) | 4 (14·8) | 5 (9·3) | 2 (8·7) | 1 (10·0) | 1 (7·7) | 1·0 |
| Dual (amphotericin B + triazole) | 16 (59·3) | 16 (59·3) | 32 (59·3) | 10 (43·5) | 6 (60·0) | 4 (30·8) | 0·31 |
| Combination (dual + echinocandin) | 10 (37·0) | 1 (3·7) | 11 (20·4) | 4 (17·4) | 1 (10·0) | 3 (23·1) | 1·0 |
| Systemic antifungal agents, n (%) | |||||||
| Amphotericin B (liposomal) | 26 (96·3) | 19 (70·4) | 45 (83·3) | 15 (65·2) | 7 (70·0) | 8 (61·5) | 0·13 |
| Voriconazole | 25 (92·6) | 19 (70·4) | 44 (81·5) | 15 (65·2) | 8 (80·0) | 7 (53·8) | 0·15 |
| Posaconazole | 12 (44·4) | 2 (7·4) | 14 (25·9) | 3 (13·0) | 1 (10·0) | 2 (15·4) | 0·25 |
| Caspofungin | 10 (37·0) | 1 (3·7) | 11 (20·4) | 4 (17·4) | 1 (10·0) | 3 (23·1) | 1·0 |
| Micafungin | 1 (3·7) | 0 | 1 (1·9) | 2 (8·7) | 0 | 2 (15·4) | 0·21 |
| Antifungal duration, median days (IQR) | |||||||
| Amphotericin B (liposomal) | 25 (17–42) | 19 (11–32) | 23 (13–38) | 17 (8–21) | 18 (15–20) | 11 (6–22) | 0·01* |
| Voriconazole | 14 (5–26) | 18 (14–28) | 18 (9–27) | 16 (9–20) | 16 (10–23) | 15 (6–19) | 0·54 |
| Total antifungal treatment | 26 (13–41) | 18 (4–30) | 22 (9–34) | 11 (6–22) | 21 (8–23) | 10 (6–16) | 0·06 |
| Hospitalization, median (IQR) | |||||||
| US MTF hospitalization (days) | 59 (38–73) | 46 (30–61) | 51 (35–70) | 37 (27–53) | 47 (32–50) | 31 (26–56) | 0·08 |
| Total hospitalization (days)§ | 61 (40–76) | 48 (34–63) | 53 (36–71) | 40 (29–57) | 50 (37–54) | 33 (27–59) | 0·12 |
| US MTF duration in ICU (days) | 14 (5–23) | 6 (3–17) | 9 (4–19) | 8 (4–11) | 9 (4–11) | 7 (4–10) | 0·41 |
| Total duration in ICU (days)§ | 16 (7–24) | 9 (5–20) | 11 (5–20) | 9 (6–16) | 11 (7–16) | 9 (6–14) | 0·60 |
| Overall OR visits | 17 (12–24) | 14 (9–18) | 16 (11–21) | 13 (8–16) | 8 (7–12) | 14 (8–15) | 0·03* |
| IFI-related timeline, median days (IQR) | |||||||
| Injury to first positive mould culture | 9 (5–12) | 4 (3–7) | 6 (3–10) | 7 (3–12) | 4 (2–7) | 8 (5–12) | 0·84 |
| Injury to IFI diagnosis | 9 (5–11) | 5 (3–9) | 7 (3–9) | 6 (3–12) | 3 (2–6) | 8 (5–12) | 0·70 |
| Injury to antifungal treatment | 9 (8–12) | 10 (6–14) | 10 (7–13) | 8 (7–11) | 7 (6–7) | 11 (8–12) | 0·28 |
| Overall outcome, n (%) | |||||||
| High-level amputations¶ | 7 (25·9) | 5 (18·5) | 12 (22·2) | 3 (13·0) | 0 | 3 (23·1) | 0·53 |
| Deaths | 4 (14·8) | 1 (3·7) | 5 (9·3) | 1 (4·3) | 0 | 1 (7·7) | 0·66 |
ICU, Intensive care unit; IQR, interquartile range; MTF, military treatment facility; OR, operating room.
P value represents comparison between proven/probable and possible/unclassifiable groups.
Indicates statistical significance.
Tissue specimens sent for histopathological analysis and no fungal elements were reported.
Tissue specimens were not sent for histopathological analysis.
Total hospitalization/duration in ICU combines data from Landstuhl Regional Medical Center and US MTFs.
High-level amputations are defined as total hip disarticulation or hemipelvectomy.
Table 5.
Case series of invasive fungal infection (IFI) cases not treated with antifungals among military personnel injured in combat (2009–2011)
| IFI cases | Lower extremity amputation | High-level amputation* | Injury severity score | PRBC transfusion in-theatre (units) | Maximum WBC count at US MTFs (109 cells/l) | Total hospitalization (days) | Total ICU duration (days) | Number of OR visits | Death |
|---|---|---|---|---|---|---|---|---|---|
| Probable | |||||||||
| 1 | No | No | 14 | 5 | 6·4 | 16 | 2 | 6 | No |
| 2 | Yes | No | 34 | 16 | 12·6 | 71 | 6 | 11 | No |
| 3 | No | No | 50 | 30 | 9·2 | 64 | 20 | 18 | No |
| 4 | Yes | No | 21 | 12 | 9·2 | 20 | 4 | 3 | No |
| 5 | No | No | 10 | 4 | 8·8 | 15 | 6 | 5 | No |
| 6 | Yes | No | 24 | 17 | n.a. | 48 | 3 | 1 | No |
| Possible | |||||||||
| 7 | Yes | No | 11 | n.a. | 5·0 | 29 | 2 | 7 | No |
| 8 | Yes | No | 24 | 33 | 7·4 | 38 | 13 | 8 | No |
| Unclassifiable | |||||||||
| 9 | No | No | 14 | 10 | 16·2 | 59 | 8 | 15 | No |
| 10 | Yes | No | 26 | 13 | 11·1 | 28 | 6 | 19 | No |
| 11 | No | No | 10 | 2 | 20·3 | 32 | 7 | 8 | No |
| 12 | Yes | Yes | 29 | 22 | 7·4 | 12 | 11 | 7 | Yes |
ICU, Intensive care unit; MTFs, military treatment facilities; OR, operating room; PRBC, packed red blood cells; WBC, white blood cell; n.a., not applicable due to missing data.
High-level amputations are defined as total hip disarticulation or hemipelvectomy.
Use of a single antifungal agent (monotherapy) during the treatment regimen was roughly 9% for both groups (Table 4). About 59% of cases in the P/P group were prescribed dual antifungal agents compared to 44% in the P/U group (P = 0·31). For both of the groups, amphotericin B (liposomal) and voriconazole were the primary antifungal agents utilized; however, the P/U cases had significantly shorter duration of amphotericin B use (P = 0·01). However, when considering the possible cases alone, there was no significant difference in duration of amphotericin B use between the possible and P/P cases (P = 0·16). Patients in the P/U group had a median duration of total antifungal therapy of 11 days compared to 22 days for the P/P cases; however, the difference was not statistically significant (P = 0·06, Table 4). P/U cases also had a statistically reduced number of operating-room visits compared to the P/P group (P = 0·03). When the unclassifiable cases were excluded, the possible cases continued to have fewer operating-room visits (P = 0·002).
Clinical outcomes
An evaluation of clinical outcomes observed few differences between the groups (Table 4). Total days hospitalization among the P/P cases ranged (interquartile) from 36 to 71 days, while it was 29–57 days for patients in the P/U group. Although 22% of P/P cases resulted in high-level amputations (i.e. total hip disarticulation or hemipelvectomy) compared to 13% in the P/U group, the difference was not statistically significant (P = 0·53). Overall, six patients with IFI died [one P/U (4·3%), five P/P (9·3%), P = 0·66) yielding a crude mortality rate of 7·8% for the cohort.
Unclassifiable cases
All 13 unclassifiable cases occurred prior to December 2010. The unclassifiable cases received significantly fewer blood transfusions (P = 0·04) and had lower injury severity scores (P = 0·05) compared to the P/P cases, but their clinical characteristics were otherwise similar. They received shorter durations of amphotericin B (P = 0·03) compared to P/P cases; however, their outcomes were not different. Three cases (two with an ISS of 21 and the third, an ISS of 29) required high-level amputations. The two surviving high-level amputation cases received antifungal treatment for 10 and 16 days and visited the operating room 11 and 14 times, respectively. Additionally, the surviving cases respectively reported 9 and 25 days length of stay in the ICU and 33 and 40 days of total hospitalization. The third case was not prescribed any antifungals because the IFI was diagnosed port-mortem.
DISCUSSION
Combat-related IFI has emerged as an important cause of morbidity and mortality among US service members during recent military conflicts [15–17]. This is the largest case series of trauma-related IFIs reported to date and determined an overall incidence of 6·8% IFI cases among combat casualties. An earlier case investigation of IFIs in US combat casualties [17] proposed modified IFI case definitions in order to identify groups of patients with similar degrees of certainty of IFI diagnoses to allow for clinical and epidemiological research on trauma-related IFI. The goal of the current report was to apply those definitions to a larger series of patients to investigate the similarities and differences between the groups which may affect management and/or prognosis of future cases.
Overall, this investigation found few differences between the IFI classification groups. The possible cases were similar to the P/P cases in their presenting clinical characteristics and management. Although they had fewer operating-room visits, their clinical outcomes did not differ. This suggests that possible cases with negative histopathology either require fewer debridement efforts because of less extensive tissue invasion or that some cases of wound contamination with environmental moulds were included as true cases. The requirement of recurrent tissue necrosis after at least two debridements makes the latter less likely; however, there are other reasons for recurrent necrosis, such as poor vascular supply due to the traumatic injury. Another possibility is that the earlier initiation of antifungal therapy (7 vs. 10 days) led to equivalent outcomes with the P/P group despite fewer operative procedures.
The unclassifiable cases occurred early in the investigation period (prior to December 2010) when sending tissue for histopathology from wounds with recurrent necrosis was not standard practice. Compared to the P/P cases, the unclassifiable cases had less severe injuries, required fewer blood transfusions, and received less amphotericin B, but had similar overall outcomes. Despite this, there were several patients in the unclassifiable group who required high level amputations because of IFI. It is likely that the unclassifiable group represents both possible cases along with cases which would have been classified as P/P if tissue had been sent for histopathology.
Due to the broad surveillance of laboratory and pathological data, antifungal use, and case records from the infectious disease and surgical services, the potential for failure in detecting IFI cases in this investigation was low. If IFI cases were not recognized, these would presumably have been less severe; thus, not triggering the collection of diagnostic specimens or initiation of antifungal therapy. It is possible that less severe cases exist, which are cured with surgical therapy alone.
If there were any inclusion of false-positives (or over-diagnosing), it would most likely occur among the cases in the possible and unclassifiable groups where there is a lack of histopathological evidence of fungal invasion. Despite fewer operating-room visits (possible cases) and shorter durations of amphotericin B (unclassifiable cases), the clinical outcomes of these cases were similar to the P/P cases. As mentioned earlier, this may suggest some misclassification of non-IFI cases as cases; however, given the similarities in clinical characteristics, as well as durations of hospitalization and ICU stay, it is not certain how often this may have occurred. There were also 12 patients who did not receive any antifungal agents. Half of these patients were in the probable group and, therefore, had fungal elements seen on histopathology. It is possible that the fungal elements were contamination and not truly invasive. The outcomes of these patients were not worse than those who received antifungals, therefore, it is uncertain if these patients were misclassified or if they were successfully treated with surgery alone.
Our data indicate that the combat trauma-specific IFI case definition and classification has utility for epidemiological surveillance and clinical research. While our IFI case definition relies on culture and histopathology results, molecular diagnostic assays (i.e. polymerase chain reaction-based) also exist for fungal identification [21, 22]. However, they are not readily available at most clinical laboratories and, therefore, not yet an effective tool for early diagnosis. Classification across the case definition does not provide prognostic value; although this may in part be limited by number of cases across each definition category. In addition to utility within combat casualty care, the IFI case definition may prove to be applicable to civilian IFI cases resulting from agricultural accidents or natural disasters, such as the tornado in Joplin, Missouri [11], and allow comparison to other outbreaks and future analysis of clinical outcomes relative to therapeutic strategies. Ongoing surveillance coupled with continued clinical investigation is needed to support early interventions to prevent IFI, enhance timely identification and diagnosis, and improve treatment strategies.
ACKNOWLEDGEMENTS
We are indebted to the Infectious Disease Clinical Research Program Trauma Infectious Disease Outcomes Study team of clinical coordinators, microbiology technicians, data managers, clinical site managers, and administrative support personnel for their tireless hours to ensure the success of this project. Support for this work (IDCRP-024) was provided by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense program executed through the Uniformed Services University of the Health Sciences. This project was funded by the National Institute of Allergy and Infectious Diseases, National Institute of Health, under Inter-Agency Agreement Y1-AI-5072, and the Department of the Navy under the Wounded, Ill, and Injured Program.
The views expressed are those of the authors do not necessarily reflect the official views of the Uniformed Services University of the Health Sciences, the National Institute of Health or the Department of Health and Human Services, the Department of Defense, or the Departments of the Army, Navy or Air Force. Mention of trade names, commercial products, or organization does not imply endorsement by the U.S. Government.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Eucker J, et al. Mucormycoses. Mycoses 2001; 44: 253–260. [PubMed] [Google Scholar]
- 2.Lanternier F, et al. A global analysis of mucormycosis in France: the RetroZygo Study (2005–2007). Clinical Infectious Diseases 2012; 54 (Suppl. 1): S35–S43. [DOI] [PubMed] [Google Scholar]
- 3.Roden MM, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clinical Infectious Diseases 2005; 41: 634–653. [DOI] [PubMed] [Google Scholar]
- 4.Steinbach WJ, et al. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. Journal of Infection 2012; 65: 453–464. [DOI] [PubMed] [Google Scholar]
- 5.Hajdu S, et al. Invasive mycoses following trauma. Injury 2009; 40: 548–554. [DOI] [PubMed] [Google Scholar]
- 6.Vitrat-Hincky V, et al. Severe filamentous fungal infections after widespread tissue damage due to traumatic injury: six cases and review of the literature. Scandinavian Journal of Infectious Diseases 2009; 41: 491–500. [DOI] [PubMed] [Google Scholar]
- 7.Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clinical Microbiology Reviews 2000; 13: 236–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Notes from the field: fatal fungal soft-tissue infections after a tornado – Joplin, Missouri, 2011. Morbidity and Mortality Weekly Report 2011; 60: 992. [DOI] [PubMed] [Google Scholar]
- 9.Skiada A, Petrikkos G. Cutaneous zygomycosis. Clinical Microbiology and Infection 2009; 15 (Suppl. 5): 41–45. [DOI] [PubMed] [Google Scholar]
- 10.Patino JF, et al. Necrotizing soft tissue lesions after a volcanic cataclysm. World Journal of Surgery 1991; 15: 240–247. [DOI] [PubMed] [Google Scholar]
- 11.Neblett Fanfair R, et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. New England Journal of Medicine 2012; 367: 2214–2225. [DOI] [PubMed] [Google Scholar]
- 12.Skiada A, et al. Global epidemiology of cutaneous zygomycosis. Clinics in Dermatology 2012; 30: 628–632. [DOI] [PubMed] [Google Scholar]
- 13.Petrikkos G, et al. Epidemiology and clinical manifestations of mucormycosis. Clinical Infectious Diseases 2012; 54 (Suppl. 1): S23–S34. [DOI] [PubMed] [Google Scholar]
- 14.Evriviades D, et al. Shaping the military wound: issues surrounding the reconstruction of injured servicemen at the Royal Centre for Defence Medicine. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences 2011; 366: 219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paolino KM, et al. Invasive fungal infections following combat-related injury. Military Medicine 2012; 177: 681–685. [DOI] [PubMed] [Google Scholar]
- 16.Radowsky JS, et al. Invasive mucormycosis and aspergillosis in a healthy 22-year-old battle casualty: case report. Surgical Infection (Larchmont) 2011; 12: 397–400. [DOI] [PubMed] [Google Scholar]
- 17.Warkentien T, et al. Invasive mold infections following combat-related injuries. Clinical Infectious Diseases 2012; 55: 1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Pauw B, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clinical Infectious Diseases 2008; 46: 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tribble DR, et al. Infection-associated clinical outcomes in hospitalized medical evacuees after traumatic injury: Trauma Infectious Disease Outcome Study. Journal of Trauma 2011; 71 (Suppl 1): S33–S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eastridge BJ, et al. Trauma system development in a theater of war: experiences from Operation Iraqi Freedom and Operation Enduring Freedom. Journal of Trauma 2006; 61: 1366–1372; discussion 1372–1373. [DOI] [PubMed] [Google Scholar]
- 21.Perfect JR. Fungal diagnosis: how do we do it and can we do better? Current Medical Research and Opinion 2013; 29 (Suppl. 4): 3–11. [DOI] [PubMed] [Google Scholar]
- 22.Massire C, et al. PCR Followed by electrospray ionization mass spectrometry for broad-range identification of fungal pathogens. Journal of Clinical Microbiology 2013; 51: 959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]