Abstract
Nanoparticle-aided radiation therapy is emerging as a promising modality to enhance radiotherapy via the radiosensitizing action of high atomic number (Z) nanoparticles. However, the delivery of sufficiently potent concentrations of such nanoparticles to the tumor remain a challenge. This study investigates the dose enhancement to lung tumors due to high-Z nanoparticles (NPs) administered via inhalation during external beam radiotherapy. Here NPs investigated include: cisplatin nanoparticles (CNPs), carboplatin nanoparticles (CBNPs), and gold nanoparticles (GNPs).
Using Monte Carlo–generated megavoltage energy spectra, a previously employed analytic method was used to estimate dose enhancement to lung tumors due to radiation-induced photoelectrons from the NPs administered via inhalation route (IR) in comparison to intravenous (IV) administration. Previous studies have indicated about 5% of FDA-approved cisplatin concentrations reach the lung via IV. Meanwhile recent experimental studies indicate that 3.5–14.6 times higher concentrations of NPs can reach the lung by IR compared to IV. Taking these into account, the dose enhancement factor (DEF) defined as the ratio of the radiotherapy dose with and without nanoparticles was calculated for a range of NPs concentrations and tumor sizes. The DEF for IR was then compared with that for IV.
For IR with 3.5 times higher concentrations than IV, and 2 cm diameter tumor, clinically significant DEF values of up to 1.19, 1.26, and 1.51 were obtained for CNPs, CBNPs and GNPs. In comparison values of 1.06, 1.08, and 1.15 were obtained via IV administration. For IR with 14.6 times higher concentrations, even higher DEF values were obtained e.g. 1.81 for CNPs. Results also showed that the DEF increased with increasing field size or decreasing tumor volume, as expected.
The results of this work indicate that IR administration of targeted high-Z CNPs/CBNPs/GNPs could enable clinically significant DEF to lung tumors compared to IV administration during external beam radiotherapy. For FDA approved concentrations of CNPs or CBNPs considered, this could allow for additional dose enhancement to tumors via photoelectric mechanism during concomitant chemoradiotherapy.
Keywords: inhalation, cisplatin nanoparticles, carboplatin nanoparticles, radiotherapy, dose Enhancement, chemoradiotherapy
Introduction
Lung cancer is one of the leading causes of cancer related mortality with a relatively short overall survival rate of 16% for five years (Komaki et al 2011). Clinical studies indicate that radiation boosting leads to significant increase in survival for lung cancer patients (Keall et al 2006, Machtay et al 2012), with every 1 Gy boost in biologically effective dose associated with 4% relative improvement in survival (Machtay et al 2010). However, current modalities for radiation boosting are critically limited by normal tissue toxicity, compounded by respiratory motion (Keall et al 2006). The issue of normal tissue toxicity is of increasing importance due to the growing use of concomitant chemoradiotherapy (CCRT). An American medical task group report notes that new treatment strategies that can overcome these limitations, allowing an increased dose to the tumor while sparing normal tissue, will significantly improve the balance between complications and cure (Keall et al 2006).
Meanwhile, nanoparticle-aided radiation therapy is emerging as a promising modality for highly localized radiation boosting due to the photoelectric interaction of radiotherapy photons with high atomic number (Z) nanoparticles such as gold nanoparticles (GNPs) employed during brachytherapy or external beam radiotherapy (Ngwa et al 2014). Such an approach could enable radiation boosting with minimal increase in toxicities to normal tissue. However, the delivery of sufficiently potent concentrations of such nanoparticles (NPs) to the tumor remain a challenge (Ngwa et al 2014). Studies show that only up to 5% NPs reach the lung via customary intravenous (IV) administration route (Taratula et al 2011). Many studies have, thus, concluded that radiation boosting from high-Z NPs would not be clinically significant for clinical 6 MV radiotherapy, partly due to consideration of low concentrations of NPs accumulating in tumor when NPs are administered intravenously (Rousseau et al 2010). Therefore, it would be useful to develop new approaches to deliver higher concentrations of nanoparticles to the tumor site.
Taratula et al recently developed a special drug delivery system (DDS) for delivery of nanoparticles to lung tumors via inhalation. Their animal based experimental results showed that delivery of nanoparticles via inhalation route (IR) provide 3.5–14.6 times higher NPs concentrations compared to IV (Taratula et al 2011, 2013). These studies included nanoparticles of chemotherapy drugs like cisplatin, which have a high-Z platinum component.
In this study, we hypothesize that the administration of FDA approved concentrations of such platinum-based chemotherapy drugs via nanoparticle inhalation/instillation will allow delivery of sufficiently potent concentrations to the tumor to elicit significant dose enhancement via photoelectric mechanism during external beam radiotherapy (EBRT). Such delivery could be achieved by the special DDS. This work tests this hypothesis for nanoparticles of FDA approved platinum-based chemotherapy drugs: cisplatin and carboplatin in comparison to gold nanoparticles.
Material and methods
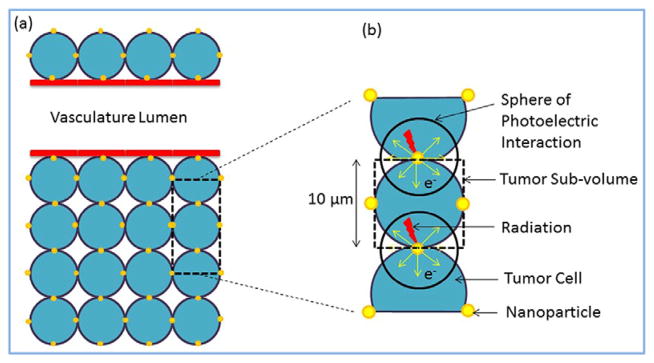
Using Monte Carlo–generated megavoltage energy spectra (Liu and Verhaegen 2002, Parsons et al 2014), a previously employed analytic method (Ngwa et al 2010, Berbeco et al 2011, Altundal et al 2015, Sinha et al 2015) was used to estimate the dose enhancement to lung tumors due to radiation-induced photoelectrons from the NPs administered via IR in comparison to IV administration. As in the previous work the tumor voxel was modeled with dimensions 10 μm × 10 μm × 10 μm (figure 1). This model assumes that the nanoparticles are distributed uniformly over the tumor sub-volume. The implications of these assumptions and others are discussed in the discussion section. The number or concentration of nanoparticles was determined under the condition of different drug concentrations, up to the maximum FDA allowed concentration. The FDA allowed concentrations of cisplatin and carboplatin are 100 mg m−2 (Boulikas 2009) and 300 mg (FDA 2010), respectively. The unit mg m−2 represents mg per human body surface area, which corresponds to 1.79 m2 for average human body. Based on this, FDA allowed concentrations of 43 mg g−1 and 72 mg g−1 were used for single concentrations of cisplatin and carboplatin, respectively. It is important to note that the amount of platinum contained in cisplatin and carboplatin are different. Cisplatin and carboplatin contain 65% and 54% of platinum, respectively. For IV administration, 5% of these concentrations are assumed to arrive the tumor compared to, 3.5–14.6 times higher for IR as highlighted in the experimental studies (Taratula et al 2011, 2013). The dose enhancement factor (DEF) was then calculated for these given range of NP concentrations.
Figure 1.
Schematic showing tumor section with vasculature and NPs.
When the NPs are exposed to radiation, photoelectrons are emitted as a result of photoelectric interaction. The energy of these photoelectrons is equal to the difference of the photon incident energy and corresponding edge energy. In this work, only the contribution of photoelectrons was taken into account for the calculation of dose enhancement (Van den Heuvel et al 2010). Step by step explanation of the tumor voxel analytical calculations can be found in the previous publications (Ngwa et al 2010, Berbeco et al 2011, Altundal et al 2015).
Briefly, the number of emitted photoelectrons per NP was derived from the multiplication of the probability for photoelectric interaction by the number of incident photons. Photoelectrons emitted from the NPs deposit kinetic energy locally. Statistically, the energy will be released in a ‘sphere of photoelectron interaction’ centered on the nanoparticle (figure 1). The range of these electrons is the radius of this sphere (Rtot). Equation (1) (Cole 1969) shows the experimentally derived relation between the deposited kinetic energy (E) and the photoelectron residual range (R) with the unit of keV and μm, respectively.
| (1) |
The total deposited energy per photoelectron to the tumor voxel was obtained by integrating equation (1), from the surface (Rn) of the NP to the distal side of the tumor voxel (Rn+ DE).
| (2) |
In equation (2), r (=Rtot − R) is the distance of the photoelectron traveled from the center of the NP, Shellhemisphere is the surface area of the hemisphere, and Shellentire sphere is the surface area of the whole sphere. The factor of 2 is necessary in the integral to add the contribution of the NP on the other side of the tumor voxel, given the isotropic emission of photoelectron and the homogenous distribution of NPs.
From the calculated total deposited energy, absorbed dose due to photoelectrons was found by dividing total energy deposited by the mass. Finally, dose enhancement factor which is defined as,
| (3) |
was calculated for a range of NPs concentrations. DEF for IR was then compared with that for IV administration. The NP size was chosen to be 2 nm. The default tumor size employed was 2 cm diameter tumor. Other tumor sizes with diameter range from 1 cm to 5 cm were also investigated. Two megavoltage photon spectra have been investigated in this study. One spectra was generated using EGS4 Monte Carlo code to simulate 6 MV clinical source (Liu and Verhaegen 2002). The fluence (MeV−1 cm−2 mGy−1) was calculated for various field sizes at 1.5 cm depths (4 cm × 4 cm field size) and 20 cm depths (10 cm × 10 cm field size) for standard (STD) beams. Another spectra considered was for a 2.35 MV carbon target in a 2100 EX Clinac using BEAMnrc (Parsons et al 2014).
Results
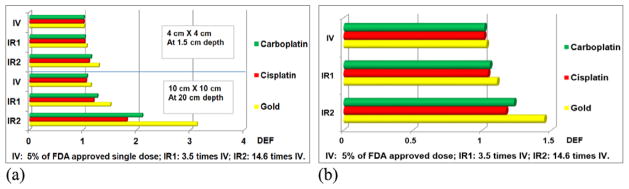
Figures 2(a) shows dose enhancement factor for 6 MV photon beams, while figure 2(b) is for the 2.35 MV photon beam. Figure 2(a) shows the DEF values for representative sample field sizes for GNPs, CNPs and CBNPs when irradiated by 6 MV x-rays source. We assumed the GNPs have the same concentration as CBNPs, given that GNPs are relatively less toxic. Taking the delivery efficiency of IV (5% of FDA concentration), IR1 (3.5 times of IV) and IR2 (14.6 times of IV) into account, the corresponding single cycle local concentrations of CNPs are 2.2 mg g−1, 7.5 mg g−1 and 31.4 mg g−1, respectively. For CBNPs, three discrete single cycle concentrations are 3.6 mg g−1, 12.6 mg g−1 and 52.6 mg g−1, respectively. The results show that substantial dose enhancement can be achieved for the same kind of NPs with different field sizes, and for same field size with different type of NPs. According to the calculation, for standard EBRT simulated by a 10 cm × 10 cm field, DEF values of GNPs, CNPs and CBNPs with 3.5 times higher concentrations than IV can be up to 1.51, 1.19 and 1.26 correspondingly. As a result of high prescription dose of carboplatin, CBNPs have better dose enhancement effect compared to CNPs.
Figure 2.
The DEF of NPs for IR and IV for both 6 MV and 2.35 MV spectra. (a) DEF for single cycle of three different NPs for 6 MV photon, (b) DEF for single cycle of three different NPs for 2.35 MV photon.
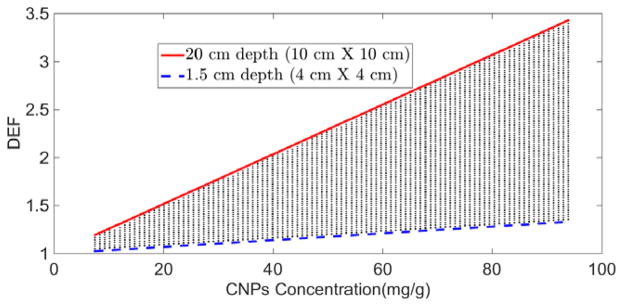
The top and bottom boundary lines in figure 3 shows DEF as function of CNPs concentration for 6 MV photon beam for the two extreme calculation points, 1.5 cm and 20 cm depth. Our results are consistent with previous studies (Berbeco et al 2011) that used Monte Carlo simulations and revealed a general trend of increasing dose enhancement with increasing concentration of NPs. In general, the lung tumor will probably be somewhere between these depths. For depth values between 1.5 cm and 20 cm, DEF values are expected to fall into the shaded area.
Figure 3.
DEF as a function of cisplatin concentration during CCRT with 6 MV external beam.
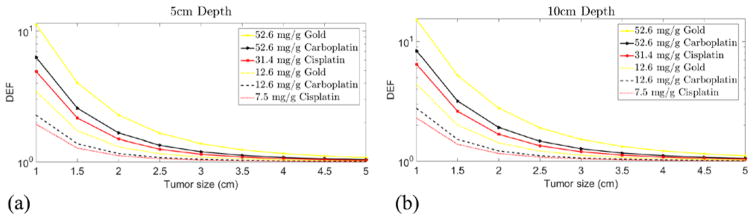
Figures 4(a) and (b) show the DEF results for 6 MV photon beam for single cycle CNPs, GNPs and CBNPs via inhalation at depth of 5 cm and 10 cm, for a range of tumor sizes. All of the curves show a downward trend. For larger tumor diameters like 5 cm, no significant dose enhancement has been obtained from our calculations. In our model, DEF is proportional to the local NPs concentration. As the result of increasing the tumor size, the average local NPs concentration reduces, and hence the DEF.
Figure 4.
DEF as a function of tumor size for single doses of NPs delivered by inhalation during CCRT with 6 MV external beam. (a) DEF at 5 cm depth, (b) DEF at 10 cm depth.
Discussion
Many studies have concluded that radiation boosting from high-Z NP, including platinum-based chemotherapy drugs would not be clinically significant for clinical 6 MV radiotherapy, partly due to practical consideration of low concentrations of NP accumulating in tumor when NP are administered intravenously (Rousseau et al 2010). Berbeco et al (Berbeco et al 2011) recently demonstrated that targeting GNP, administered intravenously, to the tumor vasculature could enable sufficiently potent concentrations of the GNP to boost radiation dose to the tumor vasculature. This innovative approach capitalizes on the fact that even the small concentration of IV administered GNP reaching the tumor vasculature would be sufficiently potent if only the tumor vasculature is targeted, as opposed to the whole tumor. Hence the GNPs could be employed as vascular disrupting agents (VDAs) during EBRT. The results in the current study indicate that if the nanoparticles are administered via IR, sufficiently potent concentrations of the NPs may be realized for significant boosting of tumors/tumor sub-volumes. This provides another potentially feasible approach besides the use of GNPs as VDAs.
Based on our calculation, platinum based NPs like cisplatin nanoparticles delivered via IR may also provide significant dose enhancement via photoelectric mechanism as well. Given the growing use of CCRT, and associated toxicity limitations, such an dditional mechanism of highly localized dose boosting could be of significant benefit.
After the nanoparticles are inhaled by the patient, they pass through the upper respiratory tract. NPs may collide with the respiratory wall and part of them may be deposited into the pharynx regions. To increase the efficiency, NPs could be delivered through a flexible bronchoscope, as is used for fiducial placement. PEGylated NPs with sufficient mass, suitable size and other relative characteristics as described recently (Kumar et al 2013) may keep travelling through trachea, primary bronchi, secondary bronchi, bronchioles, and finally, the alveoli. For tumor located inside the respiratory tract, NPs can be directly and efficiently delivered to the tumor site. Given the higher concentration of NPs reaching the lung via IR, it should also be possible for NPs to reach the tumor located on the external surface of respiratory tract by diffusion.
Lung cancer often metastasizes to the lymph nodes before spreading to other parts of the body. Choi et al demonstrated that noncationic NPs smaller than ~34 nm translocate rapidly from the lung to mediastinal lymph nodes (Choi et al 2010). NPs smaller than ~34 nm and larger than ~6 nm can provide higher efficiency of NP administration to lymph nodes, which could be used to boost radiotherapy treatment of lung tumor metastases. Taking advantage of our targeted, third generation gold nanoparticle platforms (Kumar et al 2013), the efficiency of NPs delivery can be greatly improved, as well as DEF.
Our findings show a significant increase on DEF with gold and platinum based NPs delivered by IR compared to IV administration. For dose enhancement calculation, we assume that the NPs distributed homogeneously inside the entire tumor. Inhalation administration may not result in uniform distribution of NPs as assumed in the model and certain sub-volumes will likely have higher DEF than others. But as Berbeco et al have shown theoretically and in vitro, sufficient concentrations could still lead to major dose enhancement during external beam RT. The work focused on targeting the endothelial cells lining the tumor vasculature when the nanoparticles are administered intravenously, e.g. dose enhancement factor in the endothelial cells with NPs concentration region 7 mg g−1 to 50 mg g−1 ranges from1.2 to 2.2 at 20 cm depth, if NPs are more trapped around the blood vessels (Berbeco et al 2011). Here this study demonstrates another approach that could be developed to enable significant radiation boosting to tumors/tumor sub-volumes if the NPs are administered via IR.
It is important to note that dose enhancement is sensitive to beam quality. The use of flattening filter free (FFF) quality beams have recently been considered with potential to increase the DEF (Tsiamas et al 2013) including for in vitro studies (Berbeco et al 2012). Beams with larger component of low energy photons lead to higher dose enhancement than for smaller component of low energy photons. The highest clinical impact of the IR delivery approach is anticipated in significantly increasing the survival and quality of life for early stage lung cancer patients due to the ability to boost radiation dose to the tumor without increase in normal tissue toxicity. The new approach would be particularly useful in treating populations of medically inoperable early-stage non–small cell lung cancer patients with central lesions, given that the associated normal tissue toxicity for such tumors is significant when using stereotactic body radiation therapy (SBRT) (Timmerman et al 2006, Song et al 2009). As a more effective initial treatment option, this new strategy would also help prevent cancer recurrence, which has been shown to impose a substantial burden of suffering on patients, including mental suffering.
Additional advantages for choosing GNPs, and CNPs include the fact that GNPs can inherently provide useful imaging contrast and are suitable for attaching other drugs, or moieties that would drive future expansion of the clinical benefits of the high-Z NP treatment strategy. Potential use of CNPs or CBNPs via the new approach could also maximize the benefits of CCRT, whose promise for major improvement in cancer treatment has hitherto been hampered by systemic toxicity from current IV delivery approach of drugs like cisplatin. Targeted IR would enable far less systemic toxicity.
Conclusion
Our preliminary results indicate that major dose enhancement to lung tumors can be achieved by using GNPs, CNPs and CBNPs administered via IR, in contrast to IV administration during EBRT. These findings provide further impetus for the development of an optimal IR delivery approach for nanoparticle-aided radiotherapy boosting to lung tumors using GNPs, CNPs or CBNPs. This would, especially, be beneficial during concomitant chemoradiotherapy, potentially allowing for highly localized radiation boosting via photoelectric mechanism, with minimal toxicities to healthy tissue.
Acknowledgments
This study was supported by National Institutes of Health National Cancer Institute grant 1 K01 CA172478-01.
References
- Altundal Y, Cifter G, Detappe A, Sajo E, Tsiamas P, Zygmanski P, Berbeco R, Cormack RA, Makrigiorgos M, Ngwa W. New potential for enhancing concomitant chemoradiotherapy with FDA approved concentrations of cisplatin via the photoelectric effect. Phys Medica. 2015;31:25–30. doi: 10.1016/j.ejmp.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbeco RI, Korideck H, Ngwa W, Kumar R, Patel J, Sridhar S, Johnson S, Price BD, Kimmelman A, Makrigiorgos GM. DNA damage enhancement from gold nanoparticles for clinical MV photon beams. Radiat Res. 2012;178:604–8. doi: 10.1667/RR3001.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbeco RI, Ngwa W, Makrigiorgos GM. Localized dose enhancement to tumor blood vessel endothelial cells via megavoltage x-rays and targeted gold nanoparticles: new potential for external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2011;81:270–6. doi: 10.1016/j.ijrobp.2010.10.022. [DOI] [PubMed] [Google Scholar]
- Boulikas T. Clinical overview on Lipoplatin: a successful liposomal formulation of cisplatin. Expert Opin Investig Drugs. 2009;18:1197–218. doi: 10.1517/13543780903114168. [DOI] [PubMed] [Google Scholar]
- Choi HS, Ashitate Y, Lee JH, Kim SH, Matsui A, Insin N, Bawendi MG, Semmler-Behnke M, Frangioni JV, Tsuda A. Rapid translocation of nanoparticles from the lung airspaces to the body. Nat Biotechnol. 2010;28:1300–3. doi: 10.1038/nbt.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole A. Absorption of 20 eV to 50 000 eV electron beams in air and plastic. Radiat Res. 1969;38:7–33. [PubMed] [Google Scholar]
- FDA. About the center for drug evaluation and research—carboplatin dosing. 2010 ( www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm228974.htm)
- Keall PJ, et al. The management of respiratory motion in radiation oncology report of AAPM task group 76. Med Phys. 2006;33:3874–900. doi: 10.1118/1.2349696. [DOI] [PubMed] [Google Scholar]
- Komaki R, et al. Phase I study of celecoxib with concurrent irinotecan, cisplatin, and radiation therapy for patients with unresectable locally advanced non-small cell lung cancer. Front Oncol. 2011;1:52. doi: 10.3389/fonc.2011.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Korideck H, Ngwa W, Berbeco RI, Makrigiorgos GM, Sridhar S. Third generation gold nanoplatform optimized for radiation therapy. Trans Cancer Res. 2013;2:228–39. doi: 10.3978/j.issn.2218-676X.2013.07.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HH, Verhaegen F. An investigation of energy spectrum and lineal energy variations in mega-voltage photon beams used for radiotherapy. Radiat Prot Dosim. 2002;99:425–7. doi: 10.1093/oxfordjournals.rpd.a006824. [DOI] [PubMed] [Google Scholar]
- Machtay M, Bae K, Movsas B, Paulus R. Higher biologically effective dose of radiotherapy is associated with improved outcomes for locally advanced non-small cell lung carcinoma treated with chemoradiation: an analysis of the radiation therapy oncology group. Int J Radiat Oncol Biol Phys. 2010;82:425–34. doi: 10.1016/j.ijrobp.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machtay M, Bae K, Movsas B, Paulus R, Gore EM, Komaki R, Albain K, Sause WT, Curran WJ. Higher biologically effective dose of radiotherapy is associated with improved outcomes for locally advanced non-small cell lung carcinoma treated with chemoradiation: an analysis of the radiation therapy oncology group. Int J Radiat Oncol Biol Phys. 2012;82:425–34. doi: 10.1016/j.ijrobp.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngwa W, Kumar R, Sridhar S, Korideck H, Zygmanski P, Cormack RA, Berbeco R, Makrigiorgos GM. Targeted radiotherapy with gold nanoparticles: current status and future perspectives. Nanomedicine (Lond) 2014;9:1063–82. doi: 10.2217/nnm.14.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngwa W, Makrigiorgos GM, Berbeco RI. Applying gold nanoparticles as tumor-vascular disrupting agents during brachytherapy: estimation of endothelial dose enhancement. Phys Med Biol. 2010;55:6533–48. doi: 10.1088/0031-9155/55/21/013. [DOI] [PubMed] [Google Scholar]
- Parsons D, Robar JL, Sawkey D. A Monte Carlo investigation of low-Z target image quality generated in a linear accelerator using varian’s virtualinac. Med Phys. 2014;41:021719. doi: 10.1118/1.4861818. [DOI] [PubMed] [Google Scholar]
- Rousseau J, Barth RF, Fernandez M, Adam JF, Balosso J, Esteve F, Elleaume H. Efficacy of intracerebral delivery of cisplatin in combination with photon irradiation for treatment of brain tumors. J Neurooncol. 2010;98:287–95. doi: 10.1007/s11060-009-0074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N, Cifter G, Sajo E, Kumar R, Sridhar S, Nguyen PL, Cormack RA, Makrigiorgos GM, Ngwa W. Brachytherapy application with in situ dose painting administered by gold nanoparticle eluters. Int J Radiat Oncol. 2015;91:385–92. doi: 10.1016/j.ijrobp.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SY, et al. Fractionated stereotactic body radiation therapy for medically inoperable stage I lung cancer adjacent to central large bronchus. Lung Cancer. 2009;66:89–93. doi: 10.1016/j.lungcan.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Taratula O, Garbuzenko OB, Chen AM, Minko T. Innovative strategy for treatment of lung cancer: targeted nanotechnology-based inhalation co-delivery of anticancer drugs and siRNA. J Drug Target. 2011;19:900–14. doi: 10.3109/1061186X.2011.622404. [DOI] [PubMed] [Google Scholar]
- Taratula O, Kuzmov A, Shah M, Garbuzenko OB, Minko T. Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA. J Control Release. 2013;171:349–57. doi: 10.1016/j.jconrel.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman R, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–9. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- Tsiamas P, et al. Impact of beam quality on megavoltage radiotherapy treatment techniques utilizing gold nanoparticles for dose enhancement. Phys Med Biol. 2013;58:451–64. doi: 10.1088/0031-9155/58/3/451. [DOI] [PubMed] [Google Scholar]
- Van den Heuvel F, Locquet J-P, Nuyts S. Beam energy considerations for gold nano-particle enhanced radiation treatment. Phys Med Biol. 2010;55:4509–20. doi: 10.1088/0031-9155/55/16/S06. [DOI] [PubMed] [Google Scholar]