Abstract
The transcriptional repressor B lymphocyte-induced maturation protein-1 (Blimp-1) has crucial roles in the control of plasma cell differentiation and in maintaining survival of plasma cells. However, how Blimp-1 ensures the survival of plasma cell malignancy, multiple myeloma (MM), has remained elusive. Here we identified Aiolos, an anti-apoptotic transcription factor of MM cells, as a Blimp-1-interacting protein by mass spectrometry. ChIP coupled with DNA microarray was used to profile the global binding of Aiolos and Blimp-1 to endogenous targets in MM cells, which revealed their co-binding to a large number of genes, including apoptosis-related genes. Accordingly, Blimp-1 and Aiolos regulate similar transcriptomes in MM cells. Analysis of the binding motifs for Blimp-1 and Aiolos uncovered a partial motif that was similar across sites for both proteins. Aiolos promotes the binding of Blimp-1 to target genes and thereby enhances Blimp-1-dependent transcriptional repression. Furthermore, treatment with an anti-MM agent, lenalidomide, caused ubiquitination and proteasomal degradation of Blimp-1, leading to the de-repression of a new Blimp-1 direct target, CULLIN 4A (CUL4A), and reduced Aiolos levels. Accordingly, lenalidomide-induced cell death was partially rescued by reintroduction of Blimp-1 or knockdown of CUL4A. Thus, we demonstrated the functional impacts and underlying mechanisms of the interaction between Aiolos and Blimp-1 in maintaining MM cell survival. We also showed that interruption of Blimp-1/Aiolos regulatory pathways contributes to lenalidomide-mediated anti-MM activity.
The differentiation of plasma cells is controlled by a transcriptional repressor, B lymphocyte-induced maturation protein-1 (Blimp-1).1 Blimp-1 suppresses mature B-cell gene expression including genes responsible for B-cell identity or activation, thereby allowing the activation of the plasma cell gene expression program.1 Blimp-1 is not only essential for the generation of plasma cells but also important for ensuring their survival.2, 3 Accordingly, antigen-specific long-lived plasma cells in the bone marrow cannot be maintained when Prdm1, the Blimp-1 gene, is ablated in immunized mice.4
The action of Blimp-1 in regulating gene repression has been revealed by the identification of several histone-modifying enzymes or co-repressors that physically interact with Blimp-1. For example, histone methyltransferase G9a associates with Blimp-1 and participates in Blimp-1-mediated suppression of IFN-β by adding methyl groups to lysine 9 of histone 3 (H3K9) of the IFN-β promoter.5 The proline-rich domain of Blimp-1 is a major contributor to its transcriptional repression activity via interactions with several co-repressors, including Groucho family proteins,6 histone deacetylase 2,7 and lysine-specific demethylase 1.8 Blimp-1 also interacts with PRMT5, an arginine-specific histone methyltransferase, in primordial germ cells.9
Here we sought to identify additional Blimp-1-interacting proteins via mass spectrometric analysis of Blimp-1-containing immunoprecipitates from a plasma cell line to acquire further insights into the molecular actions of Blimp-1. The transcription factor Aiolos was identified using this approach. Aiolos, an Ikaros family protein, contains four N-terminal zinc fingers in the DNA-binding domain and two C-terminal zinc fingers for protein–protein dimerization.10 Aiolos interacts with Ikaros family proteins, including Ikaros, in lymphoid cells.11 Downregulation of Aiolos contributes to the cytotoxic effects of an effective anti-malignant plasma cell (multiple myeloma, MM) agent, lenalidomide.12, 13 Lenalidomide targets Cereblon (CRBN), a component of the CULLIN 4 (CUL4)-containing E3 ligase complex (CRL4) that mediates the turnover of proteins,14 thereby resulting in the proteolysis of Ikaros family proteins and apoptosis of MM cells.12, 13 The target genes of Aiolos responsible for maintaining MM cell survival have yet to be characterized. Interactions between two or more transcription factors often provide the combinatorial control of gene expression that is needed for regulating a complex biological response. Thus, we hypothesized that the mode of action of Blimp-1 in maintaining the survival of MM cells may involve interaction with Aiolos. We show here that Aiolos assists Blimp-1 binding to target genes to jointly control the survival of MM cells and that the Blimp-1/Aiolos regulatory axis controls the responsiveness to lenalidomide treatment in MM cells.
Results
Identification of the Blimp-1-interacting protein Aiolos
We sought to identify the interacting partners of Blimp-1 that may contribute to the maintenance of the survival of MM cells, where Blimp-1 is expressed.3 Nuclear extracts from the human MM line H929 were used to immunoprecipitate Blimp-1-interacting complexes using polyclonal anti-Blimp-1. Mass spectrometric analysis of differentially expressed proteins derived from Blimp-1 immunoprecipitates relative to immunoprecipitates from a control antibody revealed 10 peptide sequences that corresponded to Aiolos (Supplementary Figure 1). The interaction of Blimp-1 and Aiolos was confirmed by co-immunoprecipitation (co-IP) with H929 nuclear extracts and anti-Blimp-1. Indeed, Aiolos in H929 cells was present in the anti-Blimp-1 immunoprecipitates (Figure 1a, upper panel). In a reciprocal experiment, Blimp-1 in H929 cells was co-IP with anti-Aiolos (Figure 1a, lower panel). Blimp-1 and Aiolos were also co-IP in another MM line, U266 (Figure 1b). More importantly, their interaction was further validated in primary MM cells isolated from bone marrow aspirate of patients (Figure 1c).
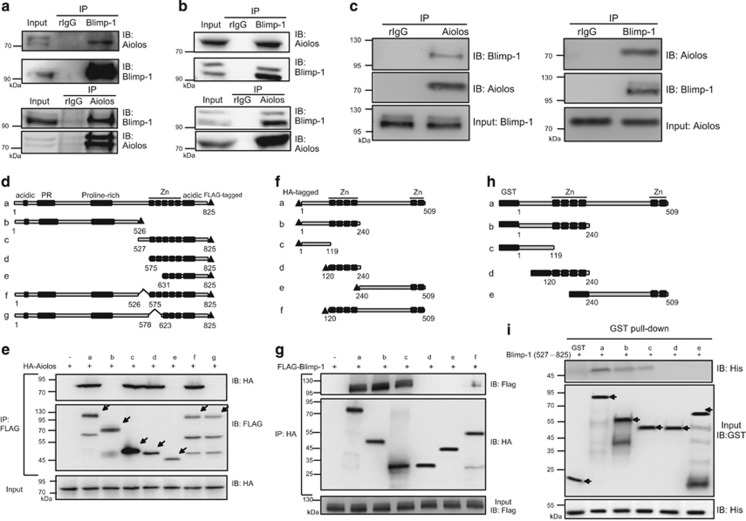
Figure 1.
Blimp-1 directly interacts with Aiolos. (a and b) H929 (a) and U266 (b) nuclear extracts were used for immunoprecipitation (IP) with anti-Blimp-1 (upper panels) or anti-Aiolos (lower panels). Immunoprecipitates and input lysates were analyzed with immunoblotting (IB) using the indicated antibodies. Rabbit IgG (rIgG) was used as the control IP antibody. (c) Co-IP using anti-Aiolos (left panel) or anti-Blimp-1 (right panel) demonstrated the interaction of Blimp-1 and Aiolos in bone marrow aspirates of MM patients. Results are one representative from two independent experiments. (d) Schematics of constructs for domain mapping of FLAG-tagged full-length Blimp-1 (a) and its various deletion mutants (b–g). Known motifs: acidic, acidic domain; PR, PR domain; proline-rich, proline-rich domain; Zn, the five zinc-finger motifs. (e) The HA-tagged Aiolos expression plasmid (HA-Aiolos) was co-transfected into 293T cells with the plasmids encoding various lengths of FLAG-tagged Blimp-1 or empty vector (−). Cell lysates were prepared for IP using anti-FLAG followed by IB. Arrows indicate FLAG-tagged Blimp-1 and its variants. (f) Schematics of constructs for domain mapping of HA-tagged full-length Aiolos (a) and its deletion mutants (b–f). (g) FLAG-tagged Blimp-1 was co-transfected with HA-Aiolos, its deletion constructs or empty vector (−) into 293T cells followed by co-IP and IB as described in e. (h) Schematics of constructs of GST-fused full-length Aiolos (a) and various deletion mutants of Aiolos (b–e). (i) A GST pull down assay was performed using recombinant His-tagged △1–526 Blimp-1 and GST or various forms of GST-Aiolos fusion proteins followed by IB. Arrows indicate GST or GST-fused Aiolos fragments
We next determined the regions in Blimp-1 and Aiolos that are required for their interaction. Several constructs encoding various forms of FLAG-tagged Blimp-1 with deletions (Figure 1d), along with HA-tagged Aiolos, were co-transfected into HEK293T cells. Lysates from transiently transfected cells were subjected to co-IP with anti-FLAG. Of note, Blimp-1 lacking the first two zinc fingers (constructs b, e and g) failed to interact with Aiolos (Figure 1e). Similarly, several HA-tagged Aiolos deletion constructs (Figure 1f) were co-transfected individually with a construct encoding FLAG-tagged Blimp-1. Aiolos lacking the N-terminal 119 amino-acid residues (constructs d, e and f) had reduced ability to pull down Blimp-1 (Figure 1g). The importance of these regions for the interaction was confirmed with glutathione S-transferase (GST) pull down assays in which several forms of bacterially expressed GST-fused Aiolos fragments (Figure 1h) were used to pull down His-tagged C-terminal Blimp-1 that contains the two essential zinc fingers for interaction with Aiolos. Results in Figure 1i confirmed that the N-terminal 119 residues of Aiolos (constructs a, b and c) are sufficient for the direct interaction with Blimp-1.
Blimp-1 and Aiolos associate with each other and bind to genes expressed in MM cells
To further study the mechanistic insights into the interaction between Blimp-1 and Aiolos, we performed chromatin IP coupled with promoter DNA microarray (ChIP–chip) to assess the direct binding of both transcription factors to genes in H929 cells (Supplementary Table 1). The genes occupied by Aiolos or Blimp-1 were obtained from the analysis with stringent criteria from two independent ChIP–chip experiments. We found that a set of genes was occupied by both Blimp-1 and Aiolos in H929 cells. Two hundred thirty-four (234/636=36.79%) Blimp-1-binding regions were also bound by Aiolos (Figure 2a). Of note, the differences in the proportion of 10 selected gene ontology (GO) terms of genes bound by Blimp-1 alone, Aiolos alone, or both, as compared with the remaining sites (which neither Blimp-1 nor Aiolos occupied), indicated that apoptosis-related genes were enriched in Aiolos-binding sites and Aiolos/Blimp-1 common targets (Figure 2b), suggesting that Aiolos and Blimp-1 may regulate apoptosis in cooperation. In particular, apoptosis-related genes such as apoptosis signal-regulating kinase 1 (ASK1) and CASP8 were bound by Aiolos and Blimp-1 in H929 cells. Moreover, the proportion of genes falling into the category of ‘intracellular signaling' and ‘transcription' is significantly enriched in only Aiolos- and Blimp-1-bound sites, respectively, as compared with non-target sites (Figure 2b).
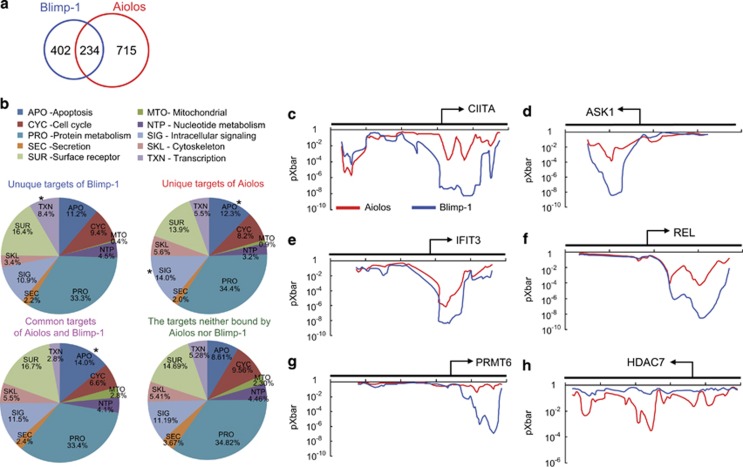
Figure 2.
Identification of direct binding sites of Aiolos and Blimp-1 in the MM cell line H929. (a and b) Chromatin from H929 cells was subjected to a ChIP assay with anti-Aiolos or anti-Blimp-1. (a) The Venn diagram shows the number of overlapping Aiolos target genes and Blimp-1 target genes in H929 cells. (b) Pie charts show the results of GO analysis of the unique targets of Blimp-1 (upper left panel), the unique targets of Aiolos (upper right panel), their common targets (lower left panel) and genes that were not bound by Blimp-1 or Aiolos (lower right panel); (*P<0.05). (c–h) The graphs are the average values of the binding probability (pXbar) of Aiolos (red line) or Blimp-1 (blue line) in H929 cells from two ChIP–chip experiments. The x axis represents the probe regions in the target genes, and the y axis shows the pXbar value. The position of transcription start sites and the direction of transcription are indicated
We next examined the probabilities of probe binding (pXbar) of Blimp-1 or Aiolos across the 5′ region of certain genes, which were calculated based on the average P-value of the central probe and its two neighboring probes according to Whitehead Neighbourhood Model.15 pXbar plots revealed that Blimp-1 and Aiolos both occupied near the promoter regions of target genes including class II, major histocompatibility complex, transactivator (CIITA), ASK1, IFIT3 and REL (Figures 2c–f). We also found some genes that were bound by either Blimp-1 (e.g., PRMT6) or Aiolos (e.g., HDAC7) (Figure 2g and h).
Two distinct motifs are recognized by Aiolos
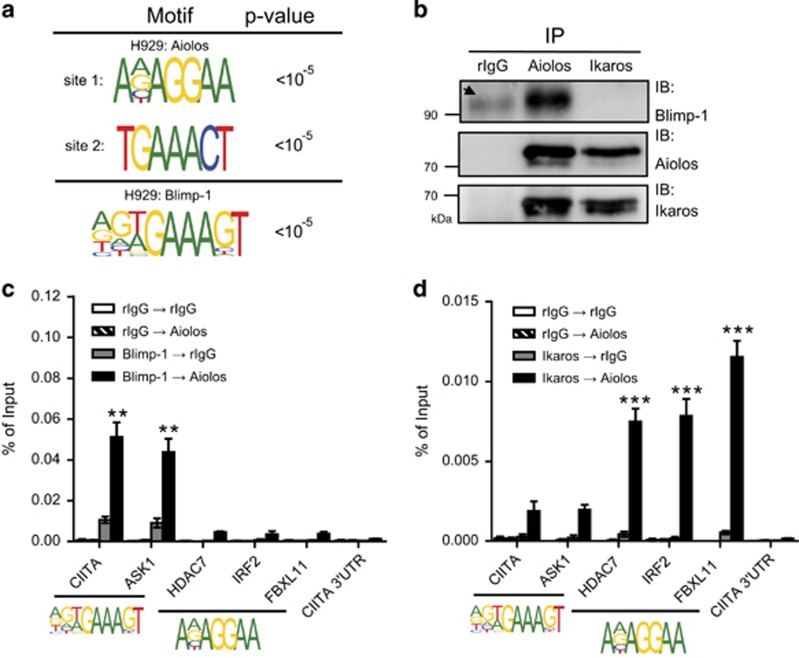
As Aiolos shares a portion of its target genes with Blimp-1, we next deciphered the consensus binding motif of Blimp-1 and Aiolos to further elucidate the detailed mode of action resulting from their interactions. We used our ChIP–chip data to analyze Blimp-1- and Aiolos-binding motifs in H929 cells. (N)(N)(T/A)GAAAGT was found as a Blimp-1-binding motif (Figure 3a). Two motifs that appeared most frequently as the consensus binding sites for Aiolos were A(N)AGGAA (site 1) and TGAAACT (site 2) (Figure 3a). Interestingly, site 1 is similar to the Ikaros/Aiolos heterodimer-binding motif, (A/G)CAGGAA(G/A), identified in mouse T cells.16 To our surprise, site 2 for Aiolos binding shares a ‘GAAA' motif with the partial Blimp-1 consensus binding motif (Figure 3a). As site 1 of Aiolos can be bound by Ikaros, we wondered if Ikaros is part of the Blimp-1/Aiolos complex. Results from co-IP showed that Blimp-1 associated only with Aiolos, but not Ikaros, in H929 cells (Figure 3b). Therefore, we propose that site 1 of the Aiolos consensus binding motif is likely occupied by Aiolos/Ikaros and that Blimp-1 binds with Aiolos on site 2 in MM cells.
Figure 3.
Aiolos/Blimp-1 and Aiolos/Ikaros heterodimers bind to two distinct motifs. (a) Sequences shown are the Blimp-1- and Aiolos-binding consensus motifs analyzed with the motif discovery web server eTFBS. (b) Lysates from H929 cells were used to perform IP with the indicated antibodies, followed by IB. Arrow indicates a nonspecific band. (c and d) Chromatin prepared from H929 cells was subjected to a ChIP and re-ChIP assay using anti-Blimp-1 (c) or anti-Ikaros (d) in the first ChIP and anti-Aiolos or rabbit IgG in the re-ChIP. Results in (c) and (d) represent the mean±S.E.M. (n=3). **P<0.01; ***P<0.005
To further test if Blimp-1 and Aiolos both bind to site 2 but not site 1 of the Aiolos-binding motif, we performed a sequential chromatin IP assay (ChIP and re-ChIP) using either anti-Blimp-1, anti-Ikaros, or control rabbit IgG in the first ChIP, followed by either anti-Aiolos or rabbit IgG in the re-ChIP. Indeed, Blimp-1 and Aiolos co-bound only to the promoter regions of CIITA and ASK1, which contain site 2, but not to HDAC7, IRF2 or FBXL11, which contain site 1 (Figure 3c). Conversely, HDAC7, IRF2 and FBXL11, but not CIITA and ASK1, were bound by the Ikaros/Aiolos complex (Figure 3d).
Aiolos facilitates the binding of Blimp-1 to target genes and its transcriptional repression
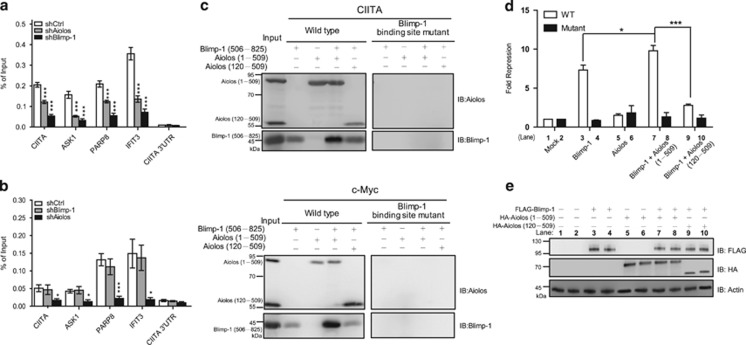
We next used ChIP to determine whether Blimp-1 and Aiolos influence each other to bind target genes containing TGAAACT (site 2). As expected, Blimp-1 binding to its target genes was diminished after knockdown of Blimp-1 in H929 cells (Figure 4a). However, the binding of Aiolos to these sites was not affected when Blimp-1 was depleted (Figure 4b). We note that the binding of Blimp-1 was significantly reduced once Aiolos was depleted (Figure 4a). An in vitro DNA pull down assay validated that Aiolos per se increased the binding of Blimp-1 to the oligonucleotides containing site 2. As shown in Figure 4c, in the presence of recombinant Aiolos, recombinant tBlimp-1, a truncated form of Blimp-1 that retains the five zinc-finger motifs for DNA binding,17 could be pulled down more efficiently by oligonucleotides derived from the CIITA and c-Myc promoters, whereas a mutant Aiolos lacking the first 119 amino acids required for the interaction with Blimp-1 did not enhance the binding of Blimp-1 to CIITA and c-Myc sites. Oligonucleotides that contained mutated sites for Blimp-1 binding failed to pull down tBlimp-1 or Aiolos.
Figure 4.
Aiolos enhances the binding of Blimp-1 to DNA. (a and b) H929 cells, transduced with shCtrl, shAiolos, or shBlimp-1 for 4 days, were subjected to the ChIP assay using anti-Blimp-1 (a) or anti-Aiolos (b). All data represent the mean±S.E.M. (n=3). (c) Immunoblot of Blimp-1 or Aiolos captured in DNA pull down assays with biotinylated CIITA (upper panel) or c-Myc (lower panel) probes containing the Blimp-1-binding site and recombinant His6-tBlimp-1 (506−825) and/or His6-Aiolos (1−509 or 120−509). (d) Luciferase reporter assay using lysates from 293T cells transfected with plasmids encoding Blimp-1 and/or Aiolos (1−509 or 120−509) or empty vector alone (mock) together with wild-type (WT) or Blimp-1 binding site-mutated (Mutant) CIITA-pIII-Luc and RL-tk. (e) Immunoblots show the expression of the indicated proteins from corresponding transfectants described in d. Results represent the mean±S.D. (n=3). *P<0.05; ***P<0.005
We further compared the transcriptional repression activity of Blimp-1 in suppressing a luciferase reporter driven by the CIITA promoter III (CIITA-pIII-Luc) in the presence or absence of Aiolos. We found that Aiolos alone did not significantly affect CIITA pIII activity. However, Aiolos, but not mutant Aiolos lacking the N-terminal 119 amino acids, promoted the transcriptional repression activity of Blimp-1 (Figures 4d and e). This effect depended on Blimp-1 binding because a mutant reporter carrying mutated Blimp-1-binding sequences failed to show the Blimp-1-dependent and Blimp-1-Aiolos-dependent repression (Figure 4d).
Aiolos cooperates with Blimp-1 to mediate gene repression that ensures the survival of MM cells
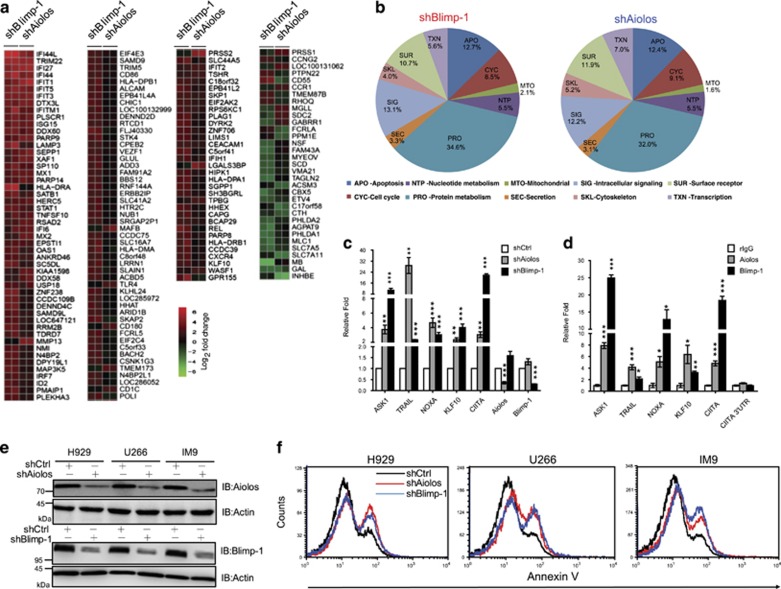
We next assessed whether occupancy by both Blimp-1 and Aiolos at target genes contributes to the regulation of expression of these genes. To obtain a global picture of the gene expression that is repressed by Blimp-1 and Aiolos, we performed a cDNA microarray to dissect the changes in mRNA after depletion of either Aiolos or Blimp-1. Genes whose expression changed by >4-fold after depletion of Aiolos or Blimp-1 (Supplementary Table 2) compared with control shRNA-transduced cells were identified with heatmap analysis (Figure 5a). Of note, most genes were up- or downregulated with a similar trend regardless of whether Aiolos or Blimp-1 was depleted in H929 cells (Figure 5a), showing that knockdown of Aiolos affects a similar transcriptome as knockdown of Blimp-1. In addition, GO analysis illustrated that Aiolos or Blimp-1 knockdown affected genes with a similar distribution among the functional categories (Figure 5b). Several pro-apoptosis genes in MM cells that were bound by Blimp-1 and Aiolos, including ASK1, TRAIL, NOXA, and KLF10,18, 19, 20, 21 were validated to be up-regulated after Aiolos or Blimp-1 knockdown (Figure 5c) and bound by both as shown by ChIP assay (Figure 5d). Consistently, knockdown of Aiolos or Blimp-1 in three MM cell lines, H929, U266 and IM9 (Figure 5e), promoted apoptosis, as determined by annexin V staining (Figure 5f).
Figure 5.
Aiolos and Blimp-1 collaboratively regulate gene expression and maintain the survival of MM cells. (a) Heatmap of microarray data from the H929 line transduced with shCtrl, shAiolos or shBlimp-1 for 4 days. The changes in expression for each gene were calculated as the ratio of expression in shAiolos- or shBlimp-1-transduced cells versus shCtrl-transduced cells. Microarray experiments were performed in duplicate. Each row represents a significantly induced (red) or repressed (green) gene following knockdown of Aiolos or Blimp-1. (b) Pie charts show the results of GO analysis of Blimp-1-dependent genes (left panel) or Aiolos-dependent genes (right panel) in H929 cells. (c) RT-QPCR analysis with samples from (a) validated the expression of apoptosis-related genes in shRNA-expressing cells. (d) Chromatin from H929 cells was subjected to the ChIP assay using anti-Blimp-1 or anti-Aiolos, followed by QPCR to quantify the binding of Blimp-1 or Aiolos to the indicated genes. (e) Immunoblots show the knockdown efficiency of shBlimp-1 and shAiolos in three MM cell lines expressing indicated shRNA for 3 days. (f) Flow cytometric analysis of annexin V staining shows the increased apoptosis in MM cells expressing shAiolos or shBlimp-1 for 3 days. Data in c and d represent the mean±S.E.M. (n=3). *P<0.05; **P<0.01; ***P<0.005
Blimp-1 directly suppresses CUL4A and is downregulated after lenalidomide treatment in MM cells
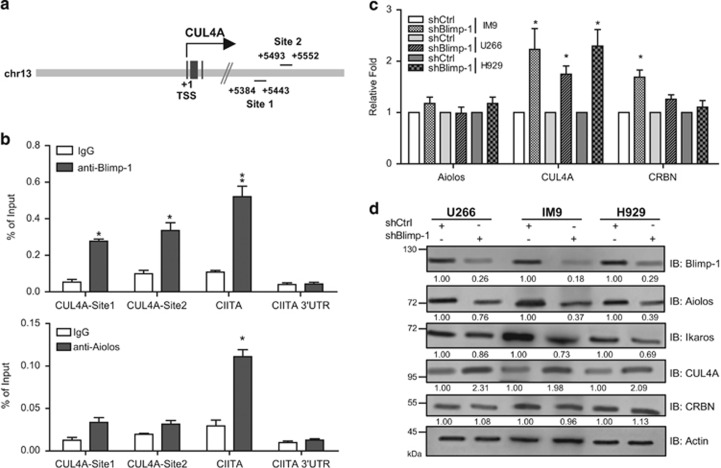
CRBN, a member of the CRL4 complex, was recently identified as the target of the anti-myeloma drug lenalidomide, which is a derivative of thalidomide.22, 23, 24 In search of the target genes of Blimp-1 or Aiolos in MM cells from our ChIP–chip data, we found that CUL4A, encoding a scaffold protein of the CRL complex, is directly bound by Blimp-1 at two potential binding sites (Figure 6a). A ChIP assay further confirmed that Blimp-1 binds to both sites in CUL4A intron 3 in MM cells (Figure 6b, upper panel). Aiolos may not bind to CUL4A, although a slight enrichment of Aiolos binding at these sites was found (Figure 6b, lower panel). Accordingly, knockdown of Blimp-1 in three MM cell lines led to increased levels of CUL4A mRNA, whereas Aiolos mRNA levels were not changed in any of the three MM lines (Figure 6c). CUL4A levels were also upregulated when Blimp-1 was depleted in all three MM lines (Figure 6d). It is noted that knockdown of Blimp-1 did not alter the levels of CRBN protein, but the levels of Aiolos and Ikaros were slightly reduced (Figure 6d).
Figure 6.
Blimp-1 directly suppresses CUL4A. (a) Schematic representation of two potential Blimp-1-binding sites on CUL4A. The transcriptional start site (+1) is indicated. (b) ChIP shows that Blimp-1 (upper panel), but not Aiolos (lower panel), binds to intron 3 of CUL4A in H929 cells. IgG was used as an isotype control antibody. CIITA promoter III region and its the 3′ untranslated regions (UTR) region were served as the positive and negative control loci for Blimp-1 binding. (c) RT-QPCR shows the increased CUL4A mRNA in shBlimp-1-expressing MM cells. (d) Immunoblots show the reduced expression of Aiolos and Ikaros but increased expression of CUL4A in three MM cell lines depleted of Blimp-1. Data in b and c represent the mean±S.D. (n=3). *P<0.05; **P<0.01
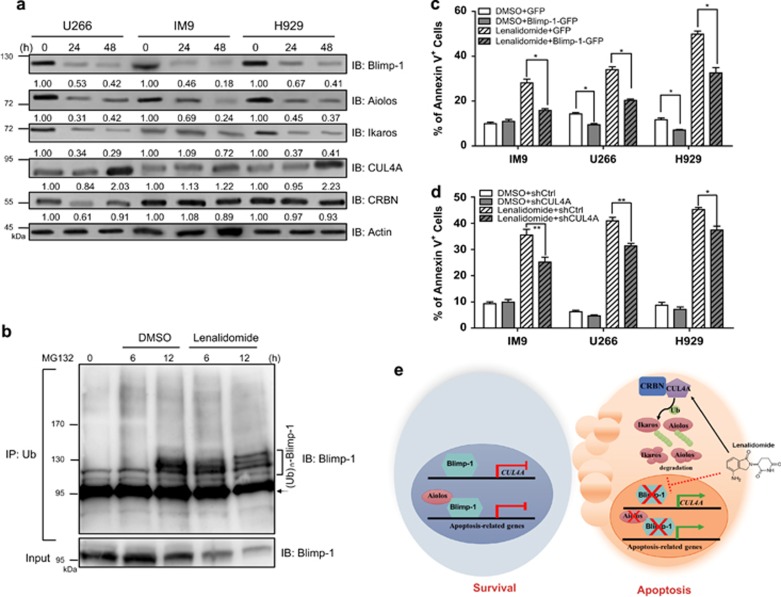
Treatment of MM cells with lenalidomide causes ubiquitination and proteolysis of Aiolos and Ikaros via altering substrate selectivity of CRBN in the CLR4 complex.12, 13 On the basis of our findings that Blimp-1 transcriptionally suppresses one component of the CLR4 complex, CUL4A, we proposed that Blimp-1 may participate in the lenalidomide-mediated anti-MM activity. We examined whether lenalidomide treatment altered the expression of Blimp-1. Indeed, as shown in Supplementary Figure 2a, lenalidomide treatment not only reduced the amounts of Aiolos and Ikaros proteins in a dose-dependent manner but also downregulated Blimp-1 levels in MM cells. Similarly, lenalidomide treatment diminished Blimp-1, Aiolos and Ikaros protein levels but induced CUL4A in three MM cell lines in a time-dependent manner (Figure 7a). However, Blimp-1 mRNA levels were not altered (Supplementary Figure 2b). The downregulation of Blimp-1 in lenalidomide-treated MM cells is mediated through the ubiquitin/proteasome pathway, because more poly-ubiquitinated Blimp-1 was immunoprecipitated by anti-Ub in the presence of the proteasome inhibitor MG132 (Figure 7b).
Figure 7.
The cytotoxic effects of lenalidomide in MM cells require proteolysis of Blimp-1. (a) Immunoblots show the effects of lenalidomide (20 μM) on the expression of the indicated proteins in three MM cell lines at the indicated time points. (b) Co-IP shows the effect of lenalidomide on ubiquitination of Blimp-1. Pre-cleared nuclear extract from H929 cells treated with lenalidomide and MG132 (10 μM) at indicated time points was used for IP with anti-Ub, followed by immunoblot analysis with anti-Blimp-1. Arrow indicates a nonspecific protein. (c) Percentage of annexin V+ cells in MM cells that expressed Blimp-1–EGFP or EGFP and were treated with lenalidomide or DMSO for 24 h. (d) Percentage of annexin V+ cells in shCUL4A#2- or shCtrl-expressing cells treated with lenalidomide or DMSO for 24 h. (e) Working model of the Blimp-1/CUL4A/Aiolos regulatory axis in inducing apoptosis after lenalidomide treatment. Data in c and d represent the mean±S.D. (n=3). *P<0.05; **P<0.01
Given that depletion of Blimp-1 leads to apoptosis of MM cells,3, 18 we next examined whether the anti-MM effects of lenalidomide could result from degradation of Blimp-1 and de-repression of the Blimp-1 direct target CUL4A. Overexpression of Blimp-1–EGFP by lentiviral transduction led to partial rescue of lenalidomide-induced apoptosis in three MM cell lines and slightly reduced the basal level of apoptosis in U266 and H929 cells treated with the solvent control DMSO (Figure 7c), which was associated with the reduced CUL4A mRNA and protein (Supplementary Figure 2c and Supplementary Figure 2d). The protein levels, but not mRNA levels, of Aiolos and Ikaros were concomitantly increased in MM cells that expressed Blimp-1–EGFP and were treated with lenalidomide (Supplementary Figures 2c and 2d). Furthermore, the elevated levels of CUL4A may contribute to the anti-MM effects of lenalidomide, because knocking down CUL4A partly reduced lenalidomide-induced apoptosis as compared with cells expressing the control shRNA shCtrl (Figure 7d), whereas depletion of CUL4A with a specific shRNA did not affect apoptosis in the cells treated with the solvent control DMSO. We note that Aiolos and Ikaros levels were not downregulated when CUL4A was depleted in lenalidomide-treated MM cells, but depletion of CUL4A barely affected Blimp-1 (Supplementary Figure 2e). Together, these results indicate that the Blimp-1/CUL4A regulatory axis controls the expression of Aiolos and Ikaros as well as lenalidomide-induced apoptosis in MM cells.
Discussion
Using ChIP–chip analysis, we were able to discover direct target genes of Blimp-1 and Aiolos in MM cells. Specifically, we identified several genes relevant to apoptosis, which are important for understanding the functional roles of Blimp-1 and Aiolos in maintaining MM cell survival. Furthermore, the sequences of the chromatin fragments occupied by Blimp-1 or Aiolos in the ChIP–chip analysis also allowed us to predict their consensus binding sites. As expected, the predicted Blimp-1-binding motif, (N)(N)(T/A)GAAAGT, is similar to the previously identified sites from two independent studies, (A/C)AG(T/C)GAAAG(T/C)(G/T) 25 and GTGAAAGT and G(N)GAAAGT.26 In addition, we found that, in MM cells, a large proportion of the genes that Aiolos bound directly had the consensus binding sequence TGAAACT (site 2), which is similar to the Blimp-1 consensus site. Another consensus binding sequence, A(N)AGGAA (site 1), was also found and is similar to that reported for Aiolos, ACAGGAAGT, in T cells.16 In MM cells, we detected an association between Aiolos and Ikaros in a complex without a requirement for Blimp-1, indicating that in addition to aiding Blimp-1, the Aiolos/Ikaros complex may regulate different sets of genes.
Blimp-1 and Aiolos may modulate similar cellular properties of plasma cells, although most of their targets are not in common. This could result from independent interactions with other transcription factors, such as Ikaros for Aiolos, and the regulation of genes that may not be identical but may possess similar biological functions. This finding is in agreement with studies of Blimp-1 and Aiolos gene knockout mice. Mice lacking Blimp-1 in B cells show severe defects in plasma cell formation and in the production of all subtypes of antibodies, but the generation of antigen-specific memory B cells is not affected.2 In addition, Blimp-1 maintains long-lived plasma cells in mice, likely because of its role in suppressing apoptosis of plasma cells.3, 4 Similarly, Aiolos knockout mice display diminished generation of long-lived plasma cells, but the generation of memory B cells is not altered.27 These studies further support our notion that Blimp-1 and Aiolos co-regulate many genes, particularly apoptosis-related genes. In contrast, some phenotypic discrepancies are present in plasma cell differentiation between Blimp-1- and Aiolos-deficient mice. For instance, somatic hypermutation and generation of short-lived plasma cells are not affected in Aiolos knockout mice. The expression of homing-related genes is also comparable between Aiolos knockout and control mice,27 which differs from a previous finding that Blimp-1 regulates chemokine-mediated homing genes, including CXCR5, to direct the exit of plasma cells from secondary lymphoid organs.1 These phenotypic discrepancies are supported by our ChIP–chip results showing that many genes were occupied by only Aiolos or Blimp-1, but not by both proteins. Our GO analysis also revealed the unique function of Aiolos and Blimp-1 when they did not target to the same sites.
Among those pro-apoptosis genes bound by Aiolos and Blimp-1 in MM cells, we previously showed that ASK1 blocks the formation of long-lived plasma cells in bone marrow because long-lived plasma cells generated by immunization accumulate in Ask1 knockout mice.18 We thus speculate that cooperative suppression of ASK1 by Blimp-1 and Aiolos may be one of the mechanisms contributing to the survival of MM cells. We here also identified several other apoptosis-related genes that are co-regulated by Blimp-1 and Aiolos, including TRAIL, NOXA and KLF10. TRAIL triggers apoptosis in MM cells.28 NOXA is a BH3-only BCL-2 family protein that mediates apoptosis.29 KLF10 induces apoptosis via transcriptionally suppressing BAX inhibitor-1.30 We thus uncovered several apoptosis-related genes that are suppressed by Blimp-1 and Aiolos and that maintain MM cell survival; however, it requires further experiments to validate if these identified genes indeed contribute to the anti-apoptosis effects of Blimp-1 and Aiolos in MM cells. In addition to MM cells, Blimp-1 and Aiolos may also regulate the survival of normal plasma cells. We found that knockdown of Blimp-1 or Aiolos consistently promoted apoptosis of CD138+ plasma cells derived from lipopolysaccharide-stimulated mouse splenic B cells (Supplementary Figure 3).
Lenalidomide binds to CRBN, and the abundance of CRBN proteins in MM cells is associated with the responsiveness of these cells to lenalidomide treatment.22 Lenalidomide-bound CRBN gains the ability to target Aiolos and Ikaros to proteasomal degradation.12, 13 We revealed here another action of lenalidomide, which triggers the proteolysis of Blimp-1 through the ubiquitin/proteasome pathway. Proteolysis of Blimp-1 is most likely to be regulated by a protein other than CUL4A, because knockdown of CUL4A in MM cells did not markedly change Blimp-1 levels after lenalidomide treatment (Supplementary Figure 2e). In agreement, knockdown of CUL4A did not change the ratio of ubiquitinated Blimp-1 to total Blimp-1 (Supplementary Figure 2f). Recently, Blimp-1 was shown to be ubiquitinated by a ubiquitin ligase, Hrd1, in dendritic cells.31 The precise ubiquitination sites on Blimp-1 and whether Hrd1 is also involved in Blimp-1 ubiquitination and degradation in MM cells await further studies. Although we here show that downregulation of Aiolos and Ikaros by CUL4A is important for the anti-MM effects of lenalidomide, we could not exclude the possibility that other targets of CUL4 may also have a role in this context. For instance, suppression of CRL4 stabilizes Myc protein.32 Thus, upregulation of CUL4A after lenalidomide treatment may result in the downregulation of Myc and apoptosis because inhibition of Myc activity causes MM cell death.33 In addition to regulating the turnover of proteins, CUL4A also controls transcription. CUL4 binds to the promoter of a tumor-suppressor gene, CDK inhibitor p16INK4a, thereby activating p16INK4a.34 Upregulation of p16INK4a is associated with apoptosis in MM cells.35 Nevertheless, our results showing the direct transcriptional repression of CUL4A by Blimp-1 extend the current understandings of the mode of action of anti-myeloma drugs. We hypothesized that Blimp-1 and Aiolos cooperatively suppress apoptosis genes in MM cells, which ensures the survival of those cells. Blimp-1 suppresses the transcription of CUL4A, resulting in the maintenance of the expression of Aiolos and Ikaros. In lenalidomide-treated MM cells, de-repression of CUL4A resulting from proteasomal degradation of Blimp-1 cooperates with CRBN to downregulate Aiolos and Ikaros, leading to apoptosis of MM cells (Figure 7e).
Materials and Methods
Cells and reagents
NCI-H929, U266 and IM9 human MM cells were cultured in RPMI medium supplemented with 10% fetal bovine serum. 293T and 3T3 cells were maintained in DMEM medium supplemented with 10% fetal bovine serum. Bone marrow aspirates from MM patients were obtained at the National Taiwan University Hospital whose consent procedure was approved by the National Taiwan University Hospital Research Ethics Committee (NTUHREC: 201505162RINC), and written informed consent was obtained from study participants in accordance with the Declaration of Helsinki and kept in their medical records. All reagents for cell culture studies were purchased from Invitrogen (Carlsbad, CA, USA) unless otherwise indicated. Lenalidomide and MG132 were purchased from Selleckchem (Houston, TX, USA) and EMD Millipore (Billerica, MA, USA), respectively.
Plasmids
Detailed information on plasmid constructions, including pFLAG-CMV5-Blimp-1 and its deletion constructs (△527−825, △1−526, △1−574, △1−630, △527−574 and △579−622 fragments), HA-Aiolos and its deletion constructs (△241−509, △1−239, △120−509, △1−119 and 120–240 fragments), pGEX4T3-GST-Aiolos and its deletion constructs (△241−509, △1−239, △120−509, △1−119 and 120−240 fragments), pRSET-His-Aiolos and its deletion constructs (△120−509 and △1−119) were available on request.
ChIP coupled with promoter DNA microarray
The ChIP on chip assays were performed essentially according to the Agilent-supported protocol. Briefly, 2 × 108 H929 cells were used per Blimp-1 or Aiolos ChIP. DNA in the IP was amplified using the GenomePlex Whole Genome Amplification (WGA) kits (Sigma, St Louis, MO, USA). The amplified DNA was labeled and hybridized to microarray chips (Agilent Technologies, Santa Clara, CA, USA), with subsequent analysis by the WELGENE Company (Taipei, Taiwan). The Agilent human promoter array chips contain ~17 000 of the best-defined transcripts covering −6-kb upstream to +3-kb downstream of the transcriptional start sites. The probe length of the array is 45–60 nucleotides, and the probe spacing is ~200 bp. The results from the Agilent microarray were further analyzed with DNA Analytics software (Agilent Technologies). GO analysis was done using BiNGO software.36 To assess whether the change of the proportion in a specific GO term is significant or not, hypergeometric test was adopted by using 'phyper' function of 'stats' package in R version 3.0.2 (https://www.r-project.org/). For example, the proportion of genes bound by Aiolos alone falling in the group ‘Apoptosis' is compared with the proportion of non-target genes (neither Aiolos nor Blimp-1 was occupied) in the same group. A P-value <0.05 is considered significant.
De novo identification of the transcription factor binding motif
The Blimp-1 and Aiolos motifs were identified using methods described previously.37 Briefly, a probe showed >4-fold binding on human promoter was obtained. Two probes were considered successive probes if the distance between them was <250 bp. Next, a potential binding region was identified by looking for a series of at least three successive probes. Afterward, a sequence of 1000 bp centered at the probe locations belonging to a potential binding region was retrieved. Using this method, we collected sequence data to feed into the motif discovery web server eTFBS (http://biominer.bime.ntu.edu.tw/etfbs/). P-values are calculated by randomly generating 100 000 sets of sequences for constructing the background distribution of motif appearances.
Flow cytometry
Flow cytometric analysis was performed essentially according to a previous report,8 by using APC-conjugated annexin V (BD PharMingen, San Diego, CA, USA). Cells were then analyzed by FACSCanto (Becton Dickinson, Franklin Lakes, NJ, USA) and FCS Express 3.0 software (Glendale, CA, USA).
RNA isolation and RT-QPCR analysis
Total RNA isolation, cDNA synthesis and subsequent reverse transcription (RT)-QPCR analysis in an ABI Prism 7300 sequence detection system were essentially performed according to a published protocol.38
Acknowledgments
We thank Dr. Wen-Hwa Lee for discussion. This work was supported by grants from Academia Sinica (AS-99-CDA-L12) and Ministry of Science and Technology (104-2320-B-001-016-MY3), Taiwan.
Author contributions
K-HH and S-TS performed the experiments and analyzed the data. C-YC, M-JMC and P-CW analyzed the data. S-YH contributed critical samples. W-JW, H-YC and F-RL performed the experiments. P-HH and M-DT contributed critical analytical tool. K-IL designed the research, analyzed the data and wrote the paper.
Glossary
- ASK1
apoptosis signal-regulating kinase 1
- Blimp-1
B lymphocyte-induced maturation protein-1
- ChIP
chromatin immunoprecipitation
- ChIP–chip
ChIP coupled with DNA microarray
- CIITA
class II, major histocompatibility complex, transactivator
- co-IP
co-immunoprecipitation
- CRBN
Cereblon
- GO
gene ontology
- GST
glutathione S-transferase
- MM
multiple myeloma
- Myc
myelocytomatosis oncogene
- RT-QPCR
quantitative real-time polymerase chain reaction.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by S Fulda
Supplementary Material
References
- Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, Rosenwald A et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 2002; 17: 51–62. [DOI] [PubMed] [Google Scholar]
- Shapiro-Shelef M, Lin KI, McHeyzer-Williams LJ, Liao J, McHeyzer-Williams MG, Calame K. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity 2003; 19: 607–620. [DOI] [PubMed] [Google Scholar]
- Lin FR, Kuo HK, Ying HY, Yang FH, Lin KI. Induction of apoptosis in plasma cells by B lymphocyte-induced maturation protein-1 knockdown. Cancer Res 2007; 67: 11914–11923. [DOI] [PubMed] [Google Scholar]
- Shapiro-Shelef M, Lin KI, Savitsky D, Liao J, Calame K. Blimp-1 is required for maintenance of long-lived plasma cells in the bone marrow. J Exp Med 2005; 202: 1471–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyory I, Wu J, Fejer G, Seto E, Wright KL. PRDI-BF1 recruits the histone H3 methyltransferase G9a in transcriptional silencing. Nat Immunol 2004; 5: 299–308. [DOI] [PubMed] [Google Scholar]
- Ren B, Chee KJ, Kim TH, Maniatis T. PRDI-BF1/Blimp-1 repression is mediated by corepressors of the Groucho family of proteins. Genes Dev 1999; 13: 125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Angelin-Duclos C, Greenwood J, Liao J, Calame K. Transcriptional repression by blimp-1 (PRDI-BF1) involves recruitment of histone deacetylase. Mol Cell Biol 2000; 20: 2592–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ST, Ying HY, Chiu YK, Lin FR, Chen MY, Lin KI. Involvement of histone demethylase LSD1 in Blimp-1-mediated gene repression during plasma cell differentiation. Mol Cell Biol 2009; 29: 1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancelin K, Lange UC, Hajkova P, Schneider R, Bannister AJ, Kouzarides T et al. Blimp1 associates with Prmt5 and directs histone arginine methylation in mouse germ cells. Nat Cell Biol 2006; 8: 623–630. [DOI] [PubMed] [Google Scholar]
- Morgan B, Sun L, Avitahl N, Andrikopoulos K, Ikeda T, Gonzales E et al. Aiolos, a lymphoid restricted transcription factor that interacts with Ikaros to regulate lymphocyte differentiation. EMBO J 1997; 16: 2004–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Sif S, Jones B, Jackson A, Koipally J, Heller E et al. Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity 1999; 10: 345–355. [DOI] [PubMed] [Google Scholar]
- Kronke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 2014; 343: 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G, Middleton RE, Sun HH, Naniong M, Ott CJ, Mitsiades CS et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science 2014; 343: 305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Nag A. CUL4A ubiquitin ligase: a promising drug target for cancer and other human diseases. Open Biol 2014; 4: 130217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HY, Yauk CL, Rowan-Carroll A, You SH, Zoeller RT, Lambert L et al. Identification of thyroid hormone receptor binding sites and target genes using ChIP-on-Chip in developing mouse cerebellum. Plos One 2009; 4: e4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JW, Jackson AF, Naito T, Dose M, Seavitt J, Liu FF et al. Harnessing of the nucleosome-remodeling-deacetylase complex controls lymphocyte development and prevents leukemogenesis. Nat Immunol 2012; 13: 86–U132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KI, Angelin-Duclos C, Kuo TC, Calame K. Blimp-1-dependent repression of Pax-5 is required for differentiation of B cells to immunoglobulin M-secreting plasma cells. Mol Cell Biol 2002; 22: 4771–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Huang SY, Hung KH, Su ST, Chung CH, Matsuzawa A et al. ASK1 promotes apoptosis of normal and malignant plasma cells. Blood 2012; 120: 1039–1047. [DOI] [PubMed] [Google Scholar]
- Wang SL, El-Deiry WS. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene 2003; 22: 8628–8633. [DOI] [PubMed] [Google Scholar]
- Gomez-Bougie P, Wuilleme-Toumi S, Menoret E, Trichet V, Robillard N, Philippe M et al. Noxa up-regulation and Mcl-1 cleavage are associated to apoptosis induction by bortezomib in multiple myeloma. Cancer Res 2007; 67: 5418–5424. [DOI] [PubMed] [Google Scholar]
- Jin W, Di GH, Li JJ, Chen Y, Li WF, Wu J et al. TIEG1 induces apoptosis through mitochondrial apoptotic pathway and promotes apoptosis induced by homoharringtonine and velcade. FEBS Lett 2007; 581: 3826–3832. [DOI] [PubMed] [Google Scholar]
- Lopez-Girona A, Mendy D, Ito T, Miller K, Gandhi AK, Kang J et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 2012; 26: 2326–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YX, Braggio E, Shi CX, Schmidt J, Bruins L, Schuster SR et al. Cereblon expression is required for the anti-myeloma activity of lenalidomide and pomalidomide. Blood 2011; 118: 61–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan BQ, Lentzsch S. The application and biology of immunomodulatory drugs (IMiDs) in cancer. Pharmacol Therapeut 2012; 136: 56–68. [DOI] [PubMed] [Google Scholar]
- Kuo TC, Calame KL. B lymphocyte-induced maturation protein (Blimp)-1, IFN regulatory factor (IRF)-1, and IRF-2 can bind to the same regulatory sites. J Immunol 2004; 173: 5556–5563. [DOI] [PubMed] [Google Scholar]
- Doody GM, Care MA, Burgoyne NJ, Bradford JR, Bota M, Bonifer C et al. An extended set of PRDM1/BLIMP1 target genes links binding motif type to dynamic repression. Nucleic Acids Res 2010; 38: 5336–5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes M, Georgopoulos K. Aiolos is required for the generation of high affinity bone marrow plasma cells responsible for long-term immunity. J Exp Med 2004; 199: 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazitt Y, Shaughnessy P, Montgomery W. Apoptosis-induced by trail and TNF-alpha in human multiple myeloma cells is not blocked by bcl-2. Cytokine 1999; 11: 1010–1019. [DOI] [PubMed] [Google Scholar]
- Shibue T, Takeda K, Oda E, Tanaka H, Murasawa H, Takaoka A et al. Integral role of Noxa in p53-mediated apoptotic response. Gene Dev 2003; 17: 2233–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CF, Sui CL, Wu WC, Wang JJ, Yang DHA, Chen YC et al. Klf10 induces cell apoptosis through modulation of BI-1 expression and Ca2+ homeostasis in estrogen-responding adenocarcinoma cells. Int J Biochem Cell B 2011; 43: 666–673. [DOI] [PubMed] [Google Scholar]
- Yang H, Qiu Q, Gao B, Kong S, Lin Z, Fang D. Hrd1-mediated BLIMP-1 ubiquitination promotes dendritic cell MHCII expression for CD4 T cell priming during inflammation. J Exp Med 2014; 211: 2467–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Wright JB, Gerber SA, Cole MD. Myc protein is stabilized by suppression of a novel E3 ligase complex in cancer cells. Gene Dev 2010; 24: 1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holien T, Vatsveen TK, Hella H, Waage A, Sundan A. Addiction to c-MYC in multiple myeloma. Blood 2012; 120: 2450–2453. [DOI] [PubMed] [Google Scholar]
- Kotake Y, Zeng YX, Xiong Y. DDB1-CUL4 and MLL1 mediate oncogene-induced p16(INK4a) activation. Cancer Res 2009; 69: 1809–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SH, Suvannasankha A, Crean CD, White VL, Chen CS, Farag SS. The novel histone deacetylase inhibitor, AR-42, inhibits gp130/Stat3 pathway and induces apoptosis and cell cycle arrest in multiple myeloma cells. Int J Cancer 2011; 129: 204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maere S, Heymans K, Kuiper M. BiNGO: a Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics 2005; 21: 3448–3449. [DOI] [PubMed] [Google Scholar]
- Chen CY, Tsai HK, Hsu CM, Chen MJM, Hung HG, Huang GTW et al. Discovering gapped binding sites of yeast transcription factors. Proc Natl Acad Sci USA 2008; 105: 2527–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KI, Kao YY, Kuo HK, Yang WB, Chou A, Lin HH et al. Reishi polysaccharides induce immunoglobulin production through the TLR4/TLR2-mediated induction of transcription factor Blimp-1. J Biol Chem 2006; 281: 24111–24123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.