Abstract
Mesenchymal stem cells (MSCs), a non-hematopoietic stem cell population first discovered in bone marrow, are multipotent cells capable of differentiating into mature cells of several mesenchymal tissues, such as fat and bone. As common progenitor cells of adipocytes and osteoblasts, MSCs are delicately balanced for their differentiation commitment. Numerous in vitro investigations have demonstrated that fat-induction factors inhibit osteogenesis, and, conversely, bone-induction factors hinder adipogenesis. In fact, a variety of external cues contribute to the delicate balance of adipo-osteogenic differentiation of MSCs, including chemical, physical, and biological factors. These factors trigger different signaling pathways and activate various transcription factors that guide MSCs to commit to either lineage. The dysregulation of the adipo-osteogenic balance has been linked to several pathophysiologic processes, such as aging, obesity, osteopenia, osteopetrosis, and osteoporosis. Thus, the regulation of MSC differentiation has increasingly attracted great attention in recent years. Here, we review external factors and their signaling processes dictating the reciprocal regulation between adipocytes and osteoblasts during MSC differentiation and the ultimate control of the adipo-osteogenic balance.
Bone is a rigid organ that provides support and physical protection to various vital organs of the body. Throughout the life, bone is in the dynamic balance involving a complex coordination of multiple bone marrow cell types. It is estimated that in adult human body, the entire skeleton is renewed every 7 years. Bone formation by osteoblasts and resorption by osteoclasts are tightly regulated processes responsible for continuous bone remodeling. Osteoclasts originate from hematopoietic stem cell precursors (HSCs) along the myeloid differentiation lineage;1 whereas osteoblasts are derived from a common progenitor cell with adipocytes, bone marrow mesenchymal stem cells (MSCs).2, 3 The imbalance between bone formation and resorption results in various diseases, such as osteopetrosis, osteopenia, and osteoporosis.1 These bone malformations also participate in other diseases such as cancer and autoimmunity. As a common progenitor, the tightly controlled lineage commitment of MSCs has a critical role in the maintenance of bone homeostasis. Although a variety of cell types can be derived from MSCs, the commitment of MSCs to adipocytes and osteoblasts has been specially implicated in pathological conditions of abnormal bone remodeling.4, 5, 6 For example, increased marrow fat content has been observed in osteoporosis patients, the most common bone remodeling disorder worldwide.7, 8 Actually, the increase in bone marrow adiposity has been observed in most bone loss conditions, including aging,8, 9 and various pathological conditions.10, 11, 12, 13, 14, 15, 16, 17 Therefore, modulating lineage commitment of MSCs could provide effective therapeutic regime for related bone diseases.
The lineage commitment of MSCs to adipocytes and osteoblasts definitely warrants further detailed studies, not only because they share a common precursor, but also for the critical roles they play in the bone marrow microenvironment. Investigations in these directions will undoubtedly offer insights into various metabolic and hematological abnormalities during conditions such as obesity, osteoporosis, cancer, and aging. Here, we will review the signaling mechanisms involved in adipogenesis and osteogenesis and discuss the factors that determine the lineage commitment of MSCs.
Mesenchymal Stem Cells
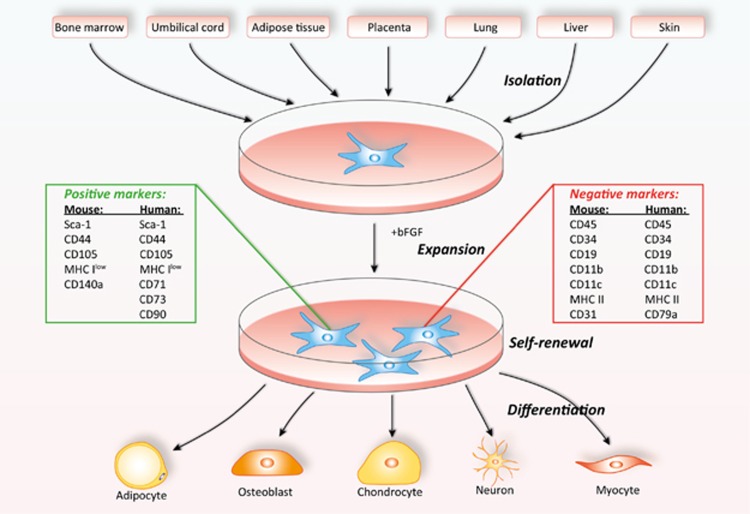
Friedenstein et al.18 first discovered mesenchymal stem cells as spindle-shaped, adherent, non-hematopoietic stem cells in bone marrow. Decades of studies have offered significant in-depth understanding of these cells. MSCs can be easily obtained from many tissues, such as bone marrow, umbilical cord, placenta, fat, lung, liver, and skin.19, 20 The most intensely studied MSCs are those derived from adult bone marrow. In fact, in bone marrow, MSCs are a minimal fraction of nucleated cells, representing 0.001–0.01% of nucleated cells.21, 22 They are typically isolated from whole bone marrow aspiration after removing the non-adherent cells. The adherent mononuclear layer of bone marrow is often cultured in DMEM supplemented with 10% fetal bovine serum and basic fibroblast growth factor (bFGF).23 When a lag phase is broken, the enriched MSCs will proliferate rapidly, reaching confluence at time intervals related to plating density and origins, but often in less than 5 days.24 After expansion and serial passaging, the enriched MSCs are usually heterogeneous, though in most culture, more than 95% are MSCs. Individual MSC clones can be obtained through seeding cells in 96-well plates by limited dilution.25 These homogenous MSC clones can be picked and expanded for studies. As no specific markers have been identified, the purified MSCs are characterized by a combination of positive markers (for human: Sca-1, CD44, CD71, CD73, CD90, and CD105;26, 27 for murine: Sca-1, CD44, CD105, and CD140a28) and negative markers, such as the hematopoietic and endothelial markers (CD45, CD34, CD19, CD11b, CD11c, CD79a, and CD31), costimulatory molecules (CD80, CD86, and CD40), and MHC molecules (negative for class II and low for class I).21, 29, 30 Another important criterion for defining MSCs is their multipotency. MSCs have been confirmed to be induced to differentiate into mature cells of several cell lineages of other types of tissue, such as cartilage, bone, tendon, ligament, and adipose tissue.21, 22 In most laboratories, the differentiation into adipocytes, chondrocytes, and osteoblasts has been used to define MSCs,30 though in vivo bone formation has been urged to be adapted as gold standard for MSC designation (Figure 1).
Figure 1.
Isolation, expansion, and differentiation of MSCs. MSCs can be isolated from various tissues of either human or mouse. This minor population of cells can be isolated, expanded, and enriched after serial passages in vitro. A combination of positive and negative markers can be used to determine the purity of MSCs. In addition to self-renewal, these multipotent MSCs can also undergo differentiation in culture. One of the gold standards for defining MSCs is their differentiation ability to cell lineages such as adipocytes and osteoblasts
Molecular Regulation of the Adipo-Osteogenic Differentiation of MSCs
Signaling pathways in adipo-osteogenic differentiation of MSCs
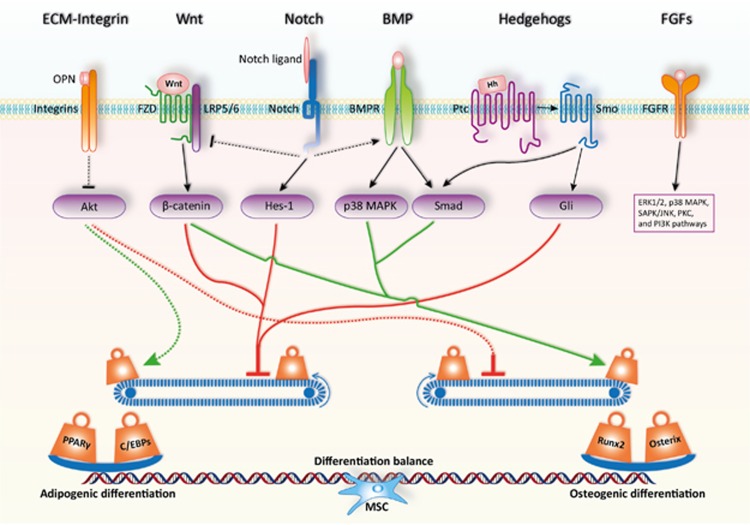
The differentiation of MSCs is a two-step process, lineage commitment (from MSCs to lineage-specific progenitors) and maturation (from progenitors to specific cell types). Intensive studies in recent decades have demonstrated that a number of critical signaling pathways are involved in regulating the lineage commitment of MSCs, including transforming growth factor-beta (TGFβ)/bone morphogenic protein (BMP) signaling, wingless-type MMTV integration site (Wnt) signaling, Hedgehogs (Hh), Notch, and fibroblast growth factors (FGFs). As these pathways are well-established, we only briefly review their roles in MSC differentiation (Figure 2).
Figure 2.
Signaling pathways and key transcription factors in regulating the adipo-osteogenic differentiation of MSCs. The fine balance of adipogenic and osteogenic differentiation of MSCs is achieved by the actions of critical signaling pathways and key transcription factors. MSCs exist in specific microenvironments or niches, which is composed of various extracellular matrix components, growth factors, cytokines, and chemokines. Upon interaction with MSCs, these components activate or inhibit the lineage commitment of MSCs. In addition, the initiated cellular signaling pathways can also interfere each other to form a fine regulatory network. Ultimately, this signaling network maintains a delicate differentiation balance through regulating key transcription factors such as PPARγ and C/EBPs or Runx2 and Osterix for adipogenesis or osteogenesis respectively. OPN, osteopontin; FZD, Frizzled receptor; Hh, Hedgehog; Ptc, Patched; Smo, Smoothened
TGFβ/BMPs family
The TGFβ superfamily consists of more than 30 members, which are widely involved in regulating cell proliferation, cell differentiation, and embryonic development.31 The TGFβ superfamily is divided into three subtypes: TGFβ1, TGFβ2, and TGFβ3 and BMPs belong to TGFβ1 family.32 Different members exert various functions, being dose dependent for some of them,33 in MSC differentiation.34 For example, BMP4 alone can promote adipogenic differentiation of MSCs,35 while BMP2 needs to work together with rosiglitazone to induce adipogenic differentiation.36 Furthermore, low dose of BMP2 promotes C3H10T1/2 to differentiate into adipocytes. However, high dose of BMP2 accelerates osteogenic and chondrogenic differentiation of C3H10T1/2.33 The TGFβ/BMPs signaling pathway has been generally recognized to have dual roles in regulating adipogenic and osteogenic differentiation of MSCs.34 By binding to their transmembrane serine-threonine kinase receptors (type I and type II), TGFβ/BMPs activate canonical Smad-dependent pathways (TGFβ/BMP ligands, receptors, and Smads) and non-canonical Smad-independent signaling pathway (e.g., p38 mitogen-activated protein kinase (MAPK) pathway).37 Upon TGFβ/BMPs stimulation, the expression of runt-related gene 2 (Runx2/Cbfa1)32 and peroxisome proliferator-activated receptor-γ (PPARγ)34 can be regulated by either the Smad or the p38 MAPK pathway. The altered expression level of lineage-specific transcription factors directly control MSC differentiation. Therefore, the composition and concentration of cytokines in the microenvironment of the MSC niche are critical for MSC lineage commitment.
Wnt
The Wnt family consists of a large number of secreted glycoproteins, which function in either paracrine or autocrine manner.38 As a highly conserved signaling pathway during the evolution of multicellular organisms, Wnt signaling is involved in many critical biological processes, including development,38 metabolism,39 and maintenance of stem cells.40 Through binding to the 7-transmembrane domain-spanning Frizzled receptor (FZD) and LRP5/6 coreceptors, Wnt ligands stabilize β-catenin via preventing its phosphorylation.41 Unphosphorylated β-catenin translocates into the nucleus and regulates target genes expression.38 Increasing evidence suggests that Wnt signaling may have an important role in regulating MSC differentiation.42, 43 The activation of Wnt signaling has been reported to facilitate osteogenic differentiation44 and inhibit adipogenic differentiation of MSCs.43 Wnt3a has been specifically shown to stimulate osteogenic differentiation through activation of TAZ by PP1A-mediated dephosphorylation.45 Most recently, it has been demonstrated that YAP/TAZ could mediate alternative Wnt signaling-induced osteogenesis.44 Animal studies showed that activation of Wnt signaling by overexpression of Wnt10b or supplementation of lithium could increase the thickness of trabecular bone.46 Accordingly, deficiency of Wnt10b leads to decrease in bone density.47 Aging-associated increase in adipocytes is also thought to be related to the reduction of Wnt10b.46, 47 In addition, the loss of β-catenin in the mesenchyme of the developing mouse uterus was found to be a switch to adipogenesis in the myometrium.48 These studies provide strong evidence for the role of Wnt signaling in regulating the balance between adipogenic and osteogenic differentiation of MSCs.
Notch
The Notch signaling pathway involves Notch, Notch ligand (Delta/Serrate/LAG-2, DSL protein), and CBF1/Su (H)/Lag-1 (CSL, DNA binding protein).49 Both Notch and Notch ligands are single transmembrane proteins, which involve cell–cell communication to regulate various cell differentiation processes. Like a double-edged sword, Notch showed an inhibitory role and an absolute necessary role in adipogenic differentiation, as demonstrated by studies of the 3T3-L1 model. The expression of PPARγ and C/EBPα was blocked by exposure to Notch ligand jagged1 or overexpression of the Notch target gene Hes-1 in 3T3-L1 cells. Surprisingly, the adipogenic differentiation capability can be reduced in these cells by knockdown of Hes-1 using siRNA.50 Recently, it has been demonstrated that blocking Notch signaling promotes autophagy-mediated adipogenic differentiation of MSCs via the PTEN-PI3K/AKT/mTOR pathway.51 Besides its role in adipogenic differentiation, Notch signaling has also been shown to suppress osteogenic differentiation via inhibiting Wnt/β-catenin signaling.37 However, other studies showed that Notch signaling could also promote osteogenic differentiation through cross-talk with BMP2 signaling.52 Therefore, Notch signaling pathway regulates both adipogenesis and osteogenesis of MSCs in a complex manner through direct targeting related genes or interacting with other signaling pathways.
Hedgehogs
Hedgehogs are secreted proteins consisting of three orthologs: Sonic Hedgehog (SHh), Indian Hedgehog (IHh), and Desert Hedgehog (DHh). Hedgehog precursor is cleaved to produce an active 19 kD N-terminal fragment, which binds to membrane proteins, Patched (Ptc) and Smoothened (Smo). With the ligation of Hedgehog, Smo is released, resulting in activation of the transcription factor Cubitus Interruptus in fly (vertebrate orthologs Gli1, Gli2, and Gli3) to regulate the expression of Hedgehog targeted genes.37 The components of Hedgehog signaling pathway such as SHh, IHh, and DHh as well as Gli are highly expressed in MSCs. During adipogenic differentiation of MSCs, Hedgehog signaling is downregulated due to the decreased expression of Gli. Consistent with this observation, activation of Hedgehog signaling blocked adipogenic differentiation by inhibiting PPARγ and C/EBPα expression and lipid accumulation in 3T3-L1and C3H10T1/2 cells. In addition, inhibition of Gli could promote adipogenic differentiation.53 Regarding osteogenic differentiation, the Hedgehog pathway has a positive role.54, 55, 56 Furthermore, the cross-talk between Hedgehog signal and BMP signal has also been shown to promote osteogenic differentiation through modulating Smad.57 In conclusion, these studies clearly demonstrate that the Hedgehog signaling pathway is pro-osteogenic and anti-adipogenic.
Other signaling molecules involved in MSC differentiation
Several other signaling pathways have also been implicated in regulating adipogenic and osteogenic differentiation of MSCs, including FGFs, PDGF, EGF, and IGF.58, 59, 60 Their roles in MSC differentiation mainly exert through regulating signaling pathways we discussed previously, such as Wnt and TGFβ/BMP pathways.
FGFs have been implicated in both adipogenesis and osteogenesis.61 The FGF family consists of 23 structurally related members that are ubiquitously expressed in almost all tissue types. After the binding of FGF, FGF receptors dimerize and set off the downstream signaling cascade. The FGF receptor signaling cascade has been shown to involve ERK1/2, p38 MAPK, SAPK/JNK, PKC, and PI3K pathways,61, 62 which all have been shown to play important roles in regulating MSC differentiation. FGF members exert different effects on adipogenic and osteogenic differentiation of MSCs. For example, the osteogenic transcription factor Runx2 can be upregulated by FGF2, FGF4, and FGF8.63 In addition, FGF2 could induce alkaline phosphatase activity in rat bone marrow precursors,64 and promote mineralization during the late phase of osteogenic differentiation, together with FGF9 and FGF18. In terms of adipogenic differentiation, FGF1, FGF2, and FGF10 have been shown to possess strong adipogenic effect under the adipogenic condition.65, 66, 67 Accumulating evidence has clearly shown that FGF2 exerts dual roles in regulating adipogenic and osteogenic differentiation. This is probably due to the interactions between FGF-initiated signaling pathways and other differentiation-related signaling pathways.
It is important to emphasize that the signaling pathways discussed above do not function in isolation. The lineage commitment of MSCs is determined by a network of various signaling pathways (Figure 2) that can be activated simultaneously by stimuli in specific microenvironment. For example, BMP2 signaling can interact with Wnt pathway through β-catenin and N-cadherin,68 which may explain the dual roles of BMP2 in the adipogenic and osteogenic differentiation of MSCs.
microRNAs
Compared with well-known molecular signaling pathways, microRNAs involved in the lineage commitment of MSCs have just been caught on. It is found that various microRNAs are related to the regulation of differentiation of MSCs. Some of them have roles in lineage commitment while others are critical for terminal differentiation.69, 70, 71 Here, we summarize the microRNAs involved in lineage determination of MSCs.
Huang et al.72 demonstrated that miR-204 promoted adipogenic differentiation of MSCs via targeting Runx2, an osteogenic transcription factor. Overexpression of miR-204 and its human homolog miR-211 suppressed osteogenic differentiation and enhanced adipogenic differentiation. By targeting Osterix, another important osteogenic transcription factor, miR-637 promoted adipogenesis while inhibited osteogenesis.73 miR-27b was reported to inhibit adipogenesis by blunting PPARγ and C/EBPα, key adipogenic transcription factors.74 In addition, miR-21 has been suggested as a negative regulator of TGFβ signaling. Overexpression of miR-21 can restore the inhibition effect of TGFβ on adipogenic differentiation of MSCs. Further study showed that miR-21 was transiently upregulated after adipogenic differentiation along with the decreased TGFβR2 expression. miR-21 blocked the TGFβ signaling via inhibiting the phosphorylation of Smad3. Therefore, miR-21 might have a negative role in osteogenic differentiation via inhibiting TGFβ signaling.75, 76
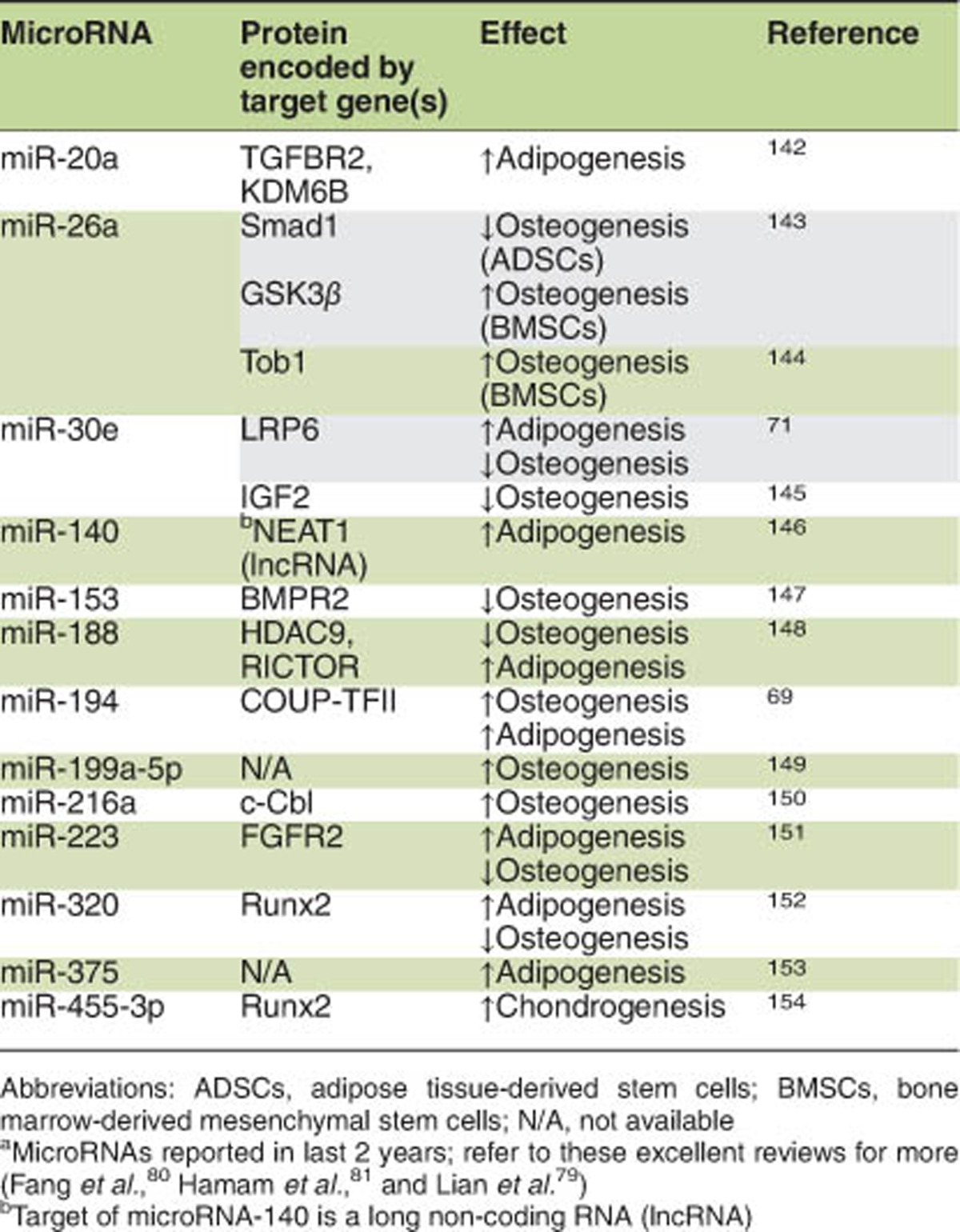
Besides controlling the balance of adipo-osteogenic differentiation in MSCs, there are some other microRNAs that exert a parallel effect on adipogenic and osteogenic differentiation. The expression of miR-335, high level in quiescent human MSCs (hMSCs), decreased during osteogenesis. However, overexpression of miR-335 inhibited both osteogenic and adipogenic differentiation ability of hMSCs. Further studies showed that miR-335 regulated the differentiation of hMSCs through direct targeting Runx2.70 Similarly, miR-138 has also been reported to inhibit both adipogenic and osteogenic differentiation of MSCs.77, 78 The regulation of MSC differentiation by microRNAs has recently been reviewed elsewhere. The bone homeostasis controlled by microRNAs was summarized recently.79 In addition, detailed lists of target genes of various microRNAs and related signaling pathways during osteogenic and adipogenic differentiation have also been published.80, 81 Interestingly, during the last 2 years, along with the understanding of the MSC biology, remarkable progress has been accomplished in this exciting field. Hereby, we present a summary of latest identified microRNAs with the capacity to regulate MSC differentiation (Table 1).
Table 1. Role of microRNAsa in the regulation of MSC differentiation.
Transcription factors involved in osteogenic and adipogenic differentiation of MSCs
Transcription factors that help to initiate and promote the differentiation process are direct or indirect targets of various signaling pathways. Multiple transcription factors have been demonstrated to be critical for the differentiation of MSCs to adipocytes or osteoblasts. The PPARγ and C/EBPs are involved in adipogenic differentiation of MSCs,82, 83, 84, 85 while Runx2 and Osterix are required for osteogenic differentiation.68 Here, we provide detailed descriptions of transcriptional cascades for adipogenic and osteogenic differentiation of MSCs.
During adipogenic differentiation, the level of cyclic AMP is elevated in the adipogenic condition, which results in phosphorylation of cyclic AMP response element-binding protein (CREB).86 The phosphorylated CREB induces the expression of C/EBPβ, a member of the C/EBP family. The other two C/EBP family members (C/EBPα and C/EBPδ) have also been implicated in adipogenic differentiation of MSCs.83 C/EBPβ and C/EBPδ are rapidly (within 4 h) upregulated following the induction of adipogenic differentiation; however, C/EBPβ is inactive and unable to bind to DNA. C/EBPβ requires activation through phosphorylation on Thr188 by MAP kinase and on Thr179 or Ser184 by GSK3β. Then, the transcription of PPARγ and C/EBPα is activated after the binding of C/EBPβ to regulatory elements in their proximal promoters.86 Once expressed, C/EBPα maintains the continuous expression of both PPARγ and C/EBPα through binding to their respective C/EBP regulatory elements. PPARγ and C/EBPα work together to regulate large group of genes that induce the adipocyte phenotype.87 Unlike the downregulation of C/EBPβ in later stage of differentiation process, the expression of PPARγ and C/EBPα maintains a high level through the entire differentiation process and continue the expression throughout the whole life of adipocytes.
Runx2 and Osterix are considered as master transcription factors in regulating osteogenic differentiation of MSCs.88, 89 During osteoblast differentiation, most signaling pathways investigated so far are targeted at Runx2.88 Upregulation of Runx2 in MSCs promotes their differentiation potential into immature osteoblasts, while inhibits their lineage commitment to the adipocytes.90 In addition, Runx2 has been shown to be required for the induction of major bone matrix genes in immature osteoblasts, while unnecessary for the maintenance of these genes in mature osteoblasts.91 Indeed, one recent study has demonstrated that the feedforward regulation between Runx2 and Glut1 (a glucose transporter) facilitates the initiation of osteoblast differentiation.92 On the other hand, Osterix and β-catenin are required for the maturation of osteoblasts,89, 90 while Runx2 is decreased during the maturation process.93 Although great progress has been made in the past few years, further studies are required for better understanding of the transcriptional network regulating osteogenic differentiation compared with the well-established transcriptional cascade during adipogenic differentiation.
Regulators Controlling the Balance Between Adipogenic and Osteogenic Differentiation of MSCs
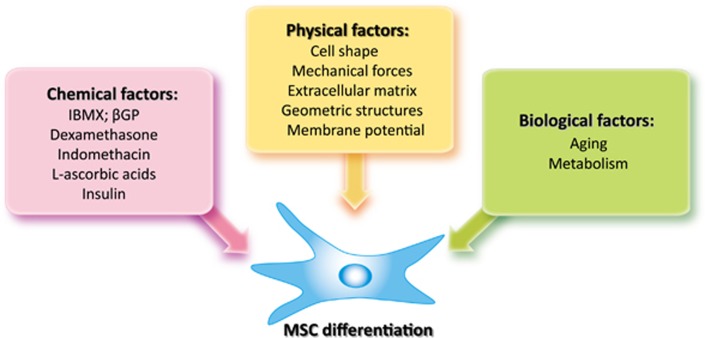
Meunier et al.7 reported that there's a replacement of cell populations of the bone marrow by adipose tissue in osteoporosis patients. The balance between adipocytes and osteoblasts in bone marrow has attracted significant attention ever since. As the prevalence of obesity and osteoporosis increases in the past few years, the commitment of MSCs has been intensely studied. Accumulating information clearly shows that the lineage commitment of MSCs is directed by a multitude of cues. Here, we will discuss the cues controlling the balance between adipogenic and osteogenic differentiation of MSCs, including chemical, physical, and biological factors (Figure 3).
Figure 3.
Multiple factors control MSC differentiation. The lineage commitment of MSCs can be regulated by three major cues, including chemical, physical, and biological factors. Chemical factors have been proven to be important in directing adipogenesis and osteogenesis of MSCs in vitro through regulating key transcription factors during MSC differentiation. In vivo, the differentiation of MSCs can also be altered by physical factors in the stem cell niche. Investigations into the regulation of MSC differentiation commitment by cell shape, external mechanical forces, extracellular matrix or geometric structures have provided very useful information for stem cell-based bone tissue regeneration/engineering. Meanwhile, tilted differentiation balance of MSCs is also observed during aging or other pathological processes, arguing for the roles of biological factors in lineage commitment of MSCs. Taken together, these three types of factors likely work closely and cooperate with each other to regulate MSC differentiation. IBMX, isobutylmethylxanthine; βGP, β-glycerophosphate
Chemical factors
The stemness of freshly isolated MSCs is determined using well-established assays in differentiation medium containing several chemicals. For adipogenic differentiation, MSCs are usually cultured in medium supplemented with isobutylmethylxanthine (IBMX), indomethacin, dexamethasone (Dex), and insulin.21, 25 IBMX and Dex are important for the initiation of adipogenic differentiation. It is reported that IBMX can inhibit phosphodiesterases, which causes an elevation of intracellular cAMP.94 Elevated cAMP then leads to the alteration in transcription factors through protein kinase A. At the same time, IBMX can directly induce C/EBPβ expression as well. Similarly, Dex activates C/EBPδ expression through binding to intracellular glucocorticoid receptor.83 Indomethacin is a well-known inhibitor of COX1/2 though its adipogenic activity is not due to the inhibition of COX, but the activation of PPARγ.95 Insulin functions to promote the uptake of glucose for the synthesis of triglycerides in adipocytes.
To differentiate into osteoblasts, MSCs are usually cultured in osteogenic medium containing Dex, l-ascorbic acid (AA), and β-glycerophosphate (βGP).21, 25 To analyze the effects of these chemicals on the differentiation of MSCs, Coelho and Fernandes96 cultured MSCs in standard medium supplemented with AA, βGP, or Dex alone, or two combinations: AA+βGP and AA+βGP+Dex. AA was found to initiate the formation of a collagenous extracellular matrix (ECM), which further led to the upregulation of alkaline phosphatase (ALP) and osteocalcin. Similar observation was also made previously by another group.97 Dex, however, promoted the cell proliferation,98 which resulted in the induction of ALP activity99 and mineral deposition.96 βGP, on the other hand, was hydrolyzed by ALP, and thus provided high level of phosphate ions for mineral deposition of ECM.96
The differentiation of MSCs is driven by several biological processes, such as proliferation, morphological changes, expression of lineage-specific markers, lipid accumulation, and mineral deposition. The chemicals mentioned above undertake the work mutually, cooperate closely, and regulate the MSC differentiation interactively.
Physical factors
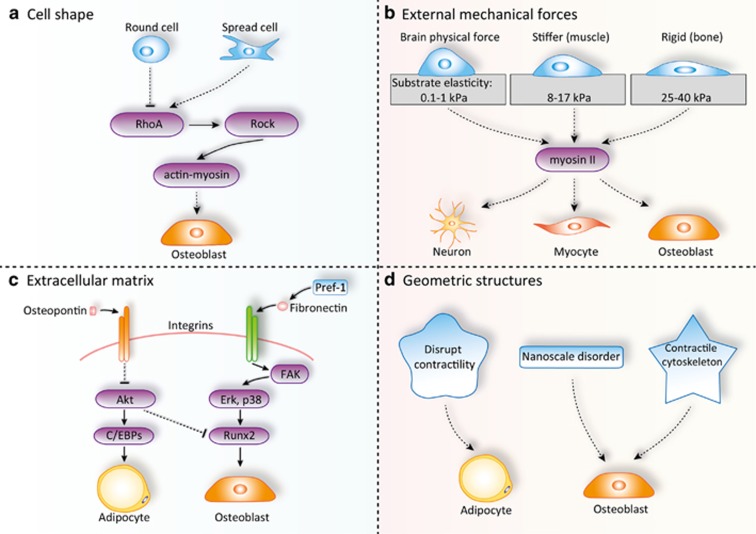
In vivo, MSCs are not in isolation, but physically interact with components in the microenvironment. For several decades, physical factors including cell shape, external mechanical forces, ECM, and geometric structures have been implicated in stem cell fate decision (Figure 4). MSCs exist in almost all adult tissues and have been isolated from a variety of tissues, such as muscle, umbilical cord, bone marrow, brain, and amniotic fluid.19, 20 Thus, MSCs physically exist in diverse microenvironment. Engler et al.100 documented that ECM controls the lineage commitment of naive MSCs. Matrices mimicking the brain physical force support neurogenic differentiation. Stiffer matrices promote myogenic differentiation, while rigid matrices support osteogenic differentiation. It was found that non-muscle myosin II is the key mechano-transducer of this ECM physical property-dependent control of MSCs fate decision.
Figure 4.
Physical factors regulating lineage commitment of MSCs. MSCs physically interact with various components in the tissue microenvironment in vivo. The physical factors including cell shape, external mechanical forces, extracellular matrix, and geometric structures are involved in stem cell fate decision. By regulating RhoA-ROCK signaling pathway, spread cells tend to differentiate into osteoblasts while round cells tend to become adipocytes (a). Different physical forces can also direct MSCs to differentiate into different lineages via controlling myosin II activity (b). Meanwhile, components of extracellular matrix, such as osteopontin and fibronectin, can regulate the adipo-osteogenic balance of MSCs through binding to integrin receptors (c). In addition, geometric cues such as nanoscale changes can also effectively dictate the differentiation of MSCs (d)
Integrins are transmembrane receptors mediating cell–matrix and cell–cell interactions. Integrins are a family of transmembrane heterodimer adhesion molecules that transduce signals to and from the cytoplasm across the plasma membrane. Ligands binding to integrins lead to its activation, which results in phosphorylation of focal adhesion kinase (FAK) and followed by activation of a series of signaling proteins including phosphatidylinositol 3-kinase (PI3K), MAPK ERK1/2, protein kinase C (PKC), and GTPases of the Rho family.101 FAK-mediated activation of ERK1/2 and p38 has been reported to phosphorylate and activate Runx2, resulting in increased osteogenic differentiation of MC3T3-E1 cells.102 Pref-1/DLK1, a known inhibitor of adipocyte differentiation, was reported to be involved in skeletal malformations, growth retardation, and obesity during development.103 Initially, it was believed to regulate adipogenic differentiation via Notch signaling. Recent study has proved that Pref-1 interacts with fibronectin to inhibit adipogenesis.104, 105 Fibronectin is an important component of the ECM, which interacts with various integrin receptors and results in suppression of known transcription factors of adipogenesis.106
Integrins can be sensors of mechanical forces through transducing mechanical signals to the actin cytoskeleton.107 It has been shown that mechanical forces could facilitate osteogenic differentiation and inhibit adipogenic differentiation of MSCs.108 Interestingly, modulation of actin using depolymerizing drugs cytochalasin D or latrunculin A, and stabilizing drug jasplakinolide during mechanical loading, was demonstrated to regulate ERK and AKT-mediated signal transduction and mechanical force-induced MSC differentiation.107 Meanwhile, the role of mTORC2 in mechanically induced signaling transduction and MSC differentiation was also investigated. It was found that Fyn, a Src family kinase, mediated the mechanical activation of mTORC2 and phosphorylation of FAK, an enhancer for mTORC2 activation. This mechanically induced Fyn/FAK/mTORC2 signaling pathway decreases adipogenic differentiation of MSCs via enhancing β-catenin signaling and regulates cytoskeleton by activating RhoA.109 In addition, a recent study showed that mTORC2 is also involved in cytoskeleton reconstruction. Deficiency of mTORC2 in MSCs has been found to abolish strain-induced cytoskeletal reorganization, and impair osteogenic differentiation while facilitate adipogenic differentiation of these cells.110 These studies provide important information for the understanding of exercise therapy regimens in treating osteogenesis and adipogenesis related diseases, such as osteoporosis.
Recently, osteopontin (OPN) has been demonstrated to inhibit adipogenic differentiation and promote osteogenic differentiation in MSCs through interacting with integrin αvβ1 and regulating C/EBPs expression.111 Blockade of OPN by neutralizing antibody or siRNA knockdown of OPN promotes robust adipogenic differentiation, while inhibiting osteogenic differentiation. Its role in MSC differentiation is further verified in a hydroxyapatite-tricalcium phosphate-based implantation model in vivo. Although the OPN-deficient mice develop normally, these mice show an increase ratio of both subcutaneous and visceral fat tissue to body weight.111 It indicates that OPN has a critical role in regulating the balance between adipogenesis and osteogenesis during the development.
Geometric cues showed dramatic effects on MSC lineage commitment. Nanoscale disorders have been shown to stimulate MSC differentiation into osteoblasts in the absence of osteogenic inducers.112 In addition, by culturing geometrically patterned MSCs in medium containing both adipogenic and osteogenic chemical inducers, it has been shown that geometric cues of native contractile cytoskeleton were osteogenic, while those disrupting contractility were adipogenic.113
A micro-patterning technique was developed to study the effect of geometric cues on MSC differentiation. By using this technology, researchers were able to precisely monitor MSC attachment by depositing specific proteins (cell resistant) on substrate and control the cell culture substrates geometrically.114 Therefore, the interaction between MSCs and patterned substrates can be specifically analyzed through applying this technique. Chen et al.115 reported that geometrically patterned substrates controlled the cell growth and viability of endothelial cells. Recently, McBeath and Chen used this technique to control the cell shape and found that spread cells tend to differentiated into osteoblasts whereas round cells tend to differentiate into adipocytes. This cell shape-controlled lineage commitment was exerted through activating the RhoA-ROCK signaling, which was activated by actin-myosin-generated tension. Moreover, as the culture density determines the spreading degree of cells,116 it might be a potential explanation for the different requirement of cell density during the differentiation of MSCs into adipocytes, osteoblasts, or chondrocytes.21
To better mimic the cell biology properties of physical factors in vivo, three-dimensional culture systems have been constructed. This is a millstone for the cell biology moving from in vitro to in vivo. These three-dimensional culture systems could better imitate the in vivo microenvironment, so that scientists are able to control the cell shape artificially in three dimensions.117 Usually, three-dimensional systems are built relying on poly (ethylene glycol)-based hydrogels. In hydrogels, the cells are more rounded than those cultured in standard two-dimensional systems. Recently, it was reported that the shape of MSCs could be modulated dynamically through creating photodegradable poly (ethylene glycol)-based hydrogels.118 This system makes it possible to study the dynamic physical interactions between ECM and MSCs as well as the effect of these dynamic interactions on MSC differentiation.
In addition to the physical contact with ECM, the membrane potential also has important roles in controlling the differentiation of MSCs. Interestingly, depolarization suppresses the adipogenic and osteogenic differentiation of MSCs while hyper-polarization promotes osteogenic differentiation.119 Moreover, uniaxial mechanical tension and fluid flow-induced shear stress have been shown to significantly increase alkaline phosphatase activity and the expression of osteogenic genes in MSCs.120, 121 Therefore, in order to better understand the role of physical factors in the differentiation of MSCs, models better mimicking the in vivo situations are awaiting to be developed.
Other biological factors
Aging
It has been known for a long time that bone loss during aging and some pathological processes are accompanied by increased bone marrow adiposity due to the shift of differentiation balance between osteoblasts and adipocytes of bone marrow mesenchymal stem cells.7, 8, 9 However, detailed mechanisms underlying this balance shift are poorly understood. Sun et al.122 had examined the effects of aging on osteogenic differentiation of MSCs by using proteomics analysis. Several molecules associated with this age-related loss of osteogenic potential were identified in MSCs. Chloride intracellular channel 1 (CLIC1) and prohibitin were found to be decreased in aged MSCs, while LIM and SH3 domain protein 1 (LASP1) and annexin V were increased. As aging progressing, reactive oxygen species (ROS) and oxidative stress have been shown to be increased and to play important roles in age-related bone loss and adipo-osteogenic differentiation through forkhead homeobox type O (FOXO), Wnt, and PPARγ.123, 124, 125
PPARγ, as the central transcription factor in adipogenic differentiation, suppresses osteoblast differentiation. Moerman et al.9 demonstrated that the expression of PPARγ was increased in aged bone marrow MSCs by unknown PPARγ activators. The increased PPARγ expression promoted adipogenesis and inhibited osteogenesis of bone marrow MSCs in old animals. Rosiglitazone, an activator of PPARγ for type II diabetes therapy, was reported to cause side effects on bone metabolism, such as osteoporosis. It was also found to induce ROS accumulation specifically in osteoblasts resulting in PPARγ-dependent apoptosis.126 Interestingly, adipocytes were protected from rosiglitazone-induced ROS-related apoptosis. Therefore, aging alters the elaborate balance system between osteogenic differentiation and adipogenic differentiation in MSCs.
Metabolism
Accumulative evidence shows that altered metabolic processes, such as mitochondrial metabolism,127 oxidative stress,128 and glucose uptake,92 have been implicated to affect MSC differentiation. An increase in mitochondrial metabolism and ROS generation is a key property of MSCs undergoing adipogenic differentiation.129, 130 However, it is unknown whether this increase is a causal factor or a consequence of adipogenic differentiation. It has been demonstrated that mitochondrial-targeted antioxidants could decreased the adipogenic differentiation of MSCs, while exogenous hydrogen peroxide could restore it. In addition, it has been showed that ROS generated by mitochondrial complex III is essential for the activation of adipogenic transcription factors.130 These results implicate that the increased mitochondrial metabolism is an early causal factor for adipogenesis. Indeed, increased mitochondrial metabolism has been shown to be prerequisite of adipogenic differentiation demonstrated by specific blocking the mitochondrial respiratory pathways.127 On the other hand, hypoxia signaling that shifts metabolism from oxidative to glycolysis has been shown to inhibit both osteogenic131, 132, 133 and adipogenic134 differentiation of MSCs. However, it has also been demonstrated that hypoxia pretreatment of human adipose tissue MSCs could facilitate both adipogenic and osteogenic differentiation under normoxic condition.135 In addition, there is another report shows that the osteogenic and adipogenic differentiation of MSCs is not affected by either hypoxia or normoxic conditions.136 There are several possibilities for these contradictory findings: (1) variation in the standards of hypoxia and normoxia; (2) differences in culture time under hypoxia (short-term, long-term, or transient); and (3) different regimes of hypoxia and normoxic culture conditions, such as pretreatment with hypoxia for a while then transfer into normoxia conditions for differentiation assay, or first normoxic conditions then transfer into hypoxic conditions. Although considerable progress have been made in deciphering the role of metabolism in regulating MSC differentiation, criteria should be put forward to standardize the experiment system and reasonable care should be taken when performing a direct extrapolation of in vitro findings to the situations in vivo.137
Reciprocality Between Adipogenesis and Osteogenesis
Over decades of study, it is more and more clear that the adipogenesis and osteogenesis of MSCs are competing and reciprocal. For example, the BMP signaling pathway has a dual role in regulating the adipogenic and osteogenic differentiation of MSCs. BMP4 subjects MSCs to adipogenic differentiation.35 Interestingly, BMP2 promotes osteogenic differentiation at high concentrations while favors adipogenic differentiation at low concentrations.33
Usually, adipose tissue is recognized as an organ of energy storage. Recently, accumulating studies have identified adipose tissue as an active endocrine organ because of the secretion of various active molecules (adipokines), such as leptin, adiponectin, IL-6, and TNF-α.138 Similarly, bones have also been recognized as endocrine organs besides their role in supporting the body. They secrete a variety of active cytokines (osteokines), including osteopontin, osteocalcin, and osteoprotegerin.139 These adipokines and osteokines have key roles in bone and fat metabolism reciprocally.
It has been reported that PKA stimulators can promote adipogenesis and inhibit osteogenesis through leptin expression and secretion.140 This effect of PKA stimulators on MSCs differentiation can be blocked by adding leptin exogenously. In addition, leptin can restore skeletal ossification in IBMX-treated developing zebrafish. Recently, it has been confirmed that overexpression of leptin in MSCs upregulates osteocalcin expression and promotes ALP activity. Cbfα1 and Cbfβ, key osteogenic transcription factors, were also upregulated in those MSCs.141 In summary, adipogenesis and osteogenesis are reciprocally regulated processes of MSC differentiation. They modulate each other through secreting various active adipokines and osteokines.
Conclusions and Future Directions
Investigations from various groups in different systems have demonstrated that biological, chemical, and physical cues can exert their effects via a batch of signaling pathways on the balance between adipogenesis and osteogenesis of MSCs by affecting initiation, commitment, and differentiation. These signals finally converge at a tightly controlled cascade of transcription events, including C/EBPs and PPARγ for adipogenesis and Runx2 and TAZ for osteogenesis.86 The chemical cues provide us a well-established system to identify and study the commitment of MSCs. The physical cues, especially those from the ECM, promise MSCs a future in biomaterial-based regenerative medicine. The critical roles of biological factors, including various types of cytokines and microRNAs, provide us a better understanding of pathophysiology control of MSC differentiation. These remarkable advances in understanding the fate decision of MSCs are still preliminary and more intense studies including ‘MSC omics' are urgently needed to fully understand the mechanisms underlying the balance between adipogenic and osteogenic differentiation of MSCs. These new data will be of great value to identify the pathogenic causes of fat and bone marrow related diseases, to develop novel therapies for these diseases, and to better clinical application of MSCs in tissue engineering and regenerative medicine.
Acknowledgments
We are indebted to those whose work is not discussed due to space limitations. This work is supported by grants from Scientific Innovation Project of the Chinese Academy of Sciences (XDA01040107), the Ministry of Science and Technology of China (2015CB964500), National Natural Science Foundation of China (81330046, 81530043, 81273316, 81571612), and Shanghai Rising-Star Program (14QA1404200).
Glossary
- MSCs
mesenchymal stem cells
- FGF
fibroblast growth factor
- TGFβ
transforming growth factor-β
- BMP
bone morphogenic protein
- Wnt
wingless-type MMTV integration site
- Hh
Hedgehog
- Runx2
runt-related gene 2
- PPARγ
peroxisome proliferator-activated receptor-γ
- ALP
alkaline phosphatase
- OPN
osteopontin
- ROS
reactive oxygen species
The authors declare no conflict of interest.
Footnotes
Edited by R De Maria
References
- Teitelbaum SL. Bone resorption by osteoclasts. Science 2000; 289: 1504–1508. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Mesenchymal stem-cells. J Orthop Res 1991; 9: 641–650. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005; 7: 393–395. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WWK, Gordon PL, Neel M et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 1999; 5: 309–313. [DOI] [PubMed] [Google Scholar]
- Pino AM, Rosen CJ, Rodriguez JP. In osteoporosis, differentiation of mesenchymal stem cells (MSCs) improves bone marrow adipogenesis. Biol Res 2012; 45: 279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, de Paula FJ, Rosen CJ. New insights into osteoporosis: the bone-fat connection. J Intern Med 2012; 272: 317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and replacement of cell populations of marrow by adipose tissue - a quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res 1971; 80: 147–154. [DOI] [PubMed] [Google Scholar]
- Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2001; 2: 165–171. [DOI] [PubMed] [Google Scholar]
- Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma 2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell 2004; 3: 379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV et al. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity 2011; 19: 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra M, Klibanski A. Anorexia nervosa, obesity and bone metabolism. Pediatr Endocrinol Rev 2013; 11: 21–33. [PMC free article] [PubMed] [Google Scholar]
- Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res 2011; 6: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AV, Sigurdsson S, Hue TF, Lang TF, Harris TB, Rosen CJ et al. Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults. J Clin Endocrinol Metab 2013; 98: 2294–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Dempster DW, Stein EM, Nickolas TL, Zhou H, McMahon DJ et al. Increased marrow adiposity in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab 2012; 97: 2782–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley-Javoroski S, Shields RK. Dose estimation and surveillance of mechanical loading interventions for bone loss after spinal cord injury. Phys Ther 2008; 88: 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee-Lawrence ME, Carpio LR, Schulze RJ, Pierce JL, McNiven MA, Farr JN et al. Hdac3 deficiency increases marrow adiposity and induces lipid storage and glucocorticoid metabolism in osteochondroprogenitor cells. J Bone Miner Res 2016; 31: 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motyl KJ, Raetz M, Tekalur SA, Schwartz RC, McCabe LR. CCAAT/enhancer binding protein beta-deficiency enhances type 1 diabetic bone phenotype by increasing marrow adiposity and bone resorption. Am J Physiol Regul Integr Comp Physiol 2011; 300: R1250–R1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet 1970; 3: 393–403. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002; 13: 4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirelles LDS, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 2006; 119: 2204–2213. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143–147. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002; 418: 41–49. [DOI] [PubMed] [Google Scholar]
- Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc 2009; 4: 102–106. [DOI] [PubMed] [Google Scholar]
- Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem 1997; 64: 278–294. [DOI] [PubMed] [Google Scholar]
- Ren GW, Zhang LY, Zhao X, Xu GW, Zhang YY, Roberts AI et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008; 2: 141–150. [DOI] [PubMed] [Google Scholar]
- Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells 2014; 32: 1408–1419. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8: 315–317. [DOI] [PubMed] [Google Scholar]
- Boxall SA, Jones E. Markers for characterization of bone marrow multipotential stromal cells. Stem Cells Int 2012; 2012: 975871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs SA, Roobrouck VD, Verfaillie CM, Van Gool SW. Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunol Cell Biol 2013; 91: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L. Immunological characterization of multipotent mesenchymal stromal cells—The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy 2013; 15: 1054–1061. [DOI] [PubMed] [Google Scholar]
- Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol 2012; 13: 616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Deng C, Li YP. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci 2012; 8: 272–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Nieden NI, Kempka G, Rancourt DE, Ahr HJ. Induction of chondro-, osteo- and adipogenesis in embryonic stem cells by bone morphogenetic protein-2: effect of cofactors on differentiating lineages. BMC Dev Biol 2005; 5: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Q, Song WX, Luo Q, Tang N, Luo J, Luo X et al. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev 2009; 18: 545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang QQ, Otto TC, Lane MD. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci USA 2004; 101: 9607–9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottile V, Seuwen K. Bone morphogenetic protein-2 stimulates adipogenic differentiation of mesenchymal precursor cells in synergy with BRL 49653 (rosiglitazone). FEBS Lett 2000; 475: 201–204. [DOI] [PubMed] [Google Scholar]
- Deng ZL, Sharff KA, Tang N, Song WX, Luo J, Luo X et al. Regulation of osteogenic differentiation during skeletal development. Front Biosci 2008; 13: 2001–2021. [DOI] [PubMed] [Google Scholar]
- Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014; 346: 1248012. [DOI] [PubMed] [Google Scholar]
- Sherwood V. WNT signaling: an emerging mediator of cancer cell metabolism? Mol Cell Biol 2015; 35: 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland JD, Klaus A, Garratt AN, Birchmeier W. Wnt signaling in stem and cancer stem cells. Curr Opin Cell Biol 2013; 25: 254–264. [DOI] [PubMed] [Google Scholar]
- Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci 2009; 66: 236–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CN, Ross SE, Longo KA, Bajnok L, Hemati N, Johnson KW et al. Regulation of Wnt signaling during adipogenesis. J Biol Chem 2002; 277: 30998–31004. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Li Q, Luo S, Liu Z, Luo D, Zhang B et al. PPARgamma and Wnt signaling in adipogenic and osteogenic differentiation of mesenchymal stem cells. Curr Stem Cell Res Ther 2015; 10: 1–10. [DOI] [PubMed] [Google Scholar]
- Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW et al. Alternative Wnt signaling activates YAP/TAZ. Cell 2015; 162: 780–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun MR, Hwang JH, Kim AR, Kim KM, Hwang ES, Yaffe MB et al. Canonical Wnt signalling activates TAZ through PP1A during osteogenic differentiation. Cell Death Differ 2014; 21: 854–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CN, Ouyang H, Ma YL, Zeng Q, Gerin I, Sousa KM et al. Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation. J Bone Miner Res 2007; 22: 1924–1932. [DOI] [PubMed] [Google Scholar]
- Stevens JR, Miranda-Carboni GA, Singer MA, Brugger SM, Lyons KM, Lane TF. Wnt10b deficiency results in age-dependent loss of bone mass and progressive reduction of mesenchymal progenitor cells. J Bone Miner Res 2010; 25: 2138–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango NA, Szotek PP, Manganaro TF, Oliva E, Donahoe PK, Teixeira J. Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol 2005; 288: 276–283. [DOI] [PubMed] [Google Scholar]
- Lin GL, Hankenson KD. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J Cell Biochem 2011; 112: 3491–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross DA, Rao PK, Kadesch T. Dual roles for the Notch target gene Hes-1 in the differentiation of 3T3-L1 preadipocytes. Mol Cell Biol 2004; 24: 3505–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song BQ, Chi Y, Li X, Du WJ, Han ZB, Tian JJ et al. Inhibition of Notch signaling promotes the adipogenic differentiation of mesenchymal stem cells through autophagy activation and PTEN-PI3K/AKT/mTOR pathway. Cell Physiol Biochem 2015; 36: 1991–2002. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Tanaka T, Iso T, Matsui H, Ooyama Y, Kawai-Kowase K et al. Notch signaling pathway enhances bone morphogenetic protein 2 (BMP2) responsiveness of Msx2 gene to induce osteogenic differentiation and mineralization of vascular smooth muscle cells. J Biol Chem 2011; 286: 19138–19148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine C, Cousin W, Plaisant M, Dani C, Peraldi P. Hedgehog signaling alters adipocyte maturation of human mesenchymal stem cells. Stem Cells 2008; 26: 1037–1046. [DOI] [PubMed] [Google Scholar]
- Kim WK, Meliton V, Bourquard N, Hahn TJ, Parhami F. Hedgehog signaling and osteogenic differentiation in multipotent bone marrow stromal cells are inhibited by oxidative stress. J Cell Biochem 2010; 111: 1199–1209. [DOI] [PubMed] [Google Scholar]
- James AW, Pang S, Askarinam A, Corselli M, Zara JN, Goyal R et al. Additive effects of sonic hedgehog and Nell-1 signaling in osteogenic versus adipogenic differentiation of human adipose-derived stromal cells. Stem Cells Dev 2012; 21: 2170–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Dong Q, Wang Y, Feng Q, Zhou P, Ou X et al. Hedgehog signaling is involved in the BMP9-induced osteogenic differentiation of mesenchymal stem cells. Int J Mol Med 2015; 35: 1641–1650. [DOI] [PubMed] [Google Scholar]
- Spinella-Jaegle S, Rawadi G, Kawai S, Gallea S, Faucheu C, Mollat P et al. Sonic hedgehog increases the commitment of pluripotent mesenchymal cells into the osteoblastic lineage and abolishes adipocytic differentiation. J Cell Sci 2001; 114: 2085–2094. [DOI] [PubMed] [Google Scholar]
- Krampera M, Pasini A, Rigo A, Scupoli MT, Tecchio C, Malpeli G et al. HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: inducing cell expansion and reversibly preventing multilineage differentiation. Blood 2005; 106: 59–66. [DOI] [PubMed] [Google Scholar]
- Gruber R, Karreth F, Kandler B, Fuerst G, Rot A, Fischer MB et al. Platelet-released supernatants increase migration and proliferation, and decrease osteogenic differentiation of bone marrow-derived mesenchymal progenitor cells under in vitro conditions. Platelets 2004; 15: 29–35. [DOI] [PubMed] [Google Scholar]
- Kratchmarova I, Blagoev B, Haack-Sorensen M, Kassem M, Mann M. Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science 2005; 308: 1472–1477. [DOI] [PubMed] [Google Scholar]
- Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev 2005; 16: 139–149. [DOI] [PubMed] [Google Scholar]
- Jackson RA, Nurcombe V, Cool SM. Coordinated fibroblast growth factor and heparan sulfate regulation of osteogenesis. Gene 2006; 379: 79–91. [DOI] [PubMed] [Google Scholar]
- Woei NgK, Speicher T, Dombrowski C, Helledie T, Haupt LM, Nurcombe V et al. Osteogenic differentiation of murine embryonic stem cells is mediated by fibroblast growth factor receptors. Stem Cells Dev 2007; 16: 305–318. [DOI] [PubMed] [Google Scholar]
- Ling L, Murali S, Dombrowski C, Haupt LM, Stein GS, van Wijnen AJ et al. Sulfated glycosaminoglycans mediate the effects of FGF2 on the osteogenic potential of rat calvarial osteoprogenitor cells. J Cell Physiol 2006; 209: 811–825. [DOI] [PubMed] [Google Scholar]
- Neubauer M, Fischbach C, Bauer-Kreisel P, Lieb E, Hacker M, Tessmar J et al. Basic fibroblast growth factor enhances PPARgamma ligand-induced adipogenesis of mesenchymal stem cells. FEBS Lett 2004; 577: 277–283. [DOI] [PubMed] [Google Scholar]
- Neubauer M, Hacker M, Bauer-Kreisel P, Weiser B, Fischbach C, Schulz MB et al. Adipose tissue engineering based on mesenchymal stem cells and basic fibroblast growth factor in vitro. Tissue Eng 2005; 11: 1840–1851. [DOI] [PubMed] [Google Scholar]
- Sakaue H, Konishi M, Ogawa W, Asaki T, Mori T, Yamasaki M et al. Requirement of fibroblast growth factor 10 in development of white adipose tissue. Genes Dev 2002; 16: 908–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augello A, De Bari C. The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther 2010; 21: 1226–1238. [DOI] [PubMed] [Google Scholar]
- Jeong BC, Kang IH, Hwang YC, Kim SH, Koh JT. MicroRNA-194 reciprocally stimulates osteogenesis and inhibits adipogenesis via regulating COUP-TFII expression. Cell Death Dis 2014; 5: e1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tome M, Lopez-Romero P, Albo C, Sepulveda JC, Fernandez-Gutierrez B, Dopazo et al. miR-335 orchestrates cell proliferation, migration and differentiation in human mesenchymal stem cells. Cell Death Differ 2011; 18: 985–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Guan X, Guo F, Zhou J, Chang A, Sun B et al. miR-30e reciprocally regulates the differentiation of adipocytes and osteoblasts by directly targeting low-density lipoprotein receptor-related protein 6. Cell Death Dis 2013; 4: e845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zhao L, Xing L, Chen D. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells 2010; 28: 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JF, Fu WM, He ML, Wang H, Wang WM, Yu SC et al. MiR-637 maintains the balance between adipocytes and osteoblasts by directly targeting Osterix. Mol Biol Cell 2011; 22: 3955–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbiener M, Fischer C, Nowitsch S, Opriessnig P, Papak C, Ailhaud G et al. microRNA miR-27b impairs human adipocyte differentiation and targets PPARgamma. Biochem Biophys Res Commun 2009; 390: 247–251. [DOI] [PubMed] [Google Scholar]
- Liu X, Sun Y, Constantinescu SN, Karam E, Weinberg RA, Lodish HF. Transforming growth factor beta-induced phosphorylation of Smad3 is required for growth inhibition and transcriptional induction in epithelial cells. Proc Natl Acad Sci USA 1997; 94: 10669–10674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Hwang SJ, Bae YC, Jung JS. MiR-21 regulates adipogenic differentiation through the modulation of TGF-beta signaling in mesenchymal stem cells derived from human adipose tissue. Stem Cells 2009; 27: 3093–3102. [DOI] [PubMed] [Google Scholar]
- Yang Z, Bian CJ, Zhou H, Huang S, Wang SH, Liao LM et al. MicroRNA hsa-miR-138 inhibits adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells through adenovirus EID-1. Stem Cells Dev 2011; 20: 259–267. [DOI] [PubMed] [Google Scholar]
- Eskildsen T, Taipaleenmaki H, Stenvang J, Abdallah BM, Ditzel N, Nossent AY et al. MicroRNA-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo. Proc Natl Acad Sci USA 2011; 108: 6139–6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian JB, Stein GS, van Wijnen AJ, Stein JL, Hassan MQ, Gaur T et al. MicroRNA control of bone formation and homeostasis. Nat Rev Endocrinol 2012; 8: 212–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S, Deng Y, Gu P, Fan X. MicroRNAs regulate bone development and regeneration. Int J Mol Sci 2015; 16: 8227–8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamam D, Ali D, Kassem M, Aldahmash A, Alajez NM. microRNAs as regulators of adipogenic differentiation of mesenchymal stem cells. Stem Cells Dev 2015; 24: 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefterova MI, Zhang Y, Steger DJ, Schupp M, Schug J, Cristancho et al. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev 2008; 22: 2941–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev 1991; 5: 1538–1552. [DOI] [PubMed] [Google Scholar]
- Kushwaha P, Khedgikar V, Gautam J, Dixit P, Chillara R, Verma et al. A novel therapeutic approach with Caviunin-based isoflavonoid that en routes bone marrow cells to bone formation via BMP2/Wnt-beta-catenin signaling. Cell Death Differ 2014; 5: e1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Ko J. A novel PPARgamma2 modulator sLZIP controls the balance between adipogenesis and osteogenesis during mesenchymal stem cell differentiation. Cell Death Differ 2014; 21: 1642–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang QQ, Lane MD. Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem 2012; 81: 715–736. [DOI] [PubMed] [Google Scholar]
- Lin FT, Lane MD. CCAAT/enhancer binding protein alpha is sufficient to initiate the 3T3-L1 adipocyte differentiation program. Proc Natl Acad Sci USA 1994; 91: 8757–8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T. Regulation of osteoblast differentiation by transcription factors. J Cell Biochem 2006; 99: 1233–1239. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002; 108: 17–29. [DOI] [PubMed] [Google Scholar]
- Komori T. Regulation of osteoblast differentiation by Runx2. Adv Exp Med Biol 2010; 658: 43–49. [DOI] [PubMed] [Google Scholar]
- Komori T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res 2010; 339: 189–195. [DOI] [PubMed] [Google Scholar]
- Wei J, Shimazu J, Makinistoglu MP, Maurizi A, Kajimura D, Zong H et al. Glucose uptake and Runx2 synergize to orchestrate osteoblast differentiation and bone formation. Cell 2015; 161: 1576–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Z, Yoshida CA, Furuichi T, Amizuka N, Ito M, Fukuyama R et al. Runx2 determines bone maturity and turnover rate in postnatal bone development and is involved in bone loss in estrogen deficiency. Dev Dyn 2007; 236: 1876–1890. [DOI] [PubMed] [Google Scholar]
- Brindle PK, Montminy MR. The CREB family of transcription activators. Curr Opin Genet Dev 1992; 2: 199–204. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem 1997; 272: 3406–3410. [DOI] [PubMed] [Google Scholar]
- Coelho MJ, Fernandes MH. Human bone cell cultures in biocompatibility testing. Part II: effect of ascorbic acid, beta-glycerophosphate and dexamethasone on osteoblastic differentiation. Biomaterials 2000; 21: 1095–1102. [DOI] [PubMed] [Google Scholar]
- Franceschi RT, Iyer BS, Cui Y. Effects of ascorbic acid on collagen matrix formation and osteoblast differentiation in murine MC3T3-E1 cells. J Bone Miner Res 1994; 9: 843–854. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Peperzak V, van Rijn L, Borst J, de Bruijn JD. Dexamethasone treatment during the expansion phase maintains stemness of bone marrow mesenchymal stem cells. J Tissue Eng Regen Med 2010; 4: 374–386. [DOI] [PubMed] [Google Scholar]
- Kim CH, Cheng SL, Kim GS. Effects of dexamethasone on proliferation, activity, and cytokine secretion of normal human bone marrow stromal cells: possible mechanisms of glucocorticoid-induced bone loss. J Endocrinol 1999; 162: 371–379. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 2006; 126: 677–689. [DOI] [PubMed] [Google Scholar]
- Marie PJ, Hay E, Saidak Z. Integrin and cadherin signaling in bone: role and potential therapeutic targets. Trends Endocrinol Metab 2014; 25: 567–575. [DOI] [PubMed] [Google Scholar]
- Ge C, Yang Q, Zhao G, Yu H, Kirkwood KL, Franceschi RT. Interactions between extracellular signal-regulated kinase 1/2 and p38 MAP kinase pathways in the control of RUNX2 phosphorylation and transcriptional activity. J Bone Miner Res 2012; 27: 538–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon YS, Smas CM, Lee K, Villena JA, Kim KH, Yun EJ et al. Mice lacking paternally expressed Pref-1/Dlk1 display growth retardation and accelerated adiposity. Mol Cell Biol 2002; 22: 5585–5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sul HS. Minireview: Pref-1: role in adipogenesis and mesenchymal cell fate. Mol Endocrinol 2009; 23: 1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhao L, Smas C, Sul HS. Pref-1 interacts with fibronectin to inhibit adipocyte differentiation. Mol Cell Biol 2010; 30: 3480–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleb S, Cancello R, Clement K, Lacasa D. Cathepsin S promotes human preadipocyte differentiation: possible involvement of fibronectin degradation. Endocrinology 2006; 147: 4950–4959. [DOI] [PubMed] [Google Scholar]
- Muller P, Langenbach A, Kaminski A, Rychly J. Modulating the actin cytoskeleton affects mechanically induced signal transduction and differentiation in mesenchymal stem cells. PLoS One 2013; 8: e71283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Liang L, Dou Y, Huang Z, Mo H, Wang Y et al. Mechanical strain regulates osteogenic and adipogenic differentiation of bone marrow mesenchymal stem cells. Biomed Res Int 2015; 2015: 873251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WR, Guilluy C, Xie Z, Sen B, Brobst KE, Yen SS et al. Mechanically activated Fyn utilizes mTORC2 to regulate RhoA and adipogenesis in mesenchymal stem cells. Stem Cells 2013; 31: 2528–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen B, Xie Z, Case N, Thompson WR, Uzer G, Styner M et al. mTORC2 regulates mechanically induced cytoskeletal reorganization and lineage selection in marrow-derived mesenchymal stem cells. J Bone Miner Res 2014; 29: 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Shou P, Zhang L, Xu C, Zheng C, Han Y et al. An osteopontin-integrin interaction plays a critical role in directing adipogenesis and osteogenesis by mesenchymal stem cells. Stem Cells 2014; 32: 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater 2007; 6: 997–1003. [DOI] [PubMed] [Google Scholar]
- Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci USA 2010; 107: 4872–4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Arcangelo E, McGuigan AP. Micropatterning strategies to engineer controlled cell and tissue architecture in vitro. Biotechniques 2015; 58: 13–23. [DOI] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science 1997; 276: 1425–1428. [DOI] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 2004; 6: 483–495. [DOI] [PubMed] [Google Scholar]
- Pampaloni F, Reynaud EG, Stelzer EH. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 2007; 8: 839–845. [DOI] [PubMed] [Google Scholar]
- Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 2009; 324: 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundelacruz S, Levin M, Kaplan DL. Membrane potential controls adipogenic and osteogenic differentiation of mesenchymal stem cells. PLoS One 2008; 3: e3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Fu Y, Tong W, Geng Y, Lui PP, Tang T et al. Uniaxial mechanical tension promoted osteogenic differentiation of rat tendon-derived stem cells (rTDSCs) via the Wnt5a-RhoA pathway. J Cell Biochem 2012; 113: 3133–3142. [DOI] [PubMed] [Google Scholar]
- Yourek G, McCormick SM, Mao JJ, Reilly GC. Shear stress induces osteogenic differentiation of human mesenchymal stem cells. Regen Med 2010; 5: 713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HJ, Bahk YY, Choi YR, Shim JH, Han SH, Lee JW. A proteomic analysis during serial subculture and osteogenic differentiation of human mesenchymal stem cell. J Orthop Res 2006; 24: 2059–2071. [DOI] [PubMed] [Google Scholar]
- Almeida M, Ambrogini E, Han L, Manolagas SC, Jilka RL. Increased lipid oxidation causes oxidative stress, increased peroxisome proliferator-activated receptor-gamma expression, and diminished pro-osteogenic Wnt signaling in the skeleton. J Biol Chem 2009; 284: 27438–27448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousteni S. FoxO1, the transcriptional chief of staff of energy metabolism. Bone 2012; 50: 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev 2008; 129: 163–173. [DOI] [PubMed] [Google Scholar]
- Bruedigam C, Eijken M, Koedam M, van de Peppel J, Drabek K, Chiba H et al. A new concept underlying stem cell lineage skewing that explains the detrimental effects of thiazolidinediones on bone. Stem Cells 2010; 28: 916–927. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Marsboom G, Toth PT, Rehman J. Mitochondrial respiration regulates adipogenic differentiation of human mesenchymal stem cells. PLoS One 2013; 8: e77077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atashi F, Modarressi A, Pepper MS. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a review. Stem Cells Dev 2015; 24: 1150–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Fritch L, Burkart A, Bell G, Mendelson K, Leszyk J, Nicoloro S et al. Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone. Mol Cell Biol 2003; 23: 1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B et al. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab 2011; 14: 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N, Liu H, Qu F, Fan J, Mao K, Yin Y et al. Hypoxia inhibits the differentiation of mesenchymal stem cells into osteoblasts by activation of Notch signaling. Exp Mol Pathol 2013; 94: 33–39. [DOI] [PubMed] [Google Scholar]
- Yang DC, Yang MH, Tsai CC, Huang TF, Chen YH, Hung SC. Hypoxia inhibits osteogenesis in human mesenchymal stem cells through direct regulation of RUNX2 by TWIST. PLoS One 2011; 6: e23965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SH, Chen CT, Wei YH. Inhibitory effects of hypoxia on metabolic switch and osteogenic differentiation of human mesenchymal stem cells. Stem Cells 2013; 31: 2779–2788. [DOI] [PubMed] [Google Scholar]
- Beegle J, Lakatos K, Kalomoiris S, Stewart H, Isseroff RR, Nolta JA et al. Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival, and promotes cell retention in vivo. Stem Cells 2015; 33: 1818–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valorani MG, Montelatici E, Germani A, Biddle A, D'Alessandro D, Strollo R et al. Pre-culturing human adipose tissue mesenchymal stem cells under hypoxia increases their adipogenic and osteogenic differentiation potentials. Cell Prolif 2012; 45: 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos F, Andrade PZ, Boura JS, Abecasis MM, da Silva CL, Cabral JM. Ex vivo expansion of human mesenchymal stem cells: a more effective cell proliferation kinetics and metabolism under hypoxia. J Cell Physiol 2010; 223: 27–35. [DOI] [PubMed] [Google Scholar]
- Buravkova LB, Andreeva ER, Gogvadze V, Zhivotovsky B. Mesenchymal stem cells and hypoxia: where are we? Mitochondrion 2014. (19 Pt A): 105–112. [DOI] [PubMed]
- Peng XD, Xie H, Zhao Q, Wu XP, Sun ZQ, Liao EY. Relationships between serum adiponectin, leptin, resistin, visfatin levels and bone mineral density, and bone biochemical markers in Chinese men. Clin Chim Acta 2008; 387: 31–35. [DOI] [PubMed] [Google Scholar]
- Gomez-Ambrosi J, Rodriguez A, Catalan V, Fruhbeck G. The bone-adipose axis in obesity and weight loss. Obes Surg 2008; 18: 1134–1143. [DOI] [PubMed] [Google Scholar]
- Yang DC, Tsay HJ, Lin SY, Chiou SH, Li MJ, Chang TJ et al. cAMP/PKA regulates osteogenesis, adipogenesis and ratio of RANKL/OPG mRNA expression in mesenchymal stem cells by suppressing leptin. PLoS One 2008; 3: e1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han GS, Jing YY, Zhang YH, Yue ZJ, Hu XW, Wang LX et al. Osteogenic differentiation of bone marrow mesenchymal stem cells by adenovirus-mediated expression of leptin. Regul Pept 2010; 163: 107–112. [DOI] [PubMed] [Google Scholar]
- Zhou J, Guo F, Wang G, Wang J, Zheng F, Guan X et al. miR-20a regulates adipocyte differentiation by targeting lysine-specific demethylase 6b and transforming growth factor-beta signaling. Int J Obes (Lond) 2015; 39: 1282–1291. [DOI] [PubMed] [Google Scholar]
- Su X, Liao L, Shuai Y, Jing H, Liu S, Zhou H et al. MiR-26a functions oppositely in osteogenic differentiation of BMSCs and ADSCs depending on distinct activation and roles of Wnt and BMP signaling pathway. Cell Death Dis 2015; 6: e1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Fan L, Hu J, Zhang L, Liao L, Liu S et al. MiR-26a rescues bone regeneration deficiency of mesenchymal stem cells derived from osteoporotic mice. Mol Ther 2015; 23: 1349–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Li J, Singh J, Alif R, Vazquez-Padron RI, Gomes SA et al. miR-30e targets IGF2-regulated osteogenesis in bone marrow-derived mesenchymal stem cells, aortic smooth muscle cells, and ApoE-/- mice. Cardiovasc Res 2015; 106: 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernapudi R, Wolfson B, Zhang Y, Yao Y, Yang P, Asahara H et al. miR-140 promotes expression of long non-coding RNA NEAT1 in adipogenesis. Mol Cell Biol 2015: MCB.00702–00715. [DOI] [PMC free article] [PubMed]
- Cao Y, Lv Q, Lv C. MicroRNA-153 suppresses the osteogenic differentiation of human mesenchymal stem cells by targeting bone morphogenetic protein receptor type II. Int J Mol Med 2015; 36: 760–766. [DOI] [PubMed] [Google Scholar]
- Li CJ, Cheng P, Liang MK, Chen YS, Lu Q, Wang JY et al. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J Clin Invest 2015; 125: 1509–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Gu S, Chen BF, Shen WL, Yin Z, Xu GW et al. Nanoparticle delivery of stable miR-199a-5p agomir improves the osteogenesis of human mesenchymal stem cells via the HIF1a pathway. Biomaterials 2015; 53: 239–250. [DOI] [PubMed] [Google Scholar]
- Li H, Li T, Fan J, Li T, Fan L, Wang S et al. miR-216a rescues dexamethasone suppression of osteogenesis, promotes osteoblast differentiation and enhances bone formation, by regulating c-Cbl-mediated PI3K/AKT pathway. Cell Death Differ 2015; 22: 1935–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Gao Y, Zhou J, Wang J, Zheng F, Guo F et al. miR-223 regulates adipogenic and osteogenic differentiation of mesenchymal stem cells through a C/EBPs/miR-223/FGFR2 regulatory feedback loop. Stem Cells 2015; 33: 1589–1600. [DOI] [PubMed] [Google Scholar]
- Hamam D, Ali D, Vishnubalaji R, Hamam R, Al-Nbaheen M, Chen L et al. microRNA-320/RUNX2 axis regulates adipocytic differentiation of human mesenchymal (skeletal) stem cells. Cell Death Dis 2014; 5: e1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus M, Greither T, Wenzel C, Brauer-Hartmann D, Wabitsch M, Behre HM. Inhibition of adipogenic differentiation of human SGBS preadipocytes by androgen-regulated microRNA miR-375. Mol Cell Endocrinol 2015; 414: 177–185. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Hou C, Meng F, Zhao X, Zhang Z, Huang G et al. MiR-455-3p regulates early chondrogenic differentiation via inhibiting Runx2. FEBS Lett 2015; 589: 3671–3678. [DOI] [PubMed] [Google Scholar]