Abstract
The content of spermidine and spermine in mammalian cells has important roles in protein and nucleic acid synthesis and structure, protection from oxidative damage, activity of ion channels, cell proliferation, differentiation, and apoptosis. Spermidine is essential for viability and acts as the precursor of hypusine, a post-translational addition to eIF5A allowing the translation of mRNAs encoding proteins containing polyproline tracts. Studies with Gy mice and human patients with the very rare X-linked genetic condition Snyder-Robinson syndrome that both lack spermine synthase show clearly that the correct spermine:spermidine ratio is critical for normal growth and development.
Keywords: eukaryotic initiation factor 5A (eIF5A); hearing; N-methyl-D-aspartate receptor (NMDA receptor, NMDAR); polyamine; potassium channel; spermidine; Snyder-Robinson syndrome; Spermine
Introduction
There is very strong experimental evidence that maintenance of a normal polyamine content is essential for a wide variety of basic cellular functions. (a) Their content is very tightly controlled with the key enzymes in biosynthesis and interconversion having multiple levels of regulation in response to hormonal stimulation and polyamine content. (b) With the use of specific inhibitors of polyamine biosynthesis, it is relatively easy to substantially deplete cellular polyamine content, and striking effects have been observed on numerous critical cell functions including growth, differentiation, apoptosis, motility, and resistance to oxidative and other stresses. (c) The interaction of polyamines with nucleic acids and proteins can affect both structure and stability. Binding of polyamines to DNA affects its structure and stability (1). The importance of the effects on DNA stability is emphasized by the presence in acute thermophiles of high levels of polyamines, including branched chain molecules and longer linear amines than those found in mammals. These allow their survival at elevated temperatures (2). The majority of cellular polyamines are bound to RNA, and the changes in structure produced by binding to ribosomes, tRNA, and some mRNAs with particular sequences influence protein synthesis in multiple ways (3). Interactions of polyamines with proteins forming microtubules can influence their assembly and shape (4), and interaction with protein membrane receptors can have profound effects on crucial receptors.
It should be stressed that these observations, although confirming that polyamines are essential cellular components, do not indicate that the polyamines necessarily play regulatory roles. Experimental support for such regulation would require the demonstration that physiological rather than pharmacological changes in polyamine content are associated with alterations in function, and this has rarely been demonstrated rigorously.
It is not possible in a brief review to describe all of the actions that have been assigned to polyamines. The focus is on those functions that have been demonstrated to influence animal growth and development with particular emphasis on the phenotypes revealed by experimental animals and humans with an inborn error of metabolism leading to a reduction in spermine.
Polyamine Content and Metabolism
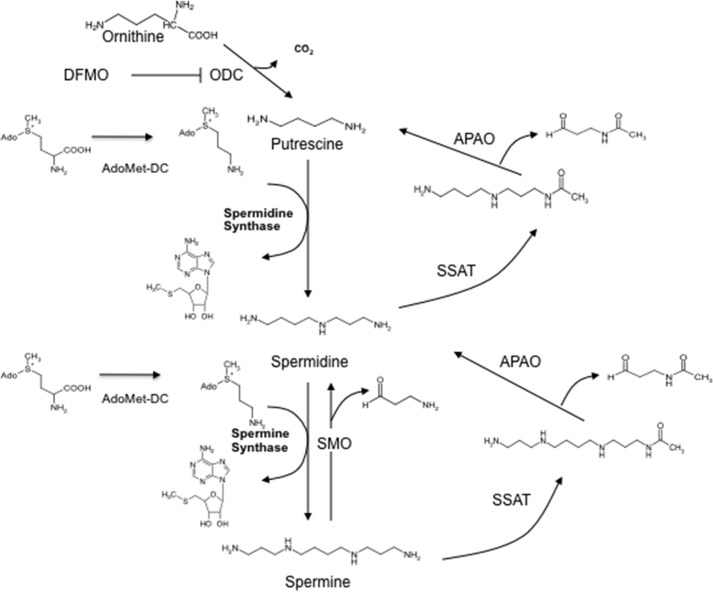
The polyamines synthesized by mammals are the triamine spermidine, the tetramine spermine, and their precursor putrescine (Fig. 1). Traces from dietary sources of other polyamines such as agmatine may be present in human tissues, but there is no convincing evidence that these play any physiological role. In contrast, the native polyamines are essential for viability. The polyamine biosynthetic and interconversion pathway in mammals is well established and has been described in multiple reviews (5, 6). Putrescine is formed by ornithine decarboxylase (ODC),2 and S-adenosylmethionine decarboxylase (AdoMetDC) produces dcAdoMet (Fig. 1). Inactivation of the ODC or AdoMetDC genes or treatment with ODC inhibitors results in lethality early in embryonic development (7–9). The contents of ODC and AdoMetDC are very highly regulated at multiple levels in response to stimuli controlling polyamine levels. The supply of dcAdoMet limits the formation of the higher polyamines by spermidine synthase and spermine synthase (Fig. 1). In mammals, these two aminopropyltransferases are highly specific with regard to their amine substrate (10, 11). Restrictions in the active site in spermidine synthase will not allow the binding of the larger spermidine at the putrescine substrate site (12), and the corresponding site in spermine synthase exclusively favors spermidine as substrate over putrescine (13). The aminopropyltransferase reactions are effectively irreversible, but the polyamines can be interconverted by oxidative degradation directly via spermine oxidase or by acetylpolyamine oxidase after acetylation via spermidine/spermine-N1-acetyltranferase (SSAT) (6, 14). The latter pathway is effectively controlled by the content of SSAT, a highly regulated cytosolic enzyme, which responds to high polyamine levels (14).
FIGURE 1.
Polyamine structures, biosynthesis, and interconversion. APAO, acetylpolyamine oxidase.
Proliferation, Differentiation, and Apoptosis
Polyamines are essential for cell proliferation. Polyamine content is higher in rapidly growing tissues, and regenerative and growth-promoting hormonal stimuli enhance polyamine synthesis and content (15–17). Treatment of cultured cells with ODC inhibitors such as α-difluoromethylornithine (DFMO) led to a virtually complete loss of putrescine and spermidine but little change in spermine and halted cell proliferation (18). Cytostasis was reversed by the provision of exogenous putrescine or spermidine, which restored a normal spermidine content. Even after long exposure, the effects of DFMO were cytostatic rather than cytotoxic. This may be due to the presence of residual spermine and hypusinated eIF5A (see below), which both decline very slowly in quiescent cells. When treatments were used that depleted both spermidine and spermine, there were progressive decreases in both proliferation and viability and increased apoptosis, which were prevented by the provision of exogenous polyamines (19–21). Studies with cultured cells from rodents lacking spermine synthase confirmed that a normal growth rate was maintained in cells with elevated spermidine levels but no spermine (22, 23).
Hypusine and eIF5A
Spermidine acts as the aminobutyl group donor for post-translational modification of a specific lysine residue of translation factor eIF5A by deoxyhypusine synthase. Subsequent hydroxylation by deoxyhypusine hydroxylase results in formation of Nϵ-(4-amino-2-hydroxybutyl)lysine (hypusine) (24, 25). This modification is essential for eIF5A activity. The genes for eIF5A-1, deoxyhypusine synthase, and deoxyhypusine hydroxylase are essential for viability in mice (26–28). eIF5A may contribute to transcription, mRNA turnover, nucleocytoplasmic transport, and apoptosis (25, 29), but its best understood function is to allow the translation of mRNAs encoding proteins containing polyproline tracts or triplets of PPX (where X may be Gly, Trp, Asp, or Asn) (30, 31). Ribosomes arrest on such nascent polyproline stretches. The ribosome-bound hypusinylated eIF-5A reaches toward the peptidyltransferase center of the ribosome and stabilizes and orients the CCA end of the peptidyl-tRNA to allow synthesis through these regions (32). Proteins containing such proline repeats include proteins regulating key functions in growth and development including actin/cytoskeleton-associated functions, RNA splicing/turnover, DNA binding/transcription, and cell signaling (27, 33). eIF5A is a relatively stable protein, but it can be acetylated either at Lys 47 (a residue essential for activity) by certain histone acetyltransferases or on the deoxyhypusine residue by SSAT (34), suggesting that its function may be regulated by acetylation/deacetylation.
Vertebrates have a second gene encoding eIF5A2, which is not widely expressed and is not essential but is present in several cancers where it is associated with aggressive growth and a poor prognosis (27, 29). Prevention of hypusine formation in eIF5A2 inhibits tumor growth and reduces expression of the oncogenic tyrosine kinase PEAK1 (35).
Inhibitors of hypusine synthesis such as N1-guanyl-1,7-diaminoheptane (GC7) block growth in a similar way to DFMO (35). Studies of the temporal effects in cells treated with both DFMO and N1-guanyl-1,7-diaminoheptane show a reduction of proliferation prior to the loss of hypusinated eIF5A, suggesting that spermidine is also required for other proliferative functions (36). Interactions of polyamines with RNA can influence the content of individual proteins in multiple ways including alterations in ribosomal structure, facilitation of the formation of initiation complexes, and allowing readthrough of inefficient initiation complexes and enhancing frameshifting (3, 37–39). Polyamines can also affect protein content via direct and indirect effects on post-translational protein processing (40, 41) and on protein degradation (41–43).
The role of polyamines in affecting the level of key regulatory proteins is likely to underlie their importance in wound healing, cell migration, and tissue remodeling, which involves regulated apoptosis. Specific examples of such polyamine-regulated proteins involved in gastrointestinal mucosal growth and self-renewal have been described (44–46). Polyamine content is critical for stem cell differentiation including adipogenesis (47, 48), osteogenesis (49, 50), and muscle development (51).
Polyamines and Ion Channels
Kir Channels
Potassium flux through Kir channels affects the resting membrane potential, cardiac and neuronal electrical activity, and electrolyte balance. In 1994, the Nichols laboratory showed that binding of polyamines caused rectification in the HRK1 Kir channel expressed in Xenopus oocytes (52). Subsequent work has extended the observation to a large family of such Kir channels. There is a very steep voltage dependence of the polyamine-mediated block, which is consistent with significant increases in excitability being associated with small changes in polyamine levels. Because spermine is more potent than spermidine (52, 53), a change in the ratio of polyamines may also bring about significant alterations in activity.
Structural studies of the Kir1–7 subfamilies have shown that polyamines bind first to a “shallow” binding site at the cytoplasmic pore with weak voltage dependence and then migrate through a long pore to the deep position where they interact with an acidic residue described as the “rectification controller” to generate steep voltage dependence (54–56) (Fig. 2A). Variants corresponding to various SNPs of the KCNJ10 gene show differences in spermine binding and response (57).
FIGURE 2.
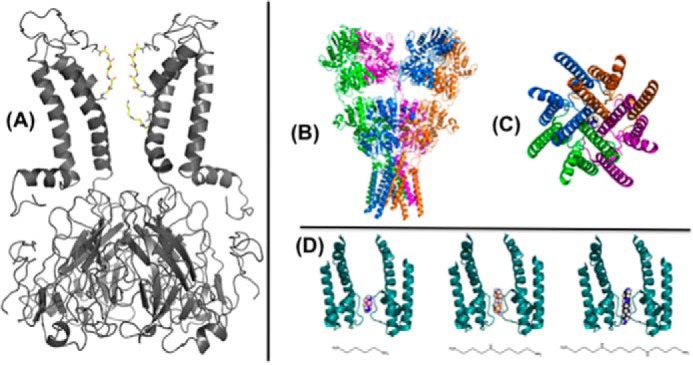
Polyamine interaction with ion channels. A, spermine is depicted in a deep binding site in the inner cavity of a molecular model of the Kir6.2[N160D] mutant channel, generated as a homology model based on an open conformation model of KirBac1.1 (98) with this blocker configuration generated using AutoDock as described previously (54), and selected based on functional studies of Kir6.2[N160D] and Kir2.1 channel (55). B, side view of the crystal structure of the full-length ionotropic glutamate receptor, GluA2 (Protein Data Bank (PDB) ID 3KG2), with a single spermine molecule manually positioned in the transmembrane pore region. C, view of the GluA2 transmembrane domain from the intracellular side showing a single spermine in the pore region. D, two subunits (A/C) of the Bacillus cereus NaK channel pore (PDB ID 3E86) with putrescine (left), spermidine (middle), and spermine (right) docked in the selectivity filter (66).
Ionotropic Glutamate Receptors
The activities of gated ion channels that allow passage of cations through the cellular membrane and that respond to the binding of a ligand such as glutamate are involved in synaptic transmission and synaptic plasticity underlying learning and memory. There are three groups of these receptors each with multiple members based on their modifying agents: NMDA; AMPA; and kainate. The activities of some members of all three groups can be influenced by polyamines (3, 58–60).
These include some NMDA receptors that act as both ligand-gated and voltage-dependent channels and control synaptic plasticity (3, 58, 60). Spermine is more potent than spermidine, and a wide variety of longer synthetic polyamines have been shown to have potent pharmacological effects. The effects of polyamines involve at least two sites and can lead to both stimulation and a weak voltage-dependent inhibition representing an open channel block. This complexity of effects reflects both the varied subunit composition of the receptors and their stimulatory agent and the presence of multiple sites at which polyamines can bind (61, 62). Some of the effects of polyamines on NMDA receptors involve binding to extracellular sites on these channels. It is unclear to what extent extracellular concentrations of polyamines are sufficient to bring about these actions under physiological circumstances. However, some flux of polyamines through these channels may occur.
Polyamines affect members of the family of AMPA receptors that lack the GluA2 subunit (59, 63). AMPA receptors are critical neurotransmitters responsible for fast excitatory neurotransmission in the CNS and regulate synaptic strength. These channels are subject to a block by endogenous intracellular polyamines (most potently spermine) that confers profound rectification on the responses and influences frequency-dependent facilitation at synapses expressing them (64, 65). Binding of spermine occurs within the pore region of the channel and is usage voltage-dependent (Fig. 2, B–D). Thus, polyamines may regulate the amount of Ca2+ flux and the excitability threshold at developing synapses. Repetitive activation of these receptors results in facilitation due to polyamine unblocking (63).
Spermine has a similar effect on some kainate receptors (which have roles in sensing pain, neuronal development, and synaptogenesis), producing a voltage-dependent block (59, 66). Extracellular spermine may also potentiate kainate receptors with certain subunit compositions by relieving proton inhibition of the receptor (67).
TRPC Channels and Connexins
TRPCs constitute a seven-member family of calcium-permeable, nonselective cation channels that function as store-operated as well as second messenger-operated channels and regulate gastrointestinal smooth muscle excitability and contractility. TRPC4 and -5 are strongly inhibited by intracellular polyamines, particularly spermine, via interactions with two glutamate residues (68). Communication between astrocytes was enhanced by spermine (69), and intracellular spermine increased gap junction communication and prevented uncoupling at low pH of connexin Cx43 channels (70).
Toxicity of Polyamines
Very early studies on polyamines revealed significant toxicity when solutions containing spermine were injected into experimental animals (71). The LD50 of spermine was 10 times higher when administered orally. Acute toxic effects included hypotension, neurotoxicity, diuresis, and a potentially lethal nephrotoxicity. Spermidine was much less potent in producing such renal damage. These results focused attention on the potential role of oxidation products in damaging the kidneys, and many subsequent experiments have confirmed the toxicity of such metabolites toward isolated cells and organisms. Several Cu2+-dependent amine oxidases that attack the terminal amine groups of polyamines can form highly toxic products from spermine including hydrogen peroxide, reactive aldehydes, and acrolein (72, 73). It seems probable that such metabolites may be responsible for the renal damage if high levels of spermine accumulate in the kidney. There is evidence that the bovine plasma amine oxidase could actually protect from injected spermine, presumably by reducing the renal level (71). The content of such plasma amine oxidases is highly species-dependent, and it is possible that maximal tolerated doses of spermine could differ substantially with species. The toxicity of polyamine oxidation products produced as described above and from the internal cleavage of spermine by spermine oxidase is well documented and has been implicated in the causation of stroke, other neurological diseases, renal failure, liver disease, and cancer (73, 74).
Mice Lacking Spermine Synthase
In 1986, a mutant mouse strain was obtained after X-irradiation in which male offspring developed hypophosphatemia with rickets/osteomalacia and other abnormalities including a circling behavior that led to their description as Gyro or Gy (75). These other alterations were not seen in Hyp mice, which have a similar hypophosphatemia due to inactivation of the phosphate-regulating gene Phex. In 1998, two groups (76, 77) showed that the Gy mice had a deletion of the part of the X chromosome that includes the spermine synthase gene as well as part of the adjacent Phex gene, suggesting that the additional changes, which include a greatly reduced size, sterility, deafness, neurological abnormalities, and a propensity to sudden death causing a very short life span, were due to the loss of spermine.
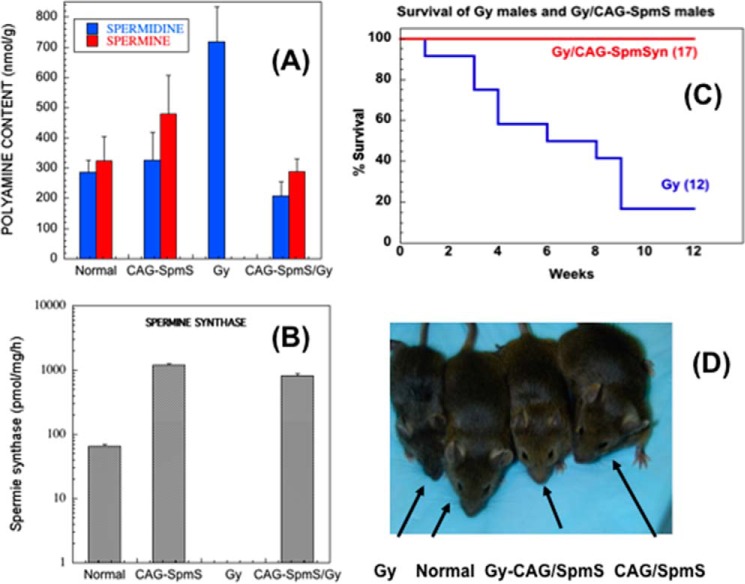
Subsequent studies confirmed that spermine synthase activity was absent from all tissues of the male Gy mice and that these tissues contained elevated levels of spermidine and lacked spermine (22). Proof that the phenotype was due to the absence of spermine synthase was obtained by breeding with a transgenic mouse line CAG-SpmS that expresses spermine synthase from a ubiquitous and unregulated promoter (78). Offspring with the transgene had normal brain function, hearing, balance, fertility, and life span despite the Gy mutation (79, 80) (Fig. 3). The hypophosphatemia was not corrected, and there was a reduction in body weight due to a loss of bone mass because of the loss of the Phex gene product (Fig. 3D). Spermine is therefore needed for normal neurological activity, growth, viability, and fertility in male mice. However, tight regulation of spermine synthase content is not required because there was a very large increase in spermine synthase above that found in normal control mice in all of the tissues studied (Fig. 3B) (79). This increase was not reflected in the spermine content, which was restored to levels only moderately above normal, but the substantial elevation of spermidine in Gy mice was abolished (Fig. 3A). These findings indicate that the spermine:spermidine ratio is critical for normal growth and development.
FIGURE 3.
Effect of transgenic spermine synthase expression in Gy mice. A, polyamine content in brain. B, spermine synthase activity in brain (note log scale). C, survival. D, size. Results in panels A and B are shown as the mean ± S.E. for at least six animals. See Ref. 79 for details. This figure was modified from research originally published in the Journal of Biological Chemistry. Wang, X., Ikeguchi, Y., McCloskey, D. E., Nelson, P., and Pegg, A. E. Spermine synthesis is required for normal viability, growth and fertility in the mouse. J. Biol. Chem. 2004. 279, 51370–51375. © The American Society for Biochemistry and Molecular Biology.
All tissues from Gy mice had undetectable levels of spermine despite a diet that contains substantial amounts of this polyamine (Fig. 3A) (22, 79). Even after greatly increasing the spermine content in the diet or after administration of spermine by injection of the maximally tolerated dose, there was only a very limited uptake (80).
A good case can be made that the impaired hearing, problems in balance, circling behavior, and short life span in Gy mice are related to malfunctions in ion channels due to the loss of spermine (80–82). The Gy mice were totally deaf and had an almost complete loss of the endocochlear potential; they also had serious problems with balance (80). These effects, which were abolished when spermine synthase was restored, can be explained by malfunction of the cochlear lateral wall-specific Kir4.1 channel (83), which maintains the endocochlear potential and is known to be one of the channels subject to strong inward rectification by polyamines. One of the side effects of clinical treatment of humans with high doses of DFMO is a high frequency hearing loss (84), but in general, DFMO has been found to be remarkably non-toxic in both clinical and animal studies. However, the Gy mice showed a striking toxic response to treatment with this ODC inhibitor (80). Within 2–3 days of oral treatment with DFMO, they suffered a catastrophic loss of motor function resulting in death within 5 days. The inability to maintain normal balance is consistent with an important role for polyamines in maintaining normal functions of Kir and glutamate receptor channels.
Impaired Kir channel activity is also likely to explain the restricted life span of the Gy mice (Fig. 3C), which showed significant irregularities in cardiac electrical activity with arrhythmias leading to sudden death (82). The steepness of rectification of cardiac Kir channels was reduced in myocytes isolated from such Gy mice (81). In humans, loss of inward rectification in Kir2.1 channel due to an inherited mutation at one of the spermine-binding sites (Asp-172) caused short QT syndrome, which predisposes patients to life-threatening arrhythmias (85).
Histological examination of the testicular morphology in Gy mice showed a reduction in Leydig cells and the almost complete absence of mature spermatozoa with most of the cells in the seminiferous tubules remaining as spermatogonia or early stage primary spermatocytes (79). Levels of polyamines are known to be markedly lower in seminal plasma of infertile men. It is possible that brain-related endocrine alterations also influence sperm development in Gy mice via changes in gonadotrophins, which are known to influence ODC activity in Leydig and Sertoli cells via changes in cAMP (86).
The small size and poor muscle development in Gy mice are consistent with the role of polyamines in protein synthesis, cell proliferation, and differentiation. However, cultured fibroblasts lacking spermine derived from these mice grew at a normal rate, although they differed in sensitivity to some DNA-damaging agents including UV radiation (22, 23, 87). This suggests that the high levels of spermidine are sufficient to allow normal cellular growth and proliferation and that the defect in Gy mice is also related to the critical role of the spermine:spermidine ratio in myocyte differentiation (51).
Unfortunately, the Gy model cannot be used to study the effects of spermine on osteogenesis because of the simultaneous deletion of the Phex gene. Attempts to generate mice with a specific inactivation of the SMS gene have not led to viable progeny in the 129/SVJ strain tested (87). The Gy mice are only viable on the B6C3H background. Attempts to move the phenotype by breeding to a more defined background were unsuccessful. It is therefore likely that this mixed strain has some other genes that allow survival in the absence of spermine. The identification of these genes would be very useful in the understanding of polyamine function.
Humans Lacking Spermine Synthase
In 2003, Schwartz and colleagues (88) discovered that a very rare X-linked recessive condition termed Snyder-Robinson syndrome (SRS) was due to a mutation in the spermine synthase (SMS) gene located at Xp.21.3-p22.12. This mutation causes incorrect splicing that inserts a premature stop codon, resulting in an inactive truncated protein. A small level of correct splicing produces some spermine synthase activity (10–15%). SRS syndrome in the original family presented as an X-linked recessive trait associated with cause mild-to-moderate mental retardation and a variety of other characteristics described below.
Subsequent genetic and biochemical analysis of other males with potential SRS has identified other point mutations at 13 different sites (Fig. 4) in the coding region of the SMS gene (89–94). Most of these mutations have been tested after expression of the mutant protein in vitro, and all alterations lead to loss of most spermine synthase activity (>90%), and in many cases, significantly decreased protein stability and protein content. The expansion of cases of SRS has led to a fuller understanding of the typical features of this syndrome. All affected males show intellectual disability, speech abnormalities, muscle hypoplasia, diminished body bulk, hypotonia, and some form of osteoporosis. Kyphoscoliosis, facial dysmorphism, long great toes, and an unsteady gait and seizures are common; renal abnormalities and myopia occur in some patients. The physical signs evolve from childhood to adulthood.
FIGURE 4.
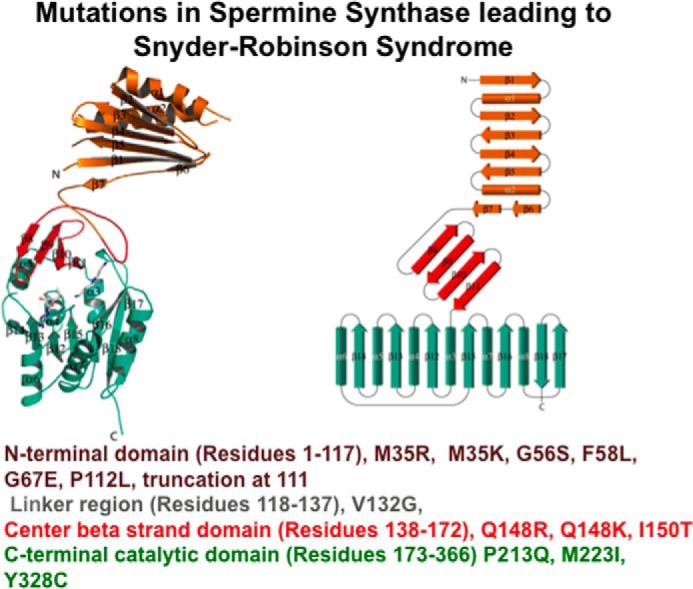
Mutations causing Snyder Robinson Syndrome. A monomer of human spermine synthase (13) is shown as a ribbon (left) and topology diagram (right) (PDB IDs 3C6K and 3C6M). The N-terminal, central, and C-terminal domains are shown in brown, red, and green, respectively. The loop connecting the N-terminal and central domains is in gray. Known mutations causing SRS are shown (88–94).
In addition to genetic analysis, SRS can be diagnosed by LC-MS/MS measurement of spermine/spermidine ratio in patient lymphoblasts (89). Plasma content of N8-acetylspermidine may also be a valuable marker (94). Cultured lymphoblasts or fibroblasts from SRS males show a major reduction in immunoreactive spermine synthase and a large decrease in the spermine:spermidine ratio. Spermine is not absent but is significantly reduced, whereas spermidine is substantially increased. The connection between the spermine synthase mutation and SRS is clearly firmly established, but the severity of symptoms is not well correlated with the extent of the alteration in polyamine content. This could be related to the putative modifying genes suggested above in connection with the Gy mice, but it is hard to reach definite conclusions without more information on polyamine content in target cells. Polyamine levels in bone marrow stromal cells from two brothers caused by a Q148R mutation were analyzed, and there was a greater reduction in spermine and increase in spermidine than seen in fibroblasts (90). It is therefore possible that cell types other than the readily obtained lymphoblasts or fibroblasts may have more pronounced alterations in polyamines, although it should be noted that levels vary with growth rate.
The skeletal abnormalities in SRS males correlate with a severe deficiency of calcium phosphate mineralization and a depletion of osteoblasts, and there is convincing experimental evidence that polyamines promote osteogenic differentiation (49, 50). These effects could also occur in Gy mice but be masked by the Phex deletion. The neurological and behavioral changes and the propensity to stroke in SRS can readily be associated with changes in polyamine-regulated ion channels. Volumetric neuroimaging analyses revealed changes in the brains of SRS (95).
It is possible that some of the changes in Gy mice related to Kir channels such as deafness and cardiac arrhythmias leading to sudden death are not seen in humans due to the continued presence of some spermine. The source of this spermine is unclear. It may be due to some limited spermine synthase activity because none of the mutations totally eliminates the hSMS gene, but the activity of the these proteins is very low when expressed in a recombinant form and they are also very unstable, leading to a much lower protein content. Another possibility is that human spermidine synthase may have a greater capacity to make spermine than the mouse equivalent. It is not likely to be due to dietary uptake as significant spermine is seen in SRS cells grown in culture using polyamine-free media.
Spermine synthase is a homodimer containing two active sites, and dimerization is essential for activity. Each monomer is made up of three domains: a C-terminal domain, which contains the active site; a central domain made up of four β-strands and an N-terminal domain needed for dimerization (Fig. 4) (13). Mutations of spermine synthase leading to SRS are found in all of these regions and in linker sequences (Fig. 4). Some mutations prevent dimerization. The possibility of using small molecules to restore activity and stability to these mutants by interacting with the dimer interface is under active investigation (96).
An alternative approach to providing more general therapy for SRS would be to attempt to restore spermine levels by direct administration. This is not a simple procedure because, as described above, there is significant toxicity of direct spermine administration, whereas dietary manipulation to increase total spermine intake did not raise spermine levels in tissues of Gy mice due to impaired uptake. Membrane transport of polyamines in mammals is blocked by antizyme, a protein that also promotes the degradation of ODC and is induced by spermidine (43, 97). The frameshifting mechanism that underlies this induction is quite well understood (38), and it may be possible to design small molecules to interfere with this. These would have the disadvantage of also increasing ODC, but contrary to many statements in the literature, the limiting step in the synthesis of spermidine is not ODC but the availability of dcAdoMet. Another approach would be to test membrane-permeable derivatives of spermine that could then be converted to spermine inside the cell. A pilot molecule for such studies would be N1,N12-diacetylspermine. Although it is possible that very early intervention would be needed to restore a totally normal phenotype, the progressive nature of the conditions does suggest that a way to normalize the spermine:spermidine ratio would have beneficial effects. Despite the differences between the phenotypes of SRS patients and Gy mice, these mice do provide a model for the examination of such approaches to increasing intracellular spermine.
Acknowledgments
I apologize that because of space constraints, many important contributions could not be included. I am very grateful to Dr. Charles Schwarz for help with the section on SRS, to Dr. Derek Bowie and Dr. Harley Kurata for advice on ion channels and Fig. 2, and to the late Dr. H. G. Williams-Ashman who, many years ago, persuaded me to study polyamines.
The author declares that he has no conflicts of interest with the contents of this article.
- ODC
- ornithine decarboxylase
- AdoMetDC
- S-adenosylmethionine decarboxylase
- dcAdoMet
- decarboxylated S-adenosylmethionine
- SSAT
- spermidine/spermine-N1-acetyltranferase
- DFMO
- α-difluoromethylornithine
- hypusine
- Nϵ-(4-amino-2-hydroxybutyl)lysine
- SRS
- Snyder-Robinson syndrome
- TRPC
- transient receptor potential cation.
References
- 1. Iacomino G., Picariello G., and D'Agostino L. (2012) DNA and nuclear aggregates of polyamines. Biochim. Biophys. Acta 1823, 1745–1755 [DOI] [PubMed] [Google Scholar]
- 2. Terui Y., Ohnuma M., Hiraga K., Kawashima E., and Oshima T. (2005) Stabilization of nucleic acids by unusual polyamines produced by an extreme thermophile. Biochem. J. 388, 427–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Igarashi K., and Kashiwagi K. (2010) Modulation of cellular function by polyamines. Int. J. Biochem. Cell Biol. 42, 39–51 [DOI] [PubMed] [Google Scholar]
- 4. Ojeda-Lopez M. A., Needleman D. J., Song C., Ginsburg A., Kohl P. A., Li Y., Miller H. P., Wilson L., Raviv U., Choi M. C., and Safinya C. R. (2014) Transformation of taxol-stabilized microtubules into inverted tubulin tubules triggered by a tubulin conformation switch. Nat. Mater. 13, 195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pegg A. E. (2009) Mammalian polyamine metabolism and function. IUBMB Life 61, 880–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Battaglia V., DeStefano Shields C., Murray-Stewart T., and Casero R. A. Jr. (2014) Polyamine catabolism in carcinogenesis: potential targets for chemotherapy and chemoprevention. Amino Acids 46, 511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fozard J. R., Part M. L., Prakash N. J., Grove J., Schechter P. J., Sjoerdsma A., and Koch-Weser J. (1980) l-Ornithine decarboxylase: an essential role in early mammalian embryogenesis. Science 208, 505–508 [DOI] [PubMed] [Google Scholar]
- 8. Pendeville H., Carpino N., Marine J. C., Takahashi Y., Muller M., Martial J. A., and Cleveland J. L. (2001) The ornithine decarboxylase gene is essential for cell survival during early murine development. Mol. Cell. Biol. 21, 6549–6558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nishimura K., Nakatsu F., Kashiwagi K., Ohno H., Saito T., and Igarashi K. (2002) Essential role of S-adenosylmethionine decarboxylase in mouse embryonic development. Genes Cells 7, 41–47 [DOI] [PubMed] [Google Scholar]
- 10. Pegg A. E., and Michael A. J. (2010) Spermine synthase. Cell. Mol. Life Sci. 67, 113–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pegg A. E. (2014) The function of spermine. IUBMB Life 66, 8–18 [DOI] [PubMed] [Google Scholar]
- 12. Wu H., Min J., Ikeguchi Y., Zeng H., Dong A., Loppnau P., Pegg A. E., and Plotnikov A. N. (2007) Structure and mechanism of spermidine synthases. Biochemistry 46, 8331–8339 [DOI] [PubMed] [Google Scholar]
- 13. Wu H., Min J., Zeng H., McCloskey D. E., Ikeguchi Y., Loppnau P., Michael A. J., Pegg A. E., and Plotnikov A. N. (2008) Crystal structure of human spermine synthase: implications of substrate binding and catalytic mechanism. J. Biol. Chem. 283, 16135–16146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pegg A. E. (2008) Spermidine/spermine N1-acetyltransferase: a key metabolic regulator. Am. J. Physiol. Endocrinol. Metab. 294, E995–1010 [DOI] [PubMed] [Google Scholar]
- 15. Raina A., Jänne J., and Siimes M. (1966) Stimulation of polyamine synthesis in relation to nucleic acids in regenerating rat liver. Biochim. Biophys. Acta 123, 197–201 [DOI] [PubMed] [Google Scholar]
- 16. Russell D., and Snyder S. H. (1968) Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc. Natl. Acad. Sci. U.S.A. 60, 1420–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pegg A. E., Lockwood D. H., and Williams-Ashman H. G. (1970) Concentrations of putrescine and polyamines and their enzymic synthesis during androgen-induced prostatic growth. Biochem. J. 117, 17–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mamont P. S., Böhlen P., McCann P. P., Bey P., Schuber F., and Tardif C. (1976) α-Methyl ornithine, a potent competitive inhibitor of ornithine decarboxylase, blocks proliferation of rat hepatoma cells in culture. Proc. Natl. Acad. Sci. U.S.A. 73, 1626–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mamont P. S., Siat M., Joder-Ohlenbusch A. M., Bernhardt A., and Casara P. (1984) Effect of (2R, 5R)-6-heptyne-2, 5-diamine, a potent inhibitor of l-ornithine decarboxylase, on rat hepatoma cells cultured in vitro. Eur. J. Biochem. 142, 457–463 [DOI] [PubMed] [Google Scholar]
- 20. He Y., Shimogori T., Kashiwagi K., Shirahata A., and Igarashi K. (1995) Inhibition of cell growth by combination of α-difluoromethylornithine and an inhibitor of spermine synthase. J. Biochem. 117, 824–829 [DOI] [PubMed] [Google Scholar]
- 21. Mandal S., Mandal A., and Park M. H. (2015) Depletion of the polyamines spermidine and spermine by overexpression of spermidine/spermine N1-acetyltransferase 1 (SAT1) leads to mitochondria-mediated apoptosis in mammalian cells. Biochem. J. 468, 435–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mackintosh C. A., and Pegg A. E. (2000) Effect of spermine synthase deficiency on polyamine biosynthesis and content in mice and embryonic fibroblasts and the sensitivity of fibroblasts to 1,3-bis(2-chloroethyl)-N-nitrosourea. Biochem. J. 351, 439–447 [PMC free article] [PubMed] [Google Scholar]
- 23. Nilsson J., Gritli-Linde A., and Heby O. (2000) Skin fibroblasts from spermine synthase-deficient hemizygous gyro male (Gy/Y) mice overproduce spermidine and exhibit increased resistance to oxidative stress but decreased resistance to UV irradiation. Biochem. J. 352, 381–387 [PMC free article] [PubMed] [Google Scholar]
- 24. Park M. H., Cooper H. L., and Folk J. E. (1981) Identification of hypusine, an unusual amino acid, in a protein from human lymphocytes and of spermidine as its biosynthetic precursor. Proc. Natl. Acad. Sci. U.S.A. 78, 2869–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Caraglia M., Park M. H., Wolff E. C., Marra M., and Abbruzzese A. (2013) eIF5A isoforms and cancer: two brothers for two functions? Amino Acids 44, 103–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nishimura K., Lee S. B., Park J. H., and Park M. H. (2012) Essential role of eIF5A-1 and deoxyhypusine synthase in mouse embryonic development. Amino Acids 42, 703–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pällmann N., Braig M., Sievert H., Preukschas M., Hermans-Borgmeyer I., Schweizer M., Nagel C. H., Neumann M., Wild P., Haralambieva E., Hagel C., Bokemeyer C., Hauber J., and Balabanov S. (2015) Biological relevance and therapeutic potential of the hypusine modification system. J. Biol. Chem. 290, 18343–18360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sievert H., Pällmann N., Miller K. K., Hermans-Borgmeyer I., Venz S., Sendoel A., Preukschas M., Schweizer M., Boettcher S., Janiesch P. C., Streichert T., Walther R., Hengartner M. O., Manz M. G., Brümmendorf T. H., et al. (2014) A novel mouse model for inhibition of DOHH-mediated hypusine modification reveals a crucial function in embryonic development, proliferation and oncogenic transformation. Dis. Model. Mech. 7, 963–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mathews M. B., and Hershey J. W. (2015) The translation factor eIF5A and human cancer. Biochim. Biophys. Acta 1849, 836–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gutierrez E., Shin B. S., Woolstenhulme C. J., Kim J. R., Saini P., Buskirk A. R., and Dever T. E. (2013) eIF5A promotes translation of polyproline motifs. Mol. Cell 51, 35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dever T. E., Gutierrez E., and Shin B. S. (2014) The hypusine-containing translation factor eIF5A. Crit. Rev. Biochem. Mol. Biol. 49, 413–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schmidt C., Becker T., Heuer A., Braunger K., Shanmuganathan V., Pech M., Berninghausen O., Wilson D. N., and Beckmann R. (2016) Structure of the hypusinylated eukaryotic translation factor eIF-5A bound to the ribosome. Nucleic Acids Res. 44, 1944–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mandal A., Mandal S., and Park M. H. (2014) Genome-wide analyses and functional classification of proline repeat-rich proteins: potential role of eIF5A in eukaryotic evolution. PLoS ONE 9, e111800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee S. B., Park J. H., Folk J. E., Deck J. A., Pegg A. E., Sokabe M., Fraser C. S., and Park M. H. (2011) Inactivation of eukaryotic initiation factor 5A (eIF5A) by specific acetylation of its hypusine residue by spermidine/spermine acetyltransferase 1 (SSAT1). Biochem. J. 433, 205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fujimura K., Wright T., Strnadel J., Kaushal S., Metildi C., Lowy A. M., Bouvet M., Kelber J. A., and Klemke R. L. (2014) A hypusine-eIF5A-PEAK1 switch regulates the pathogenesis of pancreatic cancer. Cancer Res. 74, 6671–6681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nishimura K., Murozumi K., Shirahata A., Park M. H., Kashiwagi K., and Igarashi K. (2005) Independent roles of eIF5A and polyamines in cell proliferation. Biochem. J. 385, 779–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sakamoto A., Terui Y., Yoshida T., Yamamoto T., Suzuki H., Yamamoto K., Ishihama A., Igarashi K., and Kashiwagi K. (2015) Three members of polyamine modulon under oxidative stress conditions: two transcription factors (SoxR and EmrR) and a glutathione synthetic enzyme (GshA). PLoS ONE 10, e0124883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ivanov I. P., Atkins J. F., and Michael A. J. (2010) A profusion of upstream open reading frame mechanisms in polyamine-responsive translational regulation. Nucleic Acids Res. 38, 353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yamashita T., Nishimura K., Saiki R., Okudaira H., Tome M., Higashi K., Nakamura M., Terui Y., Fujiwara K., Kashiwagi K., and Igarashi K. (2013) Role of polyamines at the G1/S boundary and G2/M phase of the cell cycle. Int. J. Biochem. Cell Biol. 45, 1042–1050 [DOI] [PubMed] [Google Scholar]
- 40. Tolbert W. D., Zhang Y., Cottet S. E., Bennett E. M., Ekstrom J. L., Pegg A. E., and Ealick S. E. (2003) Mechanism of human S-adenosylmethionine decarboxylase proenzyme processing as revealed by the structure of the S68A mutant. Biochemistry 42, 2386–2395 [DOI] [PubMed] [Google Scholar]
- 41. Pegg A. E. (2009) S-Adenosylmethionine decarboxylase. Essays Biochem. 46, 25–45 [DOI] [PubMed] [Google Scholar]
- 42. Mitchell J. L. A., Choe C. Y., and Judd G. G. (1996) Feedback repression of ornithine decarboxylase synthesis mediated by antizyme. Biochem. J. 320, 755–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pegg A. E. (2006) Regulation of ornithine decarboxylase. J. Biol. Chem. 281, 14529–14532 [DOI] [PubMed] [Google Scholar]
- 44. Rao J. N., Rathor N., Zhuang R., Zou T., Liu L., Xiao L., Turner D. J., and Wang J. Y. (2012) Polyamines regulate intestinal epithelial restitution through TRPC1-mediated Ca2+ signaling by differentially modulating STIM1 and STIM2. Am. J. Physiol. Cell Physiol. 303, C308–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gao J. H., Guo L. J., Huang Z. Y., Rao J. N., and Tang C. W. (2013) Roles of cellular polyamines in mucosal healing in the gastrointestinal tract. J. Physiol. Pharmacol. 64, 681–693 [PubMed] [Google Scholar]
- 46. Ray R. M., Guo H., Patel M., Jin S., Bhattacharya S., and Johnson L. R. (2007) Role of myosin regulatory light chain and Rac1 in the migration of polyamine-depleted intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G983–G995 [DOI] [PubMed] [Google Scholar]
- 47. Ishii I., Ikeguchi Y., Mano H., Wada M., Pegg A. E., and Shirahata A. (2012) Polyamine metabolism is involved in adipogenesis of 3T3-L1 cells. Amino Acids 42, 619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brenner S., Bercovich Z., Feiler Y., Keshet R., and Kahana C. (2015) Dual regulatory role of polyamines in adipogenesis. J. Biol. Chem. 290, 27384–27392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee M. J., Chen Y., Huang Y. P., Hsu Y. C., Chiang L. H., Chen T. Y., and Wang G. J. (2013) Exogenous polyamines promote osteogenic differentiation by reciprocally regulating osteogenic and adipogenic gene expression. J. Cell. Biochem. 114, 2718–2728 [DOI] [PubMed] [Google Scholar]
- 50. Yeon J. T., Ryu B. J., Choi S. W., Heo J. C., Kim K. J., Son Y. J., and Kim S. H. (2014) Natural polyamines inhibit the migration of preosteoclasts by attenuating Ca2+-PYK2-Src-NFATc1 signaling pathways. Amino Acids 46, 2605–2614 [DOI] [PubMed] [Google Scholar]
- 51. Luchessi A. D., Cambiaghi T. D., Hirabara S. M., Lambertucci R. H., Silveira L. R., Baptista I. L., Moriscot A. S., Costa-Neto C. M., and Curi R. (2009) Involvement of eukaryotic translation initiation factor 5A (eIF5A) in skeletal muscle stem cell differentiation. J. Cell. Physiol. 218, 480–489 [DOI] [PubMed] [Google Scholar]
- 52. Lopatin A. N., Makhina E. N., and Nichols C. G. (1994) Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature 372, 366–369 [DOI] [PubMed] [Google Scholar]
- 53. Stanfield P. R., and Sutcliffe M. J. (2003) Spermine is fit to block inward rectifier (Kir) channels. J. Gen. Physiol. 122, 481–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kurata H. T., Diraviyam K., Marton L. J., and Nichols C. G. (2008) Blocker protection by short spermine analogs: refined mapping of the spermine binding site in a Kir channel. Biophys. J. 95, 3827–3839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kurata H. T., Zhu E. A., and Nichols C. G. (2010) Locale and chemistry of spermine binding in the archetypal inward rectifier Kir2.1. J. Gen. Physiol. 135, 495–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kurata H. T., Akrouh A., Li J. B., Marton L. J., and Nichols C. G. (2013) Scanning the topography of polyamine blocker binding in an inwardly rectifying potassium channel. J. Biol. Chem. 288, 6591–6601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Méndez-González M. P., Kucheryavykh Y. V., Zayas-Santiago A., Vélez-Carrasco W., Maldonado-Martínez G., Cubano L. A., Nichols C. G., Skatchkov S. N., and Eaton M. J. (2016) Novel KCNJ10 gene variations compromise function of inwardly rectifying potassium channel 4.1. J. Biol. Chem. 291, 7716–7726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Williams K., Romano C., and Molinoff P. B. (1989) Effects of polyamines on the binding of [3H]MK-801 to the N-methyl-d-aspartate receptor: pharmacological evidence for the existence of a polyamine recognition site. Mol. Pharmacol. 36, 575–581 [PubMed] [Google Scholar]
- 59. Bowie D., and Mayer M. L. (1995) Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron 15, 453–462 [DOI] [PubMed] [Google Scholar]
- 60. Williams K. (1997) Modulation and block of ion channels: a new biology of polyamines. Cell. Signal. 9, 1–13 [DOI] [PubMed] [Google Scholar]
- 61. Han X., Tomitori H., Mizuno S., Higashi K., Füll C., Fukiwake T., Terui Y., Leewanich P., Nishimura K., Toida T., Williams K., Kashiwagi K., and Igarashi K. (2008) Binding of spermine and ifenprodil to a purified, soluble regulatory domain of the N-methyl-d-aspartate receptor. J. Neurochem. 107, 1566–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jin L., Miyazaki M., Mizuno S., Takigawa M., Hirose T., Nishimura K., Toida T., Williams K., Kashiwagi K., and Igarashi K. (2008) The pore region of N-methyl-d-aspartate receptors differentially influences stimulation and block by spermine. J. Pharmacol. Exp. Ther. 327, 68–77 [DOI] [PubMed] [Google Scholar]
- 63. Bowie D. (2012) Redefining the classification of AMPA-selective ionotropic glutamate receptors. J. Physiol. 590, 49–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bowie D., Lange G. D., and Mayer M. L. (1998) Activity-dependent modulation of glutamate receptors by polyamines. J. Neurosci. 18, 8175–8185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shin J., Shen F., and Huguenard J. (2007) PKC and polyamine modulation of GluR2-deficient AMPA receptors in immature neocortical pyramidal neurons of the rat. J. Physiol. 581, 679–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Brown P. M., Aurousseau M. R., Musgaard M., Biggin P. C., and Bowie D. (2016) Kainate receptor pore-forming and auxiliary subunits regulate channel block by a novel mechanism. J. Physiol. 594, 1821–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mott D. D., Washburn M. S., Zhang S., and Dingledine R. J. (2003) Subunit-dependent modulation of kainate receptors by extracellular protons and polyamines. J. Neurosci. 23, 1179–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kim J., Moon S. H., Shin Y. C., Jeon J. H., Park K. J., Lee K. P., and So I. (2016) Intracellular spermine blocks TRPC4 channel via electrostatic interaction with C-terminal negative amino acids. Pflugers. Arch. 468, 551–561; Correction (2016) 10.1007/s00424-016-1824-7 [DOI] [PubMed] [Google Scholar]
- 69. Benedikt J., Inyushin M., Kucheryavykh Y. V., Rivera Y., Kucheryavykh L. Y., Nichols C. G., Eaton M. J., and Skatchkov S. N. (2012) Intracellular polyamines enhance astrocytic coupling. Neuroreport 23, 1021–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Skatchkov S. N., Bukauskas F. F., Benedikt J., Inyushin M., and Kucheryavykh Y. V. (2015) Intracellular spermine prevents acid-induced uncoupling of Cx43 gap junction channels. Neuroreport 26, 528–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tabor C. W., and Rosenthal S. M. (1956) Pharmacology of spermine and spermidine; some effects on animals and bacteria. J. Pharmacol. Exp. Ther. 116, 139–155 [PubMed] [Google Scholar]
- 72. Tabor C. W., Tabor H., and Bachrach U. (1964) Identification of the aminoaldehydes produced by the oxidation of spermine and spermidine with purified plasma amine oxidase. J. Biol. Chem. 239, 2194–2203 [PubMed] [Google Scholar]
- 73. Pegg A. E. (2013) Toxicity of polyamines and their metabolic products. Chem. Res. Toxicol. 26, 1782–1800 [DOI] [PubMed] [Google Scholar]
- 74. Park M. H., and Igarashi K. (2013) Polyamines and their metabolites as diagnostic markers of human diseases. Biomol. Ther. (Seoul) 21, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lyon M. F., Scriver C. R., Baker L. R., Tenenhouse H. S., Kronick J., and Mandla S. (1986) The Gy mutation: another cause of X-linked hypophosphatemia in mouse. Proc. Natl. Acad. Sci. U.S.A. 83, 4899–4903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lorenz B., Francis F., Gempel K., Böddrich A., Josten M., Schmahl W., Schmidt J., Lehrach H., Meitinger T., and Strom T. M. (1998) Spermine deficiency in Gy mice caused by deletion of the spermine synthase gene. Hum. Mol. Genet. 7, 541–547 [DOI] [PubMed] [Google Scholar]
- 77. Meyer R. A. Jr., Henley C. M., Meyer M. H., Morgan P. L., McDonald A. G., Mills C., and Price D. K. (1998) Partial deletion of both the spermine synthase gene and the Pex gene in the x-linked hypophosphatemic, Gyro (Gy) mouse. Genomics 48, 289–295 [DOI] [PubMed] [Google Scholar]
- 78. Ikeguchi Y., Wang X., McCloskey D. E., Coleman C. S., Nelson P., Hu G., Shantz L. M., and Pegg A. E. (2004) Characterization of transgenic mice with widespread overexpression of spermine synthase. Biochem. J. 381, 701–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang X., Ikeguchi Y., McCloskey D. E., Nelson P., and Pegg A. E. (2004) Spermine synthesis is required for normal viability, growth and fertility in the mouse. J. Biol. Chem. 279, 51370–51375 [DOI] [PubMed] [Google Scholar]
- 80. Wang X., Levic S., Gratton M. A., Doyle K. J., Yamoah E. N., and Pegg A. E. (2009) Spermine synthase deficiency leads to deafness and a profound sensitivity to α-difluoromethylornithine. J. Biol. Chem. 284, 930–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lopatin A. N., Shantz L. M., Mackintosh C. A., Nichols C. G., and Pegg A. E. (2000) Modulation of potassium channels in the hearts of transgenic and mutant mice with altered polyamine biosynthesis. J. Mol. Cell. Cardiol. 32, 2007–2024 [DOI] [PubMed] [Google Scholar]
- 82. Pegg A. E., and Wang X. (2009) Mouse models to investigate the function of spermine. Commun. Integr. Biol. 2, 271–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kubo Y., Adelman J. P., Clapham D. E., Jan L. Y., Karschin A., Kurachi Y., Lazdunski M., Nichols C. G., Seino S., and Vandenberg C. A. (2005) International Union of Pharmacology. LIV. Nomenclature and molecular relationships of inwardly rectifying potassium channels. Pharmacol. Rev. 57, 509–526 [DOI] [PubMed] [Google Scholar]
- 84. McLaren C. E., Fujikawa-Brooks S., Chen W. P., Gillen D. L., Pelot D., Gerner E. W., and Meyskens F. L. Jr. (2008) Longitudinal assessment of air conduction audiograms in a phase III clinical trial of difluoromethylornithine and sulindac for prevention of sporadic colorectal adenomas. Cancer Prev. Res. (Phila.) 1, 514–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Priori S. G., Pandit S. V., Rivolta I., Berenfeld O., Ronchetti E., Dhamoon A., Napolitano C., Anumonwo J., di Barletta M. R., Gudapakkam S., Bosi G., Stramba-Badiale M., and Jalife J. (2005) A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ. Res. 96, 800–807 [DOI] [PubMed] [Google Scholar]
- 86. Lefèvre P. L., Palin M. F., and Murphy B. D. (2011) Polyamines on the reproductive landscape. Endocr. Rev. 32, 694–712 [DOI] [PubMed] [Google Scholar]
- 87. Korhonen V.-P., Niiranen K., Halmekytö M., Pietilä M., Diegelman P., Parkkinen J. J., Eloranta T., Porter C. W., Alhonen L., and Jänne J. (2001) Spermine deficiency resulting from targeted disruption of the spermine synthase gene in embryonic stem cells leads to enhanced sensitivity to antiproliferative drugs. Mol. Pharmacol. 59, 231–238 [DOI] [PubMed] [Google Scholar]
- 88. Cason A. L., Ikeguchi Y., Skinner C., Wood T. C., Holden K. R., Lubs H. A., Martinez F., Simensen R. J., Stevenson R. E., Pegg A. E., and Schwartz C. E. (2003) X-Linked spermine synthase gene (SMS) defect: the first polyamine deficiency syndrome. Eur. J. Hum. Genet. 11, 937–944 [DOI] [PubMed] [Google Scholar]
- 89. Sowell J., Norris J., Jones K., Schwartz C., and Wood T. (2011) Diagnostic screening for spermine synthase deficiency by liquid chromatography tandem mass spectrometry. Clin. Chim. Acta 412, 655–660 [DOI] [PubMed] [Google Scholar]
- 90. Albert J., Schwartz C., Boerkoel C., and Stevenson R. (2013) Snyder-Robinson Syndrome. in GeneReviews®, June 27, 2013 Ed., University of Washington, Seattle, WA: [PubMed] [Google Scholar]
- 91. Peron A., Spaccini L., Norris J., Bova S. M., Selicorni A., Weber G., Wood T., Schwartz C. E., and Mastrangelo M. (2013) Snyder-Robinson syndrome: a novel nonsense mutation in spermine synthase and expansion of the phenotype. Am. J. Med. Genet. A 161A, 2316–2320 [DOI] [PubMed] [Google Scholar]
- 92. Zhang Z., Norris J., Kalscheuer V., Wood T., Wang L., Schwartz C., Alexov E., and Van Esch H. (2013) A Y328C missense mutation in spermine synthase causes a mild form of Snyder-Robinson syndrome. Hum. Mol. Genet. 22, 3789–3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Peng Y., Norris J., Schwartz C., and Alexov E. (2016) Revealing the effects of missense mutations causing Snyder-Robinson syndrome on the stability and dimerization of spermine synthase. Int. J. Mol. Sci. 17, E77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Abela L., Simmons L., Steindl K., Schmitt B., Mastrangelo M., Joset P., Papuc M., Sticht H., Baumer A., Crowther L. M., Mathis D., Rauch A., and Plecko B. (2016) N-Acetylspermidine as a potential plasma biomarker for Snyder-Robinson syndrome identified by clinical metabolomics. J. Inherit. Metab. Dis. 39, 131–137 [DOI] [PubMed] [Google Scholar]
- 95. Kesler S. R., Schwartz C., Stevenson R. E., and Reiss A. L. (2009) The impact of spermine synthase (SMS) mutations on brain morphology. Neurogenetics 10, 299–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhang Z., Martiny V., Lagorce D., Ikeguchi Y., Alexov E., and Miteva M. A. (2014) Rational design of small-molecule stabilizers of spermine synthase dimer by virtual screening and free energy-based approach. PLoS ONE 9, e110884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mitchell J. L. A., Judd G. G., Bareyal-Leyser A., and Ling S. Y. (1994) Feedback repression of polyamine transport is mediated by antizyme in mammalian tissue culture cells. Biochem. J. 299, 19–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Domene C., Doyle D. A., and Vénien-Bryan C. (2005) Modeling of an ion channel in its open conformation. Biophys. J. 89, L01–03 [DOI] [PMC free article] [PubMed] [Google Scholar]