Abstract
Leptin and TNFα can individually work in the brain to affect blood pressure; however, it remains unknown whether these two cytokines might have an interactive role in this process and, if so, how. In this work, we found that leptin stimulation led to TNFα production under both in vitro and in vivo conditions, and diurnal fluctuation of leptin concentrations in the cerebrospinal fluid predicted the circadian changes of TNFα gene expression in the hypothalamus. Signaling analysis showed that leptin stimulation led to a rapid and strong STAT3 activation followed by a second-phase moderate STAT3 activation, which was selectively abolished by anti-inflammatory chemical PS1145 or TNFα antagonist WP9QY. Physiological study in normal mice revealed that diurnal rise of blood pressure was abrogated following central administration of PS1145 or a leptin receptor antagonist. Central TNFα pretreatment was found to potentiate the effect of leptin in elevating blood pressure in normal mice. In pathophysiology, dietary obesity mimicked TNFα pretreatment in promoting leptin-induced blood pressure rise, and this effect was blocked by central treatment with either PS1145 or WP9QY. Hence, central leptin employs TNFα to mediate the diurnal blood pressure elevation in physiology while enhancement of this mechanism can contribute to hypertension development.
Keywords: cytokine, hypertension, hypothalamus, leptin, tumor necrosis factor (TNF)
Introduction
In coordination with physical and physiological requirements, blood pressure (BP)2 levels are tightly regulated to rise and fall in circadian manners, while alterations in these regulatory processes can chronically lead to the development of hypertension. From both physiological and pathological perspectives, BP changes involve peripheral contributions as well as neural inputs. Recently, there was an increasing amount of research in addressing the molecular and neuronal types in the central nervous system (CNS) mechanisms of hypertension (1–5). Leptin, an adipose tissue-derived cytokine which is overproduced in obesity, has been shown to increase BP when chronically administrated centrally (4, 6–9), and the underlying physiological basis involves up-regulation in the sympathetic outflow from the brain to the peripheral tissues (6, 10). Interestingly, the central effects of leptin on BP versus feeding do not necessarily follow the same line (8, 11, 12), for example, while leptin's regulation on feeding is compromised (namely “leptin resistance”) in obesity, its action in raising BP is well reserved, leading to a notion that leptin resistance is physiologically selective (8, 9, 13). Taken together, the action of leptin on BP is probably mediated by certain mechanisms which are less clear compared with the mechanisms in mediating metabolic regulation. Also, an acute excess of leptin, e.g. via a single injection, has a prominent effect on feeding but little influences on the BP in normal animals (14, 15), leaving it unsolved regarding if leptin is involved in BP control under normal physiology and if so, how.
In addition to leptin, inflammatory cytokines were recently found to play crucial roles in the neural and hypothalamic mechanisms of hypertension (16). These findings agree with the general appreciation that inflammatory cytokines can interact with many important BP-regulating factors, such as renin-angiotensin system and sympathetic nervous system in participating in the development of hypertension (17, 18) and related cardiovascular diseases (19). Our recent research indicated that the hypothalamus is crucial for the action of pro-inflammatory cytokine TNFα in increasing BP in light of promoting the development of obesity-related hypertension (20, 21). In this background and also hinted by evidence showing that leptin can influence immune response (22), we carried out the current study to investigate if there exists an interactive action between leptin and TNFα in the central control of BP in normal physiology and also whether this potential mechanism is important for the development of obesity-related hypertension.
Results
Leptin-stimulated Hypothalamic TNFα and the Association with Diurnal Rhythms
Considering that leptin can influence immune response (22), we recently questioned that leptin could have a direct effect on hypothalamic TNFα production. Indeed, based on human monocytes, it has been reported that leptin stimulation led to synthesis of immune cytokines such as TNFα (23). However, it remains unclear if this relationship might be biologically important in a non-immune system. We were interested in the hypothalamus since it is classically known as a major site for exerting the actions of leptin. But, before directly analyzing the hypothalamus, we did an in vitro experiment to evaluate if leptin could affect TNFα production in cultured cells, using a cell line which is unrelated to the immune system. In this experiment, we employed HEK293 cells which do not express leptin receptor but were genetically engineered with the long form of leptin receptor, a suitable in vitro model established for studying leptin signaling in the research (24). These cells were treated with leptin or the vehicle, and TNFα mRNA levels were determined at various time points during the treatment. We obtained data confirming that leptin stimulation led to increases in TNFα mRNA levels for ∼5 h, which we followed up while the peak effects occurred at 1–3 h during the stimulation (Fig. 1A).
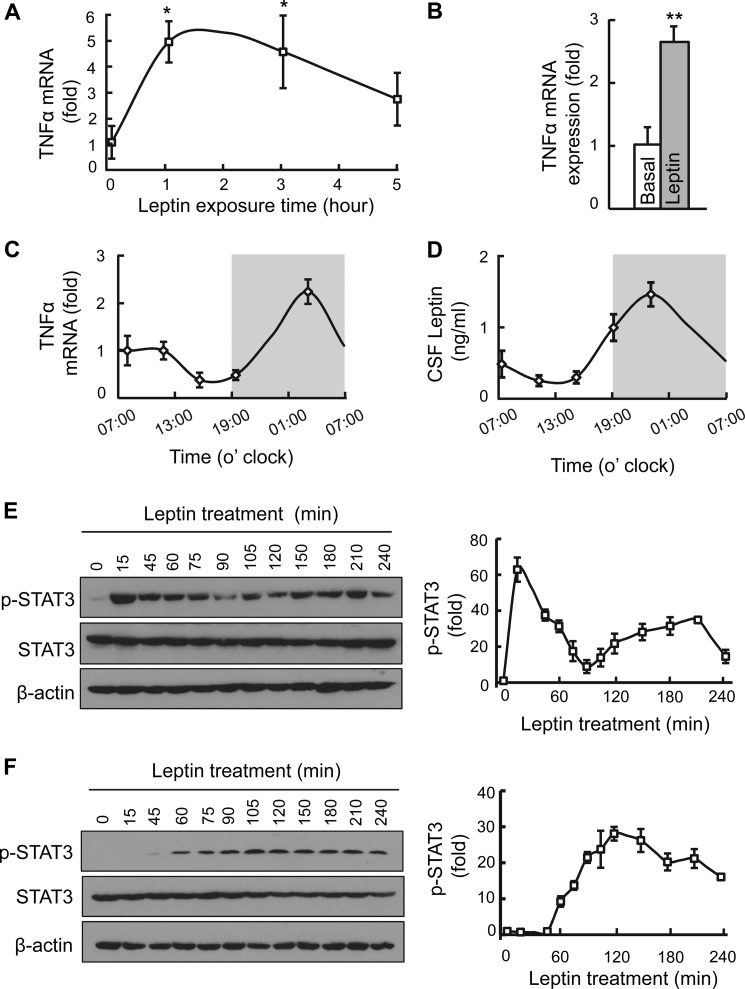
FIGURE 1.
Leptin-induced hypothalamic TNFα production in vitro and in vivo. A, time-dependent change of TNFα mRNA levels in HEK293LEPR cells in response to leptin (10 ng/ml) incubation over 5 h. B, normal adult C57BL/6 mice (chow-fed males) after overnight fasting were treated with leptin (2.5 μg) or vehicle into the third ventricle, and hypothalami were collected 2 h later for measuring TNFα mRNA levels. C and D, circadian patterns of hypothalamic TNFα mRNA levels (C) and CSF leptin concentrations (D). White area represents daytime phase (07:00–19:00), and gray area represents nighttime phase (19:00–7:00). E and F, STAT3 phosphorylation (p-STAT3) levels in HEK293LEPR cells in response to leptin exposure. Cells were deprived of serum for an overnight period and were treated with leptin at a standard dose (100 ng/ml, E) or a low dose (10 ng/ml, F) and analyzed for p-STAT3 levels at the indicated time points. Western blot analysis represented in E and F was quantified and presented in graphs on the right. Each bar graph reflects at least three independent experiments. Error bars reflect mean ± S.E. *, p < 0.05; **, p < 0.01; n = 5–6 samples per group (A), n = 4–6 mice per group (B–D), and n = 3 samples per time point (E, F right). Error bars reflect mean ± S.E.
Next, using mice, we examined if leptin-induced TNFα production might occur in the hypothalamus. To do so, we injected a single dose of leptin (2.5 μg) into the hypothalamic third ventricle through pre-implanted cannula, and 2 h later, the hypothalami were harvested for measuring TNFα mRNA. Data showed that hypothalamic TNFα mRNA increased >2-fold by leptin stimulation (Fig. 1B). Also, to gain an insight into the potential physiological relevance of this relationship, we studied if hypothalamic TNFα mRNA levels might physiologically correlate with the circadian profile of leptin release, since the release of leptin fluctuates according to the circadian rhythms of feeding behaviors (25, 26). To do so, we collected the hypothalamus as well as the cerebrospinal fluid (CSF) from normal C57BL/6 mice at different circadian time points over a 24-h period. We found that circadian rhythms displayed both in hypothalamic expression of TNFα and in leptin concentrations in the CSF (Fig. 1, C and D). Also, there was 3∼4-h phase shift between the curves of leptin and TNFα, with the peak concentration of leptin occurring 3∼4 h before the peak level of hypothalamic TNFα. In summary, leptin-induced hypothalamic TNFα production in the hypothalamus is associated with nighttime phase (active phase for mice) of the circadian rhythms in physiology.
Biphasic STAT3 Phosphorylation in Response to Leptin Stimulation
Subsequently, we profiled if the classical molecular signaling of leptin might have a component that is affected by leptin-induced TNFα. As established, leptin-induced STAT3 phosphorylation is critical for many functions of leptin (27–30); however, there seems to be a lack of sufficient details on the long-term kinetics of STAT3 phosphorylation in response to leptin. Here, we examined leptin-induced STAT3 phosphorylation over frequent time points during a 4-h period in HEK293LEPR cells. We treated HEK293LEPR cells with leptin at the concentration of 100 ng/ml, a standard dose for studying leptin signaling established in previous research of using this in vitro model (24, 31, 32). As expected, leptin stimulation strongly and rapidly increased STAT3 phosphorylation within 15 min following the treatment (Fig. 1E). Compared with the baseline level, leptin-induced STAT3 phosphorylation was robust at 15 min, declined at 45–75 min, and substantially dropped at ∼90 min during leptin stimulation. Interestingly, as we continued to monitor STAT3 phosphorylation, we observed that there was a second-phase elevation of STAT3 phosphorylation which was evident from 105 to 210 min (Fig. 1E). Also, as revealed in this experiment, the second-phase induction in STAT3 phosphorylation was moderate and tonic, in contrast with the dramatic and rapid induction of STAT3 phosphorylation in the first phase. Therefore, leptin-induced STAT3 activation include an acute-phase and a tonic, second-phase stage of effects.
Second-phase STAT3 Activation via Chronic Action of Low-dose Leptin
As established in research, leptin rapidly activates the JAK2-STAT3 cascade, which is immediately downstream of leptin receptor, and this process is the molecular basis for leptin-induced acute phosphorylation of STAT3 (30). In light of second-phase STAT3 phosphorylation triggered by leptin shown in Fig. 1E, we predicted that it was likely a result of a molecular event that is secondary to leptin stimulation. To test this hypothesis, we decreased the dose of leptin to a low concentration (10 ng/ml) which was insufficient to result in an acute induction of STAT3 activation. As shown in Fig. 1F, we barely detected STAT3 phosphorylation over 15 to 45 min of leptin treatment at this low dose. In contrast, after 60 min of this treatment, a moderate but chronic induction of STAT3 phosphorylation was observed (Fig. 1F). This time course of STAT3 activation by low-dose leptin agreed with the second-phase profile of leptin-induced STAT3 phosphorylation shown in Fig. 1F. By comparing these two sets of observations, it can be suggested that leptin can induce an acute phase followed by a second phase of STAT3 activation, and these two processes involve different molecular mediators.
Reduction of Second-phase STAT3 Activation of Leptin by Inhibiting TNFα or Downstream
Given the biphasic STAT3 activation of leptin, we re-considered the results that the circadian changes of leptin concentrations in the CSF were followed by the circadian changes of hypothalamic TNFα in mice (Fig. 1, C and D), and also the results that leptin increased hypothalamic TNFα (Fig. 1, A and B), and thus questioned how TNFα might be involved in biphasic STAT3 activation induced by leptin. To justify this question, we confirmed that TNFα stimulation indeed induces an appreciable level of STAT3 phosphorylation in these cells (data not shown), similarly as shown in the literature although based on other cell types (33, 34). To specifically address if there exists a cause-effect relationship, we did experiments to test if leptin-induced STAT3 activation could be reduced by inhibiting TNFα signaling pathway. As known, PS1145 is a potent anti-inflammatory chemical through inhibiting the IKKβ/NF-κB pathway (35) which is an important downstream of TNFα. We found that, while PS1145 did not affect acute-phase STAT3 phosphorylation of leptin (data not shown), it substantially abrogated leptin from inducing the second-phase STAT3 activation (Fig. 2A, left panel). In addition, using a TNFα antagonist, WP9QY (36, 37), we found that it similarly diminished chronic STAT3 activation of leptin (Fig. 2A, right panel). Thus, according to STAT3 signaling, leptin can exert an acute-phase and a chronic-phase action, the latter process was mediated at least by TNFα which, of note, is also produced from leptin simulation.
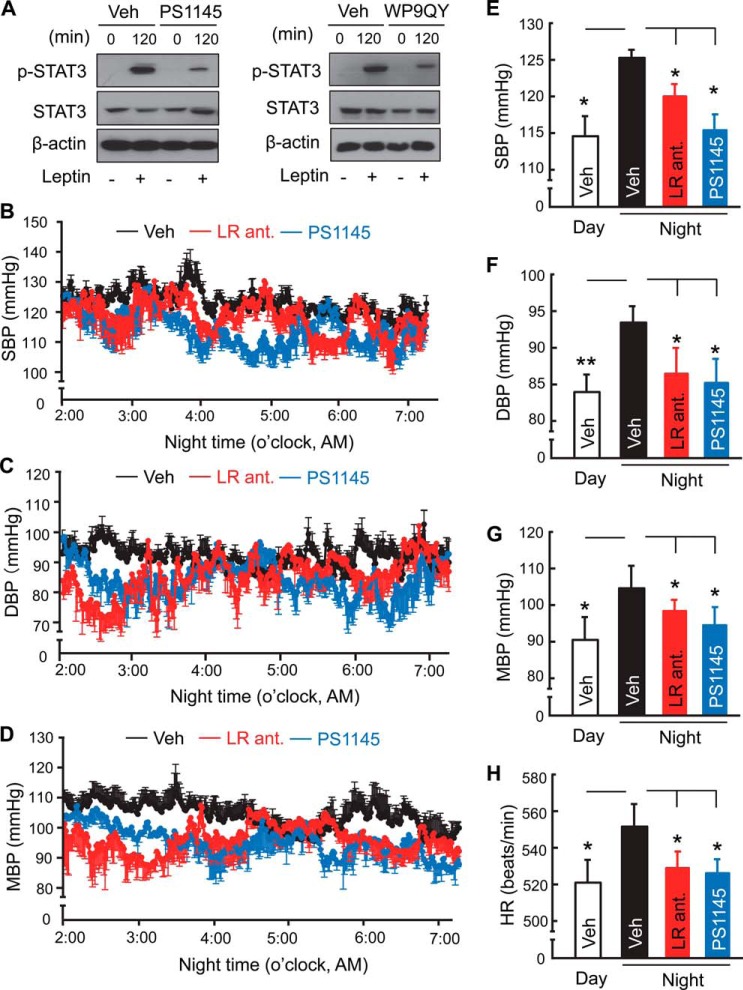
FIGURE 2.
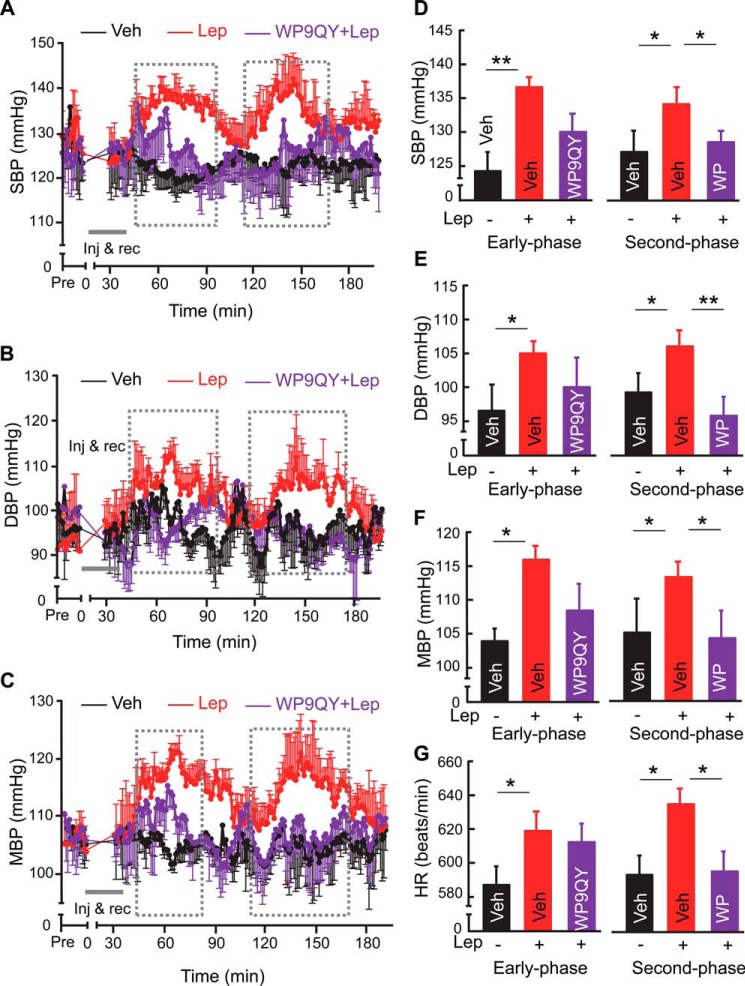
Abrogation of diurnal BP rise by central inhibition of leptin or TNFα. A, after an overnight period of serum deprivation, HEK293LEPR cells were treated with leptin (100 ng/ml) in the presence or absence of PS1145 (10 μm) or WP9QY (50 μm) for 120 min and analyzed for p-STAT3 levels. Results represent at least two independent experiments. B–H, standard C57BL/6 mice (chow-fed males, ∼3 month-old) were implanted with a BP radio transmitter in the carotid artery and an injection cannula in the hypothalamic ventricle. After post-surgery recovery, mice received a single injection of PS1145 (1 μg) or three injections of a leptin receptor antagonist (LR ant., 2.5 μg, one injection per night) at midnight (∼0:00 AM). Vehicle injection performed at the midnight as well as mid-daytime was used for comparisons. All mice were continuously recorded under telemetry for SBP (B, E), DBP (C, F), MBP (D, G), and HR (H) prior to and post injection. Bar graphs in B–D present 5-h BP levels of vehicle-, LR ant-, and PS1145-treated mice averaged from 2:00 to 7:00 AM, compared with the daytime average BP of mice that were treated with vehicle. *, p < 0.05; **, p < 0.01; n = 4–5 mice per group (B–H). Error bars reflect mean ± S.E. SBP: systolic BP; DBP: diastolic BP; MBP: mean BP.
Role of Central Leptin and TNFα in Mediating Diurnal BP Rise in Mice
Considering that central leptin and TNFα both increased during the active phase (nighttime) in mice (Fig. 1), and this diurnal phase features elevations in many physiological and physical functions supported by BP rise, we wondered if central leptin and TNFα are both required for the diurnal rhythm of BP rise occurring during the active phase of a diurnal cycle. To answer this question, we investigated if blocking TNFα or leptin in the brain could affect BP rise during the active phase (nighttime) in normal mice. Standard, chow-fed C57BL/6 mice were implanted with a telemetric BP probe in the carotid artery and an injection cannula in the third ventricle, using the surgical procedure described previously (20). Following post-implantation recovery, we injected PS1145 to therefore inhibit the action of central TNFα during the nighttime or daytime. While this treatment did not affect daytime BP levels (data not shown), it prevented the nighttime rise of BP levels over ∼4 h (Fig. 2, B–G). Also, nighttime increases in heart rates were abrogated by PS1145 treatment (Fig. 2H). In analogy, we employed the similar design but centrally injecting a leptin receptor antagonist to inhibit the action of central leptin. However, a single injection of leptin receptor antagonist did not evidently affect BP, probably because this peptide-based intervention is less efficient compared with the chemical approach of administering PS1145; thus we decided to repeat the injection over 3 diurnal cycles to potentially increase the efficacy of the treatment. Despite that the treatment did not change the daytime BP, as similarly appreciated in the literature (10), we found that 3-midnight treatment indeed prevented the nighttime rises across systolic, diastolic and mean BP levels and heart rates (Fig. 2, B–H). Also, the effect from 3-midnight injections of leptin receptor antagonist occurred earlier after the last injection, compared with the effect from a single midnight injection of PS1145 (Fig. 2, B–H). Through superimposing the time-course curves of BP levels from mice that received a single midnight PS1145 treatment versus 3-midnight leptin receptor antagonist treatment (Fig. 2, B–G), it further supported the point that central inhibition of leptin and TNFα can similarly blunt the BP rise during the nighttime in normal mice.
Central TNFα Potentiates BP-elevating Effect of Leptin
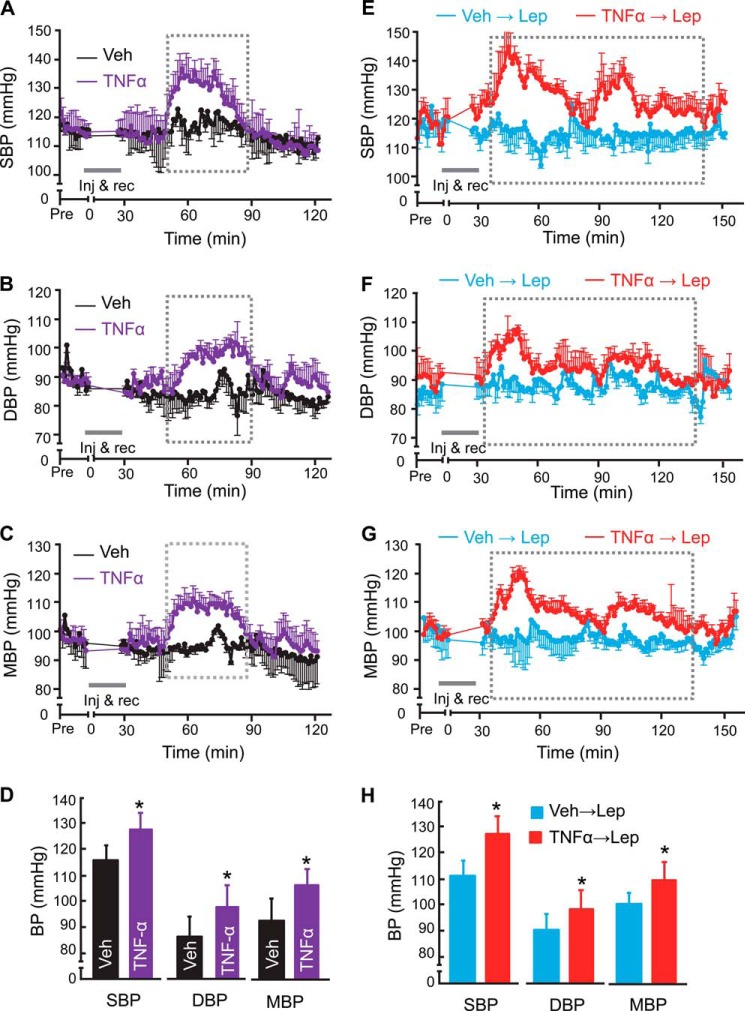
Following the loss-of-function results above suggesting that leptin and TNFα are involved in BP rise during the nighttime, we asked if this relationship of leptin and TNFα in affecting BP could be experimentally separated from nighttime condition. To answer this question, standard C57BL/6 mice received a single injection of either leptin or TNFα in the hypothalamic third ventricle via the pre-implanted cannula during the daytime. To target the daytime was more meaningful for studying if exogenously delivered cytokines have an effect on BP, because loss-of-function study suggested that daytime BP is not affected by the endogenous levels of these cytokines. The injection dose of leptin was set at 2.5 μg, as it represents an established dosage condition for studying leptin effects on metabolic physiology as well as BP (8, 38, 39). Injection dose of TNFα was set at 10 pg, a low-dose option which we employed previously to show that it led to BP increase (20), although it was unclear whether an acute action of this low dose could have a role in normal physiology of BP control. We found that a single injection of TNFα resulted in BP increases which sustained ∼30 min (Fig. 3, A–D), a single injection of leptin did not evidently increase BP (Fig. 3, E–H). The lack of an evident effect from single leptin injection was consistent with the literature, which suggested that the hypertensive effect of leptin relied on repeated injections (14, 40). Given the results in Figs. 1 and 2, we wondered if a single injection of leptin might be insufficient to induce the amount of TNFα production and its signaling required for leading to a detectable effect on BP. To explore this possibility, we pre-treated mice with low-dose TNFα (10 pg), and 2 h later after BP returned the normal baseline levels (but TNFα downstream signaling was mobilized), we performed an injection of leptin (2.5 μg) and continued to monitor BP in these mice. As shown in Fig. 3, E–H, leptin injection led to strong and sustained BP increases in mice that were pretreated with TNFα. These data indicate that central TNFα is an important mediator for central leptin in mediating physiological BP rise of normal mice.
FIGURE 3.
Central TNFα potentiates the effect of leptin in increasing BP. Normal adult C57BL/6 mice (chow-fed males) received a hypothalamic third-ventricle injection of TNFα (10 pg), leptin (Lep, 2.5 μg), both cytokines (TNFα pretreated at 2 h prior to leptin injection, labeled as “TNFα→Lep”), or vehicle (Veh) during the daytime, were continuously monitored via pre-implanted telemetric BP probes prior to injection and following the period of injection and recovery (inj & rec). Data present average BP changes of mice injected with TNFα (A–D) or mice pre-treated with TNFα at 2 h before leptin injection (E–H). Curves show the time-course changes over a ∼3-h follow-up according to SBP (A, E), DBP (B, F) and MBP (C, G). Bar graphs in D and H show average SBP, DBP, and MBP over the duration outlined by broken lines in the curves shown in A–G. *, p < 0.05; n = 4–5 mice per group (A–H). Error bars reflect mean ± S.E.
Central Leptin Excess Potentiates Hypertension Development in Obesity
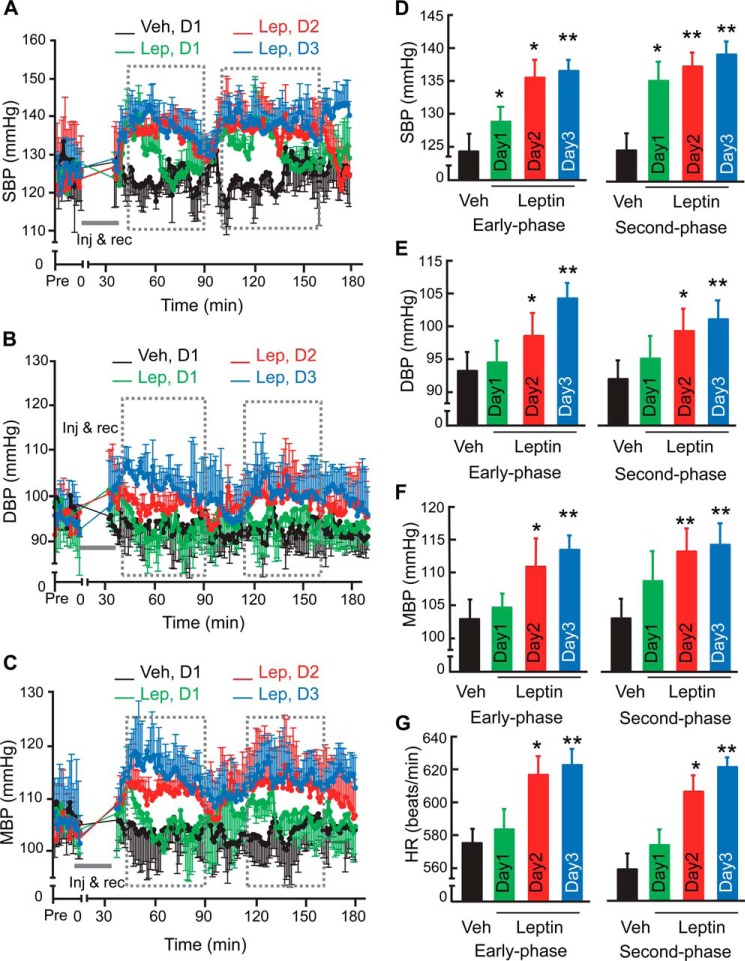
While results in Figs. 1 to 3 suggested that central TNFα and leptin are involved in mediating the diurnal BP rise in physiology, we further studied if the augmented relationship of these two cytokines could be a basis for the development of obesity-related hypertension, especially since obesity is associated not only with circadian disorders but with excess of both cytokines. Also, because the effect of single leptin administration in increasing BP is modest (14), it suggests that leptin probably require other factor(s) to act together for a potential role in hypertension. To address this question, we profiled the effect of central leptin administration on BP in mice with a moderate degree of obesity induced by ∼3-month high-fat diet (HFD) feeding; these mice were still normotensive, but expression of pro-inflammatory genes including TNFα already increased in the hypothalamus as appreciated in recent research (20, 42). These HFD-fed mice were pre-implanted with a telemetric BP probe in the carotid artery and a cannula in the third ventricle. After post-surgery recovery, mice were injected centrally with leptin (2.5 μg) or the vehicle via the cannula, and BP signals were monitored continuously prior to and post injection, as presented in Fig. 4, A–G. As discussed above, all these injections were performed during the daytime, which was more suitable for studying the effect of exogenously delivered leptin. On Day 1, we found that systolic BP increased at ∼40 min following the completion of leptin injection, and this increase remained about 45 min and then declined (Fig. 4, A and D). Notably, systolic BP resurged after an interval (∼30 min), seemingly leading to a second-phase increase, which was more long-lasting than the increases in the initial phase (Fig. 4, A and D). Leptin injection on Day 1 did not significantly increase diastolic or mean BP or heart rate (Fig. 4, B–G). On Day 2 following the injection, the BP-raising effects of leptin injection became evident across systolic, diastolic and mean BP as well as heart rate (Fig. 4, A–G), and the effect on diastolic BP further increased on Day 3 following the injection (Fig. 4, B and E). Bi-phasic BP increases continued to be observed (Fig. 4, A–F), suggesting that leptin employs different mechanisms to affect BP over time. Altogether, in contrast to that the acute effect of leptin excess in increasing BP is modest in normal physiology, obesity condition can potentiate the effect of central leptin excess in elevating BP, which can contribute to hypertension development.
FIGURE 4.
Rapid BP-raising effect of central leptin in mice with dietary obesity. C57BL/6 mice (adult males) with moderate dietary obesity through ∼3-month HFD feeding were implanted with a BP radio transmitter in the carotid artery and an injection cannula in the hypothalamic ventricle. After post-surgery recovery, mice received an injection of leptin (Lep, 2.5 μg) during the daytime per day for 3 days. All mice were continuously recorded under telemetry for SBP (A, D), DBP (B, E), MBP (C, F), and HR (G) prior to and post injection. Curves show the time-course changes over a ∼3-h follow-up according to SBP (A), DBP (B), and MBP (C). Bar graphs show the levels of SBP (D), DBP (E), MBP (F), and HR (G) averaged over the early phase or second phase outlined by broken lines following leptin injection on each day. BP profiles of mice injected with vehicle (Veh) on each day were comparable, and data presented were obtained from day 1. *, p < 0.05; **, p < 0.01, n = 4–5 mice per group. Error bars reflect mean ± S.E.
Reduction of Leptin-mediated Hypertension in Obesity by Central WP9QY Treatment
To further evaluate if TNFα was important for the BP-elevating effect of central leptin in obesity condition, we carried out a study in which mice received 2-day injection of leptin (2.5 μg) in the hypothalamic third ventricle. Since 2-day leptin injections during the daytime was sufficient to increase BP (Fig. 4), we performed 2-day injections of leptin in the daytime, each preceded by an injection of TNFα antagonist, WP9QY (5 μg) versus the vehicle at 2 h before leptin administration. Mice were continuously recorded under telemetry for BP. As shown in Fig. 5, WP9QY treatment significantly prevented leptin from increasing BP. These protective effects by WP9QY against the second-phase action of leptin seemed to be more evident compared with the effect against the first-phase action of leptin. In addition, WP9QY treatment blunted the effect of leptin in increasing the heart rate of these mice (Fig. 5G). Taken together, these results supported that central excess of leptin and TNFα act together to play a critical role in hypertension development in obesity condition.
FIGURE 5.
Reduction of leptin-mediated hypertension in obese mice by central TNFα inhibition. C57BL/6 mice (adult males) that were maintained HFD feeding for ∼3 months received 2-day injection of leptin (2.5 μg) in the hypothalamic third ventricle, each preceded by an injection of WP9QY (WP, 5 μg) versus vehicle (Veh) at 2 h before leptin administration. Mice were continuously recorded under telemetry for SBP (A, D), DBP (B, E), MBP (C, F), and HR (G) prior to and post injection. Curves show the time-course changes over a ∼3-h follow-up according to SBP (A), DBP (B), and MBP (C) following leptin injection on Day 2. Bar graphs show the levels of SBP (D), DBP (E), MBP (F), and heart rate (HR) (G) averaged over the early phase or second phase outlined by broken lines following leptin injection on Day 2. *, p < 0.05; **, p < 0.01, n = 4–5 mice per group. Error bars reflect mean ± S.E.
Prevention of Leptin-mediated Hypertension in Obesity by Central PS1145 Treatment
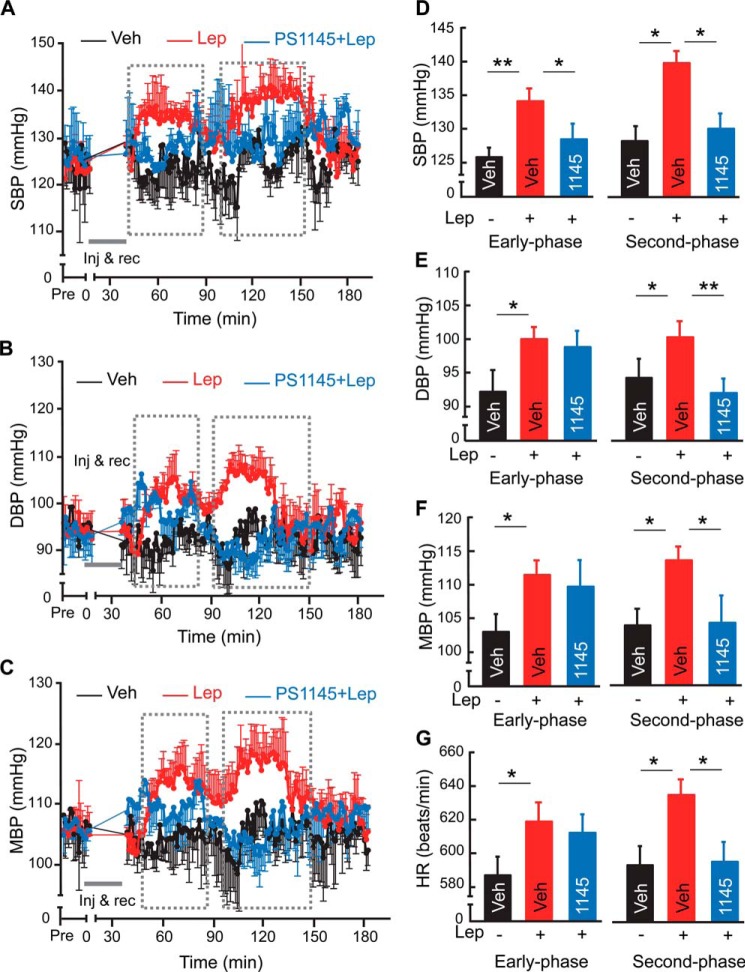
Next, we investigated if a general inhibition of hypothalamic inflammation by PS1145 could also be useful to counteract against leptin-mediated BP increase in the condition of obesity. As similarly described in Fig. 5, we employed a group of C57BL/6 mice with moderate obesity induced through ∼3-month HFD feeding. Under telemetric monitoring, these mice were pre-injected with PS1145 (1 μg) at 2 h before each of 2-day leptin injections in the daytime. Telemetric recording revealed that PS1145 pretreatment significantly prevented leptin from increasing BP in these obese mice, and these protective effects of PS1145 were observed across systolic, diastolic and mean BP (Fig. 6, A–F). In addition, PS1145 pretreatment blocked the effect of central leptin excess from increasing heart rate in these mice (Fig. 6G). Hence, these results corroborated the therapeutic effects of WP9QY treatment presented above, further supporting the point that suppression of hypothalamic inflammation is generally valuable for treating obesity-related hypertension.
FIGURE 6.
Reversal of obesity-associated hypertensive effect of leptin by PS1145 treatment. C57BL/6 mice (adult males) that were maintained HFD feeding for ∼3 months received 2-day injection of leptin (2.5 μg) in the hypothalamic third ventricle, each preceded by an injection of PS1145 (1145, 1 μg) versus vehicle (Veh) at 2 h before leptin administration. All mice were continuously recorded under telemetry for SBP (A, D), DBP (B, E), MBP (C, F), and HR (G) prior to and post injection. Curves show the time-course changes over a ∼3-h follow-up according to SBP (A), DBP (B), and MBP (C) following leptin injection on Day 2. Bar graphs show the levels of SBP (D), DBP (E), MBP (F), and heart rate (HR) (G) averaged over the early phase or second phase outlined by broken lines following leptin injection on Day 2. *, p < 0.05; **, p < 0.01; n = 4–5 mice per group. Error bars reflect mean ± S.E.
Discussion
In this work, we investigated the molecular relationship between leptin and TNFα, examined their role through the CNS in the physiological control of diurnal BP rise, and also studied the potential contribution of central leptin-TNFα connection, when augmented, to obesity-related hypertension (Fig. 7). Indeed, leptin and TNFα are both released significantly from the fat, and cell culture experiments have shown that leptin stimulation led to increased TNFα mRNA and protein levels in cells such as macrophages and monocytes (43, 44). Furthermore, TNFα inhibition was shown to diminish the effect of leptin in up-regulating CD11b expression in neutrophils (45). However, it is still unclear regarding whether there is a relationship between these two cytokines in the brain and especially in the hypothalamus for a physiological function or disease. Here, we demonstrated that increases in leptin concentrations in the CSF over a 24-h circadian period are associated with increases in hypothalamic TNFα production. Also, in vitro and in vivo models both indicated that leptin stimulation leads to TNFα production, a process mostly likely mediated by leptin-induced MAPK activation, which can sensitively increase TNFα gene expression (44). Through signaling analysis, we demonstrated that leptin-induced STAT3 activation were biphasic, including an initial, acute phase and a second, tonic phase. As known, leptin-induced STAT3 activation during the initial phase is mediate through leptin receptor activation-induced JAK2-STAT3 cascade (30). This initial phase of leptin signaling is fast and excites neurons to rapidly induce biological actions (46), some of which might be independent of STAT3 transcriptional program (47–49). In contrast to the short time window of initial leptin signaling, the second phase of leptin-activated STAT3 is moderate but tonic, presumably being involved in certain chronic functions of leptin, such as immune regulation. This second phase of STAT3 activation is attributed, at least, to leptin-mediated TNFα production, since it is reversed by not only TNFα antagonist WP9QY but also anti-inflammatory chemical PS1145.
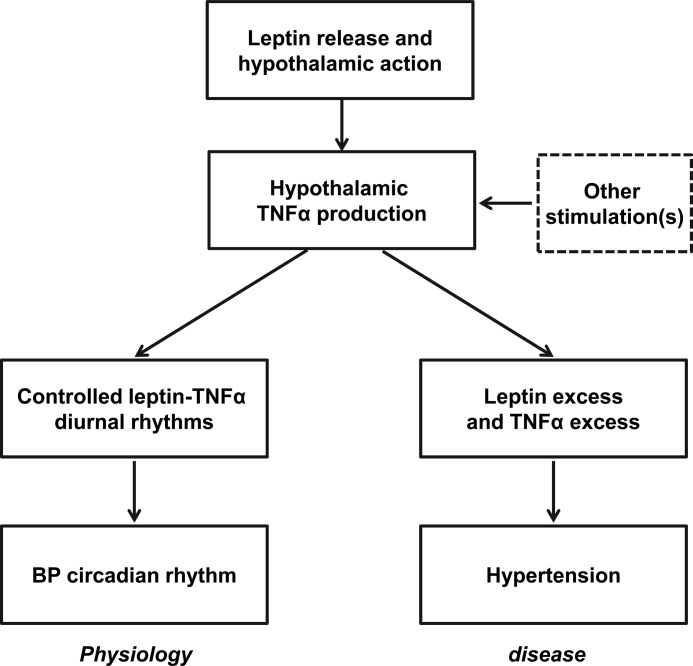
FIGURE 7.
Proposed model of central leptin and TNFα in BP control and hypertension. Increased leptin release can act in the brain to result in a controlled level of hypothalamic TNFα production, and through the central action of TNFα (which can also be contributed to by other factors in addition to leptin), it induces a moderate BP rise, which is physiologically associated with the active phase in diurnal cycles. Under conditions of obesity, which are prone to the development of hypertension, leptin, and TNFα are both chronically excess and thus induce an integrative action of chronically elevating BP, which can significantly contribute to the central mechanism of hypertension.
Our subsequent physiological experiments focused on comparatively analyzing the effects of leptin and TNFα on BP. Given these two cytokines in the CNS are associated with diurnal rhythms in a coordinated manner, we directed our attention to circadian changes of BP between daytime and night, and found that both cytokines are required to induce BP rise during the night time phase of mice. Leptin is a fat tissue-derived cytokine which is essential for the hypothalamic control over feeding and metabolic physiology, and release of leptin is associated with circadian rhythms of feeding activities (50, 51). It has been documented that central delivery of leptin can lead to BP increase in rodents (8); however, it is still unclear regarding how leptin is relevant in the control of BP in physiology. A recent study showed that patients with congenital leptin deficiency or leptin receptor deficiency manifested a decrease in systolic BP despite the condition of severe obesity (10). On the other hand, leptin treatment in normotensive patients with lipodystrophy, a disease associated with low level of leptin due to shortage of fat tissue, did not cause hypertension (52). In context of these clinical observations and supported by our findings, we speculate that in physiology, leptin has a role in inducing a normal level of BP rise during diurnal cycles, which is meant to prevent hypotension rather than causing hypertension. In light of TNFα, despite that it is often appreciated as a pro-inflammatory cytokine which participates in the inflammatory mechanism of disease, acute induction of this cytokine in a controlled manner can be physiologically relevant, and we recently reported that short-term induction of hypothalamic TNFα signaling is required for the initial phase of adaptive immune response (36). Taken together, by extending a previous view that TNFα is a mediator of metabolic syndrome including hypertension (20), the current study suggests a conceptual model (Fig. 7) that a well-regulated production of hypothalamic TNFα in the context of physiological leptin stimulation can participate in the physiological control of BP fluctuations.
From the disease perspective, our work in this study suggests that hyperleptinemia in the context of TNFα excess is chronically important for the development of obesity-related hypertension. To mimic the hypothalamic inflammatory changes in obesity, we employed central TNFα pretreatment and found that it remarkably potentiates the effect of subsequent leptin administration in elevating BP. Like leptin, TNFα is secreted prominently from the fat and is excessively produced in obesity (53). Also, it is known that early stage of obesity is already associated with sustained inflammation in the hypothalamus (20, 54), and it was elucidated that hypothalamic NF-κB pathway downstream of TNFα signaling is an important factor for the development of obesity-associated metabolic and cardiovascular disorders (20, 54). Agreeing with the fact that TNFα is an activator of NF-κB, we recently reported that TNFα excess can activate hypothalamic NF-κB to mediate the hypothalamic mechanism of obesity-related hypertension (20). The findings in this work provide information suggesting that hyperleptinemia is a chronic condition involved in inflammatory mechanism of hypertension.
Considering the recent report showing that leptin administration in patients with leptin deficiency did not increase the BP (52), we predict that central TNFα excess should be taken into account for the role of central leptin excess in hypertension development. As it has been raised in the research that the mechanisms for the hypertensive effect exerted by leptin under obesity and lean conditions are different (55, 56), our study indicates that cytokines such as TNFα could be a key point for appreciating the role of leptin in mediating pathological BP increase. This concept is also supported by our result showing that treatment with anti-inflammatory chemical PS1145 and TNFα antagonist WP9QY were both effective in reducing the hypertensive effect of leptin in obesity. Therefore, compared with the strategy of blocking leptin or leptin receptor, use of anti-inflammatory approach can represent an alternative option of breaking the leptin-TNFα mechanism to treat hypertension and related diseases.
Finally, it is worth discussing that, although high-dose TNFα is often known as a cachectic cytokine in diseases like cancers and infections, low-dose TNFα in the hypothalamus at the level of obesity-associated inflammation has been implicated to reduce the sensitivity of leptin in the control of feeding (57, 58). This effect of TNFα in decreasing leptin sensitivity in feeding is in contrast with its augmentation in the BP-raising effect of leptin. The underlying basis can be related to different formulas of how TNFα-induced STAT3 activation is involved in these two processes. As we learned in other studies of ours, STAT3 activation induced specifically by TNFα and that induced specifically by leptin (the portion that is TNFα-independent) can be added up, resulting in more levels of STAT3 activation, which have additive effects on BP. On the other hand, feeding regulation by leptin probably more relies on leptin-specific, TNFα-independent STAT3 activation, while the latter leads to a decrease in the signal to noise ratio of such leptin-specific STAT3 activation. These formulas further imply that the network as well as downstream programs of STAT3 in affecting metabolic physiology versus BP can be differential, which are also in agreement with the fact that the neuroendocrine versus sympathetic nervous system are differently required in these different physiological functions. This complexity further increases, due to the scenario that leptin can lead to TNFα production and thus contribute to TNFα-dependent STAT3 activation. Hence, future research is much needed to decipher the overlapping versus the differential programs of leptin, TNFα and their downstream molecules (including STAT3) in physiology and diseases.
Experimental Procedures
Experimental Animals
All studies were conducted in normal or obese adult male C57BL/6 mice obtained from the Jackson Laboratory. All the procedures were approved by the Institutional Animal Care and Use Committee of Albert Einstein College of Medicine. All mice were housed in a pathogen-free, standard animal facility and fed a standard chow or high-fat diet (Research Diet, Inc).
Cannulation and Infusion
As we previously described (20, 41), under an ultra-precise small animal stereotaxic apparatus (10 μm resolution, David Kopf Instruments), guide 26-gauge guide cannula (Plastics One, Inc.) were implanted into the hypothalamic third ventricle of anesthetized mice at the midline coordinates of 1.8 mm posterior to the bregma and 5.0 mm below the bregma. After 1∼2-week post-implantation recovery, mice were injected with chemicals, including leptin (2.5 μg, R&D), TNFα (10 pg, Sigma), PS1145 (1 μg, Sigma), WP9QY (5 μg, Santa Cruz Biotechnology), and moue leptin receptor antagonist (2.5 μg, Protein Laboratories). Stock solution of chemicals were finally dissolved in 0.5-μl artificial cerebrospinal fluid and infused via pre-implanted cannula over 5-min period using a 33-gauge internal injector (Plastics One, Inc.) that was connected to a 5-μl Hamilton Syringe.
Telemetric Probe Implantation and Recording
Radiotelemetric catheters (model TA11PA-C10, Data Sciences International, DSI) were implanted in the carotid artery, using the procedure we described previously (20). Briefly, mice were anesthetized, a ventral midline skin incision was made and the left common carotid artery was isolated under a binocular surgical microscope. The proximal end of the artery was ligated right below the carotid bifurcation, and the distal end was occluded with a microclip. Using a microscissor, a small incision was made near the proximal end, and pressure transmission catheter was guided into the artery, advanced to the aorta, and secured in place with sutures. The transmitter device was placed subcutaneously on the right flank, as close to the hindlimb as possible. Subsequently, neck incision was closed using 5–0 sutures (Ethicon), and mice were allowed for 1∼2 weeks of post-surgical recovery, and the pressure signals of each animal under conscious, free-moving conditions were recorded using the computerized method (DSI). BP data were sampled continuously with a sampling rate of 1,000 Hz with 1-min segment duration.
Cell Culture
HEK293LEPR cells (kindly provided by Dr. L. Rui at University of Michigan) were maintained in Dulbecco's Modified Eagle's Medium (Invitrogen) supplied with 10% fetal bovine serum (Hyclone), 100 units of penicillin, and 0.1 mg/ml streptomycin (Life Technologies) at 37 °C in a humidified atmosphere containing 5% CO2. Cells were fasted in serum-free medium for an overnight period and then were incubated with indicated chemicals including leptin, PS1145, and WP9QY for various time periods as described in the text.
Tissue Harvesting, Western Blot Analysis, and ELISA
Hypothalamus was dissected from the brain as we described previously (54). Collection of the CSF was performed in anesthetized mice at indicated circadian time points, according to an established procedure (36). Protein lysates were prepared from tissues or cultured cells, dissolved in a lysis buffer, separated by SDS-PAGE, immunoblotted with primary antibodies including rabbit anti-p-STAT3, anti-STAT3, and anti-β-actin (Cell Signaling), and reacted with HRP-conjugated secondary antibody (Pierce). Quantification of Western blots was performed with Image J, and p-STAT3 levels were normalized according to the protein levels of total STAT3. Leptin concentration in the CSF was determined using an ELISA Kit (Crystal Chem).
RNA Extraction and qPCR
Total RNA was extracted with Trizol (Invitrogen), the Moloney Leukemia Virus Reverse Transcriptase system (Promega) was used to synthesize complementary DNA, and qPCR was performed using SYBR Green MasterMix (Applied Biosystems). Data were normalized according to mRNA levels of housekeeping gene β-actin.
Statistics
Two-tailed unpaired t test was used for studies which involved only two groups. ANOVA and appropriate post-hoc test were used for experiments comprising more than two groups. All data are presented as mean ± S.E. p < 0.05 was considered statistically significant.
Author Contributions
D. C. conceived the hypothesis and designed the project and structures of experiments. C. H. co-designed and performed experiments represented in Figs. 3 to 6, data interpretation and figure preparation. W. W. and M. K. did experiments in Figs. 1 and 2. A. A. performed implantation surgeries for the study. D. C. wrote the paper, and C. H. provided assistance for writing.
Acknowledgments
We thank Cai's laboratory members for technical assistance.
This study was supported by National Institutes of Health Grants R01 DK078750, R01 AG031774, R01 HL113180, and R01 DK 099136 (all to D. C.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- BP
- blood pressure
- TNF
- tumor necrosis factor
- HFD
- high-fat diet.
References
- 1. Guyenet P. G. (2006) The sympathetic control of blood pressure. Nat. Rev. Neurosci. 7, 335–346 [DOI] [PubMed] [Google Scholar]
- 2. Iadecola C., and Davisson R. L. (2008) Hypertension and cerebrovascular dysfunction. Cell Metab. 7, 476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lifton R. P., Gharavi A. G., and Geller D. S. (2001) Molecular mechanisms of human hypertension. Cell 104, 545–556 [DOI] [PubMed] [Google Scholar]
- 4. Rahmouni K., Correia M. L., Haynes W. G., and Mark A. L. (2005) Obesity-associated hypertension: new insights into mechanisms. Hypertension 45, 9–14 [DOI] [PubMed] [Google Scholar]
- 5. Zimmerman M. C., Lazartigues E., Sharma R. V., and Davisson R. L. (2004) Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ. Res. 95, 210–216 [DOI] [PubMed] [Google Scholar]
- 6. Dunbar J. C., Hu Y., and Lu H. (1997) Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes 46, 2040–2043 [DOI] [PubMed] [Google Scholar]
- 7. Aizawa-Abe M., Ogawa Y., Masuzaki H., Ebihara K., Satoh N., Iwai H., Matsuoka N., Hayashi T., Hosoda K., Inoue G., Yoshimasa Y., and Nakao K. (2000) Pathophysiological role of leptin in obesity-related hypertension. J. Clin. Invest. 105, 1243–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rahmouni K., Haynes W. G., Morgan D. A., and Mark A. L. (2002) Selective resistance to central neural administration of leptin in agouti obese mice. Hypertension 39, 486–490 [DOI] [PubMed] [Google Scholar]
- 9. Rahmouni K., Morgan D. A., Morgan G. M., Mark A. L., and Haynes W. G. (2005) Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes 54, 2012–2018 [DOI] [PubMed] [Google Scholar]
- 10. Simonds S. E., Pryor J. T., Ravussin E., Greenway F. L., Dileone R., Allen A. M., Bassi J., Elmquist J. K., Keogh J. M., Henning E., Myers M. G. Jr., Licinio J., Brown R. D., Enriori P. J., O'Rahilly S., Sternson S. M., Grove K. L., Spanswick D. C., Farooqi I. S., and Cowley M. A. (2014) Leptin mediates the increase in blood pressure associated with obesity. Cell 159, 1404–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Myers M. G., Cowley M. A., and Münzberg H. (2008) Mechanisms of leptin action and leptin resistance. Annu. Rev. Physiol 70, 537–556 [DOI] [PubMed] [Google Scholar]
- 12. Mark A. L. (2013) Selective leptin resistance revisited. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305, R566–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lambert G. W., Straznicky N. E., Lambert E. A., Dixon J. B., and Schlaich M. P. (2010) Sympathetic nervous activation in obesity and the metabolic syndrome-causes, consequences and therapeutic implications. Pharmacol. Ther. 126, 159–172 [DOI] [PubMed] [Google Scholar]
- 14. Hall J. E., da Silva A. A., do Carmo J. M., Dubinion J., Hamza S., Munusamy S., Smith G., and Stec D. E. (2010) Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J. Biol. Chem. 285, 17271–17276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. da Silva A. A., do Carmo J. M., and Hall J. E. (2013) Role of leptin and central nervous system melanocortins in obesity hypertension. Curr. Opin. Nephrol. Hypertens. 22, 135–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shi P., Raizada M. K., and Sumners C. (2010) Brain cytokines as neuromodulators in cardiovascular control. Clin. Exp. Pharmacol. Physiol. 37, e52–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cai D., and Liu T. (2011) Hypothalamic inflammation: a double-edged sword to nutritional diseases. Ann. N.Y. Acad. Sci. 1243, E1–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tang Y., and Cai D. (2013) Hypothalamic inflammation and GnRH in aging development. Cell Cycle 12, 2711–2712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cai D. (2013) Neuroinflammation in overnutrition-induced diseases. Vitam. Horm. 91, 195–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Purkayastha S., Zhang G., and Cai D. (2011) Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-β and NF-κB. Nat. Med. 17, 883–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shin J. I., and Cai D. (2014) Hypertension in obesity: the role of hypothalamic inflammation. Nat. Rev. Endocrinol 10, 760. [DOI] [PubMed] [Google Scholar]
- 22. Loffreda S., Yang S. Q., Lin H. Z., Karp C. L., Brengman M. L., Wang D. J., Klein A. S., Bulkley G. B., Bao C., Noble P. W., Lane M. D., and Diehl A. M. (1998) Leptin regulates proinflammatory immune responses. FASEB J. 12, 57–65 [PubMed] [Google Scholar]
- 23. Santos-Alvarez J., Goberna R., and Sánchez-Margalet V. (1999) Human leptin stimulates proliferation and activation of human circulating monocytes. Cell. Immunol. 194, 6–11 [DOI] [PubMed] [Google Scholar]
- 24. Ren D., Li M., Duan C., and Rui L. (2005) Identification of SH2-B as a key regulator of leptin sensitivity, energy balance, and body weight in mice. Cell Metab. 2, 95–104 [DOI] [PubMed] [Google Scholar]
- 25. de Wardener H. E. (2001) The hypothalamus and hypertension. Physiol. Rev. 81, 1599–1658 [DOI] [PubMed] [Google Scholar]
- 26. Bodosi B., Gardi J., Hajdu I., Szentirmai E., Obal F. Jr., and Krueger J. M. (2004) Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R1071–R1079 [DOI] [PubMed] [Google Scholar]
- 27. Bates S. H., Stearns W. H., Dundon T. A., Schubert M., Tso A. W., Wang Y., Banks A. S., Lavery H. J., Haq A. K., Maratos-Flier E., Neel B. G., Schwartz M. W., and Myers M. G. Jr. (2003) STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421, 856–859 [DOI] [PubMed] [Google Scholar]
- 28. Bates S. H., Kulkarni R. N., Seifert M., and Myers M. G. Jr. (2005) Roles for leptin receptor/STAT3-dependent and -independent signals in the regulation of glucose homeostasis. Cell Metab. 1, 169–178 [DOI] [PubMed] [Google Scholar]
- 29. Vaisse C., Halaas J. L., Horvath C. M., Darnell J. E. Jr., Stoffel M., and Friedman J. M. (1996) Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat. Genet. 14, 95–97 [DOI] [PubMed] [Google Scholar]
- 30. Buettner C., Pocai A., Muse E. D., Etgen A. M., Myers M. G. Jr., and Rossetti L. (2006) Critical role of STAT3 in leptin's metabolic actions. Cell Metab. 4, 49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li Z., Zhou Y., Carter-Su C., Myers M. G. Jr., and Rui L. (2007) SH2B1 enhances leptin signaling by both Janus kinase 2 Tyr813 phosphorylation-dependent and -independent mechanisms. Mol. Endocrinol. 21, 2270–2281 [DOI] [PubMed] [Google Scholar]
- 32. Gao J., Tian J., Lv Y., Shi F., Kong F., Shi H., and Zhao L. (2009) Leptin induces functional activation of cyclooxygenase-2 through JAK2/STAT3, MAPK/ERK, and PI3K/AKT pathways in human endometrial cancer cells. Cancer Sci. 100, 389–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Toffanin S., Friedman S. L., and Llovet J. M. (2010) Obesity, inflammatory signaling, and hepatocellular carcinoma-an enlarging link. Cancer Cell 17, 115–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Theiss A. L., Simmons J. G., Jobin C., and Lund P. K. (2005) Tumor necrosis factor (TNF) α increases collagen accumulation and proliferation in intestinal myofibroblasts via TNF receptor 2. J. Biol. Chem. 280, 36099–36109 [DOI] [PubMed] [Google Scholar]
- 35. Yemelyanov A., Gasparian A., Lindholm P., Dang L., Pierce J. W., Kisseljov F., Karseladze A., and Budunova I. (2006) Effects of IKK inhibitor PS1145 on NF-κB function, proliferation, apoptosis and invasion activity in prostate carcinoma cells. Oncogene 25, 387–398 [DOI] [PubMed] [Google Scholar]
- 36. Kim M. S., Yan J., Wu W., Zhang G., Zhang Y., and Cai D. (2015) Rapid linkage of innate immunological signals to adaptive immunity by the brain-fat axis. Nat. Immunol. 16, 525–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aoki K., Saito H., Itzstein C., Ishiguro M., Shibata T., Blanque R., Mian A. H., Takahashi M., Suzuki Y., Yoshimatsu M., Yamaguchi A., Deprez P., Mollat P., Murali R., Ohya K., Horne W. C., and Baron R. (2006) A TNF receptor loop peptide mimic blocks RANK ligand-induced signaling, bone resorption, and bone loss. J. Clin. Invest. 116, 1525–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maness L. M., Kastin A. J., Farrell C. L., and Banks W. A. (1998) Fate of leptin after intracerebroventricular injection into the mouse brain. Endocrinology 139, 4556–4562 [DOI] [PubMed] [Google Scholar]
- 39. Tanida M., Yamamoto N., Morgan D. A., Kurata Y., Shibamoto T., and Rahmouni K. (2015) Leptin receptor signaling in the hypothalamus regulates hepatic autonomic nerve activity via phosphatidylinositol 3-kinase and AMP-activated protein kinase. J. Neurosci. 35, 474–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rahmouni K., Haynes W. G., Morgan D. A., and Mark A. L. (2003) Intracellular mechanisms involved in leptin regulation of sympathetic outflow. Hypertension 41, 763–767 [DOI] [PubMed] [Google Scholar]
- 41. Yan J., Zhang H., Yin Y., Li J., Tang Y., Purkayastha S., Li L., and Cai D. (2014) Obesity- and aging-induced excess of central transforming growth factor-β potentiates diabetic development via an RNA stress response. Nat. Med. 20, 1001–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Purkayastha S., and Cai D. (2013) Neuroinflammatory basis of metabolic syndrome. Mol. Metab. 2, 356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zarkesh-Esfahani H., Pockley G., Metcalfe R. A., Bidlingmaier M., Wu Z., Ajami A., Weetman A. P., Strasburger C. J., and Ross R. J. (2001) High-dose leptin activates human leukocytes via receptor expression on monocytes. J. Immunol. 167, 4593–4599 [DOI] [PubMed] [Google Scholar]
- 44. Zhao T., Hou M., Xia M., Wang Q., Zhu H., Xiao Y., Tang Z., Ma J., and Ling W. (2005) Globular adiponectin decreases leptin-induced tumor necrosis factor-α expression by murine macrophages: involvement of cAMP-PKA and MAPK pathways. Cell. Immunol. 238, 19–30 [DOI] [PubMed] [Google Scholar]
- 45. Zarkesh-Esfahani H., Pockley A. G., Wu Z., Hellewell P. G., Weetman A. P., and Ross R. J. (2004) Leptin indirectly activates human neutrophils via induction of TNF-α. J. Immunol. 172, 1809–1814 [DOI] [PubMed] [Google Scholar]
- 46. Shiraishi T., Sasaki K., Niijima A., and Oomura Y. (1999) Leptin effects on feeding-related hypothalamic and peripheral neuronal activities in normal and obese rats. Nutrition 15, 576–579 [DOI] [PubMed] [Google Scholar]
- 47. Hill J. W., Williams K. W., Ye C., Luo J., Balthasar N., Coppari R., Cowley M. A., Cantley L. C., Lowell B. B., and Elmquist J. K. (2008) Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J. Clin. Invest. 118, 1796–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xu A. W., Kaelin C. B., Takeda K., Akira S., Schwartz M. W., and Barsh G. S. (2005) PI3K integrates the action of insulin and leptin on hypothalamic neurons. J. Clin. Invest. 115, 951–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Buettner C., Muse E. D., Cheng A., Chen L., Scherer T., Pocai A., Su K., Cheng B., Li X., Harvey-White J., Schwartz G. J., Kunos G., Rossetti L., and Buettner C. (2008) Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat. Med. 14, 667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kettner N. M., Mayo S. A., Hua J., Lee C., Moore D. D., and Fu L. (2015) Circadian dysfunction induces leptin resistance in mice. Cell Metab. 22, 448–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Scheer F. A., Hilton M. F., Mantzoros C. S., and Shea S. A. (2009) Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. U.S.A. 106, 4453–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brown R. J., Meehan C. A., and Gorden P. (2015) Leptin does not mediate hypertension associated with human obesity. Cell 162, 465–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hotamisligil G. S., Shargill N. S., and Spiegelman B. M. (1993) Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science 259, 87–91 [DOI] [PubMed] [Google Scholar]
- 54. Zhang X., Zhang G., Zhang H., Karin M., Bai H., and Cai D. (2008) Hypothalamic IKKβ/NF-κB and ER stress link overnutrition to energy imbalance and obesity. Cell 135, 61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tümer N., Erdös B., Matheny M., Cudykier I., and Scarpace P. J. (2007) Leptin antagonist reverses hypertension caused by leptin overexpression, but fails to normalize obesity-related hypertension. J. Hypertens. 25, 2471–2478 [DOI] [PubMed] [Google Scholar]
- 56. Lu H., Duanmu Z., Houck C., Jen K. L., Buison A., and Dunbar J. C. (1998) Obesity due to high fat diet decreases the sympathetic nervous and cardiovascular responses to intracerebroventricular leptin in rats. Brain Res. Bull. 47, 331–335 [DOI] [PubMed] [Google Scholar]
- 57. Romanatto T., Roman E. A., Arruda A. P., Denis R. G., Solon C., Milanski M., Moraes J. C., Bonfleur M. L., Degasperi G. R., Picardi P. K., Hirabara S., Boschero A. C., Curi R., and Velloso L. A. (2009) Deletion of tumor necrosis factor-α receptor 1 (TNFR1) protects against diet-induced obesity by means of increased thermogenesis. J. Biol. Chem. 284, 36213–36222 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58. Tung Y. C., Gulati P., Liu C. H., Rimmington D., Dennis R., Ma M., Saudek V., O'Rahilly S., Coll A. P., and Yeo G. S. (2015) FTO is necessary for the induction of leptin resistance by high-fat feeding. Mol. Metab. 4, 287–298 [DOI] [PMC free article] [PubMed] [Google Scholar]