Abstract
One key to animal survival is the detection and avoidance of potentially harmful compounds by their bitter taste. Variable numbers of taste 2 receptor genes expressed in the gustatory end organs enable bony vertebrates (Euteleostomi) to recognize numerous bitter chemicals. It is believed that the receptive ranges of bitter taste receptor repertoires match the profiles of bitter chemicals that the species encounter in their diets. Human and mouse genomes contain pairs of orthologous bitter receptor genes that have been conserved throughout evolution. Moreover, expansions in both lineages generated species-specific sets of bitter taste receptor genes. It is assumed that the orthologous bitter taste receptor genes mediate the recognition of bitter toxins relevant for both species, whereas the lineage-specific receptors enable the detection of substances differently encountered by mice and humans. By challenging 34 mouse bitter taste receptors with 128 prototypical bitter substances in a heterologous expression system, we identified cognate compounds for 21 receptors, 19 of which were previously orphan receptors. We have demonstrated that mouse taste 2 receptors, like their human counterparts, vary greatly in their breadth of tuning, ranging from very broadly to extremely narrowly tuned receptors. However, when compared with humans, mice possess fewer broadly tuned receptors and an elevated number of narrowly tuned receptors, supporting the idea that a large receptor repertoire is the basis for the evolution of specialized receptors. Moreover, we have demonstrated that sequence-orthologous bitter taste receptors have distinct agonist profiles. Species-specific gene expansions have enabled further diversification of bitter substance recognition spectra.
Keywords: calcium imaging, cell signaling, G protein-coupled receptor (GPCR), human, mouse, bitter taste receptor, heterologous expression
Introduction
The plethora of natural compounds that taste bitter for humans comprises numerous chemicals with pharmacological activities that can make them powerful toxins, such as the alkaloids strychnine and colchicine or the sesquiterpene lactone picrotoxinin (1). However, compounds believed to exert health-beneficial effects such as the antioxidative phytoestrogen genistein from soy (2), the analgesic drug acetaminophen (3), or various polyphenols also taste bitter (4). To avoid ingestion of bitter substances that would pose a threat to organisms, efficient recognition and rejection mechanisms have developed throughout the animal kingdom. In bony vertebrates (Euteleostomi), the avoidance of bitter compounds is centered on taste receptors that detect potentially harmful substances with high accuracy and adequate sensitivity (5). Vertebrate bitter taste receptors, called taste 2 receptors (TAS2R (human) or Tas2r (murine)),2 are G protein-coupled receptors only remotely related to other classes of this large and enormously versatile receptor family (6–10). During evolution the first Tas2r genes appeared in the genomes of bony fish (11). In higher vertebrates frequent independent expansions and pseudogenization events resulted in differently sized Tas2r gene repertoires (12). Consequently, the number of putatively functional Tas2r genes varies considerably in vertebrates, ranging from 0 in baleen and tooth whales as well as penguins (13–16) to more than 50 in Western clawed frogs and 80 in Coelacanth (17–20). Thus, humans with ∼25 and mice with ∼35 putatively functional members possess average size Tas2r repertoires (21). The human genome not only contains fewer intact TAS2R genes than the mouse genome but also a larger number of pseudogenes (11 in human versus 7 in mice). This has been interpreted as a sign of relaxed selective constraints on the human TAS2R gene repertoire (22). The Tas2r genes of human and mouse occur clustered at few syntenic chromosomal regions (6, 22, 23). The majority of bitter taste receptor genes located on human chromosome 12 and mouse chromosome 6, respectively, occur in clusters of species-specific bitter taste receptor genes, which likely arose from gene duplications after the divergence of primate and rodent lineages. It has been speculated that these lineage-specific Tas2r recognize toxic bitter substances of particular relevance for the corresponding species (23). In contrast, the majority of Tas2r genes located on human chromosomes 5 and 7 and mouse chromosomes 2 and 15, respectively, exhibit a one-to-one orthology, suggesting that they developed prior to the divergence of primate and rodent lineages and enable the recognition of bitter substances equally important to humans and mice (23). If the above hypothesis is true, human and mouse should share Tas2r with conserved agonists, namely the one-to-one orthologs, and possess others with cognate bitter substances mostly relevant to one of the two species. In fact, when interpreting the data from rodent behavioral experiments, it is frequently argued that the murine Tas2r with highest sequence identity are true functional orthologs of their human counterparts, recognizing the same bitter compounds (cf. Refs. 24–28). However, structure-function analyses of human bitter taste receptors reveal that very few differences in the amino acid sequences of TAS2R can account for the largely deviating agonist spectra (29). Conversely, human TAS2R paralogs with pronounced amino acid sequence differences can have agonists in common even though they recognize these compounds by different binding modes (30).
The ∼25 human bitter taste receptors (TAS2R) are comparatively well characterized, with agonists identified for 21 of the ∼25 receptors (1–4, 7, 30–42). Collectively, these data indicated that humans have three very broadly tuned TAS2R “generalists” and eight receptor “specialists” that are narrowly tuned. Moreover, they have two TAS2R for compounds sharing structural motifs as well as eight moderately tuned receptors. Recently, the bitter taste receptor gene repertoires of chicken, turkey, and zebra finch, as well as the Western clawed frog, have been analyzed functionally. These studies revealed that narrowly tuned Tas2r are found only in species with larger Tas2r gene numbers, such as frog and zebra finch, whereas the three chicken and two turkey receptors are all broadly tuned (17). In mice, agonists have been reported for only two of the 35 putatively functional Tas2r, leaving the receptive range of the mouse Tas2r repertoire uncharacterized. For Tas2r105, an inhibitor of mRNA translation, cycloheximide, has been identified as a specific and potent agonist (7). The other receptor is Tas2r108, which is activated by denatonium benzoate and 6-n-propyl-2-thiouracil (PROP) with low potency (7). Thus, the scarcity of data on the functional properties of mouse Tas2r does not provide clear insight into the extent of functional orthology or whether species-specific Tas2r gene expansions have indeed resulted in specialized Tas2r for bitter compounds of species-specific relevance.
To close this gap in knowledge we investigated whether the putatively functional murine Tas2r respond to an array of 128 bitter substances in a functional heterologous expression assay. Behavioral experiments were also performed to correlate the tuning properties of mouse Tas2r with avoidance behavior of the animals assessed by brief-access taste tests.
Results
All Tas2r Genes Are Expressed in the Posterior Papillae of the Mouse Tongue
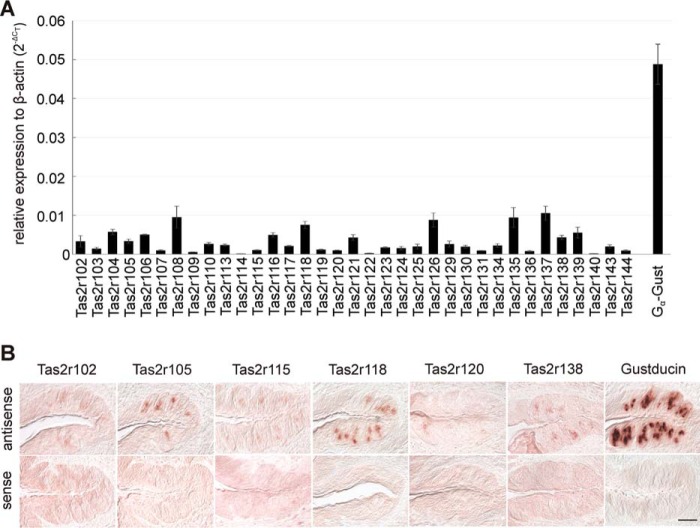
To elucidate whether all mouse Tas2r are indeed expressed in the gustatory cells of the tongue and to determine their relative expression levels and patterns, we performed quantitative RT-PCR (qRT-PCR) and in situ hybridization experiments. Quantitative RT-PCR expression analyses demonstrated that all Tas2r genes are expressed in the epithelium of the posterior tongue (Fig. 1A). Although some receptor mRNAs, e.g. those for Tas2r108, Tas2r118, Tas2r126, Tas2r135, and Tas2r137, were quite abundant, reaching ∼20% of the α-gustducin mRNA level, other Tas2r mRNAs, such as those of Tas2r114, Tas2r122, and Tas2r140, were rare, just reaching detection levels. The majority of Tas2r genes exhibited intermediate expression levels.
FIGURE 1.
Tas2r are expressed in the posterior lingual papillae. A, expression levels of all 35 Tas2r and α-gustducin (Gα-Gust) in epithelium enriched in vallate and foliate papillae of C57BL/6 mice determined by quantitative RT-PCR (means ± S.E., n = 4, normalized to β-actin). B, in situ hybridization using digoxigenin-labeled cRNA probes specific for selected Tas2r and gustducin revealed the presence of corresponding RNAs in vallate papillae of C57BL/6 mice (scale bar, 50 μm).
Differences in Tas2r gene expression for six selected receptor mRNAs were also evident on a cellular level, as shown by in situ hybridization experiments with mouse vallate papillae sections (Fig. 1B). For example, Tas2r118 mRNA was detected in a large subset of vallate taste cells with rather strong signal intensity, whereas Tas2r105 stained fewer cells but with comparable signal intensity. The Tas2r138 and Tas2r115 probes labeled cells that resemble Tas2r105 positive cells in number but not in staining intensity. Finally, cells expressing Tas2r120 or Tas2r102 were rare and showed faint staining. α-Gustducin mRNA was detected in many cells with strong signal intensity, as anticipated for a gene coding for a common signaling component of sweet, umami, and bitter taste transduction (9, 43–45). Vallate papillae sections treated with sense probes showed no staining confirming specificity of the detection method.
The results obtained by qRT-PCR and by in situ hybridization show a good correlation. The receptor Tas2r118 showing the strongest expression in the qRT-PCR experiment also exhibited the most pronounced staining in the in situ hybridization experiment. This was also true for Tas2r105 and Tas2r138, which exhibited lower mRNA levels detected by qRT-PCR and fewer cells stained in the in situ hybridization experiments. For the two receptors, Tas2r115 and Tas2r120, which showed low mRNA levels in the qRT-PCR experiment, also only faint signals were obtained by in situ hybridization. Only Tas2r102, which in the qRT-PCR experiment exhibited mRNA levels comparable to that of Tas2r105, demonstrated lower in situ hybridization signal intensity.
Tuning Breadth of Mouse Tas2r
For many decades aversive behavior has been studied in mice as model organisms to investigate taste responses elicited by compounds that taste bitter to humans (46–54). However, unlike human psychophysical observations, which can be correlated with data from the functional characterization of human TAS2R (e.g. Ref. 3), the lack of data on agonist specificities of mouse Tas2r has prevented similar correlations in the mouse with few exceptions (7).
To identify cognate agonists for mouse Tas2r, we functionally expressed 34 of the 35 putatively functional mouse Tas2r (excluding Tas2r116) in heterologous cells and screened them for responsiveness to a set of 128 predominantly naturally occurring bitter compounds with diverse chemical structures (supplemental Table 1S). The majority of the tested substances were selected from a bitter compound library used recently for the screening of human TAS2R (3, 42) and subsequently for Tas2r from avian and frog species (17). These experiments allowed us to identify cognate agonists for 21 of the 34 examined receptors (Table 1 and Fig. 2).
TABLE 1.
Threshold concentrations (mm) for Tas2r activators
Specific compounds for mouse Tas2r are shown in bold. Substances that activate only single receptors are underscored. RUA, raffinose undecaacetate; SOA, sucrose octaacetate; HSL, homoserine lactone; —, no response.
| Substance | 105 | 108 | 109 | 110 | 113 | 114 | 115 | 117 | 119 | 120 | 121 | 122 | 123 | 125 | 126 | 135 | 137 | 138 | 139 | 140 | 144 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2-Heptyl-3-hydroxy-quinolone | 0.003 | — | — | — | — | — | — | — | — | — | 0.003 | — | — | — | — | — | — | — | — | — | — |
| 5-Propyl-2-thiouracil | — | — | — | — | — | — | — | — | — | — | 0.1 | — | — | — | — | 0.1 | — | 0.1 | — | — | — |
| 6-Propyl-2-thiouracil | 0.3 | 1 | — | — | — | — | — | — | — | 0.3 | 0.3 | — | — | — | — | 0.3 | 1 | — | — | — | — |
| Absinthin | — | — | — | 0.03 | — | — | — | — | — | — | — | — | 0.1 | — | — | — | — | — | — | 0.1 | — |
| Acesulfame K | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 0.3 | — | — | — | — | — |
| Acetylpyrazine | — | — | — | — | — | — | — | — | — | — | 1 | — | — | — | — | — | — | — | — | — | — |
| Allylisothiocyanate | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 0.3 | — | — | — | — | — |
| Amarogentin | 0.0003 | 1 | — | — | — | — | — | — | — | — | — | — | 0.3 | — | — | — | — | — | — | — | 1 |
| Arborescin | — | — | — | 0.03 | — | — | — | — | 0.01 | — | 0.01 | — | — | — | — | — | — | — | — | — | — |
| Arbutin | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 30 | — | — | — | — | — | — |
| Arglabin | 0.1 | — | — | 0.03 | — | — | — | — | 0.1 | — | — | — | — | — | — | — | — | — | — | — | — |
| Artemorin | 0.03 | 0.1 | 0.1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Azathioprine | — | — | — | — | — | — | — | — | — | — | 0.3 | — | — | — | — | — | — | — | — | — | — |
| Brucine | — | — | — | — | — | — | — | 0.001 | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Caffeine | — | — | — | — | — | — | — | — | — | — | 0.01 | — | — | — | — | — | — | — | — | — | — |
| Camphor | — | — | — | — | — | — | — | — | 1 | — | — | — | — | — | — | — | 1 | — | — | — | 1 |
| Carisoprodol | 0.003 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| β-Carotene | 0.001 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Chloramphenicol | 0.03 | — | — | — | — | — | — | — | 0.3 | — | — | — | — | — | — | — | — | — | — | — | — |
| Chloroquine | —- | — | — | — | — | — | 0.1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Chlorpheniramine | — | 0.1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 0.03 | 0.1 |
| Cnicin | — | — | — | — | — | — | — | 0.1 | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Colchicine | — | — | 3 | — | — | — | — | — | — | — | — | — | — | — | 3 | — | — | — | — | — | 3 |
| Costunolide | 0.01 | — | — | — | — | — | — | — | 0.03 | 0.03 | — | — | — | — | — | — | — | — | — | — | — |
| Coumarin | 0.3 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Cucurbitacin B | 0.03 | — | — | — | — | 0.003 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Cucurbitacin D | 0.1 | — | — | — | — | 0.01 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Cucurbitacin E | 0.01 | — | — | — | — | 0.003 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Cucurbitacin I | 0.15 | — | — | — | — | 0.001 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Cycloheximide | 0.00001 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Denatonium benzoate | 0.1 | — | — | — | — | — | — | — | — | — | — | — | 0.3 | — | — | 0.1 | — | — | — | 0.3 | 3 |
| Denatonium saccharide | 0.1 | — | — | — | — | — | — | — | — | — | — | — | 0.3 | — | — | 0.1 | — | — | — | 0.3 | 3 |
| Diphenidol | 0.01 | 0.1 | — | 0.1 | — | — | — | — | — | — | — | — | 0.01 | — | — | — | 0.1 | — | — | — | 0.1 |
| Emetine | — | 0.03 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 0.03 | — |
| Epicatechin | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 | — | — | — | — | — | 1 |
| Epigallocatechin gallate | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 0.01 |
| Erythromycin | — | — | — | — | — | — | — | — | — | — | — | — | 0.3 | — | — | — | — | — | — | — | — |
| Ethylpyrazine | — | — | — | — | — | — | — | — | — | — | 3 | — | — | — | — | — | — | — | — | — | 3 |
| Falcarindiol | 0.01 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Famotidine | — | — | — | — | — | — | — | — | — | — | 0.03 | — | — | — | — | — | — | — | — | — | — |
| Haloperidol | — | — | — | 0.001 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 0.03 |
| Helicin | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 10 | — | — | — | — | — | — |
| 3-Oxo-C6-HSL | 0.1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 3-Oxo-C8-HSL | 0.1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| C4-HSL | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| C6-HSL | 0.3 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Humulone | 0.001 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Lidocaine | 0.01 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Orphenadrine | 0.03 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 0.003 | 0.03 |
| Ouabain | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 3 |
| Papaverine | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 0.01 |
| Parthenolide | 0.1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Phenanthroline | 1 | — | — | — | — | — | — | — | — | — | 1 | 1 | — | — | — | 1 | — | — | — | — | 1 |
| Phenyl-β-d-glucopyranoside | — | — | — | — | — | — | — | — | — | — | — | — | — | 3 | 10 | — | — | — | — | — | — |
| Phenylbutazone | 0.1 | — | — | — | 0.001 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Phenylethyl isothiocyanate | 0.03 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Picrotin | 3 | — | — | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Picrotoxinin | 0.3 | — | — | — | — | 3 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Progesterone | — | — | — | 0.001 | — | 0.003 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Pyrocatechin | 0.3 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 |
| Quassin | 0.3 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Quinine | 0.01 | 0.01 | — | — | — | — | 0.003 | — | — | — | — | — | — | — | 0.01 | — | 0.01 | — | — | 0.003 | 0.01 |
| RUA | — | — | 0.3 | 0.3 | — | — | — | — | — | — | — | — | 0.3 | — | — | — | — | — | — | — | — |
| Saccharin | 1 | — | 3 | — | — | — | — | — | — | — | — | — | — | — | — | 0.1 | — | — | — | — | 10 |
| d-Salicin | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 10 | — | — | — | — | — | — |
| Salicylic acid | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 0.01 | — | — | — | — | — |
| Santonin | 0.3 | — | — | — | — | 0.3 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Sinigrin | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| SOA | — | — | — | — | — | — | — | 0.03 | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Sodium benzoate | 3 | — | — | — | — | — | — | — | — | — | 10 | — | — | — | — | 0.03 | — | — | — | — | — |
| Strychnine | — | — | — | — | — | — | — | 0.01 | — | — | — | — | — | — | — | — | — | — | — | 0.01 | — |
| Sucralose | 10 | — | 30 | — | — | — | 3 | 3 | — | — | — | — | 30 | — | — | — | — | — | 30 | — | 10 |
| Tatridin A | 0.3 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Taurocholic acid | — | — | — | — | — | — | — | 0.03 | — | — | — | — | 0.3 | — | — | — | — | — | — | — | 0.3 |
| Theobromine | — | — | — | — | — | — | — | — | — | — | 0.03 | — | — | — | — | — | — | — | — | — | — |
| Theophylline | — | — | — | — | — | — | — | — | — | — | 0.03 | — | — | — | — | — | — | — | — | — | — |
| α-Thujone | 0.03 | — | — | — | — | — | — | — | 0.03 | — | — | — | — | — | — | — | — | — | — | — | — |
| Umbelliferone | 0.6 | — | — | — | — | — | — | — | — | — | — | — | — | 0.6 | — | — | — | — | — | — | — |
| Xanthotoxin | 0.01 | — | — | — | — | — | — | — | 0.1 | 0.03 | 0.1 | — | — | — | — | 0.1 | — | — | — | — | — |
| Yohimbine | 0.3 | 0.3 | — | — | — | — | — | — | — | — | — | — | 0.1 | — | — | — | — | 0.3 | — | — | 0.3 |
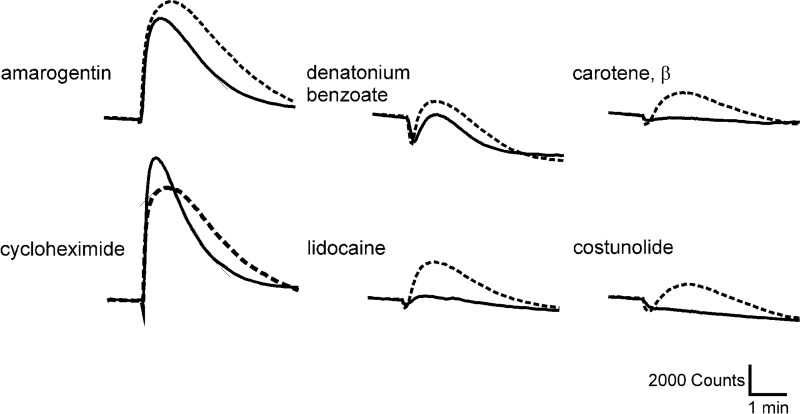
FIGURE 2.
Calcium responses of Tas2r-expressing cells challenged with different bitter compounds. A, for calcium imaging experiments, cDNA constructs coding for the indicated Tas2r or empty vector (pcDNA5 or pEAK10 = MOCK) were expressed in HEK293T-Gα16gust44 cells, seeded in 96-well microtiter plates. Calcium traces were recorded in an automated fluorescence imaging plate reader (FLIPRtetra) based on the detection of changes in fluorescence after the application of the indicated bitter compounds (first stimulus). A second application of 100 nm somatostatin-14 activating endogenous somatostatin receptors served as vitality control. Arrows point to cellular responses specific to bitter compound stimulation. Specificity of receptor activation was controlled by application of same substances on mock-transfected cells. Furthermore, the reliability of the experiment was checked every time by running parallel assays for human TAS2R with known activation patterns (right columns). B, exemplary magnified calcium traces for indicated bitter receptors and mock-transfected cells after application of denatonium saccharide (first stimulus). To demonstrate cell vitality a second application with a final concentration of 100 nm somatostatin-14 was included to activate endogenous somatostatin receptors.
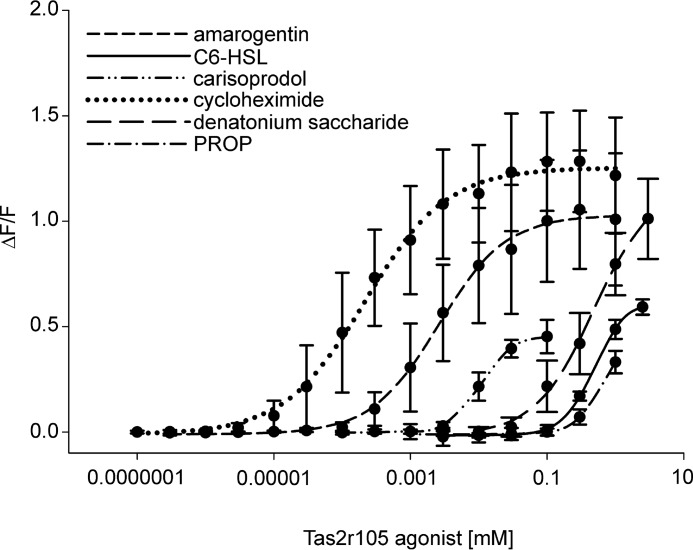
Tas2r105 appears to be a generalist receptor responding to a broad panel of 45 substances (35%) of the library (Table 1 and Fig. 3, for selected concentration response curves). Three other mouse bitter taste receptors, Tas2r121 (11%), Tas2r135 (9%), and Tas2r144 (16%), were also responsive to a large number of different bitter compounds (Table 1). Further, 10 Tas2r recognized between four and ten substances; seven receptors turned out to be specialists with strongly restricted agonist profiles responding to only one to three test compounds, whereas 13 receptors were not activated by any of the test substances. We then challenged these 13 Tas2r with additional 77 agonists (shown in italics on supplemental Table 1S) to increase the probability of deorphaning them, but no further responses were registered.
FIGURE 3.
Concentration-response relations of Tas2r105-expressing cells stimulated with increasing concentrations of the indicated compounds calculated from calcium traces acquired by FLIPR recordings. Changes in fluorescence (ΔF/F) were plotted semilogarithmically versus agonist concentrations.
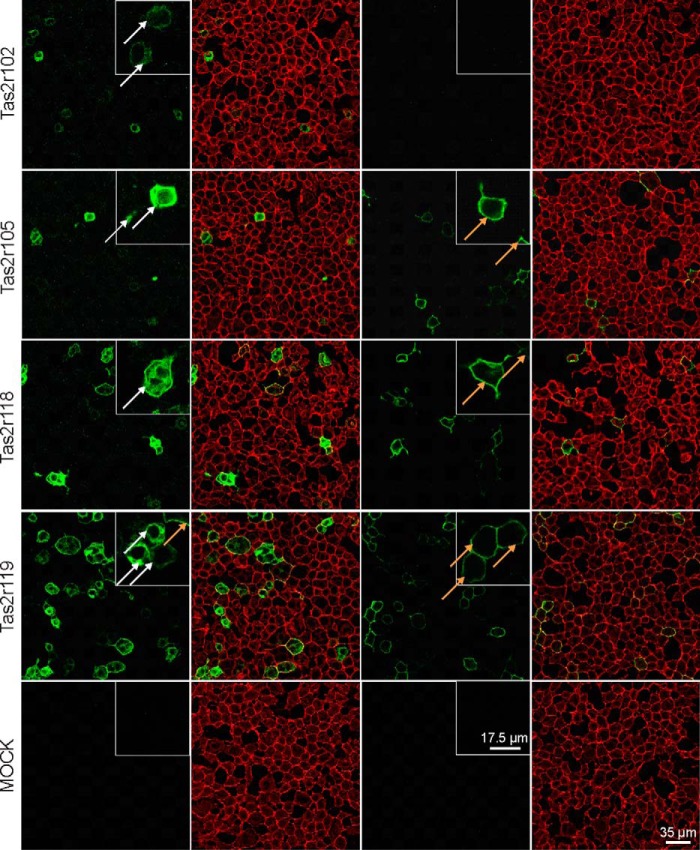
To determine whether the failure to deorphan might be due to insufficient cell surface expression, a selected subset of Tas2r was subjected to immunocytochemistry. The majority of the selected receptors show a clear external localization of Rho epitopes added to the N terminus of the recombinant Tas2r when unpermeabilized cells were subjected to staining (Fig. 4 and Table 2). Other receptors, Tas2r102 and Tas2r131, were visible only after permeabilization. As we did not identify an agonist for these receptors, insufficient cell surface expression may indeed have prevented deorphanization. However, the receptors Tas2r106, Tas2r118, and Tas2r134, for which we also did not identify agonists, exhibited clear cell surface staining of unpermeabilized cells (Fig. 4 and Table 2), indicating that problems in receptor routing are not the dominant reason for preventing functional identification of agonists. We did not find any Tas2r that lacked cell surface staining but resulted in the identification of agonists.
FIGURE 4.
Confocal fluorescence images of HEK293T-Gα16gust44 cells transiently transfected with cDNAs of mouse bitter taste receptors. Cells were transfected with Rho-tagged Tas2r constructs and underwent immunostaining with Rho antibody (green) either after (two left panels) or before fixation (two right panels). The cell surface was visualized by biotin-conjugated concanavalin A and streptavidin-conjugated Alexa Fluor 633 (red). Taste receptors located on the cell surface appear yellow in the overlay and are exemplarily indicated by orange arrows. Receptors without surface expression are exemplarily indicated by white arrows.
TABLE 2.
Cell surface localization experiments with HEK293T-Gα16gust44 cells transiently transfected with Rho-tagged cDNAs of mouse bitter taste receptors before and after permeabilization
+, expressed; −, no expression detectable.
| Receptor | Before permeabilization | After permeabilization |
|---|---|---|
| Tas2r102 | − | + |
| Tas2r105 | + | + |
| Tas2r106 | + | + |
| Tas2r108 | + | + |
| Tas2r114 | + | + |
| Tas2r118 | + | + |
| Tas2r119 | + | + |
| Tas2r120 | + | + |
| Tas2r121 | + | + |
| Tas2r123 | + | + |
| Tas2r126 | + | + |
| Tas2r129 | + | + |
| Tas2r131 | − | + |
| Tas2r134 | + | + |
| Tas2r144 | + | + |
| Mock | − | − |
Tas2r-specific Bitter Compounds
Despite their partially overlapping agonist profiles, each of the mouse Tas2r was activated by a unique subset of test substances. Moreover, regardless of their tuning breadth, eight Tas2r had specific cognate agonists not detected by any of the other identified 13 Tas2r (underlined in Table 1). For example, 16 of the 45 Tas2r105 activators were specific, including cycloheximide, which is in full agreement with the crucial role of Tas2r105 in cycloheximide avoidance (7). Interestingly, also four structurally related N-acyl homoserine lactones exclusively activated Tas2r105. These compounds are crucially involved in bacterial quorum sensing and induce antibacterial responses of the host (55).
Concentration Ranges for Activators of Mouse Tas2r
Two parameters are decisive in order to compare receptors and to predict the physiological importance of a receptor. Potency is a measure of the concentration range at which the receptor becomes responsive, whereas efficacy refers to the strength of the induced receptor's response. Our data demonstrate that different agonists activated the mouse Tas2r with widely different efficacies and potencies, as illustrated by the different maximal signal amplitudes of the activated receptors (supplemental Table 2S, A) as well as by their threshold concentrations (Table 1) and EC50 values (supplemental Table 2S, B).
Several Cognate Tas2r for the Majority of Bitter Compounds
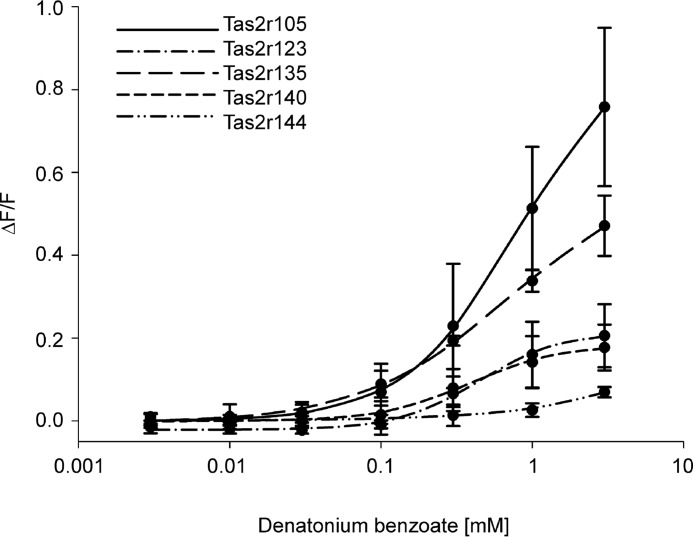
We also observed that most bitter compounds activated several mouse Tas2r. The number of Tas2r sensitive to an individual bitter compound varied considerably (Table 1). The substances that activated the highest number of Tas2r were quinine and sucralose, with seven receptors, followed by the two synthetic bitter compounds, PROP and diphenidol, which elicited responses from six receptors; and five substances, among them denatonium benzoate (Fig. 5), activated five receptors. Hence, the ability of different bitter compounds to stimulate several mouse Tas2r is similar to the response pattern seen for human TAS2R (3), although the overall promiscuity was greater in human TAS2R.
FIGURE 5.
Concentration-response relations for denatonium benzoate in cells expressing Tas2r105, Tas2r123, Tas2r135, Tas2r140, or Tas2r144. The curves have been deduced from calcium traces monitored in FLIPR experiments. Changes in fluorescence (ΔF/F) were plotted semilogarithmically versus agonist concentrations.
Screening of Human Receptors with Newly Identified Mouse Tas2r Agonists
Because we analyzed more bitter compounds in this study than in our previous characterization of the human TAS2R (3, 42), we performed an additional screening experiment with the 25 putatively functional human TAS2R and those substances not included in our previous analyses (supplemental Table 2S, C) in order to broaden the basis for comparisons. Although this screening failed to identify activators for any of the four human orphan TAS2R, we found nine, 17, and nine “new” agonists for the three most broadly tuned human TAS2R: TAS2R10, TAS2R14, and TAS2R46, respectively. We also identified additional agonists for TAS2R1 (three), TAS2R4 (two), TAS2R5 (one), TAS2R7 (two), TAS2R8 (one), TAS2R38 (one), TAS2R39 (three), TAS2R40 (two), TAS2R41 (one), TAS2R43 (three), and TAS2R31 (two).
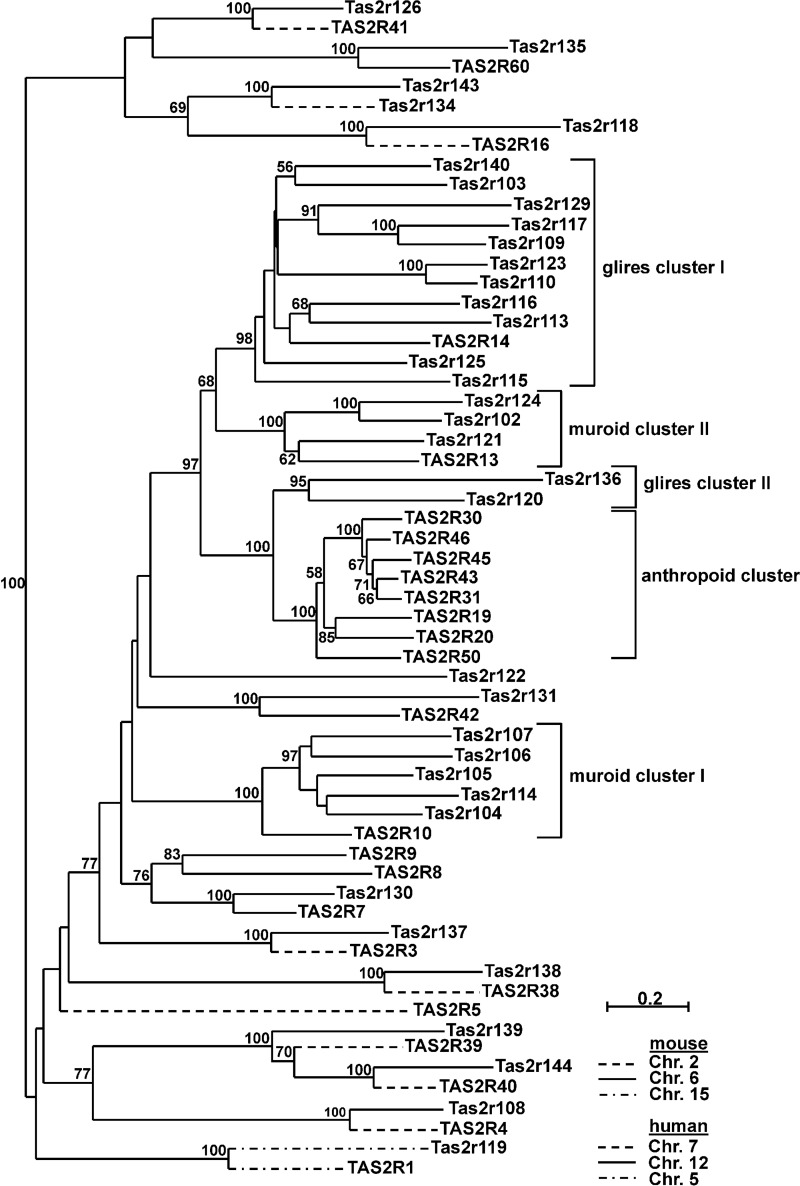
Evolutionary Relationships of Human and Mouse Bitter Taste Receptor Genes
To correlate the phylogenetic relationships of mouse and human bitter taste receptors with their cognate agonist spectra, we first constructed a phylogenetic tree of all putatively functional mouse and human bitter taste receptors (Fig. 6). As noted previously (22, 23), mouse and human bitter taste receptors do not form separate clusters, but rather they intermingle, indicating that ancestral receptor genes existed prior to the divergence of the primate and rodent lineages. Recently, a comprehensive phylogenetic analysis of the bitter taste receptor repertoires in the mammalian superorder Eurarchontoglires, which includes primates and rodents, revealed the existence of 19 one-to-one orthologs among the Tas2r genes of Euarchonta (including primates) and Glires (including rodents) as well as seven clusters of lineage-specific expansions (56), findings that confirm and expand previous observations (22, 23). By reason of pseudogenization in either of the grand orders (e.g. TAS2R12, Tas2r208, and Tas2r209), putatively functional one-to-one orthologs got lost during evolution, leaving 11 of such receptor pairs in the genomes of human and mouse (Fig. 6). Focusing on human and mouse bitter taste receptor repertoires, species-specific expansions occurring after the divergence of the Euarchontoglires lineages resulted in the formation of four Glires- and Muroidea-specific gene clusters and one Anthropoidea-specific bitter taste receptor gene cluster (56). Glires cluster I comprises Tas2r140, Tas2r103, Tas2r129, Tas2r117, Tas2r109, Tas2r123, Tas2r110, Tas2r116, Tas2r113, Tas2r125, and Tas2r115 that together with human TAS2R14 arose from a common ancestor. Muroid cluster I contains Tas2r107, Tas2r106, Tas2r105, Tas2r114, and Tas2r104 that together with human TAS2R10 have a common ancestor. Muroid cluster II encompasses Tas2r124, Tas2r102, Tas2r121, and human TAS2R13, which derive from a common ancestor. Moreover, the anthropoid cluster is composed of TAS2R30, TAS2R46, TAS2R45, TAS2R43, TAS2R31, TAS2R19, TAS2R20, and TAS2R50, together with mouse Tas2r136 and Tas2r120 (Glires cluster II).
FIGURE 6.
Phylogenetic tree based on alignment of amino acid sequences for mouse and human Tas2r and their chromosomal localization. After a multiple sequence alignment of the Tas2r manually adjusted to consider the potential tertiary structure, a neighbor-joining tree was constructed. Percentage of bootstrap values higher than 50 are displayed as node labels. Tas2r are classified into five clusters. Glires cluster I represents a species- and lineage-specific cluster of 11 mouse receptors and human TAS2R14, whereas muroid clusters I and II comprise five mouse Tas2r connected to TAS2R10 and three mouse Tas2r associated with TAS2R13, respectively. The anthropoid cluster represents human cluster composed of eight TAS2R related to the Glires cluster II comprising Tas2r120 and Tas2r136.
Role of Bitter Compounds and Their Tas2r in Avoidance Behavior
To examine whether the properties of the deorphaned Tas2r are comparable with or even predict the avoidance behavior of mice, we performed brief access taste preference tests with a subset of the bitter compounds used in the receptor assays. To this end, we used C57BL/6 mice, i.e. the same strain from which we cloned the Tas2r, and substance concentrations equivalent to those used in cell-based assays as well as at least 10-fold lower and higher concentrations. If necessary, and within the solubility range, substance concentrations were increased until the animals showed significantly reduced taste preferences relative to water. For the applied compound concentrations, the substance to water lick ratios were monitored (Table 3).
TABLE 3.
Lick ratios (mean ± S.E., n = 8) of C57BL/6 mice to concentration series of 22 bitter compounds
Statistical significance was determined by analysis of mixed models and post hoc analysis. Identical superscript italic letters (a, b, and c) indicate no variance, and variable superscript italic letters represent significant differences to at least a significance level of <0.05. SOA, sucrose octaacetate.
| Substance | Concentration | Substance/water lick ratio |
|---|---|---|
| [mm] | ||
| Acesulfame K | 1 | 0.94 ± 0.06a |
| 10 | 0.80 ± 0.13a | |
| 100 | 0.85 ± 0.06a | |
| 1000 | 0.05 ± 0.01b | |
| Aloin | 0.001 | 0.95 ± 0.08a |
| 0.01 | 1.01 ± 0.08a | |
| 0.1 | 0.71 ± 0.13b | |
| 1 | 0.41 ± 0.14b,c | |
| Amygdalin | 0.03 | 0.86 ± 0.07a |
| 0.3 | 0.78 ± 0.14a,b | |
| 3 | 0.62 ± 0.15a,b,c | |
| 30 | 0.51 ± 0.15b,c | |
| 100 | 0.33 ± 0.15c | |
| Atropine | 0.01 | 0.95 ± 0.07a |
| 0.1 | 0.85 ± 0.12a | |
| 1 | 0.31 ± 0.12b | |
| 10 | 0.06 ± 0.02c | |
| Berberine | 0.001 | 0.81 ± 0.13a |
| 0.01 | 0.84 ± 0.15a | |
| 0.1 | 0.92 ± 0.09a | |
| 1 | 0.10 ± 0.03b | |
| Brucine | 0.01 | 0.87 ± 0.12a |
| 0.1 | 0.84 ± 0.12a | |
| 1 | 0.56 ± 0.16b | |
| Caffeine | 0.1 | 0.76 ± 0.13a |
| 1 | 0.91 ± 0.11a | |
| 10 | 0.80 ± 0.14a | |
| 100 | 0.19 ± 0.05b | |
| Chloroquine | 0.1 | 0.64 ± 0.09a |
| 1 | 0.25 ± 0.07b | |
| 10 | 0.06 ± 0.02c | |
| 100 | 0.04 ± 0.01c | |
| Colchicine | 0.0003 | 0.91 ± 0.10a |
| 0.003 | 0.79 ± 0.10a | |
| 0.03 | 0.32 ± 0.10b | |
| 0.3 | 0.07 ± 0.02c | |
| 3 | 0.08 ± 0.02c | |
| 30 | 0.04 ± 0.01c | |
| Denatonium benzoate | 0.03 | 0.75 ± 0.14a |
| 0.3 | 0.82 ± 0.14a | |
| 3 | 0.06 ± 0.02b | |
| 30 | 0.05 ± 0.01b | |
| Naringin | 0.001 | 0.93 ± 0.11a |
| 0.01 | 0.80 ± 0.14a,b | |
| 0.1 | 0.64 ± 0.14b | |
| 1 | 0.52 ± 0.15b | |
| Nicotine | 0.01 | 0.94 ± 0.10a |
| 0.1 | 0.86 ± 0.14a | |
| 1 | 0.76 ± 0.13a | |
| 10 | 0.29 ± 0.08b | |
| Ouabain | 0.3 | 0.69 ± 0.16a |
| 3 | 0.28 ± 0.13b | |
| 30 | 0.12 ± 0.07b | |
| Phenanthroline | 0.1 | 0.82 ± 0.13a |
| 1 | 0.47 ± 0.13b | |
| 10 | 0.12 ± 0.04c | |
| Phenyl-β-d-glucopyranoside | 0.1 | 0.78 ± 0.11a |
| 1 | 0.67 ± 0.14a | |
| 10 | 0.35 ± 0.15b | |
| 100 | 0.07 ± 0.01c | |
| Phenylethyl isothiocyanate | 0.003 | 0.78 ± 0.12a |
| 0.03 | 0.94 ± 0.11a | |
| 0.3 | 0.69 ± 0.18a | |
| 3 | 0.66 ± 0.15a | |
| PTC | 0.01 | 1.01 ± 0.06a |
| 0.1 | 0.95 ± 0.09a | |
| 1 | 0.67 ± 0.15b | |
| 10 | 0.30 ± 0.13c | |
| Quinine | 0.001 | 0,98 ± 0.08a |
| 0.01 | 0.87 ± 0.07a | |
| 0.1 | 0.23 ± 0.08b | |
| 1 | 0.08 ± 0.02b | |
| d-(−)Salicin | 1 | 1.04 ± 0.09a |
| 10 | 0.69 ± 0.17b | |
| 100 | 0.12 ± 0.07c | |
| SOA | 0.03 | 0.84 ± 0.12a,c |
| 0.3 | 0.71 ± 0.18b,c | |
| 3 | 0.38 ± 0.31b | |
| Thiamine | 0.1 | 0.83 ± 0.11a |
| 1 | 0.96 ± 0.13a | |
| 10 | 0.26 ± 0.08b | |
| 100 | 0.04 ± 0.01c | |
| Thujone | 0.03 | 0.96 ± 0.11a |
| 0.3 | 0.90 ± 0.14a | |
| 3 | 0.47 ± 0.14b |
The data show that several bitter substances led to receptor activation and reduced substance/water lick ratios with the same potency. Ouabain and d-(−)salicin decreased lick ratios at 3.0 and 10 mm, respectively, i.e. concentrations that exactly match the threshold concentrations required to stimulate their single cognate receptors, Tas2r144 and Tas2r126 (Tables 1 and 3) and consistent with previous observations that mice are indifferent to salicin below 3.0 mm (57). Similar results were obtained for phenanthroline, which became aversive at 1.0 mm and activated all five of its cognate receptors, Tas2r105, Tas2r121, Tas2r122, Tas2r135, and Tas2r144, at a threshold of 1.0 mm. Moreover, phenyl-β-d-glucopyranoside and chloroquine induced avoidance behavior and stimulated their cognate Tas2r in a similar concentration range, but both compounds were up to 10-fold less potent in the behavioral assay. Quinine, denatonium benzoate, sucrose octaacetate, thujone, and brucine were avoided at 30–100-fold higher concentrations than those required to activate the cognate receptors in functional expression assays. For acesulfame K and caffeine the difference was several 1000-fold, whereas phenylethyl isothiocyanate did not elicit any avoidance even though its cognate receptor, Tas2r105, became responsive at 0.03 mm.
We also investigated the avoidance behavior of mice toward eight substances for which we failed to identify cognate Tas2r. Of these compounds, aloin, amygdalin, atropine, naringin, phenylthiocarbamide (PTC), and thiamine elicited aversion at 10-fold higher concentrations than those used in the receptor assays. For berberine and nicotine, the difference was 100-fold. The data suggest that a cognate Tas2r exists for these eight bitter chemicals, but we did not identify them because sufficiently high agonist concentrations could not be employed in the receptor assays. The data for colchicine differed from the former because it was 100-fold more potent to evoke avoidance than receptor activation.
Discussion
In our present work we performed a comprehensive analysis of the mouse Tas2r repertoire. In particular, we deorphaned the majority of mouse Tas2r, allowing comparisons of the pharmacological profiles with their well characterized human counterparts as well as with the plethora of behavioral data from previous sensory experiments. Although, in general, our results agreed well with observations in other species, some findings, such as functional differences among mouse and human bitter taste receptor orthologs, required some adjustment of firm beliefs in light of these data.
By in situ hybridization and qRT-PCR experiments we monitored the expression of mouse Tas2r genes in taste epithelium and compared their expression levels. Our data indicate that, similar to results obtained previously with human TAS2R (58), mouse Tas2r are indeed all expressed in gustatory tissue, confirming a role in bitter taste perception. Moreover, the variation in expression levels and numbers agree with the existence of a heterogeneous bitter taste receptor cell population in mouse (9, 59).
Recently, quantitative expression analyses of rodent Tas2r have been performed in non-gustatory tissues such as testis and heart (60, 61). In mouse testis the highest expression was observed for Tas2r113 and Tas2r124, which showed low to moderate expression levels in gustatory tissue (Fig. 1A). Moreover, one of the Tas2r genes with the lowest expression in lingual papillae, Tas2r114, exhibited robust expression in testis (61). The data suggest that Tas2r gene regulation in taste papillae differs from that in other tissues.
Central to this work was the deorphanization and functional characterization of mouse Tas2r by heterologous expression. We identified agonists for 21 of the 35 putatively functional mouse Tas2r. The number of identified agonists per receptor revealed that, like human and frog bitter taste receptors (3, 17), mouse Tas2r vary in their tuning breadth. Interestingly, this species displays only a single Tas2r, Tas2r105, that functions as a generalist (3), exhibiting an extremely broad agonist profile by recognizing >30% of the bitter compound library. Surprisingly, this Tas2r has been reported previously to be highly selective for cycloheximide (7). Of the 24 bitter compounds tested by Chandrashekar et al. (7) for activation of Tas2r105, we used 19 in the present study and found that, in addition to cycloheximide, denatonium, quinine, PROP, and yohimbine also stimulated Tas2r105-transfected cells (Figs. 3 and 5). This discrepancy appears to be due to differences in experimental methodologies. Heterologous expression analysis of Tas2r105 in HEK293T cells stably expressing Gα15 or Gα16gust44 stimulated with selected agonists indicated that low efficacy activators of Tas2r105 result in lower or even absent responses in Gα15-expressing cells (Fig. 7). Therefore, the Gα16gust44 cell system shows higher sensitivity than the Gα15-based assay (7).
FIGURE 7.
Intracellular calcium traces of Tas2r105 (non-taster variant) in HEK293T cells stable expressing Gα15 (continuous line) and Gα16gust44 (dashed line) recorded in FLIPR experiments upon stimulation with six exemplary compounds, indicating that low efficacy activators of Tas2r105 resulted in lower or even absent responses in Gα15-expressing cells. Calibration bar denotes 2000 relative light units (y) and 1 min (x).
Mice like humans show similar proportions of moderately tuned Tas2r responsive to >3–10% of the chemicals and of Tas2r specialists recognizing less than 3% of the compounds. However, the fact that we discovered activators for only 60% of the mouse Tas2r, whereas 84% of the human TAS2R were deorphaned with a comparable set of bitter chemicals previously (3, 42), suggests that mice have a higher proportion of specialist receptors relative to humans.
For 48 of the 128 compounds we failed to find a sensitive Tas2r, and for 13 Tas2r we were unable to find any bitter agonist. Low receptor expression or lack of cell surface localization in the heterologous cells as a general cause for the observed failure to identify agonists for these receptors is unlikely because transfection rates, expression levels, and cell membrane localization were not generally correlated between the groups of orphan or deorphaned Tas2r (Fig. 4 and Table 2). The inability to deorphan more Tas2r could be due to non-functional receptor variants generated by single nucleotide polymorphisms in the coding region. Nelson et al. (62) report that only two of 24 Tas2r genes showed no amino acid sequence differences when C57BL/6 and DBA/2J strains were compared. These changes in the Tas2r sequences could potentially affect ligand response profiles. Further, a lack of or inefficient G protein coupling might be another confounding feature (41, 45, 63).
The question of whether animals may recognize rather similar or different arrays of substances eliciting aversive behavior (e.g. bitter taste in human) cannot be answered conclusively as of today. Of course, one needs to assume that substances occur in nature that represent relevant toxins for some species and therefore require their recognition by bitter taste receptors, whereas other species may never encounter them and hence do not rely on receptors detecting these compounds. Answering this question would require the screening of bitter taste receptor repertoires from different species with compound libraries not preselected for their taste in humans. Thus far, such experiments have not been published, and we are aware that by screening mainly substances that taste bitter to humans our compound library was not unbiased. Indeed, some of the agonists we identified as activating mouse Tas2r, but that failed to activate human TAS2R (see below), suggest a substantial but not complete overlap among the bitter taste receptor agonists of both species. Nevertheless, from the bulk of available data, it appears that large overlaps among aversive (bitter) substances exist throughout the animal kingdom. Examples for such overlapping “bitter worlds” are plentiful and extend even to invertebrates possessing phylogenetically unrelated receptors expressed in different (neuronal) cell types. For example the nematode Caenorhabditis elegans shows aversive behavior to quinine, denatonium, and chloroquine (64), substances recognized by human and mouse as well. Such examples of agonist overlaps are even more plentiful for vertebrates, ranging from teleostean fish to primates (Refs. 17 and 65–67 and this work). In comparison, fewer reports have identified compounds that result in aversive behavior in other species but fail to activate human bitter taste perception (some compounds presented in this work (see below) and perhaps nicotine, for which we have not found a human TAS2R but a chicken Tas2r has been identified (17)). Hence, although there is considerable evidence in place for largely overlapping sets of aversive stimuli for many animals (Refs. 17 and 65–67 and this work), the assumption of the existence of large groups of species-selective bitter compounds is, even though valid, hypothetical. Nevertheless, we propose that the majority of those Tas2r that remain orphaned represent specialist receptors for compounds that are not contained in our substance library.
However, not only the number of agonists differed considerably among the receptors, but also the efficacies and potencies of the substances interacting with the various mouse receptors deviated. The highest efficacies were observed for cycloheximide (ΔF/F = 1.23 ± 0.20), denatonium saccharide (ΔF/F = 1.06 ± 0.22), and amarogentin (ΔF/F = 0.96 ± 0.24) at Tas2r105, suggesting that this receptor is critical for the recognition of these compounds in vivo when they are present in appropriate concentrations (Fig. 3 and supplemental Table 2S, A). Other compounds such as cucurbitacins B, D, E, and I demonstrate more than 10-fold lower efficacy at Tas2r105 (supplemental Table 2S, A). For the recognition of the cucurbitacins in vivo, Tas2r114 may be more relevant because they activated this receptor with substantially higher efficacies. Some substances, such as diphenidol and phenanthroline, activated their cognate receptors with similar efficacies. Thus, their overall bitterness is less likely to be dependent on a single Tas2r.
The potencies of bitter compounds also deviated largely across compounds for the same as well as different Tas2r (Table 1 and supplemental Table 2S, B). The highest potency with a threshold concentration of 10 nm and an EC50 concentration of 0.3 ± 0.2 μm was observed for cycloheximide at Tas2r105 (Fig. 3), confirming the dominant role of this receptor for the exquisite cycloheximide sensitivity of mice (7). Other agonists activated the receptor with at least ∼10-fold lower potencies, together spanning a concentration range of about 4 orders of magnitude. Most substances showed very low potencies, in the millimolar concentration range, for their cognate Tas2r. One of these substances is PROP, which activated six receptors at thresholds of only 0.3–1.0 mm (Table 1). Thus, Tas2r138 did not respond to PROP, whereas the orthologous human receptor TAS2R38 is exquisitely sensitive to PROP, showing an EC50 concentration of 2.1 μm (33). The β-d-glucopyranosides arbutin, helicin, phenyl-β-d-glucopyranoside, and d-(−)salicin all activated the receptor Tas2r126, with the highest observed threshold concentrations between 10 and 30 mm. However, in contrast to human TAS2R16 (34), the mouse Tas2r126 recognition pattern is not limited to β-d-glucopyranosides. Hence, mice appear to lack Tas2r that detect common structural configurations such as those detected by human TAS2R16 and TAS2R38.
In some cases, compounds that activated multiple Tas2r displayed similar potencies. For example, the seven receptors Tas2r105, Tas2r108, Tas2r115, Tas2r126, Tas2r137, Tas2r140, and Tas2r144 are activated by quinine at concentrations between 3.0 and 10 μm. However, for other compounds the concentrations required to activate different Tas2r are staggered. A good example for this is the artificial sweetener saccharin, which activates Tas2r135, Tas2r105, Tas2r109, and Tas2r144 with threshold concentrations of 0.1, 1.0, 3.0, and 10 mm, respectively. Hence, it is conceivable that increasing concentrations of saccharin in vivo result in a graded bitter response involving one to four Tas2r. In total, like human TAS2R (3), mouse Tas2r displayed threshold concentrations for bitter chemicals spanning 6 orders of magnitude.
The results of the present study allow a systematic comparison of the agonist spectra of mouse and human bitter taste receptors. To provide an even broader basis for such comparisons, we subjected human TAS2R to a screening with numerous substances not tested previously. These new agonist data did not change the classification of human TAS2R in broadly, moderately, or narrowly tuned receptors (3).
Our data further revealed that mice and humans detect a similar set of bitter compounds. Of the 128 substances used to challenge both the mouse and human bitter taste receptors, 80 (63%) activated mouse Tas2r and 98 (77%) human TAS2R, of which 72 substances (56%) stimulated bitter taste receptors in both species. We identified eight compounds that were selective for mouse Tas2r (shown in bold on Table 1), whereas 26 substances specifically stimulated human TAS2R (Ref. 3 and supplemental Table 2S, C). Twenty-two (17%) test substances activated neither mouse nor human Tas2r, probably because higher concentrations would be required to evoke Tas2r responses.
The ability of individual bitter compounds to activate multiple bitter taste receptors also varied between mouse and humans. Whereas quinine activated similar numbers of mouse (seven receptors) and human Tas2r (nine receptors), diphenidol stimulated more than twice as many bitter taste receptors in humans (15 receptors) as in mice (six receptors) (3). Vice versa, other substances such as PROP, with one main receptor in human, TAS2R38, acts more broadly on mouse Tas2r, being an agonist for six receptors. Thus, the response patterns of mouse and human bitter taste receptors are heterogeneous.
An important question is whether one-to-one orthologous Tas2r represent functional orthologs, e.g. show identical or at least similar agonist profiles. Although four of the one-to-one orthologous pairs could not be compared because the human, mouse, or both Tas2r remained orphan, this comparison was possible for seven orthologous pairs (Fig. 8A). Of these receptor pairs, Tas2r108 and its human ortholog TAS2R4 exhibited the highest degree of overlap in their set of agonists. Of the 18 bitter compounds for this pair, we observed that one-third of them activated both receptors. The substances capable of activating both receptors did not show apparent structural similarities. Only one of the 12 bitter substances was commonly recognized by Tas2r138 and TAS2R38. It is remarkable that the two prototypical agonists for the human TAS2R38, PROP and PTC, are not activators of the orthologous mouse receptor Tas2r138, which has been frequently, but erroneously, assumed in the past (25–28). Whereas in human the sensitivities for PROP and PTC are highly correlated to their activation of TAS2R38 (33, 38), they are not in mice, suggesting that different mouse Tas2r underlie the responsiveness to these substances (68). In fact, a polygenic control of PROP sensitivity was suggested (69), which is in good agreement with our observation that PROP activated six Tas2r (Table 1). Other pairs of orthologs share few or even not a single agonist (Fig. 8A) demonstrating that, in general, orthologous Tas2r have largely distinct agonist profiles. The little overlap is probably at the level of chance and is not unexpected given the broad tuning of Tas2r. In fact, statistical analyses (4-fold χ2 test) confirmed that the number of common agonists for all but one pair (TAS2R4/Tas2r108) of the one-to-one orthologs did not exceed chance levels. Although we cannot exclude the possibility that additional common bitter agonists for these one-to-one orthologous receptor pairs exist in nature, it seems that these receptors also contribute to species-specific bitter substance recognition.
FIGURE 8.
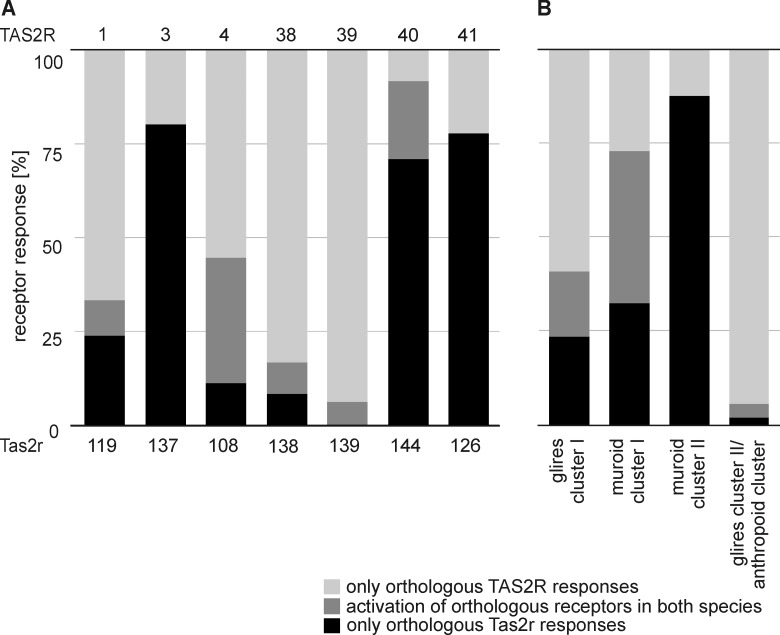
Comparisons of ligand-receptor interactions for orthologous human and mouse Tas2r for bitter compounds that activate at least one receptor. A, ligand-receptor responses of one-to-one orthologous bitter taste receptors. B, agonist spectra for species-specific Tas2r clusters and associated Tas2r in the other species. Mice show three such species- or lineage-specific expansions associated with human TAS2R14 (Glires cluster I), TAS2R10 (muroid cluster I), or TAS2R13 (muroid cluster II), whereas humans have only one cluster associated with mouse Tas2r120 and Tas2r136 (anthropoid cluster).
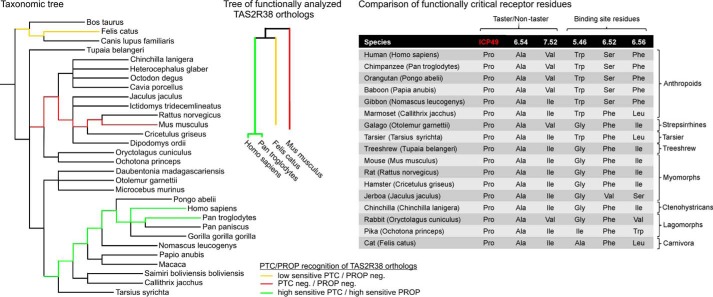
A good example of this is human TAS2R38 (70, 71). A comparison of the data from structure-function analyses, amino acid sequence homologies, and the pharmacological properties of TAS2R38 orthologues suggests that the receptor was modified differently during the evolution of Euarchontoglires, a clade including primates and rodents (cf. Ref. 56). Although primate TAS2R38 acquired sensitive PTC responsiveness as well as activation by PROP, this was not the case for the rodent ortholog Tas2r138 (Fig. 9, left and middle panels). A comparison of functionally critical residues in selected TAS2R38 orthologs of the Euarchontoglires clade revealed that all of them invariantly exhibit amino acid residues characteristic for the human taster variant TAS2R38-PAV or a variation thereof (TAS2R38-PAI), which has been experimentally validated for full PTC/PROP responsiveness (33) (Fig. 9, right panel).
FIGURE 9.
Taxonomic, phylogenetic, and functional relations of bitter taste receptor 38 in Euarchontoglires and Carnivora species. Left panel, taxonomic tree of selected Euarchontoglires and Carnivora species. The tree was generated using the Common Tree software of the Taxonomy Browser on the NCBI website with TreeView software (123). The yellow lines indicate the hypothesized evolutionary origin of TAS2R38 orthologs with low sensitivity PTC recognition and lack of PROP responsiveness. Green lines indicate the assumed evolutionary origin of high sensitivity PTC- and PROP-detecting TAS2R38 orthologs, and the red lines label the origin of PTC/PROP-insensitive TAS2R38 orthologs found in mice. Middle panel, phylogenetic tree of functionally characterized TAS2R38 orthologs. The low PTC-sensitive cat Tas2r38 cDNA (yellow) (72) and the two high PTC- and PROP-sensitive human (33) and chimpanzee (66) TAS2R38 cDNAs, as well as the PTC/PROP-insensitive Tas2r138 cDNAs of mouse (this study), were aligned with AlignX of Vector NTI software. Right panel, comparison of functionally critical residues in selected TAS2R38 orthologs. A subset of species shown in the left panel was analyzed for functionally critical amino acid positions in the corresponding TAS2R38 orthologs. The first three rows refer to amino acid positions found in human PTC/PROP taster and non-taster variants of this receptor. The first position is located in the first intracellular loop (ICP49). The second and third rows specify residues in the sixth and seventh transmembrane domain, respectively, by their position according to Ballesteros-Weinstein nomenclature (124) followed by functionally important residues in the binding pocket of human TAS2R38 contributing to PROP and PTC activation (70, 71). Among these residues were tryptophan 201, serine 260, and phenylalanine 264, with corresponding positions depicted in the 4th (5.46), 5th (6.52), and 6th (6.56) rows.
Previous in vitro mutagenesis experiments combined with functional heterologous expression assays have revealed several functionally important residues in the binding pocket of human TAS2R38 that contribute to PROP and PTC activation (70, 71). Among these residues were tryptophan 201 (5.46), serine 260 (6.52), and phenylalanine 264 (6.56). With one exception, the mutation of serine 260 to alanine, which resulted in unimpaired responsiveness to PROP but not PTC, all modifications resulted in severely reduced activation of the mutated receptors (71). In particular, exchanging tryptophan in position 201 for leucine or phenylalanine caused severely reduced PTC responsiveness and, practically, a loss of activation by PROP. Intriguingly, Trp-5.46 is found only in the haplorrhine primate clade including human and chimpanzee, which exhibit exquisitely PTC- as well as PROP-sensitive TAS2R38 receptors (33, 66). Hence, it seems that PTC/PROP-sensitive TAS2R38 evolved within the Primate order in the haplorrhine branch. Moreover, the residue at position 6.52 also showed a strict separation among the compared clades. In this case, all but the Catarrhini, which carry a serine residue at this position, have a phenylalanine (or valine in the case of Jaculus jaculus, belonging to the jerboa) at this position. Strikingly, this position affected PTC and PROP recognition in vitro as well, with serine being the preferential residue for the activation by both substances (71). Finally, position 6.56 differs among the Catarrhini, which exhibit a phenylalanine residue, and the other species with different, dominantly hydrophobic residues at this position. As experimental evidence suggests the requirement of phenylalanine at this position for full PTC/PROP responsiveness and rodent receptors differ in all three mentioned positions from the human and chimpanzee counterparts, we concluded this to be the underlying reason that the mouse receptor and perhaps all rodent receptors are incapable of interacting with PTC or PROP. This indicates that a functional divergence occurred prior to the separation of the rodent and primate lineages at the beginning of the earlier cretaceous period. Nevertheless, as the cat Tas2r38 ortholog shows insensitive PTC responsiveness and no PROP responses (72, 73) we assumed the existence of a common ancestral TAS2R38 ortholog permissive for PTC/PROP responses. Of course, the existence of further critical positions for PTC/PROP responses cannot be ruled out and may contribute significantly. This example indicates that pharmacological diversification occurs also among the group of one-to-one orthologous receptors. Therefore, the hypothesis that orthologous receptors may recognize bitter compounds important for both species (23) seems, at least in most cases, not to be true.
The persistence of these genes intact in the genomes of mice and humans and not pseudogenized could indicate that common, yet still unknown, bitter substances that pose or have posed a severe threat to the survival of both organisms throughout evolution could exist for these one-to-one orthologs. Alternatively, the corresponding receptors may fulfill another and dominant function beyond bitter taste perception by recognizing endogenous and well conserved agonists. As the number of reports on extragustatory expression of bitter taste receptors is ever increasing (24, 44), this hypothesis does not seem too farfetched. Lastly, the structure of these receptors may have allowed the rapid evolution of binding sites tailored to recognize compounds for the specific needs of mice or humans. In that case, the unique ability of bitter taste receptors to dynamically adapt their functions to the nutritional requirements of organisms may be more important than the fixation of pharmacological properties.
In contrast to the one-to-one orthologous receptor pairs, it is assumed that lineage-specific expansions possibly generated Tas2r critical for the recognition of bitter substances encountered only in the concerned species (23). If this were true, then the cluster of amplified Tas2r in one species should recognize more compounds than the related single Tas2r in the other species, some of which should be species-specific.
Glires cluster I consists of 11 mouse Tas2r and human TAS2R14, which is the most broadly tuned TAS2R in humans. In the course of our experiments, we found agonists for eight of the 11 mouse Tas2r, and three remained orphan. Of the 64 agonists activating the Tas2r of Glires cluster I, the majority, namely 38 of them, were specific for human TAS2R14; 11 activated human TAS2R14 and several mouse Tas2r, whereas only 15 substances were specific for the mouse Tas2r of this group. For muroid cluster I, which includes one broadly tuned human receptor together with five mouse Tas2r, of which we deorphaned two receptors, as well as muroid cluster II with 1 human TAS2R and three mouse Tas2r, we found similar results (Fig. 8B). In contrast to clusters showing an expansion of mouse Tas2r, in Glires cluster II/anthropoid cluster, the mouse paralogs Tas2r136 and Tas2r120 linked with eight human TAS2R. Of these receptors, mouse Tas2r136, as well as human TAS2R19 and TAS2R45, is an orphan receptor and cannot be compared. Only a single compound of the 54 activators for this cluster was specific for a murine Tas2r, and two substances activated Tas2r in both species (Fig. 8B). Remarkably, the remaining 51 chemicals were selective for the human TAS2R of this cluster.
Thus, the hypothesis that lineage-specific expansions generate Tas2r for species-specific bitter chemicals is not generally supported by our data. For example, only five of 26 human-specific compounds are recognized by members of anthropoid cluster, whereas most of the human-specific compounds are recognized by the three most broadly tuned receptors: TAS2R10, TAS2R14, and TAS2R46.
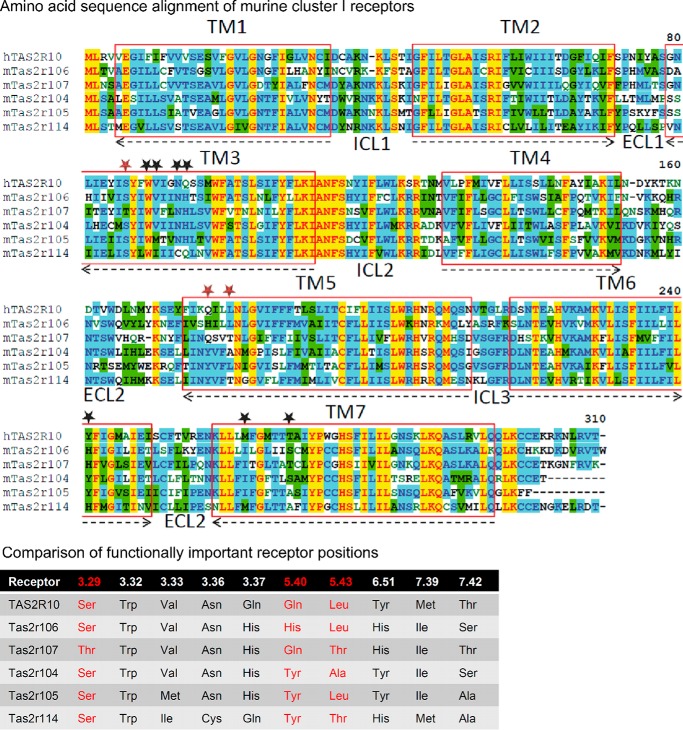
However, the bitter taste receptor gene expansions contribute to a broadening of the overall agonist profiles, which may be particularly important for the more narrowly tuned mouse receptors and, hence, may account for the fact that more frequent gene expansions occurred in mice. In fact, a closer look at the amino acid sequence of receptors of murine cluster I showed a general tendency of higher amino acid sequence homologies in intracellularly oriented transmembrane domain parts and intracellular loops compared with extracellular transmembrane domain regions and extracellular loops, which has been recognized previously (10). A detailed comparative analysis of receptor positions that constitute the binding pockets of murine cluster I receptors suggests that diversification of agonist spectra has occurred (Fig. 10). All receptors of murine cluster 1 containing human TAS2R10, which has been subjected to detailed structure-function analyses (30), exhibited a different combination of amino acid residues at positions showing pronounced agonist selectivity and, hence, their corresponding putative agonist spectrum. The concentration-response relationships for selected groups of human and mouse Tas2r indicate that the activation properties of sequence-related human and mouse Tas2r differ more substantially than is evident from sole comparisons of their agonist profiles (Fig. 11). Sequence orthologs such as TAS2R38/Tas2r138 or TAS2R1/Tas2r119 can or cannot recognize the same compounds (Fig. 11, A and B). However, even if they do so, the potencies and efficacies differ substantially (Fig. 11B). The same pronounced differences are also seen in the case of members of muroid cluster I (Fig. 11, C and D) or representatives of Glires cluster II/anthropoid cluster (Fig. 11, E and F).
FIGURE 10.
Sequence comparison of muroid cluster I. Upper panel, amino acid sequence alignment of muroid cluster I receptors. The alignment was created using the AlignX program of the Vector NTI software (Life Technologies). Residues are labeled according to the degree of conservation: yellow, identical; green, conserved; blue, identical in at least half of the sequences. The transmembrane domains (TM) are labeled by red boxes and numbered. The orientation in the lipid bilayer is shown by arrows (arrowheads point toward the extracellular site). Intracellular (ICL) and extracellular loops (ECL) are indicated and numbered. Amino acid positions demonstrated to reside in the ligand binding pocket of human TAS2R10 are indicated by stars. Red stars highlight amino acid positions with pronounced agonist selectivity, and black stars contribute to ligand binding and binding pocket constitution (30). Lower panel, comparison of functionally important receptor positions. The six receptors belonging to muroid cluster I were compared for their conservation at positions experimentally proven to contribute to agonist binding in human TAS2R10, a member of this cluster. Receptor residues are indicated by their position according to the Ballesteros-Weinstein numbering system (124). Red-labeled residues reside in positions exhibiting pronounced agonist selectivity in TAS2R10; other positions were demonstrated to constitute the receptor binding site (30).
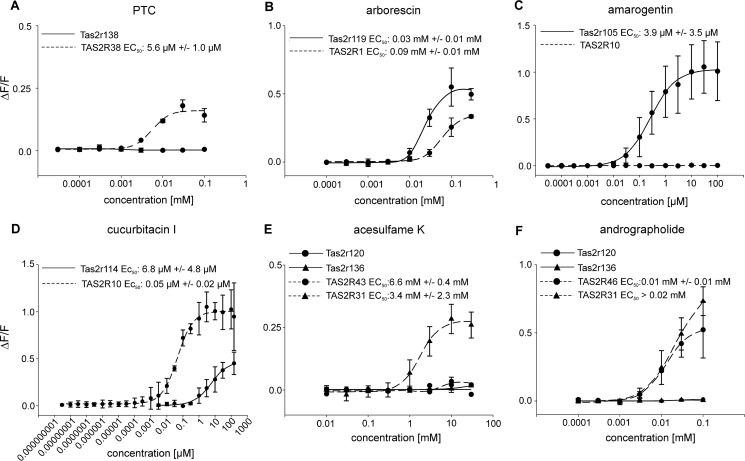
FIGURE 11.
Selected concentration-response functions of cells transiently transfected with DNA for orthologous mouse and human Tas2r. Functions are based on calcium traces acquired by FLIPR recordings. Changes in fluorescence (ΔF/F) were plotted semilogarithmically versus agonist concentrations for sequence-related human and mouse Tas2r, including one-to-one orthologs (A and B), members of the muroid (C and D), or anthropoid cluster (E and F).
Another hypothesis about the development of species-specific Tas2r gene clusters concerns tuning breadth rather than individual agonist spectra (3). Because both of the single human TAS2R corresponding to mouse Tas2r gene clusters (Glires cluster I and muroid cluster I) are extraordinarily broadly tuned receptors, one could assume that the gene expansion in the rodent lineage resulted in the development of multiple specialized receptors arising from a broadly tuned ancestral receptor or that the broad tuning of the ancestral receptor was maintained by the derived Tas2r to cover an even larger chemical space. Our results strongly suggest that multiple broadly tuned receptors were not generated but rather that a specification of several receptors occurred (Table 1).
Our analyses of bitter compounds and the corresponding mouse Tas2r for avoidance behavior revealed that in some but not all cases the sensitivity of Tas2r responses measured in vitro matched the concentration range of the substance in vitro. Whether these differences could be due to perireceptor events (74) not mimicked in our in vitro assays remains to be determined. The data suggest that receptor threshold values can, in some cases, predict bitterness avoidance of mice. However, it appears that for other bitter chemicals other possible factors such as interaction with the oral mucosa or saliva may reduce their potency of inducing aversion. However, in view of different G protein coupling of Tas2r in vitro and in vivo (41, 45, 63, 75, 76), the data on the behavioral experiments agree reasonably well with the data from the receptor assays on a qualitative level and in some cases also on a quantitative level. In the case of the substance colchicine, which showed a 100-fold higher potency when eliciting avoidance behavior in vivo compared with the receptor assays, other explanations need to be taken into account. Either the “best” receptor has not been discovered, or alternative recognition mechanisms exist that do not rely on Tas2r. However, the ability of colchicine to activate three human, one chicken, one turkey, and one frog receptor (17) suggests that Tas2r-dependent detection mechanisms likely exist for this compound.
Molecular genetics can shed light on the importance of specific Tas2r for taste-relevant behavior. The importance of Tas2r105 for cycloheximide recognition is illuminated by strain-specific differences leading to the identification of a chromosomal locus mediating cycloheximide sensitivity (51) and by Tas2r105 knock-out mice, which demonstrated loss of nerve responses and behavioral aversion to this translational inhibitor (57). However, the avoidance of denatonium, PROP, and quinine was not altered in these mice. Because all three substances activated four to six other Tas2r, it is conceivable that they suffice to evoke avoidance of those compounds. Strain-specific recognition extends to other bitter compounds, leading to the identification of chromosomal regions critical for the detection of the quinine (Qui) (49, 68, 69, 77) or sucrose octaacetate (Soa) (48, 78, 79), yet the cognate Tas2r genes remained unknown. The close genetic linkage of sucrose octaacetate and strychnine sensitivity (80) agrees well with our finding that only a single receptor, Tas2r117, responded to both compounds. Intriguingly, the Tas2r117 sequence was fully intact in C57BL/6 mice used for our analyses, but it contains missense mutations and small deletions in the DBA/2J strain (62), likely explaining the inability of DBA/2J mice to taste sucrose octaacetate, strychnine, and brucine (47, 80, 81), which are all activators of Tas2r117.
Recently, it was reported that solitary chemosensory cells in the anterior nasal epithelium express Tas2r and their signaling molecules (82) respond to bacterial quorum-sensing N-acyl homoserine lactones (82, 83). Our experiments revealed that mouse Tas2r105, as well as human TAS2R1, TAS2R10, and TAS2R14, is sensitive to various N-acyl homoserine lactones. We failed, however, to monitor N-acyl homoserine lactone responses for TAS2R38 (55), which may be explained by the use of different experimental methodologies. The ability to detect quorum-sensing molecules contributes to environmental adaptions and influences the behavior of eukaryotic organisms (84, 85). Humans homozygous for the non-taster allele of TAS2R38 are reported to be more susceptible to upper respiratory tract infections by Gram-negative bacteria than individuals carrying the taster variant of this receptor (55). It would be interesting to know if this also applies to those strains of mice harboring the less sensitive variant of Tas2r105 (7).
In light of findings showing that Tas2r are present in organs that are not at all or only partially accessible to xenobiotics, such as brain (86–89), testes (61, 90, 91), thyroid (92), and urethra (93), we also examined whether hormones could function as Tas2r activators. We found that progesterone stimulated Tas2r114 and Tas2r110. This steroid hormone is expressed in ovaries (corpus luteum), the adrenal glands, and testicular Leydig cells. It also has major effects on human sperm motility (94). Although Tas2r114 is expressed in gustatory tissue at rather low levels, its RNA is abundantly present in testis (61). Mouse spermatids and spermatozoa respond to bitter compounds with calcium signaling in an α-gustducin-dependent manner (61). Genetic ablation of bitter receptor cells in mice causes massive spermatid loss (90). Further studies are needed to elucidate the role of this and other Tas2r in testicular function.
Taken together the work presented here sheds light on the evolutionary dynamics that acted on the bitter receptor repertoires of vertebrates, resulting in the development of highly versatile G protein-coupled receptors capable of adapting to various lifestyles and habitats.
Experimental Procedures
Mice
Animal care and procedures were performed following national and institutional guidelines as approved by the local animal experimentation committee (Ministry of Environment, Health, and Consumer Protection Brandenburg, Germany; V3–2347-01-2013 and 23-2347-A-1-1-2010). Mice were housed in polycarbonate cages and kept under a 12 h light/12 h dark cycle unless otherwise noted, with water and food ad libitum.
Brief Access Tests
Behavioral studies with C57BL/6 mice (male, 8–9 weeks old; n = 8) obtained from Janvier (Le Genest St. Isle, France) were carried out using a Davis rig MS-160 (DiLog Instruments, Tallahassee, FL). Prior to testing, animals were housed individually with food available ad libitum. Mice were water-deprived for 18 h for the first 3 days of adaptation followed by restriction periods of 22.5 h in preparation for taste solution presentation. Each stimulus and concentration was presented in three independent test sessions in 5-s trials. Intertrial intervals were 7.5 s during a 20-min session as reported previously (68, 95–98). For detailed information about substances see Table 3 and supplemental Table 1S. Statistical significance was determined by analysis of mixed models and post hoc analysis using the Bonferroni test (SPSS 16.0, IBM, New York).
Tissue Preparation
Lingual epithelium was isolated from mice that were anesthetized using isoflurane (Baxter, Vienna, Austria) and sacrificed by cervical dislocation. Epithelium was removed as published earlier (91). The peeled tissue was fixed in a Sylgard dish, and epithelium containing taste papillae was dissected from the surrounding tissue. Papillae-enriched epithelium was frozen at −80 °C until finally used for RNA extraction (see “Quantitative RT-PCR”).
To prepare fixed tissue, C57BL/6 mice were anesthetized with 150 mg Narcoren/kg body weight (Merial, Hallbergmoos, Germany) and perfused transcardially with PBS followed by ice-cold 4% paraformaldehyde. Tongues were removed, postfixed for 2 h in 4% paraformaldehyde, and soaked in 30% sucrose overnight at 4 °C. Tissues were sectioned on cryostate (10 μm) and thaw-mounted onto Superfrost Plus slides (Menzel, Roth, Germany).
Quantitative RT-PCR
Epithelial preparations enriched in vallate and foliate papillae were subjected to RNA extraction using TRIzol reagent (Invitrogen). Following DNase I (Invitrogen) digestion to remove potentially contaminating genomic DNA, the cDNA was synthesized using SuperScript III (Invitrogen) according to the manufacturer's instructions.
Gene-specific primers and TaqMan probes (supplemental Table 3S) were used to amplify Tas2r and α-gustducin RNA. The mRNA for β-actin served as an internal control. Quantitative analysis was performed using the 7500 Fast Real-time PCR system (Applied Biosystems, Darmstadt, Germany). For PCR reactions cDNA corresponding to 6.25 ng of total RNA was mixed with an 0.5 μm probe, 1.25 μm each primer, and 1× TaqMan® Gene Expression Master Mix (Applied Biosystems). The following cycles were run: 50 °C for 2 min and 95 °C for 10 min followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C. Each cDNA sample was run in triplicates. For negative controls corresponding samples without prior reverse transcriptase reaction and water controls were used. Threshold cycle (CT) values were acquired with 7500, v2.0.1 Software (Applied Biosystems). Mean values of CT triplets were calculated (single values differing ≥1 CT were excluded), and ΔCT values were determined using mean CT of β-actin as reference (ΔCT = CT target − CT reference). Finally, 2−ΔCT values were calculated based on established methods (99).
In Situ Hybridization
The coding sequences of Tas2r were subcloned into vectors with an N-terminal rat somatostatin receptor subtype 3 (sst3) tag and a C-terminally located herpes simplex virus glycoprotein D epitope (HSV) (see “Calcium Imaging Analyses” for more detail). Using primers specific for the coding sequences of sst3 and HSV tags, cDNAs containing T3- and T7-RNA-polymerase promoter sequences were amplified and subsequently used as template for in vitro transcription reactions as described previously (58). All probes were hybridized to template plasmid DNA spotted onto nitrocellulose membranes to adjust sense and antisense probes for similar detection efficiencies. In situ hybridization was done as described previously (91) with 10 μm tissue sections of vallate papillae from C57BL/6 mice.
Taste Compounds
All analyzed compounds have been described previously in the literature as bitter tasting (100–105) or as representing small molecules responsible for cell-to-cell communications that have been conjectured to activate bitter taste receptors outside of the oral cavity (55, 106). Bitter chemicals were purchased mainly from Sigma-Aldrich, Fluka (Oberhaching, Germany), ABCR (Karlsruhe, Germany), ICN Biochemicals (Aurora, USA), BioChemica (Darmstadt, Germany), ChromaDex (LGC Standard, Wesel, Germany), CPS Chemie+Service GmbH (Aachen, Germany), Cfm Oskar Tropitzsch (Marktredwitz, Germany), Carl Roth (Karlsruhe, Germany), and HWI Analytik (Rülzheim, Germany). Homoserine lactones were acquired from Darren Furniss (University of Nottingham, United Kingdom). Noncommercial bitter terpenoids were obtained by isolation from their plant sources (107–112). All other substances were available from previous studies (32, 37). For detailed information about manufacturers and applied concentrations, see supplemental Table 1S.
Test compounds for calcium imaging experiments were dissolved directly in C1 buffer (130 mm NaCl, 5 mm KCl, 10 mm Hepes, 2 mm CaCl2, 10 mm glucose, pH 7.4) or dimethyl sulfoxide solution. Dimethyl sulfoxide stock solutions were further diluted in C1 buffer, not exceeding a final dimethyl sulfoxide concentration of 1%.
For behavioral studies, taste solutions were prepared in water freshly distilled every day and presented at room temperature. Substances were identical to those used in functional expression analyses with the exception of quinine, which was applied as quinine hydrochloride for behavioral studies (Table 3).
Calcium Imaging Analysis
Coding sequences of mouse bitter taste receptors (excluding the stop codon; for accession number see supplemental Table 3S) were amplified from genomic DNA from C57BL/6 animals and subcloned into expression plasmids based on pcDNA5/FRT (Invitrogen; Tas2r105, Tas2r114, Tas2r119, Tas2r138, Tas2r143, and Tas2r144) or pEAK10 (Edge BioSystems, Gaithersburg, MD; all other receptors (113)). Receptor sequences correspond to published data (for NCBI accession numbers see supplemental Table 3S) with one exception: the sequence of Tas2r105 is identical to that contained in GenBankTM except for a synonymous CCA to CCG transition at position 219. The Tas2r coding sequences in the expression plasmids are flanked at their amino termini by the first 45 amino acids of the rat somatostatin receptor subtype 3 (114) to improve plasma membrane targeting and by the HSV epitope at their carboxyl termini for immunohistochemical detection (34). Despite substantial cloning effort, generation of Tas2r116 was not possible.
Functional expression analysis was performed as described earlier for human, frog, and chicken bitter taste receptors (e.g. Refs. 3, 17, 29, 32, 33, and 115). Briefly, receptor cDNA constructs were transiently transfected in human embryonic kidney HEK293T cells stably expressing the chimeric G protein Gα16gust44 using Lipofectamine 2000 (Invitrogen) (33, 115, 116). Twenty-three to 26 h later the cells were loaded with Fluo-4 AM (Molecular Probes, Karlsruhe, Germany) in the presence of probenecid (2.5 mm, Sigma-Aldrich) in serum-free medium. Next, the loading solution was removed, and cells were incubated in C1 solution until the beginning of the experiment. For functional expression analyses, taste solutions were automatically applied to the cells. To avoid false positive signals, substances were screened at concentrations not eliciting artificial signals in empty vector (mock)-transfected cells (supplemental Table 1S), which were used as negative controls. To demonstrate cell vitality, a second application with a final concentration of 100 nm somatostatin-14 (Bachem, Weil am Rhein, Germany) was included to activate endogenous somatostatin receptors. Data were collected from at least two independent experiments carried out in triplicates. Calcium responses were baseline-corrected and expressed as ΔF/F by subtracting fluorescence changes of mock-transfected cells from those of receptor-transfected cells. For dose-response curves, two to four experiments were averaged, and the fluorescence changes of corresponding mock-transfected cells were subtracted. Signals were normalized to background fluorescence. For the calculation of EC50 values the plots of amplitude versus log concentrations were prepared in SigmaPlot 11.0 (SPSS Inc.) by nonlinear regression of the plots using the function f(x) = (a − d)/{1 + [(x/EC50)nH]} + d, with a = max, d = min, x = agonist concentration, and nH = Hill coefficient. The lowest concentration generating a calcium signal was specified as the threshold concentration.
Immunocytochemisty
To determine the cellular expression levels of mouse Tas2r and their cell surface localization, selected receptor coding sequences (excluding the stop codon) were cloned in pcDNA5/FRT expression vector resulting in Tas2r sequences flanked by the first 60 amino acids of rhodopsin (Rho tag) (117), which can be detected with suitable antisera in vivo (118), and an HSV-tagged epitope at the amino and carboxyl termini, respectively. HEK293T-Gα16gust44 cells were grown and transfected with Rho-tagged receptor plasmids on poly-d-lysine-coated glass coverslips as published previously (1, 30, 31). To visualize the cells, their surfaces were stained with 5 μg/ml biotin-conjugated concanavalin A (Sigma-Aldrich) and streptavidin-conjugated Alexa Fluor 633 (1:1,000, Molecular Probes). The Rho-tagged receptors were visualized by staining with an anti-Rho primary antibody (1:500, Abcam, Rho 4D2, Cambridge, United Kingdom) and an Alexa Fluor 488-labeled goat anti-mouse secondary antibody (1:2,000, Molecular Probes). Rho antibody was applied either before or after fixation to the cells in 3% normal horse serum in 1× PBS and left for 1 h on ice (if added prior to fixation) or at room temperature (if added after fixation), respectively. Fixation was done by acetone/methanol (1:1, v/v) treatment for 2 min and subsequent washing with 1× PBS. Receptor expression was recorded using the confocal laser-scanning microscope Leica TCS-SP8 (Leica, Wetzlar, Germany).
Phylogenetic Tree
Coding nucleotide sequences of mouse Tas2r genes were obtained from the genomic scaffolds NC_000068.7, NC_000072.6, and NC_000081.6 of the mouse genome assembly GRCm38.p2 released by the Genome Reference Consortium (119). Coding nucleotide sequences of human TAS2R genes were obtained from the genomic scaffolds 1103279188109, 1103279188381, 1103279188228, and 1103279188408 of the whole genome assembly released by the Venter Institute as Human Reference Genome (120).
A global multiple sequence alignment was performed for amino acid sequences corresponding to mouse and human bitter taste receptor genes using a modified version of the Feng-Doolittle (121) progressive alignment algorithm (Align X, Vector NTI, Life Technologies). A neighbor-joining tree (ClustalX (122)) was constructed after manual adjustment while considering previous in silico and in vitro experiments (29, 30). Bootstrap values computed using 10,000 iterations were subsequently inferred.
Author Contributions
K. L. designed the research, constructed vectors for functional expression, performed the calcium imaging analysis, immunocytochemistry, and brief-access tests, and prepared the manuscript. S. H. carried out qRT-PCR, prepared in situ hybridization probes, and performed corresponding experiments. N. R. conducted phylogenetic tree and statistical analysis. J. P. S. contributed to the design of the study. F. P. isolated and/or repurified several sequiterpene lactones. M. B. and M. W. designed research and prepared the manuscript. All authors read and approved of the final version of the manuscript.
Supplementary Material
Acknowledgments
We thank Elke Chudoba, Josefine Würfel, Florian Padberg, Lisa Oldorff, Julia Freydank, Alexandra Semmler, and Eva-Katharina Hage (Nuthetal) for expert technical assistance.
This study was supported by the Federal Ministry for Education and Research and the Ministry for Science, Research, and Culture of the state of Brandenburg, Germany, as well as the European Union's Seventh Framework Programme for research, technological development, and demonstration (SynSignal Grant 613879). J. P. S. is an employee of Givaudan Flavors Corp.

This article contains supplemental Tables 1S–3S.
- TAS2R
- human bitter taste receptor class 2
- Tas2r
- murine bitter taste receptor class 2
- FLIPR
- fluorometric imaging plate reader
- HSV
- herpes simplex virus glycoprotein D epitope
- PROP
- 6-n-propyl-2-thiouracil
- PTC
- phenylthiocarbamide
- qRT-PCR
- quantitative RT-PCR.
References
- 1. Behrens M., Brockhoff A., Kuhn C., Bufe B., Winnig M., and Meyerhof W. (2004) The human taste receptor hTAS2R14 responds to a variety of different bitter compounds. Biochem. Biophys. Res. Commun. 319, 479–485 [DOI] [PubMed] [Google Scholar]
- 2. Roland W. S., Vincken J. P., Gouka R. J., van Buren L., Gruppen H., and Smit G. (2011) Soy isoflavones and other isoflavonoids activate the human bitter taste receptors hTAS2R14 and hTAS2R39. J. Agric. Food Chem. 59, 11764–11771 [DOI] [PubMed] [Google Scholar]
- 3. Meyerhof W., Batram C., Kuhn C., Brockhoff A., Chudoba E., Bufe B., Appendino G., and Behrens M. (2010) The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses 35, 157–170 [DOI] [PubMed] [Google Scholar]
- 4. Soares S., Kohl S., Thalmann S., Mateus N., Meyerhof W., and De Freitas V. (2013) Different phenolic compounds activate distinct human bitter taste receptors. J. Agric. Food Chem. 61, 1525–1533 [DOI] [PubMed] [Google Scholar]
- 5. Behrens M., and Meyerhof W. (2013) Bitter taste receptor research comes of age: from characterization to modulation of TAS2Rs. Semin. Cell Dev. Biol. 24, 215–221 [DOI] [PubMed] [Google Scholar]
- 6. Adler E., Hoon M. A., Mueller K. L., Chandrashekar J., Ryba N. J., and Zuker C. S. (2000) A novel family of mammalian taste receptors. Cell 100, 693–702 [DOI] [PubMed] [Google Scholar]
- 7. Chandrashekar J., Mueller K. L., Hoon M. A., Adler E., Feng L., Guo W., Zuker C. S., and Ryba N. J. (2000) T2Rs function as bitter taste receptors. Cell 100, 703–711 [DOI] [PubMed] [Google Scholar]
- 8. Fredriksson R., Lagerström M. C., Lundin L. G., and Schiöth H. B. (2003) The G-protein-coupled receptors in the human genome form five main families: phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 63, 1256–1272 [DOI] [PubMed] [Google Scholar]
- 9. Matsunami H., Montmayeur J. P., and Buck L. B. (2000) A family of candidate taste receptors in human and mouse. Nature 404, 601–604 [DOI] [PubMed] [Google Scholar]
- 10. Meyerhof W. (2005) Elucidation of mammalian bitter taste. Rev. Physiol. Biochem. Pharmacol 154, 37–72 [DOI] [PubMed] [Google Scholar]
- 11. Grus W. E., and Zhang J. (2009) Origin of the genetic components of the vomeronasal system in the common ancestor of all extant vertebrates. Mol. Biol. Evol. 26, 407–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dong D., Jones G., and Zhang S. (2009) Dynamic evolution of bitter taste receptor genes in vertebrates. BMC Evol. Biol. 9, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang P., Josue J., Li X., Glaser D., Li W., Brand J. G., Margolskee R. F., Reed D. R., and Beauchamp G. K. (2012) Major taste loss in carnivorous mammals. Proc. Natl. Acad. Sci. U.S.A. 109, 4956–4961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao H., Li J., and Zhang J. (2015) Molecular evidence for the loss of three basic tastes in penguins. Curr. Biol. 25, R141–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu K., Zhou X., Xu S., Sun D., Ren W., Zhou K., and Yang G. (2014) The loss of taste genes in cetaceans. BMC Evol. Biol. 14, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feng P., Zheng J., Rossiter S. J., Wang D., and Zhao H. (2014) Massive losses of taste receptor genes in toothed and baleen whales. Genome Biol. Evol. 6, 1254–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Behrens M., Korsching S. I., and Meyerhof W. (2014) Tuning properties of avian and frog bitter taste receptors dynamically fit gene repertoire sizes. Mol. Biol. Evol. 31, 3216–3227 [DOI] [PubMed] [Google Scholar]
- 18. Li D., and Zhang J. (2014) Diet shapes the evolution of the vertebrate bitter taste receptor gene repertoire. Mol. Biol. Evol. 31, 303–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shi P., and Zhang J. (2006) Contrasting modes of evolution between vertebrate sweet/umami receptor genes and bitter receptor genes. Mol. Biol. Evol. 23, 292–300 [DOI] [PubMed] [Google Scholar]
- 20. Syed A. S., and Korsching S. I. (2014) Positive Darwinian selection in the singularly large taste receptor gene family of an “ancient” fish, Latimeria chalumnae. BMC Genomics 15, 650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Go Y., Satta Y., Takenaka O., and Takahata N. (2005) Lineage-specific loss of function of bitter taste receptor genes in humans and nonhuman primates. Genetics 170, 313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Conte C., Ebeling M., Marcuz A., Nef P., and Andres-Barquin P. J. (2003) Evolutionary relationships of the Tas2r receptor gene families in mouse and human. Physiol. Genomics 14, 73–82 [DOI] [PubMed] [Google Scholar]
- 23. Shi P., Zhang J., Yang H., and Zhang Y. P. (2003) Adaptive diversification of bitter taste receptor genes in mammalian evolution. Mol. Biol. Evol. 20, 805–814 [DOI] [PubMed] [Google Scholar]
- 24. Behrens M., and Meyerhof W. (2011) Gustatory and extragustatory functions of mammalian taste receptors. Physiol. Behav. 105, 4–13 [DOI] [PubMed] [Google Scholar]
- 25. Chen M. C., Wu S. V., Reeve J. R. Jr., and Rozengurt E. (2006) Bitter stimuli induce Ca2+ signaling and CCK release in enteroendocrine STC-1 cells: role of L-type voltage-sensitive Ca2+ channels. Am. J. Physiol. Cell Physiol. 291, C726–C739 [DOI] [PubMed] [Google Scholar]
- 26. Janssen S., Laermans J., Verhulst P. J., Thijs T., Tack J., and Depoortere I. (2011) Bitter taste receptors and α-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc. Natl. Acad. Sci. U.S.A. 108, 2094–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jeon T. I., Zhu B., Larson J. L., and Osborne T. F. (2008) SREBP-2 regulates gut peptide secretion through intestinal bitter taste receptor signaling in mice. J. Clin. Invest. 118, 3693–3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu S. V., Chen M. C., and Rozengurt E. (2005) Genomic organization, expression, and function of bitter taste receptors (T2R) in mouse and rat. Physiol. Genomics 22, 139–149 [DOI] [PubMed] [Google Scholar]
- 29. Brockhoff A., Behrens M., Niv M. Y., and Meyerhof W. (2010) Structural requirements of bitter taste receptor activation. Proc. Natl. Acad. Sci. U.S.A. 107, 11110–11115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Born S., Levit A., Niv M. Y., Meyerhof W., and Behrens M. (2013) The human bitter taste receptor TAS2R10 is tailored to accommodate numerous diverse ligands. J. Neurosci. 33, 201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Behrens M., Brockhoff A., Batram C., Kuhn C., Appendino G., and Meyerhof W. (2009) The human bitter taste receptor hTAS2R50 is activated by the two natural bitter terpenoids andrographolide and amarogentin. J. Agric. Food Chem. 57, 9860–9866 [DOI] [PubMed] [Google Scholar]
- 32. Brockhoff A., Behrens M., Massarotti A., Appendino G., and Meyerhof W. (2007) Broad tuning of the human bitter taste receptor hTAS2R46 to various sesquiterpene lactones, clerodane and labdane diterpenoids, strychnine, and denatonium. J. Agric. Food Chem. 55, 6236–6243 [DOI] [PubMed] [Google Scholar]
- 33. Bufe B., Breslin P. A., Kuhn C., Reed D. R., Tharp C. D., Slack J. P., Kim U. K., Drayna D., and Meyerhof W. (2005) The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr. Biol. 15, 322–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bufe B., Hofmann T., Krautwurst D., Raguse J. D., and Meyerhof W. (2002) The human TAS2R16 receptor mediates bitter taste in response to β-glucopyranosides. Nat. Genet. 32, 397–401 [DOI] [PubMed] [Google Scholar]
- 35. Kuhn C., Bufe B., Winnig M., Hofmann T., Frank O., Behrens M., Lewtschenko T., Slack J. P., Ward C. D., and Meyerhof W. (2004) Bitter taste receptors for saccharin and acesulfame K. J. Neurosci. 24, 10260–10265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dotson C. D., Zhang L., Xu H., Shin Y. K., Vigues S., Ott S. H., Elson A. E., Choi H. J., Shaw H., Egan J. M., Mitchell B. D., Li X., Steinle N. I., and Munger S. D. (2008) Bitter taste receptors influence glucose homeostasis. PLoS One 3, e3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Intelmann D., Batram C., Kuhn C., Haseleu G., Meyerhof W., and Hofmann T. (2009) Three TAS2R bitter taste receptors mediate the psychophysical responses to bitter compounds of hops (Humulus lupulus L.) and beer. Chemosens. Percept. 2, 118–132 [Google Scholar]
- 38. Kim U. K., Jorgenson E., Coon H., Leppert M., Risch N., and Drayna D. (2003) Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science 299, 1221–1225 [DOI] [PubMed] [Google Scholar]
- 39. Maehashi K., Matano M., Wang H., Vo L. A., Yamamoto Y., and Huang L. (2008) Bitter peptides activate hTAS2Rs, the human bitter receptors. Biochem. Biophys. Res. Commun. 365, 851–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pronin A. N., Tang H., Connor J., and Keung W. (2004) Identification of ligands for two human bitter T2R receptors. Chem. Senses 29, 583–593 [DOI] [PubMed] [Google Scholar]
- 41. Sainz E., Cavenagh M. M., Gutierrez J., Battey J. F., Northup J. K., and Sullivan S. L. (2007) Functional characterization of human bitter taste receptors. Biochem. J. 403, 537–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thalmann S., Behrens M., and Meyerhof W. (2013) Major haplotypes of the human bitter taste receptor TAS2R41 encode functional receptors for chloramphenicol. Biochem. Biophys. Res. Commun. 435, 267–273 [DOI] [PubMed] [Google Scholar]
- 43. He W., Yasumatsu K., Varadarajan V., Yamada A., Lem J., Ninomiya Y., Margolskee R. F., and Damak S. (2004) Umami taste responses are mediated by α-transducin and α-gustducin. J. Neurosci. 24, 7674–7680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Prandi S., Bromke M., Hübner S., Voigt A., Boehm U., Meyerhof W., and Behrens M. (2013) A subset of mouse colonic goblet cells expresses the bitter taste receptor Tas2r131. PLoS One 8, e82820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wong G. T., Gannon K. S., and Margolskee R. F. (1996) Transduction of bitter and sweet taste by gustducin. Nature 381, 796–800 [DOI] [PubMed] [Google Scholar]
- 46. Boughter J. D. Jr., Raghow S., Nelson T. M., and Munger S. D. (2005) Inbred mouse strains C57BL/6J and DBA/2J vary in sensitivity to a subset of bitter stimuli. BMC Genet. 6, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Boughter J. D. Jr., and Whitney G. (1997) Behavioral specificity of the bitter taste gene Soa. Physiol. Behav. 63, 101–108 [DOI] [PubMed] [Google Scholar]
- 48. Capeless C. G., Whitney G., and Azen E. A. (1992) Chromosome mapping of Soa, a gene influencing gustatory sensitivity to sucrose octaacetate in mice. Behav. Genet. 22, 655–663 [DOI] [PubMed] [Google Scholar]
- 49. Lush I. E. (1984) The genetics of tasting in mice. III. Quinine. Genet. Res. 44, 151–160 [DOI] [PubMed] [Google Scholar]
- 50. Lush I. E. (1986) The genetics of tasting in mice. IV. The acetates of raffinose, galactose, and β-lactose. Genet. Res. 47, 117–123 [DOI] [PubMed] [Google Scholar]
- 51. Lush I. E., and Holland G. (1988) The genetics of tasting in mice. V. Glycine and cycloheximide. Genet. Res. 52, 207–212 [DOI] [PubMed] [Google Scholar]
- 52. Warren R. P., and Lewis R. C. (1970) Taste polymorphism in mice involving a bitter sugar derivative. Nature 227, 77–78 [DOI] [PubMed] [Google Scholar]
- 53. Whitney G., and Harder D. B. (1986) Single-locus control of sucrose octaacetate tasting among mice. Behav. Genet. 16, 559–574 [DOI] [PubMed] [Google Scholar]
- 54. Whitney G., and Harder D. B. (1994) Genetics of bitter perception in mice. Physiol. Behav. 56, 1141–1147 [DOI] [PubMed] [Google Scholar]
- 55. Lee R. J., Xiong G., Kofonow J. M., Chen B., Lysenko A., Jiang P., Abraham V., Doghramji L., Adappa N. D., Palmer J. N., Kennedy D. W., Beauchamp G. K., Doulias P. T., Ischiropoulos H., Kreindler J. L., et al. (2012) T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J. Clin. Invest. 122, 4145–4159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hayakawa T., Suzuki-Hashido N., Matsui A., and Go Y. (2014) Frequent expansions of the bitter taste receptor gene repertoire during evolution of mammals in the Euarchontoglires clade. Mol. Biol. Evol. 31, 2018–2031 [DOI] [PubMed] [Google Scholar]
- 57. Mueller K. L., Hoon M. A., Erlenbach I., Chandrashekar J., Zuker C. S., and Ryba N. J. (2005) The receptors and coding logic for bitter taste. Nature 434, 225–229 [DOI] [PubMed] [Google Scholar]
- 58. Behrens M., Foerster S., Staehler F., Raguse J. D., and Meyerhof W. (2007) Gustatory expression pattern of the human TAS2R bitter receptor gene family reveals a heterogenous population of bitter responsive taste receptor cells. J. Neurosci. 27, 12630–12640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Caicedo A., and Roper S. D. (2001) Taste receptor cells that discriminate between bitter stimuli. Science 291, 1557–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Foster S. R., Porrello E. R., Purdue B., Chan H. W., Voigt A., Frenzel S., Hannan R. D., Moritz K. M., Simmons D. G., Molenaar P., Roura E., Boehm U., Meyerhof W., and Thomas W. G. (2013) Expression, regulation and putative nutrient-sensing function of taste GPCRs in the heart. PLoS One 8, e64579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xu J., Cao J., Iguchi N., Riethmacher D., and Huang L. (2013) Functional characterization of bitter-taste receptors expressed in mammalian testis. Mol. Hum. Reprod. 19, 17–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nelson T. M., Munger S. D., and Boughter J. D. Jr. (2005) Haplotypes at the Tas2r locus on distal chromosome 6 vary with quinine taste sensitivity in inbred mice. BMC Genet. 6, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ozeck M., Brust P., Xu H., and Servant G. (2004) Receptors for bitter, sweet, and umami taste couple to inhibitory G protein signaling pathways. Eur. J. Pharmacol. 489, 139–149 [DOI] [PubMed] [Google Scholar]
- 64. Hilliard M. A., Bergamasco C., Arbucci S., Plasterk R. H., and Bazzicalupo P. (2004) Worms taste bitter: ASH neurons, QUI-1, GPA-3, and ODR-3 mediate quinine avoidance in Caenorhabditis elegans. EMBO J. 23, 1101–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Oike H., Nagai T., Furuyama A., Okada S., Aihara Y., Ishimaru Y., Marui T., Matsumoto I., Misaka T., and Abe K. (2007) Characterization of ligands for fish taste receptors. J. Neurosci. 27, 5584–5592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wooding S., Bufe B., Grassi C., Howard M. T., Stone A. C., Vazquez M., Dunn D. M., Meyerhof W., Weiss R. B., and Bamshad M. J. (2006) Independent evolution of bitter-taste sensitivity in humans and chimpanzees. Nature 440, 930–934 [DOI] [PubMed] [Google Scholar]
- 67. Imai H., Suzuki N., Ishimaru Y., Sakurai T., Yin L., Pan W., Abe K., Misaka T., and Hirai H. (2012) Functional diversity of bitter taste receptor TAS2R16 in primates. Biol. Lett. 8, 652–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nelson T. M., Munger S. D., and Boughter J. D. Jr. (2003) Taste sensitivities to PROP and PTC vary independently in mice. Chem. Senses 28, 695–704 [DOI] [PubMed] [Google Scholar]
- 69. Harder D. B., and Whitney G. (1998) A common polygenic basis for quinine and PROP avoidance in mice. Chem. Senses 23, 327–332 [DOI] [PubMed] [Google Scholar]
- 70. Biarnés X., Marchiori A., Giorgetti A., Lanzara C., Gasparini P., Carloni P., Born S., Brockhoff A., Behrens M., and Meyerhof W. (2010) Insights into the binding of Phenyltiocarbamide (PTC) agonist to its target human TAS2R38 bitter receptor. PLoS One 5, e12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Marchiori A., Capece L., Giorgetti A., Gasparini P., Behrens M., Carloni P., and Meyerhof W. (2013) Coarse-grained/molecular mechanics of the TAS2R38 bitter taste receptor: experimentally validated detailed structural prediction of agonist binding. PLoS One 8, e64675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sandau M. M., Goodman J. R., Thomas A., Rucker J. B., and Rawson N. E. (2015) A functional comparison of the domestic cat bitter receptors Tas2r38 and Tas2r43 with their human orthologs. BMC Neurosci. 16, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lei W., Ravoninjohary A., Li X., Margolskee R. F., Reed D. R., Beauchamp G. K., and Jiang P. (2015) Functional analyses of bitter taste receptors in domestic cats (Felis catus). PLoS One 10, e0139670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Matsuo R. (2000) Role of saliva in the maintenance of taste sensitivity. Crit. Rev. Oral Biol. Med. 11, 216–229 [DOI] [PubMed] [Google Scholar]
- 75. Ming D., Ruiz-Avila L., and Margolskee R. F. (1998) Characterization and solubilization of bitter-responsive receptors that couple to gustducin. Proc. Natl. Acad. Sci. U.S.A. 95, 8933–8938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ruiz-Avila L., McLaughlin S. K., Wildman D., McKinnon P. J., Robichon A., Spickofsky N., and Margolskee R. F. (1995) Coupling of bitter receptor to phosphodiesterase through transducin in taste receptor cells. Nature 376, 80–85 [DOI] [PubMed] [Google Scholar]
- 77. Boughter J. D., Harder D. B., Capeless C. G., and Whitney G. (1992) Polygenic determination of quinine aversion among mice. Chem. Senses 17, 427–434 [Google Scholar]
- 78. Bachmanov A. A., Li X., Li S., Neira M., Beauchamp G. K., and Azen E. A. (2001) High-resolution genetic mapping of the sucrose octaacetate taste aversion (Soa) locus on mouse chromosome 6. Mamm. Genome 12, 695–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lush I. E., Hornigold N., King P., and Stoye J. P. (1995) The genetics of tasting in mice. VII. Glycine revisited, and the chromosomal location of Sac and Soa. Genet. Res. 66, 167–174 [DOI] [PubMed] [Google Scholar]
- 80. Lush I. E. (1982) The genetics of tasting in mice. II. Strychnine. Chem. Senses 7, 93–98 [Google Scholar]
- 81. Shingai T., and Beidler L. M. (1985) Response characteristics of three taste nerves in mice. Brain Res. 335, 245–249 [DOI] [PubMed] [Google Scholar]
- 82. Tizzano M., Gulbransen B. D., Vandenbeuch A., Clapp T. R., Herman J. P., Sibhatu H. M., Churchill M. E., Silver W. L., Kinnamon S. C., and Finger T. E. (2010) Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc. Natl. Acad. Sci. U.S.A. 107, 3210–3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sbarbati A., Tizzano M., Merigo F., Benati D., Nicolato E., Boschi F., Cecchini M. P., Scambi I., and Osculati F. (2009) Acyl homoserine lactones induce early response in the airway. Anat. Rec. (Hoboken) 292, 439–448 [DOI] [PubMed] [Google Scholar]
- 84. Williams P. (2007) Quorum sensing, communication, and cross-kingdom signalling in the bacterial world. Microbiology 153, 3923–3938 [DOI] [PubMed] [Google Scholar]
- 85. Zhang L. H., and Dong Y. H. (2004) Quorum sensing and signal interference: diverse implications. Mol. Microbiol. 53, 1563–1571 [DOI] [PubMed] [Google Scholar]
- 86. Ansoleaga B., Garcia-Esparcia P., Llorens F., Moreno J., Aso E., and Ferrer I. (2013) Dysregulation of brain olfactory and taste receptors in AD, PSP and CJD, and AD-related model. Neuroscience 248, 369–382 [DOI] [PubMed] [Google Scholar]
- 87. Garcia-Esparcia P., Schlüter A., Carmona M., Moreno J., Ansoleaga B., Torrejón-Escribano B., Gustincich S., Pujol A., and Ferrer I. (2013) Functional genomics reveals dysregulation of cortical olfactory receptors in Parkinson disease: novel putative chemoreceptors in the human brain. J. Neuropathol. Exp. Neurol. 72, 524–539 [DOI] [PubMed] [Google Scholar]
- 88. Dehkordi O., Rose J. E., Fatemi M., Allard J. S., Balan K. V., Young J. K., Fatima S., Millis R. M., and Jayam-Trouth A. (2012) Neuronal expression of bitter taste receptors and downstream signaling molecules in the rat brainstem. Brain Res. 1475, 1–10 [DOI] [PubMed] [Google Scholar]
- 89. Singh N., Vrontakis M., Parkinson F., and Chelikani P. (2011) Functional bitter taste receptors are expressed in brain cells. Biochem. Biophys. Res. Commun. 406, 146–151 [DOI] [PubMed] [Google Scholar]
- 90. Li F., and Zhou M. (2012) Depletion of bitter taste transduction leads to massive spermatid loss in transgenic mice. Mol. Hum. Reprod. 18, 289–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Voigt A., Hübner S., Lossow K., Hermans-Borgmeyer I., Boehm U., and Meyerhof W. (2012) Genetic labeling of Tas1r1 and Tas2r131 taste receptor cells in mice. Chem. Senses 37, 897–911 [DOI] [PubMed] [Google Scholar]
- 92. Clark A. A., Dotson C. D., Elson A. E., Voigt A., Boehm U., Meyerhof W., Steinle N. I., and Munger S. D. (2015) TAS2R bitter taste receptors regulate thyroid function. FASEB J. 29, 164–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Deckmann K., Filipski K., Krasteva-Christ G., Fronius M., Althaus M., Rafiq A., Papadakis T., Renno L., Jurastow I., Wessels L., Wolff M., Schütz B., Weihe E., Chubanov V., Gudermann T., Klein J., Bschleipfer T., and Kummer W. (2014) Bitter triggers acetylcholine release from polymodal urethral chemosensory cells and bladder reflexes. Proc. Natl. Acad. Sci. U.S.A. 111, 8287–8292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Correia J. N., Conner S. J., and Kirkman-Brown J. C. (2007) Non-genomic steroid actions in human spermatozoa: “persistent tickling from a laden environment”. Semin. Reprod. Med. 25, 208–219 [DOI] [PubMed] [Google Scholar]
- 95. Boughter J. D. Jr., St John S. J., Noel D. T., Ndubuizu O., and Smith D. V. (2002) A brief-access test for bitter taste in mice. Chem. Senses 27, 133–142 [DOI] [PubMed] [Google Scholar]
- 96. Dotson C. D., and Spector A. C. (2004) The relative affective potency of glycine, l-serine and sucrose as assessed by a brief-access taste test in inbred strains of mice. Chem. Senses 29, 489–498 [DOI] [PubMed] [Google Scholar]
- 97. Glendinning J. I., Gresack J., and Spector A. C. (2002) A high-throughput screening procedure for identifying mice with aberrant taste and oromotor function. Chem. Senses 27, 461–474 [DOI] [PubMed] [Google Scholar]
- 98. Zhang Y., Hoon M. A., Chandrashekar J., Mueller K. L., Cook B., Wu D., Zuker C. S., and Ryba N. J. (2003) Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112, 293–301 [DOI] [PubMed] [Google Scholar]
- 99. Livak K. J., and Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔC(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 100. Barratt-Fornell A., and Drewnowski A. (2002) The taste of health: nature's bitter gifts. Nutr. Today 37, 144–150 [DOI] [PubMed] [Google Scholar]
- 101. Belitz H. D., and Wieser H. (1985) Bitter compounds: occurrence and structure-activity relationships. Food Reviews International 1, 271–354 [Google Scholar]
- 102. Drewnowski A., and Gomez-Carneros C. (2000) Bitter taste, phytonutrients, and the consumer: a review. Am. J. Clin. Nutr. 72, 1424–1435 [DOI] [PubMed] [Google Scholar]
- 103. Maga J. A. (1990) Compound structure versus bitter taste, in Bitterness in Foods and Beverages: Developments in Food Science (Rousseff R. L., ed) Vol. 25, pp. 35–48, Elsevier, Amsterdam [Google Scholar]
- 104. Rouseff R. L. (1990) Bitterness in food products: an overview, in Bitterness in Foods and Beverages: Developments in Food Science (Rousseff R. L., ed) Vol. 25, pp. 1–14, Elsevier; Amsterdam [Google Scholar]
- 105. Wiener A., Shudler M., Levit A., and Niv M. Y. (2012) BitterDB: a database of bitter compounds. Nucleic Acids Res. 40, D413–D419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Tizzano M., Cristofoletti M., Sbarbati A., and Finger T. E. (2011) Expression of taste receptors in solitary chemosensory cells of rodent airways. BMC Pulm. Med. 11, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Appendino G., Belliardo F., Nano G. M., and Stefenelli S. (1982) Sesquiterpene lactones from artemisia genipi Weber: isolation and determination in plant-material and in liqueurs. J. Agric. Food Chem. 30, 518–521 [Google Scholar]
- 108. Appendino G., Gariboldi P., and Menichini F. (1991) The stereochemistry of arglabin, a cytotoxic guaianolide from Artemisia myriantha. Fitoterapia 62, 275–276 [Google Scholar]
- 109. Appendino G., Nano G. M., Calleri M., and Chiari G. (1986) The crystal structures of spiciformin acetate and tatridin-B dibenzoate. Gazz. Chim. Ital. 116, 57–61 [Google Scholar]
- 110. Fattorusso E., Taglialatela-Scafati O., Campagnuolo C., Santelia F. U., Appendino G., and Spagliardi P. (2002) Diterpenoids from cascarilla (Croton eluteria Bennet). J. Agric. Food Chem. 50, 5131–5138 [DOI] [PubMed] [Google Scholar]
- 111. Wyrembek P., Negri R., Kaczor P., Czyżewska M., Appendino G., and Mozrzymas J. W. (2012) Falcarindiol allosterically modulates GABAergic currents in cultured rat hippocampal neurons. J. Nat. Prod. 75, 610–616 [DOI] [PubMed] [Google Scholar]
- 112. Beauhaire J., Fourrey J. L., Vuilhorgne M., and Lallemand J. Y. (1980) Dimeric sesquiterpene lactones: structure of absinthin. Tetrahedron Lett. 21, 3191–3194 [Google Scholar]
- 113. Kohl S., Behrens M., Dunkel A., Hofmann T., and Meyerhof W. (2013) Amino acids and peptides activate at least five members of the human bitter taste receptor family. J. Agric. Food Chem. 61, 53–60 [DOI] [PubMed] [Google Scholar]
- 114. Ammon C., Schäfer J., Kreuzer O. J., and Meyerhof W. (2002) Presence of a plasma membrane targeting sequence in the amino-terminal region of the rat somatostatin receptor 3. Arch. Physiol. Biochem. 110, 137–145 [DOI] [PubMed] [Google Scholar]
- 115. Slack J. P., Brockhoff A., Batram C., Menzel S., Sonnabend C., Born S., Galindo M. M., Kohl S., Thalmann S., Ostopovici-Halip L., Simons C. T., Ungureanu I., Duineveld K., Bologa C. G., Behrens M., Furrer S., Oprea T. I., and Meyerhof W. (2010) Modulation of bitter taste perception by a small molecule hTAS2R antagonist. Curr. Biol. 20, 1104–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ueda T., Ugawa S., Yamamura H., Imaizumi Y., and Shimada S. (2003) Functional interaction between T2R taste receptors and G-protein α subunits expressed in taste receptor cells. J. Neurosci. 23, 7376–7380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Krautwurst D., Yau K. W., and Reed R. R. (1998) Identification of ligands for olfactory receptors by functional expression of a receptor library. Cell 95, 917–926 [DOI] [PubMed] [Google Scholar]
- 118. Yu Y., de March C. A., Ni M. J., Adipietro K. A., Golebiowski J., Matsunami H., and Ma M. (2015) Responsiveness of G protein-coupled odorant receptors is partially attributed to the activation mechanism. Proc. Natl. Acad. Sci. U.S.A. 112, 14966–14971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Mouse Genome Sequencing Consortium, Waterston R. H., Lindblad-Toh K., Birney E., Rogers J., Abril J. F., Agarwal P., Agarwala R., Ainscough R., Alexandersson M., An P., Antonarakis S. E., Attwood J., Baertsch R., Bailey, et al. (2002) Initial sequencing and comparative analysis of the mouse genome. Nature 420, 520–562 [DOI] [PubMed] [Google Scholar]
- 120. Levy S., Sutton G., Ng P. C., Feuk L., Halpern A. L., Walenz B. P., Axelrod N., Huang J., Kirkness E. F., Denisov G., Lin Y., MacDonald J. R., Pang A. W., Shago M., Stockwell T. B., et al. (2007) The diploid genome sequence of an individual human. PLoS Biol. 5, e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Feng D. F., and Doolittle R. F. (1987) Progressive sequence alignment as a prerequisitetto correct phylogenetic trees. J. Mol. Evol. 25, 351–360 [DOI] [PubMed] [Google Scholar]
- 122. Jeanmougin F., Thompson J. D., Gouy M., Higgins D. G., and Gibson T. J. (1998) Multiple sequence alignment with ClustalX. Trends Biochem. Sci. 23, 403–405 [DOI] [PubMed] [Google Scholar]
- 123. Huerta-Cepas J., Dopazo J., and Gabaldón T. (2010) ETE: a python environment for tree exploration. BMC Bioinformatics 11, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ballesteros J. A., and Weinstein H. (1995) Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 25, 366–428 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.