Abstract
Increasing evidence suggests that Toll-like receptor 4 (TLR4) contributes importantly to spinal cord glial activation and chronic pain sensitization; however, its unique role in acute and chronic itch is unclear. In this study, we investigated the involvement of TLR4 in acute and chronic itch models in male mice using both transgenic and pharmacological approaches. Tlr4−/− mice exhibited normal acute itch induced by compound 48/80 and chloroquine, but these mice showed substantial reductions in scratching in chronic itch models of dry skin, induced by acetone and diethyether followed by water (AEW), contact dermatitis, and allergic contact dermatitis on the neck. Intrathecal (spinal) inhibition of TLR4 with lipopolysaccharide Rhodobacter sphaeroides (LPS-RS) did not affect acute itch but suppressed AEW-induced chronic itch. Compound 48/80 and AEW also produced robust alloknesis, a touch-elicited itch in wild-type mice, which was suppressed by intrathecal LPS-RS and Tlr4−/− deletion. AEW induced persistent upregulation of Tlr4 mRNA and increased TLR4 expression in GFAP-expressing astrocytes in spinal cord dorsal horn. AEW also induced TLR4-dependent astrogliosis (GFAP upregulation) in spinal cord. Intrathecal injection of astroglial inhibitor L-α-aminoadipate reduced AEW-induced chronic itch and alloknesis without affecting acute itch. Spinal TLR4 was also necessary for AEW-induced chronic itch in the cheek model. Interestingly, scratching plays an essential role in spinal astrogliosis, since AEW-induced astrogliosis was abrogated by putting Elizabethan Collars on the neck to prevent scratching the itchy skin. Our findings suggest that spinal TLR4 signaling is important for spinal astrocyte activation and astrogliosis that may underlie alloknesis and chronic itch.
Keywords: Alloknesis (touch-evoked itch), astrogliosis, dry skin, innate immunity, lipopolysaccharide (LPS), Toll-like receptor 4 (TLR4)
1. Introduction
Toll-like receptors (TLRs) are evolutionarily conserved type I transmembrane proteins that can mediate innate and adaptive immunity via recognition of exogenous ligands, pathogen-associated molecular patterns (PAMPs) following viral and bacterial infection as well as detection of endogenous ligands, danger-associated molecular patterns (DAMPs) produced after tissue injury [2;50]. Different TLRs detect distinct PAMPs and DAMPs. For example, TLR3 and TLR7/8 sense double-stranded and single-stranded RNAs, respectively and TLR4 binds to lipopolysaccharide (LPS) [33]. Most TLRs (except TLR3) signal through the intracellular adaptor protein MyD88 [3;6]. In immune or glial cells, activation of TLRs produces a wide array of pro-inflammatory mediators, including cytokines and chemokines, as well as reactive oxygen/nitrogen intermediates, via activation of NF-κB and MAP kinase pathways [33]. TLR4 is the best studied member of the TLR family. Emerging evidence supports an important role of TLR4 in spinal cord glial activation in neuropathic pain [60] and chronic arthritis pain [9]. Additionally, spinal TLR4 was implicated in opioid-induced glial activation [20]. TLR4/MyD88 in DRG neurons was also involved in chemotherapy-induced neuropathic pain [31]. However, the role of TLR4 in different chronic itch conditions has not been investigated.
Glial cells such as microglia and astrocytes have been shown to play an important role in promoting chronic pain [11;16;39]. Chronic pain could be a result of gliopathy [25]. It is generally believed that glia promote pain by producing proinflammatory and pronociceptive mediators (e.g., proinflammatory cytokines and chemokines and growth factors) to activate and sensitize spinal cord nociceptive neurons [26;27;63]. Despite a prominent role of glial cells in the genesis of chronic pain, it is unclear how glial cells regulate itch. During the submission of the manuscript for this study, Shiratori-Hayashi et al. demonstrated an important role of reactive spinal cord astrocytes in chronic itch via astrocytic activation of the transcriptional factor STAT3 [53].
Itch and pain are two distinct somatic sensations, but they also share many similarities [4;18;29;34;36;47;68;69]. Itch evokes scratching response, while pain elicits withdrawal reflex [22]. Like acute pain, acute itch serves to protect our body against harmful irritants [24]. However, whether chronic itch is operated by different mechanisms or shares similar mechanisms with chronic pain is elusive [34;66;67]. In the present study, we investigated the distinct role of TLR4 in acute and chronic itch. We found that spinal TLR4 signaling is dispensable for acute itch but indispensable for chronic itch and alloknesis, the itch induced by touch. We also found that TLR4 is both sufficient and necessary for spinal astrocyte activation and astrogliosis in chronic itch.
2. Materials and methods
2.1. Animals
Male Tlr4 knockout mice (Tlr4−/−; stock#007227) and Myd88−/− (stock#009088) mice and their wild-type control (C57BL/6) male mice were purchased from The Jackson Laboratory and maintained at Duke University Animal Facility. Tlr4−/− mice have a homozygous deletion of 74 kb at the tlr4 locus with all three exons removed. For pharmacological studies, we also used CD1 mice (male, 8–10 weeks) from Charles River. Young mice (4–6 weeks of both sexes) were used for electrophysiological studies in DRG neurons. All other studies used adult male mice unless otherwise noted. Animals were maintained at Duke Animal Facility with ambient temperature and humidity and under a 12-hour light/12 hour dark cycle with ad libitum access to food and water. All the animal procedures were approved by the Institutional Animal Care and Use Committees of Duke University.
2.2. Drugs and administration
We purchased compound 48/80, chloroquine, diphenylcyclopropenone (DCP), 2,4-dinitrofluorobenzene (DNFB), L-α-aminoadipate (L-AA), LPS (Escherichia coli serotype 0111:B4) from Sigma-Aldrich and LPS Rhodobacter sphaeroides (LPS-RS) from R&D Systems. We injected the pruritic agents (compound 48/80 and chloroquine) intradermally with a 28-Gauge needle in the nape (back of the neck, 50 μl) or cheek (10 μl). According to previous reports [7;15;19;55], we also injected the following reagents intrathecally to target spinal cord cells: LPS-RS (20 μg) and L-AA (100 nmol) [70]. Intrathecal injection was performed by a lumbar puncture to deliver reagent into cerebral spinal fluid. A valid spinal puncture was confirmed by a brisk tail-flick after the needle entry into subarachnoid space [21]. We injected LPS or L-AA 24 hours before itch assay and injected LPS-RS 30 min prior to itch assay. Reagents were dissolved in sterile saline as vehicle if not specified.
2.3. Behavioral analysis
2.3.1. Neck models of acute itch
Mice were habituated to testing environment daily for two days before analysis. The back of the neck of animals were shaved on the day before testing. Mice were put in plastic chambers (14 × 18 × 12 cm) on an elevated metal mesh floor and allowed 30 min for habituation. We intradermally injected 50 μl of pruritic agents in the nape of neck and video-recorded scratching behavior for 30 min in the absence of any observer. A scratch was counted when a mouse lifted its hindpaw to scratch the shaved region and returned the paw to the floor or to the mouth for licking. The following doses for pruritic agents were chosen: 100 μg for compound 48/80 and 200 μg for chloroquine. The spontaneous itch was video-recorded and assessed blindly.
2.3.2. Cheek models of acute itch
The cheek model was developed to distinguish itch and pain [52]. We shaved mouse cheek (approx. 5×8 mm) two days before experiments. On the day of experiment, after brief anesthesia with isoflurane, we injected 10 μl of reagent (100 μg compound 48/80 or 200 μg chloroquine) into the cheek and counted the number of wipes and the number of scratches for 30 min. We counted the unilateral wipes with the forelimb. We also counted scratches, which were defined as a lifting of the hind paw toward the injection site on the cheek and then returning the paw to the floor or to the mouth.
2.3.3. Dry skin-induced chronic itch in the neck and cheek
We produced a dry skin model to induce chronic itch, as described previously [42], by painting the neck or cheek skin with acetone and diethyether (1:1) following by water (AEW) twice a day (9:00 am and 16:00 pm) for 5 days. The spontaneous scratching was video recorded for 1 hour on day 6 and total number of scratches was counted blindly. As previously reported [23], we let mouse wear an Elizabethan Collar on the neck to prevent mouse from scratching the cheek skin so that we can test the effect of scratching on AEW-induced spinal cord astrogliosis.
2.3.4. DNFB-induced allergic contact dermatitis in neck skin
We generated the allergic contact dermatitis (ACD) model of chronic itch by applying the hapten 1-fluoro-2, 4-dinitrobenzene (DNFB) onto the back skin as previously described [30]. DNFB was dissolved in a mixture of acetone:olive oil (4:1). The surface of abdomen and the nape of neck were shaved 1 day before sensitization. Mice were sensitized with 50 μl 0.5% DNFB solution by topical application to a 2 cm2 area of shaved abdomen skin. 5 days later, mice were challenged with 30 μl 0.25% DNFB solution by painting the nape of neck, then on day 1, 3, 5, and 7. Spontaneous scratching behaviors were video-recorded on day 8 for 60 min.
2.3.5. DCP-induced contact dermatitis in neck skin
To induce contact dermatitis, we shaved the back of neck and painted the back with 0.2 ml of diphenylcyclopropenone (DCP; 1% dissolved in acetone) [58]. Seven days after the sensitization, we challenged mice by painting the back skin with 0.2 ml of 0.5% DCP. Mouse itch behavior was video-recorded for 60 minutes immediately after each DCP application.
2.3.6. Alloknesis assay in neck and cheek models
According to a previous report [5], alloknesis after acute itch and chronic itch was evaluated. For testing alloknesis after acute itch, a von Frey filament (0.7 mN) was applied to the skin area 5 mm outside the injection site and 30 min after the compound 48/80 injection. A scratch bout directed to the site of mechanical stimulation was considered as a positive response. The alloknesis score was determined by calculating the total number of scratches elicited by five mechanical stimuli and was evaluated at 10-min intervals post-injection. For testing alloknesis in dry skin model, von Frey stimuli were applied at the border of the AEW treatment area 12 hours after each treatment to elicit scratching response.
2.3.7. Hot plate test
The hot plate was set at 52 °C. A mouse was put on the plate and the latency for the mouse to lick a hindpaw or jump from the hot plate was recorded.
2.4. Patch clamp recordings in DRG neurons
DRGs were removed aseptically from mice (4–6 weeks) and incubated with collagenase (1.25mg/ml, Roche)/dispase-II (2.4 units/ml, Roche) at 37°C for 90 min, then digested with 0.25% trypsin for 8 min at 37°C, followed by 0.25% trypsin inhibitor. Cells were mechanically dissociated with a flame polished Pasteur pipette in the presence of 0.05% DNAse I (Sigma). DRG cells were plated on glass cover slips and grown in a neurobasal defined medium (with 2% B27 supplement, Invitrogen) with 5 μM AraC and 5% carbon dioxide at 36.5°C. DRG neurons were grown for 24 hours before use. As we previously reported [45], whole-cell patch clamp recordings were performed at room temperature using an Axopatch-200B amplifier (Axon Instruments). The patch pipettes were pulled from borosilicate capillaries (Chase Scientific Glass Inc.). Pipette resistance was 4–6 MΩ.
2.5. Immunohistochemistry
As we previously reported [8], mice were terminally anesthetized with isoflurane and perfused through the ascending aorta with PBS followed by 4% paraformaldehyde. After the perfusion, the cervical spinal cords segments (C1–C2 in the cheek model and C3–C4 in the neck model) were collected and post-fixed in the same fixative overnight. The spinal cord sections (30 μm; free floating) from the cervical spinal cord after nape treatment or lumbar spinal cord after hindpaw treatment were cut in a cryostat and processed for immunohistochemistry as we previous described. Briefly, the tissue sections were blocked with 2% goat serum, and incubated over night at 4°C with the primary antibodies: mouse GFAP antibody (1:2000, Millipore), rabbit IBA-1 antibody (1:5000, Wako), rabbit anti-TLR4 antibody (1:200, Boster, China). The sections were then incubated for 1 h at room temperature with Cy3- or FITC-conjugated secondary antibodies. Immunostained tissue sections were examined under a Nikon fluorescence microscope, and images were captured with a high resolution CCD Spot camera (Diagnostic Instruments Inc.) and analyzed with NIH Image software or Adobe PhotoShop. Five nonadjacent spinal cord sections were randomly selected from a cervical spinal cord segment (C3–C4 from the back model and C1–C2 from the cheek model) and 4–5 mice were included for each group. The intensity of GFAP and IBA1 staining in the superficial dorsal horn (laminae I–III) was measured with a computer-assisted imaging analysis system (Image J, NIH).
2.6. Skin histology
Mice were terminally anesthetized with isoflurane and the back hairy skins were collected to perform histological examination. Tissues were postfixed in 4% paraformaldehyde overnight and skin sections were cut (14 μm) in a cryostat. The sections were stained with toluidine blue (TB) for mast cells and also processed for hematoxylin & eosin (H&E) staining. The stained sections were then dried, cleared, and covered for observation and photomicrography. The number of mast cells and hair follicles at different cycle stages was quantified by individuals that are blinded for the genotype using 10 sections per mouse and 4 mice per group [32].
2.7. Real-time quantitative RT-PCR
We rapidly collected cervical spinal cord in RNase-free conditions and isolated total RNAs using RNeasy Plus Mini kit (Qiagen, Valencia, CA). RNA (1 μg) was reverse-transcribed for each sample using SuperScript III RT (Invitrogen). The sequences of the forward and reverse primers for
| Iba1: | Forward: GGACAGACTGCCAGCCTAAG; |
| Reverse: GACGGCAGATCCTCATCATT; | |
| Gfap: | Forward: GAATCGCTGGAGGAGGAGAT; |
| Reverse: GCCACTGCCTCGTATTGAGT; | |
| Tlr4: | Forward: AAACTTGCCTTCAAAACCTGGC; |
| Reverse: ACCTGAACTCATCAATGGTCACATC; | |
| Gapdh: | Forward: AGGTCGGTGTGAACGGATTTG |
| Reverse: GGGGTCGTTGATGGCAACA |
Quantitative PCR amplification reactions contained the same amount of RT product in a final volume of 15 μl. The thermal cycling conditions comprised 3 minutes of polymerase activation at 95 °C, 45 cycles of 10 second denaturation at 95 °C, and 30 second annealing and extension at 60 °C, and a DNA melting curve was included to test the amplicon specificity. Triplicate qPCR analyses were performed using the SYBR Green master mix (KAPA) and Opticon real-time PCR Detection System (Bio-Rad, Hercules, CA) as described previously [7].
2.10. Statistical analysis
All the data were expressed as mean ± SEM. Behavioral data were analyzed using Student’s t test or one-way ANOVA followed by Dunn’s post hoc test. Two-Way repeated measured ANOVA with post hoc Bonferroni’s test was used to analyze two group data with multiple time points. The criterion for statistical significance was set at P<0.05.
3. Results
3.1. Acute itch is not affected after Tlr4 deletion and spinal inhibition of TLR4
We first employed genetic and pharmacological approaches to assess the involvement of TLR4 in acute itch sensation. Based on the sensitivity to antihistamines, acute itch can be characterized as histaminergic itch, induced by compound 48/80 and nonhistaminergic itch, induced by chloroquine (CQ). Notably, compound 48/80 or CQ-induced acute itch was not affected in Tlr4−/− mice (Fig. 1A). MyD88 is a scaffold protein and mediates canonical signaling of the majority of TLRs including TLR4 [33]. Interestingly, acute itch was also intact in mice lacking Myd88 (Fig. 1B). Furthermore, intrathecal injection of the TLR4 antagonist LPS-RS (20 μg) did not affect compound 48/80 and CQ-induced scratching in wild-type (WT) mice (Fig. 1C). To further determine the possible role of TLR4 in pain and itch, we used a cheek model that can distinguish pain versus itch, as indicated by distinct pain-like wiping by forelimbs and itch-like scratching by the hind limbs [52]. Tlr4−/− mice displayed comparable responses in the compound 48/80-and CQ-induced wiping and scratching (Fig. 1D). Thus, TLR4 signaling is dispensable for acute itch, regardless of histaminergic and nonhistaminergic itch.
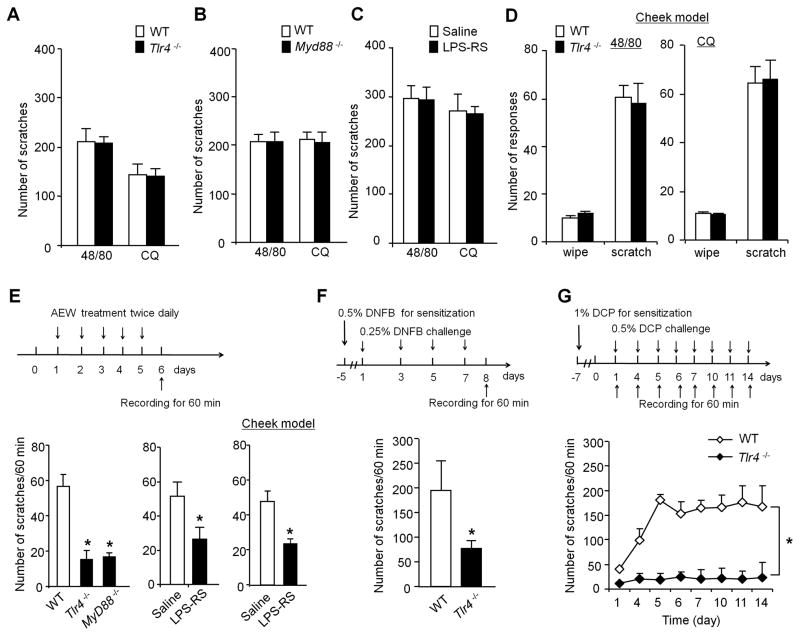
Figure 1. TLR4 signaling is required for chronic itch but not acute itch in the back and cheek models.
(A, B) Acute itch induced by intradermal injection on the back skin of compound 48/80 (48/80, 100 μg) and chloroquine (CQ, 200 μg) is not altered in Tlr4−/− mice (A) and Myd88−/− mice (B). n = 5 mice per group. (C) Intrathecal LPS-RS (20 μg) does not affect 48/80 and CQ-induced acute itch. n = 5 mice per group. (D) Acute itch and pain in mouse cheek model. Note that intradermal injection of 48/80 or CQ induced comparable wiping and scratching in WT and Tlr4−/− mice. n=5–7 mice per group. (E) Dry skin after treatment with AEW (acetone and diethyether followed by water) induces chronic itch, which is substantially reduced in Tlr4−/− mice and Myd88−/− mice and also suppressed by intrathecal LPS-RS. Top, paradigm of AEW treatment. Low right, intrathecal LPS-RS administered 5 d after AEW treatment in either neck model or cheek model suppressed AEW-evoked scratching on day 6. *P< 0.05, compared with WT mice or corresponding vehicle groups, n = 5–7 mice per group. (F–G) Chronic itch in the neck models after contact allergic dermatitis, evoked by 2,4-dinitrofluorobenzene (DNFB, F), and atopic dermatitis, evoked by diphenylcyclopropenone (DCP, G) is substantially reduced in Tlr4−/− mice. Top panels, paradigms of DNFB and DCP treatment. *P<0.05, compared with WT control mice, student t-test (F) and Two-way ANOVA (G); n = 5–6 mice per group. Note that the neck models were tested for all the experimental conditions but the cheek models were only used for some conditions and specifically indicated in each graph. Data are presented as means ± S.E.M.
3.2. Tlr4 and spinal TLR4 are required for the development of chronic itch
Dry skin, caused by skin dehydration, is associated with several chronic itch conditions, such as atopic dermatitis and xerosis [18]. We treated mice with acetone and diethyether followed by water (AEW) on neck or cheek skin for 5 days to mimic the symptoms of dry skin in patients [42]. Five days after AEW treatment, WT mice showed robust spontaneous scratching on day 6 (56.7±6.6 scratches/hour; Fig. 1E), which was drastically decreased in Tlr4−/− mice (15.2±5.0 scratches/hour, Fig. 1E). Notably, AEW-induced chronic itch on day 6 was also compromised in mice lacking Myd88 (16.8±2.3 scratches/hour; Fig. 1E). AEW-induced chronic itch in both neck and cheek models was also largely inhibited by intrathecal injection of LPS-RS (Fig. 1E).
To validate a critical role of TLR4 in chronic itch, we generated an allergic contact dermatitis mouse model by application of hapten 2,4-dinitrofluorobenzene (DNFB). After initial sensitization, treatment of DNFB on day 1, 3, 5, and 7 induced robust spontaneous itch on day 8, which was substantially reduced in Tlr4−/− mice (Fig. 1F). Additionally, we tested the involvement of TLR4 in dermatitis-induced chronic itch. Treatment with diphenylcyclopropenone (DCP) was shown to induce contact dermatitis-associated chronic itch [64]. DCP-treated WT mice exhibited robust and persistent itch for more than 2 weeks, but the development of persistent itch was compromised in Tlr4−/− mice (P<0.05, two-way ANOVA, Fig. 1G). Collectively, our results suggest that TLR4 is critically involved in chronic itch after dry skin injury, allergic contact dermatitis, and atopic dermatitis.
3.3. TLR4 is required for compound 48/80 and AEW-induced alloknesis
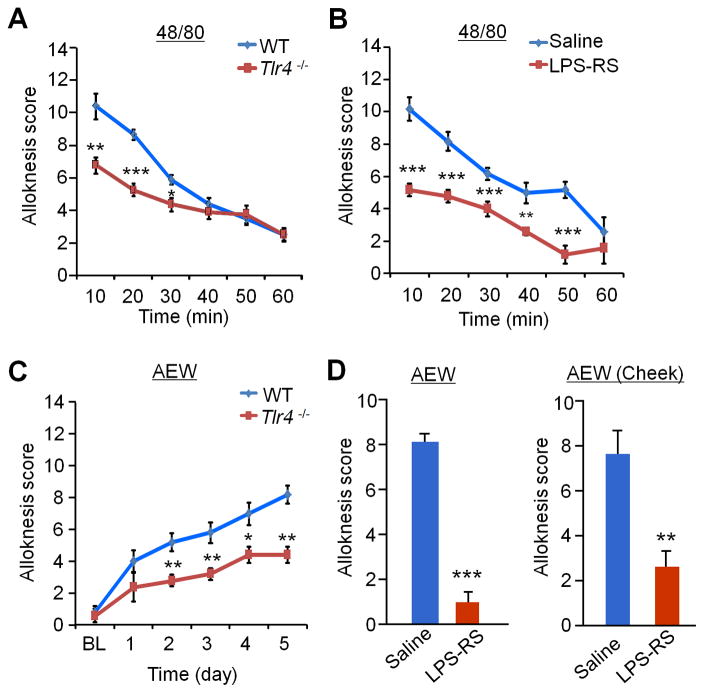
Alloknesis (touch-evoked itch) is induced by light touch at the skin near an itchy site. Scratching responses following intradermal injection of compound 48/80 declined over a 30-min period. Immediately after that period, alloknesis score was assessed by counting the number of bouts of scratching following application of 0.7 mN von Frey stimuli [5]. Tlr4−/− mice displayed a significant reduction in alloknesis score during a 60 min period (i.e., 30–90 min after the compound 48/80 injection, Fig. 2A). Intrathecal LPS-RS inhibited compound 48/80-induced alloknesis (Fig. 2B). Alloknesis was also induced after AEW treatment (dry skin) in WT mice, beginning on day 1 and reaching to a high level on day 5 (Fig. 2C). Notably, AEW-induced development of alloknesis was reduced in Tlr4−/− mice (Fig. 2C). Furthermore, dry skin-induced alloknesis in both neck and cheek models was substantially inhibited by intrathecal LPS-RS (Fig. 2D). Together, these data demonstrate that TLR4 is critically involved in alloknesis after acute itch and also during chronic itch.
Figure 2. Alloknesis under both acute and chronic itch conditions is impaired in Tlr4−/− mice and suppressed by intrathecal LPS-RS in the back and cheek models.
(A) Alloknesis, induced 30 min after compound 48/80 (48/80) injection, is partially reduced in Tlr4−/− mice. (B) Alloknesis, induced 30 min after 48/80 injection, is suppressed by intrathecal injection of LPS-RS (20 μg). (C) Alloknesis, induced after AEW-induced dry skin, is partially reduced in Tlr4−/− mice. (D) AEW-induced alloknesis at Day 6 in either neck model or cheek model is suppressed by intrathecal LPS-RS (20 μg). Data are presented as means ± S.E.M. *P<0.05, **P<0.01, ***P<0.001, compared with WT mice or saline vehicle group, Student’s t test; n = 5–6 mice per group.
3.4. Intrathecal LPS enhances acute pain, alloknesis, and chronic itch but suppresses acute itch
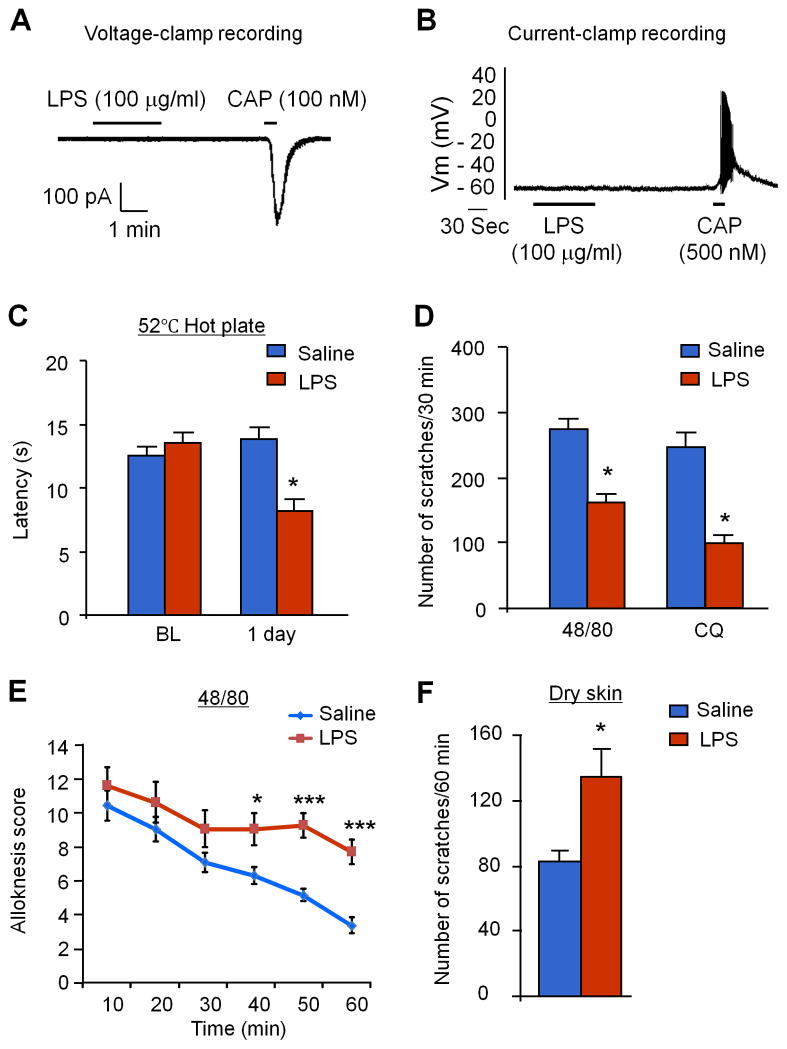
To study the relationship between pain and itch, we further tested whether direct activation of peripheral and spinal TLR4 with LPS would affect pain or itch. Whole cell patch-clamp recordings in dissociated small-sized DRG neurons showed that LPS, even at a very high concentration (100 mg/ml), failed to induce inward currents (Fig. 3A) and action potentials (Fig. 3B). As positive control, capsaicin (100 nM) induced marked inward currents and actions potentials in the same recorded neurons (Fig. 3A,B). Therefore, LPS cannot directly excite nociceptive/pruriceptive neurons in the peripheral nervous system. Consistently, intradermal injection of LPS (10 μg) did not induce scratching behavior in mice (data not shown).
Figure 3. LPS has no direct effects on neuronal excitability in cultured DRG neurons after bath application but enhances acute pain and chronic itch and suppresses acute itch in the back model after intrathecal injection.
(A, B) LPS (100 μg/ml) fails to induce inward currents (A, n=25 neurons) and action potentials (B, n=8 neurons) in dissociated small-sized DRG neurons. Note that capsaicin (100 nM) induces inward currents and action potentials in these neurons. (C) Intrathecal LPS (10 μg) induces heat hyperalgesia 1 day after the injection. BL, baseline. (D) Intrathecal LPS (10 μg) suppresses 48/80 and CQ-induced acute itch. (E) Intrathecal LPS (10 μg) increases compound 48/80-induced alloknesis. (F) Intrathecal LPS (10 μg) potentiates AEW-induced chronic itch. Data are presented as means ± S.E.M. *P< 0.05, **P< 0.01, ***P< 0.001, compared with vehicle group, Student’s t test; n = 6–8 mice per group.
Next, we examined the central effects of LPS on pain and itch. Intrathecal LPS (10 μg) induced heat hyperalgesia, as revealed by a reduction in response latency 24 h after the LPS injection (Fig. 3C). Moreover, intrathecal LPS did not evoke scratching behavior (data not shown). Interestingly, intrathecal LPS suppressed compound 48/80 and CQ-induced acute itch (Fig. 3D), but enhanced compound 48/80-induced alloknesis (Fig. 3E). Further, intrathecal LPS enhanced AEW-induced chronic itch (Fig. 3F). Together, these data suggest that activation of spinal TLR4 can (1) evoke pain but not itch, (2) suppress acute itch, and (3) enhance alloknesis and chronic itch.
3.5. AEW-induced skin pathology is not compromised after Tlr4 deletion
We also examined whether AEW-induced skin pathology would be affected in Tlr4−/− mice. Histological evaluation in the AEW-treated nape skin revealed that the thickness of epidermis and dermis significantly increased in both WT and Tlr4−/− mice following AEW treatment, and no difference in skin thickness was found between genotypes (Fig. S1A,B). The total number of immune cells in the skin (including both epidermis and dermis) was also significantly increased in dry skins of both WT and Tlr4−/− mice (Fig. S1A,B). We also checked the number of mast cells with Toluidine blue staining in control and dry skins and found that the number of mast cells was unaltered following dry skin injury, in agreement of a previous report [42]. Neither did Tlr4−/− mice show changes in mast cells before or after skin injury (Fig. S1A,B). Together, these data suggest that TLR4 plays no major role in skin pathology. However, Tlr4 expression was increased in dry skins 5 days after the AEW treatment (Fig. S1C), indicating that peripheral TLR4 might regulate chronic itch via a different mechanism.
3.6. TLR4 is upregulated and essential for spinal astrocyte activation in chronic itch
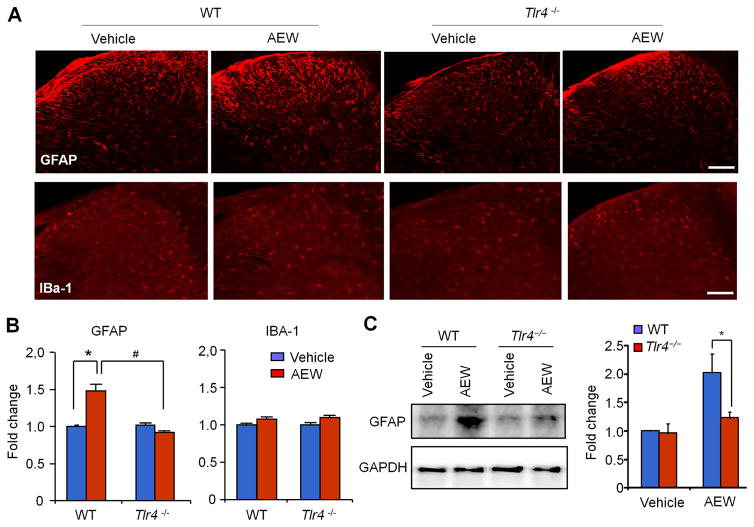
To assess glial changes in chronic itch, we employed immunohistochemistry to examine microglia and astrocyte activation (gliosis, a robust and persistent activation state of glial cells) in the spinal cord dorsal horn in a dry skin condition. GFAP and IBA-1 are two of the most frequently used markers for astrogliosis (GFAP) and microgliosis (IBA-1) in the spinal cord dorsal horn and show dramatic changes in neuropathic pain [25]. AEW treatment in nape for 5 days significantly increased the expression of GFAP but not IBA-1 in the cervical dorsal horn, and moreover, the GFAP upregulation was abolished in Tlr4−/− mice (Fig. 4A,B). Western blot analysis in cervical dorsal horn tissues further confirmed the TLR4-dependent GFAP increase 5 days after the AEW treatment (Fig. 4C). Of interest, basal GFAP expression was not affected in Tlr4−/− mice (Fig. 4A–C). In contrast, compound 48/80 failed to increase GFAP expression in both WT and Tlr4−/− mice (Fig. S2A–C). Furthermore, intrathecal LPS-RS also reduced AEW-induced GFAP expression (Fig. S3A–C). Collectively, these results suggest that chronic itch but not acute itch is associated with TLR4-dependent astrogliosis in spinal cord.
Figure 4. AEW treatment for 5 days in the back model induces astrogliosis but not microgliosis in cervical spinal cord dorsal horn (C3–C4) via TLR4.
(A) Immunohistochemical staining showing the expression of astrogliosis marker GFAP and microgliosis marker IBA-1 following AEW treatment for 5 Days in WT and Tlr4−/− mice. Scale bars, 100 μm. (B) Quantitative analysis of GFAP and IBA-1 immuofluorescence intensity (as fold of WT vehicle control) in spinal cord dorsal horn of WT and Tlr4−/− mice 5 days after AEW treatment. *P<0.05; #P<0.05, n = 5 mice per group. (C) Western blot analysis of GFAP expression in spinal cord dorsal horn of WT and Tlr4−/− mice 5 days after AEW treatment. Right, quantitative analysis of GFAP western band intensity (as fold of WT control, normalized to GAPDH). *P<0.05, Student’s t test; n = 5 mice per group.
To determine whether scratching is essential for astrogliosis in chronic itch, we used Elizabethan collar to protect mice from scratching the AEW-treated cheek skin. Dry skin-induced astrogliosis in the cervical spinal cord (C1–C2) was prevented by mouse wearing of Elizabethan Collar (Fig 5A–C). Thus, scratching could promote chronic itch by inducing astrogliosis.
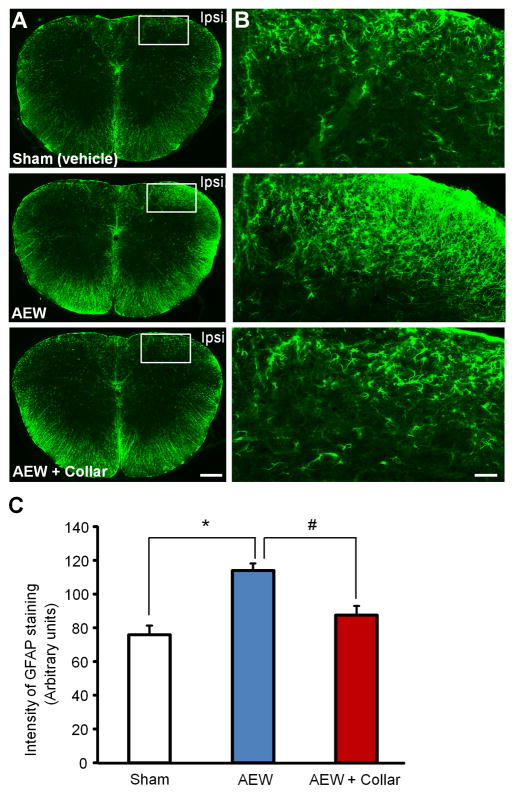
Figure 5. Scratching the dry skin is necessary for spinal cord astrogliosis in the cheek model.
(A) Representative pictures of immunohistochemical staining showing the expression of astrogliosis marker GFAP in C1–C2 cervical spinal cord following AEW treatment for 5 days in cheek skin. Scale, 100 μm. (B) Enlarged images in the boxes of (A). Scale, 50 μm. (C) Quantitative analysis of GFAP immuofluorescence intensity (Arbitrary Units) in spinal cord dorsal horn of CD-1 mice 5 days after AEW treatment. Note that AEW-induced GFAP expression in the C1–C2 cervical spinal cord following AEW treatment was blocked by mouse wearing of an Elizabethan Collar that can prevent mouse from scratching the itchy skin. *P<0.05; #P<0.05, compared with corresponding group; Student’s t test; n = 4 mice per group.
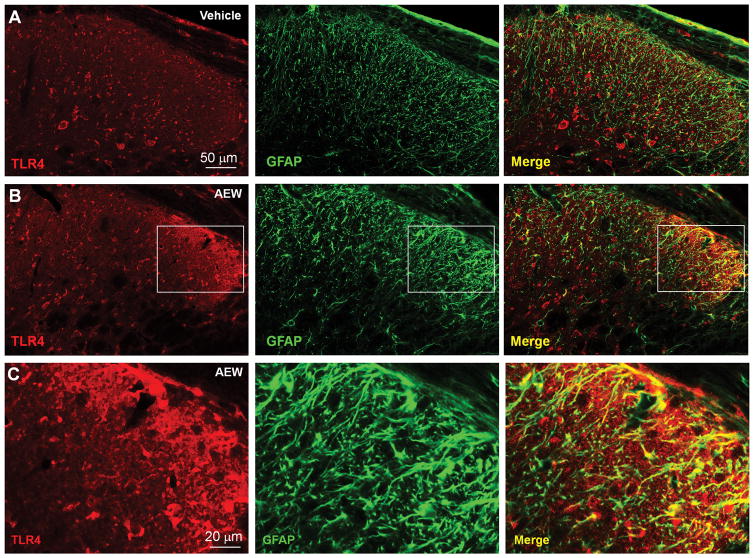
To localize TLR4 expression in the dorsal horn, we conducted double immunostaining in the spinal cord of AEW-treated mice. Fig. 6 shows that both TLR4 and GFAP-immuno-reactivity (IR) were increased in cervical spinal cord (C3–C4) 5 days after AEW treatment, especially in the ipsilateral side of the dorsal horn (Fig. 6A,B). Notably, there was more co-localization of TLR4 and GFAP in the dry skin condition. TLR4 was primarily localized in GFAP-expressing astrocytes after AEW treatment, although TLR4 was also expressed in other cell types (Fig. 6C).
Figure 6. AEW treatment for 5 days in the back model increases TLR4 expression in spinal cord astrocytes.
(A,B) Double immunostaining of TLR4 with GFAP in the spinal cord dorsal horn (C3–C4) of vehicle (A) and AEW (5 d)-treated mice. Note that AEW increases GFAP-IR and TLR4-IR. Also note there is much more GFAP/TLR4 co-localization in chronic itch. Scale bar, 50 μm. (C) High magnification images from the boxes in B. Scale bars, 20 μm.
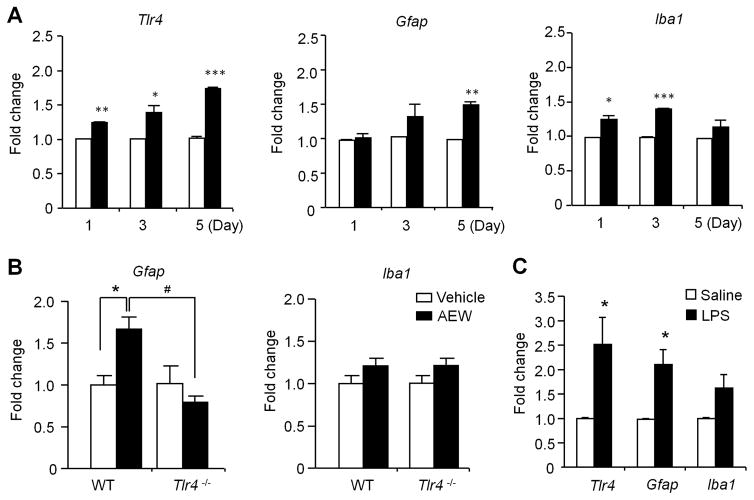
We employed quantitative PCR (qPCR) analysis to examine the time courses of glia-related gene expression in the spinal dorsal horn 1, 3, and 5 days after AEW treatment. AEW induced rapid and persistent Tlr4 mRNA expression at all the time points (Fig. 7A). AEW also induced delayed Gfap expression and rapid but transient Iba1 expression (Fig. 7A). Together, these data further support the involvement of spinal TLR4 and astrocytes in chronic itch. As expected, AEW-evoked Gfap expression was also abrogated after Tlr4 deletion (Fig. 7B).
Figure 7. Quantitative RT-PCR shows the expression of Tlr4 and glia-related genes in cervical spinal cord dorsal horn (C3–C4) after AEW treatment on the back skin or intrathecal LPS injection.
(A) Relative expression levels of Tlr4, Gfap, and Iba1 1, 3, and 5 days after AEW treatment. *P<0.05, **P<0.01, **P<0.001, compared with corresponding vehicle control, n = 4 mice per group. (B) Relative expression levels of Gfap and Iba1 in the dorsal horn of WT and Tlr4−/− mice 5 d after AEW treatment. *P<0.05; #P<0.05, n = 5 mice per group. Note that dry skin induces Tlr4-dependent Gfap expression. (C) Relative expression of Tlr4, Gfap, and Iba1 in cervical spinal cord dorsal horn 24 h after intrathecal LPS (10 μg) injection. Data are presented as means ± S.E.M. *P<0.05, compared with corresponding vehicle control, Student’s t test; n = 4 mice per group.
Next, we tested if TLR4 activation is sufficient to produce spinal glial changes. Intrathecal LPS resulted in significant increases in Tlr4 and Gfap mRNA expression in cervical spinal cord (Fig. 7C). However, the increase in Iba1 expression after LPS treatment was statistically insignificant (P=0.09, Fig. 7C).
3.7. Spinal astrocyte activation contributes to chronic itch and alloknesis not acute itch
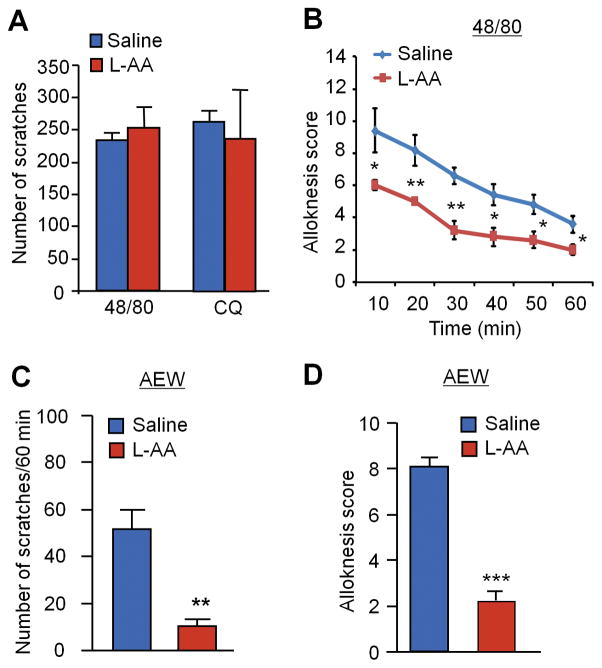
L-alpha aminoadipate (L-AA) is an astroglial toxin and has been shown to inhibit astrogliosis and neuropathic pain [70]. Intrathecal administration of L-AA had no effect on compound 48/80 and CQ-induced acute itch (Fig. 8A). However, intrathecal L-AA effectively inhibited compound 48/80-induced induced alloknesis (Fig. 8B) and AEW-induced chronic itch and alloknesis (Fig. 8C, D). Together, these results support an active role of spinal cord astrocytes in alloknesis and chronic itch.
Figure 8. Intrathecal astroglial inhibitor L-alpha aminoadipate (L-AA, 100 nmol) reduces chronic itch and alloknesis without affecting acute itch.
(A) Acute itch induced by compound 48/80 (48/80, 100 μg) and chloroquine (CQ, 200 μg) is not affected intrathecal L-AA. n = 6 mice per group. (B) 48/80-induced alloknesis is inhibited by intrathecal L-AA. *P<0.05, **P<0.01, compared with saline control, n = 6 mice per group. (C–D) AEW-induced chronic itch (C) and alloknesis (D) is suppressed by intrathecal L-AA. **P<0.01, ***P<0.001, compared with saline control, Student’s t test; n = 6 mice per group. Data are presented as means ± S.E.M.
4. Discussion
4.1. TLR3, TLR4, and TLR7 play distinct roles in itch
In the past decade, great progress has been made in identifying itch-specific receptors and neural circuit [4;18;29;34;36;41;48;57;65]. However, it is still unclear whether acute itch and chronic itch are operated by different mechanisms. In this study we have demonstrated that TLR4 specifically regulates chronic itch but not acute itch. Strikingly, chronic itch was substantially reduced in Tlr4 KO mice in three different models after dry skin, atopic dermatitis, and allergic contact dermatitis. Dry skin-induced chronic itch was also abrogated by intrathecal pharmacological antagonism of TLR4 with LPS-RS.
Our previous work and current study have clearly shown that TLR3, TLR4, and TLR7 are all involved in itch but act through different mechanisms. TLRs are typically expressed by immune cells and glial cells and signal through MyD88 (except TLR3 signaling) [33;44]. Notably, functional TLR3 and TLR7 are present in DRG pruriceptive neurons, and TLR3 agonist poly(I:C) and TLR7 agonist imiquimod not only cause direct activation of pruriceptive neurons but also induce scratching in mice [32;35]. Intriguingly, certain microRNAs such as Let-7b act as endogenous ligands of TLR7 to directly activate primary sensory neurons via TLR7/TRPA1 coupling [45]. Although both TLR3 and TLR7 were implicated in acute itch, Tlr3 deficiency lead to impairment in both histaminergic and non-histaminergic itch whereas Tlr7 deficiency only resulted in reduction in non-histaminergic itch [32;35]. Of interest acute itch (histaminergic or non-histaminergic) was intact in Myd88 KO mice. MyD88 is well known to mediate canonical signaling of TLR7 via gene transcription in immune cells (2). However, Myd88 is not required for non-canonical signaling of TLR7 in neurons. TLR7 may modulate acute itch via activation of TRPA1, since there is functional interaction between TLR7 and TRPA1 in mouse DRG neurons [45]. TLR4 is also expressed by DRG and trigeminal neurons and modulates TRPV1 expression and sensitization [13;38;40]. Unlike TLR3 and TLR7 agonists, TLR4 agonist LPS failed to induce inward currents and action potentials in DRG neurons (Fig. 3A,B) and evoke scratching behavior in mice. However, it was also shown that Tlr4 deficiency lead to a reduction in histamine-induced itch due to reduced TRPV1 expression in primary sensory neurons [40].
4.2. Spinal astrocytes have different roles in acute and chronic itch
Although spinal astrocytes have been strongly implicated inflammatory and neuropathic pain [17;25;59;62], their involvement in acute and chronic itch remains largely unknown. Our results indicated that intrathecal injection of astrocyte inhibitor L-AA did not modulate compound 48/80 and chloroquine induced acute itch but substantially reduced dry skin-induced chronic itch, supporting an involvement of spinal astrocytes in chronic itch. During the submission of this manuscript, Shiratori-Hayashi M et al. reported that signal transducer and activator of transcription 3 (STAT3)-dependent astrogliosis contributes to atopic dermatitis-induced chronic itch in mice [53], which further supports an important role of astrocytes in chronic itch in a different chronic itch condition. Our data revealed that AEW treatment caused persistent TLR4 upregulation in spinal astrocyte, which is consistent with a recent study showing astrocyte induction of TLR4 in rat spinal cord after chemotherapy-induced neuropathy [31]. Our data also showed that chronic itch is associated with delayed astrogliosis (GFAP upregulation) in spinal cord, which was abolished after Tlr4 deletion or intrathecal LPS-RS treatment. On the other hand, spinal TLR4 activation by intrathecal LPS was sufficient to increase Tlr4 and Gfap expression. However, we only saw mild and transient microgliosis (e.g., Iba1 upregulation) in the spinal dorsal horn after AEW treatment. This is consistent with the observations that (1) microgliosis is often associated with deep tissue injury (e.g., joint) or nerve injury and (2) astrogliosis is more persistent than microgliosis in chronic pain states [10;25;56]. However, we should not exclude a role of microglia in chronic itch due to the absence of microgliosis. An active role of spinal microglia in chronic itch deserves further investigation. High mobility group box-1 protein (HMGB1) was regarded as one of the endogenous ligands of TLR4. Spinal HMGB1 induced TLR4-mediated long-lasting pain hypersensitivity and glial activation in experimental arthritis [1]. It will also be of great interest to investigate the possible role of HMGB1 in chronic itch. An interesting observation of this study is that AEW-induced astrogliosis in spinal dorsal horn could be blocked by preventing mouse from scratching using Elizabethan Collar. Thus, scratching-induced sensory input (presumably nociceptive afferent input) is essential for astrogliosis in the dry skin model. Consistently, cutting the nails of mouse can also prevent astrogliosis in atopic dermatitis [53], suggesting that scratching induced noxious afferent input might be critical to drive astrogliosis (reactive changes of astrocytes). These results also suggest that scratching could promote chronic itch by inducing astrogliosis, as an important mechanism underlying itch-scratch-itch cycle.
4.3. Involvement of TLR4 and astrocyte signaling in alloknesis
Alloknesis, i.e. touch-evoked itch in itchy skin, is commonly seen in chronic itch patients [54]. Alloknesis may result from spinal cord modulation (central sensitization), since activation of low-threshold mechanoreceptors excites sensitized itch-signaling neurons in the dorsal horn [5]. We are the first to demonstrate that spinal cord astrocytes play a role in alloknesis. Our data showed that TLR4 and astroglial activation were both required for alloknesis after compound 48/80-induced acute itch and during AEW-induced chronic itch. This study also showed that AEW but not compound 48/80 induced GFAP upregulation, indicating that chronic itch but not acute itch is associated with astrogliosis in spinal cord. However, intrathecal injection of astrocyte inhibitor L-AA still suppressed alloknesis, suggesting that astrocyte activation could also be involved in the development of alloknesis. Thus, we speculated that distinct activation states of astrocytes may differentially regulate acute and chronic itch: astrocyte activation without astrogliosis regulates alloknesis after acute itch, whereas astrogliosis is involved in chronic itch. Further studies are necessary to dissect out signaling pathways that mediate different activation states in astrocytes. Compared to mechanical allodynia, a painful response induced by low-threshold mechanical stimulation, both alloknesis and mechanical allodynia can be operated by central sensitization, which is driven by activation of TLR4 and astrocytes. On the other hand, alloknesis and allodynia are distinct behaviors: mechanical allodynia is a withdrawal response, whereas alloknesis is a scratching response.
4.4. Concluding remarks
Our findings demonstrate that spinal cord TLR4 and astroglial signaling play an important role in chronic itch and allokensis without affecting acute itch. Given a well-known role of TLR4 and astrocyte signaling in chronic pain, our results suggest that chronic pain and chronic itch share similar mechanisms such as central sensitization. While scratching can relieve acute itch via a spinal cord circuit [12], it can also provoke chronic itch, and an itch-scratch-itch cycle can often be seen in chronic itch patients. Notably, scratching-induced astrogliosis can promote chronic itch. Although our data support a central mechanism of TLR4 signaling in itch control, we should not ignore the peripheral role of TLR4 expressed by other cell types in diverse skin diseases, such as mast cells, keratinocytes, and immune cells [14;46]. Despite the fact that scratching is substantially reduced in Tlr4 KO mice, the residue scratching may still be sufficient to cause skin pathology after AEW treatment in KO mice, since we did not put Elizabethan Collar on these mice to prevent them from scratching the itchy skin. Future studies are needed to fully investigate the pathological changes as well as biochemical changes (expression of itch mediators and inflammatory mediators such as cytokines and chemokines) in skins of WT and KO mice with and without Elizabethan Collars. Since TLR4 is upregulated in dry skin, it is possible that TLR4 may regulate the expression of certain cytokines and chemokines to facilitate chronic itch, despite the fact we did not see obvious changes in AEW-induced skin pathology in Tlr4−/− mice. In future study, conditional deletion of Tlr4 in keratinocytes and mast cells will help to address this question.
Chronic itch remains a major health problem in skin diseases such as atopic dermatitis (AD), contact dermatitis, allergic contact dermatitis and xerosis [66]. It also occurs in systemic diseases such as cholestatic liver diseases, kidney diseases (e.g. uraemia), and diabetes [28]. To date, there are still lack of mechanism-based therapies for chronic itch [66] and antihistamines do not work for most chronic itch conditions [61]. Therefore, targeting TLR4 and glial signaling may provide novel anti-itch therapies. Notably, there are established links between Tlr polymorphisms and individual susceptibility to chronic itch diseases in human [43;49], and glial activation has been implicated in human pain conditions [37;51], supporting translational potential of our preclinical findings.
Supplementary Material
Acknowledgments
This study was supported by NIH R01 grants DE17794, DE22743, NS87988, and NS89479 to R.-R.Ji. T. Liu was supported by grants from National Natural Science Foundation of China (31371179 and 81300968) and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Footnotes
Conflict of interest
All the authors have no competing financial interest in this study.
References
- 1.Agalave NM, Larsson M, Abdelmoaty S, Su J, Baharpoor A, Lundback P, Palmblad K, Andersson U, Harris H, Svensson CI. Spinal HMGB1 induces TLR4-mediated long-lasting hypersensitivity and glial activation and regulates pain-like behavior in experimental arthritis. Pain. 2014;155:1802–1813. doi: 10.1016/j.pain.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Akiyama T, Carstens E. Neural processing of itch. Neuroscience. 2013;250:697–714. doi: 10.1016/j.neuroscience.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akiyama T, Carstens MI, Ikoma A, Cevikbas F, Steinhoff M, Carstens E. Mouse model of touch-evoked itch (alloknesis) J Invest Dermatol. 2012;132:1886–1891. doi: 10.1038/jid.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 7.Berta T, Park CK, Xu ZZ, Xie RG, Liu T, Lu N, Liu YC, Ji RR. Extracellular caspase-6 drives murine inflammatory pain via microglial TNF-alpha secretion. J Clin Invest. 2014;124:1173–1186. doi: 10.1172/JCI72230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen G, Park CK, Xie RG, Berta T, Nedergaard M, Ji RR. Connexin-43 induces chemokine release from spinal cord astrocytes to maintain late-phase neuropathic pain in mice. Brain. 2014;137:2193–2209. doi: 10.1093/brain/awu140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christianson CA, Dumlao DS, Stokes JA, Dennis EA, Svensson CI, Corr M, Yaksh TL. Spinal TLR4 mediates the transition to a persistent mechanical hypersensitivity after the resolution of inflammation in serum-transferred arthritis. Pain. 2011;152:2881–2891. doi: 10.1016/j.pain.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J Neuroimmunol. 1997;79:163–175. doi: 10.1016/s0165-5728(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 11.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 12.Davidson S, Zhang X, Khasabov SG, Simone DA, Giesler GJ., Jr Relief of itch by scratching: state-dependent inhibition of primate spinothalamic tract neurons. Nat Neurosci. 2009;12:544–546. doi: 10.1038/nn.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diogenes A, Ferraz CC, Akopian AN, Henry MA, Hargreaves KM. LPS Sensitizes TRPV1 via Activation of TLR4 in Trigeminal Sensory Neurons. J Dent Res. 2011 doi: 10.1177/0022034511400225. [DOI] [PubMed] [Google Scholar]
- 14.Ermertcan AT, Ozturk F, Gunduz K. Toll-like receptors and skin. J Eur Acad Dermatol Venereol. 2011;25:997–1006. doi: 10.1111/j.1468-3083.2011.04049.x. [DOI] [PubMed] [Google Scholar]
- 15.Gao YJ, Zhang L, Ji RR. Spinal injection of TNF-alpha-activated astrocytes produces persistent pain symptom mechanical allodynia by releasing monocyte chemoattractant protein-1. Glia. 2010;58:1871–1880. doi: 10.1002/glia.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gosselin RD, Suter MR, Ji RR, Decosterd I. Glial cells and chronic pain. Neuroscientist. 2010;16:519–531. doi: 10.1177/1073858409360822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014 doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han L, Dong X. Itch mechanisms and circuits. Annu Rev Biophys. 2014;43:331–355. doi: 10.1146/annurev-biophys-051013-022826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutchinson MR, Coats BD, Lewis SS, Zhang Y, Sprunger DB, Rezvani N, Baker EM, Jekich BM, Wieseler JL, Somogyi AA, Martin D, Poole S, Judd CM, Maier SF, Watkins LR. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav Immun. 2008;22:1178–1189. doi: 10.1016/j.bbi.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, Hughes TS, Landgraf KE, Chan S, Fong S, Phipps S, Falke JJ, Leinwand LA, Maier SF, Yin H, Rice KC, Watkins LR. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24:83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 22.Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- 23.Inan S, Dun NJ, Cowan A. Inhibitory effect of lidocaine on pain and itch using formalin-induced nociception and 5′-guanidinonaltrindole-induced scratching models in mice: behavioral and neuroanatomical evidence. Eur J Pharmacol. 2009;616:141–146. doi: 10.1016/j.ejphar.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeffry J, Kim S, Chen ZF. Itch signaling in the nervous system. Physiology (Bethesda ) 2011;26:286–292. doi: 10.1152/physiol.00007.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji RR, Berta T, Nedergaard M. Glia and pain: Is chronic pain a gliopathy? Pain. 2013;154(Suppl 1):S10–28. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13:533–548. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kremer AE, Feramisco J, Reeh PW, Beuers U, Oude Elferink RP. Receptors, cells and circuits involved in pruritus of systemic disorders. Biochim Biophys Acta. 2014;1842:869–892. doi: 10.1016/j.bbadis.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 29.LaMotte RH, Dong X, Ringkamp M. Sensory neurons and circuits mediating itch. Nat Rev Neurosci. 2014;15:19–31. doi: 10.1038/nrn3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JH, Park CK, Chen G, Han Q, Xie RG, Liu T, Ji RR, Lee SY. A monoclonal antibody that targets a NaV1. 7 channel voltage sensor for pain and itch relief. Cell. 2014;157:1393–1404. doi: 10.1016/j.cell.2014.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Li Y, Zhang H, Zhang H, Kosturakis AK, Jawad AB, Dougherty PM. Toll-like receptor 4 signaling contributes to Paclitaxel-induced peripheral neuropathy. J Pain. 2014;15:712–725. doi: 10.1016/j.jpain.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu T, Berta T, Xu ZZ, Park CK, Zhang L, Lu N, Liu Q, Liu Y, Gao YJ, Liu YC, Ma Q, Dong X, Ji RR. TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. J Clin Invest. 2012;122:2195–2207. doi: 10.1172/JCI45414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu T, Gao YJ, Ji RR. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull. 2012;28:131–144. doi: 10.1007/s12264-012-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu T, Ji RR. New insights into the mechanisms of itch: are pain and itch controlled by distinct mechanisms? Pflugers Arch. 2013 doi: 10.1007/s00424-013-1284-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu T, Xu ZZ, Park CK, Berta T, Ji RR. Toll-like receptor 7 mediates pruritus. Nat Neurosci. 2010;13:1460–1462. doi: 10.1038/nn.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, Abdel SO, Zhang L, Duan B, Tong Q, Lopes C, Ji RR, Lowell BB, Ma Q. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron. 2010;68:543–556. doi: 10.1016/j.neuron.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, Hill E, Hsu S, Izquierdo-Garcia D, Ji RR, Riley M, Wasan AD, Zurcher NR, Albrecht DS, Vangel MG, Rosen BR, Napadow V, Hooker JM. Evidence for brain glial activation in chronic pain patients. Brain. 2015;138:604–615. doi: 10.1093/brain/awu377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meseguer V, Alpizar YA, Luis E, Tajada S, Denlinger B, Fajardo O, Manenschijn JA, Fernandez-Pena C, Talavera A, Kichko T, Navia B, Sanchez A, Senaris R, Reeh P, Perez-Garcia MT, Lopez-Lopez JR, Voets T, Belmonte C, Talavera K, Viana F. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat Commun. 2014;5:3125. doi: 10.1038/ncomms4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Min H, Lee H, Lim H, Jang YH, Chung SJ, Lee CJ, Lee SJ. TLR4 enhances histamine-mediated pruritus by potentiating TRPV1 activity. Mol Brain. 2014;7:59. doi: 10.1186/s13041-014-0059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science. 2013;340:968–971. doi: 10.1126/science.1233765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyamoto T, Nojima H, Shinkado T, Nakahashi T, Kuraishi Y. Itch-associated response induced by experimental dry skin in mice. Jpn J Pharmacol. 2002;88:285–292. doi: 10.1254/jjp.88.285. [DOI] [PubMed] [Google Scholar]
- 43.Mrabet-Dahbi S, Dalpke AH, Niebuhr M, Frey M, Draing C, Brand S, Heeg K, Werfel T, Renz H. The Toll-like receptor 2 R753Q mutation modifies cytokine production and Toll-like receptor expression in atopic dermatitis. J Allergy Clin Immunol. 2008;121:1013–1019. doi: 10.1016/j.jaci.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 44.Nicotra L, Loram LC, Watkins LR, Hutchinson MR. Toll-like receptors in chronic pain. Exp Neurol. 2011 doi: 10.1016/j.expneurol.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park CK, Xu ZZ, Berta T, Han Q, Chen G, Liu XJ, Ji RR. Extracellular MicroRNAs Activate Nociceptor Neurons to Elicit Pain via TLR7 and TRPA1. Neuron. 2014;82:47–54. doi: 10.1016/j.neuron.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paus R, Schmelz M, Biro T, Steinhoff M. Frontiers in pruritus research: scratching the brain for more effective itch therapy. J Clin Invest. 2006;116:1174–1186. doi: 10.1172/JCI28553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ross SE. Pain and itch: insights into the neural circuits of aversive somatosensation in health and disease. Curr Opin Neurobiol. 2011 doi: 10.1016/j.conb.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 48.Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, Tolias C, Corfas R, Chen S, Inquimbert P, Xu Y, McInnes RR, Rice FL, Corfas G, Ma Q, Woolf CJ, Greenberg ME. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–898. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salpietro C, Rigoli L, Miraglia Del GM, Cuppari C, Di BC, Salpietro A, Maiello N, La RM, Marseglia GL, Leonardi S, Briuglia S, Ciprandi G. TLR2 and TLR4 gene polymorphisms and atopic dermatitis in Italian children: a multicenter study. Int J Immunopathol Pharmacol. 2011;24:33–40. doi: 10.1177/03946320110240S408. [DOI] [PubMed] [Google Scholar]
- 50.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 51.Shi Y, Gelman BB, Lisinicchia JG, Tang SJ. Chronic-pain-associated astrocytic reaction in the spinal cord dorsal horn of human immunodeficiency virus-infected patients. J Neurosci. 2012;32:10833–10840. doi: 10.1523/JNEUROSCI.5628-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain. 2008;139:681–687. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shiratori-Hayashi M, Koga K, Tozaki-Saitoh H, Kohro Y, Toyonaga H, Yamaguchi C, Hasegawa A, Nakahara T, Hachisuka J, Akira S, Okano H, Furue M, Inoue K, Tsuda M. STAT3-dependent reactive astrogliosis in the spinal dorsal horn underlies chronic itch. Nat Med. 2015;21:927–931. doi: 10.1038/nm.3912. [DOI] [PubMed] [Google Scholar]
- 54.Simone DA, Alreja M, LaMotte RH. Psychophysical studies of the itch sensation and itchy skin (“alloknesis”) produced by intracutaneous injection of histamine. Somatosens Mot Res. 1991;8:271–279. doi: 10.3109/08990229109144750. [DOI] [PubMed] [Google Scholar]
- 55.Sorge RE, LaCroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin JS, Ritchie J, Chanda ML, Graham AC, Topham L, Beggs S, Salter MW, Mogil JS. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci. 2011;31:15450–15454. doi: 10.1523/JNEUROSCI.3859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun S, Cao H, Han M, Li TT, Pan HL, Zhao ZQ, Zhang YQ. New evidence for the involvement of spinal fractalkine receptor in pain facilitation and spinal glial activation in rat model of monoarthritis. Pain. 2007;129:64–75. doi: 10.1016/j.pain.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 57.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 58.Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Svensson CI, Brodin E. Spinal astrocytes in pain processing: non-neuronal cells as therapeutic targets. Mol Interv. 2010;10:25–38. doi: 10.1124/mi.10.1.6. [DOI] [PubMed] [Google Scholar]
- 60.Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. 2005 doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tey HL, Yosipovitch G. Targeted treatment of pruritus: a look into the future. Br J Dermatol. 2011;165:5–17. doi: 10.1111/j.1365-2133.2011.10217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 63.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 64.van der Steen P, van de Kerkhof P, der KD, van VI, Happle R. Clinical and immunohistochemical responses of plantar warts to topical immunotherapy with diphenylcyclopropenone. J Dermatol. 1991;18:330–333. doi: 10.1111/j.1346-8138.1991.tb03093.x. [DOI] [PubMed] [Google Scholar]
- 65.Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, Pellegrino M, Estandian DM, Bautista DM. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155:285–295. doi: 10.1016/j.cell.2013.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med. 2013;368:1625–1634. doi: 10.1056/NEJMcp1208814. [DOI] [PubMed] [Google Scholar]
- 67.Yosipovitch G, Carstens E, McGlone F. Chronic itch and chronic pain: Analogous mechanisms. Pain. 2007;131:4–7. doi: 10.1016/j.pain.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 68.Yu J, Fang Q, Lou GD, Shou WT, Yue JX, Tang YY, Hou WW, Xu TL, Ohtsu H, Zhang SH, Chen Z. Histamine modulation of acute nociception involves regulation of Nav 1. 8 in primary afferent neurons in mice. CNS Neurosci Ther. 2013;19:649–658. doi: 10.1111/cns.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu J, Lou GD, Yue JX, Tang YY, Hou WW, Shou WT, Ohtsu H, Zhang SH, Chen Z. Effects of histamine on spontaneous neuropathic pain induced by peripheral axotomy. Neurosci Bull. 2013;29:261–269. doi: 10.1007/s12264-013-1316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR, Decosterd I, Ji RR. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci. 2006;26:3551–3560. doi: 10.1523/JNEUROSCI.5290-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.