INTRODUCTION
Vancomycin, a tricyclic glycopeptide,1 is one of the oldest and most effective antibiotics used to treat gram positive aerobic species. Vancomycin is the optimal parenteral treatment for many infections, including septicemia, pneumonia, cellulitis, endocarditis, and meningitis caused by methicillin-resistant Staphylococcus aureus. Orally, vancomycin is first-line treatment for the growing healthcare associated infection, Clostridium difficile pseudomembranous colitis.2 Vancomycin is also commonly used for treating infections in hospitalized patients and surgical patients with prior hypersensitivity to penicillins and/or cephalosporins.3
Clinicians are largely familiar with the adverse drug reactions (ADRs) that occur with vancomycin use, including nephrotoxicity, ototoxicity, and hematologic toxicity.4 These ADRs are most pronounced among patients receiving extended courses of parenteral vancomycin therapy as outpatients, where the observed frequency of ADRs approaches 10% .5 Vancomycin is also known for causing red man syndrome an immediate reaction that is IgE-independent, or pseudoallergic, in nature. Red man syndrome can affect between 4 and 47% of patients treated with vancomycin,6 and symptoms range from erythema to cardiovascular compromise/shock.
Vancomycin also has the potential to cause immune-mediated reactions, or hypersensitivity reactions (HSRs), which may be less frequently recognized. Vancomycin HSRs include immediate, type I HSR (immunoglobulin [Ig]E)-mediated); organ specific reactions such as acute interstitial nephritis (AIN), typically a Type II HSR (antibody dependent); and other non-immediate HSRs, commonly type IV HSRs (cell-mediated, delayed-type). These HSRs include maculopapular rash, drug rash eosinophilia and systemic symptoms (DRESS) syndrome, linear IgA bullous dermatosis (LABD), and Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN).
While syntheses of vancomycin ADRs have been reported,4 to our knowledge, no formal review of immune-mediated HSRs to vancomycin is available. Therefore, we aimed to identify the most commonly reported vancomycin HSRs through systematic case review.
METHODS
We performed a systematic case report and case series review, with a protocol that adhered closely to the Preferred Reporting Items for Systematic Review and Meta-Analyses statement.7 The literature search was performed during January, 2015 and included Ovid MEDLINE (1982-present), PubMed (1982- present), the Cochrane Library (1982-present). The base of the search used the medical subject heading vancomycin with a subheading limiter of “adverse effects.” Additionally, text word searches for vancomycin were matched against a list of HSR key words: hypersensitivity, allergic, urticaria, hives, rash, drug induced hypersensitivity syndrome, DIHS drug rash eosinophilia and systemic symptoms, eosinophilia, , bullous, dermatosis, IgA, IgE, anaphylaxis, nephritis, acute interstitial nephritis, and AIN.8 We identified additional articles by reviewing references of included manuscripts. The last date searched was July 31, 2015.
Inclusion criteria and exclusion criteria were specified in advance. We included case reports and case series of vancomycin HSRs in English, or original work that was translated into English, from 1982 until present. In order to synthesize clinical data, only publications with available full texts were included. Each case was identified and screened by one physician (JM). Inclusion of only convincing HSR reports was determined by a board-certified allergist/immunologist (KGB), with final case inclusion approved by all board-certified allergist co-investigators (KGB, PGW, AAL, AB). Final inclusion necessitated a clear presentation of a patient with signs and symptoms of the HSR with appropriate causal logic in attribution of HSR to vancomycin. We excluded cases of ADRs that were possibly toxicities (e.g., cytopenias) and cases reporting immediate symptoms that were more likely red man syndrome than IgE. To distinguish from red man syndrome, and to be included in possible IgE-mediated HSR, cases of immediate reactions needed to have at least one of the following: (1) positive skin testing using a non-irritating concentration,9,10 (2) immediate symptoms consistent with anaphylaxis11 despite a red man syndrome protocol (slowed infusion and antihistamine premedication),12,13,14 or (3) symptoms consistent with IgE-mediated reaction during a vancomycin desensitization15,16,17.
Clinical data were collected from each case, and included patient characteristics, infection being treated, and reaction details including timing of HSR onset, available subjective and objective clinical data confirming the HSR, and subsequent treatment and course. For each HSR, we performed summary descriptive statistics, including frequenciesand medians with interquartile ranges.
RESULTS
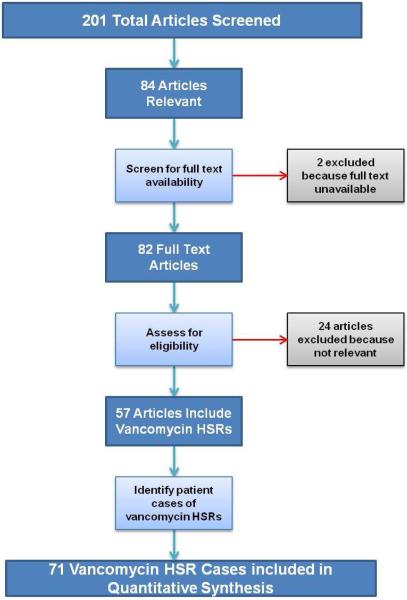
The search identified 201 possible publications, of which 84 were relevant to our topic and screened (Figure 1). Of these, 82 full-text articles were available of which 57 demonstrated vancomycin HSRs and met inclusion quality standards for qualitative and quantitative analysis. The 57 articles included 71 cases of patients with a vancomycin HSR (Supplemental Table 1).
Figure 1.
Flow chart of methodology for studies chosen for the review
Legend. Of 201 identified publications; 84 were screened and 58 met inclusion criteria. The 58 articles included 71 HSR cases.
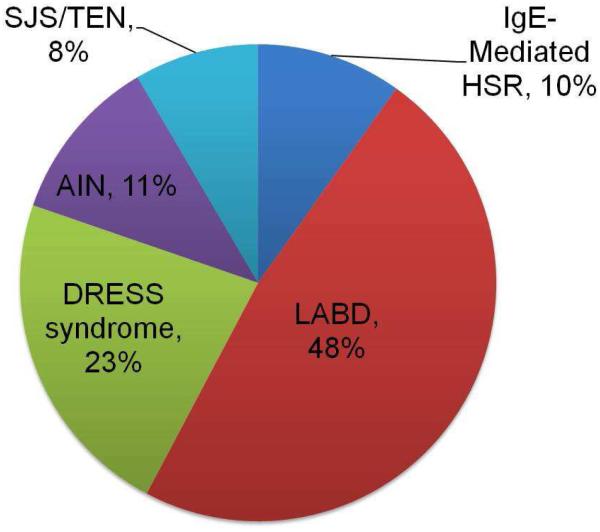
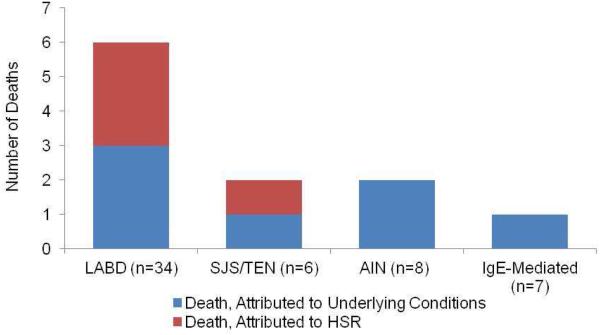
Overall, patients had a median age 60 years [IQR 46 years, 71 years] and 40 (56%) male sex. HSRs were immediate (IgE/anaphylaxis, n=7) and non-immediate (n=64). Non-immediate HSRs identified were LABD ( n=34), DRESS syndrome (n=16), AIN (n=8), and SJS/TEN (n=6, Figure 2). HSR timing varied by HSR, with median latency of 7 days [IQR 4 days, 10 days] for LABD, 9 days [IQR 9 days, 22 days] for SJS/TEN, 21 days [IQR 17 days, 28 days] for DRESS syndrome and 26 days [IQR 7 days, 29 days] for AIN (Figure 3a). Overall, 11 (16%) of patients died, with 4 (6%) dying from HSR complications (Figure 3b).
Figure 2.
Cases of Immune-Mediated Hypersensitivity Reactions to Vancomycin
Legend. HSRs were immediate (n=7) and non-immediate (n=64). Non-immediate hypersensitivity reactions included LABD ( n=34), DRESS syndrome (n=16), acute interstitial nephritis (n=8), and SJS/ TEN (n=6).
Figure 3.
Immune-mediated Hypersensitivity Reactions to Vancomycin. (a) Timing of Hypersensitivity Reactions (b) Observed fatalities by Hypersensitivity Reaction
Legend: (A) Median time to onset of hypersensitivity reactions varied by type. (B) Overall 11 (16%) of patients with vancomycin HSRs died, with 4(6%) of deaths attributed to HSR.
We identified seven cases of presumed IgE-mediated HSR to vancomycin (Table 1). Patients had median age of 43 years [IQR 39 years, 46 years] and females represented a majority (57%). Each patient exhibited immediate symptoms meeting definition of anaphylaxis. While skin findings were commonly present (n=5, 71%), 5 patients (71%) had respiratory symptoms and 4 patients (57%) had hypotension. In five patients, there was documentation of prior exposure to vancomycin. Patients had onset of reaction a median of two minutes [IQR 1 minute, 5 minutes] into the dose. Treatment included steroids and antihistamines, with epinephrine used in 4 patients (5 7%). Two of the reported cases required intubation, with one fatality, attributed to bilateral pneumonia and overwhelming sepsis.18,19 After experiencing a HSR, 4 (57%) of patients underwent skin testing or a re-challenge. One patient had a positive skin test, that was defined by a wheal diameter of 3mm larger than the negative control to vancomycin (wheal 10 mm / flare 35 mm) using 50 mg/ml of vancomycin solution.10 One patient had symptoms despite a red man syndrome protocol20.21 Two patients were desensitized to vancomycin with breakthrough symptoms. 19,22
Table 1.
Clincal data for cases of IgE-mediated reactions to vancomycin (n=7)
| Age (yrs) |
Gender (M/F) |
Number of vancomycin doses previously |
Infection treated |
Reaction timing* (minutes) |
Symptoms | Treatment | Outcome | Skin- testing or re- challenge |
Justification for IgE |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Otani et al. 2015 |
60 | F | NA |
Enterococcus
faecalis |
8m | Dyspnea Hypotension Skin erythema Flushing |
Steroids Antihistamines Epinephrine |
Improved | Yes | Skin testing positive |
| Bosse et al. 2013 |
35 | M | 1 |
Clostridium
difficile colitis |
35m | Dyspnea Throat tightness, laryngeal edema, Facial erythema |
Steroids IVF Antihistamines Epinephrine Switched therapy |
Improved | No | Convincing clinical diagnosis |
| Kupsta ie et al. 2010 |
23 | M | 1 | Polymicrobia l bactermia including Enterococcus faecalis and MRSA |
1m | Dyspnea Hypotension (80/60) Tachycardia Facial flushing, cold sweat, tremor |
Steroids IVF Antihistamines Switched therapy |
Improved, negative repeat culture |
No | Convincing clinical diagnosis |
| Kitaza wa et al. 2005 |
43 | M | 1 |
MRSA
abscess |
1m | Wheezing Flushing |
Antihistamines | Improved | Yes | Breakthrough symptoms despite premedication and desensitization |
| Hassab alla et al. 2000 |
45 | F | NA |
Enterococcus
wound infection |
1m | Cardiac arrest Hypotension Flushing Tongue swelling Emesis, Diaphoresis |
Intubated Epinephrine Steroids Antihistamines Desensitized to vancomycin |
Improved | No | Convincing clinical diagnosis |
| Chopra et al. 2000 |
46 | F | 1 |
MRSA
dialysis catheter infection |
2m | Respiratory distress, Wheezing, Cyanosis, Pruritus, Erythema, |
Steroids Antihistamines |
Deceased, from sepsis |
Yes | Severe reaction despite red man syndrome prophylactic administration |
| Vilavic encio et al. 1998 |
43 | F | 1 |
MRSA wound infection |
2m | Hypotension, Facial swelling, Lip Swelling |
CPR Intubation Epinephrine |
Improved | Yes | Severe reaction with breakthrough symptoms despite premedication and desensitization |
Timing from vancomycin dose to reaction onset
Abbreviations: NA: not available; IVF: intravenous fluids; MRSA: methicillin-resistant Staphylococcus aureus; CPR: cardiopulmonary resuscitation
Thirty-four cases of LABD from vancomycin wereidentified(Table 2). Patients median age was 70 years [IQR 61 years, 76 years)] and men represented over half (56%) of cases. Patients reported with LABD developed the rash a median of 7 days [IQR 4 days, 10 days] into vancomycin course. Thirty-two patients (94%) had bullous eruptions. Two patients (6%) had macules and papules only. The rash among cases of LABD included skin sloughing (n=5, 15%), mucosal involvement (n=7, 21%), urticaria (n=2, 6%), vesicles (n=6, 18%), target lesions (n=3, 9%), and involvement of palms and soles (n=5, 15%) All patients had biopsies with characteristic subepidermal bulla with neutrophilic abscesses in the papillary dermis with direct immunofluorescence confirming a linear pattern of IgA deposition along the basement membrane zone. Treatment included topical and/or oral steroids. Three patients (9%) were treated with dapsoneThirty patients (88%) improved. The median time to resolution among 17 cases was 14 days [IQR 14 days, 21 days]. Sixpatients 18%) died, with 3 deaths directly related to LABD.
Table 2.
Clinical data for cases of Linear IgA bullous dermatosis to vancomycin (LABD, n=34). All patients had characteristic skin biopsy findings and direct immunoflourescence with linear IgA deposition along the basement membrane/dermal-epidermal junction.
| Age (yrs) |
Sex (M/ F) |
Infection treated |
Reactio n timing |
Skin Sloughing |
Mucosal Involvement |
Other rash atypical feature |
Treatment | Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| Kakar et al. 2013 |
91 | F | Acute cholecystitis Sepsis |
2d | 40% BSA | Oral | Vesicles | Comfort measures only |
Deceased, from HSR |
| Selvaraj et al. 2013 |
70 | F | Post- operative sepsis |
5d | Oral | Vesicles Palms affected |
Discontinuation of vancomycin |
Full resolution within 14d |
|
| Jawitz et al. 2011 |
78 | F | HAP post-op | 28d | Topical steroids | Full resolution in 10d |
|||
| O’Brien et al. 2011 |
45 | M |
Clostridium
dificile colitis |
2d | Discontinuation of vancomycin Camphorylated moisturizer |
Full resolution | |||
| McDonal d et al. 2010 |
32 | M | VAP | 10d | Discontinuation of vancomycin |
Full resolution | |||
| Walshe et al. 2009 |
76 | M |
Staphylococ
cus bacteremia |
9d | Oral steroids Discontinuation of vancomycin |
Full resolution | |||
| Khan et al. 2008 |
57 | M | Liver and splenic abscesses Laparotomy |
35d | 75% BSA | Palms and Soles affected |
Topical steroids HD IVIG |
Deceased, from HSR |
|
| Senanaya ke et al. 2008 |
68 | M | Post-op wound infection |
4d | Oral | Palms affected |
Oral steroids Discontinuation of vancomycin |
Full resolution | |
| Billet et al. 2008 |
70 | M | Post-op perihepatic abscess, MRSA, Enterococcu s |
5d | No bullae | Oral steroids Dapsone |
Clinically improved within 3d |
||
| Billet et al. 2008 |
61 | F | Post-op wound abdominal infection |
13d | No bullae | Topical steroids Discontinuation of vancomycin |
Clinically improved in 6d |
||
| Navi et al. 2006 |
73 | M | ICD placement |
3d | Vesicles | Discontinuation of vancomycin |
Full resolution in 2 weeks |
||
| Waldman et al. 2004 |
77 | M | CABG, complicated post-op course |
6d | 46% BSA | Silvadene dressing changes q12h Dapsone |
Complete re- epithelialization within 3 weeks Deceased, from cardiac complications |
||
| Joshi et al. 2004 |
48 | F | TVH c/b pelvic abscess |
10d | Discontinuation of vancomycin |
Full resolution | |||
| Armstron g et al. 2004 |
81 | M | AAA repair c/b wound infection |
3d | Target lesions |
Discontinuation of vancomycin |
Full resolution in 3 weeks |
||
| Dellavalle et al. 2003 |
74 | M | Pneumonia | 4d | 90% BSA | Oral | Discontinuation of vancomycin |
Deceased, from septic shock |
|
| Neughbau er et al. 2002 |
52 | F |
Escherichia
coli urosepsis |
<1d | Vesicles | Discontinuation of vancomycin |
Full resolution in 2 weeks |
||
| Palmer et al. 2001 |
75 | F | Infected varicose ulcer |
6d | Urticaria | Oral steroids Dapsone |
Full resolution | ||
| Palmer et al. 2001 |
86 | F | Fracture of femur s/p repair |
4d | “Diffuse” | Oral steroids | Deceased, from pneumonia and complications of HSR |
||
| Palmer et al. 2001 |
78 | F | CABG c/b MRSA wound infection |
15d | Urticaria | Topical steroids Discontinuation of vancomycin |
Resolution of rash Deceased, from renal failure |
||
| Klein et al. 2000 |
65 | M | Sepsis due to Klebsiella pneumoniae, P.aeruginos a, Staphylococ cus species |
14d | Discontinuation of vancomycin |
Full resolution in 4 weeks |
|||
| Mofid et al. 2000 |
87 | F | Urinary tract infection |
11d | Oral | IV steroids Discontinuation of vancomycin |
Full resolution | ||
| Danielsen et al. 1999 |
68 | M | Culture negative endocarditis |
9d | Vesicles | Topical steroids Discontinuation of vancomycin |
Full resolution within 10d |
||
| Bernstein et al. 1998 |
60 | F | Enterocutan eous fistula |
10d | Oral steroids Discontinuation of vancomycin |
Full resolution | |||
| Nousari et al. 1998 |
65 | F |
P.aeruginos
a S.epidermidi s sepsis |
7d | Discontinuation of vancomycin |
Full resolution within 30 days |
|||
| Whitwort h et al. 1996 |
63 | M | Cardiac catherization |
1d | Oral steroids Discontinuation of vancomycin |
Full resolution within 3 weeks |
|||
| Richard et al. 1995 |
72 | F | Total pelvic ex- enteration for TCC of the bladder |
2d | Oral and genital |
Target lesions Papules |
Discontinuation of vancomycin |
Full resolution of eruptions over 2 weeks |
|
| Geismann et al. 1995 |
79 | M |
S. aureus
cellulitis |
8d | Oral and genital |
Discontinuation of vancomycin |
Full resolution | ||
| Kuechle et al. 1994 |
69 | M | Draining sinus tract status-post CABG |
14d | Discontinuation of vancomycin |
Full resolution within 3 weeks |
|||
| Kuechle et al. 1994 |
74 | F | Sternal wound infection status-post CABG |
5d | Target lesions Palms affected |
Discontinuation of vancomycin |
Full resolution | ||
| Kuechle et al. 1994 |
67 | M | Sternal wound infection status-post CABG |
1d | Vesicles | Discontinuation of vancomycin |
Full resolution | ||
| Carpenter et al. 1992 |
54 | M | Bowel perforation |
10d | Discontinuation of vancomycin |
Full resolution within 9 months |
|||
| Carpenter et al. 1992 |
72 | F | Intra- abdominal abscess |
7d | Palms and soles affected |
Discontinuation of vancomycin |
Full resolution within 2 weeks |
||
| Carpenter et al. |
54 | M | Osteomyeliti s |
21d | Vancomycin continued for an additional 3 weeks without worsening |
Full resolution | |||
| Baden et al. 1988 |
68 | M |
E.coli
urosepsis Post-op (CABG) |
9d | Discontinuation of vancomycin Twice daily compresses Bacitracin |
Resolution within 2 weeks |
* Timing from vancomycin dose to reaction onset
Abbreviations: d: days; BSA: body surface area; HSR: hypersensitivity reaction; HAP: hospital acquired pneumonia; VAP: ventilator associated pneumonia; HD: hemodialysis; IVIG: intravenous immunoglobulins; MRSA: methicillin-resistant Staphylococcus aureus; ICD; implantable cardioverter defibrillator; CABG: coronary artery bypass graft; h: hours; TVH: total vaginal hysterectomy; c/b: complicated by; AAA: abdominal aortic aneurysm; s/p: status-post; IV: intravenous; TCC: transitional cell carcinoma
We identified 16 cases of DRESS syndrome from vancomycin (Table 3). Median age was 52 years [IQR 47 years, 61 years] and men represented a majority (56%). HSR occurred a median of 21 days [IQR 17 days, 28 days] into the patient’s treatment course. Symptoms included edema (63%), lymphadenopathy (19%), and fever (81%). Cases with liver involvement (n=13, 81%), reported median peak alanine aminotransferase of 163 mg/dL [113 mg/dL, 337 mg/dL) and aspartate aminotransferase of 157 mg/mL [IQR 91 mg/mL, 313 mg/mL]. Of nine (56%) cases with renal involvement, eight reported peak Creatinine, with a median of 2.2 mg/dL [IQR 0.7 mg/dL, 4.2 mg/dL). The median absolute eosinophil count (AEC) among 16 cases was 3,180/mL [IQR 1,883/mL, 6,001/mL]. Four cases commented on atypical lymphocytes with three of them reporting they were present and one reported that they were absent. HHV6 reactivation was tested for in five cases, and elevated in only one case (1:320, Tamagawa).23 Registry of Severe Cutaneous Adverse Reactions (regiSCAR) scoring methods were included in only four (25%) of reported cases. Thirteen patients (81%) were treated with steroids (intravenous and/or oral). Other agents used for treatment included cyclosporine (n=1)24 and IVIG (n=1).25 All patients experienced complete resolution of the HSR; among five cases reporting time to resolution, there was a median time to resolution of 7 days [IQR 5 days, 60 days]
Table 3.
Clinical data for cases of Drug Rash Eosinophilia and Systemic Symptoms (DRESS) syndrome to vancomycin (n=16)
| Age (yrs) |
Gender (m/f) |
Infection treated | Reaction timing* |
Clinical signs and symptoms |
Laboratory findings |
Treatment | Outcome | |
|---|---|---|---|---|---|---|---|---|
| Young et al. 2014 |
24 | M |
Corynebacterium
jeikeium septic arthritis |
21d | MP rash, arthralgia, lymphadenopathy, fever |
AEC: 2,900/L AST M/ALT 270mg/dL No nephritis RegiSCAR score 7 |
IV steroids with a prednisone taper |
Clinically improved with resolution of symptoms |
| Young et al 2014 |
48 | F | L5/S1 osteomyelitis |
14d | MP rash, facial edema, odynophagia, fever, chills |
AEC: 2,200/L AST M/ALT 337mg/dL No nephritis RegiSCAR score 6 |
IV steroids with prolonged prednisone taper |
Clinically improved within 5 days |
| Young et al. 2014 |
59 | F | MRSA wound infection |
21d | MP rash, fever and facial edema |
AEC: 10,400/L AST M/ALT 113mg/dL No nephritis RegiSCAR score 6 |
Oral and topical steroids Antihistamines |
Clinically improved |
| Della-Torre et al. 2013 |
75 | M | Culture negative endocarditis |
27d | MP rash, fever | AEC: 0.6 × 109 AST 45/ALT 264mg/dL Cr. (bl M, max 1.31mg/dL) RegiSCAR score > 7 |
IV steroids Antihistamines IVIG |
Clinically improved, labs normalized |
| Blumenthal et al. 2013 |
65 | M | β-hemolytic Streptococcus group B empyema |
12d | MP rash | AEC: 3460/L AST 440mg/dL/AL T 105mg/dL Cr. (bl 0.5, max 2.1mg/dL) Atypical lymphocytes: none |
IV Steroids | Clinical improvement within 48h DRESS resolved after 2 months |
| Blumenthal et al. 2013 |
40 | M |
Propionibacterium
and Peptostreptococcus prosthetic joint infection |
28d | MP rash, fever, cervical lymphadenopathy, splenomegaly, pitting edema |
AEC:3,890/L AST 178mg/dL/AL T 122mg/dL Cr (bl 0.8mg/dL,max 2.2mg/dL) HHV6 IgG <1:20 |
IV steroids, followed by 6 month oral taper |
Clinical improvement within 2 days |
| Blumenthal et al. 2013 |
48 | F | Coagulase negative S.aureus prosthetic joint infection |
28d | MP rash, fever | AEC:1,900/L AST 85mg/dL/ALT 137mg/dL No nephritis HHV6 <1:20 |
No steroids used in management Antihistamines |
Gradual clinical improvement with supportive care |
| Blumenthal et al. 2013 |
74 | M | Gram positive cocci cellulitis after traumatic hand injury |
21d | MP rash, fever, facial and peripheral edema, hypotension, tachycardia |
AEC: 6,550/L AST 75mg/dL/ALT 170mg/dL Cr (bl 1.4 mg/dL,max 2.3mg/dL) HHV6 <1:20 |
No steroids used in management IVF Switched therapy |
Clinically improved |
| Blumenthal et al. 2013 |
51 | M | Osteomyelitis | 21d | MP rash, periorbital edema, chest tightness, nausea, fever, chills, lightheadedness |
AEC: 1,620/L AST 107 mg/dL/ALT 347mg/dL No nephritis HHV6 DNA <600 |
IV steroids Switched therapy |
Clinically improved |
| Dauby et al. 2012 |
54 | F | Methicillin- resistant Staphylococcus epidermis catheter associated bacteremia (febrile neutropenia in setting of chemotherapy for breast cancer) |
7d | MP rash, fever, chills |
AEC: 6,380/L AST 31mg/dL /ALT 45 mg/dL No nephritis |
Topical steroids Antihistamines Antipyretic Switched therapy |
Clinically improved |
| O’Meara et al. 2011 |
66 | M | ORIF c/b MRSA | 28d | MP rash, fever, facial edema, lymphadenopathy |
AEC: 3,620/L AST 163mg/dL /ALT 144mg/dL Cr (bl 1.3mg/dL,max 4.9mg/dL) |
IV steroids | Clinically improved after prolonged treatment course |
| Boet et al. 2009 |
38 | F |
Streptococcus
oralis endocarditis |
30d | MP rash, fever, facial edema |
AEC: 2820/L No nephritis |
IV steroids | Clinically improved, discharged within few weeks |
| Vauthey et al. 2008 |
60 | F | MRSA cellulitis after amputation |
18d | MP rash, fever, periorbital edema |
AEC: 1,251/L Cr Cl 30mL/min |
IV steroids Topical steroids Antihistamines |
Gradually improved, discharged after 2 months |
| Tamagawa- Mineoka et al. 2007 |
52 | M | Cholesteatoma s/p tymanoplasty (MRSA infection from ear wound) |
4d | MP rash, fever, facial edema |
AEC: 1,832/L AST 358 mg/dL/ALT 547mg/dL Cr (bl NA, max 3.58 mg/dL) HHV6 DNA 1:320 |
IV steroids followed by prednisone taper |
Clinically resolved |
| Zuliani et al. 2005 |
45 | F | Coagulase negative endocarditis |
18d | MP rash, fever, facial edema |
AEC: 1,474/L AST 385 mg/dL/ALT 599 mg/dL Cr (bl 0.8 mg/dL,max 5.3 mg/dL) |
IV steroids with prednisone taper Antihistamines Hemodialysis Cyclosporine |
After two cutaneous relapses, Clinically improved |
| Marik et al. 1997 |
51 | M | Culture negative endocarditis |
30d | MP rash, palpitations, malaise, dyspnea and rigors |
AEC: 5,875/L Cr (bl 1.0 mg/dL, max 7.8 mg/dL) |
IV steroids | Clinically improved in 1 week. Hospital course c/b urosepsis |
Timing from vancomycin dose to reaction onset
Abbreviations: d: days; MP: maculopapular; AEC: absolute neutrophil count; M: missing; RegiSCAR: registry of severe cutaneous adverse reactions; IV: intravenous; MRSA: methicillin-resistant Staphylococcus aureus; Cr: creatinine; bl: baseline; IVIG: intravenous immunoglobins; DRESS: drug rash eosinophilia with systemic symptoms; HHV6: human herpesvirus 6; IVF: intravenous fluids; ORIF: open reduction internal fixation; c/b: complicated by; Cl: clearance;
Among 8 AIN cases patients had median age of 58 years [IQR 42 years, 68 years]and majority (75%) were male (Table 4). The median treatment time prior to HSRwas 26 days [IQR 7 days, 29 days]. Rash was present in six patients (75%), with more than half of the rashes described as maculopapular. Median peak Creatinine was a 6.6 mg/dL [IQR 3.5 mg/dL, 8.8 mg/dL].. Peripheral blood eosinophilia was quantified in 5 cases with a median peak AEC of 936/mL [IQR 861/mL, 979/mL]. All biopsied cases were proven AIN by kidney biopsy including interstitial edema, with eosinophils, and mononuclear infiltrations. Five patients(63%) received steroid treatment and five patients alsorequired renal replacement therapy. One patient received steroid-sparing agents cyclosporine and mycophenolate mofetilafter failing steroid treatment.26 Complete resolution occurred in 6/8 (75%) of the patients, with four cases quantifying the median time to resolution (60 days [IQR 49 days, 165 days]). There were two deaths, both attributed to underlying infections..
Table 4.
Clinical data for cases of acute interstitial nephritis (AIN) to vancomycin (n=8)
| Age (yrs) |
Gender (m/f) |
Infection treated |
Reaction timing* |
Symptoms | Lab data | Biopsy | Treatment | Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| Htike et al. 2012 |
79 | F | Coagulase-neg Staphylococcus Bacteremia |
7d | Malaise, fatigue |
Cr (bl 0.9 mg/dL,max 11.7 mg/dL, 92.3% change) No urine eosinophils |
ATN changes: Loss of tubular cells tubular dilatation AIN changes: Interstitial edema Eosinophils Mononuclear infiltrate |
Oral steroids for 2 weeks |
Renal function resolved over 4 weeks |
| Salazar et al. 2010 |
51 | M | MRSA osteomyelitis |
28d | Rash | Cr (bl 0.9 mg/dL, max 2.2,59.0% change) AEC: 2318/L |
Interstitial edema Eosinophils Mononuclear infiltrations |
Oral steroids | One recurrence Followed by resolution |
| Michail et al. 2009 |
35 | M |
S. aureus
empyema |
4d | MP rash, arthralgia |
Cr (bl normal, max 6.5 mg/dL) No urine eoisinophils |
Mononuclear inflammatory infiltration |
Furosemide HD Switched therapy |
Renal function resolved over 10m |
| Hong et al. 2007 |
44 | M | Polymicrobial wound infection with Staphylococcus aureus, Group B Streptococci, and S.mitis |
28d | Rash, fever, hypotensio n |
Cr (bl 3.1 mg/dL, max 8.5 mg/dL, 63.5% change AEC: 640/L |
Giant cell granulomas Mononuclear interstitial infiltration |
After failed IV and oral steroids x 1w, switched to cyclosporine and MMF HD Switched therapy |
Renal function improved with cyclosporine, MMF and HD for 2m |
| Hsu et al. 2001 |
70 | M | MRSA abscess | 23d | Fever, MP rash |
Cr (bl 2.0 mg/dL, max 3.5 mg/dL,42.9 % change) AEC: 936 |
Interstitial edema Eosinophils Mononuclear infiltrations |
Oral steroids CVVH Switched therapy |
Several readmissions followed by death from polymicrobial sepsis |
| Wai et al. 1998 |
64 | M | MRSA sternal wound dehiscence s/p CABG |
39d | Fever, MP rash |
Cr (bl 1.1 mg/dL,max 9.5 mg/dL,88.4 % change) AEC: 979/L |
Interstitial mononuclear infiltrations Granulomata |
Oral steroids HD over 2 weeks Switched therapy |
Renal function improved Several readmissions and complicated post- operative course |
| Codding et al. 1989 |
67 | M |
Staphylococcus
aureus endocarditis |
30d | Fever, MP rash |
Cr (bl 1.5 mg/dL, max 6.6mg/dL) AEC: 861 |
Interstitial mononuclear infiltration Granulomata |
HD Switched therapy |
Clinically deteriorated Deceased from septic shock |
| Bergman et al. 1988 |
34 | F | Endometritis, Staphylococcus aureus |
6d | Fever, pedal edema |
Cr (bl 1.5 mg/dL,max 3.4 mg/dL) AEC: normal |
Refused renal biopsy |
Discontinued vancomycin |
Renal function resolved within 15 days |
Timing from vancomycin dose to reaction onset
Abbreviations: d: days; Cr: Creatinine; bl: baseline; max: maximum; ATN: acute tubular nephrosis; AIN: allergic interstitial nephritis; MRSA: methicillin-resistant Staphylococcus aureus; AEC: absolute eosinophil count; MP: maculopapular; HD: hemodialysi; IV: intravenous; w: weeks; MMF: mycofenolate mofetil; m: months; CVVH: continuous-veno-venous hemofiltration; s/p: status-post; CABG: coronary artery by-pass graft
Of the six cases of vancomycin-induced SJS or TEN, .patients had a median age of 38 years [IQR 36 years, 46 years]and half were male (Table 5). The median treatment time prior to HSR was 9 days IQR [9 days, 22days]. All cases had biopsies consistent with SJS or TEN (e.g., revealing epidermal necrosis and blisters along the dermal-epidermal junction). Steroids were used for treatment in two cases. Resolution occurred in four (67%) of cases, with time to resolution (reported in three cases), a median of 56 days [IQR 29 days, 57 days].Two patients (33%) died with one death attributed to HSR complications, and the other death being attributed to terminal illness
Table 5.
Clinical data for cases of Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) to vancomycin (n=6)
| Age (yrs ) |
Sex (m/f ) |
Infection treated |
Reactio n timing* |
Rash description | Lab data | Biopsy | Treatment | Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| Changela et al. 2013 |
35 | M | MRSA abscess |
9d | Dusky purpuric plaques and multiple fluid filled blisters on the trunk, upper and lower extremities. 30% of BSA |
IgA: 154mg/dL |
Necrotic epidermis Severe blisters at DE junction Necrotic keratocytes |
IV steroids for 4d Oral steroid taper |
Clinically improved |
| O’Brien et al. 2011 |
46 | F | Respiratory failure |
“few” d | Epidermal sloughing, erosions and blisters over 40% BSA. Nikolsky sign positive |
Epidermal necrosis, minimal interface, and perivascular lymphocytic infiltrate |
Discontinuatio n of vancomycin |
Terminally ill, deceased |
|
| Bouaziz et al. 2006 |
38 | F | Fistula | 9d | MP rash, fever, oral, ocular and mucous membrane erosions, diffuse blisters, Nikolsky sign positive, 50% epidermal skin detachment |
Serum drug level 20mg/L |
Follicular necrosis DE detachment |
IV fluids Anti-infectious therapy Nutritional support Skin care |
Deceased day 13, from HSR |
| Chan- Tack 2000 |
46 | F | Infected hip joint prosthesis |
8d | Diffuse flaccid bullae covering 50% BSA, necrotic epidermis. |
Epidermal necrosis and detachment |
Whirlpool therapy Topical antimicrobial Nutritional support Pain control |
Clinically improved, discharged after 8 weeks |
|
| Alexande r et al. 1996 |
36 | M | Endocarditi s |
17d | MP rash involving torso, abdomen, legs and arms. Lymphadenopathy , pharyngreal irritation, lip swelling, and conjunctival irritation. |
ANC: 1;911/mm3 Eosinophili a (13-28%) |
Epidermal necrosis DE junction blisters Dermal infiltration |
Steroid therapy | Clinical improvemen t within 24h |
| Vidal et al. 1992 |
28 | M |
S. aureus
sepsis (history of AIDS) |
27d | MP rash, fever, oral mucosa and genitals involved. Nikolsky’s sign positive |
ESR: 78mm/hour ANC: 1,349/mm3 |
Lymphocyti c and neutrophilic subdermal infiltration |
Switched therapy |
Recovered, discharged after 57d |
Timing from vancomycin dose to reaction onset
Abbreviations: MRSA: methicillin-resistant staphylococcus aureus; d: days; BSA: body surface area; DE: dermal-epidermal; IV: intravenous; MP: maculopapular; HSR: hypersensitivity reaction; ANC: absolute neutrophil count; AIDS: acquired immunodeficiency syndrome; ESR: erythrocyte sedimentation rate
DISCUSSION
We performed a systematic case review of vancomycin HSRs and found 71 cases representing a variety of HSRs, including IgE-mediated, LABD, DRESS syndrome, AIN, and SJS/TEN. The most frequently observed HSRs from vancomycin were non-immediate (n=64) with LABD the most frequently reported vancomycin HSR (n=34). We were able to appreciate some variability in case diagnosis and treatment that may inform future care of these patients and encourage standard case reporting or rare HSRs. We observed a high frequency of case fatality, which in some cases was directly related to the HSR, indicating a need for increased awareness of vancomycin HSRs.
The most challenging HSR to identify in our review was IgE-mediated vancomycin HSRs because it was difficult to diagnose and distinguish from red man syndrome. There are no serum tests that exist, skin testing is not validated, and vancomycin is known to be a direct mast cell activator.27 The current accepted skin testing protocol for IgE-mediated vancomycin allergy includes using a non-irritating concentration with skin prick concentration 50mg/mL and intradermal concentration 0.01 and 0.1 mcg/mL.28,10 Although only one of our IgE-mediated HSRs to vancomycin cases used skin testing, other included cases had clinical courses that supports possible existence of an IgE mechanism; many had prior vancomycin exposure to account for sensitization and many failed red man syndrome protocols or desensitization. While severe red man syndrome is clinically indistinguishable from IgE-mediated anaphylaxis, IgE-independent reactions can often be overcome with premedication. Additionally, drug hypersensitivity literature previously reported that it is often patients with IgE-mediated reactions who have breakthrough reactions during desensitization procedures.12,13 We believe that this case review highlights the need for allergist involvement when patients fail red man syndrome protocols. Such patients may benefit from vancomycin skin testing and future doses may need to be given by a desensitization procedure.
LABD was the most commonly identified vancomycin HSR, although overall LABD incidenceranges from 0.2 to 2.3 cases per million individuals per year.29,30 Our data was similar to previous data, with patients commonly over 60 years old; however, our cases included eight patients who were 60 years old or younger.29,31 This review highlights why this HSR can be confused with other HSRs, including SJS/TEN, erythema multiforme, maculopapular rash, and others. While only two cases did not present with a typical bullous eruption, cases included other exam features such as target lesions, vesicles, skin desquamation and/or mucous membrane involvement. Our findings reinforce the importance of skin biopsy in the diagnosis of cutaneous HSRs.
DRESS syndrome is a morbid, systemic HSR that includes rashes, hematologic abnormalities, lymphadenopathy, and internal organ involvement, most commonly the liver and/or kidneys. DRESS syndrome is a clinical diagnosis, usually of exclusion, and is ideally diagnosed using the regiSCAR clinical score developed by European investigators in surveillance studies.32,33 However, among the vancomycin DRESS cases synthesized, the majority did not report the regiSCAR score. Beyond its usefulness in the clinical care of patients with potential DRESS syndrome, using objective scoring is the best available method to convey diagnostic clinical certainty when publishing a case report. Finally, although DRESS syndrome has a reported mortality rate from 4-10%,34,35 our described DRESS cases had no fatalities. While there are no experimental trials evaluating the use of corticosteroids for the treatment of DRESS syndrome, our DRESS patients largely received steroids for DRESS syndrome and all experienced clinical recovery.
Because renal toxicity is at the forefront of a clinician’s mind when treating patients with vancomycin who develop an acute change in creatinine,36,37 AIN to vancomycin is likely underdiagnosed. However AIN is the cause of 10-27% of acute kidney injury without a clear cause.38 AIN clinically presents with rash, peripheral eosinophilia, and/or eosinophiliuria. By microscopy, the urine may have white blood cell casts. However, the diagnosis of AIN is made with a kidney biopsy, and because there is rarely a clinical need to perform a biopsy, there are few cases of biopsy-proven acute interstitial nephritis.39 Several risk factors for developing vancomycin-induced AIN have been identified: concurrent treatment with aminoglycosides, elevated vancomycin trough >10mg/L, and prolonged treatment (>21 days).40 We similarly found the median number of days of vancomycin therapy prior to AIN was >21 days (26 days); therefore. it may be useful to consider vancomycin AIN when patients develop a change in creatinine after a prolonged vancomycin course. There is limited prognostic data available for vancomycin-induced AIN. One case series found that patients’ serum creatinine remained elevated in approximately 40% of patients and the mean recovery time of renal function was 1.5 months.41 We found that 75% of the cases had recovery of their renal function, although five (63%) required renal replacement therapy and, overall, recovery generally took a number of months.
Our review has a number of important limitations. The first limitation concerns bias within the studies themselves. Although we established clinical case standards, all cases of HSRs to vancomycin are naturally limited by our clinical diagnostic tools in drug allergy. This is less important for HSRs that were biopsy-proven (e.g., LABD) and more important for HSRs that relied on a clinical diagnosis (e.g., IgE-mediated, DRESS syndrome). The included cases may have suffered from misattribution; many of the patients were on other drugs concurrent with vancomycin and we had to rely on the primary case author’s causality assessment. Another limitation with this type of analysis is that there is bias across studies including publication bias and selective reporting. Although we found the most cases of LABD, we cannot determine that this observed frequency is related to the actual frequency of the HSR. Our findings may be due to clinicians being more inclined to write-up cases with severe or dramatic outcomes, which could also explain the high overall mortality. Nevertheless, HSRs from vancomycin are occurring and can be severe.
In summary, we identified a variety of HSRs to vancomycin that all clinicians using vancomycin should have knowledge of, especially since vancomycin is commonly used in the United States.42 Our review reveals valuable clinical pictures of these HSRs, and highlights the need for improved diagnostic and reporting tools for rare HSRs.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Niki Holtzman for her research assistance.
Funding
This work was conducted with the support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Abbreviations
- IgE
Immunnoglobulin E
- LABD
Linear IgA Bullous Dermatosis
- DRESS
drug rash eosinophilia and systemic symptoms
- AIN
acute interstitial nephritis
- SJS/TEN
Stevens-Johnson’s syndrome/toxic epidermal necrolysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: none
REFERENCES
- 1.Rubinstein E, Keynan Y. Vancomycin revisited - 60 years later. Front public Heal. 2014 Oct 2;217 doi: 10.3389/fpubh.2014.00217. doi:10.3389/fpubh.2014.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82–98. doi: 10.2146/ajhp080434. doi:10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 3.Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70(3):195–283. doi: 10.2146/ajhp120568. doi:10.2146/ajhp120568. [DOI] [PubMed] [Google Scholar]
- 4.Bruniera R. The use of vancomycin with its therapeutic and adverse effects : a review. 2015. pp. 694–700. [PubMed]
- 5.Wynn M, Dalovisio JR, Tice AD, Jiang X. Evaluation of the efficacy and safety of outpatient parenteral antimicrobial therapy for infections with methicillin-sensitive Staphylococcus aureus. South Med J. 2005;98(6):590–595. doi: 10.1097/01.SMJ.0000145300.28736.BB. doi:10.1097/01.SMJ.0000145300.28736.BB. [DOI] [PubMed] [Google Scholar]
- 6.Sivagnanam S, Deleu D. Red man syndrome. 2003:119–121. doi: 10.1186/cc1871. doi:10.1186/cc1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions : Explanation and Elaboration. 2009;6(7) doi: 10.1371/journal.pmed.1000100. doi:10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss ME, Bernstein DI, Blessing-moore J, et al. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259–273. doi: 10.1016/j.anai.2010.08.002. doi:10.1016/j.anai.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 9.PF W. Vancomycin hypersensitivity. UpToDate. http://www.uptodate.com/contents/vancomycin-hypersensitivity. Published 2016. [Google Scholar]
- 10.Otani Iris M., Kuhlen James, Jr, Blumenthal Kimberly, Autumn Guyer AB. A role for vancomycin epicutaneous skin testing in the evaluation of perioperative anaphylaxis. J Allergy Clin Immunol Pract. 2015 doi: 10.1016/j.jaip.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell RL, Li JTC, Nicklas R a, Sadosty AT. Emergency department diagnosis and treatment of anaphylaxis: a practice parameter. Ann Allergy Asthma Immunol. 2014;113(6):599–608. doi: 10.1016/j.anai.2014.10.007. doi:10.1016/j.anai.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Solensky R. Drug allergy: an updated practise parameter. Ann Allergy, Asthma Immunol. 2010;105(4):259–273. doi: 10.1016/j.anai.2010.08.002. KD. [DOI] [PubMed] [Google Scholar]
- 13.Jingu A, Fukuda J, Taketomi-Takahashi A. Breakthrough reactions of iodinated and gadolinium contrast media after oral steroid premedication protocol. BMC Med Imaging. 2014;14(34) doi: 10.1186/1471-2342-14-34. TY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renz CL, Thurn JD, Finn HA, Lynch JP. Antihistamine prophylaxis permits rapid vancomycin infusion. Crit Care Med. 1999;27(9):1732–1737. doi: 10.1097/00003246-199909000-00006. MJ. [DOI] [PubMed] [Google Scholar]
- 15.Mezzano V, Giavina-Bianchi P, Picard M, Caiado J. Drug desensitization in the management of hypersensitivity reactions to monoclonal antibodies and chemotherapy. BioDrugs. 2014;28(2):133–144. doi: 10.1007/s40259-013-0066-x. CM. [DOI] [PubMed] [Google Scholar]
- 16.Hesterberg PE, Banerji A, Oren E, et al. Risk stratification for desensitization of patients with carboplatin hypersensitivity: clinical presentation and management. J Allergy Clin Immunol. 2009;123(6):1262–1267.e1. doi: 10.1016/j.jaci.2009.02.042. doi:10.1016/j.jaci.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 17.Castells Guitart MC. Rapid drug desensitization for hypersensitivity reactions to chemotherapy and monoclonal antibodies in the 21st century. J Investig Allergol Clin Immunol. 2014;24(2):72–79. quiz 2 p following 79. http://www.ncbi.nlm.nih.gov/pubmed/24834769. [PubMed] [Google Scholar]
- 18.Hassaballa Hesham, Naveed Mallick JO. Vancomycin Anaphylaxis in a Patient with Vancomycin-induced red man syndrome. Am J Ther. 2000;7(319):320. doi: 10.1097/00045391-200007050-00010. [DOI] [PubMed] [Google Scholar]
- 19.Villavicencio Alan, Hey Lloyd, Dhavalkumar Patel PB. Acute cardiac and pulmonary arrest after infusion of vancomycin with subsequent desensitization. J Allergy Clin Immunol. 1998;100(6):853–854. doi: 10.1016/s0091-6749(97)70287-2. [DOI] [PubMed] [Google Scholar]
- 20.Chopra N, Oppenheimer J, Derimanov GS, Fine PL. Vancomycin anaphylaxis and successful desensitization in a patient with end stage renal disease on hemodialysis by maintaining steady antibiotic levels. Ann Allergy, Asthma Immunol. 2000;84(6):633–635. doi: 10.1016/S1081-1206(10)62416-7. doi:10.1016/S1081-1206(10)62416-7. [DOI] [PubMed] [Google Scholar]
- 21.Polk RE, Healy DP, Schwartz LB, Rock DT, Garson ML. Vancomycin and the red-man syndrome: pharmacodynamics of histamine release. J Infect Dis. 1988;157(3):502. doi: 10.1093/infdis/157.3.502. RK. [DOI] [PubMed] [Google Scholar]
- 22.Kitazawa T, Ota Y, Kada N, Morisawa Y, Yoshida A. Successful Vancomycin Desensitization with a Combination. 2005:317–321. doi: 10.2169/internalmedicine.45.1388. February 1998. doi:10.2169/internalmedicine.45.1388. [DOI] [PubMed] [Google Scholar]
- 23.Tamagawa-Mineoka R, Katoh N, Nara T, Nishimura Y, Yamamoto S, Kishimoto S. DRESS syndrome caused by teicoplanin and vancomycin, associated with reactivation of human herpesvirus-6. Int J Dermatol. 2007;46(6):654–655. doi: 10.1111/j.1365-4632.2007.03255.x. doi:10.1111/j.1365-4632.2007.03255.x. [DOI] [PubMed] [Google Scholar]
- 24.Zuliani E, Zwahlen H, Gilliet F, Marone C. Vancomycin-induced hypersensitivity reaction with acute renal failure: resolution following cyclosporine treatment. Clin Nephrol. 2005;64(08):155–158. doi: 10.5414/cnp64155. doi:10.5414/CNP64155. [DOI] [PubMed] [Google Scholar]
- 25.Della-Torre E, Yacoub M-R, Pignatti P, et al. Optimal management of DRESS syndrome in course of infectious endocarditis. Ann Allergy, Asthma Immunol. 2013;110(4):303–305. doi: 10.1016/j.anai.2013.01.006. doi:10.1016/j.anai.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Hsu SI. Biopsy-Proved Acute Tubulointerstitial Nephritis and. Pharmacology. p. 200AD. Ph D. [DOI] [PubMed]
- 27.Sugimoto Y1, Iba Y, Utsugi K. Influences of everninomicin, vancomycin and teicoplanin on chemical mediator release from rat peritoneal mast cells. Jpn J Pharmacol. 2000;83(4):300–305. doi: 10.1254/jjp.83.300. KC. [DOI] [PubMed] [Google Scholar]
- 28.Polk RE, Israel D, Wang J, Venitz J, Miller J, Stotka J. Vancomycin skin tests and prediction of “red man syndrome” in healthy volunteers. Antimicrob Agents Chemother. 1993;37(10):2139–2143. doi: 10.1128/aac.37.10.2139. doi:10.1128/AAC.37.10.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fortuna G, Marinkovich MP. Linear immunoglobulin A bullous dermatosis. Clin Dermatol. 2012;30(1):38–50. doi: 10.1016/j.clindermatol.2011.03.008. doi:10.1016/j.clindermatol.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Mintz EM, Morel KD. Clinical features, diagnosis, and pathogenesis of chronic bullous disease of childhood. Dermatol Clin. 2011;29(3):459–462. doi: 10.1016/j.det.2011.03.022. ix. doi:10.1016/j.det.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 31.Wojnarowska F, Marsden RA, Bhogal B. Chronic bullous disease of childhood, childhood cicatricial pemphigoid, and linear IgA disease of adults. A comparative study demonstrating clinical and immunopathologic overlap. J Am Acad Dermatol. 1988;19:792–805. doi: 10.1016/s0190-9622(88)70236-4. BM. [DOI] [PubMed] [Google Scholar]
- 32.Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124(7):588–597. doi: 10.1016/j.amjmed.2011.01.017. doi:10.1016/j.amjmed.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 33.Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169(5):1071–1080. doi: 10.1111/bjd.12501. doi:10.1111/bjd.12501. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y-C, Chiu H-C, Chu C-Y. Drug reaction with eosinophilia and systemic symptoms: a retrospective study of 60 cases. Arch Dermatol. 2010;146(12):1373–1379. doi: 10.1001/archdermatol.2010.198. doi:10.1001/archdermatol.2010.198. [DOI] [PubMed] [Google Scholar]
- 35.Wongkitisophon P, Chanprapaph K, Rattanakaemakorn P, Vachiramon V. Six-year retrospective review of drug reaction with eosinophilia and systemic symptoms. Acta Derm Venereol. 2012;92(2):200–205. doi: 10.2340/00015555-1222. doi:10.2340/00015555-1222. [DOI] [PubMed] [Google Scholar]
- 36.Van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57(2):734–744. doi: 10.1128/AAC.01568-12. doi:10.1128/AAC.01568-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeffres MN, Isakow W, Doherty J a, Micek ST, Kollef MH. A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care-associated methicillin-resistant Staphylococcus aureus pneumonia. Clin Ther. 2007;29(6):1107–1115. doi: 10.1016/j.clinthera.2007.06.014. doi:10.1016/j.clinthera.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 38.Perazella M a. Diagnosing drug-induced AIN in the hospitalized patient: a challenge for the clinician. Clin Nephrol. 2014;81(6):381–388. doi: 10.5414/CN108301. doi:10.5414/CN108301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Htike NL, Santoro J, Gilbert B, Elfenbein IB, Teehan G. Biopsy-proven vancomycin-associated interstitial nephritis and acute tubular necrosis. Clin Exp Nephrol. 2012;16(2):320–324. doi: 10.1007/s10157-011-0559-1. doi:10.1007/s10157-011-0559-1. [DOI] [PubMed] [Google Scholar]
- 40.Rybak MJ1, Albrecht LM, Boike SC. Nephrotoxicity of vancomycin, alone and with an aminoglycoside. J Antimicrob Chemother. 1990;25(4):679–687. doi: 10.1093/jac/25.4.679. CP. [DOI] [PubMed] [Google Scholar]
- 41.Rossert J1. Drug-induced acute interstitial nephritis. Kidney Int. 2001;60:804–817. doi: 10.1046/j.1523-1755.2001.060002804.x. [DOI] [PubMed] [Google Scholar]
- 42.Kirst Herbert A., Thompson Diane G., TIN Historical Yearly Usage of Vancomycin. Antimicrob Agents Chemother. 1998;42(5):1303–1304. doi: 10.1128/aac.42.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.