Abstract
Relative to European Americans, evidence supports that African Americans with end-stage renal disease (ESRD) survive longer on dialysis. Renal-risk variants in the apolipoprotein L1 gene (APOL1), associated with non-diabetic nephropathy and less subclinical atherosclerosis, may contribute to dialysis outcomes. Here, APOL1 renal-risk variants were assessed for association with dialytic survival in 450 diabetic and 275 non-diabetic African American hemodialysis patients from Wake Forest and Emory School of Medicine outpatient facilities. Outcomes were provided by the ESRD Network 6-Southeastern Kidney Council Standardized Information Management System. Dates of death, receipt of a kidney transplant, and loss to follow-up were recorded. Outcomes were censored at the date of transplantation or through July 1, 2015. Multivariable Cox proportional hazards models were computed separately in patients with non-diabetic and diabetic ESRD, adjusting for the covariates age, gender, comorbidities, ancestry, and presence of an arteriovenous fistula or graft at dialysis initiation. In non-diabetic ESRD, patients with two (vs. zero/one) APOL1 renal-risk variants had significantly longer dialysis survival (hazard ratio 0.57); a pattern not observed in patients with diabetes-associated ESRD (hazard ratio 1.29). Thus, two APOL1 renal-risk variants are associated with longer dialysis survival in African Americans without diabetes, potentially relating to presence of renal-limited disease or less atherosclerosis.
Keywords: African Americans, APOL1, diabetes mellitus, hemodialysis, end-stage kidney disease, survival
Introduction
In the general population, African Americans have higher rates of cardiovascular disease (CVD) and myocardial infarction than European Americans.1 In contrast, African Americans with end-stage renal disease (ESRD) on dialysis live significantly longer and have fewer myocardial infarctions than their European American counterparts, an effect that persists after accounting for the earlier age at development of chronic kidney disease (CKD) in this population.2-5 Kurcika et al. reported this observation only in older individuals.6 This paradoxical observation may be related to equalization of access to healthcare, because previously uninsured patients in the general population become eligible for Medicare based on their ESRD diagnosis.
In the Veterans Health Administration (VA) and Kaiser Permanente healthcare systems where patients presumably have access to equivalent care, African Americans have approximately 50% lower rates of myocardial infarction than European Americans.7;8 This reversal from the difference seen in the general population supports lack of healthcare access as an important confounding factor in the study of biologic (inherited) susceptibility to CVD. In support of this hypothesis, African Americans have been shown to have substantially less subclinical age-adjusted atherosclerosis (lower amounts of calcified atherosclerotic plaque [CP]) than European Americans, despite exposure to more severe conventional CVD risk factors.9-13 African Americans are an admixed population with approximately 80% African and 20% European ancestry.14;15 There is evidence to suggest higher levels of European ancestry impart increased risk for subclinical atherosclerosis in African Americans, whereas African ancestry is protective.14;15
In contrast to CVD, sub-Saharan African ancestry at the apolipoprotein L1 gene (APOL1) is associated with heightened risk for non-diabetic etiologies of CKD.16-18 The effects of APOL1 renal-risk variants (RRVs) on CVD susceptibility remain controversial.19 Both the Jackson Heart Study (JHS) and African American-Diabetes Heart Study (AA-DHS) detected lower levels of CP in African Americans with increasing numbers of APOL1 RRVs; however AA-DHS reported improved survival in those with lower CP; whereas JHS reported paradoxically higher rates of incident CVD.20;21 APOL1 RRVs also associated with incident CVD in the Women's Health Initiative (WHI) and Cardiovascular Health Study (CHS);22, however, APOL1 associations with kidney disease in JHS, WHI, and CHS could have confounded associations with CVD. There was no effect of APOL1 on kidney disease in AA-DHS participants with diabetes, potentially explaining differential results. The present analyses were performed to test the hypothesis that APOL1 RRVs are associated with longer age-adjusted survival in African American patients with ESRD.
Results
The study population consisted of 725 African Americans with ESRD. Table 1 displays their demographic and clinical characteristics, according to etiology of ESRD (diabetic and non-diabetic) and the number of APOL1 RRVs. There were more females than males with diabetes-attributed ESRD (regardless of APOL1 genotype) and more males than females in the non-diabetic ESRD groups. Among African American study participants with non-diabetic etiologies of ESRD (diseases in the focal segmental glomerulosclerosis (FSGS) and focal global glomerulosclerosis (FGGS) spectrum, Methods contain exclusion criteria), 46.9% (129/275) had two APOL1 RRVs. Compared to African American study participants with non-diabetic ESRD and zero/one APOL1 RRVs (and to African American study participants with diabetes and ESRD), the age at dialysis initiation was younger and fewer co-morbidities were present in African Americans with non-diabetic ESRD who had two APOL1 RRVs. Perhaps due to earlier age at ESRD onset, more of the non-diabetic African American patients with ESRD and two APOL1 RRVs were either lost to follow-up or underwent kidney transplantation; however, their median survival on dialysis was 103.8 months, markedly longer than non-diabetic African American patients with zero/one RRVs (83.6 month median survival) or African American patients with diabetic ESRD (median survival 77.5 months with two and 79.5 months with zero/one APOL1 RRVs, respectively).
Table 1.
Demographic data in African Americans with ESRD, based on APOL1 renal risk variants
| Diabetes-attributed ESRD (N=450) | Non-diabetic ESRD (N=275) | |||
|---|---|---|---|---|
| APOL1 0,1 RRVs (N=368) | APOL1 2 RRVs (N=82) | APOL1 0,1 RRVs (N=146) | APOL1 2 RRVs (N=129) | |
| Female, N (%) | 198 (53.8%) | 46 (56.1%) | 64 (43.8%) | 54 (41.9%) |
| Age at first dialysis, years | 60.4 ± 10.8 (61.1) | 57.4 ± 11.9 (57.0) | 55.4 ± 13.7 (55.6) | 43.7 ± 14.0 (42.7) |
| Survival time, months | 85.7 ± 43.4 (79.5) | 83.3 ± 39.1 (77.5) | 95.9 ± 50.1 (83.6) | 109.2 ± 66.0 (103.8) |
| Number of comorbidities, N | 2.43 ± 1.67 (2) | 2.18 ± 1.60 (2) | 1.92 ± 1.61 (1) | 1.22 ± 0.89 (1) |
| Status at study end: | ||||
| Living | 179 (48.6%) | 36 (43.9%) | 86 (58.9%) | 76 (58.9%) |
| Discharged from study,* N (%) | 23 (6.3%) | 8 (9.8%) | 10 (6.8%) | 30 (23.3%) |
| Deceased, N (%) | 166 (45.1%) | 38 (46.3%) | 50 (34.2%) | 23 (17.8%) |
| Age at death, † years | 68.4 ± 10.2 (69.4) | 64.1 ± 11.1 (65.3) | 65.9 ± 13.3 (69.2) | 60.1 ± 12.9 (61.5) |
| Time to kidney transplant, ^ months | 53.6 ± 27.7 (57.5) (N=20) | 50.3 ± 21.9 (38.8) (N=7) | 64.6 ± 25.4 (58.6) (N=7) | 64.9 ± 51.4 (45.5) (N=26) |
| Dialysis vintage at recruitment, months | 35.9 ± 29.0 (29.1) | 36.5 ± 29.8 (27.7) | 36.3 ± 31.5 (27.1) | 48.4 ± 34.6 (40.1) |
RRVs – renal risk variant numbers (0/1 or 2). Data presented as mean ± SD (median) or N (%).
Discharged due to kidney transplant or loss to follow up.
Among participants who died during the study period.
Among participants who had a kidney transplant during the study period.
Table 2 presents results of a sensitivity analysis where survival time on dialysis was evaluated based on age at dialysis initiation (before versus after 50 years) and considering the presence or absence of diabetes as the etiology of ESRD. Despite far smaller numbers within each cell, non-diabetic African Americans aged <50 or ≥50 years at the initiation of renal replacement therapy survived longer in the presence of two APOL1 RRVs (similar results were seen using 45 years as the cut-off, data not shown). Significant differences in hemodialysis survival were not observed among African American patients with diabetes-attributed ESRD based on APOL1 genotype.
Table 2.
Survival time (months) as a function of diabetes status, age at ESRD (<50, ≥50 years), and APOL1 G1/G2 status
| Attributed cause of ESRD | Age at ESRD (years) | Two APOL1 RRVs | Death/Censored | N | Survival (Censor) Time Mean ± SD (Median) |
|---|---|---|---|---|---|
| Diabetes | <50 | No | Dead | 20 | 93.4 ± 50.2 (87.9) |
| Censored | 38 | 90.3 ± 47.5 (78.4) | |||
| Yes | Dead | 8 | 82.2 ± 53 (72.4) | ||
| Censored | 12 | 86.4 ± 58 (85.9) | |||
| ≥50 | No | Dead | 146 | 77.6 ± 44.2 (74.9) | |
| Censored | 164 | 91 ± 40.1 (85.2) | |||
| Yes | Dead | 30 | 79.6 ± 39 (74.3) | ||
| Censored | 32 | 85.9 ± 26.7 (82.4) | |||
| Non-diabetic | <50 | No | Dead | 12 | 102.1 ± 65.3 (77.4) |
| Censored | 35 | 107.9 ± 61 (84.6) | |||
| Yes | Dead | 10 | 130.4 ± 82.5 (136.8) | ||
| Censored | 77 | 113.1 ± 72.2 (114.6) | |||
| ≥50 | No | Dead | 38 | 91 ± 51.3 (87) | |
| Censored | 61 | 90.9 ± 37.8 (83.4) | |||
| Yes | Dead | 13 | 109.7 ± 48.8 (113.9) | ||
| Censored | 29 | 91.5 ± 44.9 (76.4) |
Table 3 presents the results of Cox proportional hazards models of numbers of APOL1 RRVs on survival time after initiation of hemodialysis therapy in each group. After adjustment for age at dialysis initiation, gender, number of comorbidities on the Centers for Medicare and Medicaid Services (CMS) Medical Evidence Report (CMS-2728 form), African ancestry proportion, and presence of either an arteriovenous fistula or graft at first dialysis (reflecting access to nephrology care prior to dialysis), non-diabetic African American participants with two APOL1 RRVs had significantly longer survival than those with zero/one RRVs (hazard ratio: HR=0.57, p=0.039). Of the 25 comorbidities on the CMS-2728 form in patients with non-diabetic ESRD, congestive heart failure, chronic obstructive pulmonary disease, smoking, and alcohol use were more frequent (p<0.05) in APOL1 two RRV carriers (vs. zero/one); however, study conclusions were unchanged with inclusion of these four comorbidities in the full model (data not shown). In contrast, the fully-adjusted model HR for dialytic survival in African Americans with two APOL1 RRVs and diabetes-attributed ESRD yielded non-significant results in the opposite direction (HR=1.29; p=0.165). The effect of APOL1 RRVs on dialysis survival in patients with diabetic-ESRD and non-diabetic ESRD were statistically different (diabetes status–APOL1 RRV interaction p=0.008); suggesting different clinical courses and genetic etiologies of ESRD in African Americans with and without diabetes, as reported.23
Table 3.
Cox proportional hazards modeling of the APOL1 renal risk variant associations with survival time
| Diabetic (N=450)1 | ||||
|---|---|---|---|---|
| Recessive | Additive | |||
| Covariates | P-value | Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) |
| Sex, Age at first dialysis, Number of comorbidities* | 0.3484 | 1.19 (0.83-1.69) | 0.4283 | 1.08 (0.89-1.31) |
| Sex, Age at first dialysis, Number of comorbidities*, Fistula/graft** | 0.1832 | 1.28 (0.89-1.83) | 0.3005 | 1.11 (0.91-1.35) |
| Sex, Age at first dialysis, Admixture, Number of comorbidities* | 0.3325 | 1.19 (0.84-1.70) | 0.5036 | 1.07 (0.88-1.30) |
| Sex, Age at first dialysis, Admixture, Number of comorbidities*, Fistula/graft** | 0.1653 | 1.29 (0.90-1.85) | 0.3319 | 1.10 (0.90-1.35) |
| Non-Diabetic (N=275)1 | ||||
|---|---|---|---|---|
| Recessive | Additive | |||
| Covariates | P-value | Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) |
| Sex, Age at first dialysis, Number of comorbidities* | 0.0259 | 0.55 (0.32-0.93) | 0.0163 | 0.70 (0.52-0.94) |
| Sex, Age at first dialysis, Number of comorbidities*, Fistula/graft** | 0.0277 | 0.55 (0.32-0.94) | 0.0149 | 0.69 (0.51-0.93) |
| Sex, Age at first dialysis, Admixture, Number of comorbidities* | 0.0358 | 0.56 (0.33-0.96) | 0.0260 | 0.71 (0.53-0.96) |
| Sex, Age at first dialysis, Admixture, Number of comorbidities*, Fistula/graft** | 0.0385 | 0.57 (0.35-0.91) | 0.0235 | 0.70 (0.52-0.95) |
Not all subjects had available admixture (African ancestry proportion) estimates; in analyses adjusting for admixture there are 439 Diabetics and 273 Non-Diabetics.
Winsorized to 6 comorbidities (i.e., if comorbidities were greater than 6, then the number of comorbidities was set to 6).
Accounted for missing dialysis access data in a large proportion of samples using a dummy variable (180 individuals with a Fistula/graft, 153 without a Fistula/graft, and 392 without available data).
Table 4 shows stratified results of the Cox proportional hazards model for patients who initiated hemodialysis before versus after the age of 50 years. In participants with non-diabetic ESRD, fully adjusted model results were in the same direction for the 134 younger patients at hemodialysis initiation (HR=0.37, p=0.021) and the 141 older patients at hemodialysis initiation (HR=0.73, p=0.351). There was not sufficient power to determine whether the magnitude of the effect was different in the younger versus older patients; however, given the magnitude of the difference this question merits further investigation in larger samples. Differences in age at hemodialysis initiation by APOL1 genotypes did not influence dialysis survival in African American patients with ESRD attributed to diabetes.
Table 4.
Cox proportional hazards modeling of the APOL1 renal risk variant associations with survival time, stratified at age 50 years for age at dialysis initiation
| Diabetic (N=78 with age at dialysis < 50, N=372 with age at dialysis ≥ 50) | |||||
|---|---|---|---|---|---|
| Covariates | Recessive | Additive | |||
| Age at dialysis | P-value | Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | |
| Sex, Number of comorbidities* | <50 | 0.7366 | 1.16 (0.50-2.67) | 0.8271 | 0.95 (0.57-1.56) |
| ≥50 | 0.4685 | 1.16 (0.78-1.72) | 0.5195 | 1.07 (0.87-1.32) | |
| Sex, Number of comorbidities*, Fistula/graft** | <50 | 0.8899 | 1.06 (0.46-2.46) | 0.7633 | 0.93 (0.57-1.51) |
| ≥50 | 0.2193 | 1.29 (0.86-1.92) | 0.3651 | 1.10 (0.89-1.37) | |
| Non-Diabetic (N=134 with age at dialysis < 50, N=141 with age at dialysis ≥ 50) | |||||
|---|---|---|---|---|---|
| Covariates | Recessive | Additive | |||
| Age at dialysis | P-value | Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | |
| Sex, Number of comorbidities* | <50 | 0.0210 | 0.37 (0.16-0.86) | 0.0452 | 0.61 (0.37-0.99) |
| ≥50 | 0.3650 | 0.74 (0.39-1.42) | 0.3506 | 0.84 (0.58-1.21) | |
| Sex, Number of comorbidities*, Fistula/graft** | <50 | 0.0211 | 0.37 (0.16-0.86) | 0.0455 | 0.61 (0.37-0.99) |
| ≥50 | 0.3512 | 0.73 (0.38-1.41) | 0.3142 | 0.83 (0.57-1.20) | |
Winsorized to 6 comorbidities (i.e., if comorbidities were greater than 6, then the number of comorbidities was set to 6).
The analysis accounted for missing dialysis access data in a large proportion of samples using a dummy variable (180 individuals with a Fistula/graft, 153 without a Fistula/graft, and 392 without available data).
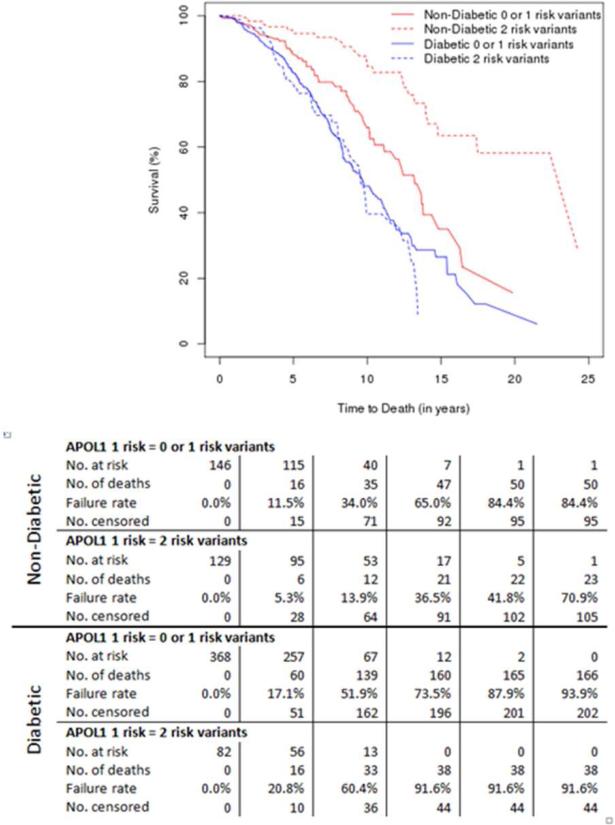
Figure 1 displays adjusted Kaplan-Meier survival curves based on numbers of APOL1 RRVs and cause of ESRD. The longest hemodialysis survivals were observed in patients with non-diabetic ESRD who had two APOL1 RRVs. There were only 2 individuals that died within 90 days of starting dialysis, one was diabetic and one non-diabetic; both had APOL1 renal-risk genotypes. Significant differences in the rates of specific causes of death within the diabetic and non-diabetic ESRD groups were not observed (data not shown).
Figure 1.
Adjusted Kaplan-Meier survival curves based on numbers of APOL1 renal-risk variants and cause of end-stage renal disease. Data below reflects numbers at risk and numbers with each outcome at specified time points, by cause of end-stage renal disease and genotype.
Discussion
The striking association of APOL1 with a spectrum of non-diabetic forms of nephropathy in populations with recent African ancestry has altered the classification of CKD.24;25 APOL1 RRVs are also consistently associated with lower levels of CP, supporting extra-renal effects on the systemic vasculature.20;21 Unfortunately, extension of APOL1 results to myocardial infarctions and CVD mortality has yielded conflicting results, perhaps relating to confounding from APOL1 association with the recognized CVD risk factor “chronic kidney disease”.19-21;26 The present analyses are the first to assess a role for APOL1 RRVs in hemodialysis survival in African Americans with non-diabetes-attributed and diabetes-attributed ESRD. African American patients with two APOL1 RRVs and non-diabetic ESRD survived significantly longer after initiating hemodialysis than did those with zero/one APOL1 RRVs. In the full sample of 275 African Americans with non-diabetic ESRD, median survival was 20.2 months longer in those with two APOL1 RRVs, compared to those with zero/one RRVs. This overall pattern held when examined in patients initiating hemodialysis both before and after the age of 50 years. There were no significant differences in time to kidney transplantation based on APOL1 status in the patients with non-diabetic ESRD (data not shown). Whether the magnitude of the length of survival between earlier and later initiation of hemodialysis differs, APOL1 RRV-by-age interactions merit further investigation in larger samples. In contrast to patients with non-diabetic causes of ESRD, patients with diabetes as the primary cause of ESRD did not exhibit a significant APOL1 RRV relationship with dialysis survival. In fact, the non-significant effect was toward shorter survival with two APOL1 RRVs, compared to zero/one RRVs.
At least two factors may contribute to the longer survival on hemodialysis for non-diabetic individuals with two APOL1 RRVs. First, APOL1-associated nephropathies are often renal-limited forms of glomerulosclerosis, FSGS and FGGS without other organ involvement; progression of CKD in patients with diabetes from the Chronic Renal Insufficiency Cohort (CRIC) has also been observed.25;27;28 As such, dialysis patients with these disorders are less likely to have extra-renal atherosclerosis and this may prolong survival on hemodialysis. Second, the lower levels of CP in African Americans with two APOL1 RRVs may directly contribute to longer dialytic survival, relative to African Americans with fewer than two APOL1 RRVs.2;20;21 We note that although the majority of African American Study of Kidney Disease and Hypertension Cohort (AASK) participants with two APOL1 RRVs met the primary study end-point of death, dialysis, or doubling of serum creatinine concentration, most AASK end-points were renal in origin and few deaths were recorded.29;30 As such, results in the AASK, albeit underpowered, may support those in the present analysis.
We previously evaluated the role of “family history of ESRD” as a surrogate for genetic susceptibility to renal-limited forms of nephropathy in survival of African Americans on dialysis. Significant effects of family history on dialysis survival were not observed.31 This demonstrates that APOL1 genotypes provide improved discriminatory capability for dialytic survival in this population. The presence of diabetes clearly alters genetic susceptibility to development of ESRD; APOL1 is not associated with classic diabetic kidney disease32;33 and the survival outcome in patients with diabetes and ESRD was different from those in non-diabetic cases. How hyperglycemia impacts APOL1 genetic risk and why the previously reported lower level of CP in African Americans with diabetes lacking advanced nephropathy differs from the results in this study of prevalent dialysis patients requires further study.
This study has several strengths. Patients on hemodialysis recruited from two large university-owned dialysis programs were directly linked to an ESRD Network 6 database for precise determination of date of dialysis initiation, numbers of co-morbidities on Medical Evidence Reports, dialysis outcomes, and placement of either an arteriovenous fistula or graft for dialysis access at initiation, an important surrogate for pre-dialysis nephrology care. Due to the relatively long survival times and because the vast majority of patients currently receiving hemodialysis receive adequate therapy (as indicated by the Kt/V; K, dialyzer clearance of urea; t, dialysis time; V, volume of distribution of urea), we did not adjust for dialysis adequacy and other variables that change over time (serum phosphorus, dialysis catheters, etc). In addition, since patients were identified using unique Social Security Numbers linked to Network 6's patient database, there was little chance of misidentification. There are also limitations to this study. Our sample sizes were relatively small, since we precisely linked patients with ESRD Network 6 data to minimize potential misidentification by using patient names. The ESRD Network 6 database lacked patient weights and socioeconomic status, important determinants of survival on dialysis. In addition, the causes of ESRD in this report were clinically determined; misclassification is possible.25;34 Although analysis of prevalent patients introduced the potential for survivor bias, this would require early deaths to correlate with APOL1 risk variants with reversal of effects after the initial period; an unlikely scenario. Finally, patients on peritoneal dialysis were not included. Due to these limitations, we encourage others to assess dialysis survival based on APOL1 genotypes in robustly characterized populations.
We conclude that non-diabetic African Americans receiving chronic hemodialysis therapy have a marked survival advantage when they possess two APOL1 RRVs, compared with zero or one. Although the reasons for this finding are not known, the presence of renal-limited forms of glomerulosclerosis and the potential for less severe subclinical atherosclerosis are likely to play roles in this result.
Methods
Self-identified African Americans with ESRD were recruited from Wake Forest and Emory School of Medicine affiliated outpatient hemodialysis facilities in northwestern North Carolina and Atlanta, Georgia. The study was approved by the Institutional Review Boards at both universities and all participants provided written informed consent. Based on medical history from participants and medical record and kidney biopsy review, causes of ESRD were adjudicated at Wake Forest as previously described.28 Patients with non-diabetic ESRD included those with hypertension-attributed nephropathy, FSGS or FGGS, HIV-associated nephropathy, non-specific glomerulosclerosis, or unknown cause in the absence of a kidney biopsy, without other known risk factors for CKD. Inclusion criteria for patients with type 2 diabetes-attributed ESRD were diabetes onset after the age of 25 years and a diabetes duration that exceeded 5 years prior to dialysis initiation. Many of these patients also had diabetic retinopathy and/or overt proteinuria. Individuals with ESRD due to urologic disease, surgical nephrectomy, cystic kidney disease, Alport's Syndrome, Immunoglobulin A nephropathy, or membranous or membranoproliferative glomerulonephritis were not recruited.
Participants were linked to the ESRD Network 6-Southeastern Kidney Council Standardized Information Management System (SIMS) via Social Security Numbers to obtain dates of dialysis initiation, number of co-morbidities at dialysis initiation listed on the CMS Medical Evidence Report (CMS-2728 form), and dates of death, kidney transplantation, elective withdrawal from dialysis, or loss to follow-up.
Two single nucleotide polymorphisms (SNPs) in the APOL1 G1 nephropathy risk allele (rs73885319; rs60910145) and an insertion/deletion for the G2 risk allele (rs71785313) were genotyped using a custom assay designed at Wake Forest School of Medicine on the Sequenom platform (San Diego, California).35 G1 and G2 genotype calls were visually inspected for quality control. Genotyping of three blind duplicates resulted in a concordance rate of 100% and the genotyping efficiency for the three SNPs was >99% in all samples.
Clinical and demographic data were examined for expected ranges and outliers. Outcomes were provided by the ESRD Network 6-Southeastern Kidney Council SIMS database. Patients were at risk from the initiation of dialysis. Dates of death (including [but not limited to] within 30 days of elective withdrawal from dialysis), kidney transplantation, and loss to follow-up were recorded; outcomes were censored at the date of transplantation or through study end (July 1, 2015). APOL1 genotype data was examined for allele frequencies expected in African American populations. Multivariable Cox proportional hazards models were computed separately for participants with non-diabetic and diabetic ESRD. These Cox models included age at dialysis initiation, gender, number of comorbidities, African ancestry proportion, and presence of either an arteriovenous fistula or graft at dialysis initiation as covariates. Hazard ratios for the recessive and additive genetic models of the APOL1 risk genotype (two vs. one or zero copies of renal risk alleles, and two vs. one vs. zero) were computed from the Cox model. Parallel to these models, Kaplan-Meier curves for survival were computed separately for non-diabetic and diabetic ESRD patients, contrasting the APOL1 two-renal-risk-variant versus non-risk genotype (zero or one copy). Because the age of dialysis initiation in non-diabetic patients tended to be earlier than that in diabetic ESRD patients, analyses were repeated stratifying at the age of hemodialysis initiation 50 years, in order to verify that any potential difference in impact on survival was not due to a younger, healthier population.
Acknowledgements
Wake Forest University Health Sciences and Barry I. Freedman have filed for a patent related to APOL1 genetic testing. Dr. Freedman receives research funding from Novartis and is a consultant for Ionis Pharmaceuticals. None of the other authors reports a conflict of interest related to this work. This research was supported, in part, by NIH grants F30 DK098836 (JAB), R01 DK53591 (DWB), R01 DK070941 (BIF), and DK084149 (BIF). Computing was, in part, provided by the Center for Public Health Genomics. Support for the preparation of this document was provided in whole or part by contract Number HHSM-500-2013-NW006C, ESRD Network 6, funded by the Centers for Medicare & Medicaid Services, an agency of the U.S. Department of Health and Human Services. The content of this publication does not necessarily reflect the policies or positions of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. The authors assume full responsibility for the accuracy and completeness of the ideas presented.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Clark LT, Ferdinand KC, Flack JM, et al. Coronary heart disease in African Americans. Heart Dis. 2001;3(2):97–108. doi: 10.1097/00132580-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Young BA, Rudser K, Kestenbaum B, et al. Racial and ethnic differences in incident myocardial infarction in end-stage renal disease patients: The USRDS. Kidney Int. 2006;69:1691–1698. doi: 10.1038/sj.ki.5000346. [DOI] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K, Kovesdy CP, Derose SF, et al. Racial and survival paradoxes in chronic kidney disease. Nat Clin Pract Nephrol. 2007;3:493–506. doi: 10.1038/ncpneph0570. [DOI] [PubMed] [Google Scholar]
- 4.Kovesdy CP, Quarles LD, Lott EH, et al. Survival advantage in black versus white men with CKD: effect of estimated GFR and case mix. Am J Kidney Dis. 2013;62:228–235. doi: 10.1053/j.ajkd.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovesdy CP, Norris KC, Boulware LE, et al. Association of Race With Mortality and Cardiovascular Events in a Large Cohort of US Veterans. Circulation. 2015;132:1538–1548. doi: 10.1161/CIRCULATIONAHA.114.015124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kucirka LM, Grams ME, Lessler J, et al. Association of Race and Age With Survival Among Patients Undergoing Dialysis. JAMA: The Journal of the American Medical Association. 2011;306:620–626. doi: 10.1001/jama.2011.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karter AJ, Ferrara A, Liu JY, et al. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287:2519–2527. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 8.Young BA, Maynard C, Boyko EJ. Racial differences in diabetic nephropathy, cardiovascular disease, and mortality in a national population of veterans. Diabetes Care. 2003;26:2392–2399. doi: 10.2337/diacare.26.8.2392. [DOI] [PubMed] [Google Scholar]
- 9.Newman AB, Naydeck BL, Whittle J, et al. Racial differences in coronary artery calcification in older adults. Arterioscler Thromb Vasc Biol. 2002;22(3):424–430. doi: 10.1161/hq0302.105357. [DOI] [PubMed] [Google Scholar]
- 10.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2005;111(10):1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 11.Lee TC, O'Malley PG, Feuerstein I, et al. The prevalence and severity of coronary artery calcification on coronary artery computed tomography in black and white subjects. J Am Coll Cardiol. 2003;41(1):39–44. doi: 10.1016/s0735-1097(02)02618-9. [DOI] [PubMed] [Google Scholar]
- 12.Freedman BI, Hsu FC, Langefeld CD, et al. The impact of ethnicity and sex on subclinical cardiovascular disease: the Diabetes Heart Study. Diabetologia. 2005;48:2511–2518. doi: 10.1007/s00125-005-0017-2. [DOI] [PubMed] [Google Scholar]
- 13.Carnethon MR, Bertoni AG, Shea S, et al. Racial/Ethnic differences in subclinical atherosclerosis among adults with diabetes: the multiethnic study of atherosclerosis. Diabetes Care. 2005;28:2768–2770. doi: 10.2337/diacare.28.11.2768. [DOI] [PubMed] [Google Scholar]
- 14.Wassel CL, Pankow JS, Peralta CA, et al. Genetic ancestry is associated with subclinical cardiovascular disease in African-Americans and Hispanics from the multi-ethnic study of atherosclerosis. Circ Cardiovasc Genet. 2009;2:629–636. doi: 10.1161/CIRCGENETICS.109.876243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Divers J, Palmer ND, Lu L, et al. Admixture mapping of coronary artery calcified plaque in african americans with type 2 diabetes mellitus. Circ Cardiovasc Genet. 2013;6:97–105. doi: 10.1161/CIRCGENETICS.112.964114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tzur S, Rosset S, Shemer R, et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010;128:345–350. doi: 10.1007/s00439-010-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freedman BI, Kopp JB, Langefeld CD, et al. The Apolipoprotein L1 (APOL1) Gene and Nondiabetic Nephropathy in African Americans. J Am Soc Nephrol. 2010;21:1422–1426. doi: 10.1681/ASN.2010070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipkowitz MS. Apolipoprotein L1: from obscurity to consistency to controversy. Kidney Int. 2015;87:14–17. doi: 10.1038/ki.2014.319. [DOI] [PubMed] [Google Scholar]
- 20.Ito K, Bick AG, Flannick J, et al. Increased burden of cardiovascular disease in carriers of APOL1 genetic variants. Circ Res. 2014;114:845–850. doi: 10.1161/CIRCRESAHA.114.302347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freedman BI, Langefeld CD, Lu L, et al. APOL1 associations with nephropathy, atherosclerosis, and all-cause mortality in African Americans with type 2 diabetes. Kidney Int. 2015;87:176–181. doi: 10.1038/ki.2014.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukamal KJ, Tremaglio J, Friedman DJ, et al. APOL1 Genotype, Kidney and Cardiovascular Disease, and Death in Older Adults. Arterioscler Thromb Vasc Biol. 2015 doi: 10.1161/ATVBAHA.115.305970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez Suarez ML, homas DB, Barisoni L, et al. Diabetic nephropathy: Is it time yet for routine kidney biopsy? World J Diabetes. 2013;4:245–255. doi: 10.4239/wjd.v4.i6.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruzel-Davila E, Wasser WG, Aviram S, et al. APOL1 nephropathy: from gene to mechanisms of kidney injury. Nephrol Dial Transplant. 2015 doi: 10.1093/ndt/gfu391. [DOI] [PubMed] [Google Scholar]
- 25.Freedman BI, Cohen AH. Hypertension-attributed nephropathy: what's in a name? Nat Rev Nephrol. 2015 doi: 10.1038/nrneph.2015.172. [DOI] [PubMed] [Google Scholar]
- 26.Langefeld CD, Divers J, Pajewski NM, et al. Apolipoprotein L1 gene variants associate with prevalent kidney but not prevalent cardiovascular disease in the Systolic Blood Pressure Intervention Trial. Kidney Int. 2015;87:169–175. doi: 10.1038/ki.2014.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skorecki KL, Wasser WG. Hypertension-misattributed kidney disease in African Americans. Kidney Int. 2013;83:6–9. doi: 10.1038/ki.2012.369. [DOI] [PubMed] [Google Scholar]
- 28.Parsa A, Kao WH, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369:2183–2196. doi: 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Appel LJ, Wright JT, Jr., Greene T, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363:918–929. doi: 10.1056/NEJMoa0910975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alves TP, Wang X, Wright JT, Jr., et al. Rate of ESRD exceeds mortality among African Americans with hypertensive nephrosclerosis. J Am Soc Nephrol. 2010;21:1361–1369. doi: 10.1681/ASN.2009060654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freedman BI, Soucie JM, Kenderes B, et al. Family history of end-stage renal disease does not predict dialytic survival. Am J Kidney Dis. 2001;38(3):547–552. doi: 10.1053/ajkd.2001.26851. [DOI] [PubMed] [Google Scholar]
- 32.Freedman BI, Langefeld CD, Lu L, et al. Differential Effects of MYH9 and APOL1 Risk Variants on FRMD3 Association with Diabetic ESRD in African Americans. PLoS Genet. 2011;7:e1002150. doi: 10.1371/journal.pgen.1002150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iyengar SK, Sedor JR, Freedman BI, et al. Genome-Wide Association and Trans-ethnic Meta-Analysis for Advanced Diabetic Kidney Disease: Family Investigation of Nephropathy and Diabetes (FIND). PLoS Genet. 2015;11:e1005352. doi: 10.1371/journal.pgen.1005352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zarif L, Covic A, Iyengar S, et al. Inaccuracy of clinical phenotyping parameters for hypertensive nephrosclerosis. Nephrol Dial Transplant. 2000;15:1801–1807. doi: 10.1093/ndt/15.11.1801. [DOI] [PubMed] [Google Scholar]
- 35.Freedman BI, Langefeld CD, Turner J, et al. Association of APOL1 variants with mild kidney disease in the first-degree relatives of African American patients with non-diabetic end-stage renal disease. Kidney Int. 2012;82:805–811. doi: 10.1038/ki.2012.217. [DOI] [PMC free article] [PubMed] [Google Scholar]