Abstract
This study tested the hypothesis that estrogen programs mechanisms within the primate fetus that promote insulin sensitivity and glucose homeostasis in offspring. Glucose tolerance tests were performed longitudinally in prepubertal offspring of baboons untreated or treated on days 100 to 165/175 of gestation (term is 184 days) with the aromatase inhibitor letrozole which decreased fetal estradiol levels by 95%. Basal plasma insulin levels were over 2-fold greater in offspring delivered to letrozole-treated than untreated animals. Moreover, the peak 1 min, average of the 1, 3 and 5 min, and area under the curve blood glucose and plasma insulin levels after an iv bolus of glucose were greater (P<0.05 and P<0.01, respectively) in offspring deprived of estrogen in utero than in untreated animals and partially or completely restored in letrozole plus estradiol-treated baboons. The value for the homeostasis model assessment of insulin resistance was 2.5-fold greater (P<0.02) and quantitative insulin sensitivity check index lower (P<0.01) in offspring of letrozole-treated versus untreated animals and returned to almost normal in letrozole plus estradiol-treated animals. The exaggerated rise in glucose and insulin levels after glucose challenge in baboon offspring deprived of estrogen in utero indicates that pancreatic beta cells had the capacity to secrete insulin, but that peripheral glucose uptake and/or metabolism were impaired, indicative of insulin resistance and glucose intolerance. We propose that estrogen normally programs mechanisms in utero within the developing primate fetus that lead to insulin sensitivity, normal glucose tolerance and the capacity to metabolize glucose after birth.
Keywords: Estrogen, Insulin, Sensitivity, Offspring, Primate
Introduction
Estrogen has a fundamentally important role in the adult in regulating pancreatic islet beta-cell integrity and function and consequently the secretion and action of insulin important for the control of metabolism of glucose (reviewed in Liu & Mauvais-Jarvis, 2010; Mauvis-Jarvis et al., 2013; Gupte et al., 2015). Thus, estrogen stimulates proliferation (Choi et al. 2005) and protects against apoptosis (LeMay et al. 2006) of beta-cells in adult rodents and improves glucose-stimulated insulin secretion (Godsland 2005) and decreases the incidence of diabetes (Margolis et al. 2004) in postmenopausal women. Indeed, a sex-specific effect exists in which the incidence of diabetes mellitus is lower in women than in men, a benefit that is lost after menopause (Gale & Gillespie 2001, Moran et al. 2008, Louet et al. 2004, Geer & Shen 2009). Moreover, in several animal models estradiol at physiological levels inhibits, and androgens stimulate, oxidative stress-induced pancreatic beta-cell glucolipotoxicity and apoptosis (Le May et al. 2006, Paik et al., 1982, Nadal et al. 2009).
In addition to its effects on pancreatic beta-cell integrity and insulin secretion, estrogen also promotes insulin action. Thus, mice lacking estrogen receptor (ER) α exhibit insulin resistance within skeletal muscle and liver (Bryzgalova et al. 2006, Ribas et al. 2011, Manrique et al. 2012). In humans and rodents, mutation of the aromatase gene results in insulin insensitivity and glucose intolerance (Jones et al. 2000, Takeda et al. 2003, Rochira et al. 2000, Belgorosky et al. 2009). ERα is highly expressed in insulin-sensitive target tissues, including skeletal muscle (Deroo & Korach 2006, Heldring et al. 2007, Wiik et al. 2009). Estradiol stimulates insulin sensitivity and enhances glucose tolerance in skeletal muscle of adult mice (Gao et al. 2006, Lundholm et al. 2008, Riant et al. 2009) and protects ovariectomized mice from high fat diet-induced insulin resistance (Camporez et al. 2013, Jelenik & Roden 2013). Administration of the ERα agonist propylpyrazoletriyl and estradiol activation of Akt (Vasconsuelo et al. 2008, Rogers et al. 2009), an essential step in insulin receptor signaling, increases GLUT4 receptor protein transcription and glucose uptake in skeletal muscle of adult rats (Barros et al. 2006, 2009, Gorres et al. 2011). Estrogen replacement therapy reverses the increased incidence of insulin resistance which occurs in postmenopausal women (Margolis et al. 2004, Karjalainen et al. 2001; Manson et al., 2013). Estrogen also restores insulin sensitivity and glucose metabolism in ovariectomized rhesus monkeys fed a high-fat diet (Wagner et al. 1998).
Although the vast majority of studies of the effects of estrogen on insulin secretion and insulin action have been conducted in the adult, very little is known about the role of the hormonal milieu in utero and the mechanisms integral to fetal development that prepare the offspring for controlling insulin secretion and action and glucose homeostasis after birth. We have shown that the baboon provides a superb nonhuman primate translational model for the study of placental, developmental and perinatal biology (Albrecht & Pepe 1990, Pepe & Albrecht 1995). In the present study, therefore, we used the baboon and a highly specific aromatase inhibitor, letrozole, to suppress placental estrogen production and levels within the fetus during the second half of gestation to test the hypothesis that estrogen programs mechanisms within the developing fetus which promote insulin secretion and action and glucose homeostasis in offspring after birth. Moreover, basal fasting levels of the insulin receptor signaling components within skeletal muscle, where over 80% of total insulin-directed glucose uptake and metabolism occur (DeFronzo et al. 1981), were also quantified in the baboon fetus near term to determine the activity of the insulin receptor signaling pathway prior to delivery of the offspring and postnatal life.
Materials and Methods
Animals
Pregnant baboons
Female baboons (Papio anubis), originally obtained from the Southwest National Primate Research Center, San Antonio, TX, were housed individually in large primate cages in air-conditioned rooms with a 12h/12h light/dark lighting cycle and fed standard primate chow (Harlan Primate Diet, Madison, WI) twice daily, fresh fruit and vitamins daily and water ad libitum. Female baboons were paired with male baboons for 5 days at mid cycle and pregnancy confirmed by ultrasound. Pregnant baboons were then either untreated or treated between days 100 and 165–175 of gestation (term = 184 days) with the aromatase inhibitor letrozole (4,4-[1,2,3-triazol-1 yl-methylene]bis-benzonitrate, Novartis Pharma AG, Basel, Switzerland; 115 μg/kg body weight/day, via maternal sc injection in 1.0 ml sesame oil) or with letrozole (115 μg/kg body weight/day) plus estradiol benzoate (beginning at 25 μg/kg on day 100 and increasing to maximum of 115 μg/kg body weight between days 120 and 165–175). Blood samples (2–3 ml) were obtained at 1–3 day intervals during the second half of gestation from a peripheral maternal saphenous vein after brief restraint and sedation with ketamine HCl (10 mg/kg body weight, im) and serum estradiol levels quantified by immulite RIA (Albrecht et al. 2000). The use of baboons for this study was approved by the Institutional Animal Care and Use Committees of the University of Maryland School of Medicine and Eastern Virginia Medical School.
Fetal development
On day 165 of gestation, 5 of the fetuses (1 female, 4 males) from untreated baboons and 5 of the fetuses (1 female, 4 males) from letrozole-treated animals were delivered via cesarean section during isoflurane anesthesia, a 2 ml blood sample obtained from the umbilical artery for glucose and insulin assay, and fetuses immediately euthanized by an iv injection of pentobarbital (100 mg/kg body weight). A section (10 mm × 10 mm) of vastus lateralis skeletal muscle was then excised, frozen on dry ice and stored at −80 C until assayed for insulin signaling molecules.
Postnatal development
On days 165–175 of gestation, the remaining offspring from baboon mothers that had been untreated or treated with letrozole ± estradiol were either delivered spontaneously or were anesthetized with isoflurane and after obtaining an umbilical artery blood sample (2 ml) delivered by cesarean section to synchronize the timing of delivery. Baboon newborns that had been untreated (7 females, 3 males) or treated in utero with letrozole (3 females, 2 males) or letrozole plus estradiol (2 females, 2 males) were then left with and nursed by their mothers for 8 months at which time they were weaned and placed in pairs in cages immediately adjacent to their respective mothers and fed standard primate chow (Harlan Primate Diet) twice daily, fresh fruit and vitamins daily and water ad libitum. Every 2–6 months thereafter baboon offspring were briefly sedated with ketamine HCl (10 mg/kg body weight, im) and body weight and blood pressure (Dinamap Pro 400, GE Medical Systems, Milwaukee, WI) measured with animals in the supine position. At this time a blood sample (2–3 ml) was also withdrawn from a peripheral saphenous vein for the purpose of quantifying serum estradiol and testosterone levels by immulite RIA.
Glucose tolerance test
To avoid the potential impact on insulin action/glucose metabolism of the rise in estrogen and testosterone levels associated with the onset of puberty, an iv glucose tolerance test was performed, according to the established method of Overkamp et al (1997), sequentially, i.e. 3–5 times, on each baboon offspring at 6–12 month intervals between the postnatal ages of 1 and 3¼ years of age, i.e. prior to puberty which occurs at 3½ years in females and 4½ years of age in males within our baboon colony. The data obtained from the several glucose tolerance tests performed was averaged to yield a single value for each animal. Baboons were fasted overnight and at 08:00 h the following morning sedated with ketamine HCl (initially 5–10 mg/kg body weight, im; then 2 mg/kg body weight, iv) and positioned on their left side on a 37 C heating pad. Dextrose (0.25 g/kg body weight) was administered as a bolus injection via a 21 gauge needle into an antecubital vein at time 0 h. Blood samples (2.5 ml each) were obtained via a sterile catheter (21 gauge) inserted into a peripheral saphenous vein at −2 (i.e. basal fasting), 1, 3, 5, 10, 20, 40, 60 and 90 min before/after dextrose administration.
To determine potential impact of estrogen deprivation on glucose metabolism in maternal baboons prior to delivery, an iv glucose test was also performed as described above between days 151 and 163 of gestation in ketamine-sedated untreated (n=4), letrozole-treated (n=6) and letrozole plus estradiol-treated (n=4) baboon mothers.
Glucose and insulin assay
Blood glucose levels were determined via an iStat Portable Clinical Analyzer (Model #210003, Abbott Labs, East Windsor, NJ) on 0.1 ml of blood. The remainder of the blood sample was collected into a heparinized tube on ice, centrifuged at 3,500 × g for 15 min and the plasma stored at −20 C for insulin determination by solid-phase chemiluminescent immunometric assay via an Immulite System (Siemens Healthcare Diagnostics, Tarrytown, NY). The insulin assay employed a monoclonal murine anti-insulin antibody and internal insulin standard curve, displayed a sensitivity of 2 μIU/ml and intra- and inter-assay coefficients of variation of 5.7% and 5.9%, respectively, and exhibited no cross reactivity with other peptides.
Western immunoblot
Samples of fetal skeletal muscle were homogenized and lysed on ice in PBS containing 1% cholic acid, 0.1% SDS, 1 mM EDTA (Sigma-Aldrich, St Louis, MO) and a protease inhibitor cocktail, essentially as described previously (Zachos et al. 2004). Briefly, after determination of protein concentration using the bicinchoninic acid method (Sigma-Aldrich), samples (50 μg protein) were mixed with 5X Laemmli buffer, heated to 95 C for 5 min, cooled on ice for 2 min and centrifuged (800 × g). Samples were then loaded onto 7.5% (IRS-1, AS160) or 10% (Akt/pAkt, GLUT1, GLUT4/pGLUT4) SDS-polyacrylamide gels (PAGE) and electrophoresed using a Bio-Rad Mini-Protean electrophoresis chamber (Bio-Rad Laboratories, Richmond, CA) and SDS-PAGE running buffer comprised of 25 mM Tris (pH 8.3), 192 mM glycine and 0.1% SDS. Proteins were wet-transferred onto an Immobilon-P membrane (Millipore Corp., Bedford, MA), blocked 1 h at room temperature with 5% BSA in 10 mM Tris-HCl, pH 7.5, 150 mM NaCl and 0.2% Tween 20 buffer (TBST), and then incubated overnight at 4 C with the following primary rabbit polyclonal antibodies diluted in TBST containing 5% BSA: anti-Akt (1:500 dilution, Cell Signaling, Danvers, MA), anti-pAkt-S473 (1:500, Cell Signaling), anti-pAkt-T308 (1:500, Abcam, Cambridge, MA), anti-AS160 (1:1,000, Cell Signaling), anti-GLUT1 (1:500, Abcam), anti-pGLUT4-S488 (1:1,000, Abcam), anti-IRS-1 (1:5,000, Thermo Fisher, Pittsburgh, PA), and anti-GAPDH (1:5,000, Abcam), and mouse monoclonal anti-GLUT4 (1:1,000, Abcam). After three washes (6 min) in TBST, membranes were incubated for 1 h at room temperature with horseradish peroxidase (HRP)-labeled secondary antibody (Serotec UK) in TBST containing 5% BSA. After washing in TBST, membranes were developed with enhanced chemiluminescence (GE Healthcare, Pittsburgh, PA) according to manufacturer’s instructions and membranes exposed to Fuji Super RX medical x-ray film (Fujifilm Medical Systems, USA, Inc., Roselle, IL) and band intensities quantified by densitometry using Image J software (National Institutes of Health). Blots were then stripped and re-probed using HRP-conjugated GAPDH as an internal loading control and results, arbitrary densitometric units/μg protein expressed as a ratio to GAPDH. Specificity of the primary antibodies was determined by incubation of samples without primary antibody.
Statistical analysis
Baboons were randomly assigned to the treatment groups and data expressed as means ± SE. Data were analyzed by ANOVA with post hoc comparison of the means by either Tukey-Kramer multiple comparisons test or Kruskal-Wallis nonparametric test using SAS statistical software (SAS Institutes).
Results
Serum steroid hormone levels
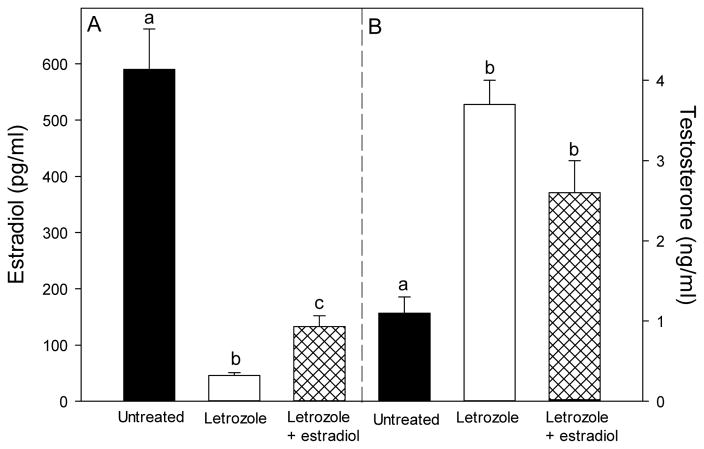
Maternal peripheral saphenous vein serum estradiol levels in untreated baboons increased from a mean ± SE of 1.0 ± 0.2 ng/ml on day 100 (i.e. midgestation) to 3.6 ± 0.4 ng/ml on days 165/175 of gestation. The administration of letrozole beginning on day 100 resulted in serum estradiol which rapidly declined within 2 days to and remained at levels of <0.1 ng/ml. Concomitant administration of letrozole and estradiol resulted in a pattern of increasing maternal peripheral serum estradiol levels that was similar to that in untreated animals. Consequently, at the time of delivery on days 165–175 of gestation, serum estradiol concentrations in blood delivered to the fetus (i.e. umbilical vein) of letrozole-treated baboons (46 ± 5 pg/ml) was only 5% of that (P<0.001) in untreated animals (590 ± 72 pg/ml, Fig. 1). Umbilical artery serum estradiol levels in letrozole plus estradiol-treated baboons were increased to a level (133 ± 19 pg/ml) almost 3-fold greater (P<0.01) than in animals treated with letrozole alone, but remained lower (P<0.001) than in untreated animals.
Fig. 1.
Umbilical artery serum estradiol and testosterone levels (means ± SE) on days 165–175 of gestation in baboons untreated or treated on days 100–165/175 with letrozole (115 μg/kg body weight/day via maternal sc injection), or letrozole (115 μg/kg body weight) plus estradiol (25 to 115 μg/kg body weight/day). Data bars marked with different letters are significantly different (P<0.01, ANOVA, Tukey-Kramer multiple comparison statistic) from one another.
Umbilical artery serum testosterone levels on days 165–175 in letrozole-treated baboons (3.7 ± 0.3 ng/ml) were over 3-fold greater (P<0.01) than in untreated controls (1.1 ± 0.2 ng/ml, Fig. 1). Serum testosterone levels remained elevated in baboons treated with both letrozole and estradiol, because of continued inhibition of aromatization of C19 steroid precursors to estrogen in these animals.
Postnatal development
Growth and serum analytes
Body weights on days 165–175 of gestation were similar in newborns delivered to untreated, letrozole-treated and letrozole plus estradiol-treated baboons (Table 1). However, placental weight was approximately 10% greater (P<0.05) in letrozole-treated than in untreated animals and returned to normal by letrozole plus estrogen treatment (Table 1).
Table 1.
Placental weight and newborn body weight and fasting blood glucose and plasma insulin levels in baboons
| Treatment | Placental wt (gm) | Body wt (gm) | Glucose (mg/dl) | Insulin (μIU/ml) |
|---|---|---|---|---|
| Untreated | 183 ± 9 | 810 ± 27 | 74 ± 6 | 3.7 ± 0.3 |
| Letrozole | 207 ± 16* | 790 ± 62 | 70 ± 8 | 4.1 ± 0.4 |
| Letrozole + estradiol | 176 ± 12 | 767 ± 59 | 75 ± 7 | 3.9 ± 0.7 |
Values are expressed as means ± SE on the day of delivery (days 165–175 of gestation) in newborns from baboons untreated (n=10) or treated on days 100–165/175 of gestation (term = 184 days) with letrozole (115 μg/kg body weight/day via maternal sc injection, n=5) or letrozole (115 μg/kg body weight) plus estradiol (25 to 115 μg/kg body weight/day, n=4).
P<0.05 versus untreated.
The body weights of baboon offspring increased (P<0.01) progressively throughout postnatal life, reaching levels at 3 years of age that were similar in animals untreated and treated in utero with letrozole or letrozole plus estrogen (Table 2). Corresponding with the normal rate of growth, mean arterial blood pressure and levels of serum chemistry analytes, including AST, creatinine, triglycerides and cholesterol, were similar at birth, throughout postnatal maturation and at 3 years of age in offspring delivered to baboons untreated or treated during the second half of gestation with letrozole or letrozole plus estradiol (Table 2).
Table 2.
Body weight, blood pressure and serum chemistry analytes in baboon offspring
| Treatment | Body weight (kg) | MABP (mmHg) | AST (U/L) | Creatinine (mg/dl) | Triglycerides (mg/dl) | Cholesterol (mg/dl) |
|---|---|---|---|---|---|---|
| Untreated | 9.6 ± 0.1 | 63 ± 9 | 34.4 ± 7.6 | 1.4 ± 0.2 | 52.1 ± 5.0 | 108 ± 10.1 |
| Letrozole | 9.9 ± 0.2 | 60 ± 10 | 37.8 ± 5.1 | 1.3 ± 0.1 | 53.5 ± 6.8 | 112 ± 9.2 |
| Letrozole + estradiol | 9.6 ± 0.1 | 62 ± 8 | 32.7 ± 4.2 | 1.5 ± 0.3 | 51.7 ± 4.9 | 109 ± 7.2 |
Values are expressed as means ± SE at 3 years of age in offspring delivered to baboons untreated (n=10) or treated on days 100–165/175 of gestation with letrozole (n=5) or letrozole plus estradiol (n=4).
Serum estradiol levels in female baboon offspring that had been untreated or treated in utero with letrozole or letrozole plus estradiol were very low (i.e. 15–30 pg/ml) throughout the first 3½ years of postnatal life. Serum testosterone also remained at basal levels (i.e. nondetectable at <0.02 ng/ml) during the first 3½ years of postnatal life in male baboon offspring untreated or treated prenatally with letrozole or letrozole plus estradiol.
Glucose and insulin metabolic parameters in offspring
Basal blood glucose and plasma insulin levels at the time of delivery near term were not significantly different in newborns delivered to baboons untreated (74 ± 6 mg/dl and 3.7 ± 0.3 μIU/ml, respectively) or treated in utero with letrozole or letrozole plus estradiol (Table 1). Basal levels of glucose immediately prior to the glucose (dextrose) tolerance test administered postnatally also were not significantly different in offspring from baboons untreated (67 ± 3 mg/dl) or treated throughout the second half of gestation with letrozole (69 ± 2 mg/dl) or with letrozole plus estradiol (69 ± 2 mg/dl). However, basal insulin levels were over 2-fold greater (P=0.06) in offspring treated prenatally with letrozole (12.3 ± 3.6 μIU/ml) than in untreated animals (5.2 ± 1.0 μIU/ml) and restored after letrozole plus estradiol treatment (6.9 ± 1.9 μIU/ml).
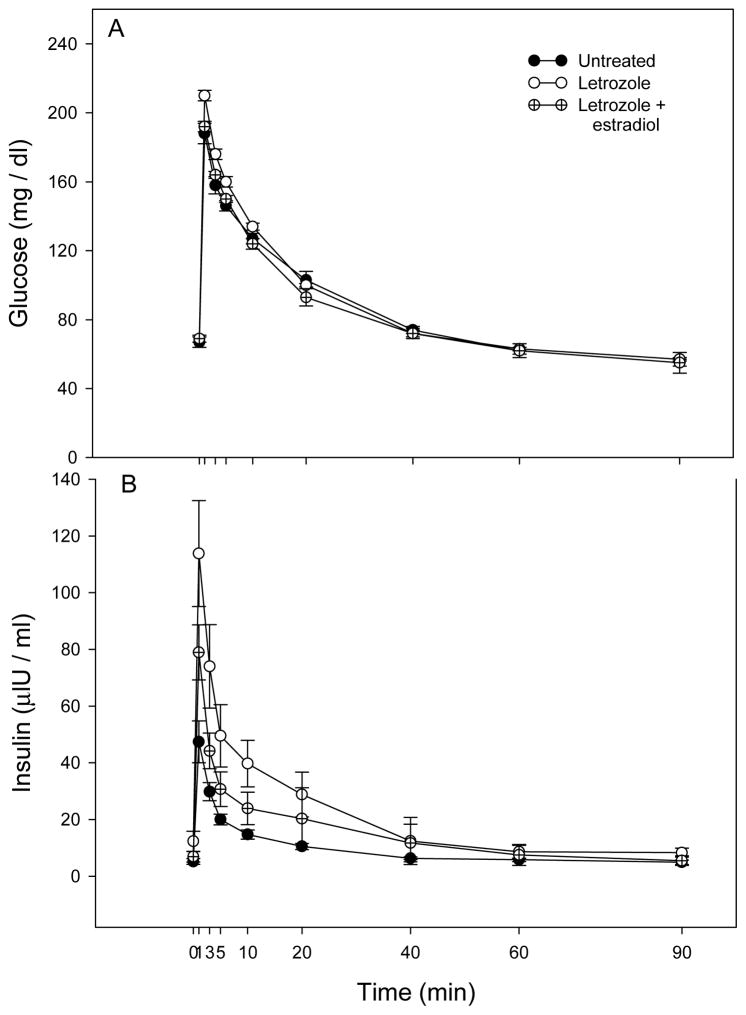
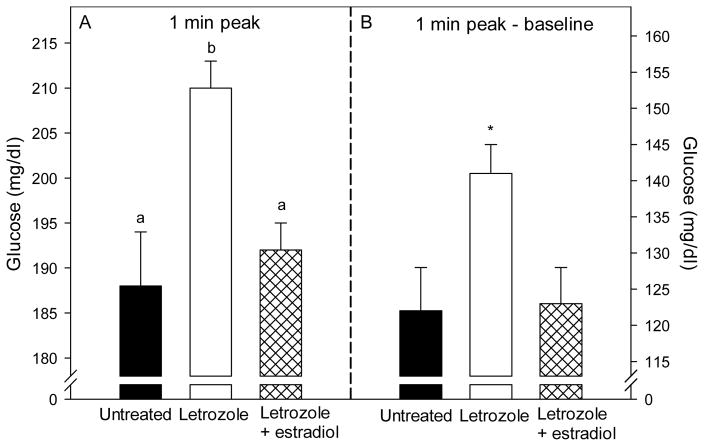
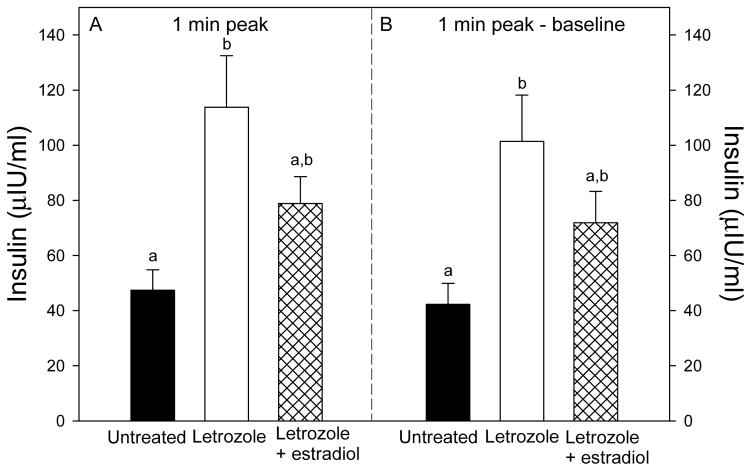
The patterns of glucose and insulin levels during the glucose challenge test in offspring delivered to untreated, letrozole-treated, and letrozole plus estradiol-treated baboons are shown in Fig. 2. Within 1 min of a bolus iv injection of dextrose, blood glucose and plasma insulin increased to peak levels, then rapidly declined and were restored within 60–90 min to pre-challenge levels. However, in baboon offspring treated in utero with letrozole, the peak 1 min post challenge levels of glucose (210 ± 3 mg/dl, Fig. 3A) and insulin (113.8 ± 18.7 μIU/ml, Fig. 4A) were greater (P<0.05 and P<0.01, respectively) than in untreated animals (188 ± 6 mg/dl and 47.4 ± 7.4 μIU/ml, respectively), and completely (glucose) or partially (insulin) returned to normal in baboons treated in utero with letrozole plus estradiol (192 ± 3 mg/dl and 78.9 ± 9.7 μIU/ml, respectively). Moreover, the net elevations (i.e. 1 min peak minus baseline) in glucose (Fig. 3B) and insulin (Fig. 4B) levels also were greater (P=0.08 and P<0.01, respectively) in letrozole-treated (141 ± 4 mg/dl and 101.4 ± 16.8 μIU/ml) than in untreated (122 ± 6 mg/dl and 42.3 ± 7.6 μIU/mg/dl) offspring and completely (glucose) or partially (insulin) restored in letrozole plus estradiol-treated animals (123 ± 5 mg/dl and 71.9 ± 11.4 μIU/ml).
Fig. 2.
Patterns of blood glucose and plasma insulin levels after iv administration at time 0 min of a bolus of dextrose during postnatal life to prepubertal offspring delivered to baboons untreated (n=10) or treated with letrozole (n=5) or letrozole plus estradiol (n=4) as detailed in the legend of Fig. 1. Values at each time point are the means ± SE of the average of several glucose challenge tests performed longitudinally (i.e. every 6–12 months) in each animal at 1–3¼ years of postnatal life (i.e. prior to puberty).
Fig. 3.
Blood glucose levels, expressed as 1 min peak (panel A) and 1 min peak-baseline (panel B) levels, after administration of an iv bolus of dextrose to baboon offspring untreated (n=10) or treated in utero with letrozole (n=5) or letrozole plus estradiol (n=4). Values of the bars in panel A with different letters are significantly different from each other (P<0.05, ANOVA, Tukey-Kramer multiple comparison test). *P=0.08 versus untreated or letrozole plus estradiol groups (panel B, ANOVA, Kruskal-Wallis nonparametric test).
Fig. 4.
Plasma insulin levels, expressed as 1 min peak (panel A) and 1 min peak-baseline (panel B) levels, after administration of an iv bolus of dextrose to the same baboon offspring in which serum glucose levels are shown in Fig. 3. Values of the bars with different letters are significantly different from each other (P<0.01, ANOVA, Tukey-Kramer multiple comparison test).
When the 1, 3 and 5 min levels in glucose and insulin were averaged, the overall mean levels of glucose (183 ± 4 mg/dl) and insulin (77.7 ± 13.6 μIU/ml) were greater (P<0.05 and P<0.01, respectively) in baboon offspring treated prenatally with letrozole than in animals untreated and partially restored by letrozole plus estradiol administration (Table 3). The overall mean levels of blood glucose (114 ± 5 mg/dl) and plasma insulin (65.4 ± 11.7 μIU/ml) at 1, 3 and 5 min minus baseline level also were greater (P=0.07 and P<0.01, respectively) in baboons treated in utero with letrozole than in untreated animals and restored by treatment with letrozole plus estradiol (Table 3). The values of area under the curve (AUC) for glucose (1,098 ± 21) and insulin (574 ± 85) in offspring from letrozole-treated baboons also were greater (P<0.05 and P<0.001, respectively) than in untreated animals and partially restored in letrozole plus estradiol-treated animals (Table 3). The ratio of glucose AUC to insulin AUC was lower (P<0.01) in offspring delivered to letrozole-treated baboons (2.04 ± 0.25) than in untreated offspring, indicative of increased capacity for insulin secretion, and restored to normal with letrozole plus estradiol treatment (Table 3).
Table 3.
Blood glucose and plasma insulin levels in baboon offspring after an iv glucose tolerance test
| Treatment | Glucose (mg/dl)
|
Glucose (AUC) | Insulin (μIU/ml)
|
Insulin (AUC) | Glucose AUC Insulin AUC | ||
|---|---|---|---|---|---|---|---|
| Mean 1+3+5 min | Mean 1+3+5 min - baseline | Mean 1+3+5 min | Mean 1+3+5 min - baseline | ||||
| Untreated | 165 ± 4a | 99 ± 5 | 977 ± 34a | 32.4 ± 3.8a | 26.6 ± 4.4a | 192 ± 23a | 5.84 ± 0.72a |
| Letrozole | 183 ± 4b | 114 ± 5* | 1098 ± 21b | 77.7 ± 13.6b | 65.4 ± 11.7b | 574 ± 85b | 2.04 ± 0.25b |
| Letrozole + estradiol | 169 ± 2a,b | 99 ± 5 | 1006 ± 10a,b | 53.2 ± 3.7a,b | 44.9 ± 2.2a,b | 318 ± 22c | 3.24 ± 0.27a |
Values are the means ± SE of the average levels of blood glucose and plasma insulin at 1, 3 and 5 min, area under curve (AUC) for glucose and insulin, and ratio of glucose AUC ÷ insulin AUC after an iv bolus of dextrose to prepubertal offspring from baboons untreated (n=10) or treated with letrozole (n=5), or letrozole plus estradiol (n=4). Values with different letters are different from each other (P<0.05, glucose: P<0.01, insulin; ANOVA, Tukey-Kramer Multiple Comparison test).
P=0.07 versus untreated or letrozole plus estradiol-treatment (ANOVA, Kruskal-Wallis test).
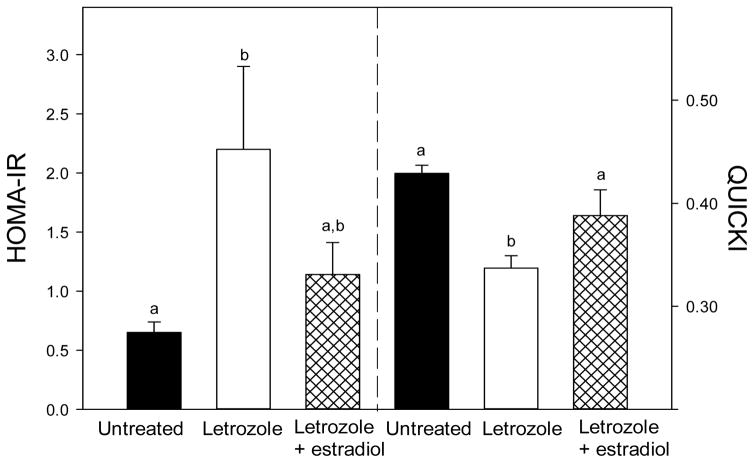
The HOMA-IR and QUICKI have not been validated for use in prepubertal and pregnant baboons. However, they are well-established as indices of insulin resistance and insulin sensitivity in the human and many animal models and therefore were employed in the present study. The value for the HOMA-IR was over 3-fold greater (P<0.05) in offspring delivered to baboons treated with the aromatase inhibitor letrozole (2.20 ± 0.70) than in untreated animals (0.65 ± 0.09) and partially restored in animals treated with letrozole plus estradiol (1.14 ± 0.27, Fig. 5A). The value for the QUICKI, an index highly correlated with assessment of insulin sensitivity using the euglycemic glucose clamp method (Katz et al. 2000, Chen et al. 2005, Lee et al. 2011), was lower (P<0.01) in offspring from letrozole-treated baboons (0.337 ± 0.012) than untreated animals (0.429 ± 0.008) and restored to normal by letrozole plus estradiol administration (0.388 ± 0.025, Fig. 5B).
Fig. 5.
(A) HOMA-IR (i.e. basal glucose x basal insulin levels ÷ 405) and (B) QUICKI (i.e. 1/[log (Io) + log (Go)], where Io is the basal insulin and Go the basal glucose levels) for the same baboon offspring in which serum glucose levels are shown in Fig. 3. Values of the bars with different letters are significantly different from each other (P<0.05 for HOMA-IR and P<0.01 for QUICKI, ANOVA, Tukey-Kramer multiple comparison test).
There was no significant change, as analyzed by repeated measures mixed-model ANOVA, in the various glucose and insulin indices obtained sequentially during postnatal development in offspring delivered to untreated, letrozole-treated or letrozole plus estradiol-treated baboons. For example, mean ± SE values for glucose AUC in untreated offspring were 929 ± 75, 1,016 ± 33 and 1,017 ± 21 at 1, 2 and 3 years of age, respectively. Mean ± SE insulin AUC values also were similar in untreated offspring at 1 (251 ± 79), 2 (203 ± 37) and 3 (219 ± 35) years of age.
Glucose and metabolic parameters in maternal baboons
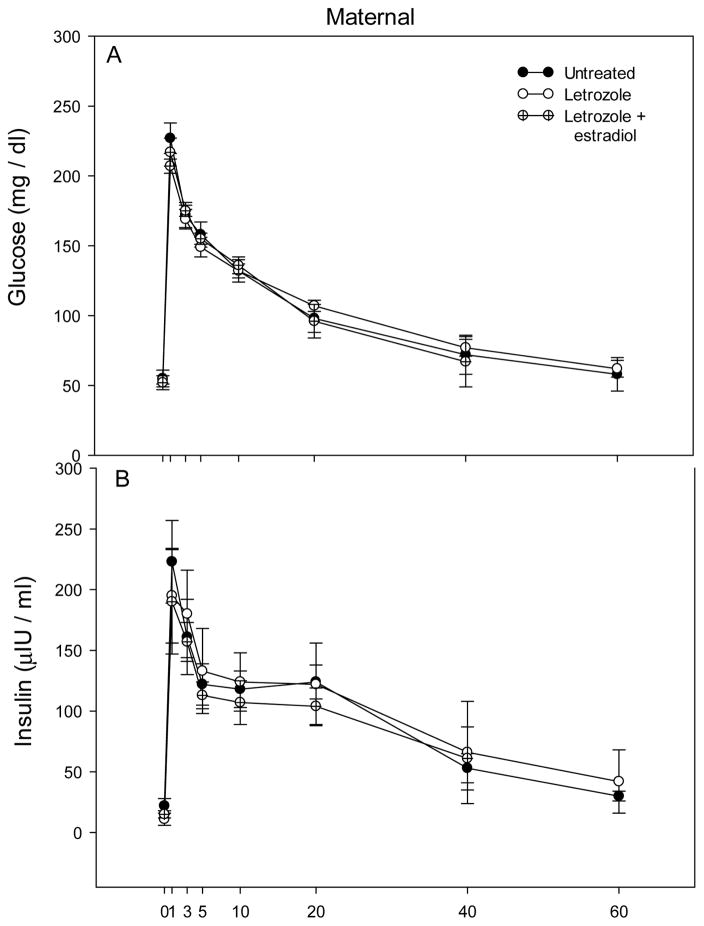
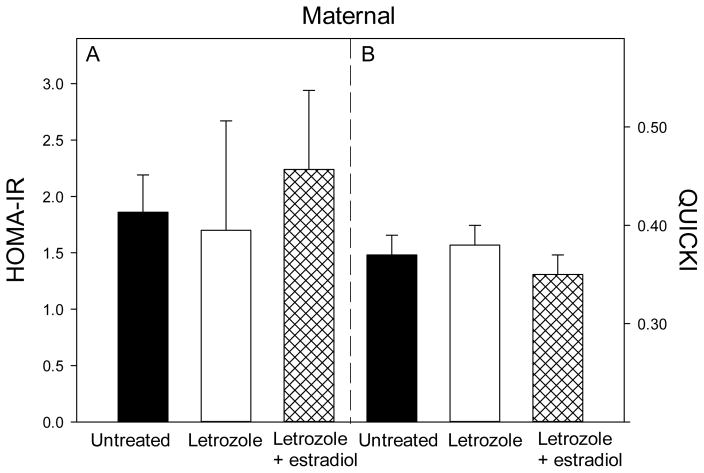
Maternal body weight near term was similar in baboons untreated (18.6 ± 1.2 kg) or treated with letrozole (18.8 ± 1.3 kg) or letrozole plus estradiol (18.6 ± 1.0 kg). The patterns of blood glucose and plasma insulin levels in maternal baboons during the glucose challenge test are shown in Fig. 6. Basal glucose (mg/dl) and insulin (μIU/dl) levels were similar in untreated (55 ± 6 and 22.5 ± 6, respectively), letrozole-treated (54 ± 3 and 10.7 ± 5) and letrozole plus estradiol-treated (52 ± 5 and 15.8 ± 4) baboons (Fig. 6). The mean (± SE) of the 1 + 3 + 5 min levels, AUC of glucose and insulin and the ratio of glucose AUC/insulin AUC also were similar in each of the groups (Table 4). Moreover, HOMA-IR and QUICKI values (Fig. 7) were similar in untreated (1.86 ± 0.33, 0.37 ± 0.02), letrozole-treated (1.70 ± 0.97, 0.38 ± 0.02) and letrozole plus estradiol-treated (2.24 ± 0.70, 0.35 ± 0.02) baboon mothers.
Fig. 6.
Mean (± SE) levels of blood glucose and plasma insulin after iv administration at time 0 min of a bolus of dextrose at 151–163 days of gestation in maternal baboons untreated (n=4) or treated with letrozole (n=6) or letrozole plus estradiol (n=4).
Table 4.
Blood glucose and plasma insulin levels in maternal baboons during an iv glucose tolerance test
| Treatment | Glucose (mg/dl)
|
Glucose (AUC) | Insulin (μlU/ml)
|
Insulin (AUC) | Glucose AUC Insulin AUC | ||
|---|---|---|---|---|---|---|---|
| Mean 1+3+5 min | Mean 1+3+5 min -basal | Mean 1+3+5 min | Mean 1+3+5 min -basal | ||||
| Untreated | 185 ± 9 | 130 ± 5 | 869 ± 44 | 175 ± 24 | 153 ± 30 | 819 ± 111 | 1.16 ± 0.27 |
| Letrozole | 179 ± 5 | 129 ± 7 | 844 ± 28 | 174 ± 34 | 166 ± 31 | 805 ± 158 | 1.19 ± 0.29 |
| Letrozole + estradiol | 182 ± 5 | 128 ± 5 | 858 ± 14 | 164 ± 21 | 145 ± 20 | 770 ± 96 | 1.18 ± 0.15 |
Values are the means ± SE of the average levels of maternal blood glucose and plasma insulin at 1, 3 and 5 min, area under curve (AUC) for glucose and insulin, and glucose AUC ÷ insulin AUC after an iv bolus of dextrose on days 151–163 of gestation to baboons untreated (n=4) or treated with letrozole (n=6), or letrozole plus estradiol (n=4).
Fig. 7.
HOMA-IR and QUICKI values for the baboon mothers in which glucose and insulin levels are shown in Fig. 6.
Fetal insulin signaling components
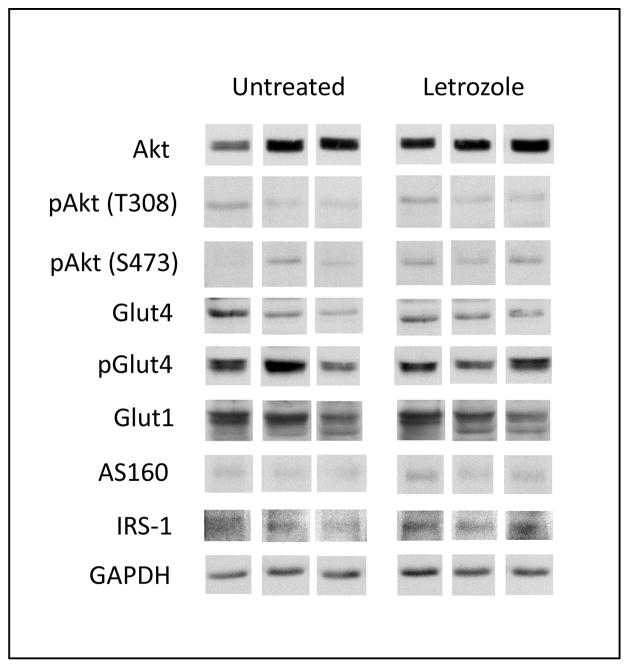
Skeletal muscle of fetuses delivered near term to untreated and letrozole-treated baboons expressed, via Western immunoblotting, the major protein components of the insulin signaling pathway, including IRS-1 (approximate molecular size of 185 kDA), total Akt, Akt phosphorylated at threonine 308 or serine 473 (each 60 kDa), Glut-1, Glut-4, Glut-4 phosphorylated at serine 488 (each 55 kDa) and total AS160 (160 kDa). Representative examples of these components of the insulin signaling pathway expressed in fetal skeletal muscle of untreated and letrozole-treated baboons are shown in Fig. 8. Quantitative densitometric analyses showed that basal levels (i.e. non-insulin stimulated) of each of these proteins, expressed as a ratio to GAPDH, were similar in fetal skeletal muscle of baboons untreated or treated with letrozole (Table 5). Moreover, in the absence of insulin challenge, the mean (± SE) ratios of p S473 Akt/total Akt (0.144 ± 0.031), p T308 Akt/total Akt (0.114 ± 0.015), and p Glut-4/total Glut-4 (2.50 ± 0.43) in fetal skeletal muscle of letrozole-treated baboons were not different from values in untreated baboons (0.112 ± 0.020; 0.113 ± 0.030; 2.69 ± 0.68, respectively).
Fig. 8.
Representative Western immunoblot of total and phosphorylated insulin receptor signaling and GLUT proteins in extracts of skeletal muscle of fetuses obtained on day 165 of gestation from baboons untreated or treated with letrozole. Protein samples from untreated and letrozole-treated animals were applied side-by-side and electrophoresed on the same membrane. For illustrative purposes, each protein band was positioned separately below the untreated and letrozole columns in Fig. 8.
Table 5.
Skeletal muscle insulin signaling molecule protein levels in fetuses delivered to baboons untreated or treated with letrozole
| IRS-1 | Akt | pAkt(T) | pAkt(S) | GLUT4 | pGLUT4 | GLUT1 | AS160 | |
|---|---|---|---|---|---|---|---|---|
| Untreated | 0.39 ± 0.15 | 2.82 ± 0.48 | 0.30 ± 0.06 | 0.22 ± 0.04 | 0.60 ± 0.16 | 3.26 ± 1.33 | 3.58 ± 1.19 | 0.16 ± 0.04 |
| Letrozole | 0.36 ± 0.20 | 3.40 ± 1.06 | 0.38 ± 0.17 | 0.27 ± 0.06 | 0.48 ± 0.10 | 2.73 ± 0.86 | 2.92 ± 0.78 | 0.25 ± 0.09 |
Values are the means (± SE) of skeletal muscle insulin signaling molecule protein levels (arbitrary densitometric units expressed as a ratio to GAPDH) quantified by Western immunoblot in fetuses obtained on day 165 of gestation from baboons untreated (n=5) or treated daily on days 100–165 with letrozole (0.115 mg/kg body weight/day, sc, n=5).
Discussion
The present study shows, for the first time in a nonhuman primate, that the peak rise in blood glucose level induced by a glucose challenge was greater in prepubertal offspring delivered to baboons in which estrogen had been suppressed throughout the second half of pregnancy than in animals exposed in utero to the normal elevation in estrogen. Moreover, the exaggerated rise in glucose levels of estrogen-suppressed baboon offspring was associated with significantly greater basal and glucose-induced levels of plasma insulin, as well as lower ratio of glucose AUC/insulin AUC, indicating that the pancreatic beta cells had the capacity to secrete insulin, but that peripheral glucose uptake and/or metabolism were impaired, a condition indicative of insulin resistance or type 2 diabetes. We propose, therefore, that estrogen normally has an important role in programming mechanisms in utero within the developing fetus that lead to insulin sensitivity and the capacity to metabolize glucose after birth.
The latter concept is consistent with previous observations of impaired insulin sensitivity and glucose metabolism in aromatase-null laboratory mice (Jones et al. 2000, Takeda et al. 2003), in the few clinical cases of aromatase deficiency that have been reported in humans (Belgorosky et al. 2009, Rochira et al. 2007, Zirilli et al. 2008, Guercio et al. 2009), and in healthy men administered anastrozole to suppress aromatase (Gibb et al., 2016). In addition, ERα-null mice develop insulin resistance, glucose intolerance and decreased glucose uptake in skeletal muscle (Bryzgalova et al. 2006, Ribas et al. 2011, Manrique et al. 2012, Couse & Korach, 1999, Heine et al. 2000). In contrast to estrogen deprivation, the administration of phytoestrogen to mice throughout gestation enhanced glucose tolerance in offspring at adulthood (Cederroth, 2009). Estrogen treatment reversed insulin resistance and normalized serum insulin levels in aromatase-deficient mouse offspring (Takeda et al. 2003). Moreover, low estrogen levels in adult laboratory rodents have been shown to result in insulin resistance in target tissues (Godsland, 2005, Nadal et al. 2009, Riant et al. 2009, Bryzgalova et al. 2008, Ropero et al. 2008).
Our study takes on heightened translational clinical significance when considering exposure of the fetus during human pregnancy to endocrine disruptors which interfere with estrogen action (Diamanti-Kandarakis et al. 2009, Robins et al. 2011, Stel & Legler, 2015) and the increasingly high incidence of premature birth in humans (Voelker, 2010), a condition that deprives the developing fetus of the normal elevation in estrogen of late gestation. However, preterm birth is also accompanied by lower than normal birth weight. Low birth weight resulting from preterm birth in humans (Barker, 2005, Ross & Beall, 2008, Gluckman et al. 2008, Thompson & Regnault 2011) and nonhuman primates (Blanco et al. 2010, 2015) and intrauterine growth restriction induced experimentally by placental insufficiency (Thorn et al. 2009), uterine artery ligation/uteroplacental insufficiency (Simmons et al. 2001), or maternal nutrient restriction (Ozanne et al. 1996, Desai et al., 1997, Kind et al. 2003, Choi et al. 2011) of laboratory animals result in insulin resistance in offspring. However, placental and fetal growth (Table 1), maternal glucose tolerance (Fig. 6 and Table 4), umbilical blood flow (Aberdeen et al. 2010), and placental villous vascularization (Robb et al. 2077), were not decreased in letrozole-treated/estrogen-deprived baboons. Overweight/obesity also predicts insulin resistance in baboons (Chavez et al., 2008, 2009), however, body weight was normal in insulin resistant offspring delivered to letrozole-treated baboons (Table 2). Therefore, the development of insulin resistance in offspring delivered to estrogen-deprived baboons of the current study was not associated with/caused by a disruption of maternal glucose metabolism, utero-placental perfusion, fetal growth restriction or overweight. We suggest that this points to the selective role of estrogen in programming processes during fetal development that promote insulin action after birth.
The mechanisms(s) by which estrogen programs processes in the developing primate fetus that prepare the offspring to respond to insulin after birth are unknown at this point. Insulin action requires a sequence of several essential steps, including development of an extensive blood vessel network to deliver circulating insulin to cells of the target tissues (Richards et al. 2010), binding of insulin to the insulin receptor, phosphorylation of IRS, expression and phosphorylation/activation of the insulin receptor signaling molecules, e.g. serine/threonine kinase Akt, phosphatidylinositol, and glucose transporters notably GLUT 4, and facilitated intracellular transport of glucose (Nystrom & Quon, 1999). Insulin receptor is expressed in high level in tissues of the human (Kaplan, 1984) and baboon (Blanco et al. 2010) fetus. Insulin target tissues, such as skeletal muscle (Wiik et al. 2009, Barros et al. 2006) and adipose (Dieudonné et al. 2004), express estrogen receptor and thus are estrogen responsive. In rodents, estrogen upregulated expression of GLUT4 (Barros et al. 2006, Moreno et al. 2010) and Akt (Vasconsuelo et al. 2008, Rogers et al. 2009) in skeletal muscle and GLUT4 expression was impaired in insulin-resistant rats (Kahn et al. 1991). In the current study, the basal total and phosphorylated levels of several components of the insulin signaling pathway, including IRS-1, Akt and GLUT4, were similar in skeletal muscle of near-term fetuses obtained from baboons untreated or treated with letrozole. However, whether the expression of these insulin signaling molecules in skeletal muscle and other insulin target tissues of fetuses or offspring delivered to estrogen-deprived baboons is altered after insulin challenge is unknown. Additional investigation is required, therefore, to determine the latter question and consequently the mechanisms that underlie the disruption of insulin sensitivity after birth induced by estrogen deprivation during fetal development.
In the present study, the glucose challenge-induced increases in blood glucose and plasma insulin in offspring delivered to baboons treated in utero with letrozole were largely but not completely prevented by concomitant administration of letrozole plus estradiol. This may have occurred because estradiol levels within the fetus of letrozole plus estradiol-treated baboons were only partially restored to normal since estradiol was administered to baboon mothers and is not readily transferred across the placenta into the fetus due to placental metabolism and selective placental secretion of estrogen into the maternal compartment during human and nonhuman primate pregnancy (Albrecht & Pepe, 1990). However, testosterone levels were elevated in baboon fetuses treated with both the aromatase inhibitor letrozole and with letrozole plus estradiol. Androgens, in high levels, have the capacity to impair insulin sensitivity (Golden et al. 2007), and administration of androgen to rhesus monkeys and sheep during early gestation disrupted insulin sensitivity in the offspring (Eisner et al. 2000, Bruns et al. 2004, Padmanabhan et al. 2010). Consequently, endogenous sex hormones may differentially regulate insulin sensitivity in men and women (Ding et al. 2006). An additional possibility, therefore, is that the insulin insensitivity and glucose intolerance which developed in baboon offspring treated prenatally with letrozole resulted from a combination of estrogen suppression and androgen excess, and thus an alteration in the ratio of estrogen and androgen.
In summary, the present study shows that the rise in serum glucose level induced by glucose challenge was greater in prepubertal offspring delivered to baboons deprived of estrogen during the second half of pregnancy than in animals exposed in utero to the normal elevation in estrogen. Since the exaggerated rise in glucose was accompanied by significantly greater levels of insulin, the results suggest a condition of insulin insensitivity and glucose intolerance. We propose, therefore, that estrogen normally has an important role in utero during primate pregnancy in programming mechanisms within the developing fetus that lead to insulin responsivity and the capacity to metabolize glucose in offspring after birth.
Acknowledgments
Funding
This work was supported by the National Institutes of Health R01 Research Grant DK93950.
The secretarial assistance of Mrs. Wanda James with computer word processing of the manuscript is greatly appreciated. We thank Novartis Pharma (Basel, Switzerland) for generously providing the aromatase inhibitor letrozole to conduct this study.
Abbreviations
- Akt (T) and Akt (S)
threonine308 and serine473 protein kinase
- pAkt
phosphorylated Akt
- AS160
Akt substrate of 160 kDa
- GLUT4 and GLUT1
glucose transporter 4 and glucose transporter 1
- AST
aspartate amino transferase
- HOMA-IR
homeostasis model assessment of insulin resistance
- QUICKI
quantitative insulin sensitivity check index
- IRS
insulin receptor substrate
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Aberdeen GW, Baschat AA, Harman CR, Weiner CP, Langenberg PW, Pepe GJ, Albrecht ED. Uterine and fetal blood flow indexes and fetal growth assessment after chronic estrogen suppression in the second half of baboon pregnancy. American Journal of Physiology. Heart and Circulatory Physiology. 2010;298:H881–H889. doi: 10.1152/ajpheart.00611.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht ED, Aberdeen GW, Pepe GJ. The role of estrogen in the maintenance of primate pregnancy. American Journal of Obstetrics and Gynecology. 2000;182:432–438. doi: 10.1016/s0002-9378(00)70235-3. [DOI] [PubMed] [Google Scholar]
- Albrecht ED, Pepe GJ. Placental steroid hormone biosynthesis in primate pregnancy. Endocrine Reviews. 1990;11:124–150. doi: 10.1210/edrv-11-1-124. [DOI] [PubMed] [Google Scholar]
- Barker DJ. The developmental origins of insulin resistance. Hormone Research. 2005;64:2–7. doi: 10.1159/000089311. [DOI] [PubMed] [Google Scholar]
- Barros RP, Gabbi C, Morani A, Warner M, Gustafsson JA. Participation of ERalpha and ERbeta in glucose homeostasis in skeletal muscle and white adipose tissue. American Journal of Physiology, Endocrinology and Metabolism. 2009;297:E124–E133. doi: 10.1152/ajpendo.00189.2009. [DOI] [PubMed] [Google Scholar]
- Barros RP, Machado UF, Gustafsson JA. Estrogen receptors: new players in diabetes mellitus. Trends in Molecular Medicine. 2006;12:425–431. doi: 10.1016/j.molmed.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Belgorosky A, Guercio G, Pepe C, Saraco N, Rivarola MA. Genetic and clinical spectrum of aromatase deficiency in infancy, childhood and adolescence. Hormone Research. 2009;72:321–330. doi: 10.1159/000249159. [DOI] [PubMed] [Google Scholar]
- Blanco CL, Liang H, Joya-Galeana J, DeFronzo RA, McCurnin D, Musi N. The ontogeny of insulin signaling in the preterm baboon model. Endocrinology. 2010;151:1990–1997. doi: 10.1210/en.2009-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco CL, McGill-Vargas LL, Gastaldelli A, Seidner SR, McCurnin DC, Leland MM, Anzueto DG, Johnson MC, Liang H, DeFronzo RA, et al. Peripheral insulin resistance and impaired insulin signaling contribute to abnormal glucose metabolism in preterm baboons. Endocrinology. 2015;156:813–823. doi: 10.1210/en.2014-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns CM, Baum ST, Colman RJ, Eisner JR, Kemnitz JW, Weindruch R, Abbott DH. Insulin resistance and impaired insulin secretion in prenatally androgenized male rhesus monkeys. Journal of Clinical Endocrinology and Metabolism. 2004;89:6218–6223. doi: 10.1210/jc.2004-0918. [DOI] [PubMed] [Google Scholar]
- Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, Dahlman-Wright K, Nilsson S, Gustafsson JA, Efendic S, et al. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice; insulin sensitivity in the liver. Diabetologia. 2006;49:588–597. doi: 10.1007/s00125-005-0105-3. [DOI] [PubMed] [Google Scholar]
- Bryzgalova G, Lundholm L, Portwood N, Gustafsson JA, Khan A, Efendic S, Dahlman-Wright K. Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. American Journal of Physiology, Endocrinologoy and Metabolism. 2008;295:E904–E912. doi: 10.1152/ajpendo.90248.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camporez JP, Jornayvaz FR, Lee HY, Kanda S, Guigni BA, Kahn M, Samuel VT, Carvalho CR, Petersen KF, Jurczak MJ, et al. Cellular mechanism by which estradiol protects female ovariectomized mice from high-fat diet-induced hepatic and muscle insulin resistance. Endocrinology. 2013;154:1021–1028. doi: 10.1210/en.2012-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederroth CR, Nef S. Fetal programming of adult glucose homeostasis in mice. PLoS ONE. 2009;4:e7281. doi: 10.1371/journal.pone.0007281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez AO, Gastaldelli A, Guardado-Mendoza R, Lopez-Alvarenga JC, Leland MM, Tejero ME, Sorice G, Casiraghi F, Davalli A, Bastarrachea RA, et al. Predictive models of insulin resistance derived from simple morphometric and biochemical indices related to obesity and the metabolic syndrome in baboons. Cardiovascular Diabetology. 2009;8:22. doi: 10.1186/1475-2840-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez AO, Lopez-Alvarenga JC, Tejero ME, Triplitt C, Bastarrachea RA, Sriwijitkamol A, Tantiwong P, Voruganti VS, Musi N, Comuzzle AG, et al. Physiological and molecular determinants of insulin action in the baboon. Diabetes. 2008;57:899–908. doi: 10.2337/db07-0790. [DOI] [PubMed] [Google Scholar]
- Chen H, Sullivan G, Quon MJ. Assessing the predictive accuracy of QUICKI as a surrogate index for insulin sensitivity using a calibration model. Diabetes. 2005;54:1914–1925. doi: 10.2337/diabetes.54.7.1914. [DOI] [PubMed] [Google Scholar]
- Choi SB, Jang JS, Park S. Estrogen and exercise may enhance beta-cell function and mass via insulin receptor substrate 2 induction in ovariectomized diabetic rats. Endocrinology. 2005;146:4786–4794. doi: 10.1210/en.2004-1653. [DOI] [PubMed] [Google Scholar]
- Choi J, Li C, McDonald TJ, Comuzzie A, Mattern V, Nathanielsz PW. Emergence of insulin resistance in juvenile baboon offspring of mothers exposed to moderate maternal nutrient reduction. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2011;301:R757–R762. doi: 10.1152/ajpregu.00051.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocrine Reviews. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Jacot E, Jequier E, Maeder, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- Deroo BJ, Korach KS. Estrogen receptors and human disease. Journal of Clinical Investigation. 2006;116:561–570. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M, Byrne CD, Meeran K, Martenz ND, Bloom SR, Hales CN. Regulation of hepatic enzymes and insulin levels in offspring of rat dams fed a reduced-protein diet. American Journal of Physiology. 1997;273:G899–G904. doi: 10.1152/ajpgi.1997.273.4.G899. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocrine Reviews. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieudonné MN, Lenevue MC, Giudicelli Y, Pecquery R. Evidence for functional estrogen receptors alpha and beta in human adipose cells: regional specificities and regulation by estrogens. American Journal of Physiology. Cell Physiology. 2004;286:C655–661. doi: 10.1152/ajpcell.00321.2003. [DOI] [PubMed] [Google Scholar]
- Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. Journal of the American Medical Association. 2006;295:1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- Eisner JR, Dumesic DA, Kemnitz JW, Abbott DH. Timing of prenatal androgen excess determines differential impairment in insulin secretion and action in adult female rhesus monkeys. Journal of Clinical Endocrinology and Metabolism. 2000;85:1206–1210. doi: 10.1210/jcem.85.3.6453. [DOI] [PubMed] [Google Scholar]
- Gale EA, Gillespie KM. Diabetes and gender. Diabetologia. 2001;44:3–15. doi: 10.1007/s001250051573. [DOI] [PubMed] [Google Scholar]
- Gao H, Bryzgalova G, Hedman E, Khan A, Efendic S, Gustafsson JA, Dahlman-Wright K. Long-term administration of estradiol decreases expression of hepatic lipogenic genes and improves insulin sensitivity in ob/ob mice: a possible mechanism is through direct regulation of signal transducer and activator of transcription 3. Molecular Endocrinology. 2006;20:1287–1299. doi: 10.1210/me.2006-0012. [DOI] [PubMed] [Google Scholar]
- Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gender Medicine. 2009;6:60–75. doi: 10.1016/j.genm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb FW, Homer NZ, Fagehi AM, Upreti R, Livingstone DE, McInnes KJ, Andrew R, Walker BR. Aromatase inhibition reduces insulin sensitivity in healthy men. Journal of Clinical Endocrinology and Metabolism. 2016 Mar 11; doi: 10.1210/jc.2015-4146. jc20154146 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. New England Journal of Medicine. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godsland IF. Oestrogens and insulin secretion. Diabetologia. 2005;48:2213–2220. doi: 10.1007/s00125-005-1930-0. [DOI] [PubMed] [Google Scholar]
- Golden SH, Dobs AS, Vaidya D, Szkio M, Gapstur S, Kopp P, Liu K, Ouyang P. Endogenous sex hormones and glucose tolerance status in postmenopausal women. Journal of Clinical Endocrinology and Metabolism. 2007;92:1289–1295. doi: 10.1210/jc.2006-1895. [DOI] [PubMed] [Google Scholar]
- Gorres BK, Bomhoff GL, Morris JK, Geiger PC. In vivo stimulation of oestrogen receptor α increases insulin-stimulated skeletal muscle glucose uptake. Journal of Physiology. 2011;589:2041–2054. doi: 10.1113/jphysiol.2010.199018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guercio G, Di Palma MI, Pepe C, Saraco NI, Prieto M, Saure C, Mazza C, Rivarola MA, Belgorosky A. Metformin, estrogen replacement therapy and gonadotropin inhibition fail to improve insulin sensitivity in a girl with aromatase deficiency. Hormone Research. 2009;72:370–376. doi: 10.1159/000249165. [DOI] [PubMed] [Google Scholar]
- Gupte AA, Pownall HJ, Hamilton DJ. Estrogen: an emerging regulator of insulin action and mitochondrial function. Journal of Diabetes Research. 2015;2015:2015. doi: 10.1155/2015/916585. 916585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, et al. Estrogen receptors: how do they signal and what are their targets. Physiological Reviews. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- Jelenik T, Roden M. How estrogens prevent from lipid-induced insulin resistance. Endocrinology. 2013;154:989–992. doi: 10.1210/en.2013-1112. [DOI] [PubMed] [Google Scholar]
- Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn BB, Rossetti L, Lodish HF, Charron MJ. Decreased in vivo glucose uptake but normal expression of GLUT1 and GLUT4 in skeletal muscle of diabetic rats. Journal of Clinical Investigation. 1991;87:2197–2206. doi: 10.1172/JCI115254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan SA. The insulin receptor. Journal of Pediatrics. 1984;104:327–336. doi: 10.1016/s0022-3476(84)81090-2. [DOI] [PubMed] [Google Scholar]
- Karjalainen A, Paassilta M, Heikkinen J, Bäckström AC, Savolainen M, Kesäniemi YA. Effects of peroral and transdermal oestrogen replacement therapy on glucose and insulin metabolism. Clinical Endocrinology. 2001;54:165–173. doi: 10.1046/j.1365-2265.2001.01208.x. [DOI] [PubMed] [Google Scholar]
- Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. Journal of Clinical Endocrinology and Metabolism. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- Kind KL, Clifton PM, Grant PA, Owens PC, Sohlstrom A, Roberts CT, Robinson JS, Owens JA. Effect of maternal feed restriction during pregnancy on glucose tolerance in the adult guinea pig. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2003;284:R140–R152. doi: 10.1152/ajpregu.00587.2001. [DOI] [PubMed] [Google Scholar]
- Lee HW, Muniyappa R, Yan X, Yue LQ, Linden EH, Chen H, Hansen BC, Quon MJ. Comparison between surrogate indexes of insulin sensitivity/resistance and hyperinsulinemic euglycemic glucose clamps in rhesus monkeys. Endocrinology. 2011;152:414–423. doi: 10.1210/en.2010-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le May C, Chu K, Hu M, Ortega CS, Simpson ER, Korach KS, Tsai MJ, Mauvais-Jarvis F. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9232–9237. doi: 10.1073/pnas.0602956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Mauvais-Jarvis F. Minireview: Estrogenic protection of beta-cell failure in metabolic diseases. Endocrinology. 2010;151:859–864. doi: 10.1210/en.2009-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louet JF, LeMay C, Mauvais-Jarvis F. Antidiabetic actions of estrogen: insight from human and genetic mouse models. Current Atherosclerosis Reports. 2004;6:180–185. doi: 10.1007/s11883-004-0030-9. [DOI] [PubMed] [Google Scholar]
- Lundholm L, Bryzgalova G, Gao H, Portwood N, Falt S, Berndt KD, Dicker A, Galuska D, Zierath JR, Gustafsson JA, et al. The estrogen receptor {alpha}-selective agonist propyl pyrazole triol improves glucose tolerance in ob/ob mice; potential molecular mechanisms. Journal of Endocrinology. 2008;199:275–286. doi: 10.1530/JOE-08-0192e. [DOI] [PubMed] [Google Scholar]
- Manrique C, Lastra G, Habibi J, Mugerfeld I, Garro M, Sowers JR. Loss of estrogen receptor α signaling leads to insulin resistance and obesity in young and adult female mice. Cardiorenal Medicine. 2012;2:200–210. doi: 10.1159/000339563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, LaCroix AZ, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. The Journal of the American Medical Association. 2013;310:1353–1358. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, Bassford T, Burke G, Torrens J, Howard BV. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia. 2004;47:1175–1187. doi: 10.1007/s00125-004-1448-x. [DOI] [PubMed] [Google Scholar]
- Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocrine Reviews. 2013;34:309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A, Jacobs DR, Jr, Steinberger J, Steffen LM, Pankow JS, Hong CP, Sinaiko AR. Changes in insulin resistance and cardiovascular risk during adolescence: establishment of differential risk in males and females. Circulation. 2008;117:2361–2368. doi: 10.1161/CIRCULATIONAHA.107.704569. [DOI] [PubMed] [Google Scholar]
- Moreno M, Ordoñez P, Alonso A, Diaz F, Tolivia J, Gonsález Chronic 17 beta-estradiol treatment improves skeletal muscle insulin signaling pathway components in insulin resistance associated with aging. AGE (Dordrecht, Netherlands) 2010;32:1–13. doi: 10.1007/s11357-009-9095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal A, Alonso-Magdalena P, Soriano S, Quesada I, Ropero AB. The pancreatic beta-cell as a target of estrogens and xenoestrogens: Implications for blood glucose homeostasis and diabetes. Molecular and Cellular Endocrinology. 2009;304:63–68. doi: 10.1016/j.mce.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Nystrom FH, Quon MJ. Insulin signalling: metabolic pathways and mechanisms for specificity. Cellular Signalling. 1999;11:563–574. doi: 10.1016/s0898-6568(99)00025-x. [DOI] [PubMed] [Google Scholar]
- Overkamp D, Gauthier JF, Renn W, Pickert A, Scheen AJ, Schmülling RM, Eggstein M, Lefèbvre PJ. Glucose turnover in humans in basal state and after intravenous glucose: a comparison of two models. American Journal of Physiology. 1997;273:E284–E296. doi: 10.1152/ajpendo.1997.273.2.E284. [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Smith GD, Tikerpae J, Hales CN. Altered regulation of hepatic glucose output in the male offspring of protein-malnourished rat dams. American Journal of Physiology. 1996;270:E559–E564. doi: 10.1152/ajpendo.1996.270.4.E559. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A, Abbott DH, Recabarren SE, Herkimer C. Developmental programming: impact of prenatal testosterone excess and postnatal weight gain on insulin sensitivity index and transfer of traits to offspring of overweight females. Endocrinology. 2010;151:595–605. doi: 10.1210/en.2009-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik SG, Michelis MA, Kim YT, Shin S. Induction of insulin-dependent diabetes by streptozotocin. Inhibition by estrogens and potentiation by androgens. Diabetes. 1982;31:724–729. doi: 10.2337/diab.31.8.724. [DOI] [PubMed] [Google Scholar]
- Pepe GJ, Albrecht ED. Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocrine Reviews. 1995;16:608–648. doi: 10.1210/edrv-16-5-608. [DOI] [PubMed] [Google Scholar]
- Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150:2109–2117. doi: 10.1210/en.2008-0971. [DOI] [PubMed] [Google Scholar]
- Ribas V, Drew BG, Le JA, Soleymani T, Daraei P, Sitz D, Mohammad L, Henstridge DC, Febbraio MA, Hewitt SC, et al. Myeloid-specific estrogen receptor alpha deficiency impairs metabolic homeostasis and accelerates atherosclerotic lesion development. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16457–16462. doi: 10.1073/pnas.1104533108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards OC, Raines SM, Attie AD. The role of blood vessels, endothelial cells, and vascular pericytes in insulin secretion and peripheral insulin action. Endocrine Reviews. 2010;31:343–363. doi: 10.1210/er.2009-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb VA, Pepe GJ, Albrecht ED. Placental villous vascular endothelial growth factor expression and vascularization after estrogen suppression during the last two-thirds of baboon pregnancy. Endocrine. 2007;31:260–267. doi: 10.1007/s12020-007-0036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins JC, Marsit CJ, Padbury JF, Sharma SS. Endocrine disruptors, environmental oxygen, epigenetics and pregnancy. Frontiers in Bioscience. 2011;3:690–700. doi: 10.2741/e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochira V, Faustini-Fustini M, Balestrieri A, Carani C. Estrogen replacement therapy in a man with congenital aromatase deficiency: effects of different doses of transdermal estradiol on bone mineral density and hormonal parameters. Journal of Clinical Endocrinology and Metabolism. 2000;85:1841–1845. doi: 10.1210/jcem.85.5.6583. [DOI] [PubMed] [Google Scholar]
- Rochira V, Madeo B, Zirilli L, Caffagni G, Maffei L, Carani C. Oestradiol replacement treatment and glucose homeostasis in two men with congenital aromatase deficiency: evidence for a role of oestradiol and sex steroids imbalance on insulin sensitivity in men. Diabetic Medicine. 2007;24:1491–1495. doi: 10.1111/j.1464-5491.2007.02304.x. [DOI] [PubMed] [Google Scholar]
- Rogers NH, Witczak CA, Hirshman MF, Goodyear LJ, Greenberg AS. Estradiol stimulates Akt, Amp-activated protein kinase (AMPK) and TBC1D1/4, but not glucose uptake in rat soleus. Biochemical and Biophysical Research Communications. 2009;382:646–650. doi: 10.1016/j.bbrc.2009.02.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropero AB, Alonso-Magdalena P, Quesada I, Nadal A. The role of estrogen receptors in the control of energy and glucose homeostasis. Steroids. 2008;73:874–879. doi: 10.1016/j.steroids.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Ross MG, Beall MH. Adult sequelae of intrauterine growth restriction. Seminars in Perinatology. 2008;32:213–218. doi: 10.1053/j.semperi.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes. 2001;50:2279–2286. doi: 10.2337/diabetes.50.10.2279. [DOI] [PubMed] [Google Scholar]
- Stel J, Leglar J. The role of epigenetics in the latent effects of early life exposure to obesogenic endocrine disrupting chemicals. Endocrinology. 2015;156:3466–3472. doi: 10.1210/en.2015-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Toda K, Saibara T, Nakagawa M, Saika K, Onishi T, Sugiura T, Shizuta Y. Progressive development of insulin resistance phenotype in male mice with complete aromatase (CYP19) deficiency. Journal of Endocrinology. 2003;176:237–246. doi: 10.1677/joe.0.1760237. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Regnault TR. In utero origins of adult insulin resistance and vascular dysfunction. Seminars in Reproductive Medicine. 2011;29:211–224. doi: 10.1055/s-0031-1275522. [DOI] [PubMed] [Google Scholar]
- Thorn SR, Regnault TR, Brown LD, Rozance PJ, Keng J, Roper M, Wilkening RB, Hay WW, Jr, Friedman JE. Intrauterine growth restriction increases fetal hepatic gluconeogenic capacity and reduces messenger ribonucleic acid translation initiation and nutrient sensing in fetal liver and skeletal muscle. Endocrinology. 2009;150:3021–3030. doi: 10.1210/en.2008-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconsuelo A, Milanesi L, Boland R. 17Beta-estradiol abrogates apoptosis in murine skeletal muscle cells through estrogen receptors: role of the phosphatidylinositol 3-kinase/Akt pathway. Journal of Endocrinology. 2008;196:385–397. doi: 10.1677/JOE-07-0250. [DOI] [PubMed] [Google Scholar]
- Voelker R. US preterm births: “D” is for dismal. Journal of the American Medical Association. 2010;303:116–117. doi: 10.1001/jama.2009.1895. [DOI] [PubMed] [Google Scholar]
- Wagner JD, Thomas MJ, Williams JK, Zhang L, Greaves KA, Cefalu WT. Insulin sensitivity and cardiovascular risk factors in ovariectomized monkeys with estradiol alone or combined with nomegestrol acetate. Journal of Clinical Endocrinology and Metabolism. 1998;83:896–901. doi: 10.1210/jcem.83.3.4628. [DOI] [PubMed] [Google Scholar]
- Wiik A, Ekman M, Johansson O, Jansson E, Esbjörnsson M. Expression of both oestrogen receptor alpha and beta in human skeletal muscle tissue. Histochemistry and Cell Biology. 2009;131:181–189. doi: 10.1007/s00418-008-0512-x. [DOI] [PubMed] [Google Scholar]
- Zachos NC, Billiar RB, Albrecht ED, Pepe GJ. Regulation of oocyte microvilli development in the baboon fetal ovary by estrogen. Endocrinology. 2004;145:959–966. doi: 10.1210/en.2003-1078. [DOI] [PubMed] [Google Scholar]
- Zirilli L, Rochira V, Diazzi C, Caffagni G, Carani C. Human models of aromatase deficiency. Journal of Steroid Biochemistry and Molecular Biology. 2008;109:212–218. doi: 10.1016/j.jsbmb.2008.03.026. [DOI] [PubMed] [Google Scholar]