Abstract
Purpose
Long-term breast cancer survivors frequently report distress (i.e. depressive symptoms) that impacts their quality of life. Previous studies have found negative social interactions (social constraints) from partners contribute to long-term, unresolved cycling of intrusive thoughts and cognitive avoidance, resulting in psychological distress. However, these relationships have not been tested in long-term breast cancer survivors. Furthermore, the affect of partners’ depressive symptoms on the survivors’ depressive symptoms have not been tested within the context of these relationships. Therefore, the purpose of this study was to test relationships between breast cancer survivors’ depressive symptoms and 1) social constraints, cognitive avoidance, and intrusive thoughts, and 2) partners’ depressive symptoms.
Methods
Data were from a cross-sectional descriptive study of breast cancer survivors (N=222) 3–8 years post diagnosis and their partners, who completed surveys assessing demographic characteristics, social constraints, intrusive thoughts, cognitive avoidance, and depressive symptoms. Structural equation modeling confirmatory path analyses were conducted to determine significant relationships between breast cancer survivors’ depressive symptoms and all other variables.
Results
Our model fit the data well. Breast cancer survivors’ depressive symptoms were predicted by social constraints and intrusive thoughts. The relationship between survivors’ depressive symptoms and partners’ depressive symptoms was close but not significant.
Conclusions
As hypothesized, depressive symptoms were predicted by social constraints and intrusive thoughts. Further research is needed to understand the possible relationship between survivors’ long-term depressive symptoms and cognitive avoidance and partners’ depressive symptoms. Our findings highlight the negative impact of social constraints from partners on psychological outcomes in long-term breast cancer survivors.
Approximately 92% of women diagnosed with breast cancer live past the 5-year survival mark as a result of earlier diagnosis and treatment [1, 2]. Almost all breast cancer survivors experience symptoms resulting from their disease and treatment that last throughout their life [1] and have a lasting impact on quality of life [3]. One of the most common symptoms is psychological distress, which often manifests as depressive symptoms [4, 5]. As many as 25% of survivors, 5 years after their breast cancer diagnosis suffer from clinically significant levels of depressive symptom [6]. Survivors who are depressed are at risk for higher side-effect burden than those who are not depressed [7], and may experience greater anxiety [8], pain, insomnia, and fatigue [9]. Depression also has consistently been linked to poorer adherence to medical care, longer hospital stays, higher mortality, and reduced overall quality of life [10, 11].
One factor contributing to depressive symptoms in breast cancer survivors is the inability to discuss cancer with a significant other because the partner blocks conversation about the event (social constraint). Social constraints discourage open communication through avoidance, denial, criticism, and minimizing concerns [12–14]. In traumatic events such as cancer diagnoses, high social constraints have been associated with greater distress [15, 16]. Without a supportive environment in which to discuss cancer, breast cancer survivors may not be able to cognitively process the trauma, hindering their psychological adjustment [17]. Unfortunately, there is a dearth of literature focusing specifically on distress in young breast cancer survivors, who suffer disproportionately compared to their older counterparts [3, 9].
The Social Cognitive Processing Theory has been used within oncology populations to predict depressive symptoms in survivors [18]. According to the theory, depressive symptoms result from one’s inability to cognitively process the cancer experience through short-term cycling of intrusive thoughts (i.e., repetitive, unbidden trauma-related thoughts or images) and cognitive avoidance (i.e., attempts to distance the individual from trauma-related thoughts and feelings) [12, 17]. Even though this process may be disruptive while it is occurring, it is imperative for later psychological adjustment [14, 15, 18]. Lepore and Helgeson propose that if a person fails to completely process the traumatic event in a timely manner, the experience may remain in active memory, causing “physiological and psychological disturbances,” (p.91) [19]. This disruptive processing becomes harmful if prolonged, leading to distress [17]. Incomplete cognitive processing has been linked to negative psychological adjustment and mediates the relationship between social constraints and psychological adjustment within the cancer experience [12]. Specifically, social constraints from a partner or spouse have been found to increase patient distress [20].
Finally, recent studies have demonstrated a direct relationship between distress (i.e. depressive symptoms, anxiety, negative affect) in breast cancer survivors and their partners [21, 22]. Distress experienced by either partner has been found to directly affect distress levels in the opposite spouse, and a negative outlook in one partner is associated with a negative outlook and higher psychological distress in the opposite partner [22]. Bigatti et al. (2012) found that survivors were more distressed when their partners were distressed as well [22]. Also, Segrin et. al. (2006) found that survivors were more affected by their partners’ anxiety than vice versa [21]. Considering the effect that partners’ distress has on survivors’ distress may add predictive power to the Social Cognitive Processing Theory.
The purpose of this study was to test the relationship between social constraints and depressive symptoms with the components of cognitive processing (both intrusive thoughts and cognitive avoidance) mediating that relationship in long-term breast cancer survivors who were 45 years or younger at diagnosis. Furthermore, the effect of partners’ depressive symptoms on survivors’ depressive symptoms was tested.
Methods
Sample
Data used for this study were part of a larger quality of life study, collected through an ECOG-ACRIN Cancer Research Group 97-site database. Results of the parent study have been reported elsewhere [3]. Women for this study were eligible if diagnosed with breast cancer stages I-IIIa at age 45 years or younger; were 3 to 8 years post diagnosis and treatment without a recurrence at the time of recruitment; treated with a chemotherapy regimen of Adriamycin, Paclitaxel, and Cyclophosphamide; and partnered. A partner was eligible if currently living with the survivor and self-identifying as a spouse or committed partner. Informed consent was obtained from all individual participants included in the study.
Measures
Personal information was collected for all survivors, including: household income, education, race, religious affiliation, current age, and time since diagnosis.
Social Constraints were measured using 14 items from the Lepore Social Constraints Scale. This instrument asks the survivor how often, on a 1–4 scale of “never” to “often,” she felt constrained by her partner in discussing cancer in the last four weeks [23]. The summed scores range from 14 to 56. Questions included, how often did your partner, “avoid you,” “seem to be hiding his/her feelings,” and “tell you not to think about your breast cancer.” Construct validity has been established previously [23]. The Cronbach alpha coefficient for this sample was α = .90.
The components of Cognitive Processing, intrusive thoughts and cognitive avoidance, were measured separately by subscales of the Impact of Event Scale [24, 25]. The Intrusive Thoughts subscale consists of 7 questions, such as “I thought about breast cancer when I didn’t mean to,” and “pictures about breast cancer popped into my mind.” Responses ranging 0–4, with higher scores indicating more intrusions. The Cognitive Avoidance subscale consists of 8 questions, such as “I stayed away from reminders about breast cancer.” Just as with the intrusions subscale, responses ranged 0–4.
Scores for each scale are summed. For this analysis, intrusive thoughts and cognitive avoidance were analyzed separately. Content, construct, and convergent validity have been previously established for the subscales as well as the total scale [24, 25]. The Cronbach alpha coefficient for survivors in this study was α= .89.
Depressive symptoms were measured using the Centers for Epidemiologic Studies-Depression Scale. This 20-item summed scale has responses ranging 0–3 for each item [26]. Scores range from 0 to 60 with scores above16 being consistent with a diagnosis of clinical depression. Concurrent and construct validity were previously established in an oncology population [27]. The Cronbach alpha coefficients for survivors and partners were α= .90 and α= .89, respectively.
Recruitment Procedures
The study was approved through the ECOG-ACRIN and the National Cancer Institute. Human subjects protection was obtained from the institutional review board of the parent site- a large Midwestern university- and from all ECOG-ACRIN sites. The statistical office for Eastern Cooperative Oncology Group identified breast cancer survivors who met eligibility criteria and forwarded the names to the breast cancer survivors’ treating physicians. Treating physicians asked for permission to forward their names and contact information to the university. If a survivor agreed, she was mailed a brochure explaining the study and contacted by a research assistant. If verbal consent was obtained, the woman was mailed the questionnaire and informed consent forms. After agreeing to participate, the survivor was asked if she had a partner who could be contacted about participation. The same procedure was followed for partner recruitment. Breast cancer survivors were eligible for enrollment even if partners declined. Because these analyses required a partner variable, only survivors whose partners also participated were used for these analyses.
Of those who agreed to participate (n= 602), 84% of breast cancer survivors returned data (n=505), and 222 had partners who returned data.
Data Analytic Plan
Descriptive statistics were used to describe the presence and severity of depressive symptoms in a sample of both young breast cancer survivors and their partners, and to describe the potentially confounding variables, social constraints, and cognitive processing characteristics (intrusive thoughts and cognitive avoidance) of breast cancer survivors. Once identified as potentially important factors from the literature, bivariate correlations between all potentially confounding variables (current age, household income, years of education, race, religious affiliation, and time since diagnosis) and depressive symptoms were analyzed. Potentially confounding variables that were related to depressive symptoms at p < .25 in bivariate analyses were entered into the model as recommended by Hosmer and Lemenshaw (1989) [28]. We used this conservative p value parameter in order to retain potentially confounding variables that may significantly affect depressive symptoms given previous literature has been mixed as to their importance in analyses. All descriptive information was computed using SPSS® statistical software, version 22.
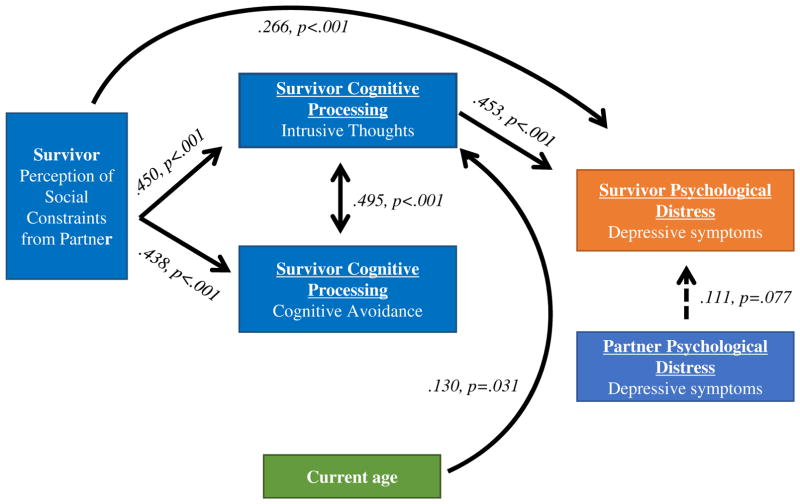
Confirmatory structural equation path model was used to simultaneously test a set of regression equations among the observed variables. Endogenous variables (dependent variables) included cognitive avoidance, intrusive thoughts, and breast cancer survivors’ depressive symptoms. Exogenous variables (independent variables) included social constraints, potentially confounding variables, and partner depressive symptoms. Additionally, the indirect effects of social constraints on depressive symptoms through intrusive thoughts and cognitive avoidance were tested. The advantage that structural equation modeling provides over linear regression for this study is that all paths are calculated simultaneously, thus all variables contributing to the variance of the outcome variable (survivors’ depressive symptoms) are accounted for in the same model. Jackson (2003) suggests a sample size of 20 cases to every model parameter for maximum likelihood analyses [29]. For this study, that would necessitate a sample of 100. Therefore, our sample of N=222 breast cancer survivors is acceptable. Mplus software was used to evaluate model fit, estimate and test path coefficients, and estimate and test indirect and total effects [30]. The chi-square fit statistic, the comparative fit index (CFI), and the root mean square error of approximation (RMSEA) were used as indices of goodness of fit [31, 32]. Indicators of a good model fit include: 1) a non-significant chi-square statistic, 2) RMSEA < .06, and 3) CFI > .95 [33]. The final model is shown in Figure 1.
Figure 1.
Standard coefficients for final model.
Results
Of the variables assessed, only current age (M=45.4 years, SD=4.7 years); annual income identified as low (<$50,000), middle ($50,000-$100,000), and high (>$100,000); years of education (M=14.9 years, SD=2.5 years), and time since diagnosis (M=5.8 years, SD=1.5 years) were significantly related to breast cancer survivors’ depressive symptoms and thus included in the model. Survivors who reported more depressive symptoms were younger (r=-.13, p<.001), less educated (r=-.05, p<.001), had lower income (rs=-.15, p=.023), and had less time since diagnosis (r=-.04, p<.001).
Descriptive information for all scales can be found in Table 1. In bivariate analyses, breast cancer survivors’ depressive symptoms were significantly related to social constraints (r=.45, p<.001), cognitive avoidance (r=.30, p<.001), intrusive thoughts (r=.52, p<.001), and were marginally related to partners’ depressive symptoms (r=.13, p=.053). Survivors who reported more depressive symptoms were more likely to report greater social constraints, more cognitive avoidance, and more intrusive thoughts.
Table 1.
Descriptive Information for all Scales.
| Measure | Mean | SD | Range | Alpha Coefficients* |
|---|---|---|---|---|
| Lepore Social Constrains Scale | 20.95 | 7.21 | 14–55 | .90 |
| Intrusive Thoughts (IES) | 5.87 | 5.55 | 0–30 | .85 |
| Cognitive Avoidance (IES) | 4.91 | 5.04 | 0–23 | .78 |
| Combined Intrusions and Avoidance subscales | 10.78 | 9.49 | 14–55 | .89 |
| CES-D Survivor | 10.16 | 8.88 | 0–47 | .90 |
| CES-D Partner | 8.80 | 8.49 | 0–42 | .89 |
Alpha coefficients are representative of the internal consistency for each scale. All numbers presented refer to survivor results with the exception of the last row, which provides partner results.
Standardized beta regression coefficients (stb) are shown with associated p-values in Figure 1. The hypothesized model showed adequate goodness of fit, with RMSEA=.02, CFI=.99, and SRMR=0.04, with a Chi-square of 13.27 (12 DF) and 19 free parameters. The final model contained only two additional paths- allowing intrusive thoughts to be regressed on current age and years out from diagnosis separately- which were allowed to be estimated on the basis of modification indices.
The final model demonstrated good fit to the data with RMSEA=.000, CFI= 1.00, and chi-square=8.41 (df=11) with 19 free parameters. Variables entered into the model included: potentially confounding variables described above, social constraints, cognitive avoidance, intrusive thoughts, partner depressive symptoms, and survivor depressive symptoms. Paths were tested between all variables. Because cognitive avoidance and intrusive thoughts were highly correlated (r=.60, p<.001) and theoretically related as pieces of cognitive processing, we allowed these subscales to co-vary in the model.
Survivors’ depressive symptomatology, the main outcome, was regressed on all variables. Significant relationships were found between depressive symptoms and several of the variables, including both a direct path (stb=.27, p<.001) and an indirect path through intrusive thoughts (stb=.16, p<.001) to social constraints, and to intrusive thoughts (stb=.45, p<.001). Table 2 lists the z-statistic and associated p-value for each indirect path. The path between partners’ depressive symptoms and survivors’ depressive symptoms was not significant but nearly so (stb=.11, p=.077). The covariance path between avoidance and intrusive thoughts was significant (stb=.50, p<.001). Furthermore, paths between social constraints and intrusive thoughts (stb=.45, p<.001), current age and intrusive thoughts (stb=-.13, p=.031), and social constraints and avoidance (stb=.44, p=<.001) were significant. For potentially confounding variables, only current age was related to intrusions. No other potentially confounding variables were related to any other variable in the model. Additionally, neither the direct nor the indirect path between survivor depressive symptoms and cognitive avoidance was significant. Therefore, experiencing social constraints and intrusive thoughts was associated with survivor depressive symptoms.
Table 2.
Indirect Effects from Social Constraints to Survivors’ Depressive Symptoms
| Effect | ||
|---|---|---|
| Z statistic | p-value | |
| Total indirect | ||
| Social constraints > intrusive thoughts > BCS depressive symptoms | 0.20 | 0.00 |
| Social constraints > cognitive avoidance > BCS depressive symptoms | −0.04 | 0.19 |
Discussion
Breast cancer survivors often struggle with significant quality of life issues throughout survivorship [3, 34], and depressive symptoms are one of the most frequent problems reported in the literature. We sought to better understand survivor depressive symptoms by using the Social Cognitive Processing Theory and partner depressive symptoms to predict depressive symptoms of young, long-term breast cancer survivors. After adjusting for potentially confounding variables, breast cancer survivors’ depressive symptoms were related to intrusive thoughts and to social constraints both directly and indirectly through intrusive thoughts. Furthermore, social constraints were significantly related to both intrusive thoughts and cognitive avoidance. Two hypothesized relationships were not supported by the model. The effect of partners’ depressive symptoms on depressive symptoms in survivors was close (p=.077), but did not meet criteria for significance. Second, no significant relationship was found between survivors’ depressive symptoms and cognitive avoidance.
This study advantageously analyzed intrusive thoughts and cognitive avoidance separately in order to determine a differential influence of these variables on depressive symptoms. While the variables are highly correlated (r=.60, p<.001), each one’s contribution to cognitive processing is unique. Previous studies have found significant relationships between avoidance and depression in both cross-sectional and longitudinal designs [35, 36]. Shand, et. al. (2015) reported a significant relationship between depressive symptoms and cognitive avoidance in a sample of ovarian cancer patients [37]. It is important to note that they described the same variables that we termed cognitive avoidance and intrusive thoughts within a posttraumatic stress disorder framework. The levels of cognitive avoidance in our study were subclinical if compared to posttraumatic stress disorder criteria [38], and may be part of the reason that we did not find a relationship between avoidance and depressive symptoms.
Intrusive thoughts, in our sample, were related to depressive symptoms. The relationship between intrusive thoughts and negative outcomes, such as depressive symptoms, is well documented [39]. A recent study by Dupont, et. al. (2014) found a significant relationship between intrusive thoughts and depressive symptoms, and that those with high intrusive thoughts at baseline had higher rates of depressive symptoms at one year than those with low [37] intrusions at baseline [36]. In accordance with Social Cognitive Processing Theory, intrusive thoughts have been consistently related to psychological distress (i.e. depressive symptoms, anxiety, negative affect) [18, 40, 41], and mediate the relationship between social constraints and depressive symptoms [18].
Consistent with Social Cognitive Processing Theory and previous literature, we found a significant relationship between social constraints from a partner and depressive symptoms in breast cancer survivors. According to the theory, negative social interactions (i.e. social constraints) lead to distress [12]. In fact, a recent meta-analysis by Adams, et. al. (2015) found a moderate, significant relationship between social constraints and both general (depression and anxiety) and cancer-specific distress in 30 studies from the oncology literature [42].
Potentially confounding variables that prior research found to be related to depressive symptoms in breast cancer survivors were included in all analyses. These variables include current age of the survivor, length of time from diagnosis, education, and income. Whether found to be significant or not, many studies have controlled for a host of socio-demographic and treatment variables [43], including age, race, education [44], treatment type (surgery and chemo, radiation, and tamoxifen), and disease stage [45].
Depressive symptoms have a profound impact on one’s general wellbeing and overall quality of life. In breast cancer survivors, depressive symptoms have been linked to poor physical, social/family, emotional and functional well-being [46]. Recently, (2014) Galiano-Castillo and colleagues found support for a symptom cluster including depression, fatigue, and pain as a large contributing factor to decreased quality of life [47]. Women with past or current depression are also at greater risk for declines in emotional well-being [48] and persistently decreased health-related quality of life [49]. Research focusing on depressive symptoms has the potential to inform interventions and increase overall quality of life.
Limitations
While this large-scale study uniquely explored the relationship between depressive symptoms and the Social Cognitive Processing Theory, including the partners’ depressive symptoms, in young, long-term breast cancer survivors, several limitations must be noted. First, data from this study are cross-sectional from a descriptive, rather than experimental, design. Therefore, our ability to draw causal conclusions is limited. Second, while the majority of potentially confounding variables typically explored in survivors were not significantly related to depressive symptoms in our structural equation model path analysis, participants in our sample were mostly Caucasian, well educated, and from middle- and high-income homes, and in these ways not representative of the general population. For these reasons, caution should be applied when relating these findings to the larger breast cancer survivor population.
Implications for Future Research
Our findings provide partial support for using the Social Cognitive Processing Theory to predict depressive symptoms in long-term breast cancer survivors. Interventions designed to decrease social constraints and promote open communication within couples that have experienced breast cancer may be useful in promoting timely cognitive processing, thus decreasing intrusive thoughts and depressive symptoms.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Acknowledgments
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported in part by Public Health Service Grants CA189828, CA180795, CA37403, CA35199, CA17145 and CA49883, and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Research reported in this publication was supported by the National Institute of Nursing Research of the National Institutes of Health under Award Number F31NR013822, and by the National Cancer Institute of the National Institutes of Health under Award Numbers K05CA175048 and R25CA117865. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, including the National Cancer Institute or the National Institute of Nursing Research.
Footnotes
None of the authors have any conflicts of interest.
References
- 1.Soerjomataram I, et al. An overview of prognostic factors for long-term survivors of breast cancer. Breast cancer research and treatment. 2008;107(3):309–30. doi: 10.1007/s10549-007-9556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howlader NNA, Krapcho M, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. N.C. Institute, editor. SEER Cancer Statistics Review, 1975–2011. Bethesda, MD: Apr, 2014. [Google Scholar]
- 3.Champion VL, et al. Comparison of younger and older breast cancer survivors and age-matched controls on specific and overall quality of life domains. Cancer. 2014 doi: 10.1002/cncr.28737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karakoyun-Celik O, et al. Depression and anxiety levels in woman under follow-up for breast cancer: relationship to coping with cancer and quality of life. Medical Oncology. 2010;27(1):108–113. doi: 10.1007/s12032-009-9181-4. [DOI] [PubMed] [Google Scholar]
- 5.Cappiello M, et al. Breast Cancer Survivors. Clinical Nursing Research. 2007;16(4):278. doi: 10.1177/1054773807306553. [DOI] [PubMed] [Google Scholar]
- 6.Kim SH, et al. Fatigue and depression in disease-free breast cancer survivors: prevalence, correlates, and association with quality of life. Journal of pain and symptom management. 2008;35(6):644–655. doi: 10.1016/j.jpainsymman.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Badger TA, et al. Depression burden, psychological adjustment, and quality of life in women with breast cancer: patterns over time. Research in nursing & health. 2004;27(1):19–28. doi: 10.1002/nur.20002. [DOI] [PubMed] [Google Scholar]
- 8.McDonald MV, et al. Nurses’ recognition of depression in their patients with cancer. 1999 [PubMed] [Google Scholar]
- 9.Reyes-Gibby CC, et al. Depressive symptoms and health-related quality of life in breast cancer survivors. Journal of Women’s Health. 2012;21(3):311–318. doi: 10.1089/jwh.2011.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newport DJ, Nemeroff CB. Assessment and treatment of depression in the cancer patient. Journal of Psychosomatic Research. 1998;45(3):215–237. doi: 10.1016/s0022-3999(98)00011-7. [DOI] [PubMed] [Google Scholar]
- 11.Spiegel D. Psychosocial aspects of breast cancer treatment. 1997 [PubMed] [Google Scholar]
- 12.Lepore SJ. A Social-Cognitive Processing Model of Emotional Adjustment to Cancer. In: Baum BLAA, editor. Psychosocial Interventions for Cancer. American Psychological Association; Washington, D. C: 2001. pp. 99–116. [Google Scholar]
- 13.Manne SL. Intrusive thoughts and psychological distress among cancer patients: The role of spouse avoidance and criticism. Journal of Consulting and Clinical Psychology. 1999;67(4):539. doi: 10.1037//0022-006x.67.4.539. [DOI] [PubMed] [Google Scholar]
- 14.Badr H, Taylor CL. Social constraints and spousal communication in lung cancer. Psycho-Oncology. 2006;15(8):673–83. doi: 10.1002/pon.996. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt JE, Andrykowski MA. The role of social and dispositional variables associated with emotional processing in adjustment to breast cancer: an internet-based study. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2004;23(3):259–66. doi: 10.1037/0278-6133.23.3.259. [DOI] [PubMed] [Google Scholar]
- 16.Song L. Couples’ communication in quality of life during prostate cancer survivorship. Wayne State University; 2009. [Google Scholar]
- 17.Lepore SJ, Revenson TA. Social constraints on disclosure and adjustment to cancer. Social and Personality Psychology Compass. 2007;1(1):313–333. [Google Scholar]
- 18.Cordova MJ, et al. Social constraints, cognitive processing, and adjustment to breast cancer. Journal of Consulting and Clinical Psychology. 2001;69(4):706–11. [PubMed] [Google Scholar]
- 19.Lepore SJ, V, Helgeson S. Social constraints, intrusive thoughts, and mental health after prostate cancer. Journal of Social and Clinical Psychology. 1998;17(1):89–106. [Google Scholar]
- 20.Manne SL, et al. Partner unsupportive responses, avoidant coping, and distress among women with early stage breast cancer: Patient and partner perspectives. Health Psychology. 2005;24(6):635. doi: 10.1037/0278-6133.24.6.635. [DOI] [PubMed] [Google Scholar]
- 21.Segrin C, Badger TA, Dorros S, Meek P, Lopez AM. Interdependent anxiety and psychological distress in women with breast cancer and their partners. Psycho-Oncology. 2006;16:634–643. doi: 10.1002/pon.1111. [DOI] [PubMed] [Google Scholar]
- 22.Bigatti SM, et al. Matched and mismatched cognitive appraisals in patients with breast cancer and their partners: implications for psychological distress. Psycho-Oncology. 2012 doi: 10.1002/pon.2028. [DOI] [PubMed] [Google Scholar]
- 23.Lepore SJI, PHG Optimism about cancer enhances mood by reducing negative social interactions. Cancer Research, Therapy, and Control. 1999;8:165–174. [Google Scholar]
- 24.Creamer M, Bell R, Failla S. Psychometric properties of the Impact of Event Scale--Revised. Behaviour Research and Therapy. 2003;41(12):1489–1496. doi: 10.1016/j.brat.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Sundin EC, Horowitz MJ. Impact of Event Scale: psychometric properties. The British Journal of Psychiatry. 2002;180(3):205–209. doi: 10.1192/bjp.180.3.205. [DOI] [PubMed] [Google Scholar]
- 26.Radloff LS. The CES-D Scale: A Self Report Depression Scale for Research in the General. Applied psychological measurement. 1977;1(3):385–401. [Google Scholar]
- 27.Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: Evaluation of the center for epidemiological studies depression scale (Ces-d) Journal of Psychosomatic Research. 1999;46(5):437–443. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 28.Lemenshaw D. Applied logistic regression. New York, NY: Wiley; 1989. [Google Scholar]
- 29.Jackson DL. Revisiting sample size and number of parameter estimates: Some support for the N: q hypothesis. Structural Equation Modeling. 2003;10(1):128–141. [Google Scholar]
- 30.Muthén LK, Muthén BO. Mplus User’s Guide: Statistical Analysis with Latent Variables: User’ss Guide. Muthén & Muthén; 2010. [Google Scholar]
- 31.Gefen D, Straub D, Boudreau MC. Structural equation modeling and regression: Guidelines for research practice. Communications of the association for information systems. 2000;4(1):7. [Google Scholar]
- 32.McDonald RP, Ho MHR. Principles and practice in reporting structural equation analyses. Psychological methods. 2002;7(1):64. doi: 10.1037/1082-989x.7.1.64. [DOI] [PubMed] [Google Scholar]
- 33.Hu Lt, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural equation modeling: a multidisciplinary journal. 1999;6(1):1–55. [Google Scholar]
- 34.Koch L, et al. Fear of recurrence in long-term breast cancer survivors—still an issue. Results on prevalence, determinants, and the association with quality of life and depression from the Cancer Survivorship—a multi-regional population-based study. Psycho-Oncology. 2014;23(5):547–554. doi: 10.1002/pon.3452. [DOI] [PubMed] [Google Scholar]
- 35.Vickberg SMJ, et al. Intrusive thoughts and psychological distress among breast cancer survivors: Global meaning as a possible protective factor. Behavioral Medicine. 2000;25(4):152–160. doi: 10.1080/08964280009595744. [DOI] [PubMed] [Google Scholar]
- 36.Dupont A, et al. Cancer-related intrusive thoughts predict behavioral symptoms following breast cancer treatment. Health Psychology. 2014;33(2):155. doi: 10.1037/a0031131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shand LK, et al. Symptoms of posttraumatic stress in Australian women with ovarian cancer. Psycho-Oncology. 2015;24(2):190–196. doi: 10.1002/pon.3627. [DOI] [PubMed] [Google Scholar]
- 38.Weiss DS, Marmar CR. In: Assessing psychological trauma and PTSD. Wilson JP, Keane TM, editors. Guilford press; 2004. [Google Scholar]
- 39.Steiner JL, et al. Depressive rumination and cognitive processes associated with depression in breast cancer patients and their spouses. Families, Systems, & Health. 2014;32(4):378. doi: 10.1037/fsh0000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mosher CE, et al. Social correlates of distress following hematopoietic stem cell transplantation: Exploring the role of loneliness and cognitive processing. Journal of health psychology. 2012:1359105311432490. doi: 10.1177/1359105311432490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myers SB, et al. Social–cognitive processes associated with fear of recurrence among women newly diagnosed with gynecological cancers. Gynecologic oncology. 2013;128(1):120–127. doi: 10.1016/j.ygyno.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams RN, Winger JG, Mosher CE. A meta-analysis of the relationship between social constraints and distress in cancer patients. Journal of behavioral medicine. 2014;38(2):294–305. doi: 10.1007/s10865-014-9601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Northouse LL. Social Support in Patients’ and Husbands’ Adjustment to Breast Cancer. Nursing research. 1988;37(2):91. [PubMed] [Google Scholar]
- 44.Ganz PA, et al. Quality of life in long-term, disease-free survivors of breast cancer: a follow-up study. Journal of the National Cancer Institute. 2002;94(1):39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- 45.Bloom JR, et al. Quality of life of younger breast cancer survivors: persistence of problems and sense of well-being. Psycho-Oncology. 2012;21(6):655–665. doi: 10.1002/pon.1965. [DOI] [PubMed] [Google Scholar]
- 46.Ho SS, et al. Anxiety, depression and quality of life in Chinese women with breast cancer during and after treatment: a comparative evaluation. European Journal of Oncology Nursing. 2013;17(6):877–882. doi: 10.1016/j.ejon.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 47.Galiano-Castillo N, et al. Depressed mood in breast cancer survivors: Associations with physical activity, cancer-related fatigue, quality of life, and fitness level. European Journal of Oncology Nursing. 2014;18(2):206–210. doi: 10.1016/j.ejon.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 48.Janz NK, et al. Emotional well-being years post-treatment for breast cancer: prospective, multi-ethnic, and population-based analysis. Journal of Cancer Survivorship. 2014;8(1):131–142. doi: 10.1007/s11764-013-0309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kenyon M, Mayer DK, Owens AK. Late and long-term effects of breast cancer treatment and surveillance management for the general practitioner. Journal of Obstetric, Gynecologic, & Neonatal Nursing. 2014;43(3):382–398. doi: 10.1111/1552-6909.12300. [DOI] [PubMed] [Google Scholar]