Abstract
Advances in our understanding of the structure and function of the lymphatic system have made it possible to identify its role in a variety of disease processes. Because it is involved not only in fluid homeostasis but also in immune cell trafficking, the lymphatic system can mediate and ultimately alter immune responses. Our rapidly increasing knowledge of the molecular control of the lymphatic system will inevitably lead to new and effective therapies for patients with lymphatic dysfunction. In this review, we discuss the molecular and physiological control of lymphatic vessel function and explore how the lymphatic system contributes to many disease processes, including cancer and lymphedema.
Keywords: lymphatic vessels, lymphedema, lymph node metastasis, lymphatic pumping, lymph transport, lymphangiogenesis
1. INTRODUCTION
Research into the development, structure, and function of the lymphatic system has been accelerating over the past decade, fueled by the seminal discoveries of the first lymphatic growth factor (1) and markers to help identify lymphatic vessels in tissue (2, 3). These critical molecular tools have allowed the exploration of the formation of the lymphatic system in the embryo (3–5); the growth, maturation, and function of lymphatics in the adult (1, 6); and the role of lymphatic vessels in disease processes (7–12). Interest in the lymphatic system has increased rapidly as its functional role in immune function has become more evident (13–15). In this review, we discuss the current understanding of the role of the lymphatic system in normal and disease processes.
2. THE LYMPHATIC SYSTEM
The lymphatic system is critical for maintaining tissue fluid balance, transporting antigen and antigen presenting cells (APCs) to lymph nodes to generate adaptive immune responses, and carrying lipids absorbed in the gut to the blood circulation. Correspondingly, disruption of the lymphatic system can lead to lymphedema, localized immune compromise, and gut malabsorption. The lymphatic system is also involved in cancer progression, as metastatic cancer cells can spread to lymph nodes through lymphatic vessels.
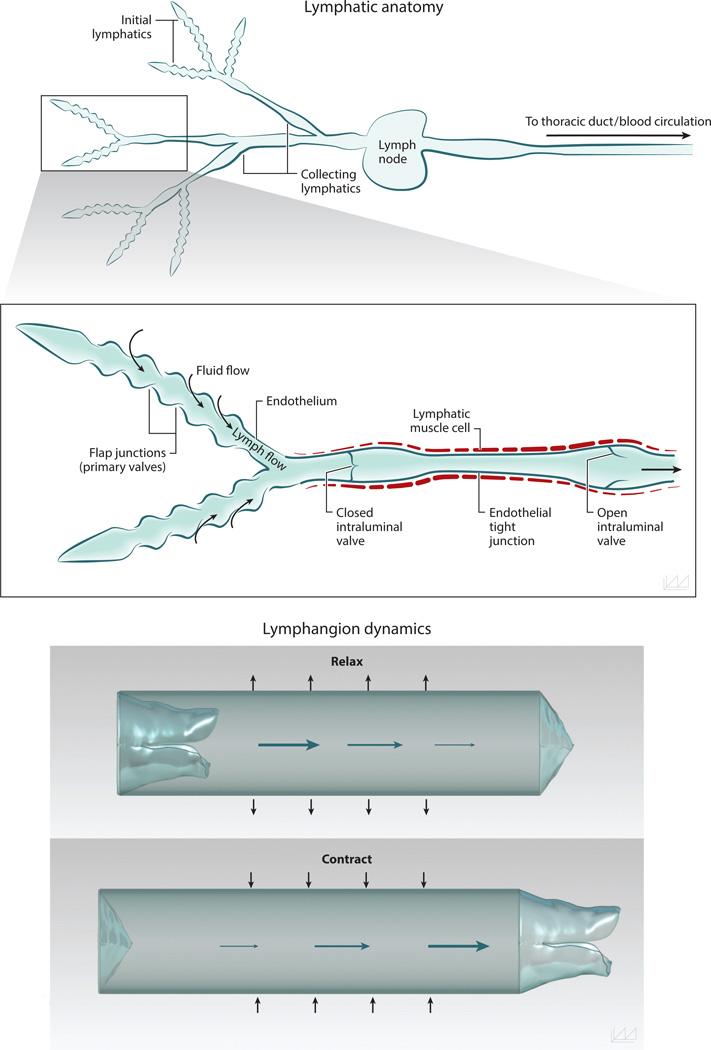
Lymph is created from a tissue’s extracellular fluid and contains unique components derived from that tissue, reflecting its current functional state. Thus, the composition of lymph differs when sampled from lymphatic vessels draining different tissues and changes with time as a tissue undergoes physiological or pathological processes. Lymph production occurs as tissue fluid enters initial lymphatic vessels (Figure 1), which consist of a single layer of overlapping endothelial cells (ECs) on a discontinuous basement membrane, typically with sparse association with perivascular cells. Initial lymphatic endothelial cells (LECs) are connected by “button-like” (16) intercellular junctions that facilitate the collection of interstitial fluid and its contents. The unique microarchitecture of these oak leaf–shaped LECs creates overlapping flaps of adjacent cells, which form primary valve structures in the wall of initial lymphatics (17). When tissue fluid pressure is greater than that in an initial lymphatic, the primary LEC valves open and extracellular fluid freely enters (17, 18). When the fluid pressure is higher inside the lymphatic vessel, the LEC valves close, trapping the newly formed lymph inside. Functionally, the primary LEC flaps act as one-way valves and are critical for the production of lymph. The primary LEC valves also enable dendritic cells (DCs) to pass through and enter the vessel without requiring integrin adhesion or pericellular proteolysis (19). DCs and other APCs are attracted to initial lymphatic vessels by local chemokine CCL21 gradients produced by LECs and interstitial flow (20–23). After entering an initial lymphatic vessel, DCs can interact with and crawl on LECs as they travel to the lymph node (15).
Figure 1.
Anatomy of the lymphatic network and valve function. The lymphatic network consists of initial lymphatic vessels, which are responsible for collecting interstitial fluid to create lymph, and collecting lymphatic vessels, which carry fluid from the peripihfery to lymph nodes. The endothelial cells of initial lymphatic vessels overlap one another to create one-way valves, enabling cell- and pressure-driven fluid entry. The collecting lymphatic vessels are invested in specialized lymphatic muscle cells that contract to drive flow. Intraluminal valves in the collecting lymphatic vessels are critical to preventing backflow. The vessel segment between two valves is called a lymphangion and is the primary pump for lymph flow.
After lymph is produced in initial lymphatic vessels, it travels toward lymph nodes and eventually back into the blood circulation. Vessels proximal to the initial lymphatics—precollecting and collecting lymphatic vessels—have an increase in coverage by specialized lymphatic muscle cells (LMCs) (24). In contrast to initial lymphatic vessels, the LECs in collecting lymphatic vessels have a continuous “zipper-like” (16) junction pattern, creating tight junctions and reducing the transport of material across the vessel wall under normal conditions. Collecting lymphatic vessels also contain intraluminal valves, which are composed primarily of ECs and matrix. These valves maintain unidirectional proximal lymph flow by preventing flow distally when closed and functioning properly (25). The vessel segment between two intraluminal valves is known as a lymphangion, which is the primary pumping structure of the lymphatic system. In physiological conditions, both active pumping by LMCs and passive forces—such as pulsatile blood flow, skeletal or smooth muscle contraction, fluid pressure gradients, and gravity—drive lymph flow (17, 26). However, in the absence of these passive mechanisms, autonomous LMC-mediated contractions of lymphatics vessels can drive lymph through the lymphatic system toward the blood circulation (6, 27). Many signaling molecules regulate lymphatic contractions, including EC-derived nitric oxide (NO), calcium signaling, and certain neurotransmitters (28).
A critical component of collected lymph fluid is the rich diversity of antigens and humoral factors that are derived from the surrounding tissue. The lymph fluid travels through collecting lymphatic vessels to the lymph nodes, where the transported antigens and APCs accumulate. During normal homeostasis, DCs and memory T cells are the most common cells transported through lymphatic vessels (29, 30). Most of the time, DCs sample self-antigens, maintain an immature status, and express low levels of costimulatory molecules after arrival in the lymph node. In this way, DCs carrying self-antigen can control self-reactive T cell activity by inducing anergy and clonal deletion, mediated by signaling molecules such as cytotoxic T lymphocyte–associated protein 4 (CTLA-4) and programmed death 1/programmed death ligand 1 (PD-1/PD-L1) (31, 32). Thus, lymph nodes help central tolerance mechanisms generated in the thymus to maintain peripheral self-tolerance. Resident lymph node stromal cells [e.g., LECs or fibroblastic reticular cells (FRCs)] also promote tolerance through their expression of peripheral tissue antigens and immune checkpoint molecules (14, 33–36). In contrast, foreign antigens stimulate robust adaptive immune responses. As foreign antigens are presented on activated DCs—which express high levels of costimulatory molecules—and arrive in the lymph node from collecting lymphatic vessels, lymphocytes are stimulated and begin differentiating into effector cells. Thus, functional transport through lymphatic vessels is necessary for maintaining the lymph node microarchitecture and supporting optimal interactions between APCs and cognate lymphocytes (37).
Numerous signaling molecules cooperate in the formation and maintenance of lymphatic vessels (1, 4, 5). Two major families of signaling pathways that govern LEC biology are the VEGF-VEGFR (vascular endothelial growth factor–VEGF receptor) family and the Ang-TIE (angiopoietin–tyrosine kinase with immunoglobulin and epidermal growth factor homology domain) family. Activation of VEGFR-2 and VEGFR-3 by VEGF-C and VEGF-D drives lymphangiogenesis, and new lymphatic vessels are maintained by these pathways during adulthood (1, 38). Angiopoietin molecules stimulate postnatal vessel growth, remodeling, and maturation (39–41). In addition, many other signaling molecules—such as ephrin-B2, hepatocyte growth factor, and platelet-derived growth factor–derived receptor-β—are critical for the growth, remodeling, and maturation of the hierarchical lymphatic vessel network (42–44). CD11b+ macrophages also play important roles in inflammation- and tumor-induced lymphangiogenesis, including by producing VEGF-C and VEGF-D (45–47).
3. LYMPHATIC TRANSPORT AND PUMPING
3.1. Lymph Drainage: An Overview
Drainage of lymph from tissues is driven by fluid pressure gradients. The gradients can be established by plasma leakage from blood microvessels (which pushes fluid into the lymphatic system) or by the active pumping of collecting lymphatic vessels (which pulls the fluid in). The pressure gradients drive fluid through the tissue and into the lymphatics, effectively flushing the extravascular space—a process thought to be important for conditioning the extracellular matrix and providing signals to tissue cells. The ability of the collecting lymphatic vessels to actively contract to create lymph flow is a key feature that maintains the pressure gradients, ensuring fluid homeostasis. The relationship between tissue fluid pressures and lymph drainage has been explored with mathematical models (48–50) and studied in vivo by tracking the movement of fluorescent tracers (51).
3.2. Physiology of Lymphatic Pumping
Because dysregulation of fluid homeostasis impairs immune function and creates pathologies such as lymphedema, the ability of lymphatic vessels to restore homeostasis by active pumping has been a topic of intense research. Although similar signaling pathways and contraction machinery are present in the blood and lymphatic systems, the lymphatic vessels are unique in their ability to act as a distributed system of pumps, as opposed to the blood system, where flow is driven by a single pump. Consequently, determining how the calcium-based contractions can be initiated and coordinated in normal physiology, and how this control might be disrupted in pathologies, is an active area of research.
3.2.1. Calcium dynamics and lymphatic muscle contractions
There is a rich literature describing the mechanisms responsible for collecting lymphatic vessel contractions (6, 24, 52). Similar to blood vessels, the muscle cells that surround lymphatic vessels respond to changes in Ca2+ concentration: When Ca2+ levels in the cytosol rise due to influx from extracellular and intracellular stores, myosin light-chain kinase (MLCK) is activated, generating force through the crossbridging of actin and myosin light chain (53, 54).
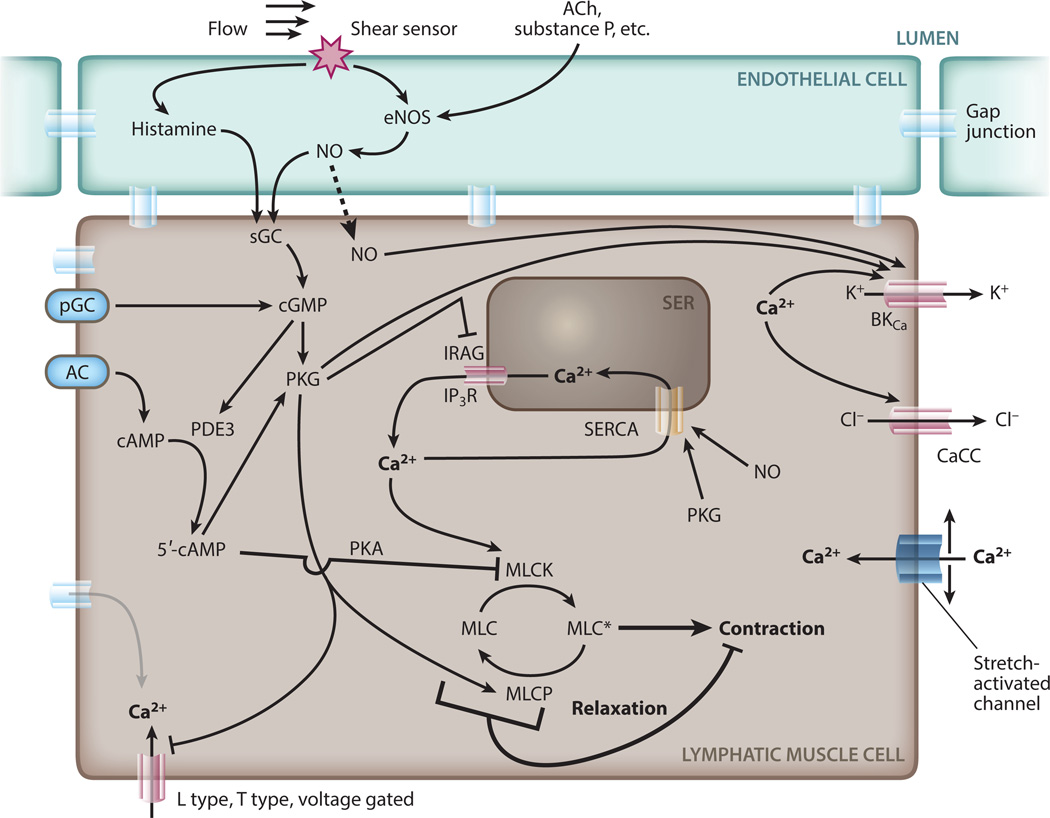
Cytosolic calcium concentrations are affected by many processes and depend on the activity of various ion channels (Figure 2). Inositol 1,4,5-trisphosphate receptors (IP3Rs) are involved in the initial Ca2+ influx, which can in turn open calcium-activated chloride channels (CaCCs), leading to further depolarization (55, 56). The calcium flux also involves voltage-gated L-type channels and ryanodine-sensitive channels, although the importance of calcium-induced calcium release (CICR) is not well established in LMCs. The forward feedback due to the opening of calcium-dependent and voltage-sensitive channels results in a rapid spike in cytosolic Ca2+ and depolarization of the cell membrane, a process that, when it occurs without an external trigger, is known as a spontaneous transient depolarization (STD). The STD, and the resulting contraction, can be blocked with Ca2+ chelators (57).
Figure 2.
Calcium dynamics in the lymphatic muscle cell. Calcium enters the cytosol through ion channels (L type, T type, stretch activated) in the plasma membrane or SER (SERCA, IP3R). Ca2+ acts through MLCK to phosphorylate MLC, allowing formation of the myosin–actin crossbridges and cell contraction. CaCCs can enhance depolarization during STD generation. Endothelial cells produce EDRFs such as histamine and NO that activate sGC, cGMP, PKG, and MLCP. In addition to these effects, which reduce intracellular Ca2+, EDRFs also dephosphorylate MLC, directly interfering with the calcium-induced contractions. Abbreviations: AC, adenylate cyclase; ACh, acetylcholine; CaCC, calcium-activated chloride channel; cGMP, cyclic guanosine monophosphate; EDRF, endothelium-derived relaxing factor; eNOS, endothelial nitric oxide synthase; IP3R, inositol 1,4,5-trisphosphate receptor; IRAG, IP3R-associated cGMP kinase substrate; MLC, myosin light chain; MLCK, MLC kinase; MLCP, MLC phosphatase; NO, nitric oxide; pGC, particulate guanylate cyclase; PKA, protein kinase A; PKG, protein kinase G; SER, smooth endoplasmic reticulum; SERCA, sarco/endoplasmic reticulum Ca2+ ATPase; sGC, soluble guanylyl cyclase; STD, spontaneous transient depolarization.
Once a cell in the vessel wall initiates a contraction, the Ca2+ wave can propagate the contraction along the vessel (58). It has been suggested that a subset of cells acts as a pacemaker to initiate the contractions (59, 60). However, the production of STDs is distinct from cardiac electrical pacemaker activity, which is established via nerve action potentials (52, 57, 59). Some larger lymphatic vessels have nerves in their walls that can alter pumping contraction frequency when stimulated (61), but it is not clear whether smaller lymphatic vessels are innervated, or how such action potentials could be coordinated throughout the network.
The process by which calcium fluctuations progress to become STDs in individual pacemaker cells is still an area of active investigation, and many biochemical pathways have been implicated. Neurotransmitters such as noradrenaline, isoproterenol (61, 62), and substance P (63) and inflammatory mediators such as histamine (54, 64, 65) can affect lymphatic contractions by altering calcium fluxes through various channels. Endothelium-derived agents such as endothelin-1 (ET-1) can also enhance lymphatic vasomotion. ET-1 acts through IP3, directly affecting the IP3R-mediated release of Ca2+ (66).
3.2.2. Mechanical activation of contractions
Much of our knowledge of lymphatic physiology derives from experimental models in which lymphatic vessels are isolated ex vivo, cannulated, and challenged with various pressure regimens (6, 64, 67). These experiments are able to quantify lymphatic function and have shown that lymphatic vessel contractions are very sensitive to physical distension of the vessel wall (6, 68, 69). For example, increasing luminal pressure without changing the axial pressure gradient increases both the frequency and force of the individual contractions (67, 70), and the contractions are preceded by transient calcium bursts (71). At higher pressures, lymph output decreases, likely due to pressure-limited wall displacement (69, 72).
Other studies have applied strain to the vessel wall directly, independently of fluid pressure, using servo-controlled wire-myograph devices (73). These devices allow isometric stretching of the vessel wall and measurement of the resulting contractions. Stretching the vessel increases the amplitude and the frequency of the contractions, and the behavior is dependent on the rate of stress application: Fast stretching induces bursts of higher-frequency contractions (74).
These studies suggest that a calcium release mechanism in lymphatic vessels is modulated by mechanical stresses. Although our understanding of mechanically activated channels lags behind that of voltage- or ion-gated channels, there are a few candidates that could be involved in the lymphatic response to mechanical distension. Stretch-activated channels (SACs) were originally identified in nerve cells, where they mediate processes such as sensation of touch, pain, and hearing. Endothelial and smooth muscle cells of blood vessels also have SACs (75). By changing conformation in response to membrane deformation, SACs can increase their permeability to Ca2+. They have been implicated in the control of cell volume and the mediation of ion exchange as the channels are opened due to membrane stretch (76). They are also involved in orchestrating Ca2+ fluxes in the filopodia of migrating epithelial cells (77). Whether SACs play a central role in driving the cyclic lymphatic contractions remains to be determined.
3.2.3. Modulation of contractions: endothelium-derived relaxing factors
Whereas some vasoactive agents enhance lymphatic muscle contractions, there are also complementary mechanisms that dampen the Ca2+ dynamics (64, 78–80). Perhaps the best characterized is NO, a vasodilator produced by the endothelium. NO diffuses to the LMCs, where it affects multiple aspects of the Ca2+ contraction machinery (60, 65, 81–83). NO modulates Ca2+ release and uptake (84), as well as the activity of key enzymes involved in force production (85, 86). By activating cytoplasmic guanylate cyclase in vascular smooth muscle, it can decrease vascular tone through cyclic guanosine monophosphate (cGMP)-dependent mechanisms (Figure 2). NO decreases in-tracellular Ca2+ by inhibiting its entry from internal stores through IP3R channels. At the same time, it can activate sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) and BKCa channels to increase Ca2+ outflux (60, 87). NO also directly induces relaxation by dismantling the myosin light-chain crossbridges through protein kinase G (PKG) and myosin light-chain phosphatase (88, 89). Together, these effects result in lymphatic vessel relaxation and decreased contraction frequency. Under normal conditions, NO is produced primarily by the endothelium in response to increased fluid flow (60, 82, 90), but during inflammation, NO can be produced by extravascular stromal cells via another enzyme, inducible nitric oxide synthase (iNOS), and the resulting excess of NO can inhibit pumping (65).
The fact that NO is produced through endothelial nitric oxide synthase (eNOS) in response to fluid shear forces allows it to establish another mechanobiological feedback system that, together with stretch-activated calcium channels, can help control the phasic contractions (91). Endothelium-derived relaxing factors such as NO provide a counterbalance to calcium-induced contractions, maintaining vessel dilation in circumstances when low resistance is needed to allow pressure-driven flow (78). Unfortunately, relatively little is known about endothelial mechanobiology and the structures in the cells that transduce shear stress signals. Although channels such as piezo1 might be involved, other structures such as cytoskeletal elements, adhesion molecules (92), and glycocalyx components (93, 94) have been implicated in vessel mechanobiology. Furthermore, it is likely that cells in the lymphatic wall use distinct pathways to discriminate fluid shear stress and pressure-induced stretch, given the distinct responses to these stimuli. Thus, more research is needed to identify and characterize the mechanosensors involved in lymphatic physiology.
3.3. Coordination of Lymphatic Pumping
It is not obvious how a series of lymphangions in a lymphatic network can be controlled so that the contractions are coordinated efficiently. In the blood system, a single pump (the heart) drives flow through the diverging arterial network, and the network itself is relatively passive (with the exception of vasodilation, which adjusts vessel diameters to distribute the flow to capillary beds according to local demand). In the lymphatic system, we have the inverse scenario: Because the network is converging rather that diverging, the individual contractions need to be coordinated along vessels and at branch points so that the system does not fight against itself.
Because of the complexity in driving such a system by long-range nervous system actuation, it seems reasonable that local feedback is primarily responsible for controlling the contractions and relaxations. Lymph flow and fluid pressure are two primary indicators of how well the lymphatic system is functioning. Therefore, it is logical that these physical properties should be central to the intrinsic control mechanisms. The question is: How do these mechanical signals integrate with the known physiology to regulate contractions?
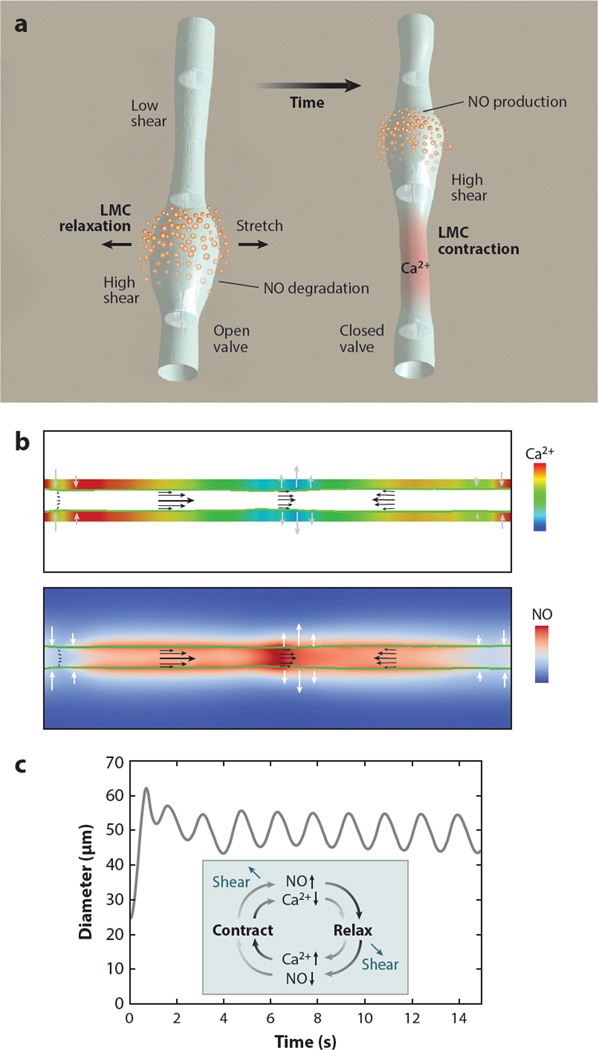
Computational modeling shows that the mechanosensitive calcium and NO dynamics can cooperate to control lymphatic transport via mechanobiological feedback loops (91): During a lymphatic contraction cycle, increased flow causes local eNOS activation, and the subsequent production of NO attenuates and/or reverses the Ca2+-dependent contraction (Figure 3). As the vessel relaxes, NO degrades rapidly and its production drops due to the reduced fluid velocity in the now-larger-diameter vessel. Meanwhile, membrane potentials and resting calcium levels are restored in preparation for another contraction. The next contraction can be triggered by any signal able to mobilize Ca2+, including opening of SACs, release of neurotransmitters, or Ca2+ flux through gap junctions of neighboring cells.
Figure 3.
Dynamics of lymphatic pumping. (a) Conceptual scheme, showing two different snapshots of the lymphatic pumping cycle. Flow direction is from bottom to top. NO relaxes the vessel wall, increasing vessel diameter and pulling fluid from upstream. As the lymphangion fills, the upstream valve is open, and the downstream valve is closed. When the lymphangion is filled, flow and shear stress decrease, and NO is degraded; a subsequent contraction can be initiated through Ca2+ influx via stretch-, voltage-, or ion-activated channels. The contraction closes the upstream valve and opens the downstream valve, increases wall shear stress and induces NO production locally, thus starting the cycle again (NO, orange; cytosolic Ca2+, red). (b) Snapshot of the simulated system at steady state. The vessel boundary is indicated by the green line. Ca2+ and NO concentrations are shown in the top and bottom color maps, respectively. The flow field is represented by the black arrows, and the current wall velocity is indicated by the gray/white arrows. Numerical check valves at the entrance/exit and midpoint enforce flow toward the right. (c) Pumping dynamics predicted by the model. At t = 0, flow is initiated by a mechanical perturbation. The system quickly stabilizes, and subsequent pumping is self-sustained. (Inset) The cyclical nature and dependency of NO and Ca2+ production during a lymphatic contraction. Abbreviations: NO, nitric oxide; LMC, lymphatic muscle cell. Modified from Reference 91.
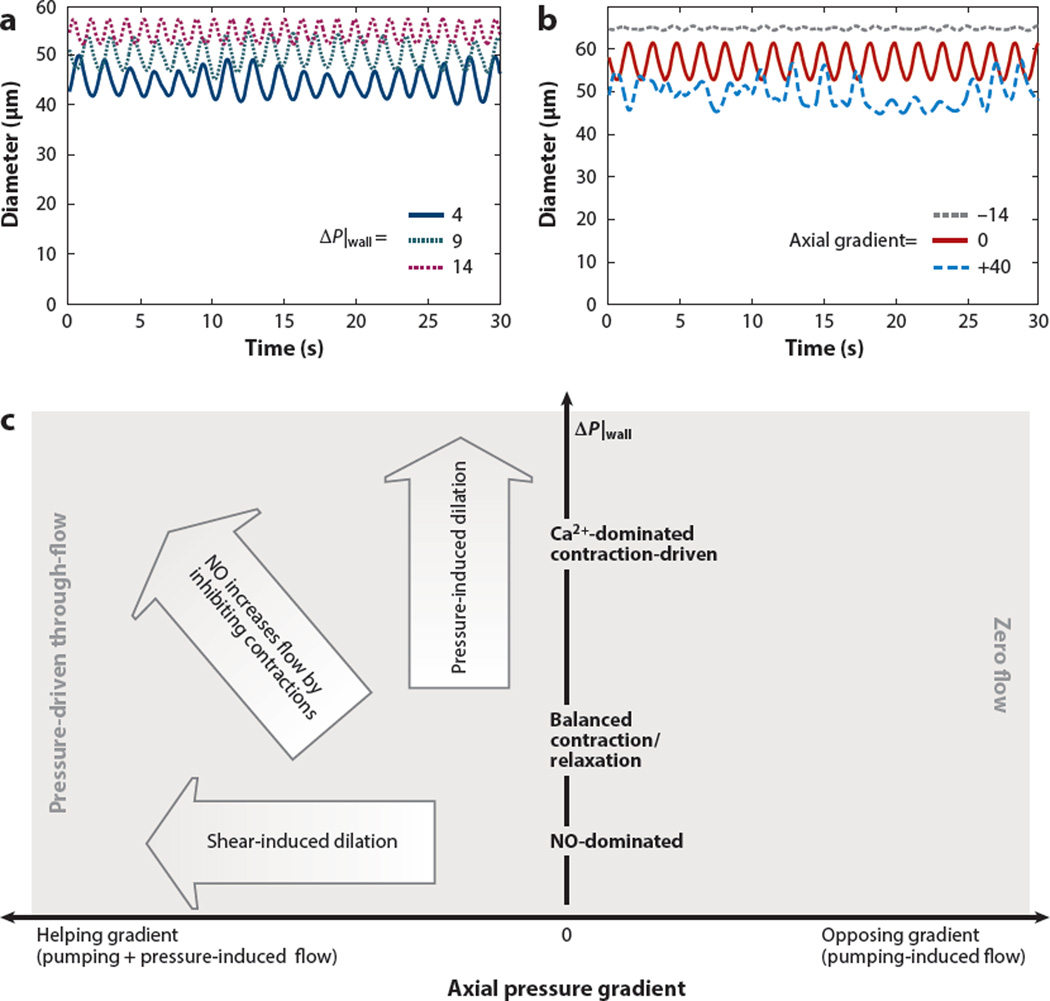
Using this scheme as a basis for mathematical simulations, one can reproduce lymphatic pumping over a range of transwall pressures, as well as a decrease in vessel tone (larger diameter) due to increased luminal pressure (Figure 4a). In these simulations, axial pressure gradients affect pumping indirectly, by changing the level of NO. In agreement with experimental studies showing that imposed flow tends to inhibit contractions (68, 78, 95, 96), the simulations predict decreasing amplitude with increasingly negative (helping) axial pressures (Figure 4b). In addition, experimental observations have shown that the system is able to pump without endothelium-derived NO (87). Our simulations suggest that this is possible for a limited range of pressures: If the pressure is too low, stretch-activated contractions cannot be maintained, and if the pressure is too high, the vessel stalls and cannot complete a contraction cycle. By contrast, with shear-induced NO, positive flow rates are possible over the full range of pressures considered due to NO steering (91).
Figure 4.
Effects of pressure and nitric oxide (NO) dynamics on lymphatic contractions. (a) Transwall pressure (P|wall) affects diastolic and systolic diameters, as well as pumping frequency. Higher pressures result in lower amplitude and larger diameters. There were no axial pressure gradients imposed for these simulations. (b) In the absence of a transwall pressure difference, frequencies are affected by flow induced by axial pressure gradients. With an opposing gradient (blue), the vessel has to pump against the hydrostatic pressure. With a helping gradient ( gray), the pressure can drive flow and create NO to inhibit contractions. (c) Behavior map of transport in a collecting lymphatic vessel. The performance of the system is summarized as a function of axial (x axis) and transwall ( y axis) pressure gradients. Modified from Reference 91.
Computer simulations based on known mechanobiological systems can reproduce the range of behaviors observed in lymphatic vessels in vivo and show how lymphatic vessels adjust their vasomotion using mechanical cues of pressure and flow. The feedback provided by NO pathways and calcium fluxes establishes a robust and autoregulated system that drives active pumping when gravity opposes flow but induces vessel relaxation when pressures are able to drive flow passively (Figure 4c).
3.4. Summary and Conclusions
To appropriately respond to changing fluid environments, the lymphatic system needs feedback mechanisms so it can correct any imbalances dynamically and locally. One way it can do so is through mechanobiological activation of biochemical pathways that control the motion of the vessel wall. Such active coupling of mechanical signals and biochemical pathways creates a complex system in which the flow of lymph, or changes in its pressure, can modulate lymphatic contractions and control fluid drainage. The reliance of lymphatic pumping on mechanosensors for controlling calcium and NO levels suggests that these might be new, selective targets for modulating lymphatic function in pathologies. Once the mechanosensors are better characterized, it should be possible to develop drugs to either enhance or block these pathways with minimal toxicity to blood vessels and other tissues.
4. ROLE OF THE LYMPHATIC SYSTEM IN CANCER PROGRESSION
4.1. Lymph Node Metastasis: Clinical Role
Lymph nodes are the most common sites of solid tumor metastases. Their presence signals a poorer prognosis and prompts a recommendation for systemic therapy in most cancer patients. However, why lymph node metastases are such strong predictors of outcome in cancer patients is the subject of much debate. On one hand, it is possible that the presence of cancer cells in lymph nodes simply reflects the ability of the primary tumor to metastasize, and the actual disease in the lymph nodes is inconsequential (97, 98). On the other hand, the strong predictive power of lymph node metastases could be due to the ability of cancer cells in the lymph node to leave and spread to distant metastatic sites (99–101). This argument suggests that lymph node metastases need to be treated in order to prevent distant metastasis and ultimately eliminate all disease from the patient (102–104). For an individual patient, the truth likely lies in between, depending on where on the spectrum of progression to distant metastasis the cancer is diagnosed (105). The status of distant metastasis in individual patients will determine how large a role lymph node metastases will play in the outcome for the patient—that is, whether there is risk of further spread from lymph nodes or whether distant spread has already occurred.
4.2. Tumor-Draining Lymphatic Vessels
Initially thought to be a passive process, lymphatic metastasis is now thought to be regulated at multiple steps, including the attraction and entry of cancer cells to lymphatic vessels and the successful penetration into draining lymph nodes (106). Solid tumors commonly induce an expansion of the surrounding lymphatic network (8, 38). However, functional lymphatic vessels are restricted to the tumor margin and peritumor regions surrounding tumors (107). As tumors lack intratumor functional lymphatic vessels, the interstitial fluid pressure is elevated, which can alter lymph flow to tumor-draining lymph nodes (108). In experimental mouse models of cancer, over-expression of the lymphangiogenic growth factors VEGF-C and VEGF-D enhanced peripheral tumor lymphatic vessel growth and increased lymph node metastasis (8, 107, 109, 110) as a result of an increased number of cancer cells arriving in the lymph node (108, 111). CCL21 production by the lymphatic endothelium can also be enhanced by VEGF-C, thereby promoting lymphatic entry of CCR7+ tumor cells (112). Thus, tumor cells that arrive at the lymphatic vessels may enter passively or by active signaling mechanisms.
After entry of tumor cells into tumor margin initial lymphatic vessels, the cancer cells travel through collecting lymphatic vessels to the lymph node (Figure 5). Tumor-derived VEGF-C and VEGF-D increase the contraction of proximal collecting lymphatic vessels (1), potentially increasing lymph flow and tumor cell dissemination (108, 113). When tumor-induced lymphatic vessel remodeling was prevented, the spread of cancer cells to lymph nodes was reduced (108, 111, 114, 115). In collecting lymphatic vessels draining melanoma, it is thought that lymph flow promotes the spread of “in-transit metastases”—cancer cells that are initially spread locally to tissues between the primary tumor and the lymph nodes—ultimately leading to lymphatic metastasis (116).
Figure 5.
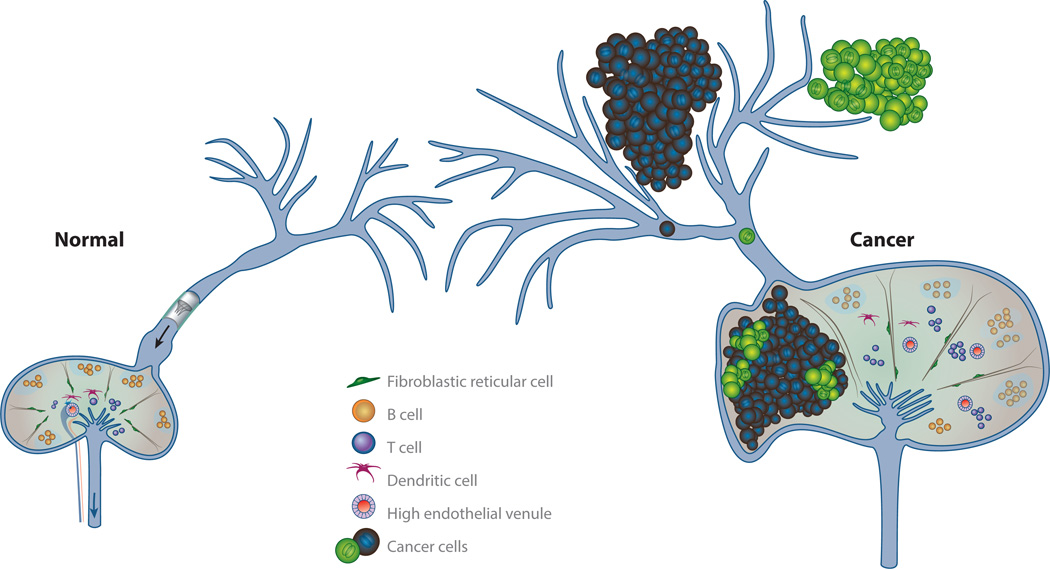
Lymph node anatomy and lymphatic metastasis. Tumor-draining lymphatic vessels are remodeled by the tumor, causing cancer to spread through the lymphatic vessels to lymph nodes. The primary tumor is also able to precondition tumor-draining lymph nodes, both altering the ability of the lymph node to mount an immune response and potentially making the lymph node microenvironment more conducive to cancer growth. Metastatic lesions that form in the lymph node lack high endothelial venules and are devoid of lymphocytes. These attributes make it challenging for the lymph node to generate and sustain antitumor immunity.
As tumors grow in lymphatic vessels or overtake a lymph node, flow resistance increases and lymph is diverted around these structures through collateral lymphatic vessels (117–119). Similarly, removal of popliteal lymph nodes in mice results in rerouting of lymph drainage from hind-limb tumors through preexisting collateral lymphatic vessels, causing corresponding changes in the patterns of lymph node metastases (120). On the basis of these data, new routes of lymph transport created after regional lymphadenectomies may allow cancer progression to additional lymph nodes.
4.3. Molecular Signals Precondition the Lymph Node Microenvironment
In 1889, Paget (121) described the interaction between the tumor cell (which he called the seed) and the microenvironment (the soil). At distant metastatic sites, the arrival and successful colonization of tumor cells are often preceded by changes in the microenvironment of the metastatic organ—referred to as a premetastatic niche. Several studies have now elucidated some distinctive features of premetastatic lymph nodes, including increased lymphangiogenesis and lymph flow (122), remodeling of high endothelial venules (HEVs) (123–125), recruitment of myeloid cells, and reduction of effector lymphocyte numbers and function (Figure 5) (126). It is thought that lymph-transported molecules that originate from the primary tumor orchestrate these events as they arrive in the sentinel lymph node. Immune cell composition can also be altered in tumor-free, nonsentinel lymph nodes, suggesting that some effects on the immune system can be propagated to multiple lymph nodes (127).
Lymph node lymphangiogenesis (LNL) is the best-characterized aspect of these premetastatic changes. LNL, but not angiogenesis, is a strong predictor of further lymph node metastasis (128). LNL is driven by VEGF-A, VEGF-C, integrin α4β1, and erythropoietin (129–131) and correlates with increased systemic metastasis in these studies. In addition to causing changes in the lymphatic vasculature in premetastatic lymph nodes, HEVs also remodel and lose surface molecules, altering their function (123, 132). The remodeling of HEVs in tumor-draining lymph nodes likely impairs the recruitment of naïve lymphocytes and the antitumor immune response. Remodeled HEVs may also increase the supply of oxygen and nutrients to a growing lymph node lesion, as lymph node metastases seem not to rely on angiogenesis (124).
In addition to ECs, other stromal cells and the immune cell populations are altered in tumor-draining lymph nodes. CCL21 production is impaired (133), whereas Stat-3/S1PR1 signaling in myeloid cells (134) and prostaglandin E2 (PGE2) production in subcapsular sinus DCs are increased (126). Blocking Stat-3/S1PR1 reduces metastasis, whereas PGE2 production leads to SDF-1α production, which is known to attract CXCR4+ tumor cells and correlate with lymph node metastasis (135).
Several cytokines play a prominent role in the immunosuppression of tumor-draining lymph nodes. Interleukin (IL)-10, transforming growth factor (TGF)-β, and granulocyte macrophage colony–stimulating factor (GM-CSF) are all elevated in tumor-draining lymph nodes (136, 137). Similarly, recruitment of myeloid immune cells from the blood also promotes an immunosuppressive microenvironment in premetastatic lymph nodes, facilitating cancer cell growth and expansion (127).
These studies show that tumor-draining lymph nodes undergo vascular and stromal remodeling, have altered immune cell recruitment, and change the normal chemokine environment. Together, these alterations create a permissive microenvironment for metastatic growth as well as suppression of antitumor immunity.
5. THE GROWTH OF LYMPH NODE METASTASES
The previous section describes how tumors can alter the draining lymph node and cancer cells can invade tumor-draining lymphatic vessels. In this section, we discuss the formation of metastasis as cancer cells arrive and grow in a lymph node, as well as potential therapeutic opportunities.
5.1. Cancer Cell Survival in the Lymphatic System
Cancer cells that enter the lymphatics need to survive in a low-oxygen environment (138, 139). Cancer cells that subsequently reach the lymph node first encounter the subcapsular sinus (SCS), which is devoid of blood vessels. Here, they experience hypoxia (124), which affects tumor cell metabolism and selects for clones that are more aggressive and metastatic. This selection and adaptation may enable cancer cells to survive in the avascular SCS, spread further in the patient, or alternatively enter a state of dormancy while in the SCS.
Angiogenesis is known to be induced in response to hypoxic environments. However, increases in blood vessel density in lymph node metastasis have not been observed (115, 124, 140, 141). Sprouting angiogenesis was not observed in a study using intravital microscopy to longitudinally follow the growth of spontaneous lymph node metastases in mouse models (124). Instead, cancer cells in the SCS invaded the lymph node, where they could utilize the native vasculature of the lymph node. In doing so, these cells no longer experienced hypoxia (124).
5.2. Chemokine Signaling in Lymph Node Metastases
In lymph nodes, chemokines help direct immune cells to new locations in order for them to perform their functions in initiating and maintaining adaptive immune responses, as well as preserving self-tolerance. Increasing evidence shows that cancer cells can utilize these pathways to spread to lymph nodes. A seminal study by Müller et al. (135) showed that expression of several chemokine receptors, including CXCR4 and CCR7on breast cancer cells from patients, correlates with lymph node metastasis. By blocking CXCR4 signaling, lung and lymph node metastases were reduced in mouse models (135). Additionally, CCR7 signaling increased lymph node metastasis in mouse models of melanoma (142) and esophageal cancers (143). Expression of CCL1 by LECs of the SCS permitted CCR8+ tumor cell escape from the SCS. Invasion of these melanoma cells into the lymph node parenchyma was blocked by inhibiting CCL1/CCR8 signaling (144). In addition to attracting tumor cells, chemokines may also establish a protumor microenvironment in the lymph node on the basis of the immune cells they attract and their role in determining the effector function of these cells. The potential to reduce cancer cell spread and boost antitumor immune function makes targeting specific chemokine signaling pathways an attractive option to add to standard cytotoxic approaches for cancer treatment.
5.3. Evading Immune Surveillance in the Lymph Node
Lymphatic vessels carry foreign, pathogenic, or self-antigens as well as antigen-loaded DCs to lymph nodes, where each antigen is surveyed. Precise control over the interaction between DCs and lymphocytes is critical to prevent immune responses to self-antigens. Lymph node stromal cells—FRCs and ECs—help facilitate these interactions (145, 146).
Tumor-draining lymph nodes commonly have an environment that suppresses immune response. For example, fewer effector T cells are found in metastatic and premetastatic lymph nodes (127). In addition, myeloid-derived suppressor cells (MDSCs) can accumulate in sentinel lymph nodes, resulting in the recruitment of inhibitory regulatory T cells (Tregs) and suppressed T cell proliferation and function (147, 148). Many similarities are observed in tumor-draining lymph nodes as those draining areas of chronic inflammation. For example, activated LECs of the afferent lymphatic vessel can present self-antigens on major histocompatibility (MHC) class I molecules. These LECs lack the costimulatory molecules necessary to activate CD8+ T cells and express the immune checkpoint inhibitory ligand PD-L1 (14). Thus, LECs can scavenge and cross-present self-antigens from lymph on MHC class I molecules, leading to the deletion of autoreactive naïve CD8+ T cells (149). This ability for LECs from afferent lymphatic vessels (the connection between the tumor and the lymph node) to cross-present antigen may promote the survival of tumor cells in lymph nodes. LECs can also alter the maturation of DCs, preventing an immune response (150).
It is in the context of these alterations in tumor-draining lymph nodes that metastatic cancer cells arrive to establish metastatic lesions and evade T cell responses. Cancer-induced remodeling of HEVs can potentially impair immune cell trafficking to the lymph node (124), promoting tumor cell survival. Among the T cells that populate a metastatic lymph node, tumor-specific T cells are functionally tolerant (151), stemming from inhibition by LECs (149) as well as inhibition by CTLA-4 or PD-1 during initial interaction with their cognate receptor on DCs or cancer cells (31, 32, 152, 153). In late-stage cancers, further evidence of immune suppression is observed in the lack of effector function in proliferating CD8+ T cells (154).
In addition to T cells, B cells also can affect the growth of lymph node metastasis (155). B cells process and present antigen to naïve CD4+ or CD8+ T cells in tumor-draining lymph nodes, activating effector T cell response to tumor antigens (156). Activated B cells also clonally expand and secrete tumor-specific immunoglobulin G (IgG) in response to tumor antigens in the sentinel lymph node (157). These studies underscore the role of B cells in generating antitumor immunity, although additional research will be critical to fully understand the importance of B cells and reveal any potential anticancer therapeutic opportunities.
5.4. Clonal Dissection of Lymph Node Metastases in Solid Tumors
Recent genomic studies have attempted to better define the sequence of disease progression from primary tumor to distant metastasis (158–160). These data show that human lymph node metastases are polyclonal and contain similar genetic compositions as the primary tumor (159, 160). In contrast, clonal lesions have been detected in distant metastases (158–160). Research in animals has also shown direct evidence that lymph node metastases originate from multiple cells that disseminate from the primary tumor (124). These data suggest that there is a fundamental difference in the spread of cancer through lymphatic vessels in comparison to blood vessels. Lymphatic vessels bring cancer cells to a common location—the lymph node—where they deposit cancer cells. As such, lymph node metastasis can be continually reinforced by the arrival of new cells as they gain a foothold in their new microenvironment (Figure 5). In contrast, the branching blood vasculature delivers metastatic cells to multiple locations in a single organ, leading to a greater chance of selecting a specific clone that will show genetic divergence from the primary tumor. These observations may have multiple implications for patients with lymph node metastasis (12). The genetic diversity in lymph node metastases makes targeting a single molecular pathway to treat lymph node metastases challenging and may not prevent progression of the disease (159).
5.5. Treating Lymph Node Metastases
Although metastasis remains the major cause of cancer mortality, the challenge of eradicating cancer cells that have spread to lymph nodes or distant organs remains. Seminal discoveries defining the molecular and cellular mechanisms that drive metastasis have yet to improve survival for many patients with metastatic disease. One limitation is that most anticancer drugs are optimized by studying the primary tumor growing in its native microenvironment. The local microenvironment in which tumor cells grow greatly affects growth rate, metabolism, vascularization, immune response, and ultimately response to therapy. Thus, drugs designed to work in the primary tumor are often less effective in treating metastasis. Preclinical and clinical studies show that lymph node metastases and primary tumors can respond differently to the same therapeutic regimen (111, 124, 161–164). Drug development needs to account for the various microenvironments in which disseminated cancer may reside to improve efficacy of therapy.
In addition to adapting drugs to the biology imposed by the lymph node microenvironment, investigators are attempting to improve the delivery of pharmaceuticals to the lymph node. Subcutaneous chemotherapy targeted to regional lymph nodes increased lymph node drug concentration compared with intravenous delivery (165), although this method has yet to be tested in a spontaneous model of lymph node metastasis. Lipid nanoparticles also increased delivery of antiretroviral drugs to lymph nodes of primates (166). Similar approaches should be tested for improved efficacy in lymph node metastasis. Nanoparticles successfully delivered adjuvant to sentinel lymph nodes in order to stimulate a response to tumor antigens already present. This strategy stimulated an immune response and reduced tumor growth (167).
In addition, novel immunomodulating agents may be beneficial to patients with lymph node metastasis. Inhibition of GM-CSF reduces tumor-induced tolerance by increasing tumor-specific CD8+ lymphocytes that produce interferon (IFN)-γ in lymph nodes (168). Similarly, inhibition of TGF-β decreases Tregs and increases the number of tumor antigen–specific CD4+ and CD8+ T cells producing IFN-γ (169), thereby inhibiting the growth of distant metastases. Methods to further activate an antitumor immune response in lymph node metastases are under development.
6. IMPAIRED LYMPHATIC FUNCTION IN LYMPHEDEMA
Lymphedema describes a progressive pathologic condition in which lymphatic vessels fail to drain fluid from the interstitial space and return it to the blood. This failure causes accumulation of protein-rich fluid combined with inflammation, adipose tissue hypertrophy, and progressive fibrosis (170), ultimately leading to reduced quality of life, functional impairment, and physical deformity. Primary lymphedema is defined as a congenital form of lymphedema caused by dysplasia of lymph vessels, with the phenotype developing at varying stages in a patient’s life. Primary lymphedema is commonly associated with a congenital syndrome (171). Secondary lymphedema is acquired during life and is mainly a consequence of trauma, infection, surgery, radiation, or malignancy (172). An estimated three million patients in the United States are currently afflicted with lymphedema (9, 173)—nearly 1% of the population. Currently, lymphedema treatments help alleviate symptoms but do not cure the underlying disease processes (174). Here, we describe the different origins of lymphedema, discuss how they develop, and present potential therapeutic opportunities for lymphedema patients.
6.1. Primary Lymphedema
Primary lymphedema is congenital and defined by the age of onset. The disease itself is rare and affects 1.2 per 100,000 patients younger than 20 years (172). Primary lymphedemas are caused by a variety of developmental and/or functional defects affecting the lymphatic vessels.In these patients, extremities are typically affected as a result of insufficient drainage, although visceral drainage can also be abnormal (171). In children, primary lymphedema is typically part of Milroy’s disease or lymphedema–distichiasis (174). Today, many of the gene mutations associated with primary lymphedema involve the VEGF-C/VEGFR-3 axis or developmental pathways for lymphatic vessel formation (175), but mutations downstream of other tyrosine kinase receptors and developmental genes have also been identified. Table 1 presents a list of congenital lymphatic abnormalities.
Table 1.
Known congenital lymphedema and lymphatic abnormality syndromes
| Lymphedema syndromes | Gene (protein) |
|---|---|
| Cantu syndrome | ABCC9, KCNJ8 |
| Cholestasis–lymphedema (Aagenaes) syndrome | Locus in 15q |
| Choanal atresia/lymphedema | PTPN14 |
| Emberger syndrome | GATA2 |
| Hennekam lymphangiectasia–lymphedema syndrome | CCBE1, FAT4 |
| Hypotrichosis–lymphedema–telangiectasia syndrome | SOX18 |
| Lymphedema–lymphangiectasia | HGF |
| Lymphedema–distichiasis syndrome | FOXC2 |
| MCLMR | KIF11 |
| Meige disease | GJC2 (CX47) |
| Milroy disease/Nonne–Milroy lymphedema | FLT4 (VEGFR-3) |
| Milroy-like disease | VEGFC, KIF11 |
| Noonan syndrome 1 | PTPN11 (SHP2), SOS1, KRAS, RAF1 |
| Oculodentodigital dysplasia/lymphedema | GJA1 (CX43) |
| OLEDAID syndrome | IKBKG (NEMO) |
| Parkes–Weber syndrome | RASA1 |
| Other lymphatic anomalies |
Responsible gene (protein) or chromosomal abnormality |
| CLOVES syndrome, Klipple–Trenaunay–Weber syndrome |
PIK3CA |
| Costello syndrome | HRAS |
| Fetal chylothorax | ITGA9 |
| Proteus syndrome, PTEN hamartoma tumor syndrome | PTEN, AKT1 |
| Turner syndrome | Monosomy X |
Data are from References 9 and 176–178. Abbreviations: CLOVES, congenital lipomatous overgrowth, vascular malformations, epidermal nevis, spinal/skeletal anomalies/scoliosis; MCLMR, microcephaly with or without chorioretinopathy, lymphedema, or mental retardation; OLEDAID, ectodermal dysplasia with immunodeficiency, osteopetrosis, and lymphedema.
6.2. Secondary Lymphedema
Secondary lymphedema is usually caused by trauma to lymphatic structures, infection, surgery, radiation, and/or malignancy (172, 174). Therefore, secondary lymphedema generally develops at a later age than primary lymphedema and may progress to a chronic condition. Lymphedema caused by the nematode parasite Wuchereria bancrofti remains the most common cause of lymphedema worldwide, responsible for 90% of cases of lymphatic filariasis (179). The remaining 10% are caused by Brugian species, Brugia malayi and Brugia timori (180). The clinical manifestations of lymphatic filariasis are varied, and clinically apparent lymphatic disease is present in 30–40% of the estimated 120 million individuals with filarial infections (180). All lymphatic filariasis patients typically show retrograde adenolymphangitis with an acute infection. Lymphedema of the lower leg, elephantiasis, and chyluria are the most common chronic conditions (181). Rather than rendering the lymphatics hyperpermeable, active lymphatic remodeling involving EC growth, migration, and proliferation is considered an important feature in early filarial infection (182). Current treatments involve targeting the endosymbiont Wolbachia in filarial nematodes with doxycycline, which improves mild to moderate—but not severe—filarial lymphedema independent of an ongoing infection.
Upper-extremity lymphedema is often associated with the treatment of breast cancer, and lower-extremity lymphedema is common in patients being treated for gynecologic or urologic malignancy, prostate cancer, melanoma, and lymphoma (170). The incidence of cancer-related secondary lymphedema after lymph node dissection and/or sentinel lymph node biopsy can be as high as 63.4%, and this condition commonly results from treatments that include surgical intervention, radiation therapy, chemotherapy, hormonal therapy, and/or targeted therapy (174, 183). Notably, a prospective study with 1,121 patients showed that taxane-based therapy does not increase the risk of developing lymphedema (184).
6.3. Clinical Lymphedema Measurements
There are many methods to clinically evaluate lymphedema and the structure and function of the lymphatic system. Limb volume (LV) measurements—made simply by using a tape measure—are the most common way to identify lymphedema. If subsequent measurements are taken by an experienced user on the basis of anatomical landmarks, this method is reliable in detecting and monitoring lymphedema (185). Alternatively, the clinician may use perometry or multifrequency bioimpedance measurements, which are automated LV scanning methods that eliminate interobserver variability, but these are more expensive. Comparison between these LV measurement methods shows that the use of a tape measure for early diagnosis of lymphedema in patients is effective, but the sensitivity of this technique leads to some uncertainty (186).
There are several imaging techniques available in the clinic to reliably evaluate lymphatic function and lymphedema. Lymphoscintigraphy is still considered the gold standard of imaging modalities (9, 174, 185) to investigate lymphatic function using two-dimensional imaging. The clearance of radioactive tracers injected intradermally or subcutaneously can quantify lymphatic clearance rates, map aberrant drainage patterns, and identify dermal backflow by imaging a location and assessing the increase in signal over time. This technique has recently been used to measure impaired lymph flow in cancer patients (187). To improve the spatial resolution, other techniques have been combined with lymphoscintigraphy to allow for three-dimensional imaging.
The combination of SPECT/CT (single-photon emission computed tomography/computed tomography) and lymphoscintigraphy allows the abnormal findings in lymphatic function to be better correlated with anatomy (188). The addition of SPECT/CT has also proven favorable in evaluating the therapeutic efficacy of lymphovenous anastomosis (188) and documenting lymph node regeneration (189).
MR (magnetic resonance) lymphangiography has better spatial resolution than nuclear medicine techniques and is a valuable modality in clinical imaging of lymphedema. MR lym-phangiography using contrast agents is able to visualize lymphatic vessels, determine backflow, and correlate lymphatic drainage patterns with disease severity in lymphedematous limbs (190, 191). These characteristics are useful in both diagnosing and surgically managing lymphedema (191). Data suggest that T2-weighted MRI (magnetic resonance imaging) has a greater sensitivity, but contrast-enhanced MR lymphangiography produces better image quality (185). However, contrast-enhanced vascular structures may confound image interpretation. Noncontrast MR lymphography using very heavily T2-weighted fast spin echo sequences detects differences in lymphatic flow and lymphedema severity in normal and lymphedematous limbs (192).
Near-infrared imaging (NIR) is also being used in the clinic for the evaluation of lymphatic function; it is more time efficient and less expensive than lymphoscintigraphy (193). In its first clinical uses, this method improved sentinel lymph node mapping and intraoperative guidance. Moreover, this test was able to detect dermal backflow, reduced clearance, and dilated lymphatic vessels (194).
In comparisons between these techniques, there is debate over the best modality to diagnose, identify causes, and plan treatment of lymphedema. The method of choice depends on the clinical question to be answered and the severity of disease in the patient. Lymphoscintigraphy and NIR can better assess lymphatic function and the severity of any dysfunction, whereas MRI and CT provide more information regarding anatomical status for surgical treatment planning in advanced disease. MRI and indocyanine green lymphography have more sensitivity than lymphoscintigraphy or CT for diagnosis of lymphedema (195). The current gold standard, lymphoscintigraphy, carries many disadvantages, including generally poor spatial resolution, added costs when combined with SPECT/CT, and exposure to radioactive compounds (187). However, lymphoscintigraphy has more sensitivity in distinguishing functional defects and altered drainage patterns, especially when combined with SPECT/CT (188, 190). MRI seems to best evaluate anatomical status, whereas NIR shows great promise in addressing functional status of lymphatics. The latter technique may be better at detecting lymphatic dysfunction at an earlier preclinical stage where current lymphedema interventions may be more successful. This point emphasizes a current diagnostic challenge: the need to improve early detection and treatment efficacy of lymphedema.
6.4. Pathogenesis of Secondary Lymphedema
Regardless of the causative event in the development of secondary lymphedema, the host responses of inflammation, irreversible lymphatic dysfunction, and fibrosis seem to be the main mechanisms in the progression of lymphedema. However, there is limited understanding of the underlying biology that permits lymphedema formation. Known risk factors for lymphedema include obesity (196), elevated blood pressure, adjuvant radiation therapy following lumpectomy, the location of removed lymph nodes, and a greater number of lymph nodes removed during surgery (185). Additionally, combinations of genetic risk factors predispose patients to developing secondary lymphedema (197).
In mouse models as well as human lymphedema specimens, lymphatic stasis induces CD4+— but not CD8+ or CD25+—T cell activation and T helper 2 (Th2) cell differentiation, necessary for the development of fibrosis, adipose deposition, and lymphatic dysfunction in lymphedema (198). Furthermore, macrophages have been hypothesized to have an important lymphangiogenic role in humans and mice, notably via transdifferentiation to macrophage-derived LEC progenitors and their incorporation into the lymphatic wall (199).
6.4.1. Fibrosis
Fibrosis is a critical inhibitor of lymphatic regeneration and an important player in lymphedema formation (200). Interestingly, increased TGF-β levels are correlated with disease severity in patients with fibrosis, cancer, and systemic sclerosis (201). TGF-β also inhibits lymphatic vessel formation (202) and promotes tissue fibrosis after radiation therapy (203). Additionally, in mice, TGF-β1 blockade promotes lymphatic regeneration and improves lymphatic function while decreasing tissue fibrosis, chronic inflammation, and Th2 cell migration (204). Radiation is a major risk factor for lymphedema development (205); however, little is known about the mechanisms responsible for this effect. Avraham et al. (173) analyzed the effects of radiation induced fibrosis on lymphatic function by blocking TGF-β after radiation in vivo. They found that radiation therapy decreases lymphatic vessels in mice and verified that radiation promotes soft tissue fibrosis. Moreover, short-term inhibition of TGF-β following radiation improved lymphatic function and decreased soft tissue fibrosis (173). Paradoxically, VEGF-C increased the amount of radiation-induced DNA damage in LECs, likely due to VEGF-C-induced mitosis (206). Thus, a local microenvironment that encourages lymphatic proliferation at the time of radiation might also impair long-term capacity for repair of the lymphatic system. Recent clinical trials have shown that adjuvant radiation therapy following lumpectomy reduced the risk of in-breast recurrence and metastasis (205), and early reports in thousands of patients from clinical trials (207) have shown a significant increase in local control, disease-free survival, and overall survival in patients receiving regional lymph node radiotherapy. Thus, the use of radiotherapy in treating breast cancer will continue to be an important therapy, increasing the risk of fibrosis and subsequent lymphedema in these patients.
6.4.2. Predictive biomarkers of secondary lymphedema
Little is known about predictive biomarkers in secondary lymphedema. Miaskowski et al. (208) evaluated genotypic characteristics involved in lymphangiogenesis and angiogenesis in women with and without lymphedema following breast cancer treatment, finding genetic associations for lymphocyte cytosolic protein 2, neuropilin-2, protein tyrosine kinase, and vascular cell adhesion molecule 1. They also found three haplotypes associated with lymphedema following breast cancer treatment—forkhead box protein C2 (haplotype A03), neuropilin-2 (haplotype F03), and VEGF-C (haplotype B03). Lin et al. (209) used tissue prospectively collected from human skin in patients with lymphedema to identify proteins involved in lymphangiogenesis, inflammation, fibrosis, and lipid metabolism. They found basic fibroblast growth factor (bFGF), IL-4, IL-10, TNF-β, TGF-β, and leptin to be potential biomarkers for lymphedema. Further exploration is needed to evaluate whether these biomarkers have clinical relevance for diagnosis of early and latent lymphedema.
6.5. Therapeutic Opportunities
Currently, lymphedema treatments—consisting of compression garments, massage, and intensive bandaging—help alleviate symptoms, but do not cure the underlying disease processes (174). For patients who are unresponsive to these interventions and continue to have an increase in the size and weight of the extremity as well as progressive impairment in function, several surgical options are available. After assessment of deep and superficial lymphatic vessel function using imaging modalities described above, surgical interventions can be evaluated in individual patients. These include debulking and liposuction, lymphaticovenular by passs urgery, and vascularized lymph node transfer. Lymphaticovenular bypass surgery and vascularized lymph node transfer are mainly used to reduce the fluid in a limb and are combined with chronic nonsurgical treatments to obtain optimal volume reduction. In order to reduce the volume of the inflamed fatty tissue, debulking and liposuction can be used, although superficial lymphatics will be damaged. These methods—and treatment combinations—have shown promising results in reducing symptoms and extremity size (210). However, treatment options differ per region, and clinicians do not necessarily agree on the best treatment of complex patients, indicating the need for more research in this area. In the case of vascularized lymph node transfer, there can be a significant risk of regional lymphedema from the site where the donor lymph node was removed, depending on the donor site (174). Therefore, more recent studies have improved the process of omental lymph node flap harvesting (211, 212). Interestingly, Alitalo and colleagues (213) have shown that lymph node transfer in mice and pigs, when combined with perinodal VEGF-C therapy, induces lymphangiogenesis and improves lymphatic vessel function. In addition, Avraham et al. (204) have demonstrated that blockade of TGF-β1 accelerates lymphatic regeneration during wound repair in mice. Moreover, insights into lymphatic pumping physiology and pathophysiology indicate that improving lymphatic pumping might identify targets in treating lymphedema. These are active areas of research in the search for new therapeutic strategies for progressive lymphedema, as are efforts to detect and prevent lymphedema.
7. ROLE OF THE LYMPHATIC SYSTEM IN DISEASE PROCESSES
7.1. Inflammation
Acute inflammation is the process by which pathogens and/or tissue injuries are dealt with in a regulated manner, normally returning the tissue to the preinflamed phenotype afterward. However, dysregulated or chronic inflammation can also occur as a result of many disease processes. Acute inflammation consists of two main phases: the initiation phase and the resolution phase. The inflammatory cascade during acute inflammation (214), regardless of whether it is sterile or nonsterile, causes lymphangiogenesis and morphological changes to existing lymphatic vessels (108). These processes are intended to create adequate fluid and antigen drainage to lymph nodes (215) so as to enable antigen processing, appropriate immune responses, and maintenance of tissue fluid homeostasis.
The resolution phase of acute inflammation, histologically marked by hypertrophy of the draining lymph nodes caused by increased cellularity and lymphangiogenesis, is also a controlled process and causes remodeling of lymphatic vessels (214). These findings illustrate the importance of the lymphatic system in transporting inflammatory cells out of a tissue in order to resolve acute inflammation.
In chronic inflammation, there is prolonged exposure to injurious stimuli or failure to resolve acute inflammation, promoting sustained immune cell infiltration. Induced by progressive accumulation of lymphocytes, tertiary lymphoid organs (TLOs) can form in chronic inflammatory disorders, creating HEV-like vessels de novo and stimulating lymphocyte homing as it would normally occur in lymph nodes (216). These TLOs are observed in many chronic inflammation conditions, including autoimmune responses, graft rejection, atherosclerosis, microbial infections, and cancer. The stromal components (217) and molecular regulation of these TLOs mimic those of lymph nodes (218). Recently, nuclear factor κB (NF-κB)-inducing ECs were suggested to be central to the formation of TLOs (219). Creation of TLOs is thought to have a role in maintaining immune responses against persistent injurious stimuli in an attempt to resolve the underlying pathology and stop the inflammatory process (220). Ongoing research will further elucidate the complete process of acute and chronic inflammation, possibly supporting therapies that alter the growth and function of lymphatic vessels.
7.2. Graft-Versus-Host Disease
Graft failure because of immune rejection is a significant problem in organ transplantation and shares many features with chronic inflammation, including the importance of lymphatic and blood vessels. In cornea transplantation research, the corneal ingrowth of lymphatic vessels facilitates the transport of APCs to lymph nodes and entry of immune effector cells into the graft, accelerating the induction of alloimmunity and subsequent graft rejection (221, 222). Therapeutically, in mice, targeting angiogenesis and lymphangiogenesis, individually or simultaneously, significantly improves graft survival (221, 223). Anti-VEGF treatment with ranibizumab and bevacizumab has been used to reduce graft rejection in high-risk human patients (10). In mice, blocking insulin receptor substrate 1 (IRS-1) with GS-101 (aganirsen) also inhibits hemangiogenesis and lymphangiogenesis (224), showing favorable results in clinical trials for progressive corneal neo-vascularization (10). These successful clinical trials mark the first translation of lymphatic-focused pharmacological treatments in patients.
In both animal (225) and human (226, 227) renal grafts, lymphangiogenesis is significantly more present in renal transplants; this literature suggests that lymphangiogenesis reduces chronic renal graft survival, although conflicting evidence exists (228). In human lung transplant biopsies (229) and rat cardiac allografts (230, 231), lymphangiogenesis also reduces graft survival, and evidence from cardiac transplantation research suggests that draining mediastinal lymph nodes have a crucial role in the immune response (232). In acute cardiac (233, 234) and liver (235) rejections, however, lymphatics seem to be organ protective by ensuring adequate lymphatic drainage postoperatively, although the literature is not extensive. In small-bowel transplantation in rats, perioperative lymphatic reconstruction improves long-term viability, resulting in better survival rates and less mucosal damage due to chronic graft rejection (236).
Thus, lymphatic vessels and lymphangiogenesis in transplantation can determine the ultimate outcome for the graft. Soon after implantation of solid organs, lymphatic formation might reduce tissue edema and inflammation by providing an exit route for lymphocytes and macrophages, as has been shown in successful therapy of acute renal (237) and liver (235) transplant rejection. In the later phase of graft rejection, the persistence of numerous lymphatic vessels might enable chronic inflammation and/or allogenic responses that disfavor allograft survival (238). There are some hints that blocking lymphangiogenesis might not be advantageous in all organs soon after transplantation; however, overgrowth of lymphatic vessels in organ grafts seems to reduce chronic graft survival. Further research in this important area is needed to develop rational strategies for improving the success of organ transplantation.
7.3. Arthritis
Arthritis is an inflammatory joint disease characterized by swelling, pain, and irreversible tissue damage. Arthritis has many forms and causes; the most common form, osteoarthritis, is caused by trauma, biomechanical alterations, infection, or age. Rheumatoid arthritis is caused by autoimmune reactions affecting the joints, leading to long-term chronic inflammation. Even though the etiologies are not fully understood, the lymphatic system is known to play a role in arthritic diseases.
In animal models, studies show that lymphangiogenesis driven by VEGF-C is an important compensatory mechanism for regulating joint inflammation during (chronic) arthritis (239). Interestingly, during arthritic progression in TNF-transgenic mice, the popliteal lymph node first expands and increases contrast uptake in combination with only mild inflammation and little bone erosion. Later, the popliteal lymph node decreases in volume (i.e., collapses), with more severe inflammation and bone erosion as well as decreased lymphatic transport to the lymph node (240). Additionally, B cells may clog the lymphatic sinuses, causing arthritic flare (239). This hypothesis is supported by data showing that B cell depletion dramatically attenuates rheumatoid arthritis (241).
In humans, lymphatic vessels are present in all zones of normal and arthritic synovial tissues. During inflammation, lymphangiogenesis facilitates immune cell trafficking throughout the inflamed synovial tissue (242). The number of lymphatic vessels is positively related to the severity of synovial inflammation in patients with spondyloarthritis and rheumatoid arthritis. TNF blockade promotes lymphangiogenesis in human inflammatory tissue, possibly playing an important part in transporting cells and fluid out of the inflamed tissue (243). In addition, increased VEGF-C expression can be observed in the synovial lining of patients with rheumatoid arthritis and anky-losing spondylitis (244, 245), whereas VEGF-D levels are extremely low. The VEGF-C receptors VEGFR-2 and -3 are also expressed to a greater extent in inflamed synovium (245). Interestingly, patients with psoriatic arthritis and rheumatoid arthritis have been diagnosed with (sometimes bilateral) lymphedema of the upper extremities (246, 247), demonstrating damage to lymphatics beyond the joints. Thus, even though the exact mechanisms have not been determined, there is strong evidence of lymphatic involvement in arthritic disease.
7.4. Hypertension
Lymphatic vessels play a role in blood pressure maintenance. In salt-induced hypertension in rats, interstitial hypertonic sodium accumulation in skin causes lymphatic hyperplasia and an increased density in the initial lymphatic network due to macrophage VEGF-C secretion (248). In humans, serum VEGF-C levels increase significantly in subjects who respond with blood pressure changes when adjusting their salt intake, supporting the idea that VEGF-C is important for blood pressure homeostasis (249). Other studies evaluating the effects of a high-salt diet in rats and mice on collecting lymph vessels show changes in mechanical activity, suggesting that a high-salt diet enhances myogenic activity and lymphatic pump efficiency both in vivo and ex vivo (249, 250).
8. CONCLUSIONS
The past few decades of research into the lymphatic system have established a fundamental understanding of how it develops, grows, matures, and functions. Importantly, we are now beginning to fully appreciate the role the lymphatic system plays in immune function and many disease processes. Now that the molecular control of many of these processes is being uncovered, we are on the verge of being able to translate our knowledge into therapeutic interventions that target the lymphatic system. The first such lymphatic-based therapy is already being used to prevent the rejection of corneal grafts (10). Further research is needed to develop therapies to alter lymphatic function in order to treat lymphedema, prevent cancer progression, and resolve inflammatory disorders. In the near future, we expect that the impact of lymphatic research on the treatment of patients with a variety of diseases will continue to increase.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Zheng W, Aspelund A, Alitalo K. Lymphangiogenic factors, mechanisms, and applications. J. Clin. Investig. 2014;124:878–887. doi: 10.1172/JCI71603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerji S, Ni J, Wang SX, Clasper S, Su J, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Corral I, Ulvmar MH, Stanczuk L, Tatin F, Kizhatil K, et al. Nonvenous origin of dermal lymphatic vasculature. Circ. Res. 2015;116:1649–1654. doi: 10.1161/CIRCRESAHA.116.306170. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y, Oliver G. Development of the mammalian lymphatic vasculature. J. Clin. Investig. 2014;124:888–897. doi: 10.1172/JCI71609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zawieja DC. Contractile physiology of lymphatics. Lymphat. Res. Biol. 2009;7:87–96. doi: 10.1089/lrb.2009.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dieterich LC, Seidel CD, Detmar M. Lymphatic vessels: new targets for the treatment of inflammatory diseases. Angiogenesis. 2014;17:359–371. doi: 10.1007/s10456-013-9406-1. [DOI] [PubMed] [Google Scholar]
- 8.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, et al. Induction of tumor lymphangio-genesis by VEGF-C promotes breast cancer metastasis. Nat. Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 9.Mortimer PS, Rockson SG. New developments in clinical aspects of lymphatic disease. J. Clin. Investig. 2014;124:915–921. doi: 10.1172/JCI71608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hos D, Schlereth SL, Bock F, Heindl LM, Cursiefen C. Antilymphangiogenic therapy to promote transplant survival and to reduce cancer metastasis: What can we learn from the eye? Semin. Cell Dev. Biol. 2015;38:117–130. doi: 10.1016/j.semcdb.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Goel S, Gupta N, Walcott BP, Snuderl M, Kesler CT, et al. Effects of vascular-endothelial protein tyrosine phosphatase inhibition on breast cancer vasculature and metastatic progression. J. Natl. Cancer Inst. 2013;105:1188–1201. doi: 10.1093/jnci/djt164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira ER, Jones D, Jung K, Padera TP. The lymph node microenvironment and its role in the progression of metastatic cancer. Semin. Cell Dev. Biol. 2015;38:98–105. doi: 10.1016/j.semcdb.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Card CM, Yu SS, Swartz MA. Emerging roles of lymphatic endothelium in regulating adaptive immunity. J. Clin. Investig. 2014;124:943–952. doi: 10.1172/JCI73316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tewalt EF, Cohen JN, Rouhani SJ, Guidi CJ, Qiao H, et al. Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood. 2012;120:4772–4782. doi: 10.1182/blood-2012-04-427013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teijeira A, Russo E, Halin C. Taking the lymphatic route: dendritic cell migration to draining lymph nodes. Semin. Immunopathol. 2014;36:261–274. doi: 10.1007/s00281-013-0410-8. [DOI] [PubMed] [Google Scholar]
- 16.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 2007;204:2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmid-Schönbein GW. Microlymphatics and lymph flow. Physiol. Rev. 1990;70:987–1028. doi: 10.1152/physrev.1990.70.4.987. [DOI] [PubMed] [Google Scholar]
- 18.Witte MH, Bernas MJ, Martin CP, Witte CL. Lymphangiogenesis and lymphangiodysplasia: from molecular to clinical lymphology. Microsc. Res. Tech. 2001;55:122–145. doi: 10.1002/jemt.1163. [DOI] [PubMed] [Google Scholar]
- 19.Pflicke H, Sixt M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J. Exp. Med. 2009;206:2925–2935. doi: 10.1084/jem.20091739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 21.Schumann K, Lämmermann T, Bruckner M, Legler DF, Polleux J, et al. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity. 2010;32:703–713. doi: 10.1016/j.immuni.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Miteva DO, Rutkowski JM, Dixon BJ, Kilarski W, Shields JD, Swartz MA. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ. Res. 2010;106:920–931. doi: 10.1161/CIRCRESAHA.109.207274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber M, Hauschild R, Schwarz J, Moussion C, de Vries I, et al. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science. 2013;339:328–332. doi: 10.1126/science.1228456. [DOI] [PubMed] [Google Scholar]
- 24.von der Weid PY, Zawieja DC. Lymphatic smooth muscle: the motor unit of lymph drainage. Int. J. Biochem. Cell Biol. 2004;36:1147–1153. doi: 10.1016/j.biocel.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Davis MJ, Rahbar E, Gashev AA, Zawieja DC, Moore JE., Jr Determinants of valve gating in collecting lymphatic vessels from rat mesentery. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H48–H60. doi: 10.1152/ajpheart.00133.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skalak TC, Schmid-Schönbein GW, Zweifach BW. New morphological evidence for a mechanism of lymph formation in skeletal muscle. Microvasc. Res. 1984;28:95–112. doi: 10.1016/0026-2862(84)90032-3. [DOI] [PubMed] [Google Scholar]
- 27.Eisenhoffer J, Kagal A, Klein T, Johnston MG. Importance of valves and lymphangion contractions in determining pressure gradients in isolated lymphatics exposed to elevations in outflow pressure. Microvasc. Res. 1995;49:97–110. doi: 10.1006/mvre.1995.1008. [DOI] [PubMed] [Google Scholar]
- 28.Gasheva OY, Zawieja DC, Gashev AA. Contraction-initiated NO-dependent lymphatic relaxation: a self-regulatory mechanism in rat thoracic duct. J. Physiol. 2006;575:821–832. doi: 10.1113/jphysiol.2006.115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mackay CR, Marston WL, Dudler L. Naïve and memory T cells show distinct pathways of lymphocyte recirculation. J. Exp. Med. 1990;171:801–817. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pugh CW, MacPherson GG, Steer HW. Characterization of nonlymphoid cells derived from rat peripheral lymph. J. Exp. Med. 1983;157:1758–1779. doi: 10.1084/jem.157.6.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 33.Lee JW, Epardaud M, Sun J, Becker JE, Cheng AC, et al. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat. Immunol. 2007;8:181–190. doi: 10.1038/ni1427. [DOI] [PubMed] [Google Scholar]
- 34.Nichols LA, Chen Y, Colella TA, Bennett CL, Clausen BE, Engelhard VH. Deletional self-tolerance to a melanocyte/melanoma antigen derived from tyrosinase is mediated by a radio-resistant cell in peripheral and mesenteric lymph nodes. J. Immunol. 2007;179:993–1003. doi: 10.4049/jimmunol.179.2.993. [DOI] [PubMed] [Google Scholar]
- 35.Cohen JN, Guidi CJ, Tewalt EF, Qiao H, Rouhani SJ, et al. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J. Exp. Med. 2010;207:681–688. doi: 10.1084/jem.20092465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swartz MA. Immunomodulatory roles of lymphatic vessels in cancer progression. Cancer Immunol. Res. 2014;2:701–707. doi: 10.1158/2326-6066.CIR-14-0115. [DOI] [PubMed] [Google Scholar]
- 37.Liao S, Ruddle NH. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J. Immunol. 2006;177:3369–3379. doi: 10.4049/jimmunol.177.5.3369. [DOI] [PubMed] [Google Scholar]
- 38.Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat. Rev. Cancer. 2014;14:159–172. doi: 10.1038/nrc3677. [DOI] [PubMed] [Google Scholar]
- 39.Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev. Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 40.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 41.Kesler CT, Pereira ER, Cui CH, Nelson GM, Masuck DJ, et al. Angiopoietin-4 increases permeability of blood vessels and promotes lymphatic dilation. FASEB J. 2015;29:3668–3677. doi: 10.1096/fj.14-268920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, et al. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kajiya K, Hirakawa S, Ma B, Drinnenberg I, Detmar M. Hepatocyte growth factor promotes lymphatic vessel formation and function. EMBO J. 2005;24:2885–2895. doi: 10.1038/sj.emboj.7600763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao R, Björndahl MA, Religa P, Clasper S, Garvin S, et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6:333–345. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 45.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Investig. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mimura T, Amano S, Usui T, Kaji Y, Oshika T, Ishii Y. Expression of vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 in corneal lymphangiogenesis. Exp. Eye Res. 2001;72:71–78. doi: 10.1006/exer.2000.0925. [DOI] [PubMed] [Google Scholar]
- 47.Schoppmann SF, Birner P, Stöckl J, Kalt R, Ullrich R, et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am. J. Pathol. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Margaris KN, Black RA. Modelling the lymphatic system: challenges and opportunities. J. R. Soc. Interface. 2012;9:601–612. doi: 10.1098/rsif.2011.0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baxter LT, Jain RK. Transport of fluid and macromolecules in tumors. I. Role of interstitial pressure and convection. Microvasc. Res. 1989;37:77–104. doi: 10.1016/0026-2862(89)90074-5. [DOI] [PubMed] [Google Scholar]
- 50.Roose T, Swartz MA. Multiscale modeling of lymphatic drainage from tissues using homogenization theory. J. Biomech. 2012;45:107–115. doi: 10.1016/j.jbiomech.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 51.Hagendoorn J, Padera TP, Kashiwagi S, Isaka N, Noda F, et al. Endothelial nitric oxide synthase regulates microlymphatic flow via collecting lymphatics. Circ. Res. 2004;95:204–209. doi: 10.1161/01.RES.0000135549.72828.24. [DOI] [PubMed] [Google Scholar]
- 52.Van Helden DF, Zhao J. Lymphatic vasomotion. Clin. Exp. Pharmacol. Physiol. 2000;27:1014–1018. doi: 10.1046/j.1440-1681.2000.03368.x. [DOI] [PubMed] [Google Scholar]
- 53.Ledvora RF, Barany M, Barany K. Myosin light chain phosphorylation and tension development in stretch-activated arterial smooth muscle. Clin. Chem. 1984;30:2063–2068. [PubMed] [Google Scholar]
- 54.von der Weid PY. Review article: Lymphatic vessel pumping and inflammation—the role of spontaneous constrictions and underlying electrical pacemaker potentials. Aliment. Pharmacol. Ther. 2001;15:1115–1129. doi: 10.1046/j.1365-2036.2001.01037.x. [DOI] [PubMed] [Google Scholar]
- 55.Toland HM, McCloskey KD, Thornbury KD, McHale NG, Hollywood MA. Ca2+-activated Clcurrent in sheep lymphatic smooth muscle. Am. J. Physiol. Cell Physiol. 2000;279:C1327–C1335. doi: 10.1152/ajpcell.2000.279.5.C1327. [DOI] [PubMed] [Google Scholar]
- 56.von der Weid PY, Rahman M, Imtiaz MS, Van Helden DF. Spontaneous transient depolarizations in lymphatic vessels of the guinea pig mesentery: pharmacology and implication for spontaneous contractility. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H1989–H2000. doi: 10.1152/ajpheart.00007.2008. [DOI] [PubMed] [Google Scholar]
- 57.Van Helden DF. Pacemaker potentials in lymphatic smooth muscle of the guinea-pig mesentery. J. Physiol. 1993;471:465–479. doi: 10.1113/jphysiol.1993.sp019910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zawieja DC, Davis KL, Schuster R, Hinds WM, Granger HJ. Distribution, propagation, and coordination of contractile activity in lymphatics. Am. J. Physiol. Heart Circ. Physiol. 1993;264:H1283–H1291. doi: 10.1152/ajpheart.1993.264.4.H1283. [DOI] [PubMed] [Google Scholar]
- 59.McCloskey KD, Hollywood MA, Thornbury KD, Ward SM, McHale NG. Kit-like immunopositive cells in sheep mesenteric lymphatic vessels. Cell Tissue Res. 2002;310:77–84. doi: 10.1007/s00441-002-0623-y. [DOI] [PubMed] [Google Scholar]
- 60.von der Weid PY, Crowe MJ, Van Helden DF. Endothelium-dependent modulation of pacemaking in lymphatic vessels of the guinea-pig mesentery. J. Physiol. 1996;493:563–575. doi: 10.1113/jphysiol.1996.sp021404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hollywood MA, McHale NG. Mediation of excitatory neurotransmission by the release of ATP and noradrenaline in sheep mesenteric lymphatic vessels. J. Physiol. 1994;481:415–423. doi: 10.1113/jphysiol.1994.sp020450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.von der Weid PY, Van Helden DF. β-Adrenoceptor-mediated hyperpolarization in lymphatic smooth muscle of guinea pig mesentery. Am. J. Physiol. Heart Circ. Physiol. 1996;270:H1687–H1695. doi: 10.1152/ajpheart.1996.270.5.H1687. [DOI] [PubMed] [Google Scholar]
- 63.Davis MJ, Lane MM, Davis AM, Durtschi D, Zawieja DC, et al. Modulation of lymphatic muscle contractility by the neuropeptide substance P. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H587–H597. doi: 10.1152/ajpheart.01029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fox JL, von der Weid PY. Effects of histamine on the contractile and electrical activity in isolated lymphatic vessels of the guinea-pig mesentery. Br. J. Pharmacol. 2002;136:1210–1218. doi: 10.1038/sj.bjp.0704820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liao S, Cheng G, Conner DA, Huanga Y, Kucherlapati RS, et al. Impaired lymphatic contraction associated with immunosuppression. PNAS. 2011;108:18784–18789. doi: 10.1073/pnas.1116152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao J, Van Helden DF. ET-1-associated vasomotion and vasospasm in lymphatic vessels of the guinea-pig mesentery. Br. J. Pharmacol. 2003;140:1399–1413. doi: 10.1038/sj.bjp.0705573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davis MJ, Scallan JP, Wolpers JH, Muthuchamy M, Gashev AA, Zawieja DC. Intrinsic increase in lymphangion muscle contractility in response to elevated afterload. Am. J. Physiol. Heart Circ. Physiol. 2012;303:H795–H808. doi: 10.1152/ajpheart.01097.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am. J. Physiol. Heart Circ. Physiol. 1989;257:H2059–H2069. doi: 10.1152/ajpheart.1989.257.6.H2059. [DOI] [PubMed] [Google Scholar]
- 69.McGeown JG, McHale NG, Roddie IC, Thornbury K. Peripheral lymphatic responses to outflow pressure in anaesthetized sheep. J. Physiol. 1987;383:527–536. doi: 10.1113/jphysiol.1987.sp016426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McHale N, Roddie I. The effect of transmural pressure on pumping activity in isolated bovine lymphatic vessels. J. Physiol. 1976;261:255–269. doi: 10.1113/jphysiol.1976.sp011557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shirasawa Y, Benoit JN. Stretch-induced calcium sensitization of rat lymphatic smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2573–H2577. doi: 10.1152/ajpheart.00002.2003. [DOI] [PubMed] [Google Scholar]
- 72.Johnston MG, Elias R. The regulation of lymphatic pumping. Lymphology. 1987;20:215–218. [PubMed] [Google Scholar]
- 73.Davis MJ, Lane MM, Scallan JP, Gashev AA, Zawieja DC. An automated method to control preload by compensation for stress relaxation in spontaneously contracting, isometric rat mesenteric lymphatics. Microcirculation. 2007;14:603–612. doi: 10.1080/10739680701436152. [DOI] [PubMed] [Google Scholar]
- 74.Davis MJ, Davis AM, Lane MM, Ku CW, Gashev AA. Rate-sensitive contractile responses of lymphatic vessels to circumferential stretch. J. Physiol. 2009;587:165–182. doi: 10.1113/jphysiol.2008.162438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davis MJ, Donovitz JA, Hood JD. Stretch-activated single-channel and whole cell currents in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 1992;262:C1083–C1088. doi: 10.1152/ajpcell.1992.262.4.C1083. [DOI] [PubMed] [Google Scholar]
- 76.Christensen O. Mediation of cell volume regulation by Ca2+ influx through stretch-activated channels. Nature. 1987;330:66–68. doi: 10.1038/330066a0. [DOI] [PubMed] [Google Scholar]
- 77.Lee J, Ishihara A, Oxford G, Johnson B, Jacobson K. Regulation of cell movement is mediated by stretch-activated calcium channels. Nature. 1999;400:382–386. doi: 10.1038/22578. [DOI] [PubMed] [Google Scholar]
- 78.Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J. Physiol. 2002;540:1023–1037. doi: 10.1113/jphysiol.2001.016642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kurtz KH, Moor AN, Souza-Smith FM, Breslin JW. Involvement of H1 and H2 receptors and soluble guanylate cyclase in histamine-induced relaxation of rat mesenteric collecting lymphatics. Microcirculation. 2014;21:593–605. doi: 10.1111/micc.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nizamutdinova IT, Maejima D, Nagai T, Bridenbaugh E, Thangaswamy S, et al. Involvement of histamine in endothelium-dependent relaxation of mesenteric lymphatic vessels. Microcirculation. 2014;21:640–648. doi: 10.1111/micc.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.von der Weid PY, Zhao J, Van Helden DF. Nitric oxide decreases pacemaker activity in lymphatic vessels of guinea pig mesentery. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2707–H2716. doi: 10.1152/ajpheart.2001.280.6.H2707. [DOI] [PubMed] [Google Scholar]
- 82.Bohlen HG, Gasheva OY, Zawieja DC. Nitric oxide formation by lymphatic bulb and valves is a major regulatory component of lymphatic pumping. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H1897–H1906. doi: 10.1152/ajpheart.00260.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scallan JP, Davis MJ. Genetic removal of basal nitric oxide enhances contractile activity in isolated murine collecting lymphatic vessels. J. Physiol. 2013;591:2139–2156. doi: 10.1113/jphysiol.2012.250662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cohen RA, Weisbrod RM, Gericke M, Yaghoubi M, Bierl C, Bolotina VM. Mechanism of nitric oxide-induced vasodilatation: refilling of intracellular stores by sarcoplasmic reticulum Ca2+ ATPase and inhibition of store-operated Ca2+ influx. Circ. Res. 1999;84:210–219. doi: 10.1161/01.res.84.2.210. [DOI] [PubMed] [Google Scholar]
- 85.Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, et al. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416:337–339. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- 86.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 87.Mizuno R, Koller A, Kaley G. Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998;274:R790–R796. doi: 10.1152/ajpregu.1998.274.3.R790. [DOI] [PubMed] [Google Scholar]
- 88.Murphy RA, Walker JS. Inhibitory mechanisms for cross-bridge cycling: the nitric oxide-cGMP signal transduction pathway in smooth muscle relaxation. Acta Physiol. Scand. 1998;164:373–380. doi: 10.1046/j.1365-201X.1998.00434.x. [DOI] [PubMed] [Google Scholar]
- 89.Blatter LA, Wier WG. Nitric oxide decreases [Ca2+]i in vascular smooth muscle by inhibition of the calcium current. Cell Calcium. 1994;15:122–131. doi: 10.1016/0143-4160(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 90.Shyy JYJ, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ. Res. 2002;91:769–775. doi: 10.1161/01.res.0000038487.19924.18. [DOI] [PubMed] [Google Scholar]
- 91.Kunert C, Baish JW, Liao S, Padera TP, Munn LL. Mechanobiological oscillators control lymph flow. PNAS. 2015;112:10938–10943. doi: 10.1073/pnas.1508330112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ross TD, Coon BG, Yun S, Baeyens N, Tanaka K, et al. Integrins in mechanotransduction. Curr. Opin. Cell Biol. 2013;25:613–618. doi: 10.1016/j.ceb.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qazi H, Palomino R, Shi ZD, Munn LL, Tarbell JM. Cancer cell glycocalyx mediates mechan-otransduction and flow-regulated invasion. Integr. Biol. Quant. Biosci. Nano Macro. 2013;5:1334–1343. doi: 10.1039/c3ib40057c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tarbell JM, Simon SI, Curry FR. Mechanosensing at the vascular interface. Annu. Rev. Biomed. Eng. 2014;16:505–532. doi: 10.1146/annurev-bioeng-071813-104908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eisenhoffer J, Elias RM, Johnston MG. Effect of outflow pressure on lymphatic pumping in vitro. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1993;265:R97–R102. doi: 10.1152/ajpregu.1993.265.1.R97. [DOI] [PubMed] [Google Scholar]
- 96.Kornuta JA, Dixon BJ. Ex vivo lymphatic perfusion system for independently controlling pressure gradient and transmural pressure in isolated vessels. Ann. Biomed. Eng. 2014;42:1691–1704. doi: 10.1007/s10439-014-1024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cady B. Regional lymph node metastases, a singular manifestation of the process of clinical metastases in cancer: Contemporary animal research and clinical reports suggest unifying concepts. Ann. Surg. Oncol. 2007;14:1790–1800. doi: 10.1245/s10434-006-9234-2. [DOI] [PubMed] [Google Scholar]
- 98.Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N. Engl. J. Med. 2002;347:567–575. doi: 10.1056/NEJMoa020128. [DOI] [PubMed] [Google Scholar]
- 99.Halsted WS. The results of radical operations for the cure of carcinoma of the breast. Ann. Surg. 1907;46:1–19. doi: 10.1097/00000658-190707000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cascinelli N, Morabito A, Santinami M, MacKie RM, Belli F. Immediate or delayed dissection of regional nodes in patients with melanoma of the trunk: a randomised trial. WHO Melanoma Programme. Lancet. 1998;351:793–796. doi: 10.1016/s0140-6736(97)08260-3. [DOI] [PubMed] [Google Scholar]
- 101.Starz H, Balda BR, Kramer KU, Buchels H, Wang H. A micromorphometry-based concept for routine classification of sentinel lymph node metastases and its clinical relevance for patients with melanoma. Cancer. 2001;91:2110–2121. [PubMed] [Google Scholar]
- 102.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 103.Falkson CB. How do I deal with the axilla in patients with a positive sentinel lymph node? Curr. Treat. Options Oncol. 2011;12:389–402. doi: 10.1007/s11864-011-0170-4. [DOI] [PubMed] [Google Scholar]
- 104.Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Nieweg OE, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N. Engl. J. Med. 2014;370:599–609. doi: 10.1056/NEJMoa1310460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hellman S. Karnofsky Memorial Lecture. Natural history of small breast cancers. J. Clin. Oncol. 1994;12:2229–2234. doi: 10.1200/JCO.1994.12.10.2229. [DOI] [PubMed] [Google Scholar]
- 106.Podgrabinska S, Skobe M. Role of lymphatic vasculature in regional and distant metastases. Microvasc. Res. 2014;95:46–52. doi: 10.1016/j.mvr.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296:1883–1886. doi: 10.1126/science.1071420. [DOI] [PubMed] [Google Scholar]
- 108.Hoshida T, Isaka N, Hagendoorn J, di Tomaso E, Chen YL, et al. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res. 2006;66:8065–8075. doi: 10.1158/0008-5472.CAN-06-1392. [DOI] [PubMed] [Google Scholar]
- 109.Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Ylä-Herttuala S, et al. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 2001;61:1786–1790. [PubMed] [Google Scholar]
- 110.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat. Med. 2001;7:186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 111.Padera TP, Kuo AH, Hoshida T, Liao S, Lobo J, et al. Differential response of primary tumor versus lymphatic metastasis to VEGFR-2 and VEGFR-3 kinase inhibitors cediranib and vandetanib. Mol. Cancer Ther. 2008;7:2272–2279. doi: 10.1158/1535-7163.MCT-08-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Issa A, Le TX, Shoushtari AN, Shields JD, Swartz MA. Vascular endothelial growth factor C and C-C chemokine receptor 7 in tumor cell-lymphatic cross-talk promote invasive phenotype. Cancer Res. 2009;69:349–357. doi: 10.1158/0008-5472.CAN-08-1875. [DOI] [PubMed] [Google Scholar]
- 113.Gogineni A, Caunt M, Crow A, Lee CV, Fuh G, et al. Inhibition of VEGF-C modulates distal lymphatic remodeling and secondary metastasis. PLOS ONE. 2013;8:e68755. doi: 10.1371/journal.pone.0068755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Karnezis T, Shayan R, Caesar C, Roufail S, Harris NC, et al. VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell. 2012;21:181–195. doi: 10.1016/j.ccr.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 115.Roberts N, Kloos B, Cassella M, Podgrabinska S, Persaud K, et al. Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res. 2006;66:2650–2657. doi: 10.1158/0008-5472.CAN-05-1843. [DOI] [PubMed] [Google Scholar]
- 116.Tammela T, Saaristo A, Holopainen T, Ylä-Herttuala S, Andersson LC, et al. Photodynamic ablation of lymphatic vessels and intralymphatic cancer cells prevents metastasis. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3001699. 69ra11. [DOI] [PubMed] [Google Scholar]
- 117.Escobar-Prieto A, Gonzalez G, Templeton AW, Cooper BR, Palacios E. Lymphatic channel obstruction. Patterns of altered flow dynamics. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1971;113:366–375. doi: 10.2214/ajr.113.2.366. [DOI] [PubMed] [Google Scholar]
- 118.Proulx ST, Luciani P, Christiansen A, Karaman S, Blum KS, et al. Use of a PEG-conjugated bright near-infrared dye for functional imaging of rerouting of tumor lymphatic drainage after sentinel lymph node metastasis. Biomaterials. 2013;34:5128–5137. doi: 10.1016/j.biomaterials.2013.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nathanson SD, Shah R, Chitale DA, Mahan M. Intraoperative clinical assessment and pressure measurements of sentinel lymph nodes in breast cancer. Ann. Surg. Oncol. 2014;21:81–85. doi: 10.1245/s10434-013-3249-2. [DOI] [PubMed] [Google Scholar]
- 120.Kwon S, Agollah GD, Wu G, Sevick-Muraca EM. Spatio-temporal changes of lymphatic contractility and drainage patterns following lymphadenectomy in mice. PLOS ONE. 2014;9:e106034. doi: 10.1371/journal.pone.0106034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;133:571–573. [PubMed] [Google Scholar]
- 122.Harrell MI, Iritani BM, Ruddell A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am. J. Pathol. 2007;170:774–786. doi: 10.2353/ajpath.2007.060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Qian CN, Berghuis B, Tsarfaty G, Bruch M, Kort EJ, et al. Preparing the “soil”: The primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Res. 2006;66:10365–10376. doi: 10.1158/0008-5472.CAN-06-2977. [DOI] [PubMed] [Google Scholar]
- 124.Jeong HS, Jones D, Liao S, Wattson DA, Cui CH, et al. Investigation of the lack of angiogenesis in the formation of lymph node metastases. J. Natl. Cancer Inst. 2015;107 doi: 10.1093/jnci/djv155. djv155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Farnsworth RH, Lackmann M, Achen MG, Stacker SA. Vascular remodeling in cancer. Oncogene. 2014;33:3496–3505. doi: 10.1038/onc.2013.304. [DOI] [PubMed] [Google Scholar]
- 126.Ogawa F, Amano H, Eshima K, Ito Y, Matsui Y, et al. Prostanoid induces premetastatic niche in regional lymph nodes. J. Clin. Investig. 2014;124:4882–4894. doi: 10.1172/JCI73530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kohrt HE, Nouri N, Nowels K, Johnson D, Holmes S, Lee PP. Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer. PLOS Med. 2005;2:e284. doi: 10.1371/journal.pmed.0020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Van den Eynden GG, Van der Auwera I, Van Laere SJ, Colpaert CG, Turley H, et al. Angiogenesis and hypoxia in lymph node metastases is predicted by the angiogenesis and hypoxia in the primary tumour in patients with breast cancer. Br. J. Cancer. 2005;93:1128–1136. doi: 10.1038/sj.bjc.6602828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J. Exp. Med. 2005;201:1089–1099. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Garmy-Susini B, Avraamides CJ, Desgrosellier JS, Schmid MC, Foubert P, et al. PI3Kαactivates integrin α4β1 to establish a metastatic niche in lymph nodes. PNAS. 2013;110:9042–9047. doi: 10.1073/pnas.1219603110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee AS, Kim DH, Lee JE, Jung YJ, Kang KP, et al. Erythropoietin induces lymph node lymphan-giogenesis and lymph node tumor metastasis. Cancer Res. 2011;71:4506–4517. doi: 10.1158/0008-5472.CAN-10-3787. [DOI] [PubMed] [Google Scholar]
- 132.Farnsworth RH, Karnezis T, Shayan R, Matsumoto M, Nowell CJ, et al. A role for bone morpho-genetic protein 4 in lymph node vascular remodeling and primary tumor growth. Cancer Res. 2011;71:6547–6557. doi: 10.1158/0008-5472.CAN-11-0200. [DOI] [PubMed] [Google Scholar]
- 133.Carriére V, Colisson R, Jiguet-Jiglaire C, Bellard E, Bouche G, et al. Cancer cells regulate lymphocyte recruitment and leukocyte-endothelium interactions in the tumor-draining lymph node. Cancer Res. 2005;65:11639–11648. doi: 10.1158/0008-5472.CAN-05-1190. [DOI] [PubMed] [Google Scholar]
- 134.Deng J, Liu Y, Lee H, Herrmann A, Zhang W, et al. S1PR1-STAT3 signaling is crucial for myeloid cell colonization at future metastatic sites. Cancer Cell. 2012;21:642–654. doi: 10.1016/j.ccr.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Müller A, Homey B, Soto H, Ge N, Catron D, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 136.Lee JH, Torisu-Itakara H, Cochran AJ, Kadison A, Huynh Y, et al. Quantitative analysis of melanoma-induced cytokine-mediated immunosuppression in melanoma sentinel nodes. Clin. Cancer Res. 2005;11:107–112. [PubMed] [Google Scholar]
- 137.Leong SP, Peng M, Zhou YM, Vaquerano JE, Chang JW. Cytokine profiles of sentinel lymph nodes draining the primary melanoma. Ann. Surg. Oncol. 2002;9:82–87. doi: 10.1245/aso.2002.9.1.82. [DOI] [PubMed] [Google Scholar]
- 138.Barankay T, Baumgärtl H, Lübbers DW, Seidl E. Oxygen pressure in small lymphatics. Pflüg. Arch. 1976;366:53–59. doi: 10.1007/BF02486560. [DOI] [PubMed] [Google Scholar]
- 139.Hangai-Hoger N, Cabrales P, Briceño JC, Tsai AG, Intaglietta M. Microlymphatic and tissue oxygen tension in the rat mesentery. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H878–H883. doi: 10.1152/ajpheart.00913.2003. [DOI] [PubMed] [Google Scholar]
- 140.Arapandoni-Dadioti P, Giatromanolaki A, Trihia H, Harris AL, Koukourakis MI. Angiogenesis in ductal breast carcinoma. Comparison of microvessel density between primary tumour and lymph node metastasis. Cancer Lett. 1999;137:145–150. doi: 10.1016/s0304-3835(98)00343-7. [DOI] [PubMed] [Google Scholar]
- 141.Naresh KN, Nerurkar AY, Borges AM. Angiogenesis is redundant for tumour growth in lymph node metastases. Histopathology. 2001;38:466–470. doi: 10.1046/j.1365-2559.2001.01061.x. [DOI] [PubMed] [Google Scholar]
- 142.Wiley HE, Gonzalez EB, Maki W, Wu MT, Hwang ST. Expression of CC chemokine receptor 7 and regional lymph node metastasis of B16 murine melanoma. J. Natl. Cancer Inst. 2001;93:1638–1643. doi: 10.1093/jnci/93.21.1638. [DOI] [PubMed] [Google Scholar]
- 143.Ding Y, Shimada Y, Maeda M, Kawabe A, Kaganoi J, et al. Association of CC chemokine receptor 7 with lymph node metastasis of esophageal squamous cell carcinoma. Clin. Cancer Res. 2003;9:3406–3412. [PubMed] [Google Scholar]
- 144.Das S, Sarrou E, Podgrabinska S, Cassella M, Mungamuri SK, et al. Tumor cell entry into the lymph node is controlled by CCL1 chemokine expressed by lymph node lymphatic sinuses. J. Exp. Med. 2013;210:1509–1528. doi: 10.1084/jem.20111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cochran AJ, Huang RR, Lee J, Itakura E, Leong SP, Essner R. Tumour-induced immune modulation of sentinel lymph nodes. Nat. Rev. Immunol. 2006;6:659–670. doi: 10.1038/nri1919. [DOI] [PubMed] [Google Scholar]
- 146.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 147.Watanabe S, Deguchi K, Zheng R, Tamai H, Wang LX, et al. Tumor-induced CD11b+Gr-1+ myeloid cells suppress T cell sensitization in tumor-draining lymph nodes. J. Immunol. 2008;181:3291–3300. doi: 10.4049/jimmunol.181.5.3291. [DOI] [PubMed] [Google Scholar]
- 148.Deng L, Zhang H, Luan Y, Zhang J, Xing Q, et al. Accumulation of Foxp3+ T regulatory cells in draining lymph nodes correlates with disease progression and immune suppression in colorectal cancer patients. Clin. Cancer Res. 2010;16:4105–4112. doi: 10.1158/1078-0432.CCR-10-1073. [DOI] [PubMed] [Google Scholar]
- 149.Lund AW, Duraes FV, Hirosue S, Raghavan VR, Nembrini C, et al. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep. 2012;1:191–199. doi: 10.1016/j.celrep.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 150.Podgrabinska S, Kamalu O, Mayer L, Shimaoka M, Snoeck H, et al. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. J. Immunol. 2009;183:1767–1779. doi: 10.4049/jimmunol.0802167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zippelius A, Batard P, Rubio-Godoy V, Bioley G, Lienard D, et al. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res. 2004;64:2865–2873. doi: 10.1158/0008-5472.can-03-3066. [DOI] [PubMed] [Google Scholar]
- 152.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 154.Hargadon KM, Brinkman CC, Sheasley-O’Neill SL, Nichols LA, Bullock TN, Engelhard VH. Incomplete differentiation of antigen-specific CD8 T cells in tumor-draining lymph nodes. J. Immunol. 2006;177:6081–6090. doi: 10.4049/jimmunol.177.9.6081. [DOI] [PubMed] [Google Scholar]
- 155.Ruddell A, Harrell MI, Furuya M, Kirschbaum SB, Iritani BM. B lymphocytes promote lym-phogenous metastasis of lymphoma and melanoma. Neoplasia. 2011;13:748–757. doi: 10.1593/neo.11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li Q, Grover AC, Donald EJ, Carr A, Yu J, et al. Simultaneous targeting of CD3 on T cells and CD40 on B or dendritic cells augments the antitumor reactivity of tumor-primed lymph node cells. J. Immunol. 2005;175:1424–1432. doi: 10.4049/jimmunol.175.3.1424. [DOI] [PubMed] [Google Scholar]
- 157.Zirakzadeh AA, Marits P, Sherif A, Winqvist O. Multiplex B cell characterization in blood, lymph nodes, and tumors from patients with malignancies. J. Immunol. 2013;190:5847–5855. doi: 10.4049/jimmunol.1203279. [DOI] [PubMed] [Google Scholar]
- 158.Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–1113. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McFadden DG, Papagiannakopoulos T, Taylor-Weiner A, Stewart C, Carter SL, et al. Genetic and clonal dissection of murine small cell lung carcinoma progression by genome sequencing. Cell. 2014;156:1298–1311. doi: 10.1016/j.cell.2014.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Naxerova K, Brachtel E, Salk JJ, Seese AM, Power K, et al. Hypermutable DNA chronicles the evolution of human colon cancer. PNAS. 2014;111:e1889–e1898. doi: 10.1073/pnas.1400179111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Akita H, Doki Y, Yano M, Miyata H, Miyashiro I, et al. Effects of neoadjuvant chemotherapy on primary tumor and lymph node metastasis in esophageal squamous cell carcinoma: additive association with prognosis. Dis. Esophagus. 2009;22:291–297. doi: 10.1111/j.1442-2050.2008.00879.x. [DOI] [PubMed] [Google Scholar]
- 162.Medich D, McGinty J, Parda D, Karlovits S, Davis C, et al. Preoperative chemoradiotherapy and radical surgery for locally advanced distal rectal adenocarcinoma: pathologic findings and clinical implications. Dis. Colon Rectum. 2001;44:1123–1128. doi: 10.1007/BF02234632. [DOI] [PubMed] [Google Scholar]
- 163.Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J. Clin. Oncol. 1999;17:460–469. doi: 10.1200/JCO.1999.17.2.460. [DOI] [PubMed] [Google Scholar]
- 164.Cox C, Holloway CM, Shaheta A, Nofech-Mozes S, Wright FC. What is the burden of axillary disease after neoadjuvant therapy in women with locally advanced breast cancer? Curr. Oncol. 2013;20:111–117. doi: 10.3747/co.20.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wu F, Tamhane M, Morris ME. Pharmacokinetics, lymph node uptake, and mechanistic PK model of near-infrared dye-labeled bevacizumab after IV and SC administration in mice. AAPS J. 2012;14:252–261. doi: 10.1208/s12248-012-9342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Freeling JP, Ho RJ. Anti-HIV drug particles may overcome lymphatic drug insufficiency and associated HIV persistence. PNAS. 2014;111:e2512–e2513. doi: 10.1073/pnas.1406554111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Thomas SN, Vokali E, Lund AW, Hubbell JA, Swartz MA. Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials. 2014;35:814–824. doi: 10.1016/j.biomaterials.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 168.Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur. J. Immunol. 2010;40:22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- 169.Fujita T, Teramoto K, Ozaki Y, Hanaoka J, Tezuka N, et al. Inhibition of transforming growth factor β-mediated immunosuppression in tumor-draining lymph nodes augments antitumor responses by various immunologic cell types. Cancer Res. 2009;69:5142–5150. doi: 10.1158/0008-5472.CAN-08-2499. [DOI] [PubMed] [Google Scholar]
- 170.Warren AG, Brorson H, Borud LJ, Slavin SA. Lymphedema: a comprehensive review. Ann. Plast. Surg. 2007;59:464–472. doi: 10.1097/01.sap.0000257149.42922.7e. [DOI] [PubMed] [Google Scholar]
- 171.Bellini C, Hennekam RC. Clinical disorders of primary malfunctioning of the lymphatic system. Adv. Anat. Embryol. Cell Biol. 2014;214:187–204. doi: 10.1007/978-3-7091-1646-3_14. [DOI] [PubMed] [Google Scholar]
- 172.Maclellan RA, Greene AK. Lymphedema. Semin. Pediatr. Surg. 2014;23:191–197. doi: 10.1053/j.sempedsurg.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 173.Avraham T, Yan A, Zampell JC, Daluvoy SV, Haimovitz-Friedman A, et al. Radiation therapy causes loss of dermal lymphatic vessels and interferes with lymphatic function by TGF-β1-mediated tissue fibrosis. Am. J. Physiol. Cell Physiol. 2010;299:C589–C605. doi: 10.1152/ajpcell.00535.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tiwari P, Coriddi M, Salani R, Povoski SP. Breast and gynecologic cancer-related extremity lymphedema: a review of diagnostic modalities and management options. World J. Surg. Oncol. 2013;11:237. doi: 10.1186/1477-7819-11-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb. Perspect. Med. 2012;2:a006502. doi: 10.1101/cshperspect.a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Alders M, Al-Gazali L, Cordeiro I, Dallapiccola B, Garavelli L, et al. Hennekam syndrome can be caused by FAT4 mutations and be allelic to Van Maldergem syndrome. Hum. Genet. 2014;133:1161–1167. doi: 10.1007/s00439-014-1456-y. [DOI] [PubMed] [Google Scholar]
- 177.Balboa-Beltran E, Fernández-Seara MJ, Pérez-Muñuzuri A, Lago R, García-Magán C, et al. A novel stop mutation in the vascular endothelial growth factor C gene (VEGFC) results in Milroy-like disease. J. Med. Genet. 2014;51:475–478. doi: 10.1136/jmedgenet-2013-102020. [DOI] [PubMed] [Google Scholar]
- 178.García-Cruz D, Mampel A, Echeverria MI, Vargas AL, Castañeda-Cisneros G, et al. Cantu syndrome and lymphoedema. Clin. Dysmorphol. 2011;20:32–37. doi: 10.1097/MCD.0b013e32833d015c. [DOI] [PubMed] [Google Scholar]
- 179.Bennuru S, Nutman TB. Lymphangiogenesis and lymphatic remodeling induced by filarial parasites: implications for pathogenesis. PLOS Pathog. 2009;5:e1000688. doi: 10.1371/journal.ppat.1000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nutman TB. Insights into the pathogenesis of disease in human lymphatic filariasis. Lymphat. Res. Biol. 2013;11:144–148. doi: 10.1089/lrb.2013.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Babu S, Nutman TB. Immunopathogenesis of lymphatic filarial disease. Semin. Immunopathol. 2012;34:847–861. doi: 10.1007/s00281-012-0346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chakraborty S, Gurusamy M, Zawieja DC, Muthuchamy M. Lymphatic filariasis: perspectives on lymphatic remodeling and contractile dysfunction in filarial disease pathogenesis. Microcirculation. 2013;20:349–364. doi: 10.1111/micc.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gebruers N, Verbelen H, De Vrieze T, Coeck D, Tjalma W. Incidence and time path of lymphedema in sentinel node negative breast cancer patients: a systematic review. Arch. Phys. Med. Rehabil. 2015;96:1131–1139. doi: 10.1016/j.apmr.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 184.Swaroop MN, Ferguson CM, Horick NK, Skolny MN, Miller CL, et al. Impact of adjuvant taxane-based chemotherapy on development of breast cancer-related lymphedema: results from a large prospective cohort. Breast Cancer Res. Treat. 2015;151:393–403. doi: 10.1007/s10549-015-3408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dixon BJ, Weiler MJ. Bridging the divide between pathogenesis and detection in lymphedema. Semin. Cell Dev. Biol. 2015;38:75–82. doi: 10.1016/j.semcdb.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bundred NJ, Stockton C, Keeley V, Riches K, Ashcroft L, et al. Comparison of multi-frequency bioimpedance with perometry for the early detection and intervention of lymphoedema after axillary node clearance for breast cancer. Breast Cancer Res. Treat. 2015;151:121–129. doi: 10.1007/s10549-015-3357-8. [DOI] [PubMed] [Google Scholar]
- 187.Burnand KM, Glass DM, Mortimer PS, Peters AM. Lymphatic dysfunction in the apparently clinically normal contralateral limbs of patients with unilateral lower limb swelling. Clin. Nucl. Med. 2012;37:9–13. doi: 10.1097/RLU.0b013e31823931f5. [DOI] [PubMed] [Google Scholar]
- 188.Iimura T, Fukushima Y, Kumita S, Ogawa R, Hyakusoku H. Estimating lymphodynamic conditions and lymphovenous anastomosis efficacy using 99mTc-phytate lymphoscintigraphy with SPECT-CT in patients with lower-limb lymphedema. Plast. Reconstr. Surg. Glob. Open. 2015;3:e404. doi: 10.1097/GOX.0000000000000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Blum KS, Radtke C, Knapp WH, Pabst R, Gratz KF. SPECT-CT: a valuable method to document the regeneration of lymphatics and autotransplanted lymph node fragments. Eur. J. Nucl. Med. Mol. Imaging. 2007;34:1861–1867. doi: 10.1007/s00259-007-0458-6. [DOI] [PubMed] [Google Scholar]
- 190.Weiss M, Burgard C, Baumeister R, Strobl F, Rominger A, et al. Magnetic resonance imaging versus lymphoscintigraphy for the assessment of focal lymphatic transport disorders of the lower limb: first experiences. Nucl. Med. 2014;53:190–196. doi: 10.3413/Nukmed-0649-14-03. [DOI] [PubMed] [Google Scholar]
- 191.Liu N, Zhang Y. Magnetic resonance lymphangiography for the study of lymphatic system in lymphedema. J. Reconstr. Microsurg. 2016;32:66–71. doi: 10.1055/s-0034-1384213. [DOI] [PubMed] [Google Scholar]
- 192.Arrivé L, Derhy S, El Mouhadi S, Monnier-Cholley L, Menu Y, Becker C. Noncontrast magnetic resonance lymphography. J. Reconstr. Microsurg. 2016;32:80–86. doi: 10.1055/s-0035-1549133. [DOI] [PubMed] [Google Scholar]
- 193.Sevick-Muraca EM, Kwon S, Rasmussen JC. Emerging lymphatic imaging technologies for mouse and man. J. Clin. Investig. 2014;124:905–914. doi: 10.1172/JCI71612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Aldrich MB, Guilliod R, Fife CE, Maus EA, Smith L, et al. Lymphatic abnormalities in the normal contralateral arms of subjects with breast cancer-related lymphedema as assessed by near-infrared fluorescent imaging. Biomed. Opt. Express. 2012;3:1256–1265. doi: 10.1364/BOE.3.001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mihara M, Hara H, Araki J, Kikuchi K, Narushima M, et al. Indocyanine green (ICG) lymphography is superior to lymphoscintigraphy for diagnostic imaging of early lymphedema of the upper limbs. PLOS ONE. 2012;7:e38182. doi: 10.1371/journal.pone.0038182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mehrara BJ, Greene AK. Lymphedema and obesity: Is there a link? Plast. Reconstr. Surg. 2014;134:e154–e160. doi: 10.1097/PRS.0000000000000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Leung G, Baggott C, West C, Elboim C, Paul SM, et al. Cytokine candidate genes predict the development of secondary lymphedema following breast cancer surgery. Lymphat. Res. Biol. 2014;12:10–22. doi: 10.1089/lrb.2013.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Avraham T, Zampell JC, Yan A, Elhadad S, Weitman ES, et al. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J. 2013;27:1114–1126. doi: 10.1096/fj.12-222695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zumsteg A, Baeriswyl V, Imaizumi N, Schwendener R, Rüegg C, Christofori G. Myeloid cells contribute to tumor lymphangiogenesis. PLOS ONE. 2009;4:e7067. doi: 10.1371/journal.pone.0007067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lynch LL, Mendez U, Waller AB, Gillette AA, Guillory RJ2nd, Goldman J. Fibrosis worsens chronic lymphedema in rodent tissues. Am. J. Physiol. Heart Circ. Physiol. 2015;308:H1229–H1236. doi: 10.1152/ajpheart.00527.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hawinkels LJ, Ten Dijke P. Exploring anti-TGF-βtherapies in cancer and fibrosis. Growth Factors. 2011;29:140–152. doi: 10.3109/08977194.2011.595411. [DOI] [PubMed] [Google Scholar]
- 202.Clavin NW, Avraham T, Fernandez J, Daluvoy SV, Soares MA, et al. TGF-β1 is a negative regulator of lymphatic regeneration during wound repair. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H2113–H2127. doi: 10.1152/ajpheart.00879.2008. [DOI] [PubMed] [Google Scholar]
- 203.Martin M, Lefaix J, Delanian S. TGF-β1 and radiation fibrosis: a master switch and a specific therapeutic target? Int. J. Radiat. Oncol. Biol. Phys. 2000;47:277–290. doi: 10.1016/s0360-3016(00)00435-1. [DOI] [PubMed] [Google Scholar]
- 204.Avraham T, Daluvoy S, Zampell J, Yan A, Haviv YS, et al. Blockade of transforming growth factor β1 accelerates lymphatic regeneration during wound repair. Am. J. Pathol. 2010;177:3202–3214. doi: 10.2353/ajpath.2010.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Warren LE, Miller CL, Horick N, Skolny MN, Jammallo LS, et al. The impact of radiation therapy on the risk of lymphedema after treatment for breast cancer: a prospective cohort study. Int. J. Radiat. Oncol. Biol. Phys. 2014;88:565–571. doi: 10.1016/j.ijrobp.2013.11.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kesler CT, Kuo AH, Wong HK, Masuck DJ, Shah JL, et al. Vascular endothelial growth factor C enhances radiosensitivity of lymphatic endothelial cells. Angiogenesis. 2014;17:419–427. doi: 10.1007/s10456-013-9400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Budach W, Kammers K, Boelke E, Matuschek C. Adjuvant radiotherapy of regional lymph nodes in breast cancer—a meta-analysis of randomized trials. Radiat. Oncol. 2013;8:267. doi: 10.1186/1748-717X-8-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Miaskowski C, Dodd M, Paul SM, West C, Hamolsky D, et al. Lymphatic and angiogenic candidate genes predict the development of secondary lymphedema following breast cancer surgery. PLOS ONE. 2013;8:e60164. doi: 10.1371/journal.pone.0060164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lin S, Kim J, Lee MJ, Roche L, Yang NL, et al. Prospective transcriptomic pathway analysis of human lymphatic vascular insufficiency: identification and validation of a circulating biomarker panel. PLOS ONE. 2012;7:e52021. doi: 10.1371/journal.pone.0052021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Leung N, Furniss D, Giele H. Modern surgical management of breast cancer therapy related upper limb and breast lymphoedema. Maturitas. 2015;80:384–390. doi: 10.1016/j.maturitas.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 211.Nguyen AT, Suami H. Laparoscopic free omental lymphatic flap for the treatment of lymphedema. Plast. Reconstr. Surg. 2015;136:114–118. doi: 10.1097/PRS.0000000000001374. [DOI] [PubMed] [Google Scholar]
- 212.Lasso JM, Pinilla C, Castellano M. New refinements in greater omentum free flap transfer for severe secondary lymphedema surgical treatment. Plast. Reconstr. Surg. Glob. Open. 2015;3:e387. doi: 10.1097/GOX.0000000000000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tervala TV, Hartiala P, Tammela T, Visuri MT, Ylä-Herttuala S, et al. Growth factor therapy and lymph node graft for lymphedema. J. Surg. Res. 2015;196:200–207. doi: 10.1016/j.jss.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 214.Headland SE, Norling LV. The resolution of inflammation: principles and challenges. Semin. Immunol. 2015;27:149–160. doi: 10.1016/j.smim.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 215.Liao S, von der Weid PY. Lymphatic system: an active pathway for immune protection. Semin. Cell Dev. Biol. 2015;38:83–89. doi: 10.1016/j.semcdb.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sakai Y, Kobayashi M. Lymphocyte ‘homing’ and chronic inflammation. Pathol. Int. 2015;65:344–354. doi: 10.1111/pin.12294. [DOI] [PubMed] [Google Scholar]
- 217.Ruddle NH. Lymphatic vessels and tertiary lymphoid organs. J. Clin. Investig. 2014;124:953–959. doi: 10.1172/JCI71611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Stranford S, Ruddle NH. Follicular dendritic cells, conduits, lymphatic vessels, and high endothelial venules in tertiary lymphoid organs: parallels with lymph node stroma. Front. Immunol. 2012;3:350. doi: 10.3389/fimmu.2012.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Noort AR, van Zoest KP, van Baarsen LG, Maracle CX, Helder B, et al. Tertiary lymphoid structures in rheumatoid arthritis: NF-κB-inducing kinase-positive endothelial cells as central players. Am. J. Pathol. 2015;185:1935–1943. doi: 10.1016/j.ajpath.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 220.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat. Rev. Immunol. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 221.Albuquerque RJ, Hayashi T, Cho WG, Kleinman ME, Dridi S, et al. Alternatively spliced vascular endothelial growth factor receptor 2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat. Med. 2009;15:1023–1030. doi: 10.1038/nm.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yamagami S, Dana MR. The critical role of lymph nodes in corneal alloimmunization and graft rejection. Investig. Ophthalmol. Vis. Sci. 2001;42:1293–1298. [PubMed] [Google Scholar]
- 223.Dohlman TH, Omoto M, Hua J, Stevenson W, Lee SM, et al. VEGF-trap aflibercept significantly improves long-term graft survival in high-risk corneal transplantation. Transplantation. 2015;99:678–686. doi: 10.1097/TP.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 224.Hos D, Regenfuss B, Bock F, Onderka J, Cursiefen C. Blockade of insulin receptor substrate 1 inhibits corneal lymphangiogenesis. Investig. Ophthalmol. Vis. Sci. 2011;52:5778–5785. doi: 10.1167/iovs.10-6816. [DOI] [PubMed] [Google Scholar]
- 225.Palin NK, Savikko J, Koskinen PK. Sirolimus inhibits lymphangiogenesis in rat renal allografts, a novel mechanism to prevent chronic kidney allograft injury. Transplant Int. 2013;26:195–205. doi: 10.1111/tri.12005. [DOI] [PubMed] [Google Scholar]
- 226.Vass DG, Shrestha B, Haylor J, Hughes J, Marson L. Inflammatory lymphangiogenesis in a rat transplant model of interstitial fibrosis and tubular atrophy. Transplant Int. 2012;25:792–800. doi: 10.1111/j.1432-2277.2012.01482.x. [DOI] [PubMed] [Google Scholar]
- 227.Oka K, Namba Y, Ichimaru N, Moriyama T, Kyo M, et al. Clinicopathological study of expression of lymphatic vessels in renal allograft biopsy after treatment for acute rejection. Transplant. Proc. 2009;41:4154–4158. doi: 10.1016/j.transproceed.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 228.Stuht S, Gwinner W, Franz I, Schwarz A, Jonigk D, et al. Lymphatic neoangiogenesis in human renal allografts: results from sequential protocol biopsies. Am. J. Transplant. 2007;7:377–384. doi: 10.1111/j.1600-6143.2006.01638.x. [DOI] [PubMed] [Google Scholar]
- 229.Dashkevich A, Heilmann C, Kayser G, Germann M, Beyersdorf F, et al. Lymph angiogenesis after lung transplantation and relation to acute organ rejection in humans. Ann. Thorac. Surg. 2010;90:406–411. doi: 10.1016/j.athoracsur.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 230.Nykänen AI, Sandelin H, Krebs R, Keränen MA, Tuuminen R, et al. Targeting lymphatic vessel activation and CCL21 production by vascular endothelial growth factor receptor 3 inhibition has novel immunomodulatory and antiarteriosclerotic effects in cardiac allografts. Circulation. 2010;121:1413–1422. doi: 10.1161/CIRCULATIONAHA.109.910703. [DOI] [PubMed] [Google Scholar]
- 231.Soong TR, Pathak AP, Asano H, Fox-Talbot K, Baldwin WM., 3rd Lymphatic injury and regeneration in cardiac allografts. Transplantation. 2010;89:500–508. doi: 10.1097/TP.0b013e3181c73c34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Brown K, Badar A, Sunassee K, Fernandes MA, Shariff H, et al. SPECT/CT lymphoscintigraphy of heterotopic cardiac grafts reveals novel sites of lymphatic drainage and T cell priming. Am. J. Transplant. 2011;11:225–234. doi: 10.1111/j.1600-6143.2010.03388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kong XQ, Wang LX, Kong DG. Cardiac lymphatic interruption is a major cause for allograft failure after cardiac transplantation. Lymphat. Res. Biol. 2007;5:45–47. doi: 10.1089/lrb.2007.5108. [DOI] [PubMed] [Google Scholar]
- 234.Rockson SG. Lymphatics and the heart: the importance of visceral lymphatic function in health and disease. Lymphat. Res. Biol. 2007;5:1–2. doi: 10.1089/lrb.2007.5101. [DOI] [PubMed] [Google Scholar]
- 235.Ishii E, Shimizu A, Kuwahara N, Arai T, Kataoka M, et al. Lymphangiogenesis associated with acute cellular rejection in rat liver transplantation. Transplant. Proc. 2010;42:4282–4285. doi: 10.1016/j.transproceed.2010.09.081. [DOI] [PubMed] [Google Scholar]
- 236.Kellersman R, Zhong R, Kiyochi H, Garcia B, Grant DR. Reconstruction of the intestinal lymphatic drainage after small bowel transplantation. Transplantation. 2000;69:10–16. doi: 10.1097/00007890-200001150-00003. [DOI] [PubMed] [Google Scholar]
- 237.Kerjaschki D. Lymphatic neoangiogenesis in renal transplants: a driving force of chronic rejection? J. Nephrol. 2006;19:403–406. [PubMed] [Google Scholar]
- 238.Seeger H, Bonani M, Segerer S. The role of lymphatics in renal inflammation. Nephrol. Dial. Transplant. 2012;27:2634–2641. doi: 10.1093/ndt/gfs140. [DOI] [PubMed] [Google Scholar]
- 239.Bouta EM, Li J, Ju Y, Brown EB, Ritchlin CT, et al. The role of the lymphatic system in inflammatory-erosive arthritis. Semin. Cell Dev. Biol. 2015;30:90–97. doi: 10.1016/j.semcdb.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhou Q, Wood R, Schwarz EM, Wang YJ, Xing L. Near-infrared lymphatic imaging demonstrates the dynamics of lymph flow and lymphangiogenesis during the acute versus chronic phases of arthritis in mice. Arthritis Rheum. 2010;62:1881–1889. doi: 10.1002/art.27464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mandik-Nayak L, Allen PM. Initiation of an autoimmune response: insights from a transgenic model of rheumatoid arthritis. Immunol. Res. 2005;32:5–13. doi: 10.1385/IR:32:1-3:005. [DOI] [PubMed] [Google Scholar]
- 242.Xu H, Edwards J, Banerji S, Prevo R, Jackson DG, Athanasou NA. Distribution of lymphatic vessels in normal and arthritic human synovial tissues. Ann. Rheum. Dis. 2003;62:1227–1229. doi: 10.1136/ard.2003.005876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Polzer K, Baeten D, Soleiman A, Distler J, Gerlag DM, et al. Tumour necrosis factor blockade increases lymphangiogenesis in murine and human arthritic joints. Ann. Rheum. Dis. 2008;67:1610–1616. doi: 10.1136/ard.2007.083394. [DOI] [PubMed] [Google Scholar]
- 244.Wauke K, Nagashima M, Ishiwata T, Asano G, Yoshino S. Expression and localization of vascular endothelial growth factor C in rheumatoid arthritis synovial tissue. J. Rheumatol. 2002;29:34–38. [PubMed] [Google Scholar]
- 245.Paavonen K, Mandelin J, Partanen T, Jussila L, Li TF, et al. Vascular endothelial growth factors C and D and their VEGFR-2 and 3 receptors in blood and lymphatic vessels in healthy and arthritic synovium. J. Rheumatol. 2002;29:39–45. [PubMed] [Google Scholar]
- 246.Böhm M, Riemann B, Luger TA, Bonsmann G. Bilateral upper limb lymphoedema associated with psoriatic arthritis: a case report and review of the literature. Br. J. Dermatol. 2000;143:1297–1301. doi: 10.1046/j.1365-2133.2000.03905.x. [DOI] [PubMed] [Google Scholar]
- 247.Kiely PD, Joseph AE, Mortimer PS, Bourke BE. Upper limb lymphedema associated with polyarthritis of rheumatoid type. J. Rheumatol. 1994;21:1043–1045. [PubMed] [Google Scholar]
- 248.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor C-dependent buffering mechanism. Nat. Med. 2009;15:545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 249.Liu F, Mu J, Yuan Z, Lian Q, Zheng S, et al. Involvement of the lymphatic system in salt-sensitive hypertension in humans. Med. Sci. Monit. 2011;17:542–546. doi: 10.12659/MSM.881978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kwon S, Agollah GD, Chan W, Sevick-Muraca EM. Altered lymphatic function and architecture in salt-induced hypertension assessed by near-infrared fluorescence imaging. J. Biomed. Opt. 2012;17:080504. doi: 10.1117/1.JBO.17.8.080504. [DOI] [PMC free article] [PubMed] [Google Scholar]