Abstract
Introduction
Tumor hypoxia causes resistance to radiation and chemotherapy. Evofosfamide (TH-302) has exhibited specific hypoxia-dependent cytotoxicity against primary acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) samples in vitro. Based on these findings, a Phase I study of evofosfamide was designed for patients with relapsed/refractory leukemia (NCT01149915).
Methods
In this open-label study, patients were treated with evofosfamide as a 30 to 60 minute/day infusion on Days 1–5 of a 21-day cycle (Arm A, n=38) or as a continuous infusion over 120 hours over Days 1–5 of a 21-day cycle (Arm B, n=11).
Results
Forty-nine patients were treated including 39 (80%) with AML and 9 (18%) with ALL. Patients had received a median of 5 prior therapies. In Arm A, the dose-limiting toxicities (DLTs) were grade 3 esophagitis, observed at a dose of 550 mg/m2. The maximum tolerated dose (MTD) was a daily dose of 460 mg/m2. In Arm B, the DLTs were grade 3 stomatitis and hyperbilirubinemia, observed at a daily dose of 460 mg/m2. The continuous infusion MTD was a daily dose of 330 mg/m2. Hypoxia markers HIF-1α and CAIX were highly expressed in leukemic bone marrow and were significantly reduced after evofosfamide therapy. The combined overall response rate in Arms A and B was 6% (2 CR/CRi and 1 PR), with all responses seen in Arm A.
Conclusion
Evofosfamide has shown limited activity in heavily pretreated leukemia patients. Further evaluation investigating evofosfamide in combination with cytotoxic or demethylating agents is warranted.
Keywords: myeloid leukemia, hypoxia-activated pro-drug, hypoxic bone marrow microenvironment
Introduction
Tumor invasiveness, metastatic behavior, and resistance to chemotherapy have been shown to be associated with tumor hypoxia.[1, 2] Hypoxia-inducible factor 1 alpha (HIF-1α) and HIF-2α are transcription factors overexpressed in cancers, and up-regulation of these proteins is associated with resistance to chemotherapy and radiotherapy. [3–7]
Even though the importance of hypoxia in malignancies was proposed several decades ago, evaluation of tumor hypoxia in hematologic malignancies is more recent.[8] This may be due to the lack of reliable methods for measuring oxygen levels in bone marrow. Using the 2-nitroimidazole hypoxia probe pimonidazole, Benito et al showed marked expansion of hypoxic bone marrow areas in immunodeficient mice engrafted with acute lymphoblastic leukemia (ALL) cells.[9] In ALL-derived cell lines, hypoxia was found to cause chemotherapy resistance. As in solid tumors, HIF-1α is frequently expressed in bone marrow of patients with acute myeloid leukemia (AML) or ALL and is associated with poor outcome.[10–12] Notably, HIF-1α expressed in bone marrow of newly diagnosed ALL patients became untraceable in remission bone marrows.[9]
Tumor hypoxia and increased expression of HIF-1α has been proposed as a mechanism of disease progression in multiple myeloma (MM) as well as leukemia. Hu et al showed higher positivity of the 2-nitroimidazole hypoxic probe in the bone marrow of mice harboring 5T33MM cells than in controls.[13] Azab et al showed that MM-related hypoxic conditions activate the epithelial-mesenchymal transition program, causing dissemination of MM cells as well as homing of circulating MM cells to the new bone marrow niche by increased expression of chemokine CXCR4.[14]
Tumor hypoxia, or low oxygen concentration, represents a compelling target for anticancer intervention. Therapeutic agents that can target hypoxic zones that are resistant to chemotherapy and radiotherapy may provide clinical benefit. One approach to targeting hypoxia is inhibition of molecular targets required for survival of hypoxic cells, particularly HIF-1.[15] A second approach is elimination of cancer cells in the hypoxic microenvironment by utilizing so-called hypoxia-activated prodrugs (HAPs).
Several HAPs for treatment of malignancies have been investigated.[16–18] Evofosfamide (TH-302), a nitroimidazole prodrug of the bromo-isophosphoramide mustard (Br-IMP), is one such HAP. The nitroimidazole group of evofosfamide is reduced by intracellular one-electron reductases and when exposed to hypoxic conditions, it preferentially releases the alkylating agent, Br-IPM. Br-IPM then acts as a DNA crosslinking agent. Evofosfamide has been designed to selectively target and become activated in hypoxic cells. It may also diffuse to adjacent cells in normoxic regions, acting as a cytotoxic agent outside the hypoxic activation zone.
Evofosfamide has exhibited specific hypoxia-dependent cytotoxicity when tested in primary ALL and AML samples in vitro.[19, 20] Similarly, in MM cells both in vitro and in vivo, evofosfamide induces cell cycle arrest and triggers apoptosis in severe hypoxic conditions, while having no effect at similar doses in normoxic conditions.[13] In preclinical and Phase I and II trials for solid malignancies, evofosfamide monotherapy was well tolerated and yielded responses.[21–24]
We conducted a Phase I study to determine the maximum tolerated dose (MTD), dose-limiting toxic effects (DLT), safety, tolerability, and clinical activity of evofosfamide in patients with advanced hematologic malignancies.
Patients and Methods
Patient cohort
Patients with a relapsed/refractory hematologic malignancy for which no standard therapies were anticipated to result in a durable response, or in whom potentially curative therapy had failed, or who were considered unsuitable for standard therapy were eligible. Other eligibility criteria included age 18 years or older, ECOG performance status of 0–2, and adequate hepatic and renal functions. Exclusion criteria included New York Heart Association class III or IV, myocardial infarction within 6 months, uncontrolled seizure disorder, severe chronic obstructive pulmonary disease, active uncontrolled infection, or a washout period of less than 2 weeks for cytotoxic agents or less than 5 half-lives for non-cytotoxic agents from prior treatment to time of entry on study.
The study protocol was approved by the Institutional Review Board at MD Anderson Cancer Center, and all patients gave written informed consent.
Study design
Evofosfamide was administered as a daily 30- to 60-minute infusion on Days 1–5 of 21-day cycles (Arm A) or as a continuous intravenous infusion (CIV) over 120 hours on Days 1–5 of 21-day cycles (Arm B). Once enrollment into Arm A was completed, eligible subjects were enrolled into Arm B to investigate if a continuous infusion could result in higher dose intensity.
Dosing was begun at level 1 (supplemental table 1). Three patients were enrolled at the initial dose level. Doses were increased to the next level in groups of 3 patients until the MTD was established. If one patient developed a DLT at a certain dose level, up to three additional patients were treated at that dose level. If two or more patients at a given dose level experienced a DLT during the first cycle, then the MTD was considered to have been exceeded and a total of six patients were enrolled at the next lower dose level. When fewer than two of those six patients experienced a DLT at this next lower dose level, this dose was declared the MTD. Once an MTD was established, 10 additional evaluable patients were enrolled at the MTD to better define the toxicity profile. MTD expansion was done only in Arm A, and not done in Arm B because the MTD was lower than in Arm A. The dose levels of evofosfamide utilized in both arms are specified in supplemental table I.
A DLT was defined as a clinically significant grade 3 or grade 4 adverse event or abnormal laboratory value unrelated to disease progression, intercurrent illness, or concomitant medications and occurring during the first cycle of therapy. Adverse events were defined according to the common terminology criteria for adverse events (CTCAE Version 4): grade 3 elevation of aspartate aminotransferase (AST; SGOT) or alanine aminotransferase (ALT; SGPT) for ≥7 days, grade 4 elevation of AST or ALT of any duration, and all other grade 3 or 4 events meeting CTCAE criteria. Nausea and vomiting grade ≤ grade 3, alopecia, study drug–related fever, and electrolyte abnormalities (including K, Na, Cl, HCO3, Mg, and Ca) were not considered DLTs. Prolonged cytopenias, defined by the U.S. National Cancer Institute criteria for leukemia from the start of Cycle 1 without evidence of leukemia, was considered an adverse event.
Response criteria
Response assessments were performed prior to Cycle 2, Cycle 4 and after Cycle 6. Complete remission (CR) was defined as disappearance of all clinical and/or radiologic evidence of disease with <5% bone marrow blasts, neutrophil count ≥1.0 × 109/L, and platelet count ≥100 × 109/L. Complete response with incomplete count recovery (CRi) was defined as per CR but with a platelet count of <100 × 109/L and/or a neutrophil count <1.0 × 109/L. Partial response (PR) required all of the hematologic values for a CR but with a decrease of bone marrow blasts by ≥50%. Stable disease (SD) was defined as failure to achieve at least a PR, but with no evidence of progression (increase in circulating or bone marrow blast) on assessment after completion of the 1st cycle, and response should be sustained for at least 4 weeks.
Statistical analysis
This Phase I study was designed to evaluate the safety and efficacy of evofosfamide. Descriptive statistics were used to define patient characteristics. Since it is a dose-escalation study, no power calculations were performed. A standard 3+3 design was utilized. Up to six subjects per dose cohort were treated. The final cohort at the MTD was expanded by enrolling an additional 10 evaluable subjects.
Hypoxia biomarker studies
Pimonidazole (PIMO) is a marker of hypoxia approved as an investigative new drug diagnostic tracer for tumor hypoxia in humans. PIMO is a 2-nitroimidazole that undergoes metabolic reduction in hypoxic cells to generate stable intracellular adducts that can be detected by immunohistochemical staining. PIMO infusion and testing was an optional procedure for this study. In subjects who consented, PIMO dissolved in 0.9% saline solution was infused over 20 minutes at a dosage of 0.5 g/m2, approximately 16 (±6) hours prior to bone marrow biopsy (prior to starting therapy and after 1 cycle to detect bone marrow hypoxia). We also studied the changes in hypoxia following treatment in selected cases. Bone marrow biopsy samples were fixed in formalin, embedded in paraffin, sectioned, and immunostained by using the Hypoxyprobe-1 MAb (Hypoxyprobe Inc., Cat #HP2-100Kit). The cells were identified by masking on the nuclear label, and the percentage of cells labeled with PIMO adducts was quantified.
HIF-1α and carbonic anhydrase IX (CAIX) expression was assessed in fixed, paraffin-embedded sections of the bone marrow biopsy specimens by using anti-HIF-1α or -CAIX antibody from Novus (catalog numbers NB100-105 and NB100-417) and immunohistochemical methods described previously. Positive and negative cells were counted in five random high-power fields (×400) and the counts averaged. A tumor was considered positive if 10% or more of cells demonstrated nuclear (HIF) or membrane (CAIX) staining.
Pimonidazole flow cytometry: Mononuclear cells from bone marrow aspirate were washed with PBS and then fixed in 250 μL of 1.6% formaldehyde (Pierce) for 15 minutes at room temperature. After spinning (5 minutes × 1200 g) cells were resuspended in 1 mL of 100% methanol and incubated at −20°C for 1 hour to overnight. Cells were washed twice in PBS supplemented with 2% fetal calf serum and stained for 1 hour at room temperature with 100 μL of a 1:200 dilution of anti-pimonidazole antibody (Hypoxyprobe Inc.) in PBS supplemented with 2% fetal calf serum, 0.002% sodium azide. After washing with PBS, fluorescence was analyzed in a Gallios flow cytometer (Beckman-Coulter).
Results
Patient characteristics
Baseline characteristics of patients in both Arms are summarized in Table I. The median age for all patients enrolled in this study was 58 years (range, 23–76). Approximately 80% of patients in both arms had AML. Overall, 23 (59%) of patients had poor risk cytogenetics. Cytogenetic risk stratification for AML was based on most commonly used European LeukemiaNet (ELN) classification.[25] The median number of prior therapies patients received in both Arms was 5 (range, 2–10), and 41 (84%) of patients had received ≥ 3 prior therapies. Eighteen (37%) patients had undergone prior stem cell transplant.
Table I.
Baseline patient and clinical characteristics
| Characteristic | Median [range], n (%) | ||||
|---|---|---|---|---|---|
| Arm A (N= 38) |
Arm B (N= 11) |
Total (N= 49) |
|||
| Age, years | 61.5 [24–76] | 56 [23–73] | 58 [23–76] | ||
| Male/Female | 20/18 | 5/6 | 25/24 | ||
| ECOG Performance Status | |||||
| 0 | 0 | 1 (9) | 1 (2) | ||
| 1 | 25 (66) | 5 (45) | 30 (61) | ||
| 2 | 10 (26) | 5 (45) | 15 (31) | ||
| 3 | 3 (8) | 0 (0) | 3 (6) | ||
| Type of Leukemia | |||||
| AML | 30 (79) | 9 (82) | 39 (80) | ||
| ALL | 7 (18) | 2 (18) | 9 (18) | ||
| CML | 1 (3) | 0 (0) | 1 (2) | ||
| Risk Status AML | CG | Mol. abn | CG | Mol. abn | |
| Favorable | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 1 (3) |
| Intermediate risk | 13 (43) | 0 (0) | 3 (33) | 0 (0) | 16 (41) |
| Poor risk | 17 (57) | 0 (0) | 6 (67) | 1 (11) | 23 (59) |
| ALL | |||||
| B cell | 4 (57) | 0 (0) | 4 (44) | ||
| T cell | 3 (43) | 2 (100) | 5 (56) | ||
| Prior Stem Cell Transplant | 12 (32) | 6 (55) | 18 (37) | ||
| No. of prior therapies | 5 [2–10] | 5 [2–9] | 5 [2–10] | ||
| ≥3 prior therapies | 33 (87) | 8 (78) | 41 (84) | ||
Arm A; Daily IV evofosfamide administration, Arm B; continuous evofosfamide administration CG, cytogenetics; Mol. abn, molecular abnormalities
Dose level and toxicity
Thirty-eight patients received evofosfamide in Arm A, at doses ranging between 120 mg/m2 and 550 mg/m2; 11 patients received continuous infusion of evofosfamide in Arm B (supplemental table II). In Arm A, two of the four patients treated at 550 mg/m2 had a DLT of grade 3 esophagitis. The MTD was established at 460 mg/m2 as a 30–60 minute daily IV infusion in Arm A and another 10 patients were treated at that dose. In Arm B, two of the three patients treated at 460 mg/m2 experienced DLTs: grade 3 stomatitis and hyperbilirubinemia. Hence, the MTD in Arm B was established at 330 mg/m2. In Arm B enrollment was stopped after the MTD was established as the MTD was established at a lower dose than in Arm A. The median number of cycles at the MTD of 460 mg/m2 in Arm A was 1 (range, 1–4) and at MTD of 330 mg/m2 in Arm B was 1 (range, 1–3). The number of cycles at each dose level, MTD and DLT are summarized in supplemental tables II and III.
Non-hematologic toxic effects
The most frequent non-hematologic adverse events regardless of relationship to study drug, reported in ≥15% of patients in Arm A and Arm B, are summarized in Table II. In general, the adverse events profiles were similar in both Arms. The adverse effects observed most frequently in Arm A were diarrhea (17, 45%), fatigue (9, 24%), peripheral edema (9, 24%), rash (9, 24%), pneumonia (8, 21%), urinary tract infection (8, 21%) and nausea (7, 18%). In Arm B, the most frequent adverse events were pneumonia (6, 55%), stomatitis (6, 55%), rash (3, 27%), diarrhea (3, 27%), nausea (3, 27%), and peripheral edema (3, 27%).
Table II.
Most frequent non-hematologic adverse events*
| Arm A (N= 38) |
Arm B (N= 11) |
|||
|---|---|---|---|---|
| Toxic effects | n (%) | |||
| Any Grade | Grade 3–4 | Any Grade | Grade 3–4 | |
| Diarrhea | 17 (45) | 2 (5) | 3 (27) | 1 (9) |
| Fatigue | 9 (24) | 2 (5) | 1 (9) | 0 (0) |
| Peripheral edema | 9 (24) | 1 (3) | 3 (27) | 0 (0) |
| Rash | 9 (24) | 0 (0) | 3 (27) | 0 (0) |
| Pneumonia | 8 (21) | 8 (21) | 6 (55) | 6 (55) |
| Urinary tract infection | 8 (21) | 6 (16) | 0 (0) | 0 (0) |
| Nausea | 7 (18) | 1 (3) | 3 (27) | 1 (9) |
| Headache | 6 (16) | 2 (5) | 0 (0) | 0 (0) |
| Stomatitis | 6 (16) | 1 (3) | 6 (55) | 4 (36) |
| Dermatitis, bullous | 2 (5) | 0 (0) | 2 (18) | 1 (9) |
| Sepsis | 3 (8) | 3 (8) | 2 (18) | 2 (18) |
| Hyperbilirubinemia | 3 (8) | 2 (5) | 2 (18) | 1 (9) |
| Cough | 2 (5) | 0 (0) | 2 (18) | 0 (0) |
| Epistaxis | 1 (3) | 0 (0) | 2 (18) | 0 (0) |
| Multi-organ failure | 0 (0) | 0 (0) | 2 (18) | 2 (18) |
| Most frequent hematologic adverse events effects | ||||
|
Arm A (N= 38) |
Arm B (N= 11) |
|||
| Toxic effects | Any Grade | Grade 3–4 | Any Grade | Grade 3–4 |
| Neutropenic fever | 17 (45) | 17 (45) | 2 (18) | 2 (18) |
| Thrombocytopenia | 2 (5) | 2 (5) | 0 (0) | 0 (0) |
| Anemia | 3 (8) | 3 (8) | 0 (0) | 0 (0) |
Arm A; Daily IV evofosfamide administration, Arm B; continuous evofosfamide administration
≥15% of patients in either Arm
Hematologic toxic effects
The most common hematologic adverse events are summarized in Table II. Seventeen (45%) patients in Arm A and two (18%) patients in Arm B had neutropenic fever. Two patients in Arm A had drug-related grade 3 thrombocytopenia and three patients in Arm A had grade 3 anemia.
Serious adverse events
Forty-six serious adverse events (SAEs) regardless of relationship to study drug were reported in 30 (61%) patients. SAEs occurring in more than two patients were pneumonia (n=9), febrile neutropenia (n=5), sepsis (n=5) and stomatitis (n=3). Six (12%) patients had SAEs related to study drug: stomatitis (n=2), esophagitis (n=2), stomatitis, multi-organ failure, dermatitis bullous and hyper bilirubinemia (n=1) and anorectal infection (n=1).
Efficacy
Responses to evofosfamide are summarized in supplemental table IV. The majority of patients had rapid cytoreduction early in Cycle 1, but these reductions in blast percentages tended to be transient.
The median initial decrease in peripheral blasts was 67%, and 12 patients had a clearing of all peripheral blasts; however, most patients experienced disease progression prior to initiating Cycle 2 (Fig. 1). The median decrease in percentage of bone marrow blasts was 14% (range, 8–48). In Arm A, three patients achieved objective response (2 CR/CRi and 1 PR, 8%); additionally eight patients had stable disease (SD, 21%). One other patient had an extramedullary response. At the MTD of 460 mg/m2, one patient had a CR and three patients had SD. In Arm B, none of the patients achieved CR and five (45%) had SD. Among the three patients in Arm A, who achieved PR or CR/CRi, one patient with PR had ALL and two patients with CR/CRi had AML. The ALL patient was 69 year old male with relapsed/refractory T-cell ALL, diploid cytogenetics, and had undergone three prior regimens hyperCVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone), nelarabine and high dose methotrexate + pegylated asparginase. The patient received evofosfamide at a dose 170 mg/m2, achieved PR after 2nd cycle but progressed after Cycle 3 despite dose escalation to 240mg/m2. One patient with AML who achieved CR was a 68 year old male with myelodysplastic syndrome transformed into AML, had t (7; 21) chromosomal abnormality, and had undergone three lines of prior therapy of standard 3+7, decitabine, and MEC (mitoxantrone, etoposide, and cytarabine) chemotherapy. The patient received four cycles of evofosfamide at a dose of 460 mg/m2 with a response lasting for four months. One patient who achieved CRi was a 62-year old female, had AML with normal cytogenetics and has undergone two lines of prior therapy of daunorubicin plus cytarabine (standard 3+7) and fludarabine plus cytarabine chemotherapy. This patient received two cycles of evofosfamide at a dose of 550 mg/m2 with a CRi response lasting for two months. Two AML patients in Arm A, who received evofosfamide at a dose of 550 mg/m2, had complete resolution of leukemia cutis; one of them had PR in the bone marrow.
Figure 1.
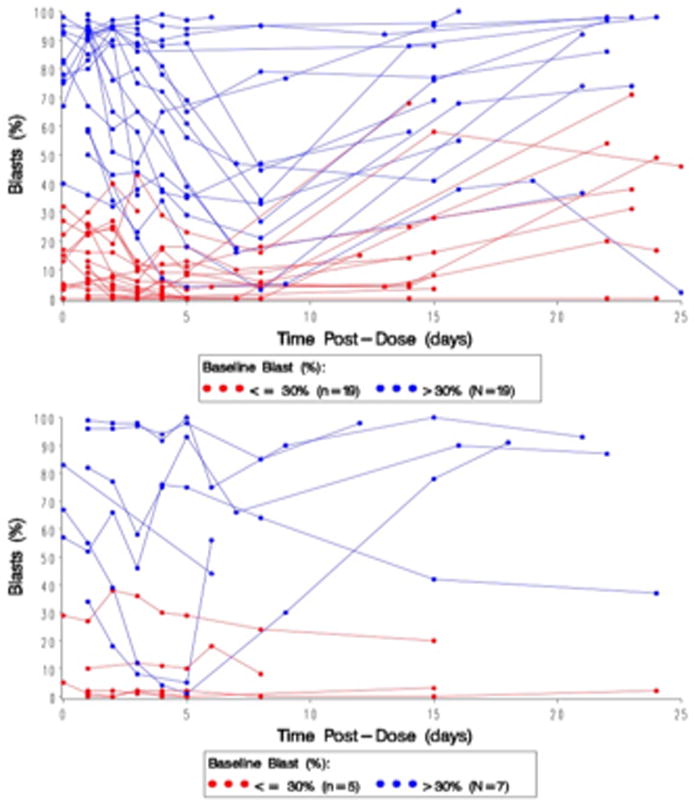
Peripheral blasts (%) versus days from initial dose of Evofosfamide for each patient in Arm A (administered daily over 30 minutes for Day 1 to Day 5) (above) and Arm B (continuous infusion over Day 1 to Day 5 for Arm B (below).
Hypoxia biomarkers
To characterize the extent of bone marrow hypoxia before and after evofosfamide treatment, we utilized the hypoxia marker PIMO and immunostaining for HIF-1α and CAIX. Eleven (22%) patients consented to undergo testing for these biomarkers; two were tested for PIMO, HIF-1α, and CAIX; three for HIF-1α and CAIX; and six for PIMO only. Thus, overall eight (16%) bone marrow samples were tested for hypoxia by PIMO. After the PIMO injection, all eight samples showed positive bone marrow signal for hypoxia by flow cytometry. Quantification of PIMO signal in the bone marrow by immunohistochemistry showed percentages of positive cells ranging between 0.65% and 92.5%, and was above 40% in seven of the eight patients studied. The fraction of bulk hypoxic cells (i.e., percentage of PIMO-positive cells) significantly correlated with the percentage of PIMO-positive cells within CD34-positive AML progenitor cells (r2 = 0.96 and p = <0.0001 [supplemental Fig. 1]). PIMO was also detected by immunohistochemistry in two patients using bone marrow clots, and the results correlated with flow cytometry (supplemental Fig. 2). In the two patients who underwent PIMO testing before and after the first cycle, minimal decrease in PIMO-positive cells (from 92% at baseline to 75% after the first cycle) was seen in one patient and no significant change was observed in the second patient, with neither of the two patients achieving an objective response.
Expression of hypoxia markers HIF-1α and CAIX was studied in bone marrow specimens at baseline and after the first cycle of evofosfamide in five patients for whom specimens were available for research studies: three patients treated at 460 mg/m2 and 2 at 550 mg/m2. HIF-1α was positive in all samples at baseline, with the percentage of positive cells ranging between 80% and 95%; CAIX was positive in four (80%) samples at baseline, and the percentage of positive cells was between 5% and 70%. There was a significant correlation between the percentage of HIF-1α–positive cells and the bone marrow blast percentage (Pearson r = 0.68, p = 0.01 [supplemental Fig. 3A]). The CAIX-positive cells also demonstrated moderately significant correlation with the bone marrow blast percentage (Pearson r = 0.52 and p = 0.09 [supplemental Fig. 3B]). In five paired specimens before and after treatment (median of two cycles of evofosfamide [range, 1–2]), fewer HIF-1α–positive cells were detectable after evofosfamide administration (mean ± SEM, 92 ± 2.0% vs. 58 ± 7.7%, p=0.003, supplemental Fig. 3C). In a comparison of CAIX-positive cells in four paired specimens, fewer CAIX-positive cells were detectable after evofosfamide administration (50 ± 15.4% vs. 35 ± 17%, p=0.32, supplemental Fig. 3D). Among these patients studied, one of them achieved CR after two cycles of treatment, with a decrease in HIF-1α–positive cells from 80% to 50% and CAIX positive cells from 5% to 0% (supplemental Fig. 3C and D). Another patient had abundant HIF-1α– and CAIX-positive myeloid blasts in skin (Fig. 2, panels A–C) and BM (Fig. 2, panels D–F) prior to therapy, which decreased after one cycle of evofosfamide at 550 mg/m2 with complete resolution of leukemia cutis, reduction in BM blasts with corresponding reduction in the fraction of HIF-1α–positive cells (Fig. 2, panels G–I).
Figure 2.
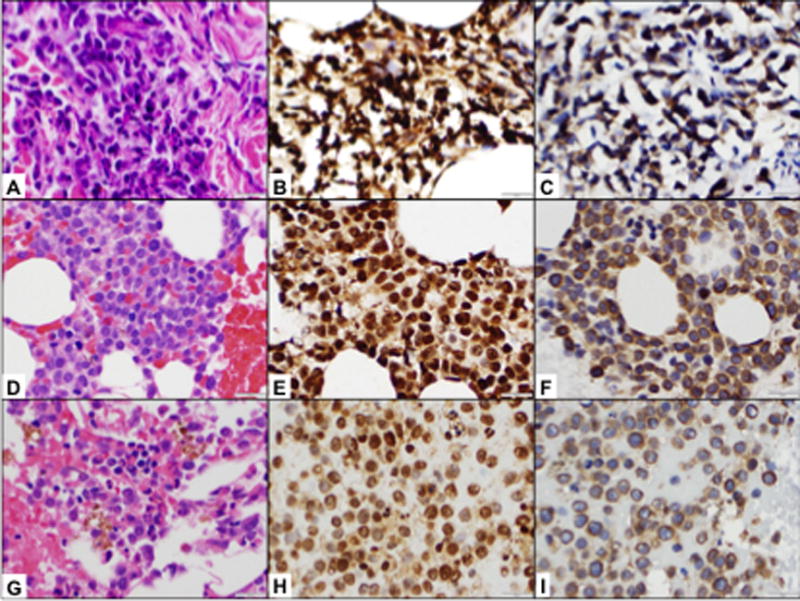
Patient #222-023. Prior to the therapy, the patient had multiple skin infiltrates. Skin biopsy detected dense perivascular infiltrate composed of intermediate to large neoplastic cells with irregular nuclear contours, slightly clumped chromatin, indistinct nucleoli, and scant to moderate pink cytoplasm (A, H&E, ×500). The neoplastic cells demonstrated strong and uniform HIF-1α expression (B, IHC, ×500) and strong CAIX expression (C, IHC, ×500). Bone marrow biopsy prior to therapy demonstrated hypercellular (70–80%) bone marrow with sheets of immature cells (D, H&E, ×500). Immunohistochemical studies detected strong and uniform HIF-1α expression (E, IHC, ×500); CAIX expression was somewhat weaker and seen in about 60–70% of cells (F, IHC, ×500). After 1 cycle of evofosfamide at 550mg/m2 (C1D40), patient had complete resolution of leukemia cutis and reduction of BM blasts from 69 to 21% (G, H&E, ×500) with corresponding decrease in fraction of BM HIF-1α-expressing cells (95% vs 60%, H, IHC, ×500) and no change in CAIX expression (70% vs 80%, I, IHC, ×500).
Pharmacokinetics
Following a single intravenous infusion of 0.5 – 1 hour, the evofosfamide maximum concentration and AUC increased linearly over the 120 – 550 mg/m2 dose range. The time of maximum concentration was generally at the end of the infusion. Evofosfamide had a moderately high geometric mean clearance of 40.7 – 64.9 L/m2. The geometric mean volume of distribution at steady state was independent of dose and ranged between 44.9 to 68.1 L/m2, suggesting that evofosfamide has a volume of distribution greater than total body water. Once the IV infusion was terminated, plasma concentrations of evofosfamide rapidly declined with a geometric mean terminal T1/2 of 0.53 to 0.80 hour. There was no evidence of accumulation of evofosfamide at Day 5. In patients receiving continuous infusions over 122 – 153 hours, evofosfamide maximum concentration and AUC increased linearly over the 330 – 460 mg/m2 dose range with a geometric mean terminal half-life of 0.51 – 0.56 hour following the termination of the infusion. As expected, at equivalent doses of evofosfamide (330–460 mg/m2), peak plasma concentrations of evofosfamide was substantially lower when administered as 5-day continuous infusion (range, 0.662 – 1.09 μg/mL) as compared to a short-term infusion (1.23 – 24.8 μg/mL), while the total AUC, corrected for the total dose administered, was comparable. Plasma concentrations of Br-IPM were below the limit of detection in the majority of the patients following the short-term as well as the 5-day continuous infusions (supplemental Fig. 4).
Discussion
Here, we report a first phase I study of evofosfamide in patients with advanced hematologic malignancies, the MTD for evofosfamide administered as brief daily infusions on Days 1–5 of a 21-day cycle (Arm A) was established at 460 mg/m2 and that for evofosfamide administered as a continuous infusion over Days 1–5 of a 21-day cycle (Arm B) was 330 mg/m2. The DLTs were primarily mucositis at the doses of 550 mg/m2 in Arm A and 460 mg/m2 in Arm B. Other adverse events were diarrhea, skin effects, peripheral edema, pneumonia, and urinary tract infection. Adverse events were similar in the 2 arms, except for mucositis, which was more frequent in the continuous infusion group (Arm B).
Although hematopoietic stem cells are believed to reside in hypoxic niches, the hematologic toxicities were not dose limiting. In another Phase I study comparing two dosing schedules of evofosfamide in advanced solid malignancies (30- to 60-minutes infusions weekly for 3 weeks followed by 1 week off versus a single infusion every 3 weeks schedule); the MTDs were 575 mg/m2 and 670 mg/m2, respectively. Grade 3 skin and mucosal toxic effects at 670 mg/m2 were dose limiting in the three-times-weekly group, and grade 3 fatigue and grade 3 vaginitis/proctitis were dose limiting at 940 mg/m2 in the every-three-week group.[22] The differences in MTD between solid malignancies and hematologic malignancies might have been due to different dosing schedules or differences in tolerability. The dose-dense daily dosing utilized in the current study to rapidly deplete blasts may limit the daily dose MTD. Alternatively, a higher incidence of gastrointestinal toxicity has been reported in patients with hematological malignancies receiving chemotherapy notably in the setting of drug-induced neutropenia.[26–29] The daily dosing regimen MTD of 460 mg/m2 for 5 days of a 21-day cycle with an associated dose density of 767 mg/m2/week is the highest dose density achieved with evofosfamide.
In our study, evofosfamide demonstrated evidence of activity in a majority of patients with clearance of circulating blast, but the responses were transient and overall objective response rate in Arms A and B was only 6% (2 CR/CRi and 1PR). Additionally, 13 patients had SD over the course of 1st cycle, and two patients had complete resolution of leukemia cutis (one of them also had bone marrow PR). There are several plausible explanations for the inferior responses observed. The patients enrolled in the study were heavily pretreated, 18(37%) patients had experienced relapse after stem cell transplant and 41 (84%) patients had received ≥ 3 prior therapies. It is possible that evofosfamide was unable to induce cytotoxicity in refractory leukemia cells possessing multiple acquired mechanisms of resistance. In addition, the alkylating agents have limited activity in AML, and a different cytotoxic payload (i.e. topoisomerase inhibitor) could have resulted in higher efficacy. Dose escalation was limited by the on-target GI toxicity, precluding the use of higher doses of evofosfamide in leukemia patients. Furthermore, most of the patients in our study were elderly with AML, and many of these are not eligible for high dose evofosfamide therapy. Although it seems counterintuitive, recent findings in MM models and our unpublished observations indicate that circulating tumor cells retain intracellular hypoxia, supporting the on-target antileukemia activity of evofosfamide.
Our findings also validate that hypoxia is predominant in human leukemic bone marrow, consistent with preclinical findings in murine leukemia models.[9, 20] In this study, use of PIMO to measure hypoxia levels in bone marrow prior to evofosfamide therapy was limited to eight (16%) patients, including two patients before and after the first treatment cycle, since the majority of patients underwent routine bone marrow testing prior to study consent. In those patients, PIMO-positive cells were significantly correlated with PIMO/CD34-positive cells, indicating hypoxic nature of AML progenitor cells. The expression of HIF-1α and CAIX showed significant correlation in pretreatment and post treatment bone marrow samples, supporting the importance of these markers in representing hypoxia. Notably, expression of HIF-1α and CAIX correlated with percentage of bone marrow blasts, indicating that stabilization of HIF-1α and CAIX strongly correlates with the extent of leukemia infiltration. These findings suggest that leukemia marrow is hypoxic, and therefore a reasonable target for the hypoxia-activated prodrug, evofosfamide. Although targeting the HIF-1α transcription factor is difficult, combining evofosfamide therapy with other chemotherapeutic agents (anthracyclines and topoisomerase inhibitors) may be a more effective therapeutic option.[30, 31]
Evofosfamide has shown activity in patients with advanced leukemia, but the refractory patient population in our study obscured the true efficacy of this novel agent. Our unpublished preclinical data support additive ant-leukemia activity of evofosfamide and hypomethylating agent in AML cells. Considering that a significant population of elderly AML patients is not suitable candidates for intensive chemotherapy, we hypothesize that combination of evofosfamide and hypomethylating agent, which has non-overlapping toxicities, will provide an attractive alternative therapy with a potential to improve outcome.
Studies in solid tumors and other hematological malignancies have shown additive advantage when evofosfamide is used with combination chemotherapy.[23, 32, 33]
In conclusion, our findings show that evofosfamide monotherapy has activity in heavily pretreated leukemia patients. Further studies investigating combinations of evofosfamide with cytotoxic or hypomethylating agents are warranted.
Supplementary Material
Acknowledgments
Grant Support
This study was funded by Threshold Pharmaceuticals in partnership with Merck KGaA, Darmstadt, Germany and in part by NIH/NCI P30 CA016672 (MK).
Footnotes
Author Contributions
Conception and Designed Research: T. Badar, M. Konopleva, H.M. Kantarjian, D. Handisides, T. Pearce, S. Kroll, D.A. Thomas
Performed Research: T. Badar, M. Konopleva, D.A. Thomas, J. Cortes
Acquisition of Data (acquired and managed patients): G. Borthakur, E. Jabbour, S. Faderl, M. Konopleva, D.A. Thomas, M.A. Richie, H.M. Kantarjian, M. Andreeff
Analyzed Data: T. Badar, M. Konopleva
Performed experiments: J. Benito, K. Harutyunyan, S. Konoplev, M. Konopleva
Writing, review, and/or revision of the manuscript: T. Badar, M. Konopleva
Disclosure of Potential Conflicts of Interest
References
- 1.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 2.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhong H, De Marzo AM, Laughner E, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- 4.Durand RE. The influence of microenvironmental factors during cancer therapy. In Vivo. 1994;8:691–702. [PubMed] [Google Scholar]
- 5.Gray LH, Conger AD, Ebert M, et al. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953;26:638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- 6.Boyle RG, Travers S. Hypoxia: targeting the tumour. Anticancer Agents Med Chem. 2006;6:281–286. doi: 10.2174/187152006777698169. [DOI] [PubMed] [Google Scholar]
- 7.Koukourakis MI, Bentzen SM, Giatromanolaki A, et al. Endogenous markers of two separate hypoxia response pathways (hypoxia inducible factor 2 alpha and carbonic anhydrase 9) are associated with radiotherapy failure in head and neck cancer patients recruited in the CHART randomized trial. J Clin Oncol. 2006;24:727–735. doi: 10.1200/JCO.2005.02.7474. [DOI] [PubMed] [Google Scholar]
- 8.Jensen PO, Mortensen BT, Hodgkiss RJ, et al. Increased cellular hypoxia and reduced proliferation of both normal and leukaemic cells during progression of acute myeloid leukaemia in rats. Cell Prolif. 2000;33:381–395. doi: 10.1046/j.1365-2184.2000.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benito J, Shi Y, Szymanska B, et al. Pronounced hypoxia in models of murine and human leukemia: high efficacy of hypoxia-activated prodrug PR-104. PLoS One. 2011;6:e23108. doi: 10.1371/journal.pone.0023108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deeb G, Vaughan MM, McInnis I, et al. Hypoxia-inducible factor-1alpha protein expression is associated with poor survival in normal karyotype adult acute myeloid leukemia. Leuk Res. 2011;35:579–584. doi: 10.1016/j.leukres.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Wellmann S, Guschmann M, Griethe W, et al. Activation of the HIF pathway in childhood ALL, prognostic implications of VEGF. Leukemia. 2004;18:926–933. doi: 10.1038/sj.leu.2403332. [DOI] [PubMed] [Google Scholar]
- 12.Frolova O, Samudio I, Benito JM, et al. Regulation of HIF-1alpha signaling and chemoresistance in acute lymphocytic leukemia under hypoxic conditions of the bone marrow microenvironment. Cancer Biol Ther. 2012;13:858–870. doi: 10.4161/cbt.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu J, Handisides DR, Van Valckenborgh E, et al. Targeting the multiple myeloma hypoxic niche with TH-302, a hypoxia-activated prodrug. Blood. 2010;116:1524–1527. doi: 10.1182/blood-2010-02-269126. [DOI] [PubMed] [Google Scholar]
- 14.Azab AK, Hu J, Quang P, et al. Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features. Blood. 2012;119:5782–5794. doi: 10.1182/blood-2011-09-380410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia Y, Choi HK, Lee K. Recent advances in hypoxia-inducible factor (HIF)-1 inhibitors. Eur J Med Chem. 2012;49:24–40. doi: 10.1016/j.ejmech.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 16.Shulman LN, Buswell L, Riese N, et al. Phase I trial of the hypoxic cell cytotoxin tirapazamine with concurrent radiation therapy in the treatment of refractory solid tumors. Int J Radiat Oncol Biol Phys. 1999;44:349–353. doi: 10.1016/s0360-3016(99)00016-4. [DOI] [PubMed] [Google Scholar]
- 17.Patterson AV, Ferry DM, Edmunds SJ, et al. Mechanism of action and preclinical antitumor activity of the novel hypoxia-activated DNA cross-linking agent PR-104. Clin Cancer Res. 2007;13:3922–3932. doi: 10.1158/1078-0432.CCR-07-0478. [DOI] [PubMed] [Google Scholar]
- 18.Albertella MR, Loadman PM, Jones PH, et al. Hypoxia-selective targeting by the bioreductive prodrug AQ4N in patients with solid tumors: results of a phase I study. Clin Cancer Res. 2008;14:1096–1104. doi: 10.1158/1078-0432.CCR-07-4020. [DOI] [PubMed] [Google Scholar]
- 19.Portwood S, Lal D, Hsu YC, et al. Activity of the hypoxia-activated prodrug, TH-302, in preclinical human acute myeloid leukemia models. Clin Cancer Res. 2013;19:6506–6519. doi: 10.1158/1078-0432.CCR-13-0674. [DOI] [PubMed] [Google Scholar]
- 20.Benito J, Ramirez MS, Millward NZ, et al. Hypoxia-activated prodrug TH-302 targets hypoxic bone marrow niches in pre-clinical leukemia models. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-14-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganjoo KN, Cranmer LD, Butrynski JE, et al. A phase I study of the safety and pharmacokinetics of the hypoxia-activated prodrug TH-302 in combination with doxorubicin in patients with advanced soft tissue sarcoma. Oncology. 2011;80:50–56. doi: 10.1159/000327739. [DOI] [PubMed] [Google Scholar]
- 22.Weiss GJ, Infante JR, Chiorean EG, et al. Phase 1 study of the safety, tolerability, and pharmacokinetics of TH-302, a hypoxia-activated prodrug, in patients with advanced solid malignancies. Clin Cancer Res. 2011;17:2997–3004. doi: 10.1158/1078-0432.CCR-10-3425. [DOI] [PubMed] [Google Scholar]
- 23.Borad MJ, Reddy SG, Bahary N, et al. Randomized Phase II Trial of Gemcitabine Plus TH-302 Versus Gemcitabine in Patients With Advanced Pancreatic Cancer. J Clin Oncol. 2015;33:1475–1481. doi: 10.1200/JCO.2014.55.7504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chawla SP, Cranmer LD, Van Tine BA, et al. Phase II study of the safety and antitumor activity of the hypoxia-activated prodrug TH-302 in combination with doxorubicin in patients with advanced soft tissue sarcoma. J Clin Oncol. 2014;32:3299–3306. doi: 10.1200/JCO.2013.54.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 26.Davila ML. Neutropenic enterocolitis: current issues in diagnosis and management. Curr Infect Dis Rep. 2007;9:116–120. doi: 10.1007/s11908-007-0006-3. [DOI] [PubMed] [Google Scholar]
- 27.Gorschluter M, Mey U, Strehl J, et al. Neutropenic enterocolitis in adults: systematic analysis of evidence quality. Eur J Haematol. 2005;75:1–13. doi: 10.1111/j.1600-0609.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- 28.Ebert EC, Hagspiel KD. Gastrointestinal manifestations of leukemia. J Gastroenterol Hepatol. 2012;27:458–463. doi: 10.1111/j.1440-1746.2011.06908.x. [DOI] [PubMed] [Google Scholar]
- 29.Hogan WJ, Letendre L, Litzow MR, et al. Neutropenic colitis after treatment of acute myelogenous leukemia with idarubicin and cytosine arabinoside. Mayo Clin Proc. 2002;77:760–762. doi: 10.4065/77.8.760. [DOI] [PubMed] [Google Scholar]
- 30.Rapisarda A, Uranchimeg B, Sordet O, et al. Topoisomerase I-mediated inhibition of hypoxia-inducible factor 1: mechanism and therapeutic implications. Cancer Res. 2004;64:1475–1482. doi: 10.1158/0008-5472.can-03-3139. [DOI] [PubMed] [Google Scholar]
- 31.Lee K, Qian DZ, Rey S, et al. Anthracycline chemotherapy inhibits HIF-1 transcriptional activity and tumor-induced mobilization of circulating angiogenic cells. Proc Natl Acad Sci U S A. 2009;106:2353–2358. doi: 10.1073/pnas.0812801106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Irene M, Ghobrial JL, Philippe Armand, et al. Phase I study of TH-302, an investigational hypoxia-targeted drug, and dexamethasone in patients with relapsed/refractory multiple myeloma. ASCO Annual Meeting Abstracts J Clin Oncol. 2013;31(suppl) abstr 8602. [Google Scholar]
- 33.Liu Q, Sun JD, Wang J, et al. TH-302, a hypoxia-activated prodrug with broad in vivo preclinical combination therapy efficacy: optimization of dosing regimens and schedules. Cancer Chemother Pharmacol. 2012;69:1487–1498. doi: 10.1007/s00280-012-1852-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


