Abstract
Investigation into the mechanisms driving cancer cell behavior and the subsequent development of novel targeted therapeutics requires comprehensive experimental models that mimic the complexity of the tumor microenvironment. Recently, our laboratories have combined a novel tissue culture model and laser direct-write, a form of bioprinting, to spatially position single or clustered cancer cells onto ex vivo microvascular networks containing blood vessels, lymphatic vessels, and interstitial cell populations. Herein, we highlight this new model as a tool for quantifying cancer cell motility and effects on angiogenesis and lymphangiogenesis in an intact network that matches the complexity of a real tissue. Application of our proposed methodology offers an innovative ex vivo tissue perspective for evaluating the effects of gene expression and targeted molecular therapies on cancer cell migration and invasion.
Keywords: Rat Mesentery Culture Model, Laser Direct Write, Bioprinting, Cancer Cell Migration, Metastasis, Angiogenesis, Lymphangiogenesis
INTRODUCTION
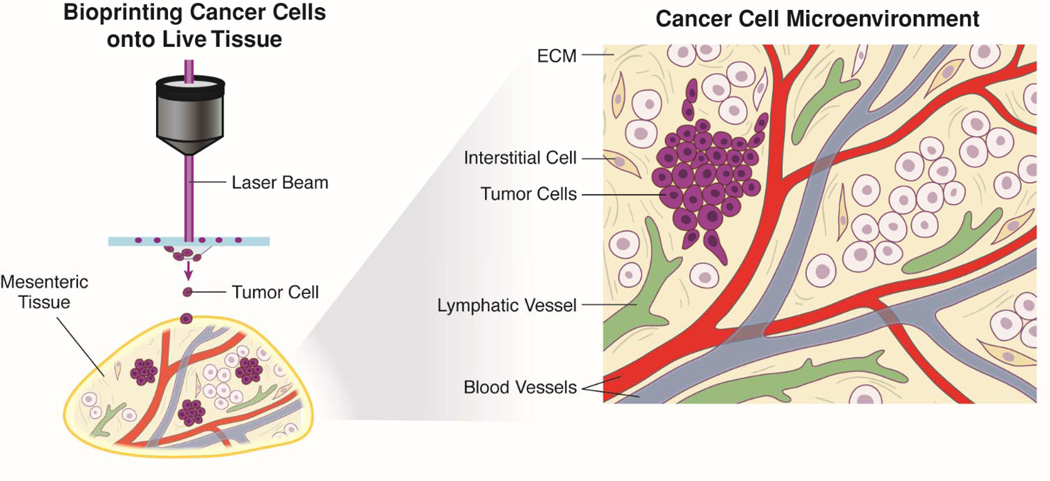
A challenge in cancer research is the lack of a physiologically responsive in vitro model that allows for the in depth study of cancer cells in a tissue-like microenvironment. Systematic investigation of the underlying mechanisms involved in cancer cell dynamics and the pre-clinical testing of novel therapeutics require experimental models that mimic the tumor-stroma microenvironment, including cancer cells, stromal cells, blood vessels, and lymphatic vessels (Corliss et al., 2016; Fukumura et al., 2010; Gomes et al., 2013; Kalluri and Zeisberg, 2006). Recently, our laboratories have combined the innovations of a novel tissue culture model with laser direct-write (LDW), a bioprinting technology, to introduce a biomimetic microenvironment that enables real-time investigation of cancer cell migration, fate, and function during microvascular network growth, including both angiogenesis and lymphangiogenesis, in an attempt to fill the scientific gap between current in vitro and in vivo experimentations and the in vivo reality (Figure 1) (Phamduy et al., 2015). In this “From the Bench” article, we highlight the novelty and potential impact of our approach as an ex vivo platform for probing the reciprocal interactions between cancer cells, fibroblasts, blood vessels, and lymphatic vessels. A unique advantage of LDW is the ability to see both the living construct and the deposited cell during printing. The effective controlled spatial deposition of cancer cells onto a live three-dimensional tissue and time-lapse imaging exemplify the integrated “bottom-up” and “top-down” methodologies and motivates the future use of the model for pre-clinical screening of molecular therapies and genetic mutations on cancer cell dynamics and observation at temporal and spatial resolutions only made possible by the presence of intact microvascular networks and stroma.
Figure 1. Bioprinting Cancer Cells onto Live Tissue as an Ex Vivo Model to Study Cell Migration.
By combining advantages of laser direct-write (LDW) and the rat mesentery culture model, cancer cells can be spatially patterned onto intact tissues and cultured. This new ex vivo model platform enables time lapse quantification of cancer cell behavior in an intact microvascular network environment with blood vessels, lymphatic vessels, extracellular matrix components and interstitial cells.
LASER DIRECT-WRITE + THE RAT MESETERY CULTURE MODEL: BIOPRINTING CANCER CELLS ON INACT TISSUES FOR THE STUDY OF CANCER CELL MIGRATION
While metastatic disease remains a significant driver of mortality in cancer patients, the exact processes underlying its progression remain poorly understood. Few therapeutic options are available which effectively target the mechanisms responsible for cancer metastasis, including cell migration and invasion into surrounding tissue as current models suffer from the reality that de novo creation of two or three-dimensional environment is an oversimplification of a tissue microenvironment. We introduced LDW on live tissues (Figure 2) in our recent work (Phamduy et al., 2015), and demonstrated that 1) printed breast cancer cells integrate with the intact mesentery tissue and remain viable after printing (Figure 3), 2) cancer cells migrate away from their initial location during angiogenesis, 3) cancer cells are capable of migrating and interacting with both blood and lymphatic vessels, and 4) printing multiple cell types allows for comparison of cell specific migratory behavior (Figure 3). The potential impact of our proposed platform to study cancer metastasis is realized when compared to traditional two-dimensional and three-dimensional assays.
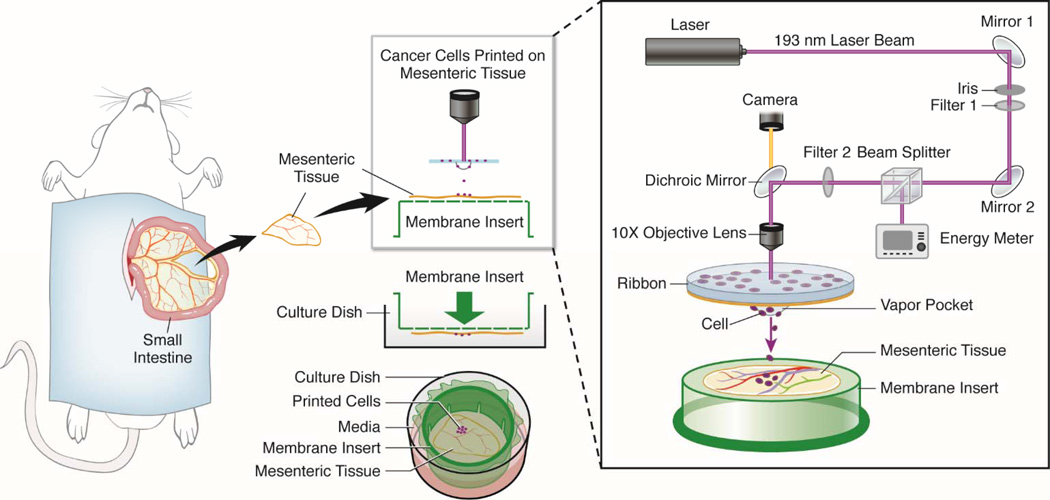
Figure 2. Laser Direct-Write Printing on Cultured Rat Mesentery Tissue.
Rat mesenteric tissue windows, defined as the thin, translucent connective tissue section between artery/vein pairs feeding the small intestine, are harvested from adult rats and spread onto inverted commercially available trans-well inserts. Prior to insertion of the insert plus tissue into a well of a 6 well culture plate, cells embedded in a gelatin coated UV-transparent quartz disc, termed “ribbon,” can be printed directly onto the tissue (Phamduy et al., 2015). A single pulse of 193 nm Arf excimer laser causes the formation of a local vapor pocket that ejected a desired amount of cells. Use of an in situ camera enables real time targeting of cells. We have demonstrated that bioprinted tissues can be cultured using normal culture conditions for at least up to 5 days.
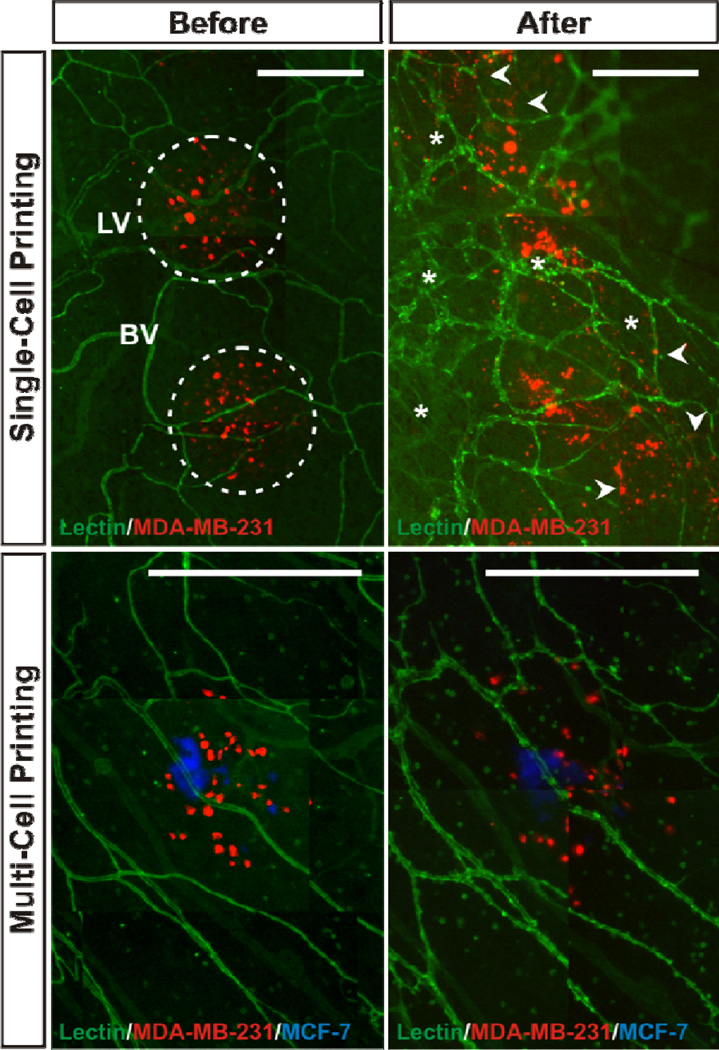
Figure 3. Time Lapse Comparison of Cancer Cell Location Before and After Culture.
A, B) Day 0 and Day 5 image of printed MDA-MB-231 metastatic breast cancer cells on rat mesenteric tissue. Cells can be fluorescently tagged prior to printing via either cell membrane labeling or transfection. Lectin labeling identifies endothelial cells along blood vessels, “bv,” and lymphatic vessels, “L.” Arterioles, venules, capillaries and lymphatics can be identified based on relative endothelial cell morphology and labeling intensity (Sweat et al., 2012); (Sweat et al., 2014); (Stapor et al., 2013). The dotted circles at day 0 (A) identify initial printed cell spots. By day 5 (B) cancer cells can be identified outside the initial location indicative of cell migration (arrow heads) and re-labeling with lectin identifies angiogenesis (i.e. new vessel formation; asterisks). C, D). Printing of metastatic breast cancer MDA-MB-231 cells and non-metastatic MCF-7 cells enables direct comparison of cell migration behavior. Comparison of relative cell locations on day 0 (C) versus day 2 (D) identifies apparent increased cell dispersion of the MDA-MB-231 cells. Lectin labeling again identifies microvessels. Scale bars = 500 µm.
Frequently utilized two-dimensional approaches using trans-well systems or scratch assays can be used to evaluate cell migration under single or co-culture conditions (Kramer et al., 2013). In scratch assays, single or heterogeneous cell types are grown together on a single surface and can be differentially labeled with fluorescent probes, seeded together, and monitored real time as they migrate across a “scratched” or bordered off region (Das et al., 2015). Conversely, in trans-well systems, two distinct cell populations are grown on separate surfaces; one on the solid well surface and the other on the porous membrane insert (Dirat et al., 2011; Gao et al., 2010). The membrane and well-bottom are spaced such that cells on the two surfaces cannot form direct cell-to-cell contacts. Thus, the interaction between the two cell cultures is mediated by secreted soluble factors, and chemotactic migration of each cell type, as well as invasion when using matrigel-coated chambers, can be monitored at endpoints. Although the trans-well system is an indispensable tool for studying paracrine signaling events, the bulk monolayers do not resemble in vivo tissue construction. To this end, micropatterned systems have been developed to mimic cell patterning seen on tissues for studying heterotypic cell-to-cell interactions (Bhatia et al., 1997). Distinct cell groups can either be grown on differential growth surfaces, which allows for formation of direct cell-cell contacts at culture interfaces, or isolated on surface adhesion islands, which limits groups of cells to specific positions in spatially-defined patterns. Cell migration away from these patterns can then be observed (Kim et al., 2013). As seen in the other two-dimensional assays, however, micropatterned cells often lack 3-D tissue geometry.
Organotypic culture models, in which cells are combined with additional cell types such as fibroblasts in native extracellular matrix scaffolds, attempt to mimic this tissue dimensionality (Shamir and Ewald, 2014). In general, cells are seeded on top of a collagen or ECM-like matrix and the depth or the pattern of the invasion can be observed with the migration of cells and quantified in one direction. Variations of this approach include hydrogel technologies, which have been similarly developed to embed or encapsulate cells in 3-D microenvironments. Using this approach, several co-culture models have been developed: bulk hydrogel encapsulation (Wang et al., 2010), microbeads (Yao et al., 2013), micro-molded hydrogel constructs (Zhu and Nelson, 2013), and layered hydrogel models (Delort et al., 2013). While these approaches attempt to offer physiologically relevant scaffolding on which to quantify migratory differences between cell types, their application is limited by the inability to spatially position cells or cell groups within the matrix. To circumvent this problem, several research groups have combined micro-scale encapsulation technique, which enables the creation of artificial cell microenvironments that resemble in vivo niches, with bottom-up tissue engineering technologies capable of assembling the cell-laden hydrogel subunits (Du et al. 2008). As an alternative approach to recapitulate multi-cellular, multi-system interactions involved in site specific metastasis, microfluidic models have emerged to investigate cancer cell migration and extravasation through endothelial lined channels (Bersini et al. 2014; Ehsan et al. 2014). For example, work by Dr. Roger Kamm’s group have demonstrated the value of such a microfluidic based approach for metastasis of breast cancer to bone by creating a vascularized osteo-cell conditioned microenvironment. Employing a tri-culture system, they were able to show preferential trans-endothelial migration of breast cancer cells toward the osteo-cell conditioned environment compared to the collagen-gel only matrices (Bersini et al. 2014). Due to fabrication of virtually any 'channel' network patterns and availability of profusion, microfluidic devices provide an attractive venue to examine cancer cell-endothelial crosstalk (Ehsan et al., 2014; Moya et al., 2013; Stroock et al., 2010). Microfluidic devices, similar to the other “bottom-up” approaches, however, do not reach the complexity of intact blood microvascular networks, lymphatic microvascular networks, fibroblasts, macrophages, and cancer cells, thus re-emphasizing the novelty of combining LDW with the rat mesentery culture model. The “top-down” nature of the rat mesentery culture model enables the maintenance of a multi-cellular, multi-system tissue microenvironment and the “bottom-up” characteristic of LDW adds the real-time patterning of the cancer cells, ranging from single cells to groups of cells (Figure 1, Figure 2).
ADVANTAGES OF LASER DIRECT-WRITE
Direct-write technologies, often called bioprinting, when “bioinks” (cells, biologics, or biomimetic material subunits) are printed, offer the ability to generate tissue analogues from spatially-ordered cell cultures in artificial microenvironments (Ringeisen et al., 2013). The “direct-write” paradigm refers to the deliberate placement of bioinks into desired pattern positions. Specific technologies, such as ink-jet printing (Mironov and Forgacs, 2007), extrusion-based bioprinting (Ozbolat and Hospodiuk, 2016), or laser direct-write (Schlie et al., 2011), incorporate computer-aided design/computer-aided manufacturing (CAD/CAM) for high-level precision in spatial positioning of voxels of bioink within a co-fabricated 3-D hydrogel matrix. Because blue-print patterns are defined by computer coding, virtually any histologically-relevant geometry can be reconstructed, though at different resolutions. Inkjet printing utilizes piezoelectric-pressure (Jakab et al., 2010) or heat (Cui et al., 2010) to deposit droplets of biological “inks” on demand, and is capable of forming meso-scale constructs from micro-scale subunits (Boland et al., 2006; Mironov et al., 2003). However, the technique is plagued by nozzle-clogging when printing viscous hydrogel solutions and variability in droplet-to-droplet cell density as cells in suspension may settle over the duration of printing. In addition, variability in droplet-to-droplet composition does not allow inkjet printing to consistently deposit single hydrogel subunits from a prepared suspension. Concomitant problems are associated with other bioprinting techniques, such as extrusion-based bioprinting.
LDW provides an attractive alternative for bioprinting multicellular constructs in spatially ordered patterns at near single cell resolution. Gelatin-based LDW (Schiele et al., 2011) is a non-contact, orifice-free technique that offers the ability to deposit biological materials with micro-scale precision (±5 µm) (Schiele et al., 2010). In addition, the system is capable of printing over 2 magnitudes of voxel size (up to 600 µm), simply by expanding the laser beam’s cross-sectional area (Phamduy et al., 2012). LDW systematically delivers low-power UV laser pulses to the “bio-inked” ribbon to generate droplets and softly transfer them to the defined pattern positions on the receiving substrate (Figure 2). Successful droplet generation and kinetic transfer of materials occur upon localized vaporization and rapid expansion of the gelatin matrix, which transfers the droplet from the ribbon–matrix interface. LDW achieves CAD/CAM capability through automated x, y, z translation of the substrate platform according to the user-generated blue-print code. Concurrently, LDW incorporates an in situ, charge-coupled device (CCD) camera placed directly above the motorized ribbon stage, providing the user with real-time vision for selective cell or cell-laden hydrogel transfer. Thus, through independent ribbon and substrate stage controls, targeted groups of cells or single hydrogel micro-droplets can be targeted in real-time and additively deposited into programmable array positions. By spatially controlling cell group placement, well-defined constructs from discrete subunits are fabricated for the study of spatial-dependent cell-cell communication and cancer cell migration and invasion.
ADVANTAGES OF THE RAT MESENTERY CULTURE MODEL
The need exists for biomimetic in vitro models of cancer cell migration/invasion that match the in vivo complexity of microvascular network growth models, including multiple cellular dynamics integrating angiogenesis and lymphangiogenesis. Over the past 4 years, our laboratories have introduced and developed the rat mesentery culture model. In comparison to other angiogenesis-motivated models, the rat mesentery culture model offers the potential for time-lapse investigation of mechanistic cell–cell interactions at specific locations across blood and lymphatic microvascular networks. We have shown that the model is advantageous because it can be used for 1) real time imaging in the same tissue, 2) quantification of endothelial cell sprouting at specific locations within a microvascular network during growth factor-induced angiogenesis, 3) investigating functional effects of pericytes on endothelial cell sprouting, 4) studying lymphangiogenesis, 5) investigation of lymphatic/blood endothelial cell interactions, and 6) anti-angiogenic drug testing (Azimi et al., 2015; Stapor et al., 2013; Sweat et al., 2014). A key advantage of our model is its simplicity - the tissue is easy to obtain, self-contained and does not need to be embedded. More importantly, the unique thinness (20–40 µm) of mesentery, which allows for observation of intact networks down to a single cell level while still maintaining three-dimensionality, makes it an ideal tissue for printing exogenous cells.
POTENTIAL APPLICATIONS AND LIMITATIONS
We envision that the LDW plus rat mesentery culture model platform can be used to evaluate cancer cell migration, intravasation, and vessel recruitment within an intact microvascular network. Using LDW cell printing, cells can be deposited onto mesenteric tissues at specific locations. The cancer cell-environment interactions can then be evaluated by spatial and temporal quantitative read-outs such as the direct effect of cancer cells on angiogenesis and lymphangiogenesis, whether these effects correlate with location within a vessel network, and whether different types of cancer cells preferentially metastasize to lymphatic versus blood vessels. Another application would be the screening of cell specific responses to molecular treatments. For example, consider that characterized breast cancer subtypes (e.g., luminal and basal) are defined by unique signaling, transcriptomic and epigenetic signatures (Network, 2012; Perou et al., 2000; Sotiriou et al., 2003). With the use of LDW bioprinting onto mesenteric tissues, cell type specific migratory dynamics can be quantified. These response measurements can further be used for the evaluation of targeted therapeutics. Specifically, cancer cells printed on mesentery can be treated with existing drugs and novel compounds as a preclinical model for testing efficacy and toxicity, both to the cells and to the surrounding components of the microenvironment. In our laboratories, as one example, we have shown that altered kinase signaling, chemokine and cytokine receptor active ion and histone deacetylase (hDAC) inhibitors suppress cancer cell invasion/migration in 2-dimensional trans-well assays, which are further supported by regulation of cancer cell metastasis in vivo (Rhodes et al., 2011; Rhodes et al., 2014; Rhodes et al., 2015). The application of our innovative model platform now offers a new method for the quantitative evaluation of molecular pathways and novel therapeutics that regulate the dynamics of cancer cell invasion/migration in distinct cancer subtypes via metrics for cell motility toward specific vessel types and cell phenotypic state during angiogenesis and lymphangiogenesis. Alternatively, primary cells expanded from clinical biopsy samples, patient-derived xenograft models, or characterized cell lines can be printed onto autologous mesentery tissue and challenged with various therapeutics. Furthermore, because the mesentery allows for single cell observation, immunohistochemical identification of cell phenotype and morphology alterations, like those associated with invadopodia formation (Leong et al., 2014), or other activated states, can be quantified in real time or at specified endpoints.
A unique advantage of LDW bioprinting on mesenteric tissues is the ability to print single or multiple cell subtypes in specific locations at specific times and in specific numbers. By defining the laser pulse beam size and controlling the size of printed cell groups, cell number can be varied and a determination can be made on critical cell number needed for the cancer cell phenotypic changes or cancer cell population initiation of tissue level responses. Furthermore, with printing single cancer cells, high-resolution models can be generated to compare single cell versus population-based dynamics and predict conditions that activate the "switch" to overcome metastatic dormancy. The use of LDW offers the potential for interrogation of dynamic interaction between multiple cell populations interacting upon stromal scaffold to define the tumorigenic and metastatic process. For example, we have demonstrated interaction between tumor cells and normal human mesenchymal stem cell populations and adipocyte stem cell populations in 2D culture and in vivo tumorigenesis models (Rhodes et al., 2010; Strong et al., 2015). The use of LDW would allow the manipulation of individual cell populations combined with coordinated cell printing to test mechanisms that regulate cell to cell crosstalk. Likewise, it has been shown that the co-printing the metastatic breast cancer cell line MDA-MB-231 with fibroblasts motivates the investigation of how fibroblasts, which are known to be play a role in cell migration (Camp et al., 2011), chaperone cancer cell migration toward specific locations across the hierarchy of a blood or lymphatic microvascular network (Duyverman et al., 2012), (Orimo et al., 2005). Moreover, the printing of metastatic and non-metastatic breast cancer cells (Figure 3) supports the use of the model for the direct comparison of different cancer cell types.
While printing cells onto live tissues represents an exciting opportunity for current cancer cell migration research, we recognize that the impact of its applications will be dependent on the model platform’s continued development. First, the characterization of all the cell types that remain present in cultured rat mesenteric tissues is incomplete. We do know that most cells remain viable including CD11b positive macrophages and other interstitial cells, yet further characterization is needed to identify the various cell populations. Moreover, we have yet to confirm cancer cell viability past 5 days in culture with rat mesenteric tissues (Phamduy et al., 2015). Additional investigation is needed to define the temporal resolution and maximum duration of experiments. Undoubtedly, these details will influence the scope of cancer cell dynamics that can be captured and related questions that can be answered. In addition, we have demonstrated the potential for bioprinting on the rat mesentery using breast cancer cells (Phamduy et al., 2015). LDW is a cell type-independent process and studies with alternative cell lines, including additional cancer cell types, are necessary. A more pathologically relevant line for mesentery tissue might be ovarian cancer cells, which have been known to home to intestinal tissue (Lengyel, 2010). Another limitation of our integrated model platform includes the relevance of the rat mesentery tissue, which arguably is not optimal to represent a tumor microenvironment. We postulate that this critique is indeed common for most in vitro models and for now we can only offer a rebuttal that our approach potentially offers a way to incorporate unmatched complexity. An alternative source for mesenteric tissues is mice. Unfortunately, mouse mesentery tissues are avascular. Nevertheless, the future use of mouse mesentery tissues derived from transgenic mice could be useful for investigating extracellular matrix-dependent cell migration behaviors or related scientific questions that do not necessitate the presence of a microvasculature. The use of the rat mesentery tissue does enable printing of cells on tissues from aged or pathological rat strains to examine the bi-directional effects of disease environmental backgrounds and cancer cells. Finally, similar to other ex vivo systems, our model currently lacks the presence of blood flow. Future studies will also be needed to include this and evaluate its necessity for specific cancer cell migration dynamics, yet despite the current limitations, critiques and unknowns, the novelty of the combined LDW bioprinting and rat mesentery culture model methods leads to an ex vivo platform that potentially enables quantification of cancer cell dynamics and effects within a complex environment at temporal and spatial resolutions not yet attained by other models.
Acknowledgments
This work was supported by the Tulane Center for Aging and funding from grants NIH 5-P20GM103629-04 to W.L.M, NIH CA125806 to M.E.B, and NSF 1258536 to D.B.C.
Footnotes
The authors have no conflicts of interest to declare.
LITERATURE CITED
- Azimi MS, Myers L, Lacey M, Stewart SA, Shi Q, Katakam PV, Mondal D, Murfee WL. An ex vivo model for anti-angiogenic drug testing on intact microvascular networks. PLoS One. 2015;10:e0119227. doi: 10.1371/journal.pone.0119227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersini S, Jeon JS, Dubini G, Arrigoni C, Chung S, Charest JL, Moretti M, Kamm RD. A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials. 2014a;35:2454–2461. doi: 10.1016/j.biomaterials.2013.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersini S, Jeon JS, Moretti M, Kamm RD. In vitro models of the metastatic cascade: from local invasion to extravasation. Drug Discov. Today. 2014b;19:735–742. doi: 10.1016/j.drudis.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia SN, Yarmush ML, Toner M. Controlling cell interactions by micropatterning in co-cultures: hepatocytes and 3T3 fibroblasts, J. Biomed. Mater. Res. 1997;34:189–199. doi: 10.1002/(sici)1097-4636(199702)34:2<189::aid-jbm8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Boland T, Xu T, Damon B, Cui X. Application of inkjet printing to tissue engineering. Biotechnol. J. 2006;1:910–917. doi: 10.1002/biot.200600081. [DOI] [PubMed] [Google Scholar]
- Camp JT, Elloumi F, Roman-Perez E, Rein J, Stewart DA, Harrell JC, Perou CM, Troester MA. Interactions with fibroblasts are distinct in Basal-like and luminal breast cancers. Mol. Cancer Res. 2011;9:3–13. doi: 10.1158/1541-7786.MCR-10-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corliss BA, Azimi MS, Munson JM, Peirce SM, Murfee WL. Macrophages: An Inflammatory Link Between Angiogenesis and Lymphangiogenesis. Microcirculation. 2016;23:95–121. doi: 10.1111/micc.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Dean D, Ruggeri ZM, Boland T. Cell damage evaluation of thermal inkjet printed Chinese hamster ovary cells. Biotechnol. Bioeng. 2010;106:963–969. doi: 10.1002/bit.22762. [DOI] [PubMed] [Google Scholar]
- Das AM, Eggermont AMM, ten Hagen TLM. A ring barrier-based migration assay to assess cell migration in vitro. Nat. Protoc. 2015;10:904–915. doi: 10.1038/nprot.2015.056. [DOI] [PubMed] [Google Scholar]
- Delort L, Lequeux C, Dubois V, Dubouloz A, Billard H. Reciprocal Interactions between Breast Tumor and Its Adipose Microenvironment Based on a 3D Adipose Equivalent Model. Development. 2013;8:1–11. doi: 10.1371/journal.pone.0066284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirat B, Bochet L, Dabek M. Cancer-Associated Adipocytes Exhibit an Activated Phenotype and Contribute to Breast Cancer Invasion Cancer-Associated Adipocytes Exhibit an Activated. Cancer Res. 2011:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- Du Y, Lo E, Ali S, Khademhosseini A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proc. Natl. Acad. Sci. United States Am. 105(28):9522–9527. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyverman AMMJ, Steller EJA, Fukumura D, Jain RK, Duda DG. Studying primary tumor-associated fibroblast involvement in cancer metastasis in mice. Nat. Protoc. 2012;7:756–762. doi: 10.1038/nprot.2012.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehsan SM, Welch-Reardon KM, Waterman ML, Hughes CCW, George SC. A three-dimensional in vitro model of tumor cell intravasation. Integr. Biol. (Camb) 2014;6:603–610. doi: 10.1039/c3ib40170g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumura D, Duda DG, Munn LL, Jain RK. Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models. Microcirculation. 2010;17:206–225. doi: 10.1111/j.1549-8719.2010.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Kim BG, Kang S, Choi YP, Park H, Kang KS, Cho NH. Stromal fibroblasts from the interface zone of human breast carcinomas induce an epithelial – mesenchymal transition-like state in breast cancer cells in vitro. J. Cell Sci. 2010 doi: 10.1242/jcs.072900. [DOI] [PubMed] [Google Scholar]
- Gomes FG, Nedel F, Alves AM, Nör JE, Tarquinio SBC. Tumor angiogenesis and lymphangiogenesis: tumor/endothelial crosstalk and cellular/microenvironmental signaling mechanisms. Life Sci. 2013;92:101–107. doi: 10.1016/j.lfs.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab K, Norotte C, Marga F, Murphy K, Vunjak-Novakovic G, Forgacs G. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication. 2010;2:022001. doi: 10.1088/1758-5082/2/2/022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat. Rev. Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- Kim M-C, Neal DM, Kamm RD, Asada HH. Dynamic modeling of cell migration and spreading behaviors on fibronectin coated planar substrates and micropatterned geometries. PLoS Comput. Biol. 2013;9:e1002926. doi: 10.1371/journal.pcbi.1002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer N, Walzl A, Unger C, Rosner M, Krupitza G, Hengstschläger M, Dolznig H. In vitro cell migration and invasion assays. Mutat. Res. 2013;752:10–24. doi: 10.1016/j.mrrev.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Lengyel E. Ovarian cancer development and metastasis. Am. J. Pathol. 2010;177:1053–1064. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong HS, Robertson AE, Stoletov K, Leith SJ, Chin CA, Chien AE, Hague MN, Ablack A, Carmine-Simmen K, McPherson VA, et al. Invadopodia are required for cancer cell extravasation and are a therapeutic target for metastasis. Cell Rep. 2014;8:1558–1570. doi: 10.1016/j.celrep.2014.07.050. [DOI] [PubMed] [Google Scholar]
- Mironov V, Forgacs G. Bioprinting living structures. Society. 2007:2054–2060. [Google Scholar]
- Mironov V, Boland T, Trusk T, Forgacs G, Markwald RR. Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003;21:157–161. doi: 10.1016/S0167-7799(03)00033-7. [DOI] [PubMed] [Google Scholar]
- Moya M, Tran D, George SC. An integrated in vitro model of perfused tumor and cardiac tissue. Stem Cell Res. Ther. 2013;4(Suppl 1):S15. doi: 10.1186/scrt376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Network TCGA. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016;76:321–343. doi: 10.1016/j.biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Phamduy TB, Raof NA, Schiele NR, Yan Z, Corr DT, Huang Y, Xie Y, Chrisey DB. Laser direct-write of single microbeads into spatially-ordered patterns. Current. 2012;025006 doi: 10.1088/1758-5082/4/2/025006. [DOI] [PubMed] [Google Scholar]
- Phamduy TB, Sweat RS, Azimi MS, Burow ME, Murfee WL, Chrisey DB. Printing cancer cells into intact microvascular networks: a model for investigating cancer cell dynamics during angiogenesis. Integr. Biol. (Camb) 2015;7:1068–1078. doi: 10.1039/c5ib00151j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes LV, Antoon JW, Muir SE, Elliott S, Beckman BS, Burow ME. Effects of human mesenchymal stem cells on ER-positive human breast carcinoma cells mediated through ER-SDF-1/CXCR4 crosstalk. Mol. Cancer. 2010;9:295. doi: 10.1186/1476-4598-9-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes LV, Short SP, Neel NF, Salvo Va, Zhu Y, Elliott S, Wei Y, Yu D, Sun M, Muir SE, et al. Cytokine receptor CXCR4 mediates estrogen-independent tumorigenesis, metastasis, and resistance to endocrine therapy in human breast cancer. Cancer Res. 2011;71:603–613. doi: 10.1158/0008-5472.CAN-10-3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes LV, Tate CR, Segar HC, Burks HE, Phamduy TB, Hoang V, Elliott S, Gilliam D, Pounder FN, Anbalagan M, et al. Suppression of triple-negative breast cancer metastasis by pan-DAC inhibitor panobinostat via inhibition of ZEB family of EMT master regulators. Breast Cancer Res. Treat. 2014;145:593–604. doi: 10.1007/s10549-014-2979-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes LV, Tate CR, Hoang VT, Burks HE, Gilliam D, Martin EC, Elliott S, Miller DB, Buechlein A, Rusch D, et al. Regulation of triple-negative breast cancer cell metastasis by the tumor-suppressor liver kinase B1. Oncogenesis. 2015;4:e168. doi: 10.1038/oncsis.2015.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringeisen BR, Pirlo RK, Wu PK, Boland T, Huang Y, Sun W, Hamid Q, Chrisey DB. Cell and organ printing turns 15: Diverse research to commercial transitions. MRS Bull. 2013;38:834–843. [Google Scholar]
- Schiele NR, Corr DT, Huang Y, Abdul Raof N, Xie Y, Chrisey DB. Laser-based direct-write techniques for cell printing. Biofabrication. 2010;2:032001. doi: 10.1088/1758-5082/2/3/032001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiele NR, Chrisey DB, Corr DT. Gelatin-Based Laser Direct-Write Technique for the Precise Spatial Patterning of Cells. Tissue Eng. Part C. 2011;17:289–298. doi: 10.1089/ten.tec.2010.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlie SDP, Deiwick A, Koch L, Wilhelmi M. Laser Printing of Three-Dimensional Multicellular Arrays for Studies of Cell – Cell and Cell – Environment Interactions. Cell. 2011;17 doi: 10.1089/ten.tec.2011.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamir ER, Ewald AJ. Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat. Rev. Mol. Cell Biol. 2014;15:647–664. doi: 10.1038/nrm3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiriou C, Neo S-Y, McShane LM, Korn EL, Long PM, Jazaeri A, Martiat P, Fox SB, Harris AL, Liu ET. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapor PC, Azimi MS, Ahsan T, Murfee WL. An angiogenesis model for investigating multicellular interactions across intact microvascular networks. Am. J. Physiol. Heart Circ. Physiol. 2013;304:H235–H245. doi: 10.1152/ajpheart.00552.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong AL, Ohlstein JF, Biagas BA, Rhodes LV, Pei DT, Tucker HA, Llamas C, Bowles AC, Dutreil MF, Zhang S, et al. Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers. Breast Cancer Res. 2015;17:112. doi: 10.1186/s13058-015-0622-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroock AD, D P, Fischbach C. Microfluidic Culture Models of Tumor Angiogenesis. Angiogenesis. 2010;16 doi: 10.1089/ten.tea.2009.0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweat RS, Stapor PC, Murfee WL. Relationships between lymphangiogenesis and angiogenesis during inflammation in rat mesentery microvascular networks. Lymphat. Res. Biol. 2012;10:198–207. doi: 10.1089/lrb.2012.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweat RS, Sloas DC, Murfee WL. VEGF-C induces lymphangiogenesis and angiogenesis in the rat mesentery culture model. Microcirculation. 2014;21:532–540. doi: 10.1111/micc.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Reagan MR, Kaplan DL. Synthetic Adipose Tissue Models for Studying Mammary Gland Development and Breast Tissue Engineering. J. Mammary Gland Biol. Neoplasia. 2010:365–376. doi: 10.1007/s10911-010-9192-y. [DOI] [PubMed] [Google Scholar]
- Yao R, Du Y, Zhang R, Lin F, Luan J. A biomimetic physiological model for human adipose tissue by adipocytes and endothelial cell cocultures with spatially controlled distribution. Biomed. Mater. 2013;8:045005. doi: 10.1088/1748-6041/8/4/045005. [DOI] [PubMed] [Google Scholar]
- Zhu W, Nelson M. Adipose and mammary epithelial tissue engineering. Breast. 2013:1–6. doi: 10.4161/biom.24630. [DOI] [PMC free article] [PubMed] [Google Scholar]