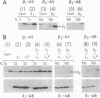
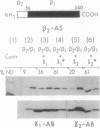
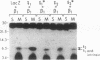
Abstract
Signal-transducing guanine nucleotide-binding proteins (G proteins) are made up of three subunits, alpha, beta, and gamma. Each of these subunits comprises a family of proteins. The rules for association between members of one family with members of another to form a multimer are not known; it is not clear whether associations are specific or nonspecific. Other than transducin (Gt), the G protein in rod photoreceptors, most purified G proteins contain more than one subtype of beta or gamma subunits. The Gt alpha subunit is associated only with beta 1 and gamma 1. It is not known whether this specificity is due to the differential expression of these subunit types in a cell type or due to intrinsically different affinities between different beta and gamma subunit types. We have used a transfected cell assay system to examine the association of the beta 1, beta 2, and beta 3 proteins with the gamma 1 and gamma 2 proteins. Results show that gamma 1 does not associate with beta 2 and that beta 3 does not associate with gamma 1 or gamma 2. Differences in affinities between types of G protein subunits will impose restrictions on the formation of certain heterotrimers and determine which G protein will be active in a cell. A chimeric molecule of beta 1 and beta 2 was used to broadly map the regions on these subunits that determine specificity of association.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amatruda T. T., 3rd, Gautam N., Fong H. K., Northup J. K., Simon M. I. The 35- and 36-kDa beta subunits of GTP-binding regulatory proteins are products of separate genes. J Biol Chem. 1988 Apr 15;263(11):5008–5011. [PubMed] [Google Scholar]
- Backlund P. S., Jr, Simonds W. F., Spiegel A. M. Carboxyl methylation and COOH-terminal processing of the brain G-protein gamma-subunit. J Biol Chem. 1990 Sep 15;265(26):15572–15576. [PubMed] [Google Scholar]
- Birnbaumer L. G proteins in signal transduction. Annu Rev Pharmacol Toxicol. 1990;30:675–705. doi: 10.1146/annurev.pa.30.040190.003331. [DOI] [PubMed] [Google Scholar]
- Bubis J., Khorana H. G. Sites of interaction in the complex between beta- and gamma-subunits of transducin. J Biol Chem. 1990 Aug 5;265(22):12995–12999. [PubMed] [Google Scholar]
- Fong H. K., Amatruda T. T., 3rd, Birren B. W., Simon M. I. Distinct forms of the beta subunit of GTP-binding regulatory proteins identified by molecular cloning. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3792–3796. doi: 10.1073/pnas.84.11.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong H. K., Hurley J. B., Hopkins R. S., Miake-Lye R., Johnson M. S., Doolittle R. F., Simon M. I. Repetitive segmental structure of the transducin beta subunit: homology with the CDC4 gene and identification of related mRNAs. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2162–2166. doi: 10.1073/pnas.83.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada Y., Takao T., Ohguro H., Yoshizawa T., Akino T., Shimonishi Y. Farnesylated gamma-subunit of photoreceptor G protein indispensable for GTP-binding. Nature. 1990 Aug 16;346(6285):658–660. doi: 10.1038/346658a0. [DOI] [PubMed] [Google Scholar]
- Gautam N., Baetscher M., Aebersold R., Simon M. I. A G protein gamma subunit shares homology with ras proteins. Science. 1989 May 26;244(4907):971–974. doi: 10.1126/science.2499046. [DOI] [PubMed] [Google Scholar]
- Gautam N., Northup J., Tamir H., Simon M. I. G protein diversity is increased by associations with a variety of gamma subunits. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7973–7977. doi: 10.1073/pnas.87.20.7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hurley J. B., Fong H. K., Teplow D. B., Dreyer W. J., Simon M. I. Isolation and characterization of a cDNA clone for the gamma subunit of bovine retinal transducin. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6948–6952. doi: 10.1073/pnas.81.22.6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Lewis D. L., Graziadei L., Neer E. J., Bar-Sagi D., Clapham D. E. G-protein beta gamma-subunits activate the cardiac muscarinic K+-channel via phospholipase A2. Nature. 1989 Feb 9;337(6207):557–560. doi: 10.1038/337557a0. [DOI] [PubMed] [Google Scholar]
- Lai R. K., Perez-Sala D., Cañada F. J., Rando R. R. The gamma subunit of transducin is farnesylated. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7673–7677. doi: 10.1073/pnas.87.19.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. A., Smallwood P. M., Moen P. T., Jr, Helman L. J., Ahn T. G. Molecular cloning of beta 3 subunit, a third form of the G protein beta-subunit polypeptide. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2329–2333. doi: 10.1073/pnas.87.6.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltese W. A., Robishaw J. D. Isoprenylation of C-terminal cysteine in a G-protein gamma subunit. J Biol Chem. 1990 Oct 25;265(30):18071–18074. [PubMed] [Google Scholar]
- Moscovici C., Moscovici M. G., Jimenez H., Lai M. M., Hayman M. J., Vogt P. K. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell. 1977 May;11(1):95–103. doi: 10.1016/0092-8674(77)90320-8. [DOI] [PubMed] [Google Scholar]
- Mumby S. M., Casey P. J., Gilman A. G., Gutowski S., Sternweis P. C. G protein gamma subunits contain a 20-carbon isoprenoid. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5873–5877. doi: 10.1073/pnas.87.15.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robishaw J. D., Kalman V. K., Moomaw C. R., Slaughter C. A. Existence of two gamma subunits of the G proteins in brain. J Biol Chem. 1989 Sep 25;264(27):15758–15761. [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Schmidt C. J., Neer E. J. In vitro synthesis of G protein beta gamma dimers. J Biol Chem. 1991 Mar 5;266(7):4538–4544. [PubMed] [Google Scholar]
- Simon M. I., Strathmann M. P., Gautam N. Diversity of G proteins in signal transduction. Science. 1991 May 10;252(5007):802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Simonds W. F., Butrynski J. E., Gautam N., Unson C. G., Spiegel A. M. G-protein beta gamma dimers. Membrane targeting requires subunit coexpression and intact gamma C-A-A-X domain. J Biol Chem. 1991 Mar 25;266(9):5363–5366. [PubMed] [Google Scholar]
- Tang W. J., Gilman A. G. Type-specific regulation of adenylyl cyclase by G protein beta gamma subunits. Science. 1991 Dec 6;254(5037):1500–1503. doi: 10.1126/science.1962211. [DOI] [PubMed] [Google Scholar]
- Yamane H. K., Farnsworth C. C., Xie H. Y., Howald W., Fung B. K., Clarke S., Gelb M. H., Glomset J. A. Brain G protein gamma subunits contain an all-trans-geranylgeranylcysteine methyl ester at their carboxyl termini. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5868–5872. doi: 10.1073/pnas.87.15.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]