Abstract
Background
The World Health Organization recently released recommendations stating that red blood cell (RBC) folate concentrations should be above 400 ng/L (906 nmol/L) for optimal prevention of folate-sensitive neural tube defects (NTDs). The objective of this study was to determine the distribution of folate insufficiency (FI) (<906 nmol/L) and potential risk of NTDs based on RBC folate concentrations among nonpregnant women of child-bearing age in Guatemala.
Methods
A national and regional multistage cluster probability survey was completed during 2009 to 2010 among Guatemalan women of child-bearing age 15 to 49 years of age. Demographic and health information and blood samples for RBC folate analyses were collected from 1473 women. Prevalence rate ratios of FI and predicted NTD prevalence were estimated based on RBC folate concentrations comparing subpopulations of interest.
Results
National FI prevalence was 47.2% [95% confidence interval, 43.3–51.1] and showed wide variation by region (18–81%). In all regions, FI prevalence was higher among indigenous (27–89%) than among nonindigenous populations (16–44%). National NTD risk based on RBC folate concentrations was estimated to be 14 per 10,000 live births (95% uncertainty interval, 11.1–18.6) and showed wide regional variation (from 11 NTDS in the Metropolitan region to 26 NTDs per 10,000 live births in the Norte region).
Conclusion
FI remains a common problem in populations with limited access to fortified products, specifically rural, low income, and indigenous populations. However, among subpopulations that are most likely to have fortified food, the prevalence of FI is similar to countries with well-established fortification programs.
Keywords: Folate, Red Blood Cell Folate Insufficiency Prevalence, Neural Tube Defects Childbearing-age Women, Guatemala
Introduction
It is estimated that each year more than 300,000 newborns worldwide are born with neural tube defects (NTDs) (Christianson et al., 2006). These devastating birth defects (including anencephaly and spina bifida) happen in the early weeks of pregnancy, often before a woman knows she is pregnant. Many of these NTDs can be prevented if women have adequate folic acid intake before conception (MRC Vitamin Study Group, 1991; Czeizel and Dudas, 1992). To promote folic acid intake, in 1992 the United States Public Health Service recommended that women of child bearing age (WCBA) who could become pregnant should consume 400 μg of folic acid daily (Centers for Disease Control and Prevention, 1992). Several countries (e.g., Canada, Chile, Costa Rica, United States) increased folic acid intake through mandatory folic acid fortification, and all observed reductions in the prevalence of NTDs, ranging from 31 to 78% (De Wals et al., 2008; Hertrampf and Cortes, 2008; Arguello and Umaña, 2011; Rosenthal et al., 2013; Williams et al., 2015). These and other data suggest increasing folic acid intake can reduce NTD rates to as low as 5 to 6 per 10,000 births (Berry et al., 1999; Arguello and Umaña, 2011; Rosenthal et al., 2013; Williams et al., 2015; Cordero et al., 2015). However, achieving such reductions depends on the effectiveness of interventions for increasing folic acid intake among WCBA before pregnancy.
To help monitor the effectiveness of folic acid interventions, the World Health Organization (WHO) has recently released a new guideline that establishes a threshold for optimal red blood cell (RBC) folate concentration for neural tube defect (NTD) prevention (>400 ng/L or 906 nmol/L) (WHO, 2015). This threshold can be used to help public health officials determine if their overall populations or subpopulations are at risk for folate-sensitive (and thus preventable) NTDs.
In this study, the objective was to apply the new WHO guideline for the first time in a developing country, to determine if the current RBC folate concentrations are consistent with optimal NTD prevention and to identify groups of women who continue to be at increased risk of a folate-sensitive NTD-affected pregnancy. To do so, we used the Guatemala Encuesta Nacional de Micronutrientes (ENMICRON) data collected in 2009 to 2010. These data were of special interest because in 2003 Guatemala increased the amount of folic acid in fortified wheat flour from 1.08 to 1.80 mg/kg (David, 2004) with the goal of providing adequate intake of folate to WCBA. As part of the study, we assessed the prevalence of folate insufficiency based on RBC folate concentrations at national and regional levels, and by population sociodemographic characteristics. Then we used the RBC folate concentrations to model the estimated risk of NTDs among nonpregnant women ages 15 to 49 years.
Participants and Methods
The ENMICRON survey was conducted in Guatemala during 2009 to 2010 among a sample of civilian, noninstitutionalized children and WCBA. Details of the ENMICRON survey, laboratory methods, and folate deficiency results for WCBA have been published previously (Rosenthal et al., 2015). The ENMICRON used a complex, multistage probability design with sufficient sample size and population coverage to provide representative estimates of RBC folate concentrations for WCBA, with national and regional representation, and stratified by urban and rural settings. The ENMICRON sample included 1473 nonpregnant WCBA who were interviewed; blood samples were obtained from 1448 of these women. The Guatemalan Ministry of Health requested that the study protocol be reviewed by the CDC Institutional Review Board in Atlanta, Georgia, United States. The protocol was approved in 2008 by the CDC Institutional Review Board.
BIOCHEMICAL ANALYSES
After the interviews were completed, blood (nonfasting) was drawn for measurement of nutrition biomarkers. Details of the field management and processing of blood samples were reported earlier (Rosenthal et al., 2015). In short, blood samples were taken by venipuncture into tubes containing EDTA, and processed to measure RBC and plasma folate concentrations as described by O’Broin at the Centers for Disease Control and Prevention in Atlanta, Georgia, United States (O’Broin et al., 1997).
Consistent with previous analyses (Pfeiffer et al., 2011; Crider et al., 2014; Tinker et al., 2015), we converted the folate insufficiency cutoff point (906 nmol/L) to adjust for differences in microbiologic assays by using the following equation:
(Pfeiffer et al., 2011; Tinker et al., 2015).
As such, <906 nmol/L (Daly microbiologic assay folate insufficiency cutoff) (Daly et al., 1995) = <748 nmol/L (CDC microbiologic assay folate insufficiency cutoff), as established previously (Pfeiffer et al., 2011; Crider et al., 2014; Tinker et al., 2015).
ANALYTICAL AND STATISTICAL METHODS
We described the variation in the prevalence estimates of RBC folate insufficiency using prevalence risk ratios (PRRs) for area type (urban or rural), age, ethnicity, level of education, wealth index, and region. We calculated point estimates and 95% confidence intervals (CIs) using average marginal predictions (Santos et al., 2008; Bieler et al., 2010). We performed a multivariable logistic regression analysis to determine independent predictors of folate insufficiency and to determine the adjusted folate insufficiency prevalence (i.e., predictive margins). We used geometric means for RBC folate because the distribution of RBC folate concentrations was skewed; we adjusted mean differences for sociodemographic and other variables using multiple linear regression models (Proc Regress SUDAAN) (SUDDAN statistical software, 2012). In all analyses, we used sample weights to reflect the complex sample survey design and Taylor series approximation to obtain the standard errors of the measurements (Walter, 2007).
We modeled NTD prevalences within subpopulations (e.g., urban, rural) based on RBC concentration data from the ENMICRON survey. We used Bayesian methods to account for both the uncertainty about the true values of the RBC concentrations and the uncertainty in predicting NTD prevalences using RBC concentrations, as follows:
To begin, for each subpopulation we generated a hypothetical population of 100,000 women, each having a value for RBC concentration derived by taking a random sample from a log normal distribution of RBC concentrations with mean and variance for that subpopulation calculated from the ENMICRON survey (Table 2). For each of these 100,000 hypothetical women, we generated 10,000 pairs of NTD risk parameters based on a published model that was created to predict NTD prevalences using RBC concentrations (Crider et al., 2014). Specifically, we obtained each pair of NTD risk parameters by taking a random sample from among the final set of 20,000 posterior samples that constituted the estimated posterior distribution of the published model.
TABLE 2.
National and Regional Geometric Means and Percentiles of Red Blood Cell (RBC) Concentrations by Sociodemographic Characteristics and Regions: ENMICRON 2009–2010
|
N (unweighted) |
RBC folatea (nmol/L) |
95% CI | Percentileb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5th | 10th | 25th | 50th | 75th | 90th | 95th | ||||
| National | 1448 | 725 | 711, 738 | 305 | 361 | 548 | 766 | 997 | 1243 | 1373 |
| Area | ||||||||||
| Rural | 773 | 664 | 624, 707 | 282 | 313 | 458 | 664 | 926 | 1176 | 1311 |
| Urban | 675 | 763c | 727, 802 | 374 | 494 | 651 | 875 | 1075 | 1299 | 1452 |
| Ethnicity | ||||||||||
| Indigenous | 559 | 702 | 665, 742 | 280 | 328 | 501 | 689 | 946 | 1209 | 1300 |
| Non-indigenous | 889 | 722 | 689, 756 | 331 | 391 | 577 | 815 | 1022 | 1279 | 1451 |
| Age (yr) | ||||||||||
| 15 – 19 | 233 | 681 | 639, 726 | 298 | 371 | 568 | 756 | 955 | 1139 | 1279 |
| 20 – 34 | 525 | 723 | 685, 762 | 315 | 387 | 535 | 764 | 977 | 1230 | 1366 |
| 35 – 49 | 690 | 734 | 698, 773 | 305 | 353 | 546 | 768 | 1041 | 1280 | 1400 |
| Education (yr) | ||||||||||
| None | 314 | 702 | 653, 755 | 287 | 366 | 474 | 665 | 929 | 1160 | 1233 |
| Primary | 713 | 694 | 658, 732 | 293 | 337 | 517 | 713 | 982 | 1240 | 1398 |
| Secondary or higher | 419 | 742 | 699, 787 | 395 | 496 | 651 | 880 | 1058 | 1316 | 1395 |
| Wealth index (terciles) | ||||||||||
| Lowest | 477 | 624d,e | 583, 668 | 279 | 307 | 438 | 584 | 812 | 1093 | 1206 |
| Middle | 538 | 731 | 690, 773 | 329 | 358 | 552 | 756 | 999 | 1264 | 1408 |
| High | 431 | 792 | 738, 851 | 415 | 510 | 718 | 889 | 1076 | 1319 | 1405 |
| Region | ||||||||||
| Metropolitan | 141 | 834f | 774, 898 | 353 | 589 | 779 | 949 | 1180 | 1347 | 1481 |
| Norte | 107 | 548g | 482, 625 | 239 | 270 | 321 | 510 | 667 | 865 | 1048 |
| Nor-Oriente | 216 | 696 | 621, 781 | 277 | 330 | 465 | 719 | 948 | 1233 | 1443 |
| Sur-Oriente | 161 | 692h | 631, 759 | 299 | 314 | 542 | 720 | 929 | 1221 | 1409 |
| Central | 219 | 658 | 592, 731 | 284 | 311 | 450 | 695 | 939 | 1234 | 1378 |
| Sur-Occidente | 431 | 723 | 644, 741 | 331 | 375 | 535 | 732 | 964 | 1204 | 1315 |
| Nor-Occidente | 101 | 807 | 722, 901 | 418 | 475 | 568 | 767 | 977 | 1221 | 1349 |
| Peten | 72 | 745 | 661, 839 | 347 | 465 | 557 | 754 | 948 | 1197 | 1370 |
Adjusted by area, ethnicity, age, education, and wealth index.
Calculated on the basis of raw blood folate data (weighted untransformed data).
Area: rural vs urban (p < 0.001).
Wealth Index: Lowest vs Middle (p< 0.001)
Wealth Index: Lowest vs High (p< 0.001)
Region: Metropolitana vs. Norte; (p< 0.001); Nor-Oriente (p <0.001); Central (p< 0.05); and Sur-Occidente (p< 0.001)
Region:: Norte vs. Nor-Occidente (p<0.001); Peten (<0.05)
Region: Sur-Oriente vs. Nor-Occidente (p<0.01)
Using the RBC concentration and a given pair of NTD risk parameters, we could calculate a probability value for NTD risk. If that value was greater than a random number drawn from a uniform distribution between zero and one, then we counted it as an NTD case. By repeating this algorithm for 10,000 pairs of NTD risk parameters, we generated the number of NTD cases out of 10,000 for a hypothetical woman with a fixed RBC concentration. We then repeated this process for each of the 100,000 hypothetical women. Finally, we used the resulting distribution of 100,000 NTD prevalence estimates to determine the median and the 95% uncertainty interval (defined by using the 2.5th and 97.5th percentiles).
We estimated differences in the NTD prevalence between two subpopulations (e.g., urban vs. rural) by calculating the difference in the NTD prevalence estimates for each of the 100,000 hypothetical women generated for each subpopulation. We summarized the uncertainty in these difference estimates by using a 95% uncertainty interval bounded by the 2.5th and 97.5th percentiles of the 100,000 difference estimates. If this 95% uncertainty interval for the NTD prevalence difference between the two subpopulations excluded the value zero, then we termed the prevalence in the subpopulation with the higher median NTD prevalence to “likely be greater” than that in the other subpopulation. Differences in estimated prevalences meeting this criteria are noted in Table 2.
Results
NATIONAL AND REGIONAL PREVALENCE OF RBC FOLATE INSUFFICIENCY
The national prevalence of RBC folate insufficiency was 47.2% (95% CI, 43.3–51.1) (Table 1). The prevalence of RBC folate insufficiency varied by region and ranged from a low of 18.8% (95% CI, 11.7–28.9) in the Metropolitana region to a high of 80.9% (95% CI, 62.7–91.4) in the Norte region. When compared with the Metropolitana region, all regions, with the exception of the Nor-Occidente, showed significantly higher adjusted PRRs for folate insufficiency, ranging from a low of 1.6 (95% CI, 1.0–2.5) in the Petén to 2.8 (95% CI, 1.9–4.1) in the Norte.
TABLE 1.
Unadjusted and Adjusted Prevalence Risk Ratios (PRR) for Red Blood Cell (RBC) Folate Insufficiency (< 748 nmol/L) by Region and Sociodemographic Characteristics: ENMICRON, 2009–2010
|
N (unweighted) |
Prevalence (95% CI) |
Unadjusted PRR (95% CI) |
Adjusteda PRR (95% CI)a |
|
|---|---|---|---|---|
| RBC folate insufficiency (< 748 nmol/L) | ||||
| National | 1448 | 47.2 (43.3, 51.1) | ||
| Region | ||||
| Metropolitana | 141 | 18.8 (11.7, 28.9) | Referent | Referent |
| Norte | 107 | 80.9 (62.7, 91.4) | 4.3 (2.6, 7.0)*** | 2.8 (1.9, 4.1)*** |
| Nor-Oriente | 216 | 52.1 (41.7, 62.3) | 2.8 (1.7, 4.5)*** | 1.8 (1.2, 2.7)* |
| Sur-Oriente | 161 | 51.6 (41.0, 62.1) | 2.7 (1.7, 4.5)*** | 1.8 (1.2, 2.7)** |
| Central | 219 | 56.8 (47.4, 65.7) | 3.0 (1.9, 4.9)*** | 2.2 (1.5, 3.2)*** |
| Sur-Occidente | 431 | 51.3 (43.7, 58.9) | 2.7 (1.7, 4.4)*** | 1.9 (1.3, 2.8)*** |
| Nor-Occidente | 101 | 46.8 (33.3, 60.8) | 2.5 (1.4, 4.3)*** | 1.4 (0.9, 2.4) |
| Petén | 72 | 47.6 (35.0, 60.6) | 2.5 (1.5, 4.3)*** | 1.6 (1.0, 2.5)* |
| Area | ||||
| Rural | 773 | 59.8 (53.9, 65.4) | 1.8 (1.5, 2.1)*** | 1.3 (1.1, 1.5)* |
| Urban | 675 | 33.8 (28.9, 39.0) | Referent | Referent |
| Ethnicity | ||||
| Indigenous | 559 | 56.0 (49.3, 62.6) | 1.3 (1.1, 1.6)*** | 1.0 (0.9, 1.2) |
| Non-Indigenous | 889 | 41.7 (37.3, 46.3) | Referent | Referent |
| Age (yr) | ||||
| 15 – 19 | 233 | 48.4 (40.8, 56.0) | 1.0 (0.9, 1.3) | 1.2 (1.0, 1.4) |
| 20 –34 | 525 | 48.0 (42.3, 53.9) | 1.0 (0.9, 1.2) | 1.1 (0.9, 1.3) |
| 35 – 49 | 690 | 46.0 (40.8, 51.3) | Referent | Referent |
| Education (yr) | ||||
| None | 314 | 59.7 (50.8, 67.9) | 1.8 (1.4, 2.3)*** | 1.2 (0.9, 1.5) |
| Primary | 715 | 52.0 (47.0, 56.9) | 1.6 (1.3, 1.9)*** | 1.1 (1.0, 1.4) |
| Secondary or higher | 419 | 32.4 (27.3, 38.8) | Referent | Referent |
| Wealth index (terciles) | ||||
| Low | 478 | 68.4 (61.6, 74.4) | 2.6 (2.0, 3.2)*** | 1.9 (1.4, 2.5)*** |
| Middle | 538 | 49.1 (43.8, 54.4) | 1.8 (1.4, 2.3)*** | 1.5 (1.1, 1.9)** |
| High | 432 | 26.8 (21.3, 33.2) | Referent | Referent |
ENMICRON, Guatemala Encuesta Nacional de Micronutrientes.
Adjusted model had RBC folate insufficiency as the outcome variable and area type, ethnicity, age, education, wealth index, and region as predictor variables.
p-Value < 0.05.
p–Value < 0.01.
p-Value < 0.001.
PREVALENCE OF FOLATE INSUFFICIENCY BY POPULATION CHARACTERISTICS
We found significantly higher folate insufficiency prevalence in rural areas than in urban areas (59.8% vs. 33.8%) (Table 1). The differences in prevalence between women in rural and urban areas remained significant after adjusting for other covariates (adjusted PRR 1.3 [95% CI, 1.1–1.6]). The prevalence of RBC folate insufficiency was higher among indigenous women than among nonindigenous women (56.0% vs. 41.7%), but the difference was not significant after adjusting for other covariates.
Women with higher educational levels were more likely to have lower folate insufficiency prevalences (Table 1), but only in the unadjusted models. We observed significant differences in folate insufficiency prevalences at different wealth indices. At the low and middle wealth indices, women were more likely to show higher folate insufficiency prevalence (68.4% and 49.1%, respectively) (adjusted PRRs, 1.9 [95% CI, 1.4–2.5] and 1.5 [95% CI, 1.1–1.9]) compared with women in the high wealth index (26.8%) (Table 1).
PREVALENCE OF FOLATE INSUFFICIENCY BY REGION STRATIFIED BY AREA, WEALTH INDEX, AND ETHNICITY
Because both area type (urban or rural) and wealth index likely affected access to fortified staples in each region, we assessed the independent effects of area and wealth index in each region (Supplementary Table S1, which is available online). Across regions, there was a consistently higher prevalence of folate insufficiency among women in rural areas than those in urban areas. Prevalence of folate insufficiency in rural areas was lowest in the Metropolitana region (26.6%), whereas the highest prevalences were found to be in the Norte (88.8%) and Central (70.9%) regions. Differences in folate insufficiency prevalence between rural and urban areas only reached statistical significance in the Norte and Central regions (adjusted PRR = 1.2 [95% CI, 1.04–1.4] and 1.5 [95% CI, 1.1–2.1], respectively).
Supplementary Table S1 also shows that the prevalence of folate insufficiency in each region varied by wealth index. The prevalence of folate insufficiency among those in the low wealth index was lowest in the Metropolitana region (22.5%); this region’s prevalence was much lower than that of any other region (55.2–94.6%). Only the Metropolitana and Petén regions did not show significant differences in the prevalence of folate insufficiency by wealth index.
The prevalence of folate insufficiency was higher among indigenous populations in all regions, with the exception of the Metropolitana region; however, statistically significant differences were demonstrated only in Norte, Nor-Oriente, and Sur-Oriente regions (Supp. Table S2).
GEOMETRIC MEANS AND PERCENTILE DISTRIBUTION OF RBC FOLATE CONCENTRATIONS
Table 2 shows that the national median (50th percentile) RBC folate concentration among women 15 to 49 years of age was 766 nmol/L which is above the cutoff point of 748 nmol/L for folate insufficiency. However, the median RBC folate concentrations for women who resided in rural settings, were indigenous, had no or only primary education, and were in the lowest wealth index were below the folate insufficiency cutoff point. Similarly, five of the eight regions showed median RBC folate concentrations below the folate insufficiency cutoff point and in the Norte region, 75% of women were folate insufficient.
PREDICTED NTD RISK BASED ON RBC FOLATE CONCENTRATION PERCENTILES
Based on the RBC folate concentration distributions, the estimated NTD risk for the overall population was 14 NTDs per 10,000 births (95% uncertainty interval [UI], 11–19). The NTD risk was higher among rural women (19; 95% UI, 14–23) than among urban women (11; 95% UI, 8–13) (Tables 2, 3; Fig. 1). Furthermore, when assessing NTD risk by region, regions with a high number of low income and indigenous women were at the highest NTD risk (e.g., Norte region: 26; 95% UI, 19–35). NTD risk was much lower in the Metropolitan region (11; 95% UI, 8–14), with other regions at intermediate risk (Tables 2, 3; Supp. Fig. S1). The high NTD risk were driven by the very high risks among those at the lower end of the distributions (5th–25th) (Fig. 1; Supp. Fig. S1; Table 3).
TABLE 3.
Predicted median neural tube defect (NTD) risk per 10,000 live births in Guatemala based on adjusted mean(s) and percentile distributions of red blood cell (RBC) concentrations by selected socio-demographic characteristics and region. ENMICRON 2009–2010
| Predicted Median NTD Risk per 10,000 | 95% CI | Percentilea | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 5th | 10th | 25th | 50th | 75th | 90th | 95th | |||
| National | 14 | 11.1, 18.6 | 48 | 35 | 16 | 9 | 6 | 4 | 3 |
| Area | |||||||||
| Rural | 19 | 13.6, 23.1 | 56 | 46 | 22 | 12 | 6 | 4 | 4 |
| Urban | 11 | 8.3, 13.5 | 33 | 20 | 12 | 7 | 5 | 4 | 3 |
| Ethnicity | |||||||||
| Indigenous | 17 | 13.3, 22.6 | 56 | 42 | 19 | 11 | 6 | 4 | 4 |
| Non-Indigenous | 12 | 9.4, 15.2 | 41 | 30 | 15 | 8 | 5 | 4 | 3 |
| Wealth index (terciles) | |||||||||
| Low | 20 | 15.2, 25.5 | 57 | 47 | 24 | 15 | 8 | 5 | 4 |
| Middle | 14 | 10.6, 17.3 | 42 | 35 | 16 | 9 | 6 | 4 | 3 |
| High | 9 | 7.2, 11.9 | 27 | 19 | 10 | 7 | 5 | 3 | 3 |
| Region | |||||||||
| Metropolitan | 11 | 8.2, 13.6 | 34 | 18 | 12 | 10 | 7 | 6 | 5 |
| Norte | 26 | 19.5, 34.8 | 59 | 50 | 39 | 21 | 15 | 11 | 8 |
| Nor-Oriente | 17 | 13.0, 22.3 | 48 | 38 | 24 | 14 | 10 | 7 | 6 |
| Sur-Oriente | 16 | 12.1, 20.2 | 43 | 40 | 20 | 14 | 10 | 7 | 6 |
| Central | 17 | 12.9, 21.9 | 46 | 41 | 25 | 14 | 10 | 7 | 6 |
| Sur-Occidente | 17 | 12.7, 21.8 | 38 | 32 | 20 | 13 | 9 | 7 | 6 |
| Nor-Occidente | 11 | 8.3, 13.5 | 27 | 23 | 18 | 12 | 9 | 7 | 6 |
| Peten | 13 | 10.0, 16.1 | 35 | 24 | 19 | 13 | 10 | 7 | 6 |
predicted percentile NTD risk calculated with RBC folate concentration raw data
FIGURE 1.
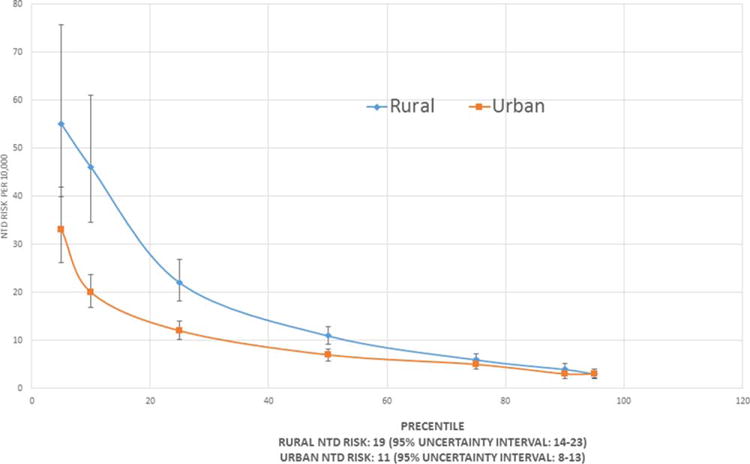
Distribution of estimated risk of neural tube defects (NTDs) of red blood cell folate concentrations for nonpregnant women of child-bearing age in urban and rural areas in Guatemala. ENMI-CRON 2009–2010.
Discussion
This is the first study that has applied the new WHO guidelines to population-based data to assess folate insufficiency prevalence and predict NTD risk outside the United States and in a low or middle income country. Our study indicates that during 2009 to 2010, folate insufficiency was a countrywide public health problem in Guatemala, with an overall prevalence of 47.2%. The study revealed major regional disparities, with seven of eight regions having high prevalence of folate insufficiency ranging from 46.8 to 80.9%. The results clearly show that folate insufficiency is widespread in rural, lower socioeconomic status, and indigenous populations. The Metropolitana region had the lowest folate insufficiency prevalence (18.8%), similar to the overall level in the United States of 22.8% (Tinker et al., 2015), indicating the success of folic acid fortification efforts in this region, that is, the population likely has access to fortified wheat flour.
In Guatemala, we found that the most vulnerable subpopulations were at highest risk of folate insufficiency. A woman’s area of residence (i.e., urban or rural), wealth index, and ethnicity were among the strongest predictors of whether she had folate insufficiency. These results were consistent with those reported by Imhoff-Kunsch et al. (2007) in which the effect of wheat flour fortification on folate status in Guatemala was examined by urbanicity, ethnicity, and socioeconomic status. The authors found that folic acid fortification contributed to the estimated average requirement for women living in rural and urban areas by 15% and 78%, respectively, and for the indigenous and nonindigenous populations by 10% and 57%, respectively. These results, as well as ours, suggest that vulnerable populations have limited access to fortified staples, likely due to lack of availability (e.g., in rural areas) and/or lack of means to purchase fortified staples (e.g., those in poverty or extreme poverty) (Popkin and Haines, 1981; Imhoff-Kunsch et al., 2007; Frohlic and Potvin, 2008; Iannotti et al., 2012).
In addition, we found that ethnicity was associated strongly with folate insufficiency in Guatemala. Two main ethnic groups account for most of the population: the indigenous people, who are descendants of Mayan and other indigenous groups and who maintain their own cultural identity and language, and Ladinos, who are of mixed indigenous and European origins and who speak Spanish. In Guatemala, ethnicity is closely tied to economic status; the indigenous population is, in general, found in the lowest wealth levels, while Ladinos are members of all economic classes (World Bank, 2009). Ethnic identities in Guatemala also may be associated with health beliefs and dietary practices that can influence whether intake of fortified staples is sought or accepted (Cosminsky, 1987; Scrimshaw and Hurtado, 1987; Leslie, 1991). Indigenous populations have a corn-based diet (tortillas), and because they grow their own corn or buy corn to process locally into corn masa flour, they are less likely to purchase fortified products. Furthermore, because women typically make the food and nutrition decisions for the household (Cosminsky, 1987), women’s acceptance of fortified foods would affect whether or not these foods are consumed. To our knowledge, no studies have researched these issues in Guatemala.
Comparisons of NTD predicted risk in the present study with other observed NTD prevalence at prefortification and postfortification periods are important because they can provide guidance on how populations or subgroups of populations are standing in regard to NTD risk. Within-country comparisons showed that our postfortification NTD predicted risk results indicated a national NTD predicted risk of 14 (95% UI, 11–19) per 10,000 live births compared to prefortification NTD prevalence of 23 per 10,000 (Chua Lopez, 2006; Acevedo et al., 2004). However, our study results showed higher NTD prevalence risk than prefortification NTD prevalence in the Norte region (26 per 10,000 vs. 23 per 10,000); Nor-Oriente (17 vs. 14 per 10,000); Sur-Oriente (16 vs. 6 per 10,000); and lower risk in the Metropolitana region (11 vs. 16 per 10,000); Central (17 vs. 19 per 10,000); Sur-Occidente (17 vs. 28 per 10,000); Nor-Occidente (11 vs. 27 per 10,000); and Petén (13 vs. 14 per 10,000) regions (Salguero Garcia et al., 2009; Chua Lopez, 2006). It is clear that the population sub-groups that bear the highest burden are among the rural, indigenous, and poorest groups.
In countries that have implemented successful fortification programs (e.g., Chile, Costa Rica, and the United States), the NTD prevalence ranges from 5.4 to 8.3 per 10,000 live births (Hertrampf and Cortes, 2008; Arguello and Umaña, 2011; Williams et al., 2015). Our results suggest that, although mandatory fortification has been successful in high- and middle-income countries where a very large proportion of the population has access to industrially produced fortified products, mandatory fortification has been only partially successful in low- and middle-income countries where vulnerable populations may have limited access to fortified foods. In such countries there is a need for studies of food preferences and availability (or daily consumption data) among vulnerable populations. These data would provide information to help identify population at risk and appropriate foods to consider for fortification.
LIMITATIONS
In accordance with the WHO recommendations, we determined folate insufficiency using population-based RBC folate concentrations measured with the microbiologic assay (ENMICRON 2009–2010 for women ages 15–49 years). Because the 906 nmol/L threshold was derived using a different calibrator for the microbiologic assay, we adjusted the threshold to 748 nmol/L in accordance with previous analyses (Tinker et al., 2015). While this conversion is based on >2600 paired samples (r = 0.92), the uncertainly associated with the conversion cannot be accounted for in the analysis. Also, we were able to model NTD risk based on RBC folate concentrations but we were not able to model the relationship between folic acid intake and RBC folate concentrations because we did not collect intake information.
In addition, the relationship between NTD risk and RBC folate concentrations is based on a study of pregnant women, and we are assuming that Guatemala women will behave in a similar way and are not directly linked to pregnancy outcomes as reported by Daly et al., 1995. Finally, at this time there are no population-based estimates of NTD prevalence in Guatemala to compare with the folate-insufficiency based NTD estimates. Predicted NTD risk estimates are not a substitute for actual NTD prevalence. However, there are two hospital-based studies that estimated NTD prevalence. Ortiz and Kestler (2006) estimated the prevalence to be 20 per 10,000 live births, and Salguero-García et al. (2009) estimated the prevalence to be 19 per 10,000 live births, estimates that are consistent with the NTD range predicted by the population-based RBC folate concentrations among the WCBA.
There were several strengths to our study. In particular, the ENMICRON survey is a nationally and regionally representative sample of the Guatemalan WCBA with low non-response rate and extensive information on different population characteristics.
PUBLIC HEALTH IMPLICATIONS
This is the first time the new WHO guideline has been applied outside the United States. This analysis shows the utility of RBC folate concentrations as part of biomarker surveys to assess the potential and realized impact of a folic acid fortification programs. RBC folate concentrations clearly show that among subpopulations that are most likely to have fortified staples, the prevalence of folate insufficiency is similar to countries with well-established fortification programs. However, folate insufficiency remains common among the poor, rural and indigenous subpopulations. At the time of the study, almost 50% of WCBA in Guatemala had blood folate concentrations that were less than the recommended WHO threshold for optimal NTD prevention. In particular, women in the Norte region are predicted to be at the greatest risk of folate sensitive NTDs, with 80.9% being folate insufficient.
Additional preventions efforts should target this high risk region to increase folic acid intake and RBC folate concentrations among WCBA. New efforts should also attempt to reach women with low household incomes and indigenous food consumption patterns. Finally, given the high prevalence of folate insufficiency among WCBA in Guatemala and the strong predicted risk of NTDs, future efforts should include population-based surveillance to monitor trends in NTD prevalence and follow-up biomarker surveys to confirm that programs are reaching the overall population at risk.
Supplementary Material
Acknowledgments
We thank Dr. C. Pfeiffer for the blood sample testing and comments to this manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention
Supported by partial funding from the Centers for Disease Control and Prevention, Fundacioen de Alimentacioen y Nutricioen de Centro America y Panama (FANCAP), Inter-American Development Bank (Public Regional Goods), and Guatemala Ministerio de Salud Pública y Asistencia Pública.
Footnotes
Additional Supporting information may be found in the online version of this article.
References
- Acevedo CR, Alvarez SY, Anzueto ER, et al. Prevalencia de Anomalias Congenitas Mayores Externas de Recien Nacidos en Hospitales Nacionales y Regionales de Guatemala 2001–2003. Guatemala City: Universidad de San Carlos de Guatemala, Facultad de Ciencias Medicas; 2004. [Google Scholar]
- Arguello MP, Umaña L. Impacto de la fortificación de alimentos con ácido fólico en los defectos del tubo neural en Costa Rica. Rev Panam Salud Pública. 2011;30:1–6. [PubMed] [Google Scholar]
- Berry RJ, Erickson JD, Li S, et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N Engl J Med. 1999;341:1485–1490. doi: 10.1056/NEJM199911113412001. [DOI] [PubMed] [Google Scholar]
- Bieler GS, Brown GG, Williams RL, Brogan DJ. Estimating model-adjusted risks, risk differences, and risk ratios from complex survey data. Am J Epidemiol. 2010;171:618–623. doi: 10.1093/aje/kwp440. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR. 1992;41:1–7. [PubMed] [Google Scholar]
- Chua Lopez CA. Anomalias del tubo neural en Guatemala. Editorial Universitaria de la Universidad de San Carlos; Guatemala City, Guatemala: 2006. [Google Scholar]
- Christianson A, Howson CP, Modell B. The hidden toll of dying and disabled children. March of Dimes Birth Defects Foundation; White Plains, New York: 2006. Global report on birth defects. [Google Scholar]
- Cordero AM, Crider KS, Rogers LM, et al. Optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects: World Health Organization guidelines. MMWR Morb Mortal Wkly Rep. 2015;64:421–423. [PMC free article] [PubMed] [Google Scholar]
- Cosminsky S. Women and Health Care on a Guatemalan Plantation. Social Science and Medicine. 1987;25:1163–1173. doi: 10.1016/0277-9536(87)90358-3. [DOI] [PubMed] [Google Scholar]
- Crider KS, Devine O, Hao L, et al. Population red blood cell folate concentrations for prevention of neural tube defects: Bayesian model. BMJ. 2014;349:g4554. doi: 10.1136/bmj.g4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- Daly LE, Kirke PN, Molloy A, et al. Folate levels and neural tube defects. Implications for prevention. JAMA. 1995;274:1698–1702. doi: 10.1001/jama.1995.03530210052030. [DOI] [PubMed] [Google Scholar]
- David JL. Wheat flour fortification in Latin America and the Caribbean Region. Rev Chil Nutr. 2004;31:336–347. [Google Scholar]
- De Wals P, Tairou F, Van Allen MI, et al. Spina bifida before and after folic acid fortification in Canada. Birth Defects Res A Clin Mol Teratol. 2008;82:622–626. doi: 10.1002/bdra.20485. [DOI] [PubMed] [Google Scholar]
- Frohlich KL, Potvin L. Transcending the known in public health practice. Am J Pub Hlth. 2008;98:216–221. doi: 10.2105/AJPH.2007.114777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertrampf E, Cortes F. National food fortification program with folic acid in Chile. Food Nutr Bull. 2008;29:S231–S237. doi: 10.1177/15648265080292S128. [DOI] [PubMed] [Google Scholar]
- Iannotti LL, Robles M, Pachon H, Chiarella C. Food prices and poverty negatively affect micronutrient intakes in Guatemala. J Nutr. 2012;142:1568–1576. doi: 10.3945/jn.111.157321. [DOI] [PubMed] [Google Scholar]
- Imhoff-Kunsch B, Flores R, Dary O, Martorell R. Wheat flour fortification is unlikely to benefit the neediest in Guatemala. J Nutr. 2007;137:1017–1022. doi: 10.1093/jn/137.4.1017. [DOI] [PubMed] [Google Scholar]
- Leslie J. Women’s nutrition: the key to improving family health in developing countries? Health Planning. 1991;6:1–19. [Google Scholar]
- MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- O’Broin SD, Kelleher BP, Davoren A, Gunter EW. Field-study screening of blood folate concentrations: specimen stability and finger-stick sampling. Am J Clin Nutr. 1997;66:1398–1405. doi: 10.1093/ajcn/66.6.1398. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Kestler E. Defectos del tubo neural en el Departamento de Guatemala. Revista del Colegio de Médicos y Cirujanos de Guatemala, Epoca IV. 2006;16:29–34. [Google Scholar]
- Pfeiffer CM, Zhang M, Lacher DA, et al. Comparison of serum and red blood cell folate microbiologic assays for national population surveys. J Nutr. 2011;141:1402–1409. doi: 10.3945/jn.111.141515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkin BM, Haines PS. Factors affecting food selection: the role of economics. J AM Diet Assoc. 1981;79:419–425. [PubMed] [Google Scholar]
- Rosenthal J, Casas J, Taren D, et al. Neural tube defects in Latin America and the impact of fortification: a literature review. Pub Hlth Nutr. 2013;17:537–550. doi: 10.1017/S1368980013000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal J, Lopez-Pazos E, Dowling NF. Folate and vitamin B12 deficiency among non-pregnant women of childbearing-age in Guatemala 2009–2010: prevalence and disparities. Mat Child Health J. 2015;19:2272–2285. doi: 10.1007/s10995-015-1746-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salguero-García EJ, Barrios-Ruiz AP, Cardona de León VK, et al. Estudio descriptivo del número de casos de recién nacidos con defectos del tubo neural atendidos en hospitales nacionales y maternidades periféricas del Ministerio de Salud Pública y Asistencia Social del departamento de Guatemala durante el perıodo de 2004–2008. Guatemala City: Universidad de San Carlos; 2009. Impacto de la norma de suplementación de ácido folicócon relación a casos de defectos del tubo neuralén recién nacidos. [Google Scholar]
- Santos CA, Fiaccone RL, Oliveira NF, et al. Estimating adjusted prevalence ratio in clustered cross-sectional epidemiological data. MMC Medical Research Methodology. 2008;8:80–89. doi: 10.1186/1471-2288-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrimshaw SCM, Hurtado E. Rapid assessment procedures for nutrition and primary health care: anthropological approaches to improving programme effectiveness. Los Angeles: UCLA Latin American Center; 1987. [Google Scholar]
- SUDAAN statistical software (version 9.3) Research triang le Institute; Research Triangle Park, NC: 2012. [Google Scholar]
- Tinker SC, Hammer HC, Qi YQ, et al. U.S. women of childbearing age who are at possible increased risk of a neural tube defect-affected pregnancy due to suboptimal red blood cell folate concentrations, National Health and Nutrition Examination Survey 2007 to 2012. Birth Defects Res A Clin Mol Teratol. 2015;103:517–526. doi: 10.1002/bdra.23378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J, Mai CT, Mulinare J, et al. Updated estimates of neural tube defects prevented by mandatory folic Acid fortification - United States, 1995–2011. MMWR Morb Mortal Wkly Rep. 2015;64:1–5. [PMC free article] [PubMed] [Google Scholar]
- WHO. Guideline. Optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- Walter KM. Introduction to variance estimation. 2nd. New York: Springer; 2007. Taylor series methods; pp. 226–271. [Google Scholar]
- World Bank. Guatemala - Evaluación de la pobreza. Washington DC: The World Bank; 2009. Available at http://documents.worldbank.org/curated/en/2009/03/10792680/guatemala-poverty-assessment-good-performance-low-levels-guatemala-evaluacion-de-la-pobreza-buen-desempeno-bajo-nivel. Accessed November 10, 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


