Abstract
Coronary atherosclerotic disease is highly prevalent in chronic kidney disease (CKD). Although revascularization improves outcomes, procedural risks are increased in CKD and unbiased data comparing bypass surgery (CABG) and percutaneous intervention (PCI) in CKD are sparse. To compare outcomes of CABG and PCI in stage 3-5 CKD, we identified randomized trials comparing these procedures. Investigators were contacted to obtain individual, patient-level data. Ten of 27 trials meeting inclusion criteria provided data. These trials enrolled 3993 patients encompassing 526 patients with stage 3-5 CKD of which 137 were stage 3b-5 CKD. Among individuals with stage 3-5 CKD survival through 5-years was not different following CABG compared with PCI (hazard ratio 0.99, 95% confidence interval: 0.67, 1.46) or stage 3b-5 CKD (1.29: 0.68, 2.46). However, CKD modified the impact on survival free from myocardial infarction: it was not different between CABG and PCI for individuals with preserved kidney function (0.97: 0.80, 1.17), but was significantly lower following CABG in stage 3-5 CKD (0.49: 0.29, 0.82) and stage 3b-5 CKD (0.23: 0.09, 0.58). Repeat revascularization was reduced following CABG compared with PCI regardless of baseline kidney function. Results were limited by unavailability of data from several trials and paucity of enrolled patients with stage 4-5 CKD. Thus, our patient-level meta-analysis of individuals with CKD randomized to CABG versus PCI suggests that CABG significantly reduces the risk of subsequent myocardial infarction and revascularization without impacting survival in these patients.
Keywords: Coronary artery disease, Chronic kidney disease, Myocardial infarction, Coronary revascularization
Introduction
More than 10% of the adult U.S. population have chronic kidney disease (CKD)1, which is associated with increased cardiovascular morbidity and mortality2, 3. Standard cardiovascular therapies have the potential to decrease morbidity and mortality, but utilization of established cardiovascular therapies including coronary angiography and revascularization procedures has remained lower in individuals with CKD than in patients with relatively preserved kidney function.4, 5
Although this selective underutilization of coronary revascularization in a population at high cardiovascular risk (“renalism”5) could represent inappropriate therapeutic nihilism, recent trials have failed to demonstrate efficacy of standard medical therapies in patients on dialysis6, 7 while the majority of large cardiovascular trials have excluded individuals with CKD raising important questions about the efficacy or safety of other accepted cardiovascular therapies in this population. Indeed, patients with CKD experience higher perioperative mortality8, 9 following coronary artery bypass grafting (CABG), are at higher risk of acute kidney injury following CABG surgery or percutaneous coronary intervention (PCI)10, 11, and have generally much higher overall mortality12, 13 compared with the subjects enrolled in landmark trials comparing CABG and PCI, in whom advanced kidney dysfunction was uncommon8. Therefore, a dedicated, CKD-specific comparison of the risks and benefits of PCI and CABG is needed to define the optimal role for each therapy in the setting of impaired kidney function.
Although several retrospective comparisons of PCI and CABG among individuals with CKD undergoing coronary revascularization for clinical indications have generally favored CABG14-16, the potential for indication bias and residual confounding remains an important concern with non-randomized studies in this area. To provide highest-level evidence, we conducted a systematic review of the literature and, subsequently, a detailed, individual-level meta-analysis of patients with moderate to severe CKD from published randomized trials comparing CABG and PCI.
Results
Study Identification and Characteristics
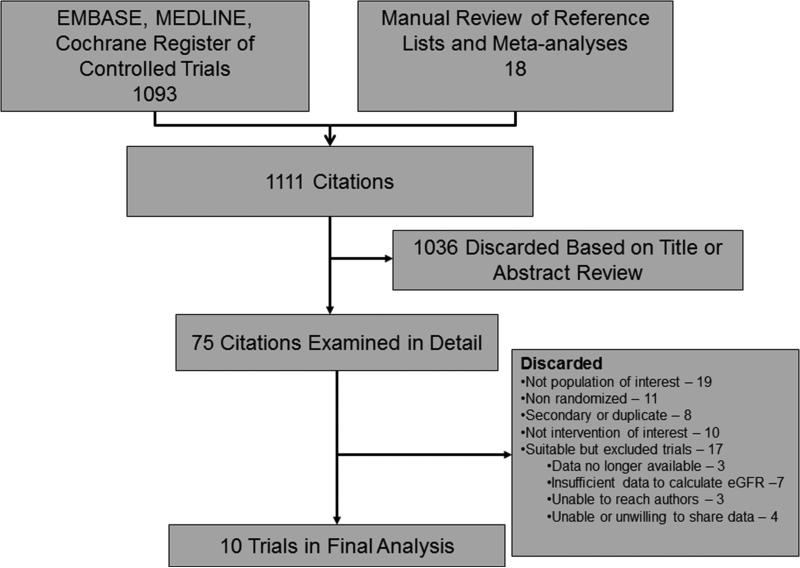
Our pre-specified literature search identified 1111 citations (Figure 1). After title and abstract review, 75 citations were examined in detail; however, 48 were excluded because they failed to meet the specified inclusion criteria. A total of 27 eligible trials were identified for inclusion, but 17 had to be excluded for the following reasons: data no longer available (n=3)17-19; insufficient data to calculate eGFR (n=7)20-26unable to contact the investigators despite multiple attempts (n=3)27-29; investigators unable (n=2)30, 31 or unwilling (n=2)32, 33 to share data.
Figure 1.
Flow diagram of study selection
The remaining 10 trials comprised the analytical dataset and included the following trials: AMIST34; Bypass Angioplasty Revascularization Investigators Trial (BARI)35; Cisowski et al. 36; Argentine Randomized Study: Coronary Angioplasty with Stenting versus Coronary Bypass Surgery in Multivessel Disease (ERACI II)37; German Angioplasty Bypass Surgery Investigation (GABI)38; Left Main Stenting (Le MANS)39; Leipzig40; Medicine, Angioplasty or Surgery Study (MASS 1)41; Medicine, Angioplasty or Surgery II Study (MASS 2)42; and Veterans Affairs Cooperative Study #385, the Angina With Extremely Serious Operative Mortality Evaluation (VA [AWESOME])43.
All studies used central and concealed randomization and intention to treat analyses of outcomes. However, in 2 studies, outcomes assessors were not blinded to treatment assignment.34, 36 Loss to follow-up was generally low, but exceeded 10% in 2 studies34, 38 (Table 1).
Table 1.
Trial characteristics
| Characteristic | AMIST | BARI | Cisowski | ERACI II | GABI | Le MANS | Leipzig | MASS 1 | MASS 2 | VA |
|---|---|---|---|---|---|---|---|---|---|---|
| Central randomization | + | + | - | + | + | + | + | + | + | + |
| Concealed randomization | + | + | + | + | + | + | + | + | + | + |
| Blinded outcomes assessment | - | + | - | + | + | + | + | + | + | - |
| Intention to treat analysis | + | + | + | + | + | + | + | + | + | + |
| Stents used | +* | + | +* | + | - | +* | +* | - | + | + |
| Off-pump bypass | +* | - | +* | - | - | + | +* | - | - | NR |
| LIMA | +* | + | +* | + | + | + | +* | +* | + | + |
| Enrollment Period | 1999-2001 | 1988-1991 | 2000-2002 | 1996-1998 | 1986-1991 | 1997-2008 | 1997-2001 | 1988-1991 | 1995-2000 | 1995-2000 |
| Single vessel disease only | + | - | + | - | - | - | +* | +* | - | - |
| Multi-vessel disease only | - | +* | - | +* | +* | - | - | - | +* | - |
| Single or multi-vessel disease | - | - | - | - | - | + | - | - | - | + |
| Left main disease | - | - | - | + | - | +* | - | - | - | - |
NR-not recorded. LIMA-left internal mammary artery.
Required by protocol.
The majority of trials completed enrollment between 1991 and 2001 with exception of a single trial that completed enrollment in 200236 and the Le Mans trial, which enrolled subjects from 1997-200839. As shown in Table 1, stents were utilized in all but 2 studies38, 41, and off-pump bypass techniques were available for CABG patients in 5 studies34, 36, 39-41. Four studies required multi-vessel disease for inclusion35, 37, 38, 42 while 4 excluded individuals with multi-vessel coronary disease34, 36, 40, 41. One study (AMIST)34 did not collect data on at least one covariate leading to systematic missingness. Eligible studies for which we were unable to obtain data were qualitatively similar to included studies in terms of sample size, year enrolled, revascularization technique, inclusion criteria and the range of relative risks of study outcomes following PCI compared with CABG (Supplementary Tables 4 & 5).
Baseline Characteristics of Study Subjects
The study cohort included 3993 randomized subjects (CABG: 1994, PCI: 1999,) with 17,131 person-years (PY) of post-intervention follow-up time (post-CABG: 8528 PY, post-PCI: 8603 PY). There were 526 individuals with stage 3 or worse CKD with 1856 PY of follow-up (CABG: 892 PY, PCI: 964 PY), and 137 with stage 3b or worse CKD (20 with stage 4-5 CKD) with 402 PY of follow-up (CABG: 195 PY, PCI: 207 PY). There were 7 individuals with stage 5 CKD. Baseline characteristics of the enrolled patients and those with CKD are shown in Tables 2 and 3. Individuals with and without CKD were mostly similar, but those with CKD tended be older and a higher percentage of those with CKD were female.
Table 2.
Baseline characteristics of trial subjects
| Characteristic N (%) | AMIST | BARI | Cisowski | ERACI II | GABI | Le MANS | Leipzig | MASS 1 | MASS 2 | VA |
|---|---|---|---|---|---|---|---|---|---|---|
| Year published | 2004 | 1996 | 2002 | 2001 | 1994 | 2008 | 2005 | 1999 | 2002 | 2001 |
| No. of subjects | 89 | 1829 | 76 | 450 | 313 | 82 | 220 | 141 | 408 | 385 |
| Age, years mean (SD) | 57.7 (9.6) | 61.0 (9.4) | 53.4 (10.0) | 60.7 (10.3) | 58.9 (7.9) | 61.0 (9.8) | 62.0 (10.1) | 56.5 (10.1) | 59.7 (9.0) | 67.4 (9.2) |
| Male | 71 (79.8) | 1340 (73.3) | 63 (82.9) | 357 (79.3) | 248 (79.2) | 56 (68.3) | 164 (74.6) | 104 (73.8) | 283 (69.4) | 381 (99.0) |
| White | 87 (97.8) | 1653 (90.4) | 76 (100.0) | 450 (100.0) | 313 (100.0) | 81 (98.8) | 220 (100.0) | 137 (97.2) | 352 (86.3) | 339 (88.1) |
| Stage 3-5 CKD | 18 (20.2) | 43 (2.4) | 11 (14.5) | 111 (24.7) | 51 (16.3) | 16 (19.5) | 30 (13.6) | 25 (17.7) | 83 (20.3) | 138 (35.8) |
| Stage 3b-5 CKD | 4 (4.5) | 23 (1.3) | 4 (5.3) | 14 (3.1) | 11 (3.5) | 5 (6.1) | 4 (1.8) | 4 (2.8) | 18 (4.4) | 50 (13.0) |
| Diabetes | -- | 353 (19.3) | 6 (7.9) | 78 (17.3) | 39 (12.6) | 14 (17.1) | 63 (29.6) | 30 (21.3) | 115 (28.2) | 125 (32.6) |
| Smoking | -- | 463 (25.3) | 39 (51.3) | 233 (51.8) | 35 (11.8) | 6 (9.0) | 54 (25.4) | 56 (39.8) | 123 (30.2) | 96 (34.0) |
| Hypertension | -- | 896 (49.0) | 42 (55.3) | 318 (70.7) | 130 (41.8) | 76 (92.7) | 152 (71.4) | 43 (30.5) | 253 (62.0) | 267 (69.5) |
| Hyperlipidemia | -- | 725 (44.0) | 59 (77.6) | 275 (61.1) | 193 (62.7) | 80 (97.6) | 152 (71.4) | 108 (76.6) | 322 (78.9) | -- |
| Prior revascularization | -- | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (7.3) | 0 (0.0) | 0 (0.0.) | 0 (0.0) | 164 (42.7) |
| Prior MI | -- | 987 (54.5) | 9 (11.8) | 126 (28.0) | 143 (46.3) | 31 (37.8) | 99 (46.5) | 0 (0.0) | 191 (46.8) | 275 (72.0) |
| CHF | -- | 161 (8.9) | 0 (0.0 | 16 (3.6) | 151 (48.7) | 1 (1.2) | -- | 0 (100.0) | 2 (0.5) | 294 (86.5) |
| Multi-vessel disease | 0 (0.0) | 1796 (98.4) | 0 (0.0) | 450 (100.0) | 313 (100.0) | 78 (95.1) | 0 (0.0) | 0 (0.0) | 408 (100.0) | 316 (82.1) |
| Left main disease | 0 (0.0) | 0 (0.0) | 0 (0.0) | 21 (4.7) | 0 (0.0) | 80 (97.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 28 (7.5) |
| Proximal LAD disease | 89 (100.0) | 1081 (59.2) | 76 (100.0) | 230 (51.1) | 90 (28.9) | 28 (34.2) | 220 (100.0) | 141 (100.0) | 381 (93.4) | 221 (58.9) |
| No. diseased vessels | 1 (0.0) | 2.4 (0.5) | 1 (0.0) | 2.4 (0.5) | 2.4 (0.5) | 3.0 (1.0) | 1.2 (0.4) | 1 (0.0) | 2.6 (0.5) | 2.3 (0.8) |
| Ejection Fraction, %, mean (SD) | 66.6 (10.6) | 57.4 (11.0) | 56.9 (4.9) | 52.9 (5.6) | 63.8 (10.6) | 54.3 (8.6) | 62.4(13.1) | 69.2 (3.2) | 67.3 (8.0) | 46.0 (14.7) |
| Elevated cardiac biomarkers on admission | -- | -- | 0 (0.0) | 87 (19.3) | 0 (0.0) | 6 (7.3) | 0 (0.0) | -- | 0 (0.0) | -- |
| MI on admission | 0 (0.0) | 58 (3.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (7.4) | 0 (0.0) | 0 (0.0) | 191 (46.8) | 134 (34.8) |
| Unstable angina on admission | 18 (21.2) | 1192 (65.2) | 8 (10.5) | 412 (91.6) | 40 (13.5) | 45 (54.9) | 37 (16.8) | 0 (0.0) | 0 (0.0) | -- |
Table 1-Baseline characteristics. Data are presented as n (%) unless otherwise noted. CKD-chronic kidney disease, CHF-congestive heart failure, LAD-left anterior descending artery, MI-myocardial infarction, No-number, SD-standard deviation. Data on diabetes was not available for subjects in AMIST and was missing for 3 subjects in GABI, and 2 subjects in the VA study. Data on smoking was not available in AMIST, and was missing in 181 subjects from BARI, for 2 subject from GABI, 15 in LE MANS, 7 in Leipzig, and 103 subjects in the VA study. Hyperlipidemia was missing all AMIST subjects, 181 in BARI, 5 in GABI, 7 in Leipzig and all VA subjects. Data on baseline hypertension was unavailable in AMIST and was missing for 2 subjects from BARI, 2 in GABI, 7 in Leipzig and 1 in the VA study. Prior MI was unavailable for AMIST, and was missing in 18 subjects from BARI, 6 subjects from GABI, 7 in Leipzig, and 3 in the VA study. CHF was not available for AMIST participants and Leipzig and was missing in 10 subjects in BARI, 3 from GABI, and 45 subjects from the VA study. Prior revascularization was unavailable for AMIST and was missing 1 subject in the VA study. Multi-vessel disease was missing in 3 subjects from BARI. Left main disease was missing in 10 subjects from the VA study. Proximal LAD was missing in 3 subjects in BARI, 2 in GABI, and 10 in the VA study. Data on number of diseased vessels was missing in 7 subjects from GABI, 3 subjects from BARI, and 6 subjects from the VA study. Ejection fraction was missing in 55 subjects in AMIST, 475 in BARI 149 in GABI, 3 in Le MANS and 98 in the VA study. Cardiac biomarkers at baseline were not available for AMIST, BARI, VA, and MASS 1 studies. MI on admission was missing in 1 subject from Le MANS. Unstable angina at admission was missing in 4 subjects from AMIST, 17 from GABI, and all subjects in the VA study.
Table 3.
Baseline characteristics of trial subjects with chronic kidney disease
| Characteristic | AMIST (n=18) | BARI (n=43) | Cisowski (n=11) | ERACI II (n=111) | GABI (n=51) | Le MANS (n=16) | Leipzig (n=30) | MASS 1 (n=25) | MASS 2 (n=83) | VA (n=138) |
|---|---|---|---|---|---|---|---|---|---|---|
| Age, years mean (SD) | 64.3 (8.9) | 63.9.(8.8) | 62.3 (10.0) | 64.6 (9.4) | 64.3 (7.0) | 68.0 (8.3) | 68.7 (7.2) | 61.8 (9.4) | 66.2 (6.5) | 72.2 (6.6) |
| Male | 11 (61.1) | 31 (72.1) | 7 (63.6) | 44 (39.6) | 26 (51.0) | 7 (43.8) | 16 (53.3) | 8 (32.0) | 3 (63.9) | 136 (98.6) |
| White | 18 (100.0) | 33 (76.7) | 11 (100.0) | 111 (100.0) | 51 (100.0) | 16 (100.0) | 30 (100.0) | 25 (100.0) | 76 (91.6) | 122 (88.4) |
| Stage 3b-5 CKD | 4 (22.2) | 23 (53.5) | 4 (36.4) | 14 (12.6) | 11 (21.6) | 5 (31.3) | 4 (13.3) | 4 (16.0) | 18 (21.7) | 50 (36.2) |
| Stage 4-5 CKD, | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.9) | 4 (7.6) | 1 (6.3) | 0 (0.0) | 0 (0.0) | 4 (4.8) | 10 (7.0) |
| eGFR mL/min/1.73m2, mean (SD) | 49.8 (8.1) | 43.0 (6.7) | 48.3 (8.1) | 50.1 (6.6) | 49.7 (11.4) | 46.9 (8.4) | 53.1 (6.5) | 52.0 (6.7) | 49.0 (10.4) | 46.9 (10.6) |
| Diabetes | -- | 20 (46.5) | 3 (27.3) | 22 (19.8) | 8 (15.7) | 6 (37.5) | 15 (5.7) | 5 (20.0) | 25 (30.1) | 40 (29.0) |
| Smoking | -- | 7 (16.3) | 3 (27.3) | 50 (45.1) | 3 (5.9) | 1 (6.7) | 4 (13.8) | 7 (28.0) | 14 (16.9) | 20 (20.2) |
| Hypertension | -- | 33 (76.7) | 8 (72.7) | 91 (82.0) | 32 (62.8) | 16 (93.8) | 28 (96.6) | 7 (28.0) | 58 (69.9) | 113 (81.9) |
| Hyperlipidemia | -- | 18 (48.7) | 5 (45.5) | 72 (64.9) | 36 (70.6) | 16 (100.) | 18 (62.1) | 19 (76.0) | 61 (73.5) | -- |
| Prior revascularization | -- | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 49 (35.5) |
| Prior MI | -- | 24 (57.1) | 2 (18.2) | 31 (27.9) | 27 (54.0) | 11 (68.8) | 17 (58.6) | 0 (0.0) | 38 (45.8) | 93 (68.4) |
| CHF | -- | 14 (32.6) | 0 (0.0) | 2 (1.8) | 30 (58.8) | 0 (0.0) | -- | 0 (0.0) | 2 (2.4) | 108 (88.5) |
| Multi-vessel disease | 0 (0.0) | 42 (97.7) | 0 (100.0) | 111 (100.0) | 51 (100.0) | 16 (100.0) | 30 (0.0) | 0 (0.0) | 83 (100.0) | 116 (84.1) |
| Left main disease | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (4.5) | 0 (0.0) | 16 (100.0) | 0 (0.0) | 0 (0.0) | 0 (.0.0) | 12 (8.9) |
| Proximal LAD disease | 18 (100.0) | 27 (62.8) | 11 (100.0) | 59 (53.2) | 14 (27.5) | 8 (50.0) | 30 (100.0) | 25 (100.0) | 78 (94.0) | 69 (51.1) |
| No. diseased vessels, mean (SD) | 1 (0.0) | 2.6 (0.5) | 1 (0.0) | 2.6 (0.5) | 2.4 (0.5) | 3.3 (0.8) | 1.2 (0.4) | 1 (0.0) | 2.6 (0.5) | 2.3 (0.8) |
| Ejection Fraction, %, mean (SD) | 61.3 (14.1) | 54.5 (12.5) | 58.1 (5.9) | 52.8 (5.3) | 64.1 (11.1) | 54.1 (5.0) | 62.6 (13.5) | 69.3 (3.2) | 67.5 (9.2) | 45.4(14.7) |
| Elevated cardiac biomarkers on admission | -- | -- | 0 (0.0) | 29 (26.1) | 0 (0.0) | 1 (6.3) | 0 (0.0) | -- | 0 (0.0) | -- |
| MI on admission | 0 (0.0) | 1 (2.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (6.3) | 0 (0.0) | 0 (0.0) | 38 (45.8) | 40 (29.0) |
| Unstable angina on admission | 3 (16.7) | 32 (74.4) | 1 (9.1) | 104 (93.7) | 10 (20.0) | 11 (68.8) | 6 (20.0) | 0 (0.0) | 0 (0.0) | -- |
Table 1-Baseline characteristics. Data are presented as n (%) unless otherwise noted. eGFR estimates for BARI were calculated as described in the methods. CKD-chronic kidney disease, CHF-congestive heart failure, LAD-left anterior descending artery, MI-myocardial infarction, No-number, SD-standard deviation. Data on diabetes was not available for subjects in AMIST. Data on smoking was not available in AMIST, and was missing for 1 subject in LE MANS, and 39 subjects in the VA study. Hyperlipidemia was missing all AMIST subjects, 6 in BARI, 1 in Leipzig, and all VA subjects. Data on baseline hypertension was unavailable in AMIST and was missing for 1 subject from Leipzig. Prior revascularization was unavailable for AMIST. Prior MI was unavailable for AMIST, and was missing in 1 subject from BARI, 1 subject from GABI, 1 in Leipzig, and 2 in the VA study. CHF was not available for AMIST participants and was missing in 16 subjects from the VA study. Data on left main disease was missing in 3 VA subjects. Proximal LAD was missing in 3 subjects in the VA study. Data on number of diseased vessels was missing in 3 subjects from the VA study. Ejection fraction was missing in 10 subjects in AMIST, 23 in GABI, 1 in Le MANS and 33 in the VA study. Cardiac biomarkers at baseline were not available for AMIST, BARI, VA, and MASS 1 studies. Unstable angina at admission was missing in 1 subject from GABI, and all subjects in the VA study.
Survival
All-cause mortality rates were similar following CABG or PCI, and were higher among individuals with CKD (CABG: 5.6/100 PY, PCI: 5.5/100 PY) compared to those with preserved kidney function (CABG: 2.1/100 PY, PCI: 2.3/100 PY).
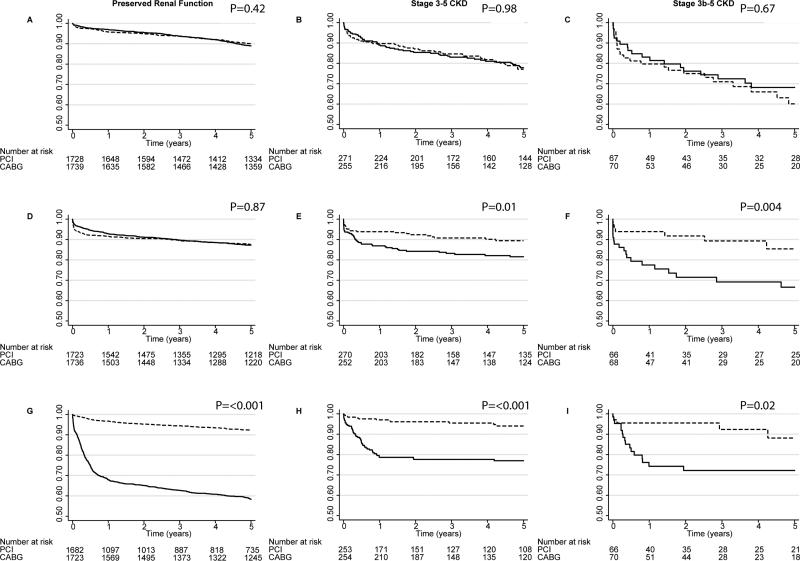
In primary multiple imputation-based analysis adjusted for all covariates of interest, mortality did not differ between patients randomized to CABG versus PCI among individuals with relatively preserved kidney function (HR 0.90, 95% CI: 0.73, 1.11), those with stage 3-5 CKD (HR 0.99, 95% CI: 0.67, 1.46), those with stage 3a CKD (HR 0.79, 95% CI: 0.47, 1.33), or those with stage 3b-5 CKD (HR 1.29, 95% CI: 0.68, 2.46; Figure 2A-C). In the overall cohort, there was no significant evidence for effect modification by the presence of CKD (Pinteraction=0.52). Among individuals with CKD there was no significant effect modification on survival according to the presence of proximal left anterior descending artery stenosis (Pinteraction=0.88) or according to the presence or absence of multi-vessel disease (Pinteraction=0.13). Results were similar in crude and adjusted analyses (Table 4). For the subgroup with stage 3-5 CKD, the I2 statistic (0.0%) was consistent with minimal between-study heterogeneity.
Figure 2.
Actuarial freedom from death, MI, or revascularization after CABG and PCI by clinical subset
Event-Free Survival after CABG and PCI calculated using the Kaplan-Meier method. (A-C) Overall survival. (D-F) Freedom from myocardial infarction. (G-I) Freedom from repeat revascularization. Unadjusted (Cox) P values stratified by trial are provided. CABG-dashed lines. PCI-solid lines.
Table 4.
Mortality risk with CABG compared to PCI
| Group | Crude HR | 95% CI | P Value | Adjusted HR | 95% CI | P Value |
|---|---|---|---|---|---|---|
| Overall (n=3993) | 0.93 | 0.77, 1.12 | 0.43 | 0.92 | 0.76, 1.11 | 0.38 |
| Preserved kidney Function (n=3467) | 0.92 | 0.74, 1.13 | 0.42 | 0.90 | 0.73, 1.11 | 0.33 |
| Stage 3-5 CKD (n=526) | 1.01 | 0.68, 1.49 | 0.98 | 0.99 | 0.67, 1.46 | 0.96 |
| Stage 3a CKD (n=389) | 0.87 | 0.52, 1.45 | 0.60 | 0.79 | 0.47, 1.33 | 0.39 |
| Stage 3b-5CKD (n=137) | 1.15 | 0.62, 2.13 | 0.67 | 1.29 | 0.68, 2.46 | 0.43 |
| CKD with multi-vessel disease* (n=419) | 1.16 | 0.77, 1.75 | 0.49 | 1.10 | 0.73, 1.67 | 0.65 |
| CKD with single-vessel disease* (n=107) | 0.33 | 0.07, 1.61 | 0.17 | 0.32 | 0.06, 1.76 | 0.19 |
| CKD proximal LAD disease* (n=342) | 0.88 | 0.54, 1.43 | 0.61 | 0.94 | 0.57, 1.54 | 0.80 |
| CKD without proximal LAD disease* (n=185) | 1.31 | 0.67, 2.56 | 0.43 | 1.15 | 0.57, 2.27 | 0.71 |
All models were stratified by trial. Multivariable models adjusted for treatment, age, diabetes, prior myocardial infarction, proximal left anterior descending artery disease, ejection fraction <40%, prior revascularization, and multi-vessel.
To avoid model overspecification, these subgroup models did not include terms for multi-vessel disease or proximal LAD disease, respectively. CKD-chronic kidney disease. LAD-left anterior descending artery.
Short-term results at 1 year were qualitatively similar to 5-year outcomes. Adjusted risks of mortality did not differ 1 year after CABG compared with PCI among individuals with preserved kidney function (HR 1.35, 95% CI: 0.95, 1.93), those with stage 3-5 CKD (HR 0.92, 95% CI: 0.54, 1.58), those with stage 3a CKD (HR 0.73, 95% CI: 0.35-1.54), or those with stage 3b-5 CKD (HR 1.28, 95% CI: 0.56, 2.95).
Myocardial Infarction
Among individuals with CKD, non-fatal MI rates were higher after PCI (5.1/100 PY) than CABG (2.7/ 100 PY, P=0.01) whereas the rates were similar after PCI (2.9/100 PY) and CABG (2.9/100 PY, P=0.95) amongst individuals with preserved kidney function. Among individuals with CKD 13.2% died within 30 days of an MI compared with 7.3% among those with preserved renal function.
In primary analysis models, the risk of non-fatal MI among individuals with preserved kidney function (HR 0.97, 95% CI: 0.80, 1.17) did not differ between the two treatments, whereas MI risk among patients was lower following CABG compared with PCI in those with stage 3-5 CKD (HR 0.49, 95% CI: 0.29, 0.82) and stage 3a CKD (HR 0.70, 95% CI: 0.36, 1.39), and was even lower among those with stage 3b-5 CKD (HR 0.23, 95% CI: 0.09, 0.58). A significant test of interaction in analyses of the full cohort was consistent with effect modification by the presence versus absence of stage 3-5 CKD (Pinteraction=0.04). Among individuals with CKD, CABG provided similar benefits between individuals with and without multi-vessel disease (Pinteraction=0.13) and between those with versus without proximal LAD disease (Pinteraction=0.32). Results were qualitatively similar in crude and adjusted analyses (Table 5). For the subgroup with stage 3-5 CKD, the I2 statistic (0.0%) was consistent with minimal between-study heterogeneity.
Table 5.
Risk of myocardial infarction with CABG compared to PCI
| Group | Crude HR | 95% CI | P Value | Adjusted HR | 95% CI | P Value |
|---|---|---|---|---|---|---|
| Overall (n=3981) | 0.90 | 0.75, 1.07 | 0.23 | 0.88 | 0.73, 1.05 | 0.16 |
| Preserved kidney Function (n=3459) | 0.98 | 0.81, 1.19 | 0.87 | 0.97 | 0.80, 1.17 | 0.72 |
| Stage 3-5 CKD (n=522) | 0.49 | 0.30, 0.81 | 0.01 | 0.49 | 0.29, 0.82 | 0.01 |
| Stage 3a CKD (n=388) | 0.68 | 0.37, 1.27 | 0.24 | 0.71 | 0.36, 1.39 | 0.31 |
| Stage 3b-5CKD (n=134) | 0.27 | 0.11, 0.66 | 0.004 | 0.23 | 0.09, 0.58 | 0.002 |
| CKD with multi-vessel disease* (n=416) | 0.45 | 0.26, 0.79 | 0.01 | 0.43 | 0.24, 0.76 | 0.004 |
| CKD with single vessel disease* (n=106) | 0.71 | 0.20, 2.47 | 0.59 | 1.09 | 0.24, 4.86 | 0.91 |
| CKD proximal LAD disease* (n=338) | 0.39 | 0.19, 0.80 | 0.01 | 0.39 | 0.18, 0.82 | 0.01 |
| CKD without proximal LAD disease* (n=183) | 0.64 | 0.31, 1.33 | 0.23 | 0.74 | 0.34, 1.64 | 0.46 |
All models were stratified by trial. Multivariable models adjusted for treatment, age, diabetes, prior myocardial infarction, proximal left anterior descending artery disease, ejection fraction <40%, prior revascularization, and multi-vessel.
To avoid model overspecification, these subgroup models did not include terms for multi-vessel disease or proximal LAD disease, respectively. CKD-chronic kidney disease. LAD-left anterior descending artery
Short-term results at 1 year were similar to 5-year outcomes. Adjusted risks of MI did not differ 1 year after CABG compared with PCI among individuals with preserved kidney function (HR 1.17, 95% CI: 0.92, 1.49), but were lower following CABG compared with PCI in those with stage 3-5 CKD (HR 0.44, 95% CI: 0.23, 0.81), those with stage 3b-5 CKD (HR 0.18, 95% CI: 0.05, 0.58), and were not significantly lower among those with stage 3a CKD (HR 0.59, 95% CI: 0.28-1.28).
Repeat Revascularization
Repeat revascularization was conducted more frequently after PCI than CABG (Figure 2) both among individuals with CKD (7.2 cases/100 PY versus 1.4 cases/100 PY, P<0.001) and those with preserved kidney function (13.7 cases/100 PY versus 1.7 cases/100 PY, P<0.001). Risk reduction associated with revascularization was similar for individuals with preserved kidney function (HR 0.14, 95% CI: 0.11, 0.17), those with stage 3-5 CKD (0.21, 95% CI: 0.11, 0.39), those with stage 3a CKD (HR 0.17, 95% CI: 0.08, 0.40) and those with stage 3b-5 CKD (HR 0.25, 95% CI: 0.09, 0.71). There was no evidence of effect modification by the presence of CKD, Pinteraction=0.26). Tests of interaction with multi-vessel disease (Pinteraction=0.93) or proximal LAD involvement (Pinteraction=0.90) were also non-significant. Results were similar in crude and adjusted models (Table 6). For the subgroup with stage 3-5 CKD, the I2 statistic (25.3%) was consistent with minimal between study heterogeneity.
Table 6.
Risk of repeat revascularization for CABG compared to PCI
| Group | Crude HR | 95% CI | P Value | Adjusted HR | 95% CI | P Value |
|---|---|---|---|---|---|---|
| Overall (n=3912) | 0.14 | 0.12, 0.17 | <0.001 | 0.14 | 0.11, 0.17 | <0.001 |
| Preserved kidney Function (n=3405) | 0.13 | 0.11, 0.16 | <0.001 | 0.13 | 0.11, 0.16 | <0.001 |
| Stage 3-5 CKD (n=507) | 0.21 | 0.11, 0.40 | <0.001 | 0.21 | 0.11, 0.39 | <0.001 |
| Stage 3a CKD (n=371) | 0.18 | 0.08, 0.41 | <0.001 | 0.17 | 0.08, 0.40 | <0.001 |
| Stage 3b-5CKD (n=136) | 0.30 | 0.11, 0.85 | 0.02 | 0.25 | 0.09, 0.71 | 0.01 |
| CKD with multi-vessel disease* (n=400) | 0.21 | 0.10, 0.46 | <0.001 | 0.21 | 0.10, 0.46 | <0.001 |
| CKD with single vessel disease* (n=107) | 0.20 | 0.07, 0.62 | 0.01 | 0.19 | 0.06, 0.61 | 0.01 |
| CKD proximal LAD disease* (n=329) | 0.19 | 0.09, 0.40 | <0.001 | 0.18 | 0.09, 0.38 | <0.001 |
| CKD without proximal LAD disease* (n=176) | 0.25 | 0.07, 0.87 | 0.03 | 0.25 | 0.07, 0.92 | 0.04 |
All models were stratified by trial. Multivariable models adjusted for treatment, age, diabetes, prior myocardial infarction, proximal left anterior descending artery disease, ejection fraction <40%, prior revascularization, and multi-vessel.
To avoid model overspecification, these models did not include terms for multi-vessel disease or proximal LAD disease, respectively. CKD-chronic kidney disease. LAD
Short-term results at 1 year were similar to 5-year outcomes. Adjusted risks of revascularization were lower 1 year after CABG compared with PCI among individuals with preserved kidney function (HR 0.08, 95% CI: 0.06, 0.11), as well as those with stage 3-5 CKD (HR 0.14, 95% CI: 0.06, 0.30), those with stage 3a CKD (HR 0.12, 95% CI: 0.04-0.33), or those with stage 3b-5 CKD (HR 0.17, 95% CI: 0.05, 0.61).
Sensitivity Analyses
Results of models with differing levels of covariate adjustment, excluding studies with systematic missingness, or using complete-case analysis rather than multiple imputation were qualitatively similar to our main findings (Supplementary Tables).
Acute Dialysis and Hospitalization
Information on dialysis was not available for GABI. In the remaining trials, there were 8 (0.5%) cases of dialysis requiring acute kidney injury (AKI) in the PCI group and 5 (0.3%) cases in the CABG group. Among individuals with stage 3-5 CKD there were 5 (2.4%) cases in the PCI group and 2 cases (1.1%) in the CABG group. The risk of dialysis-dependent AKI did not differ significantly with CABG compared to PCI overall (odds ratio [OR] 0.61, 95% CI 0.20, 1.88), those with preserved kidney function (OR 0.98, 95% CI: 0.20, 4.85), or those with stage 3-5 CKD (OR 0.41, 95% CI: 0.08, 2.15), or stage 3b CKD (OR 0.71, 95% CI: 0.10, 5.23).
Data on cardiovascular hospitalizations was available from 6 trials.36, 38, 39, 41-43 CABG was generally associated with lower risks of hospitalization than PCI. At 5-years, the adjusted risk was lower after CABG than PCI among those with preserved kidney function (HR 0.30, 95% CI: 0.23, 0.39), those with stage 3-5 CKD (0.43, 95% CI: 0.27, 0.71), and those with stage 3a CKD (HR 0.32, 0.17, 0.60). CV hospitalization rates were lower but the change in risk was not significant with stage 3b-5 CKD (HR 0.77, 95% CI: 0.35, 1.72). There was no evidence of effect modification according to the presence of CKD (Pinteraction=0.19). For the subgroup with stage 3-5 CKD, the I2 statistic (1.0%) was consistent with minimal between study heterogeneity. Results during the first year were qualitatively similar to those at 5 years (data not shown).
Discussion
Although CKD is a common condition1 with high risks of cardiovascular morbidity and mortality3, high quality evidence to guide the use of PCI versus CABG in the setting of significant kidney impairment has been lacking. To better understand the risks and benefits of coronary revascularization in individuals with CKD, we analyzed individual, patient-level data from almost four thousand individuals enrolled in 10 trials in which patients were randomized to receiving CABG or PCI. To our knowledge, the 526 individuals with CKD that we identified represent the largest randomly assigned cohort comparing the risks of benefits of CABG and PCI in the setting of CKD.
We found that for individuals with stage 3-5 CKD in whom both CABG and PCI were clinically indicated and technically feasible, there were no significant differences in mortality with either approach to revascularization. However, despite the similarities in mortality, CABG strongly reduced both the risks of MI and the need for additional revascularization procedures without evidence for significant effect modification by the presence of single compared with multi-vessel disease. The present study provides important new evidence informing the decision faced by clinicians and their patients with CKD who require coronary intervention and have to decide between CABG and PCI.
While we are unaware of any published clinical trials specifically randomizing individuals with CKD to CABG versus PCI, several observational studies have suggested that CABG was associated with lower mortality than PCI in the setting of CKD15, 44-46, and at least one suggested that the mortality benefit increased as eGFR declined15. In contrast, a study by Szczech47 was consistent with our findings. This study may more closely resemble the randomized population we studied as it specifically excluded subjects belonging to anatomic subgroups with grossly unbalanced utilization of CABG and PCI (suggesting non-comparability of the indication for revascularization), and it did not find a survival benefit from CABG among individuals with serum creatinine ≥2.5 mg/dL.
In contrast with some observational studies, our findings are mostly consistent with a prior analysis by Ix et al. of 290 randomized participants with CKD from the Arterial Revascularization Therapies Study48 in which CABG did not impact mortality (HR 0.93, 95% CI 0.54-1.60) compared with PCI, but led to a significant reduction in the need for repeat revascularization (HR 0.28, 95% CI: 0.14-0.54). Both results were confirmed by our analysis although the primary investigator of the Arterial Revascularization Therapies Study did not grant access to their data for our study. Our results differ, however, in that the former study did not demonstrate significant reductions in the risk of MI (HR 1.34, 95% CI: 0.55-3.23). However, the confidence intervals around this estimate were wide because of low the number of MI events (n=20). By contrast, we found a strong reduction in MI risk from CABG that also appeared to increase with decreasing kidney function. Therefore, owing to nearly double the number of participants, a larger number of events within the CKD population (103 deaths, 68 MIs, 65 repeat revascularizations), and a more clinically relevant duration of follow-up (5 versus 3 years), our analysis extends the findings by Ix et al. in several important ways. In particular, our cohort included subjects from multiple trials with a more generalizable set of inclusion criteria that more broadly represent the range of clinical indications for revascularization than the Arterial Revascularization Therapies Study48, which included only subjects with multi-vessel disease and excluded subjects with overt congestive heart failure. Finally, the use of an individual patient data from multiple trials allowed us to adjust for multiple covariates simultaneously, which would not have been possible using traditional meta-analytic techniques.
Taken together, our study and the one by Ix et al48 suggest that prior observational analyses showing large survival benefits may have overestimated the mortality benefits of CABG compared with PCI in the setting of CKD. In fact, observational studies have consistently demonstrated increasing risks of operative death as kidney function declines49, and our estimates do not rule out worsened survival following CABG compared with PCI among subjects with the most advanced CKD—although confidence intervals around these estimates were very wide.
Indication bias or residual confounding via selective utilization of CABG in those individuals with the best underlying prognosis or with anatomic features most clearly favorable to surgical revascularization or, conversely, selective use of PCI in patients with very high operative risk, may have driven prior findings of a survival benefit with CABG compared with PCI in the setting of CKD. Although our findings do not support a conclusion that CABG reduces the hazard of mortality compared to PCI when both CABG and PCI are anatomically and clinically feasible, we did find that among CKD patients, CABG was associated with dramatically lower risks of MI and repeat revascularization during follow-up. Thus, CABG may be the preferable procedure that reduces overall morbidity despite not conferring a survival advantage.
Our study had certain limitations that require consideration. Unfortunately, despite including data from the largest number of trials and including the largest reported number of randomized patients with CKD (particularly those with ≥stage 3b disease), numerous trials either no longer had data available or failed to collect sufficient information to calculate eGFR. We were also unable to obtain data from several additional trials despite several attempts. The majority of trials were completed before IDMS-traceable creatinine assays were in wide use, and we did not have access to the assays used for creatinine testing. The lack of standardization or calibration may have led to some imprecision in estimation of GFR, although this should be balanced in the two treatment groups. In addition, for the BARI trial35 we were unable to obtain the actual creatinine, and instead had to use a threshold value, as described above. Although we are confident with the specificity of this approach for the identification of CKD, some patients with moderate CKD may have been missed.
We were also unable to standardize outcomes or baseline variable definitions across trials. We cannot rule out the possibility that different assessments across trials could have impacted our findings. Lastly, most of the included trials were completed more than a decade ago. Whether results would differ in the context of contemporary medical therapy, newer revascularization techniques, or for subjects not meeting the entrance criteria of these trials cannot be answered by our analysis, and results should be extrapolated cautiously.
Finally, our study does not address the gaping hole in the evidence on how to best treat patients with severe kidney dysfunction who require revascularization including those with end-stage kidney disease requiring dialysis or kidney transplantation. Indeed, an important finding of our analysis is that among nearly 4000 patients included in a series of randomized trials that helped establish the standard of care for coronary artery disease only 137 had stage 3b or worse CKD, only 20 had stage 4-5 CKD, and none had ESRD. Assuming that trial practices have not changed, this finding raises serious questions about the extrapolation of standard of care practices to the care of those at the most advanced stages of CKD.
In conclusion, our study provides the highest-quality evidence to date regarding the morbidity and mortality benefits of CABG compared with PCI in the setting of CKD. While survival was similar following CABG and PCI, we found that CABG significantly reduced the risk of subsequent MI or revascularization procedures. In the absence of additional randomized data, our analysis should be reassuring to clinicians who can counsel individuals requiring coronary revascularization that benefits of CABG do not appear to be attenuated in the setting of moderate CKD and that surgical revascularization is more likely than PCI to prevent subsequent MI or revascularization without adversely impacting survival. Finally, the hypothesis generating findings indicating worse survival with CABG in the small subsample of patients with Stage 3b and 4-5 CKD should provide additional motivation for performing randomized studies specifically enrolling individuals with advanced CKD or ESRD in order to provide better answers on risks and benefits in these high risk patients.
Methods
Search Criteria and Identification of Eligible Trials
We searched MEDLINE, EMBASE and Cochrane databases (Ovid Technologies 1950-September 2010) for keywords related to coronary revascularization procedures including, “angioplasty, transluminal, percutaneous coronary, and coronary artery bypass”. The search was limited to randomized controlled trials (not valid within EMBASE), humans, and English language publications. Following automated removal of duplicate citations, results of the computerized search were independently reviewed in duplicate by 2 investigators (DMC, NMS, or WCW) to identify unique, randomized trials comparing CABG and PCI. The reference lists of identified trials and relevant meta-analyses were subsequently reviewed for studies not identified electronically. Trials that randomly allocated patients to CABG or PCI were considered for inclusion without further restriction. The manuscript reporting the primary endpoint results was used to identify trials and investigators. Additional detail on the research plan and modifications to the study protocol are provided in the Supplementary Appendix. The PRISM individual patient meta-analysis statement was used as a guideline for structuring the manuscript.50
Data Extraction
The majority of identified studies had not published CKD-specific results. Investigators from each trial were therefore contacted and asked to prepare and share data on trial characteristics and individual, patient-level data including serum creatinine, baseline characteristics, interventions, and selected outcomes for enrolled subjects. Multiple attempts were made to contact study investigators before determining investigators’ status as unreachable. Provided data sets were individually cleaned and compared against trial publications for consistency with baseline characteristics and main outcomes. Trial investigators were re-contacted and queried as needed to ensure fidelity, accuracy, and completeness of final data sets.
Kidney Function
Kidney function was determined using the estimated glomerular filtration rate (eGFR), which was calculated using the CKD-EPI equation51 from baseline serum creatinine concentrations, age, sex, and race. The Bypass Angioplasty Revascularization Investigation (BARI) trial recorded only a dichotomized kidney dysfunction variable according to whether serum creatinine was >1.5 mg/dL but did not record the actual baseline values35. Therefore the theoretical maximum value of eGFR was calculated for BARI subjects using a creatinine of 1.6 mg/dL for individuals above this threshold and 0.1 mg/dL for individuals below this threshold. Given the primary analytic goal of assessing effects of CABG versus PCI in CKD patients, this approach was adopted in order to ensure a high specificity of the CKD definition for BARI subjects despite the possibility of misclassifying some BARI subjects with less significantly elevated creatinine as having preserved kidney function. Stages of CKD were defined as stage 3a (eGFR 45-59 mL/min/1.73m2), stage 3b (eGFR 30-44 mL/min/1.73m2), stage 4 (eGFR 15-29 mL/min/1.73m2), or stage 5 CKD (eGFR <15mL/min/1.73m2 or dialysis-dependent) according to the 2012 updates of the KDOQI guidelines52.
Other Patient Characteristics
Baseline demographic and clinical characteristics were assessed according to trial-specific definitions. Covariates obtained were chosen on the basis of availability and well-established associations with outcomes and included assigned treatment, age, race, sex, history of diabetes, hypertension, hyperlipidemia, congestive heart failure, prior coronary revascularization, history of prior myocardial infarction (MI), presentation with MI, unstable angina, or elevated cardiac enzymes, ejection fraction, and coronary anatomy.
Endpoints
Given the advanced age of the population and inconsistent data capture beyond 5 years, we calculated follow-up time and examined time-to-event outcomes through 5 years for the following events: all-cause mortality, myocardial infarction (MI), and repeat coronary revascularization. MI and repeat coronary revascularization outcomes were assessed according to the definitions originally used in the individual trials. Subjects who did not experience the event of interest during the study period were censored at the date of last clinical visit or recorded activity with right censoring at 5-years.
Statistics and Analysis
Summary statistics are presented as counts (%) or mean ± standard deviation (SD) as appropriate. For the primary analyses, we used Cox proportional hazards regression models, stratified by trial, to model the hazard of each endpoint (all-cause mortality, MI, and repeat revascularization) as a function of treatment arm (PCI versus CABG), adjusting for age, diabetes, prior history of MI, proximal left anterior descending artery disease, ejection fraction <40%, prior revascularization, and multi-vessel disease. We fit models to the entire pooled dataset as well as within pre-defined subsets of clinical interest. Namely, subset analyses were conducted in subjects with: 1) preserved kidney function, 2) stage 3-5 CKD, 3) stage 3a CKD, 4) stage 3b-5 CKD, 5) CKD with multi-vessel disease, 6) CKD with single-vessel disease, 7) CKD with proximal left anterior descending [LAD] disease, or 8) CKD without proximal LAD disease. Kaplan-Meier estimates were used to graphically depict survival.
Multiple imputation was used to account for missing data. Multiple imputation is a statistical method used to address missing data by imputing values for missing observations from plausible distributions that preserve the interrelationships among the variables.53, 54 Validity of the results relies on the assumption that data are missing at random (MAR), or that missingness is related to observed features only. Specifically, for primary analyses, we imputed data using predictive mean matching to impute each row independently. It is critical to include the outcome in the imputation model to reduce bias55; we therefore included an indicator for whether the observation was censored and also included the Nelson-Aalen estimator of cumulative hazard as a co-factor within the imputation models.56 As a sensitivity analysis, we imputed under a linear multilevel model that accounts for a trial-specific underlying hazard of the event corresponding to the study's unique population. For this approach, computational limitations required the exclusion of trials (Angioplasty versus Minimally Invasive Surgery Trial, AMIST)34 with systematic missingness on any variable (meaning that a variable is completely missing within a trial).
We conducted sensitivity analyses manipulating 3 analytic choices in all possible combinations to assess the effects on point estimates of covariate adjustment, inclusion of studies with systematic missingness, and method of handling missing data. Firstly, we conducted analyses adjusting for 1) all covariates of interest, as in primary analyses, 2) a “minimal” subset of only those covariates that were not systematically missing by trial, or 3) no covariates (unadjusted estimates). Secondly, we excluded either 1) none of the 10 eligible studies, as in primary analyses, or 2) all studies with systematic missingness on any variable. Thirdly, we handled missing data either 1) via multiple imputation, as in primary analyses, or 2) via complete-case analysis.
Heterogeneity of outcomes within the CKD group was analyzed by calculating the I-squared statistic. Published meta-analyses comparing CABG and PCI have not found evidence of publication bias.8 Given our primary aim of comparing unpublished outcomes from the subset of those studies with available data on renal function and the attendant analysis of only a minority of published studies, testing for publication bias on the included studies was not repeated.
Baseline data and incidence rates and calculation of I-squared for measurement of heterogeneity were analyzed using STATA (version 13.0, STATA Corp, College Station, Texas). Multiple imputation and survival analyses were performed in R (Version 3.1.0, R Foundation for Statistical Computing, Vienna, Austria)57-61. All tests were two-sided, and we defined statistical significance using an alpha threshold of 0.05.
Supplementary Material
Acknowledgements
This study was funded by grant DK089368 from the National Institutes of Health to Dr. Charytan and Dr. Winkelmayer. Dr. Charytan was also supported by grant HL118314. Dr. Winkelmayer receives salary and research support through the endowed Gordon A. Cain Chair in Nephrology at Baylor College of Medicine. Drs. Desai and Mathur were supported by a Patient-Centered Outcomes Research Institute Grant ME-1303-5989. Dr. Reeves is supported by the UK National Institute of Health Research Bristol Biomedical Research Unit in Cardiovascular Disease. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the position or policy of the Department of Veterans Affairs (VA), the Patient-Centered Outcomes Research Institute, or the United States government. We acknowledge the help of the VA Cooperative Studies Program, as well as VA CSP Study# 385, the Angina with Extremely Serious Operative Mortality Evaluation (AWESOME).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Helsinki
This research was conducted in accordance with the declaration of Helsinki. Informed consent was obtained for subjects at the time of enrollment in the original trials.
Disclosures
No relevant conflicts of interest
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. Jama. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K, Zelenkofske S, Leimberger JD, Califf RM, Pfeffer MA. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Charytan D, Mauri L, Agarwal A, Servoss S, Scirica B, Kuntz RE. The use of invasive cardiac procedures after acute myocardial infarction in long-term dialysis patients. Am Heart J. 2006;152:558–564. doi: 10.1016/j.ahj.2006.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chertow GM, Normand SL, McNeil BJ. “Renalism”: inappropriately low rates of coronary angiography in elderly individuals with renal insufficiency. Journal of the American Society of Nephrology : JASN. 2004;15:2462–2468. doi: 10.1097/01.ASN.0000135969.33773.0B. [DOI] [PubMed] [Google Scholar]
- 6.Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Gronhagen-Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Suleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wuthrich RP, Gottlow M, Johnsson E, Zannad F. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–1407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 7.Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 8.Bravata DM, Gienger AL, McDonald KM, Sundaram V, Perez MV, Varghese R, Kapoor JR, Ardehali R, Owens DK, Hlatky MA. Systematic review: the comparative effectiveness of percutaneous coronary interventions and coronary artery bypass graft surgery. Ann Intern Med. 2007;147:703–716. doi: 10.7326/0003-4819-147-10-200711200-00185. [DOI] [PubMed] [Google Scholar]
- 9.Hannan EL, Racz MJ, Walford G, Jones RH, Ryan TJ, Bennett E, Culliford AT, Isom OW, Gold JP, Rose EA. Long-term outcomes of coronary-artery bypass grafting versus stent implantation. N Engl J Med. 2005;352:2174–2183. doi: 10.1056/NEJMoa040316. [DOI] [PubMed] [Google Scholar]
- 10.Huang TM, Wu VC, Young GH, Lin YF, Shiao CC, Wu PC, Li WY, Yu HY, Hu FC, Lin JW, Chen YS, Lin YH, Wang SS, Hsu RB, Chang FC, Chou NK, Chu TS, Yeh YC, Tsai PR, Huang JW, Lin SL, Chen YM, Ko WJ, Wu KD, National Taiwan University Hospital Study Group of Acute Renal, F Preoperative proteinuria predicts adverse renal outcomes after coronary artery bypass grafting. Journal of the American Society of Nephrology : JASN. 2011;22:156–163. doi: 10.1681/ASN.2010050553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Renal Data System . USRDS 2008 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2008. [Google Scholar]
- 13.Collins AJ, Li S, Gilbertson DT, Liu J, Chen SC, Herzog CA. Chronic kidney disease and cardiovascular disease in the Medicare population. Kidney Int Suppl. 2003:S24–31. doi: 10.1046/j.1523-1755.64.s87.5.x. [DOI] [PubMed] [Google Scholar]
- 14.Chertow GM, Normand SL, Silva LR, McNeil BJ. Survival after acute myocardial infarction in patients with end-stage renal disease: results from the cooperative cardiovascular project. Am J Kidney Dis. 2000;35:1044–1051. doi: 10.1016/s0272-6386(00)70038-2. [DOI] [PubMed] [Google Scholar]
- 15.Reddan DN, Szczech LA, Tuttle RH, Shaw LK, Jones RH, Schwab SJ, Smith MS, Califf RM, Mark DB, Owen WF., Jr. Chronic kidney disease, mortality, and treatment strategies among patients with clinically significant coronary artery disease. Journal of the American Society of Nephrology : JASN. 2003;14:2373–2380. doi: 10.1097/01.asn.0000083900.92829.f5. [DOI] [PubMed] [Google Scholar]
- 16.Chang TI, Shilane D, Kazi DS, Montez-Rath ME, Hlatky MA, Winkelmayer WC. Multivessel coronary artery bypass grafting versus percutaneous coronary intervention in ESRD. Journal of the American Society of Nephrology : JASN. 2012;23:2042–2049. doi: 10.1681/ASN.2012060554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez A, Boullon F, Perez-Balino N, Paviotti C, Liprandi MI, Palacios IF. Argentine randomized trial of percutaneous transluminal coronary angioplasty versus coronary artery bypass surgery in multivessel disease (ERACI): in-hospital results and 1-year follow-up. ERACI Group. J Am Coll Cardiol. 1993;22:1060–1067. doi: 10.1016/0735-1097(93)90416-x. [DOI] [PubMed] [Google Scholar]
- 18.Goy JJ, Eeckhout E, Moret C, Burnand B, Vogt P, Stauffer JC, Hurni M, Stumpe F, Ruchat P, von Segesser L, Urban P, Kappenberger L. Five-year outcome in patients with isolated proximal left anterior descending coronary artery stenosis treated by angioplasty or left internal mammary artery grafting. A prospective trial. Circulation. 1999;99:3255–3259. doi: 10.1161/01.cir.99.25.3255. [DOI] [PubMed] [Google Scholar]
- 19.Drenth DJ, Winter JB, Veeger NJ, Monnink SH, van Boven AJ, Grandjean JG, Mariani MA, Boonstra PW. Minimally invasive coronary artery bypass grafting versus percutaneous transluminal coronary angioplasty with stenting in isolated high-grade stenosis of the proximal left anterior descending coronary artery: six months’ angiographic and clinical follow-up of a prospective randomized study. J Thorac Cardiovasc Surg. 2002;124:130–135. doi: 10.1067/mtc.2002.122525. [DOI] [PubMed] [Google Scholar]
- 20.Goy JJ, Kaufmann U, Goy-Eggenberger D, Garachemani A, Hurni M, Carrel T, Gaspardone A, Burnand B, Meier B, Versaci F, Tomai F, Bertel O, Pieper M, de Benedictis M, Eeckhout E. A prospective randomized trial comparing stenting to internal mammary artery grafting for proximal, isolated de novo left anterior coronary artery stenosis: the SIMA trial. Stenting vs Internal Mammary Artery. Mayo Clin Proc. 2000;75:1116–1123. doi: 10.4065/75.11.1116. [DOI] [PubMed] [Google Scholar]
- 21.Henderson RA, Pocock SJ, Sharp SJ, Nanchahal K, Sculpher MJ, Buxton MJ, Hampton JR. Long-term results of RITA-1 trial: clinical and cost comparisons of coronary angioplasty and coronary-artery bypass grafting. Randomised Intervention Treatment of Angina. Lancet. 1998;352:1419–1425. doi: 10.1016/s0140-6736(98)03358-3. [DOI] [PubMed] [Google Scholar]
- 22.King SB, 3rd, Lembo NJ, Weintraub WS, Kosinski AS, Barnhart HX, Kutner MH, Alazraki NP, Guyton RA, Zhao XQ. A randomized trial comparing coronary angioplasty with coronary bypass surgery. Emory Angioplasty versus Surgery Trial (EAST). N Engl J Med. 1994;331:1044–1050. doi: 10.1056/NEJM199410203311602. [DOI] [PubMed] [Google Scholar]
- 23.First-year results of CABRI (Coronary Angioplasty versus Bypass Revascularisation Investigation). CABRI Trial Participants. Lancet. 1995;346:1179–1184. [PubMed] [Google Scholar]
- 24.Carrie D, Elbaz M, Puel J, Fourcade J, Karouny E, Fournial G, Galinier M. Five-year outcome after coronary angioplasty versus bypass surgery in multivessel coronary artery disease: results from the French Monocentric Study. Circulation. 1997;96:II–1-6. [PubMed] [Google Scholar]
- 25.SOS Investigators Coronary artery bypass surgery versus percutaneous coronary intervention with stent implantation in patients with multivessel coronary artery disease (the Stent or Surgery trial): a randomised controlled trial. Lancet. 2002;360:965–970. doi: 10.1016/S0140-6736(02)11078-6. [DOI] [PubMed] [Google Scholar]
- 26.Eefting F, Nathoe H, van Dijk D, Jansen E, Lahpor J, Stella P, Suyker W, Diephuis J, Suryapranata H, Ernst S, Borst C, Buskens E, Grobbee D, de Jaegere P. Randomized comparison between stenting and off-pump bypass surgery in patients referred for angioplasty. Circulation. 2003;108:2870–2876. doi: 10.1161/01.CIR.0000100723.50363.2C. [DOI] [PubMed] [Google Scholar]
- 27.Hong SJ, Lim DS, Seo HS, Kim YH, Shim WJ, Park CG, Oh DJ, Ro YM. Percutaneous coronary intervention with drug-eluting stent implantation vs. minimally invasive direct coronary artery bypass (MIDCAB) in patients with left anterior descending coronary artery stenosis. Catheter Cardiovasc Interv. 2005;64:75–81. doi: 10.1002/ccd.20238. [DOI] [PubMed] [Google Scholar]
- 28.Kim JW, Lim DS, Sun K, Shim WJ, Rho YM. Stenting or MIDCAB using ministernotomy for revascularization of proximal left anterior descending artery? Int J Cardiol. 2005;99:437–441. doi: 10.1016/j.ijcard.2004.08.045. [DOI] [PubMed] [Google Scholar]
- 29.Grip L, Wahrborg P, Odell A, Albertsson P, Berglin E, Brandrup-Wognesen G, Ekstron L, Johansson S, Radberg G, Wiklund L. Coronary artery bypass beating heart surgery with LIMA graft, versus coronary angioplasty with stent for patients with single left anterior descending artery - a pilot study. Eur Heart J. 2001;22:597. [Google Scholar]
- 30.Pohl T, Giehrl W, Reichart B, Kupatt C, Raake P, Paul S, Reichenspurner H, Steinbeck G, Boekstegers P. Retroinfusion-supported stenting in high-risk patients for percutaneous intervention and bypass surgery: results of the prospective randomized myoprotect I study. Catheter Cardiovasc Interv. 2004;62:323–330. doi: 10.1002/ccd.20060. [DOI] [PubMed] [Google Scholar]
- 31.Kapur A, Hall RJ, Malik IS, Qureshi AC, Butts J, de Belder M, Baumbach A, Angelini G, de Belder A, Oldroyd KG, Flather M, Roughton M, Nihoyannopoulos P, Bagger JP, Morgan K, Beatt KJ. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients. 1-year results of the CARDia (Coronary Artery Revascularization in Diabetes) trial. J Am Coll Cardiol. 2010;55:432–440. doi: 10.1016/j.jacc.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 32.Serruys PW, Unger F, Sousa JE, Jatene A, Bonnier HJ, Schonberger JP, Buller N, Bonser R, van den Brand MJ, van Herwerden LA, Morel MA, van Hout BA. Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. N Engl J Med. 2001;344:1117–1124. doi: 10.1056/NEJM200104123441502. [DOI] [PubMed] [Google Scholar]
- 33.Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Stahle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD, Mohr FW. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 34.Reeves BC, Angelini GD, Bryan AJ, Taylor FC, Cripps T, Spyt TJ, Samani NJ, Roberts JA, Jacklin P, Seehra HK, Culliford LA, Keenan DJ, Rowlands DJ, Clarke B, Stanbridge R, Foale R. A multi-centre randomised controlled trial of minimally invasive direct coronary bypass grafting versus percutaneous transluminal coronary angioplasty with stenting for proximal stenosis of the left anterior descending coronary artery. Health Technol Assess. 2004;8:1–43. doi: 10.3310/hta8160. [DOI] [PubMed] [Google Scholar]
- 35.Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. N Engl J Med. 1996;335:217–225. doi: 10.1056/NEJM199607253350401. [DOI] [PubMed] [Google Scholar]
- 36.Cisowski M, Drzewiecka-Gerber A, Ulczok R, Abu Samra R, Drzewiecki J, Guzy M, Trusz-Gluza M, Bochenek A. Primary direct stenting versus endoscopic atraumatic coronary artery bypass surgery in patients with proximal stenosis of the left anterior descending coronary artery--a prospective, randomised study. Kardiol Pol. 2004;61:253–261. discussion 262-254. [PubMed] [Google Scholar]
- 37.Rodriguez A, Bernardi V, Navia J, Baldi J, Grinfeld L, Martinez J, Vogel D, Grinfeld R, Delacasa A, Garrido M, Oliveri R, Mele E, Palacios I, O'Neill W. Argentine Randomized Study: Coronary Angioplasty with Stenting versus Coronary Bypass Surgery in patients with Multiple-Vessel Disease (ERACI II): 30-day and one-year follow-up results. ERACI II Investigators. J Am Coll Cardiol. 2001;37:51–58. doi: 10.1016/s0735-1097(00)01052-4. [DOI] [PubMed] [Google Scholar]
- 38.Hamm CW, Reimers J, Ischinger T, Rupprecht HJ, Berger J, Bleifeld W. A randomized study of coronary angioplasty compared with bypass surgery in patients with symptomatic multivessel coronary disease. German Angioplasty Bypass Surgery Investigation (GABI). N Engl J Med. 1994;331:1037–1043. doi: 10.1056/NEJM199410203311601. [DOI] [PubMed] [Google Scholar]
- 39.Buszman PE, Kiesz SR, Bochenek A, Peszek-Przybyla E, Szkrobka I, Debinski M, Bialkowska B, Dudek D, Gruszka A, Zurakowski A, Milewski K, Wilczynski M, Rzeszutko L, Buszman P, Szymszal J, Martin JL, Tendera M. Acute and late outcomes of unprotected left main stenting in comparison with surgical revascularization. J Am Coll Cardiol. 2008;51:538–545. doi: 10.1016/j.jacc.2007.09.054. [DOI] [PubMed] [Google Scholar]
- 40.Diegeler A, Thiele H, Falk V, Hambrecht R, Spyrantis N, Sick P, Diederich KW, Mohr FW, Schuler G. Comparison of stenting with minimally invasive bypass surgery for stenosis of the left anterior descending coronary artery. N Engl J Med. 2002;347:561–566. doi: 10.1056/NEJMoa013563. [DOI] [PubMed] [Google Scholar]
- 41.Hueb WA, Bellotti G, de Oliveira SA, Arie S, de Albuquerque CP, Jatene AD, Pileggi F. The Medicine, Angioplasty or Surgery Study (MASS): a prospective, randomized trial of medical therapy, balloon angioplasty or bypass surgery for single proximal left anterior descending artery stenoses. J Am Coll Cardiol. 1995;26:1600–1605. doi: 10.1016/0735-1097(95)00384-3. [DOI] [PubMed] [Google Scholar]
- 42.Hueb W, Soares PR, Gersh BJ, Cesar LA, Luz PL, Puig LB, Martinez EM, Oliveira SA, Ramires JA. The medicine, angioplasty, or surgery study (MASS-II): a randomized, controlled clinical trial of three therapeutic strategies for multivessel coronary artery disease: one-year results. J Am Coll Cardiol. 2004;43:1743–1751. doi: 10.1016/j.jacc.2003.08.065. [DOI] [PubMed] [Google Scholar]
- 43.Morrison DA, Sethi G, Sacks J, Henderson W, Grover F, Sedlis S, Esposito R, Ramanathan K, Weiman D, Saucedo J, Antakli T, Paramesh V, Pett S, Vernon S, Birjiniuk V, Welt F, Krucoff M, Wolfe W, Lucke JC, Mediratta S, Booth D, Barbiere C, Lewis D. Percutaneous coronary intervention versus coronary artery bypass graft surgery for patients with medically refractory myocardial ischemia and risk factors for adverse outcomes with bypass: a multicenter, randomized trial. Investigators of the Department of Veterans Affairs Cooperative Study #385, the Angina With Extremely Serious Operative Mortality Evaluation (AWESOME). J Am Coll Cardiol. 2001;38:143–149. doi: 10.1016/s0735-1097(01)01366-3. [DOI] [PubMed] [Google Scholar]
- 44.Ashrith G, Lee VV, Elayda MA, Reul RM, Wilson JM. Short- and long-term outcomes of coronary artery bypass grafting or drug-eluting stent implantation for multivessel coronary artery disease in patients with chronic kidney disease. Am J Cardiol. 2010;106:348–353. doi: 10.1016/j.amjcard.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 45.Charytan DM, Li S, Liu J, Herzog CA. Risks of death and end-stage renal disease after surgical compared with percutaneous coronary revascularization in elderly patients with chronic kidney disease. Circulation. 2012;126:S164–169. doi: 10.1161/CIRCULATIONAHA.111.083568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sugumar H, Lancefield TF, Andrianopoulos N, Duffy SJ, Ajani AE, Freeman M, Buxton B, Brennan AL, Yan BP, Dinh DT, Smith JA, Charter K, Farouque O, Reid CM, Clark DJ, Australia, New Zealand Society of, C, Thoracic, S, Melbourne Interventional, G Impact of renal function in patients with multi-vessel coronary disease on long-term mortality following coronary artery bypass grafting compared with percutaneous coronary intervention. Int J Cardiol. 2014;172:442–449. doi: 10.1016/j.ijcard.2014.01.096. [DOI] [PubMed] [Google Scholar]
- 47.Szczech LA, Reddan DN, Owen WF, Califf R, Racz M, Jones RH, Hannan EL. Differential survival after coronary revascularization procedures among patients with renal insufficiency. Kidney Int. 2001;60:292–299. doi: 10.1046/j.1523-1755.2001.00799.x. [DOI] [PubMed] [Google Scholar]
- 48.Ix JH, Mercado N, Shlipak MG, Lemos PA, Boersma E, Lindeboom W, O'Neill WW, Wijns W, Serruys PW. Association of chronic kidney disease with clinical outcomes after coronary revascularization: the Arterial Revascularization Therapies Study (ARTS). Am Heart J. 2005;149:512–519. doi: 10.1016/j.ahj.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 49.Yeo KK, Li Z, Yeun JY, Amsterdam E. Severity of chronic kidney disease as a risk factor for operative mortality in nonemergent patients in the California coronary artery bypass graft surgery outcomes reporting program. Am J Cardiol. 2008;101:1269–1274. doi: 10.1016/j.amjcard.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, Tierney JF, Group, P-ID Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD Statement. JAMA. 2015;313:1657–1665. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- 51.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI. KDOQI US Commentary on the 2012 KDIGO Clinical Practice Guideline for the Evaluation and Management of CKD. Am J Kidney Dis. 2014;63:713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 53.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 54.Van Buuren S. Flexible Imputation of Missing Data. Chapman & Hall/CRC Press; Boca Raton: 2012. [Google Scholar]
- 55.Moons KG, Donders RA, Stijnen T, Harrell FE., Jr. Using the outcome for imputation of missing predictor values was preferred. Journal of clinical epidemiology. 2006;59:1092–1101. doi: 10.1016/j.jclinepi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 56.Hox JJ, Roberts JK. Handbook of Advanced Multilevel Analysis. Psychology Press; New York: 2011. [Google Scholar]
- 57.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Software. 2011;45:1–67. [Google Scholar]
- 58.Therneau T. A Package for Survival Analysis in S. R package version 2.37-7. 2014 [Google Scholar]
- 59.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. Springer; New York: 2000. [Google Scholar]
- 60.Wickham H. ggplot2: elegant graphics for data analysis. Springer; New York: 2009. [Google Scholar]
- 61.Fox J, Weisberg S. An {R} Companion to applied regression. Sage; Thousand Oaks: 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.