Abstract
Background
With the exception of the head, the vertebrate embryonic body is formed progressively in an anterior-posterior direction, originating from a posteriorly located bipotential neural-mesodermal progenitor population. The T-box transcription factor Brachyury is expressed within the progenitors, and is essential for the formation of the posterior mesoderm. A novel cold-sensitive mutant of zebrafish Brachyury (ntlacs) is described that allows exploration of the temporal role of this key factor.
Results
The ntlacs mutant is used to show that Ntla has an essential role during early gastrulation, but as gastrulation proceeds the importance of Ntla declines as Ntlb acquires a capacity to form the posterior mesoderm. Remarkably, ntlacs embryos held at the non-permissive temperature just during the gastrula stages show recovery of normal levels of mesodermal gene expression, demonstrating the plasticity of the posterior progenitors.
Conclusion
ntlacs is a valuable tool for exploring the processes forming the posterior body since it allows temporally specific activation and inactivation of Brachyury function. It is used here to show the changing roles of Ntla during early development, and the dynamics of the neuromesdermal progenitors.
Keywords: Brachyury, neuromesodermal progenitors, T-box genes, early vertebrate development
INTRODUCTION
The brachyury gene is one of the major players involved in forming the early vertebrate embryonic body (reviewed in Kimelman, 2016; Wardle and Papaioannou, 2008). Mice lacking brachyury (also known as T) lack a notochord and have an almost complete absence of somites (Chesley, 1935; Gluecksohn-Schoenheimer, 1944). A brachyury mutant called no tail (ntl), identified in a mutant screen in zebrafish, has normal trunk somites but lacks a notochord and tail somites, suggesting that brachyury has a less important role in the lower vertebrates (Halpern et al., 1993; Schulte-Merker et al., 1994). However, a second ntl gene was later discovered, and loss of both alleles recapitulates the mouse phenotype, demonstrating a conserved ancestral role for brachyury (Martin and Kimelman, 2008). The original ntl is now called ntla (also ta) and the second one is ntlb (tb).
Brachyury is expressed in the progenitors found at the most posterior end of the growing vertebrate embryo, together with the essential neural factor sox2 (Garriock et al., 2015; Martin and Kimelman, 2012; Olivera-Martinez et al., 2012; Tsakiridis et al., 2014). Cells exiting this progenitor zone can either become mesodermal (brachyury+/sox2−) or neural (brachyury−/sox2+) as they begin to differentiate, thus producing the somites and neural tube (Bouldin et al., 2015; Garriock et al., 2015; Gouti et al., 2014; Jurberg et al., 2014; Martin and Kimelman, 2012; Tsakiridis et al., 2014; Tzouanacou et al., 2009; Wymeersch et al., 2016). The cells in the progenitor zone are therefore referred to as the bipotent neuromesodermal progenitor cells (reviewed in Gouti et al., 2014; Henrique et al., 2015; Kimelman, 2016).
Brachyury is a transcription factor with many embryonic targets in the notochord and mesoderm (Evans et al., 2012; Garnett et al., 2009; Gentsch et al., 2013; Morley et al., 2009). Surprisingly, cells lacking both ntla and ntlb develop normally into muscle cells when transplanted into wild-type zebrafish embryos, demonstrating that the essential role of brachyury in the somites is non-cell autonomous (Martin and Kimelman, 2008). Importantly, Brachyury activates the expression of wnt genes, which are essential for sustaining the neuromesodermal progenitors and forming the mesoderm (reviewed in Kimelman, 2016; Wymeersch et al., 2016). In zebrafish, we showed that transgenic embryos containing a heat inducible specific inhibitor of the Wnt signaling pathway rapidly cease forming the posterior body soon after the embryos are heat shocked anytime during the gastrula or somite-forming stages (Martin and Kimelman, 2008). Based on this, and results showing that Wnts activate brachyury expression (Arnold et al., 2000; Vonica and Gumbiner, 2002; Yamaguchi et al., 1999), we proposed that Wnt and Brachyury exist in an autoregulatory loop that begins in gastrulation and runs through the somitogenesis stages (Martin and Kimelman, 2008). However, because there is no completely specific inhibitor of Brachyury we were unable to analyze the temporal role of Brachyury in forming the embryo.
I report here a novel cold-sensitive mutant of ntla, ntlacs, that arises from two conservative missense mutations in the C-terminus of the protein. ntlacs embryos maintained at the non-permissive temperature have a phenotype and gene expression profile that looks very similar to ntla null mutants. Using timed temperature shifts I show that Ntla is essential during gastrulation but its role diminishes as embryos enter somitogenesis since Ntlb provides an increasingly important role. Importantly, embryos held at the non-permissive temperature only during gastrulation produce normal levels of posterior mesodermal gene expression and significantly well developed bodies compared to embryos maintained at the non-permissive temperature, revealing the inherent plasticity of the neuromesodermal progenitors.
RESULTS and DISCUSSION
Identification of a cold-sensitive mutation in ntla
In order to study zebrafish embryos during the very early stages of somitogenesis we routinely grow them at the standard temperature of ~29°C until the start of gastrulation (shield stage), place them in room temperature embryo media, and then put them in a 17°C incubator overnight, returning them to 29°C the next morning. Under these conditions, the embryos grow completely normally with no loss of viability. While crossing pairs of fish from one family, I observed that approximately one-quarter of the embryos had a variably truncated tail (Fig. 1C, D). When the same adults were crossed and the embryos were raised at 29°C, the embryos showed no phenotypic abnormalities and could be raised to the adult stage (see below).
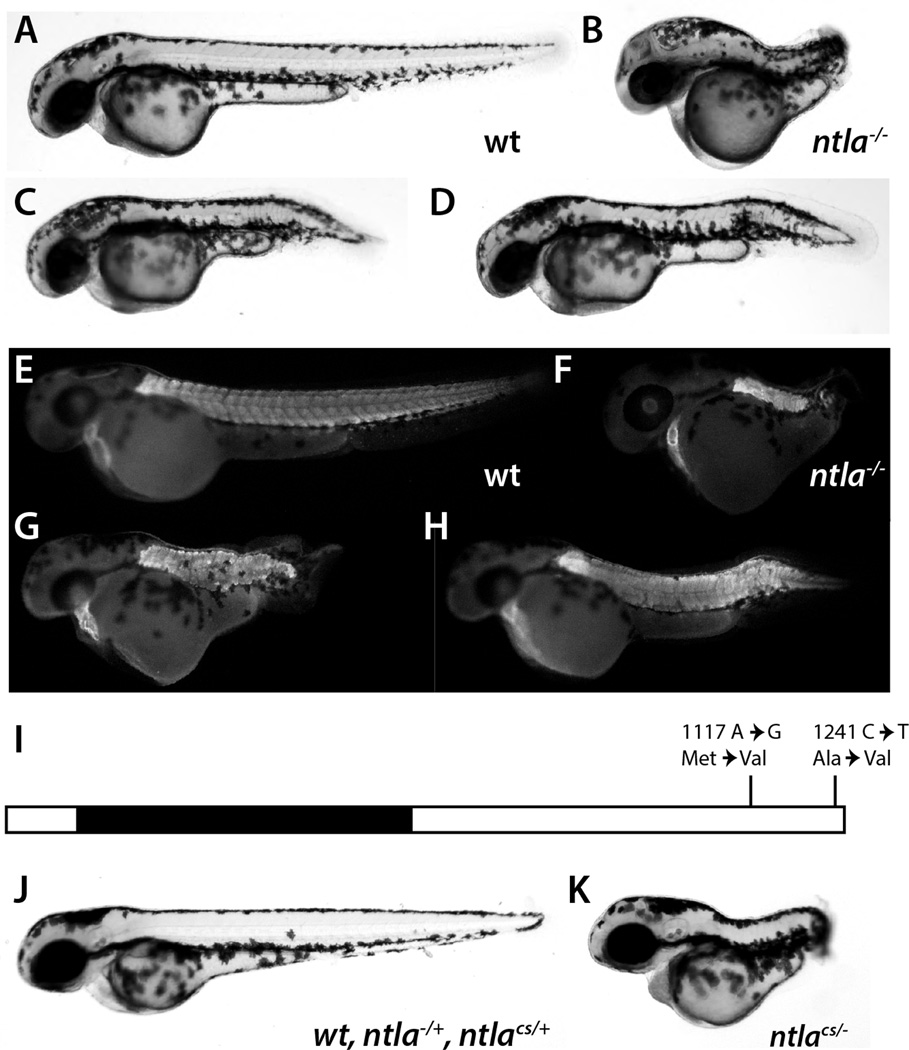
Figure 1.
Identification of a cold-sensitive mutation in ntla. (A–D) A cross from one family of fish produced embryos with a truncated tail phenotype (C, D) as well as wild-type (wt) embryos after being raised overnight at 17°C from shield stage. The phenotype is less severe than seen with the ntlab195 mutant (B, ntla−/−). (E–H) Staining with the muscle antibody MF20 shows that the embryos with the truncated tails have less somitic tissue (G, H), although with less reduction than seen with ntla−/− (F). (I) The mutant embryos have two base pair changes in the C-terminal domain of the Ntla coding region. The numbers indicate the base pair where the change occurred relative to the first base of the start codon. (J, K) A cross of ntla−/+ and ntlacs/+ adults produced phenotypically wild-type and mutant embryos after being raised overnight at 17°C from shield stage. The genotypes of both are indicated on the figure. All embryos are shown at the equivalent of two days post-fertilization.
As shown by staining with a muscle marker, the embryos grown in the cold condition had a variably reduced number of somites compared to wild-type embryos (Fig. 1E, G, H). This phenotype appeared similar to that seen in ntla hypomorphs ntlats260, ntlam550 and ntlab487 (Doyon et al., 2008; Odenthal et al., 1996; Stemple et al., 1996) but less severe than seen in the ntla null mutant, ntlab195 (Fig. 1B, F, Halpern et al., 1993), suggesting that the mutation might be in ntla or an interacting factor. DNA sequence analysis of ntla cDNA isolated from embryos with a mutant phenotype showed two conservative amino acid changes in the C-terminal region of Ntla (Fig. 1I). Based on these changes, primers for both High Resolution Melt Analysis (Talbot and Amacher, 2014) and dCAPS (Neff et al., 2002) were designed and used to test embryos. In every case, embryos that showed the mutant phenotype also had the C-terminal mutations (n=32). Based on these results, this cold-sensitive mutation of ntla was designated ntlacs (the official name of this line is ntlaw181). As a final test, ntlacs adults were crossed to ntlab195 null mutants (ntla−/−) and placed at 17°C at shield stage. 21% (26/126) of the embryos had a truncated tail-phenotype. When the wild-type and mutant embryos were genotyped (n=16), all of the mutant embryos were ntlacs/−, whereas the phenotypically wild-type embryos were wild-type, ntlacs/+ or ntla−/+ (Fig. 1J, K), further confirming that ntlacs is a novel cold-sensitive allele of ntla.
Of the two amino acid mutations (Fig. 1I), the alanine that changes to valine is a reside that is conserved among fish species including Spotted gar, Fugu, Stickleback and Medaka, although not in Coelacanth. In contrast, the methionine that changes to valine is found as a valine in some fish species such as Spotted gar and Coelacanth, although it is deleted in other fish species such as Medaka, Stickleback and Fugu. Thus, of all these fish species, Spotted gar is the only one likely to acquire the same cold-sensitive mutation, if the equivalent alanine were to change to a valine.
Ntla is required during the gastrula stages
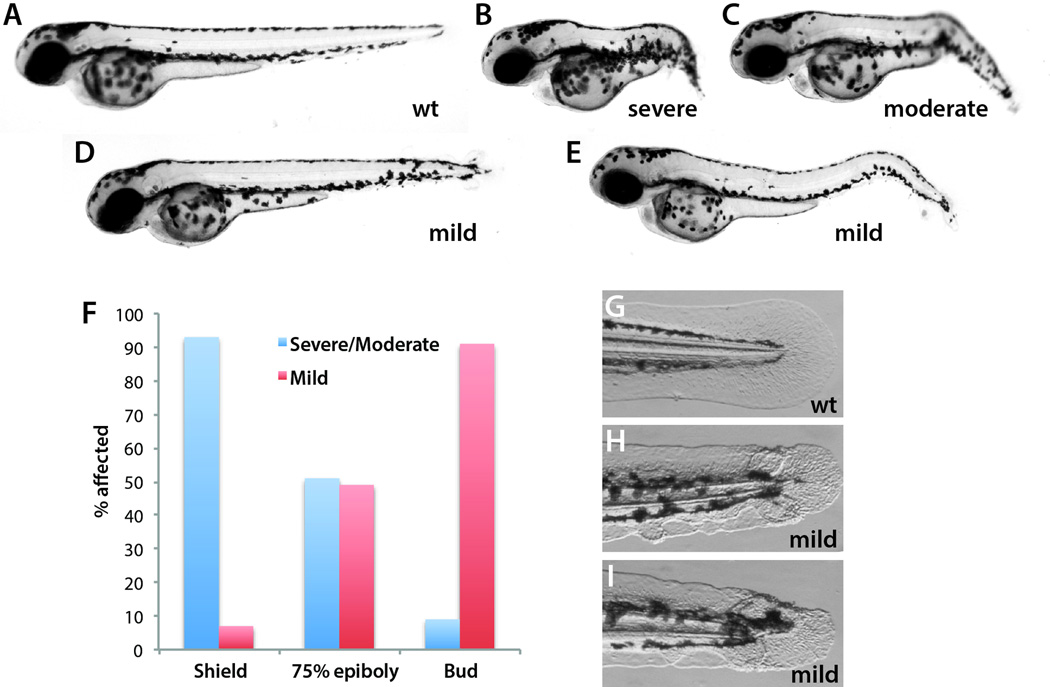
With a temperature regulated Ntla it was now possible to determine when it is required during embryogenesis without the need for specialized photo-regulated morpholino oligonucleotides, which have been used previously to study notochord and floor plate formation (Shestopalov et al., 2012; Shestopalov et al., 2007; Tallafuss et al., 2012). Because the ntlacs embryos are completely viable it was possible to isolate homozygous adults, which were used for the remainder of the experiments presented here since 100% of the progeny of a cross of two homozygous adults are mutant, obviating the need to genotype the resulting embryos. Embryos raised from a cross of homozygous ntlacs adults were placed overnight at 17°C at the start of gastrulation (shield stage), halfway through gastrulation (75% epiboly) or at the end of gastrulation (bud stage). The next morning the embryos were placed at 29°C and raised at this temperature until they reached the equivalent stage that wild-type embryos reach at two days post-fertilization (dpf). All embryos treated this way showed at least some minor defect. The embryos were then categorized as severe/moderate or mild (Fig. 2A–E). While cold treatment at the start of gastrulation resulted in embryos that were mostly severe and moderate (n=44), this effect was gradually lost during gastrulation (Fig. 2F). Cold treatment at bud stage produced almost exclusively mild embryos that had only a mild kink in the tail or often just minor defects at the most posterior end, which were recognizable by slight morphological aberrations in the most posterior tail fin (Fig. 2G–I; n=44). Thus, the major role of Ntla in forming the posterior body occurs during the gastrula stages.
Figure 2.
Ntla is essential during the gastrula stages. (A–E) ntlacs/cs embryos were categorized as severe, moderate or mild. Mild embryos had either a kinked tail (E) or an essentially normal body axis with minor tail fin defects at the most posterior end (D). (F) Quantification of the results from placing embryos in the cold at the indicated stages. Severe and moderate phenotypes were grouped together since embryos were a continuum between the phenotypes shown in panels B and C. (G–I) Close up images showing the posterior tail fin defects observed in the mild embryos.
Ntla continues to function post-gastrulation
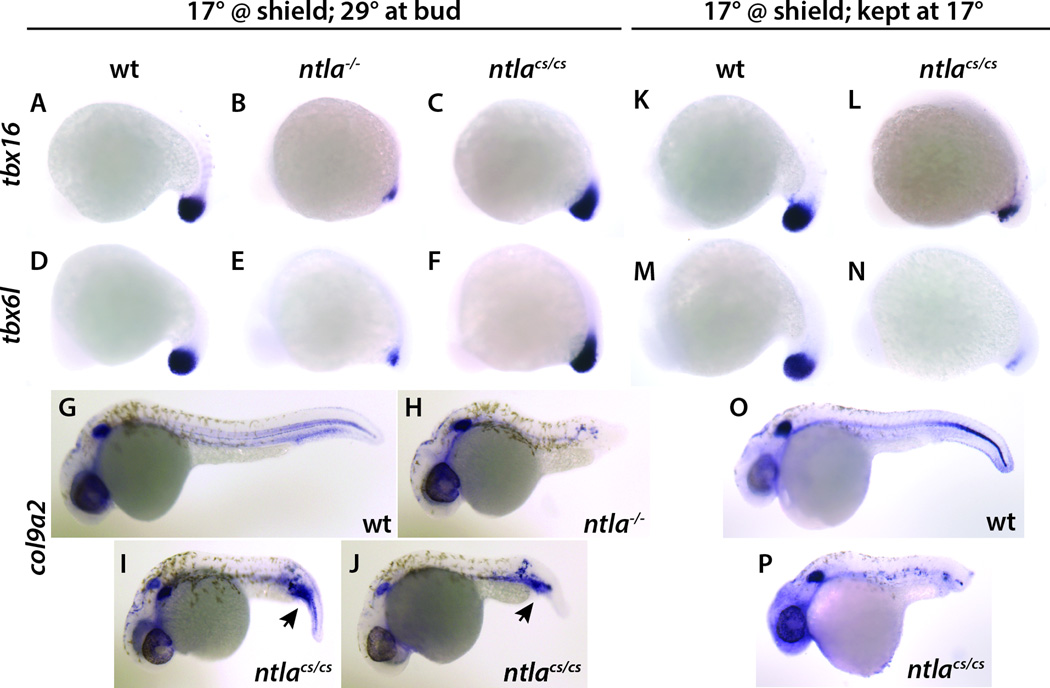
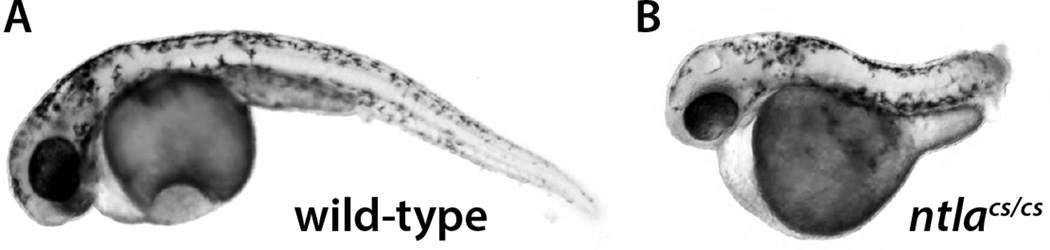
Ntlacs produces a less severe phenotype at the non-permissive temperature than is seen with the ntla null allele (Fig. 1A–H). The same effect was also seen when the expression of two mesodermal genes downstream of Ntla, tbx16/spadetail (Griffin et al., 1998; Ruvinsky et al., 1998) and tbx6l (Goering et al., 2003; Hug et al., 1997), were examined at the mid-somitogenesis stage (Fig. 3A–F), demonstrating that the increased number of somites in ntlcs mutants is due to continued production of new mesoderm during later stages of somitogenesis. These results suggested that the missense mutations in ntlacs might be unable to produce a null phenotype and instead only cause a partial loss of function. Alternatively, since the embryos placed at 17°C overnight were returned to 29°C the next morning (when they have reached the end of gastrulation and just begun somitogenesis), it was possible that when the embryos were returned to the permissive temperature the accumulated Ntlacs protein was able to provide some degree of rescue. To test this, embryos were maintained at 17°C until they had reached the equivalent of two days post-fertilization. Under this procedure, wild-type embryos developed mostly normally although the body was often curved (Fig. 4A). In contrast, ntlacs mutant embryos phenotypically resembled ntla null mutants (Fig. 4B, compare to Fig. 1B–D; 100%, n=55). Similarly, analysis of molecular markers at mid-somitogenesis revealed highly diminished expression of posterior mesodermal gene expression, similar to that seen in the ntla null mutants (Fig. 3L, N compare to B, E). Similarly, when the notochord marker col9a2 (Mangos et al., 2010) was examined, ntla null mutants showed no expression of this marker whereas ntlacs embryos raised at 29°C after an overnight 17°C incubation had strong posterior expression of col9a2 (Fig. 3G–J), indicating recovery of notochordal gene expression. In contrast, ntlacs embryos kept at 17°C had almost no col9a2 expression (Fig. 3P). These results show that ntlacs produces an essentially null phenotype when kept at 17°C, whereas shifting back to the permissive temperature allows complete recovery of the posterior mesoderm from the neuromesodermal progenitor population. In addition, these results provide further support for the model that the notochord in the fish, as in higher vertebrates, is continuously induced from a progenitor population in the tailbud during the post-gastrula stages and is not induced only during the gastrula stages (Row et al., 2016)
Figure 3.
Analysis of gene expression in ntla mutant embryos. (A–J) Wild-type, ntla−/− and ntlacs/cs embryos were placed at 17°C at shield stage, then incubated at 29°C the next morning (when the embryos were at bud stage) and raised to the 14 somite stage. Whereas ntla−/− embryos had essentially no expression of the presomitic genes tbx16 and tbx6l or the notochordal gene col9a2, ntlacs/cs embryos had strong expression of all three genes. All these embryos were hybridized with the indicated probe at the same time and for the same length of time. (K–P) In contrast, when ntlacs/cs embryos were kept continuously at 17°C, expression of all three genes was considerably diminished. Arrows show accumulation of col9a2 in the posterior of ntlacs/cs embryos.
Figure 4.
Maintaining ntlacs/cs embryos at 17°C enhances the mutant phenotype. Wild-type and ntlacs/cs embryos were maintained at 17°C from shield stage until the equivalent of 2 dpf. Whereas wild-type embryos formed a normal body (although often with a curved tail), ntlacs/cs embryos appeared morphologically the same as ntla−/− embryos (see Figure 1).
Ntlb is essential for forming the posterior body during the post-gastrula stages
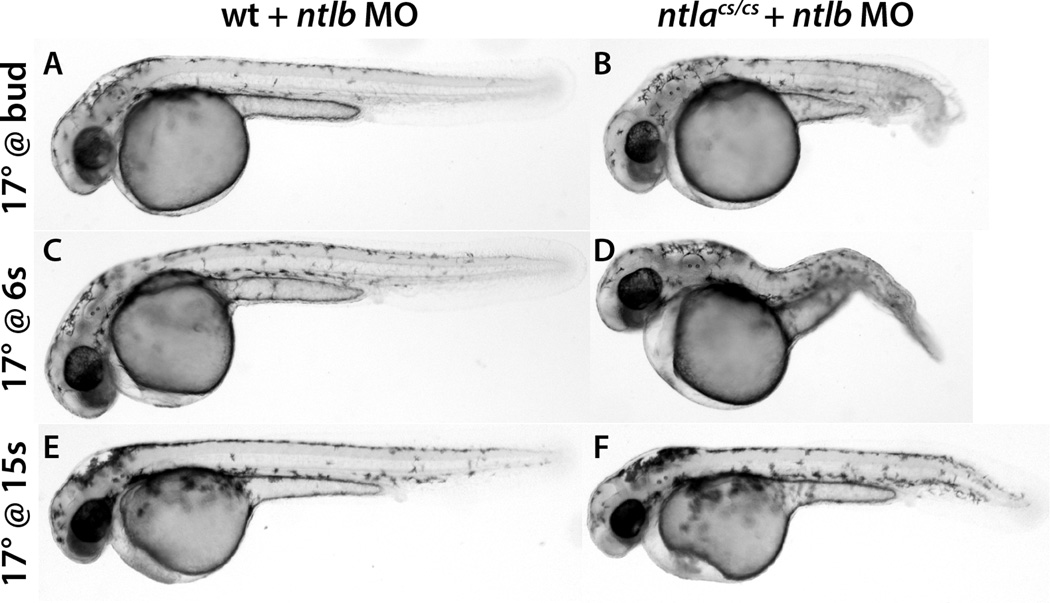
We previously showed that whereas ntla null mutant embryos produce approximately 18 well formed somites of the trunk (Halpern et al., 1993), embryos lacking both Ntla and Ntlb form only 8–12 poorly formed somites (Martin and Kimelman, 2008). These results suggested that Ntlb might function largely during formation of the trunk even though it is also expressed in the neuromesodermal progenitors during the stages when the tail somites form. Since ntlacs embryos placed at 17° at the end of gastrulation (bud stage) develop virtually normally (Figure 2), this provided an opportunity to examine the later role of Ntlb. To test the role of Ntlb, its translation was inhibited with a morpholino oligonucleotide (MO) as previously described (Martin and Kimelman, 2008). Whereas wild-type embryos injected with the ntlb MO and placed at 17° at bud stage develop normally (Fig. 5A), ntlacs embryos containing the ntlb MO and treated the same way have a truncated axis (Fig. 5B; 91%, n=22). The same effects were observed when the embryos were placed at 17° during the early somitogenesis stages (Fig. 5C, D; ntlacs 100%, n=23), with milder defects when the embryos were placed at 17° at mid-somitogenesis (Fig. 5E, F; ntlacs 82%, n=65). These results demonstrate that Ntla can continue to function in the post-gastrula stages but that its role is obscured by Ntlb, which is co-expressed with Ntla.
Figure 5.
Ntla is required for posterior development in the absence of Ntlb. Wild-type or homozygous ntlacs/cs embryos were injected with a ntlb MO and then placed at 17°C overnight at bud stage (A, B), 6 somite stage (C, D) or 15 somite stage (E, F). The embryos were then grown to the equivalent of 2 dpf at 30°C. Interfering with Ntlb function causes a truncated tail phenotype in ntlacs embryos.
Conclusions
The cold-sensitive ntla mutant described here will be a valuable tool for studies of this essential protein. Whereas light-controlled MOs have been useful for the study of the role of ntla in notochord and floor plate formation (Shestopalov et al., 2012; Shestopalov et al., 2007; Tallafuss et al., 2012), once the MO is light activated it will irreversibly activate or eliminate Ntla function. In contrast, a temperature sensitive allele can be variably inactivated and activated just by shifting the temperature of the growth medium. Thus, ntlacs is used here to show that Ntla plays an essential role during the gastrula stages, but if Ntla function is restored at the end of gastrulation, the neuromesodermal progenitors will return to producing normal levels of mesoderm as long as Ntlb is present. Thus Ntla is essential because it provides a “kick start” to get the system running, which Ntlb alone cannot provide. It is interesting to note that all the bony fish sequenced, including Coelacanth and Spotted Gar that predate the teleost genome duplication (Amemiya et al., 2013; Braasch et al., 2016), have two copies of the brachyury gene whereas other vertebrates have only one. Since, at least in zebrafish, the role of Ntlb in forming both the trunk and tail is unnecessary as long as Ntla is present, it remains an interesting mystery why all these species maintain two copies of brachyury.
EXPERIMENTAL PROCEDURES
Fish lines
All fish are Hybrid WIK/AB. Genetic analysis used either High Resolution Melt Analysis (Talbot and Amacher, 2014) or dCAPS (Neff et al., 2002). Since the dCAPS was more useful in segregating wild-type, heterozygous and homozygous genotypes, that procedure is provided here. Genomic DNA was amplified with 5’-AGTTCCTACGCGGTTCATCG-3’ and 5’-CAGTAGCTCTGAGCCACAGG-3’ using standard PCR conditions with GoTaq Flexi buffer (Promega). After amplification the PCR reaction was digested with ClaI and separated on a 2.5% agarose gel using a LiCl buffer. ClaI cuts the wild-type allele but not the ntlacs allele.
In situ hybridization, immunofluorescence and morpholino
Alkaline phosphatase in situs were performed as described (Griffin et al., 1995). The MF20 monoclonal antibody (Developmental Studies Hybridoma Bank) was used at a 1:50 dilution. The ntlb translation blocking morpholino oligonucleotide was previously described (MO2, Martin and Kimelman, 2008) and used at 2.5 ng along with 0.5 ng of a p53 morpholino (Robu et al., 2007).
Acknowledgments
I am indebted to Ben Martin for providing bra MO, Iain Drummond for providing the col9a2 plasmid, and the reviewers for their valuable contributions. D.K. was supported by a National Institutes of Health grant (RO1GM079203).
REFERENCES
- Amemiya CT, Alfoldi J, Lee AP, Fan S, Philippe H, Maccallum I, Braasch I, Manousaki T, Schneider I, Rohner N, Organ C, Chalopin D, Smith JJ, Robinson M, Dorrington RA, Gerdol M, Aken B, Biscotti MA, Barucca M, Baurain D, Berlin AM, Blatch GL, Buonocore F, Burmester T, Campbell MS, Canapa A, Cannon JP, Christoffels A, De Moro G, Edkins AL, Fan L, Fausto AM, Feiner N, Forconi M, Gamieldien J, Gnerre S, Gnirke A, Goldstone JV, Haerty W, Hahn ME, Hesse U, Hoffmann S, Johnson J, Karchner SI, Kuraku S, Lara M, Levin JZ, Litman GW, Mauceli E, Miyake T, Mueller MG, Nelson DR, Nitsche A, Olmo E, Ota T, Pallavicini A, Panji S, Picone B, Ponting CP, Prohaska SJ, Przybylski D, Saha NR, Ravi V, Ribeiro FJ, Sauka-Spengler T, Scapigliati G, Searle SM, Sharpe T, Simakov O, Stadler PF, Stegeman JJ, Sumiyama K, Tabbaa D, Tafer H, Turner-Maier J, van Heusden P, White S, Williams L, Yandell M, Brinkmann H, Volff JN, Tabin CJ, Shubin N, Schartl M, Jaffe DB, Postlethwait JH, Venkatesh B, Di Palma F, Lander ES, Meyer A, Lindblad-Toh K. The African coelacanth genome provides insights into tetrapod evolution. Nature. 2013;496:311–316. doi: 10.1038/nature12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SJ, Stappert J, Bauer A, Kispert A, Herrmann BG, Kemler R. Brachyury is a target gene of the Wnt/beta-catenin signaling pathway. Mech Dev. 2000;91:249–258. doi: 10.1016/s0925-4773(99)00309-3. [DOI] [PubMed] [Google Scholar]
- Bouldin CM, Manning AJ, Peng Y-H, Farr GHI, Hung KL, Dong A, Kimelman D. Wnt signaling and tbx16 form a bistable switch to commit bipotential progenitors to mesoderm. Development. 2015;142:2499–2507. doi: 10.1242/dev.124024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braasch I, Gehrke AR, Smith JJ, Kawasaki K, Manousaki T, Pasquier J, Amores A, Desvignes T, Batzel P, Catchen J, Berlin AM, Campbell MS, Barrell D, Martin KJ, Mulley JF, Ravi V, Lee AP, Nakamura T, Chalopin D, Fan S, Wcisel D, Canestro C, Sydes J, Beaudry FE, Sun Y, Hertel J, Beam MJ, Fasold M, Ishiyama M, Johnson J, Kehr S, Lara M, Letaw JH, Litman GW, Litman RT, Mikami M, Ota T, Saha NR, Williams L, Stadler PF, Wang H, Taylor JS, Fontenot Q, Ferrara A, Searle SM, Aken B, Yandell M, Schneider I, Yoder JA, Volff JN, Meyer A, Amemiya CT, Venkatesh B, Holland PW, Guiguen Y, Bobe J, Shubin NH, Di Palma F, Alfoldi J, Lindblad-Toh K, Postlethwait JH. The spotted gar genome illuminates vertebrate evolution and facilitates human-teleost comparisons. Nat Genet. 2016 doi: 10.1038/ng.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesley P. Development of the short-tailed mutant in the house mouse. J. Exp. Zool. 1935;70:429–435. [Google Scholar]
- Doyon Y, McCammon JM, Miller JC, Faraji F, Ngo C, Katibah GE, Amora R, Hocking TD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Amacher SL. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat Biotechnol. 2008;26:702–708. doi: 10.1038/nbt1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AL, Faial T, Gilchrist MJ, Down T, Vallier L, Pedersen RA, Wardle FC, Smith JC. Genomic targets of Brachyury (T) in differentiating mouse embryonic stem cells. PLoS One. 2012;7:e33346. doi: 10.1371/journal.pone.0033346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett AT, Han TM, Gilchrist MJ, Smith JC, Eisen MB, Wardle FC, Amacher SL. Identification of direct T-box target genes in the developing zebrafish mesoderm. Development. 2009;136:749–760. doi: 10.1242/dev.024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriock RJ, Chalamalasetty RB, Kennedy MW, Canizales LC, Lewandoski M, Yamaguchi TP. Lineage tracing of neuromesodermal progenitors reveals novel Wnt-dependent roles in trunk progenitor cell maintenance and differentiation. Development. 2015;142:1628–1638. doi: 10.1242/dev.111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch GE, Owens ND, Martin SR, Piccinelli P, Faial T, Trotter MW, Gilchrist MJ, Smith JC. In vivo T-box transcription factor profiling reveals joint regulation of embryonic neuromesodermal bipotency. Cell Rep. 2013;4:1185–1196. doi: 10.1016/j.celrep.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluecksohn-Schoenheimer S. The development of normal and homozygous Brachy (T/T) mouse embryos in the extraembryonic coelom of the chick. Proc. Natl. Acad. Sci. USA. 1944;30:134–140. doi: 10.1073/pnas.30.6.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goering LM, Hoshijima K, Hug B, Bisgrove B, Kispert A, Grunwald DJ. An interacting network of T-box genes directs gene expression and fate in the zebrafish mesoderm. Proc Natl Acad Sci U S A. 2003;100:9410–9415. doi: 10.1073/pnas.1633548100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouti M, Tsakiridis A, Wymeersch FJ, Huang Y, Kleinjung J, Wilson V, Briscoe J. In vitro generation of neuromesodermal progenitors reveals distinct roles for wnt signalling in the specification of spinal cord and paraxial mesoderm identity. PLoS Biol. 2014;12:e1001937. doi: 10.1371/journal.pbio.1001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin K, Patient R, Holder N. Analysis of FGF function in normal and no tail zebrafish embryos reveals separate mechanisms for formation of the trunk and the tail. Development. 1995;121:2983–2994. doi: 10.1242/dev.121.9.2983. [DOI] [PubMed] [Google Scholar]
- Griffin KJ, Amacher SL, Kimmel CB, Kimelman D. Molecular identification of spadetail: regulation of zebrafish trunk and tail mesoderm formation by T-box genes. Development. 1998;125:3379–3388. doi: 10.1242/dev.125.17.3379. [DOI] [PubMed] [Google Scholar]
- Halpern ME, Ho RK, Walker C, Kimmel CB. Induction of muscle pioneers and floor plate is distinguished by the zebrafish no tail mutation. Cell. 1993;75:99–111. [PubMed] [Google Scholar]
- Henrique D, Abranches E, Verrier L, Storey KG. Neuromesodermal progenitors and the making of the spinal cord. Development. 2015;142:2864–2875. doi: 10.1242/dev.119768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug B, Walter V, Grunwald DJ. tbx6, a Brachyury-related gene expressed by ventral mesendodermal precursors in the zebrafish embryo. Dev Biol. 1997;183:61–73. doi: 10.1006/dbio.1996.8490. [DOI] [PubMed] [Google Scholar]
- Jurberg AD, Aires R, Novoa A, Rowland JE, Mallo M. Compartment-dependent activities of Wnt3a/beta-catenin signaling during vertebrate axial extension. Dev Biol. 2014;394:253–263. doi: 10.1016/j.ydbio.2014.08.012. [DOI] [PubMed] [Google Scholar]
- Kimelman D. Tales of tails (and trunks): forming the posterior body in vertebrate embryos. Curr. Topics in Dev. Biol. 2016;116:517–536. doi: 10.1016/bs.ctdb.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangos S, Lam PY, Zhao A, Liu Y, Mudumana S, Vasilyev A, Liu A, Drummond IA. The ADPKD genes pkd1a/b and pkd2 regulate extracellular matrix formation. Dis Model Mech. 2010;3:354–365. doi: 10.1242/dmm.003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BL, Kimelman D. Regulation of canonical Wnt signaling by Brachyury is essential for posterior mesoderm formation. Dev Cell. 2008;15:121–133. doi: 10.1016/j.devcel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BL, Kimelman D. Canonical Wnt signaling dynamically controls multiple stem cell fate decisions during vertebrate body formation. Dev Cell. 2012;22:223–232. doi: 10.1016/j.devcel.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley RH, Lachani K, Keefe D, Gilchrist MJ, Flicek P, Smith JC, Wardle FC. A gene regulatory network directed by zebrafish No tail accounts for its roles in mesoderm formation. Proc Natl Acad Sci U S A. 2009;106:3829–3834. doi: 10.1073/pnas.0808382106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff MM, Turk E, Kalishman M. Web-based primer design for single nucleotide polymorphism analysis. Trends Genet. 2002;18:613–615. doi: 10.1016/s0168-9525(02)02820-2. [DOI] [PubMed] [Google Scholar]
- Odenthal J, Haffter P, Vogelsang E, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Warga RM, Allende ML, Weinberg ES, Nusslein-Volhard C. Mutations affecting the formation of the notochord in the zebrafish, Danio rerio. Development. 1996;123:103–115. doi: 10.1242/dev.123.1.103. [DOI] [PubMed] [Google Scholar]
- Olivera-Martinez I, Harada H, Halley PA, Storey KG. Loss of FGF-dependent mesoderm identity and rise of endogenous retinoid signalling determine cessation of body axis elongation. PLoS Biol. 2012;10:e1001415. doi: 10.1371/journal.pbio.1001415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, Farber SA, Ekker SC. p53 activation by knockdown technologies. PLoS Genet. 2007;3:e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Row RH, Tsotras SR, Goto H, Martin BL. The zebrafish tailbud contains two independent populations of midline progenitor cells that maintain long-term germ layer plasticity and differentiate in response to local signaling cues. Development. 2016;143:244–254. doi: 10.1242/dev.129015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvinsky I, Silver LM, Ho RK. Characterization of the zebrafish tbx16 gene and evolution of the vertebrate T-box family. Dev Genes Evol. 1998;208:94–99. doi: 10.1007/s004270050158. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, van Eeden FJ, Halpern ME, Kimmel CB, Nusslein-Volhard C. no tail (ntl) is the zebrafish homologue of the mouse T (Brachyury) gene. Development. 1994;120:1009–1015. doi: 10.1242/dev.120.4.1009. [DOI] [PubMed] [Google Scholar]
- Shestopalov IA, Pitt CL, Chen JK. Spatiotemporal resolution of the Ntla transcriptome in axial mesoderm development. Nat Chem Biol. 2012;8:270–276. doi: 10.1038/nchembio.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shestopalov IA, Sinha S, Chen JK. Light-controlled gene silencing in zebrafish embryos. Nat Chem Biol. 2007;3:650–651. doi: 10.1038/nchembio.2007.30. [DOI] [PubMed] [Google Scholar]
- Stemple DL, Solnica-Krezel L, Zwartkruis F, Neuhauss SC, Schier AF, Malicki J, Stainier DY, Abdelilah S, Rangini Z, Mountcastle-Shah E, Driever W. Mutations affecting development of the notochord in zebrafish. Development. 1996;123:117–128. doi: 10.1242/dev.123.1.117. [DOI] [PubMed] [Google Scholar]
- Talbot JC, Amacher SL. A streamlined CRISPR pipeline to reliably generate zebrafish frameshifting alleles. Zebrafish. 2014;11:583–585. doi: 10.1089/zeb.2014.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallafuss A, Gibson D, Morcos P, Li Y, Seredick S, Eisen J, Washbourne P. Turning gene function ON and OFF using sense and antisense photo-morpholinos in zebrafish. Development. 2012;139:1691–1699. doi: 10.1242/dev.072702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiridis A, Huang Y, Blin G, Skylaki S, Wymeersch F, Osorno R, Economou C, Karagianni E, Zhao S, Lowell S, Wilson V. Distinct Wnt-driven primitive streak-like populations reflect in vivo lineage precursors. Development. 2014;141:1209–1221. doi: 10.1242/dev.101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzouanacou E, Wegener A, Wymeersch FJ, Wilson V, Nicolas JF. Redefining the progression of lineage segregations during mammalian embryogenesis by clonal analysis. Dev Cell. 2009;17:365–376. doi: 10.1016/j.devcel.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Vonica A, Gumbiner BM. Zygotic Wnt activity is required for Brachyury expression in the early Xenopus laevis embryo. Dev Biol. 2002;250:112–127. doi: 10.1006/dbio.2002.0786. [DOI] [PubMed] [Google Scholar]
- Wardle FC, Papaioannou VE. Teasing out T-box targets in early mesoderm. Curr Opin Genet Dev. 2008;18:418–425. doi: 10.1016/j.gde.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymeersch FJ, Huang Y, Blin G, Cambray N, Wilkie R, Wong FC, Wilson V. Position-dependent plasticity of distinct progenitor types in the primitive streak. Elife. 2016;5 doi: 10.7554/eLife.10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi TP, Takada S, Yoshikawa Y, Wu N, McMahon AP. T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev. 1999;13:3185–3190. doi: 10.1101/gad.13.24.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]