Abstract
Previous studies suggest that there are sex differences in endocannabinoid function and the response to exogenous cannabinoids, though data from clinical studies comparing acute cannabinoid effects in men and women under controlled laboratory conditions are limited. To further explore these potential differences, data from 30 cannabis users (N=18 M, 12 F) who completed previous Δ9-tetrahydrocannabinol (Δ9-THC) discrimination studies were combined for this retrospective analysis. In each study, subjects learned to discriminate between oral Δ9-THC and placebo and then received a range of Δ9-THC doses (0, 5, 15 and a “high” dose of either 25 or 30 mg). Responses on a drug-discrimination task, subjective effects questionnaire, psychomotor performance tasks, and physiological measures were assessed. Δ9-THC dose-dependently increased drug-appropriate responding, ratings on “positive” visual analog scale (VAS) items (e.g., Good Effects, Like Drug, Take Again), and items related to intoxication (e.g., High, Stoned). Δ9-THC also dose-dependently impaired performance on psychomotor tasks and elevated heart rate. Sex differences on VAS items emerged as a function of dose. Women exhibited significantly greater subjective responses to oral drug administration than men at the 5 mg Δ9-THC dose, whereas men were more sensitive to the subjective effects of the 15 mg dose of Δ9-THC than women. These results demonstrate dose-dependent separation in the subjective response to oral Δ9-THC administration by sex, which might contribute to the differential development of problematic cannabis use.
Keywords: marijuana, cannabis, gender, drug-discrimination, self-report
1. Introduction
Cannabis (cannabis sativa, cannabis indica) is one of the most commonly abused drugs in the United States. Results from the 2013 Substance Abuse and Mental Health Services Administration (SAMHSA) National Survey on Drug Use and Health indicated that 24.6 million Americans aged 12 or older were recent (past month) illicit drug users, and cannabis users accounted for 80% of those individuals (19.8 million). Survey results also demonstrated that rates of recent cannabis use were greater in males (12.3 million) relative to females (7.5 million), and similarly, that rates of cannabis use disorders (CUDs) were greater in males (2.7 million) than females (1.5 million) (SAMHSA, 2014). Further, an analysis of recent Treatment Episode Data Set results (SAMHSA, Center for Behavioral Health Statistics and Quality, 2013) revealed that more males (223,026) than females (82,468) aged 12 or older were admitted to substance abuse treatment for cannabis as the primary substance of abuse.
Although males have higher prevalence rates of recent cannabis use, cannabis use disorders, and CUD treatment, females appear to be more susceptible to “telescoping,” which is the rapid progression from initial use to dependence, and from developing dependence to seeking treatment (Ehlers et al., 2010; Hernandez-Avila et al., 2004; Schepis et al., 2011; Westermeyer and Beodicker, 2000). For example, the University of California San Francisco Family Study of Alcoholism found that women had a shorter time from initial cannabis use to cannabis dependence than men (Ehlers et al., 2010). There also appear to be additional barriers to successful treatment for cannabis-dependent women. Compared to men, women abstaining from cannabis use reported more withdrawal symptoms, which have been linked to relapse (Copersino et al., 2010). Women with cannabis use disorders also present with higher rates of certain comorbid health problems. For example, upon treatment entry, females reported more mood and anxiety disorders (Agrawal et al., 2005; Khan et al., 2013). Co-occurring psychiatric and substance use disorders in women are frequently associated with poorer psychosocial functioning, medication non-compliance, worse treatment outcomes, including drop out, and higher relapse rates (Bernstein, 2000; Reed and Mowbray, 1999).
Sex differences in the underlying neurobiology of the endogenous cannabinoid system could contribute to the differential use and development of dependence observed in men and women described above, and have been observed in preclinical studies. For example, Castelli and colleagues (2013) found significantly greater cannabinoid CB1 receptor density in the prefrontal cortex and amygdala of male rats and ovariectomized female rats compared to cycling female rats and ovariectomized female rats receiving replacement estradiol. Region-specific sex differences in cannabinoid receptor mRNA and protein expression, as well as endocannabinoid concentrations, that varied as a function of gonadal sex hormones have been identified in other brain areas as well (Rodriguez de Fonseca et al., 1994; González et al., 2000). Preclinical studies have also found sex differences in the response to exogenously administered cannabinoids. For instance, using the behavioral “tetrad” assay of cannabinoid effects, Tseng and Craft (2001) demonstrated that several cannabinoid agonists including the primary active constituent of cannabis, Δ9-tetrahydrocannabinol (Δ9-THC), produced greater antinociceptive and motor effects in female rats compared to male rats. In another study, Higuera-Matas et al., (2012) found that repeated administration of the cannabinoid agonist CP 55,940 to adolescent rats disrupted the normal balance between glutamate and GABA transmission to a greater extent in females than in males. A study designed evaluate the influence of sex on the reinforcing effects of cannabinoids found that female rats acquired self-administration of the cannabinoid agonist WIN 55,212-2 at higher rates and with greater intake relative to male and ovariectomized female rats (Fattore et al., 2007).
Sensitivity to the behavioral, abuse-related effects of cannabis and cannabinoid agonists likely contributes to vulnerability to problematic use, but clinical studies that have focused on sex differences in these effects are limited. One recent report described a retrospective analysis of data combined from four prior studies that examined potential sex differences in the behavioral and physiological effects of placebo versus active (3.27%–5.50% Δ9-THC) smoked cannabis in frequent cannabis users (Cooper and Haney, 2014). That analysis indicated that females were more sensitive to some of the abuse-related subjective effects of smoked cannabis (e.g., Take Again and Good Effects) relative to males. There do not appear to be any previous studies that have evaluated potential sex differences in the abuse-related effects of orally administered Δ9-THC. Such research would be valuable because conditioned drug effects, expectancies, and/or peripheral cues could differentially impact the response to smoked cannabis in men and women. To this end, the present report combined data from seven previous studies to examine possible sex differences in the effects of a range of doses of oral Δ9-THC (Lile et al., 2015; 2014; 2012a; 2012b; 2011; 2010a; 2009).
2. Materials and Methods
In each of the studies included in the present analysis, the discriminative-stimulus, subject-rated, performance and physiological effects of a range of oral Δ9-THC doses (0, 5, 15 and a “high” dose, either 25 or 30 mg) were determined. In addition, each study tested various other drug conditions either alone or alone and in combination with Δ9-THC (see below), but only data from sessions in which Δ9-THC was administered alone were included in the present analysis. When a subject completed more than one study, only data from the first study that the subject completed were included here.
2.1 Subjects
Data from thirty subjects (18 males and 12 females) who self-reported current cannabis use were analyzed. To maximize the number of subjects who could be included, and considering the retrospective nature of the analysis, males and females were not matched on cannabis use; however, cannabis use (i.e., days per week and years of use) did not significantly differ as a function of sex (see section 2.3). Subjects were predominantly White (1 Black, 1 White-Hispanic, 1 multiracial White/Asian) adults with a history of cannabis use who were recruited from the local community. Subjects completed demographic, drug-use history, and medical history questionnaires, as well as medical screens. Individuals with current or past histories of Axis I disorders according to DSM-IV criteria (American Psychiatric Association, 2000), including substance dependence other than nicotine, were excluded from participation. Substance abuse was not an exclusion criterion. No recent illicit drug use except for cannabis was detected during screening with two exceptions; one subject tested positive for cocaine and one subject tested positive for benzodiazepines during the medical screening. The Institutional Review Board of the University of Kentucky Medical Center approved the studies and the informed consent document.
2.2 General Procedures
All subjects were enrolled as outpatients at the University of Kentucky. They completed two drug-free practice sessions to become familiarized with the procedures prior to completing the study proper. Subjects were informed that they would receive various drugs including placebo and Δ9-THC but were blind to the dose and order of administration. They were asked to abstain from illicit drugs other than cannabis, and to abstain from over-the-counter medications, other than non-steroidal anti-inflammatory analgesics, for the duration of the experiment. Subjects were also asked to abstain from any drug use on the day of experimental sessions to avoid potentially unsafe drug interactions. In addition, subjects were asked to refrain from food or caffeine intake for 4 h prior to each experimental session, or alcohol for 12 h prior to and following each experimental session. Subjects who smoked tobacco cigarettes were also asked to abstain from smoking the morning of each session, but were allowed to smoke a single tobacco cigarette upon arrival to the laboratory to avoid testing under conditions of nicotine withdrawal, but not again until the session had ended. There was no indication of nicotine withdrawal in these subjects.
Experimental sessions were conducted at a fixed time, Monday through Friday, and lasted approximately 6.5–7.5 h; subjects typically participated in 1 to 5 sessions per week. At the beginning of each session, breath and urine tests to assess substance use and pregnancy were conducted. Urine samples were negative for recent use of drugs other than cannabis and those administered experimentally. Pregnancy screening results were negative throughout the studies.
At session intake, subjects also completed a modified version of the U.S. Department of Transportation Drug Evaluation and Classification Screening (walk and turn, timed one-leg balance or Romberg balance, time interval reproduction and the finger-to-nose tests; [Toland and Green, 1991]) and were observed by trained research staff for signs of cannabis intoxication (e.g., bloodshot, glassy eyes). Subjects were also asked to self-report their cannabis use in the past 24 h. No cannabis intoxication was detected during intake throughout the studies as verified by trained research staff and self-report. Furthermore, because oral Δ9-THC was not administered for approximately 30–60 min after arrival to the laboratory and considering that plasma Δ9-THC concentrations typically peak within 10 min and return to near-baseline levels within 1 h of smoked administration (Huestis et al., 1992), the impact of any undetected cannabis use prior to the start of the session on study outcomes would have been minimal. Subjects were reassessed at the end of the session for possible intoxication and/or residual drug effects prior to release. In addition, subjects were required to report no further drug effects. Occasionally subjects were retained at the laboratory beyond the scheduled session time until residual drug effects dissipated.
2.3 Drug-Discrimination Procedure
Subjects learned to discriminate between a “Drug X” condition (i.e., 25 or 30 mg Δ9-THC) and a “Not Drug X” condition (i.e., placebo) as described previously in detail (e.g., Lile et al., 2009). During a sampling phase, subjects received the training dose of Δ9-THC (25 or 30 mg), identified by a unique letter code (e.g., Drug X). During a control phase, subjects were required to correctly identify when they received placebo (i.e., Not Drug X) or the training dose of Δ9-THC (Drug X). The criterion for having acquired the discrimination was ≥ 80% correct responding on the final assessment of the drug-discrimination task for four consecutive sessions (two sessions each for the Drug X and Not Drug X conditions). Finally, a test phase was conducted in which subjects received placebo, Δ9-THC (5, 15 and 25 or 30 mg) and a range of doses of various drugs alone (methylphenidate, hydromorphone, triazolam and nabilone) or alone and in combination with Δ9-THC (i.e., nabilone, tiagabine, baclofen, diazepam, and gabapentin). Each drug dose or dose combination was administered once, with the exception of the training conditions, which were administered throughout the test phase to monitor and provide feedback about drug-discrimination performance. The present analysis included data only from sessions in which Δ9-THC and placebo were administered alone.
2.4 Outcome measures
2.4.1. Drug-Discrimination Task
Two circles labeled Drug X and Not Drug X and associated counters were displayed on a computer screen. Button presses increased the counter for a particular circle according to a fixed-interval 1-s schedule for 60 s (no change-over delay). At the end of the final assessment, subjects were informed whether it was a control or a test session. During control sessions, points accumulated on the correct option were exchangeable for money (up to approximately $50.00/session). During test sessions, when drugs and/or doses other than the control conditions were administered, subjects earned the average from all previous sessions in which control conditions were tested. The dependent variable for this task was the percent responding on the drug-appropriate option at the final assessment of the session.
2.4.2. Visual Analog Scale (VAS) Subject-Rated Drug-Effect Questionnaire
Subjects rated 18 items (see Lile et al., 2012a) that assessed general “positive” (e.g., Good Effects, Like Drug) and “negative” (e.g., Bad Effects, Nauseated) drug effects, as well as items specific to cannabis intoxication (e.g., Stoned). Subjects rated items presented individually on the computer by marking a 100-unit line anchored on the extremes by “Not At All” and “Extremely.”
2.4.3. Performance Tasks
These tasks were chosen because prior research has found them to be sensitive to the impairing effects of oral Δ9-THC (Hart et al., 2005; Kamien et al., 1994) and smoked cannabis (Heishman et al., 1989; Kelly et al., 1993; 1990; Wilson et al., 1994). Subjects did not receive additional compensation based on task performance.
2.4.3.1. Repeated Acquisition of Response Sequences Task (RA task)
During the initial acquisition component, subjects pressed 4 keys (1, 3, 7 and 9) on a numeric keypad to learn a new, randomly-determined 10-response sequence (a “chain”) for 180-s. When a correct key in the sequence was pressed, a “position” counter on the screen increased by 1. When the tenth and final key in the sequence was pressed, a “points” counter increased by one, and the position counter reset. A 60-s performance component of this task, in which the 10-response sequence remained the same across trials, followed the acquisition component. The primary dependent measures for this task were the number of chains completed (i.e., accuracy) and the total number of responses emitted (i.e., response rate).
2.4.3.2. Digit-Symbol-Substitution Test (DSST)
A modified version of the computerized DSST was used (McLeod et al., 1982). Briefly, subjects used a numeric keypad to enter the geometric pattern associated with one of nine patterns identified on a given trial for 90s. The dependent measures were the number of patterns the subject entered correctly (i.e., trials correct; accuracy) and the total number of patterns entered (i.e., trials completed; response rate).
2.4.3.3. Time Reproduction Task
Two time periods, 30, and 60s and were presented. Subjects responded to start a timer, and held down the response key until they believed that the interval had elapsed.
2.4.4. Physiological Indices
2.4.4.1. Heart Rate and Blood Pressure
Heart rate and blood pressure were recorded using an automated monitor (DINAMAP, Johnson and Johnson, Alexandria, TX).
2.4.4.2. Temperature
An infrared thermographic scanner (Derma-Temp, Exergen Corporation, Watertown, MA) was used to measure skin temperature on the tip of the index finger. An electronic thermometer was used to measure oral temperature.
2.5. Drug Administration
All drug conditions were administered in a double-blind fashion. Doses were prepared by encapsulating commercially available capsules of dronabinol (Δ9-THC in sesame oil, Solvay Pharmaceuticals, Marietta, GA) in opaque green size 00 capsules. Cornstarch was used to fill the remainder of all capsules. Placebo capsules contained only cornstarch. Capsules were prepared by the University of Kentucky Medical Center Investigational Drug Service Pharmacy. For reference, the acute recommended Δ9-THC dosing range in adults for appetite stimulation and the prevention of nausea and vomiting is 2.5 to 20 mg (Marinol® product information).
2.6. Data Analyses
Demographic variables (weight, age, cannabis, alcohol, cigarette, and caffeine use history) were compared using two-sample t-tests. For the high dose (i.e., 25 or 30 mg) Δ9-THC and placebo conditions, data were averaged across the sessions in which these conditions were presented during the test phase. Raw data from the self-reported drug-effect questionnaires, performance tasks and physiological measures were analyzed for each drug as area-under-the curve (AUC) from data collected hourly for five hours after Δ9-THC administration. In order to 1) incorporate data from all time points, 2) more closely correspond with the analytical strategy and 3) facilitate interpretation of the figures (i.e., provide values that approximate raw data), AUC values were calculated and then divided by the number of post-drug data points used to calculate AUC for graphical representation.
AUC outcomes corresponding to performance and physiological measures were analyzed using linear mixed models. Skewed values for AUC outcomes, corresponding to items on the Visual Analog Scale, were handled by using generalized linear mixed modeling. Specifically, AUC values were rounded up to the closest integer value, and generalized poisson models for overdispersed count data were utilized. All models were mixed, as random subject effects were used to account for subjects contributing an AUC outcome at each dose.
Results for overall main effects or interactions of Δ9-THC and Sex in a given model were judged by a Type III F test. Significant effects were followed by pairwise comparisons of individual data points, with the likelihood of Type I error minimized by limiting comparisons to planned pairs. More specifically, if a main effect of Δ9-THC attained statistical significance, planned comparisons of active drug doses to placebo were conducted; if an interaction of Δ9-THC and Sex attained statistical significance, each dose of Δ9-THC was compared across Sex. For all measures, effects were considered significant for p ≤ 0.05. Analyses were conducted using SAS v9.4 (SAS Institute Inc., Cary, NC).
3. Results
3.1. Demographics
Table 1 presents the age, weight and self-reported daily caffeine and cigarette use, weekly alcohol and cannabis use, and number of years using cannabis, for male and female subjects. Men and women did not differ on demographic variables or substance use patterns, except that the men were significantly older.
Table 1.
Subject Demographic Characteristics: Means, standard deviations, and t-values from independent samples t-tests. Bold values indicate a significant difference (p < 0.05).
| Demographics | Male (N = 18) | Female (N = 12) | t-value (df = 28) |
|---|---|---|---|
| Age | 23.39 ± 0.86 | 21.08 ± 0.68 | 2.10 |
| Weight (kg) | 78.90 ± 4.25 | 68.98 ± 4.22 | 1.59 |
| Years of Cannabis Use | 7.47 ± 1.19 | 4.41 ± 0.93 | 1.41 |
| Cannabis Use (days per week) | 4.5 ± 0.54 | 4.63 ± 0.72 | 1.85 |
| Cigarette Use (# per day) | 3.28 ± 1.41 | 2.67 ± 1.65 | 0.30 |
| Alcohol Use (days per week) | 1.93 ± 0.33 | 1.40 ± 0.35 | 1.08 |
| Caffeine Use (mg per day) | 161.61 ± 60.41 | 113.17 ± 48.64 | 0.58 |
3.2. Drug-Discrimination Task
Men and women did not differ significantly in terms of the number of trials needed to acquire the Δ9-THC discrimination (mean of 4.89 and 4.92 trials, respectively). A main effect of Δ9-THC was detected for percentage of drug appropriate responding (F3, 84 = 41.56, p < 0.01). The effects of Δ9-THC on percentage of drug appropriate responding did not vary significantly as a function of Sex.
3.3. Subject Ratings
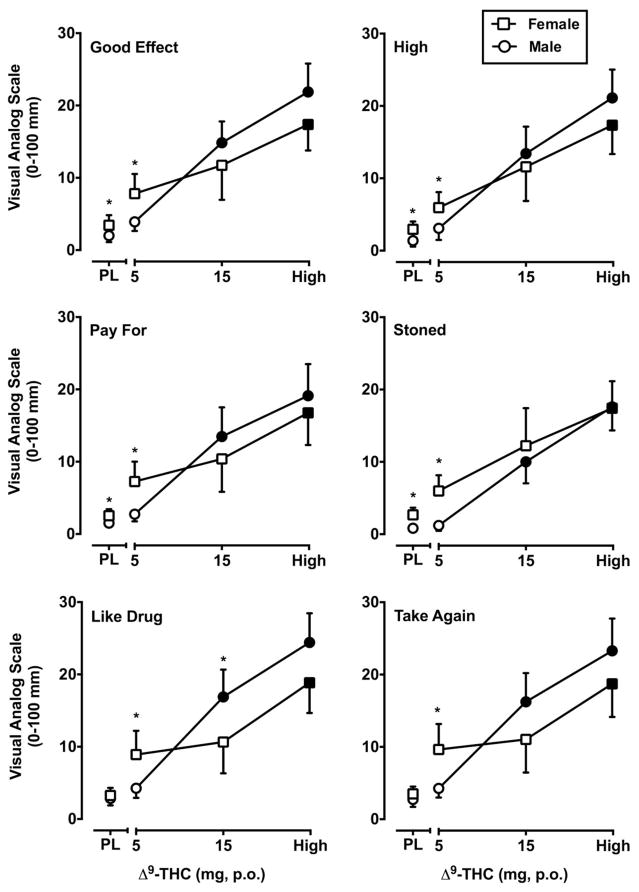
The interaction of Δ9-THC and Sex attained statistical significance for nine items from the VAS: Any Effect (F3, 84 = 5.53, p < 0.01), Confused/Difficulty Concentrating (F3, 84 = 5.21, p < 0.01), Good Effect (F3, 84 = 6.28, p < 0.01), High (F3, 84 = 7.41, p < 0.01), Like Drug (F3, 84 = 6.36, p < 0.01), Pay For (F3, 84 = 4.41, p < 0.01), Stimulated (F3, 84 = 5.12, p < 0.01), Stoned (F3, 84 = 7.56, p < 0.01) and Take Again (F3, 84 = 4.68, p < 0.01). Figure 1 presents the items Good Effect, High, Pay For, Stoned, Like Drug and Take Again. As illustrated in that figure, Δ9-THC dose-dependently increased ratings on each of these items. Comparisons of sex at each Δ9-THC dose revealed that, in general, women had a greater subjective response following administration of 5 mg Δ9-THC compared to men, although that dose did not engender subjective effects that were significantly greater than placebo in either sex. In contrast, men were more sensitive to the subjective effects of the 15 mg Δ9-THC dose. More specifically, 15 mg Δ9-THC significantly increased ratings on those VAS items in men only, and for the item Like Drug, male subjects’ ratings were significantly greater than those of female subjects. Sex differences also emerged following administration of placebo for the items Confused/Difficulty Concentrating, Good Effect, High, Pay For, and Stoned, with women reporting exhibiting a greater subjective response following placebo administration, but no differential responding by sex at active doses.
Figure 1.
Visual Analog Scale ratings of Good Effect, High, Pay For, Stoned, Like Drug and Take Again as a function of Δ9-THC dose and Sex. The x-axis represents Δ9-THC dose in mg; PL denotes placebo. Squares indicate female subjects (N=12) and circles indicate male subjects (N=18). Filled symbols indicate values that are significantly different from placebo. Asterisks indicate significant sex differences at each dose condition. The y-axis represents VAS data in area-under-the-curve (AUC) values. In order to incorporate data from all time points and more closely correspond with the analytical strategy data are presented as area-under-the-curve (AUC) values divided by the number of post-drug data points used to calculate AUC. Unidirectional brackets indicate 1 SEM.
Δ9-THC also dose-dependently increased ratings of Bad Effect, Dizzy/Lightheaded, Forgetful, Hungry, Shaky/Jittery, Suspicious, and Thirsty (F’s3,84 = 3.03–13.21, p’s < 0.05), but these effects did not vary significantly as a function of Sex.
3.4. Physiological Effects
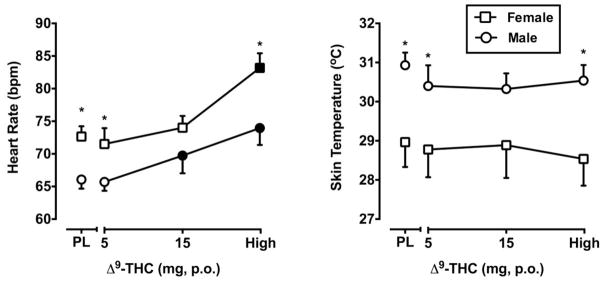
Main effects of Δ9-THC (F3, 84 = 23.69, p < 0.01) and Sex (F3, 84 = 6.80, p < 0.05) were detected for heart rate. The highest doses of Δ9-THC significantly elevated heart rate in male and female subjects relative to placebo. Heart rate was significantly higher in women after administration of the 5 mg and “high” doses of Δ9-THC, but also following placebo, suggesting that the differential heart rate was independent of Δ9-THC dose (Figure 2).
Figure 2.
Peak heart rate (maximum value) and skin temperature (minimum value) as a function of Δ9-THC dose and Sex. All other details are as in Figure 1.
A main effect of Sex was found for skin temperature (F3, 84 = 6.37, p < 0.05). Women had lower skin temperature following administration of the 5 mg and “high” doses of Δ9-THC as well as placebo. Δ9-THC did not influence skin temperature (Figure 2). Systolic blood pressure, diastolic blood pressure and oral temperature did not vary as a function of Δ9-THC or Sex.
3.5. Performance Effects
3.5.1. Digit-Symbol Substitution Task
Δ9-THC dose dependently impaired accuracy on the DSST as measured by the number of correct trials (F3, 84 = 3.25, p < 0.05); the number of trials completed (i.e., rate) was not impacted by Δ9-THC (data not shown). The effects of Δ9-THC on the DSST did not vary significantly as a function of Sex.
3.5.2. Repeated Acquisition of Response Sequences Task
Δ9-THC dose dependently impaired performance on the RA task as measured by the number of chains completed (accuracy; F3, 84 = 6.99, p < 0.01) and the total number of responses emitted (rate; F3, 84 = 6.98, p < 0.01; data not shown). The effects of Δ9-THC on the RA Task did not vary significantly as a function of Sex. In addition, Δ9-THC dose dependently impaired accuracy on the performance component of the RA task (F3, 84 = 2.86, p < 0.05), but the effects of Δ9-THC did not vary significantly as a function of Sex (data not shown). Neither Δ9-THC nor Sex impacted rate on the performance component of the RA task.
4. Discussion and Conclusions
The present study demonstrated dose dependent sex differences in the subjective response to oral Δ9-THC administration. Women exhibited greater subjective responses to oral drug administration than men at the 5 mg Δ9-THC dose, whereas men were more sensitive to the subjective effects of the 15 mg dose of Δ9-THC than women. These results differ from a prior retrospective analysis that examined sex differences in the response to acutely administered smoked cannabis in frequent cannabis smokers and found that women were more sensitive to abuse-related subjective effects relative to men on some drug effect questionnaire items (Cooper and Haney, 2014). The reasons for the discrepancies between the results of that study and the present study are unknown, but could be due to procedural factors such as route of administration, the amount of Δ9-THC delivered, testing multiple Δ9-THC doses versus a single Δ9-THC concentration per study, and/or subject differences such as cannabis use history and race/ethnicity. For example, the Cooper and Haney study administered a single active concentration of smoked cannabis (ranging from 3.27%–5.50% Δ9-THC, varying by study) containing other phytocannabinoids, whereas the present study used a range of Δ9-THC doses (5–30 mg), administered orally. In addition, participants in the prior analysis were predominantly Black and reported smoking cannabis at least four times per day, approximately 7 days a week in the four weeks prior to screening. In contrast, participants in the present analysis were predominantly White and reported smoking cannabis an average of 4.5 days per week in the month prior to screening. A prospective study to test potential sex differences of cannabinoids administered via commonly used routes (i.e., oral and smoked) in a sample with greater racial/ethnic diversity would be useful to disentangle whether these factors account for the discordant results from these retrospective analyses.
The pattern of differential sensitivity to low and high doses of Δ9-THC administration as a function of sex observed in the present analysis is reminiscent of the response to Δ9-THC observed in frequent vs. infrequent cannabis users (Kirk and de Wit, 1999). In that study, frequent users (self-reported cannabis use greater than 100 times) reported increased ratings of “feel drug” at the lower dose compared to the infrequent users (self-reported cannabis use less than 100 times). However, at the higher dose, infrequent users reported increased ratings of “feel drug” compared to frequent users. The present study suggests that, like frequent users, females may be more likely to report drug effects at lower doses but are more tolerant to higher doses. Greater tolerance to cannabinoid effects in women could result in more frequent use or use of a larger amount of cannabis, which might be a contributing factor to the telescoping effect observed in women.
The underlying factors contributing to sex differences in the abuse-related subjective effects of smoked cannabis and Δ9-THC are unknown. The differential response does not appear to be due to pharmacokinetic factors, as the absorption, disposition, metabolism and excretion of Δ9-THC following oral and intravenous administration did not differ in men and women (Wall et al., 1983). Similarly, although fixed doses were administered in each study that was included in the present analysis, male and female subjects did not differ significantly in weight, suggesting that functional dose differences cannot account for the separation in subjective effects between men and women.
Another possible explanation is that the composition of the endogenous cannabinoid system is distinct in women, as suggested by preclinical studies (e.g., Castelli et al., 2013; González et al., 2000; Rodriguez de Fonseca et al., 1994). Further, the effects of cannabinoids in women could be subject to dynamic changes as a function of fluctuating gonadal sex hormones (i.e., estrogen and progesterone). For example, CB1 receptor density in the medial basal hypothalamus and expression in the anterior pituitary varies across the estrous cycle in rodents (Rodriguez de Fonseca et al., 1994). Further, Craft and Leitl (2008) followed up on earlier work that compared cannabinoid-induced antinociception in male and female rats, and demonstrated that estrogen administration enhanced the antinociceptive effects of Δ9-THC in ovariectomized females. A more recent study that assessed the influence of gonadal sex hormones on withdrawal from repeated Δ9-THC administration in rats suggested that estradiol and progesterone could promote the development of dependence, whereas testosterone might be protective (Marusich et al., 2015).
Some previous clinical studies have also demonstrated that menstrual cycle phase, gonadal sex hormone levels and the administration of these hormones are associated with altered abuse-related behavioral effects of psychoactive drugs. For example, two studies that tested d-amphetamine across menstrual cycle phase found that women rated certain positive subject-rated drug effect questionnaire items higher during the early-to-mid follicular phase, when only estrogen levels are increasing, relative to the luteal phase, when both estrogen and progesterone are elevated (Justice and de Wit, 2000a; White et al., 2002). Similarly, another study showed that administration of exogenous estradiol when endogenous hormone levels were low enhanced the subjective and discriminative-stimulus effects of d-amphetamine (Lile et al., 2007). In a series of studies, Babalonis and colleagues found that the interoceptive effects of triazolam were affected by menstrual cycle phase (2008) and concurrent progesterone administration (2011). Only a few clinical studies have examined potential interactions of cannabis with the menstrual cycle. In one study, self-reported cannabis use did not vary with cycle phase (Griffin et al., 1986). A second study that examined the effects of controlled administration of smoked cannabis containing a low concentration (1.8%) of Δ9-THC in women on mood and heart rate did not find significant differences on any measure across menstrual cycle phases (Lex et al., 1984). Worth noting, however, is that clinical studies with abused drugs other than cannabis have not always revealed hormone-related differences in subjective effects (e.g., Justice and de Wit, 2000a, 2000b; Mendelson et al., 1999). Also worth noting is that the data in the present analysis and previous studies that have reported sex differences in the subjective response to cannabinoids (Cooper and Haney, 2009, 2014) were collected without regard to menstrual cycle phase, which might have limited the ability to detect sex differences, and more specifically, the influence of gonadal sex hormones, on cannabinoid effects. Further research would be useful to better understand the relationship between cycle phase, hormones, and cannabinoid effects.
The present analysis did not reveal sex differences in the number of trials needed to acquire the Δ9-THC discrimination or in the Δ9-THC discrimination dose-response curves. Consistent with these results, two previous retrospective analyses using drug discrimination data did not find sex differences in the discriminative-stimulus effects of triazolam (Vansickel et al., 2006) or d-amphetamine (Vansickel et al., 2007). As described above, in drug discrimination studies subjects must acquire the discrimination before continuing to a test phase in which full dose-response curves are determined. This training provides similar recent behavioral and pharmacological histories, which are important determinants of drug effects (e.g., Lile et al., 2000; Nader and Reboussin, 1994), and which might have limited the ability to detect individual differences in drug effects in the test phase (Kamien et al., 1995; Singha et al., 1999). However, the present study and the analysis of the combined d-amphetamine discrimination data (Vansickel et al., 2007), which had approximately equivalent sample sizes (N = 30 and 27, respectively), detected sex differences for some of the subject-rated drug-effects questionnaire items. Together, these results demonstrate that sex differences in the subjective response to drugs can be sufficiently robust as to emerge despite the influence of the recent discrimination training.
Sex differences were also observed for heart rate and skin temperature after administration of two active doses of Δ9-THC. However, similar differences between males and females were also found following placebo administration, suggesting that these results were due to baseline physiological differences rather than Δ9-THC. The observation that women had elevated heart rate and lower skin temperature relative to men is consistent with previously documented differences in women and men for these physiological outcomes (Christensen et al., 2012; Ryan et al., 1994; Umetani et al., 1998). Another possibility is that chronic cannabis administration might result in further sex-based separation in baseline heart rate and skin temperature, considering that these measures are sensitive to acute cannabinoid administration (e.g., Lile et al., 2010b).
A notable limitation of the present analysis is that, due to the retrospective nature of the design, groups were not matched on all cannabis use variables. At the time of study screening, subjects reported the number of days per week and the total number years of cannabis use. However, the total number of cannabis use instances per week and the total lifetime instances of use, which could impact sensitivity to the effects of oral Δ9-THC, were only available for a subset of subjects who participated in the later studies, and were therefore not included in the analysis. An exploratory analysis of potential sex differences in this smaller subset of subjects suggested that the present findings were not due to group differences in the number of weekly cannabis use instances (N = 6F, 16.83 ± 5.50 [MEAN ± SEM] uses, 13M, 9.08 ± 2.09; t = 1.62, ns) or estimated total lifetime cannabis use (N = 9F, 4330 ± 3655, 12M, 4597 ± 1825; t = 0.07, ns).
In conclusion, dose-dependent sex differences in the subjective response to oral Δ9-THC were observed. These sex differences were apparent despite the similar recent behavioral and pharmacological histories imposed by the drug-discrimination training, and despite the fact that testing was done irrespective of menstrual cycle phase. The existing clinical studies of sex differences in cannabinoid effects under controlled laboratory conditions are limited and the results are mixed, suggesting that further research is warranted. A better understanding of how differences in the subjective response to cannabis in women might contribute to their more rapid progression to dependence is needed. Further, a more comprehensive investigation into sex-related factors in the etiology of cannabis use disorders should be conducted in an effort to address the unique treatment barriers for women, such as comorbid psychiatric disorders and greater cannabis withdrawal signs and symptoms.
Highlights.
A retrospective analysis using data from prior drug discrimination studies in humans was conducted to examine potential sex differences in the acute effects of Δ9-THC.
Subjects received a range of Δ9-THC doses (0, 5, 15 and a “high” dose of either 25 or 30 mg); drug effects were assessed using drug discrimination, subjective effects, cognitive/psychomotor performance and physiological measures.
Dose-dependent sex differences in some of the subjective effects of oral Δ9-THC administration were found, which might contribute to the differential development of dependence in men and women.
Acknowledgments
This research and the preparation of this manuscript were supported by grants awarded to Dr. Joshua Lile (National Institute on Drug Abuse [NIDA] grants K01 DA018772, K02 DA031766, R01 DA025605 and R01 DA036550), a NIDA T32 training grant DA035200, and a National Center for Advancing Translational Sciences grant awarded to the University of Kentucky Center for Clinical and Translational Science (UL1TR000117). These sponsors were not directly involved in the study design, collection, analysis and interpretation of the data, the writing of the report or the decision to submit the article for publication.
The authors wish to express their gratitude to the expert technical assistance of the staff at the University of Kentucky Residential Research Facility, Investigational Pharmacy Services, and Department of Behavioral Science.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal A, Gardner CO, Prescott CA, Kendler KS. The differential impact of risk factors on illicit drug involvement in females. Social Psychiatry and Psychiatric Epidemiology. 2005;40(6):454–466. doi: 10.1007/s00127-005-0907-0. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Washington, DC: 2013. [Google Scholar]
- Babalonis S, Emurian CS, Martin CA, Lile JA, Kelly TH. Modulation of the discriminative stimulus effects of triazolam across the menstrual cycle phase in healthy pre-menopausal women. Drug and alcohol dependence. 2008;94(1):276–280. doi: 10.1016/j.drugalcdep.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babalonis S, Lile JA, Martin CA, Kelly TH. Physiological doses of progesterone potentiate the effects of triazolam in healthy, premenopausal women. Psychopharmacology. 2011;215(3):429–439. doi: 10.1007/s00213-011-2206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP. Childhood trauma and drug addiction: Assessment, diagnosis, and treatment. Alcoholism Treatment Quarterly. 2000;18(3):19–30. [Google Scholar]
- Castelli MP, Fadda P, Casu A, Spano MS, Casti A, Fratta W, Fattore L. Male and female rats differ in brain cannabinoid CB1 receptor density and function and in behavioural traits predisposing to drug addiction: effect of ovarian hormones. Current Pharmaceutical Design. 2014;20(13):2100–2113. doi: 10.2174/13816128113199990430. [DOI] [PubMed] [Google Scholar]
- Christensen J, Væth M, Wenzel A. Thermographic imaging of facial skin—gender differences and temperature changes over time in healthy subjects. Dentomaxillofacial Radiology. 2012;41(8):662–667. doi: 10.1259/dmfr/55922484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. Comparison of the subjective, physiologic and pharmacokinetic effects of marijuana smoked in cigarette paper (joints) versus cigar paper (blunts) Drug and Alcohol Dependence. 2009;103(3):107–113. doi: 10.1016/j.drugalcdep.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug and Alcohol Dependence. 2014;136:85–91. doi: 10.1016/j.drugalcdep.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, Simmons MS, Gorelick DA. Sociodemographic characteristics of cannabis smokers and the experience of cannabis withdrawal. The American Journal of Drug and Alcohol Abuse. 2010;36(6):311–319. doi: 10.3109/00952990.2010.503825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Leitl MD. Gonadal hormone modulation of the behavioral effects of delta9-tetrahydrocannabinol in male and female rats. European Journal of Pharmacology. 2008;578(1):37–42. doi: 10.1016/j.ejphar.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Gizer IR, Vieten C, Gilder DA, Stouffer GM, Lau P, Wilhelmsen KC. Cannabis dependence in the San Francisco Family Study: age of onset of use, DSM-IV symptoms, withdrawal, and heritability. Addictive Behaviors. 2010;35(2):102–110. doi: 10.1016/j.addbeh.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Angius P, Fadda W, Fratta W. Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. British Journal of Pharmacology. 2007;152(5):795–804. doi: 10.1038/sj.bjp.0707465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S, Bisogno T, Wenger T, Manzanares J, Milone A, Berrendero F, Di Marzo V, Ramos JA, Fernandez-Ruiz JJ. Sex steroid influence on cannabinoid CB 1 receptor mRNA and endocannabinoid levels in the anterior pituitary gland. Biochemical and biophysical research communications. 2000;270(1):260–266. doi: 10.1006/bbrc.2000.2406. [DOI] [PubMed] [Google Scholar]
- Griffin ML, Mendelson JH, Mello NK, Lex BW. Marihuana use across the menstrual cycle. Drug and Alcohol Dependence. 1986;18(2):213–224. doi: 10.1016/0376-8716(86)90053-0. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Comer SD, Foltin RW. Reinforcing effects of oral Δ9-THC in male marijuana smokers in a laboratory choice procedure. Psychopharmacology. 2005;181(2):237–243. doi: 10.1007/s00213-005-2234-2. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Stitzer ML, Yingling JE. Effects of tetrahydrocannabinol content on marijuana smoking behavior, subjective reports, and performance. Pharmacology Biochemistry and Behavior. 1989;34(1):173–179. doi: 10.1016/0091-3057(89)90369-9. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis-and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug and Alcohol Dependence. 2004;74(3):265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Higuera-Matas A, Miguéns M, Coria SM, Assis MA, Borcel É, del Olmo N, Ambrosio E. Sex-specific disturbances of the glutamate/GABA balance in the hippocampus of adult rats subjected to adolescent cannabinoid exposure. Neuropharmacology. 2012;62(5):1975–1984. doi: 10.1016/j.neuropharm.2011.12.028. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. Journal of Analytical Toxicology. 1992;16(5):276–282. doi: 10.1093/jat/16.5.276. [DOI] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of estradiol pretreatment on the response to d-amphetamine in women. Neuroendocrinology. 2000a;71(1):51–59. doi: 10.1159/000054520. [DOI] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of d-amphetamine during the early and late follicular phases of the menstrual cycle in women. Pharmacology Biochemistry and Behavior. 2000b;66(3):509–515. doi: 10.1016/s0091-3057(00)00218-5. [DOI] [PubMed] [Google Scholar]
- Kamien JB, Bickel WK, Higgins ST, Hughes JR. The effects Δ9-tetrahydrocannabinol on repeated acquisition and performance of response sequences and on self-reports in humans. Behavioural Pharmacology. 1994;5(1):71–78. doi: 10.1097/00008877-199402000-00008. [DOI] [PubMed] [Google Scholar]
- Kamien JB, Bickel WK, Oliveto AH, Higgins ST, Hughes JR, Richards AT, Badger GJ. Placebo-effects contribute to differences in the acquisition of drug discrimination by humans: a retrospective analysis. Behavioural Pharmacology. 1995;6(2):187–194. [PubMed] [Google Scholar]
- Kelly TH, Foltin RW, Emurian CS, Fischman MW. Multidimensional behavioral effects of marijuana. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1990;14(6):885–902. doi: 10.1016/0278-5846(90)90075-r. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Foltin RW, Emurian CS, Fischman MW. Performance-based testing for drugs of abuse: dose and time profiles of marijuana, amphetamine, alcohol, and diazepam. Journal of Analytical Toxicology. 1993;17(5):264–272. doi: 10.1093/jat/17.5.264. [DOI] [PubMed] [Google Scholar]
- Khan SS, Secades-Villa R, Okuda M, Wang S, Pérez-Fuentes G, Kerridge BT, Blanco C. Gender differences in cannabis use disorders: Results from the National Epidemiologic Survey of Alcohol and Related Conditions. Drug and Alcohol Dependence. 2013;130(1):101–108. doi: 10.1016/j.drugalcdep.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk JM, de Wit H. Responses to oral Δ9-tetrahydrocannabinol in frequent and infrequent marijuana users. Pharmacology Biochemistry and Behavior. 1999;63(1):137–142. doi: 10.1016/s0091-3057(98)00264-0. [DOI] [PubMed] [Google Scholar]
- Lex BW, Mendelson JH, Bavli S, Harvey K, Mello NK. Effects of acute marijuana smoking on pulse rate and mood states in women. Psychopharmacology. 1984;84(2):178–187. doi: 10.1007/BF00427443. [DOI] [PubMed] [Google Scholar]
- Lile JA, Morgan D, Freedland CS, Sinnott RS, Davies HM, Nader MA. Self-administration of two long-acting monoamine transport blockers in rhesus monkeys. Psychopharmacology. 2000;152(4):414–421. doi: 10.1007/s002130000554. [DOI] [PubMed] [Google Scholar]
- Lile JA, Kendall SL, Babalonis S, Martin CA, Kelly TH. Evaluation of estradiol administration on the discriminative-stimulus and subject-rated effects of d-amphetamine in healthy pre-menopausal women. Pharmacology Biochemistry and Behavior. 2007;87(2):258–266. doi: 10.1016/j.pbb.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Pinsky DJ, Hays LR. Substitution profile of Δ9-tetrahydrocannabinol, triazolam, hydromorphone, and methylphenidate in humans discriminating Δ9-tetrahydrocannabinol. Psychopharmacology. 2009;203(2):241–250. doi: 10.1007/s00213-008-1393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Hays LR. The reinforcing, self-reported, performance and physiological effects of Δ9-tetrahydrocannabinol, triazolam, hydromorphone and methylphenidate in cannabis users. Behavioural pharmacology. 2010a;21(1):29. doi: 10.1097/FBP.0b013e32833470d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Hays LR. Substitution profile of the cannabinoid agonist nabilone in human subjects discriminating Δ9-tetrahydrocannabinol. Clinical Neuropharmacology. 2010b;33(5):235–242. doi: 10.1097/WNF.0b013e3181e77428. [DOI] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Hays LR. Separate and combined effects of the cannabinoid agonists nabilone and Δ9-THC in humans discriminating Δ9-THC. Drug and Alcohol Dependence. 2011;116(1):86–92. doi: 10.1016/j.drugalcdep.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Hays LR. Separate and combined effects of the GABAB agonist baclofen and Δ9-THC in humans discriminating Δ9-THC. Drug and Alcohol Dependence. 2012a;126(1):216–223. doi: 10.1016/j.drugalcdep.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Hays LR. Separate and combined effects of the GABA reuptake inhibitor tiagabine and Δ9-THC in humans discriminating Δ9-THC. Drug and Alcohol Dependence. 2012b;122(1):61–69. doi: 10.1016/j.drugalcdep.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Hays LR. Separate and combined effects of the GABAA positive allosteric modulator diazepam and Δ9-THC in humans discriminating Δ9-THC. Drug and Alcohol Dependence. 2014;143:141–148. doi: 10.1016/j.drugalcdep.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Wesley MJ, Kelly TH, Hays LR. Separate and combined effects of gabapentin and Δ9-THC in humans discriminating Δ9-THC. 2015 doi: 10.1097/FBP.0000000000000187. Unpublished data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Craft RM, Lefever TW, Wiley JL. The impact of gonadal hormones on cannabinoid dependence. Experimental and clinical psychopharmacology. 2015;23(4):206. doi: 10.1037/pha0000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behavior Research Methods & Instrumentation. 1982;14(5):463–466. [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Kaufman MJ, Levin JM, … Cohen BM. Cocaine pharmacokinetics in men and in women during the follicular and luteal phases of the menstrual cycle. Neuropsychopharmacology. 1999;21(2):294–303. doi: 10.1016/S0893-133X(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Nader MA, Reboussin DM. The effects of behavioral history on cocaine self-administration by rhesus monkeys. Psychopharmacology. 1994;115(1–2):53–58. doi: 10.1007/BF02244751. [DOI] [PubMed] [Google Scholar]
- Reed BG, Mowbray CT. Mental illness and substance abuse: implications for women’s health and health care access. Journal of the American Medical Women’s Association. 1999;54(2):71–78. [PubMed] [Google Scholar]
- Rodriguez de Fonseca FR, Cebeira M, Ramos JA, Martin M, Fernandez-Ruiz JJ. Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life sciences. 1994;54(3):159–170. doi: 10.1016/0024-3205(94)00585-0. [DOI] [PubMed] [Google Scholar]
- Ryan SM, Goldberger AL, Pincus SM, Mietus J, Lipsitz LA. Gender-and age-related differences in heart rate dynamics: are women more complex than men? Journal of the American College of Cardiology. 1994;24(7):1700–1707. doi: 10.1016/0735-1097(94)90177-5. [DOI] [PubMed] [Google Scholar]
- Schepis TS, Desai RA, Cavallo DA, Smith AE, McFetridge A, Liss TB, Potenza MN, Krishnan-Sarin S. Gender differences in adolescent marijuana use and associated psychosocial characteristics. Journal of Addiction Medicine. 2011;5(1):65. doi: 10.1097/ADM.0b013e3181d8dc62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singha AK, McCance-Katz EF, Heck SA, Kosten TR, Oliveto A. Individual differences in humans responding under a cocaine discrimination procedure: discriminators versus nondiscriminators. Experimental and Clinical Psychopharmacology. 1999;7(4):391. doi: 10.1037//1064-1297.7.4.391. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Data Archive. 2013. Treatment Episode Data Set -- Admissions (TEDS-A), 2012. Analysis ran on 2015-01-27 (02:47 PM EST) using SDA 3.5: Tables. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. [Google Scholar]
- Toland SL, Green W. DRE field testing of drug impaired drivers. In: Watts V, editor. The effects of drugs on human performance and behavior: Drugs and driving/drugs in the workplace. American Academy of Forensic Sciences; Colorado: 1991. [Google Scholar]
- Tseng AH, Craft RM. Sex differences in antinociceptive and motoric effects of cannabinoids. European journal of pharmacology. 2001;430(1):41–47. doi: 10.1016/s0014-2999(01)01267-5. [DOI] [PubMed] [Google Scholar]
- Umetani K, Singer DH, McCraty R, Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. Journal of the American College of Cardiology. 1998;31(3):593–601. doi: 10.1016/s0735-1097(97)00554-8. [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Hays LR, Rush CR. Discriminative-stimulus effects of triazolam in women and men. The American Journal of Drug and Alcohol Abuse. 2006;32(3):329–349. doi: 10.1080/00952990500479266. [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Lile JA, Stoops WW, Rush CR. Similar discriminative-stimulus effects of D-amphetamine in women and men. Pharmacology Biochemistry and Behavior. 2007;87(2):289–296. doi: 10.1016/j.pbb.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall ME, Sadler BM, Brine D, Taylor H, Perez-Reyes M. Metabolism, disposition, and kinetics of Δ9-tetrahydrocannabinol in men and women. Clinical Pharmacology & Therapeutics. 1983;34(3):352–363. doi: 10.1038/clpt.1983.179. [DOI] [PubMed] [Google Scholar]
- Westermeyer J, Boedicker AE. Course, severity, and treatment of substance abuse among women versus men. The American journal of drug and alcohol abuse. 2000;26(4):523–535. doi: 10.1081/ada-100101893. [DOI] [PubMed] [Google Scholar]
- White TL, Justice AJ, de Wit H. Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacology Biochemistry and Behavior. 2002;73(4):729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- Wilson WH, Ellinwood EH, Mathew RJ, Johnson K. Effects of marijuana on performance of a computerized cognitive-neuromotor test battery. Psychiatry research. 1994;51(2):115–125. doi: 10.1016/0165-1781(94)90031-0. [DOI] [PubMed] [Google Scholar]