Abstract
Objective
Assess the performance characteristics of axillary ultrasound (AUS) for accurate exclusion of clinically significant axillary lymph node (ALN) disease.
Background
Sentinel lymph node biopsy (SLNB) is currently the standard of care for staging the axilla in patients with clinical T1–T2, N0 breast cancer. AUS is a noninvasive alternative to SLNB for staging the axilla.
Methods
Patients were identified using a prospectively maintained database. Sensitivity, specificity, and negative predictive value (NPV) were calculated by comparing AUS findings to pathology results. Multivariate analyses were performed to identify patient and/or tumor characteristics associated with false negative (FN) AUS. A blinded review of FN and matched true negative cases was performed by two independent medical oncologists to compare treatment recommendations and actual treatment received. Recurrence-free survival was described using Kaplan-Meier product limit methods.
Results
647 patients with clinical T1–T2, N0 breast cancer underwent AUS between January, 2008 and March, 2013. AUS had a sensitivity of 70%, NPV of 84% and PPV of 56% for the detection of ALN disease. For detection of clinically significant disease (> 2.0 mm), AUS had a sensitivity of 76% and NPV of 89%. FN AUS did not significantly impact adjuvant medical decision making. Patients with FN AUS had recurrence-free survival equivalent to patients with pathologic N0 disease.
Conclusions
AUS accurately excludes clinically significant ALN disease in patients with clinical T1–T2, N0 breast cancer. AUS may be an alternative to SLNB in these patients where axillary surgery is no longer considered therapeutic, and predictors of tumor biology are increasingly used to make adjuvant therapy decisions.
Keywords: axillary ultrasound, sentinel lymph node biopsy, sensitivity, specificity, negative predictive value, breast cancer
INTRODUCTION
Sentinel lymph node biopsy (SLNB) is currently the standard of care for staging of the axilla in patients with clinical T1–T2, N0 breast cancer. SLNB accurately predicts the pathologic status of the axilla with an overall accuracy of 93–97% and a false negative (FN) rate of 9.8%, based on large randomized trials.1–4 Although SLNB has fewer and less severe complications compared to axillary lymph node dissection (ALND),5–8 it is not a risk-free procedure. Large prospective trials such as ACOSOG Z0010, NSABP B-32, and the ALMANAC trial have documented SLNB complications including allergic reactions to isosulfan blue dye (0.1–1.0%), wound infection (1.0–10%), seroma (7.1%), paresthesias (8.6–11%), and hematoma (1.4%).6, 9–11 In addition, SLNB is associated with significant costs including the costs of lymphoscintigraphy and operating time. In an era where axillary surgery is not considered to be therapeutic in clinical T1–T2, N0 breast cancer, and tumor biology as determined by biomarker profile and molecular profiling is increasingly used as the basis for adjuvant therapy decisions,12–15 we believe that alternatives to SLNB should be considered.
Axillary ultrasound (AUS) has been investigated as a noninvasive alternative to SLNB for staging of the axilla. AUS can identify disease in axillary lymph nodes (ALN) based on the size and morphology of the lymph nodes.16, 17 Several studies have examined the accuracy of AUS with or without fine needle aspiration biopsy (FNA) with a focus on identification of ALN disease.18–23 A 2011 meta-analysis by Houssami et al. reported a 79.6% sensitivity, 98.3% specificity, and 97.1% positive predictive value for AUS with FNA or core-needle biopsy (CNB).24 Prior to publication of the results of the ACOSOG Z0011 trial, AUS was used primarily to identify patients that required ALND. A positive AUS FNA/CNB allowed surgeons to bypass SLNB and proceed directly to ALND. Publication of the ACOSOG Z0011 findings resulted in a paradigm shift in the management of patients with clinical T1–T2, N0 breast cancer.25, 26 ACOSOG Z0011 randomized women with positive SLNB to no further axillary surgery or to completion ALND. ACOSOG Z0011 demonstrated no local control or overall survival advantage with ALND suggesting that axillary surgery in this context is not therapeutic. Following publication of the ACOSOG Z0011 trial results, the role of AUS in axillary staging has become less clear.27, 28
Previously, we evaluated the performance characteristics of AUS +/− FNA with a focus on identification of ALN disease.29 In this current study, we focus on the ability of AUS to accurately exclude clinically significant disease in the axilla, as we believe that AUS has the potential to replace SLNB in patients with clinical T1–T2, N0 breast cancer. In addition to assessing the performance characteristics of AUS, we have performed additional studies to define the potential clinical impact of a false negative (FN) AUS study. A FN AUS is an AUS with no abnormal lymph nodes noted on imaging, but evidence of metastatic spread on pathology. It is important to define the clinical impact of a FN AUS study as we believe that AUS has the potential to replace SLNB in patients with clinical T1–T2, N0 breast cancer, and a FN AUS may lead to understaging and undertreatment.
METHODS
This study was approved by the Institutional Review Board at Washington University School of Medicine (WUSM). We used a prospectively maintained database to identify patients with newly diagnosed, clinical T1–T2, N0 breast cancer who underwent AUS between January, 2008 and March, 2013. All individual electronic medical records were reviewed to confirm patient demographics, tumor characteristics including histology and grade, recurrence and survival data, and treatments received. Clinical tumor size was based on breast imaging and physical examination. AUS reports were reviewed and the results compared to cytology or surgical pathology results. AUS was considered abnormal if lymph nodes were noted to be completely hypoechoic (absent hilum), or to have focal hypoechoic cortical thickening/bulging.30 For all FN AUS cases, pathology slides were reviewed by a dedicated breast pathologist, and the size of the largest ALN metastasis was recorded.
Patient clinicopathologic information was summarized using descriptive statistics as appropriate. Sensitivity and specificity were calculated for the detection of any metastatic disease, and for the detection of macrometastatic disease only. Negative predictive value (NPV) and positive predictive value were calculated, and the association between NPV and patient characteristics was assessed using Chi-square test or Fisher's exact test as appropriate. Recurrence-free survival (RFS) was defined as time from surgery to axillary, breast, or distant recurrence or death due to any cause, whichever occurred first. Patients alive and free of recurrence were censored at the date of last contact. The differences in RFS by AUS and pathology status were described using Kaplan-Meier product limit methods and compared by log-rank test. All analyses were two-sided and significance was set at a p-value of 0.05. Statistical analyses were performed using SAS 9.2 (SAS Institutes, Cary, NC). For the blinded review, the overall frequency of agreement between reviewers’ recommendations and actual treatment were calculated in FN and true negative (TN) groups separately, and the groups were compared using Fisher’s exact test. Incomplete information was available for 3 patients in the TN group and 1 patient in the FN group and these patients were excluded from the blinded review.
RESULTS
To study the role of AUS in breast cancer staging, we performed a retrospective review of a prospectively maintained database of our experience with AUS. A total of 1387 patients with newly diagnosed clinical T1–T2, N0 M0 breast cancer were evaluated and treated at WUSM between January, 2008 and March, 2013. Patients who did not undergo AUS were excluded, as were patients diagnosed with recurrent or metastatic disease, leaving 647 eligible patients who were included in the analysis.
Patient demographics and tumor characteristics are reported in Table 1. The median age at diagnosis was 58 years (range 28–88). The majority of study patients were estrogen receptor (ER)-positive (n = 490, 76%) and progesterone receptor (PR)-positive (n = 457, 71%). The most common histology was invasive ductal carcinoma (533, 82%). 108 patients (17%) were treated with neoadjuvant therapy. The axillary recurrence rate was 1% (n = 7), with a median follow-up of 38 months. Of the 7 axillary recurrences, 4 occurred in patients who were AUS-positive and pathology-negative (false positive (FP) AUS), 2 occurred in patients who were AUS-positive and pathology-positive (true positive (TP) AUS) and 1 occurred in a patient who was AUS-negative and pathology-negative (TN AUS). None of the 63 FN patients had an axillary recurrence. (Of note, in the 4 patients who recurred with a positive AUS and negative pathology, the AUS was classified as FP as the trial design considers pathology to be the gold standard. In these cases, the AUS studies might be more accurately considered TP based on the true disease state.)
Table 1.
Demographic and clinicopathologic characteristics of newly diagnosed breast cancer patients undergoing axillary ultrasound
| Characteristic | Number of patients (n = 647) n (%) |
|---|---|
| Age | |
| Median, years (range) | 58 (28–88) |
| Tumor Histology | |
| Invasive ductal | 533 (82) |
| Invasive lobular | 49 (8) |
| Other | 65 (10) |
| Tumor Size | |
| T1 | 368 (57) |
| T2 | 276 (42) |
| Tumor Grade | |
| I | 169 (26) |
| II | 231 (36) |
| III | 245 (38) |
| ER status | |
| Positive | 490 (76) |
| Negative | 156 (24) |
| Unknown | 1 (<1) |
| PR status | |
| Positive | 457 (71) |
| Negative | 189 (29) |
| Unknown | 1 (<1) |
| HER2 status | |
| Positive | 94 (15) |
| Negative | 549 (85) |
| Unknown | 4 (<1) |
| Neoadjuvant therapy | 108 (17) |
| Median F/U (months) | 38.0 (0–130) |
| Recurrence | |
| Breast | 6 (<1) |
| Axilla | 7 (1) |
| Distant | 30 (5) |
ER, estrogen receptor; PR, progesterone receptor, F/U, follow-up
AUS and pathology results are reported in Figure 1 and Tables 2A and 2B. AUS and pathology results were concordant in 84% (319/382) of cases with a normal AUS, and in 56% (148/265) of cases with an abnormal AUS (Figure 1). The sensitivity and specificity of AUS for the detection of ALN disease was 70% and 73%, respectively, with a NPV of 84% and PPV of 56%. (Table 2A). The sensitivity of AUS for the detection of macrometastatic disease (>2.0 mm) was 76%, with a NPV of 89% (Table 2B). No patient or tumor characteristics significantly impacted the sensitivity, specificity, or NPV of AUS detection of metastatic breast cancer to the axilla (Supplementary Table 1). The median size of ALN metastasis in the FN group was 2.0 mm (range 0.1–21 mm, mean 3.7 mm). Of note, there were 108 patients in our study who were treated with neoadjuvant therapy. All of the AUS studies were performed prior to the initiation of neoadjuvant therapy. SLNB was performed after neoadjuvant therapy in 44 patients. 7 of these 44 patients had a negative AUS and negative pathology, and it is possible that neoadjuvant therapy resulted in clearance of disease that was not detected by AUS.
Figure 1.
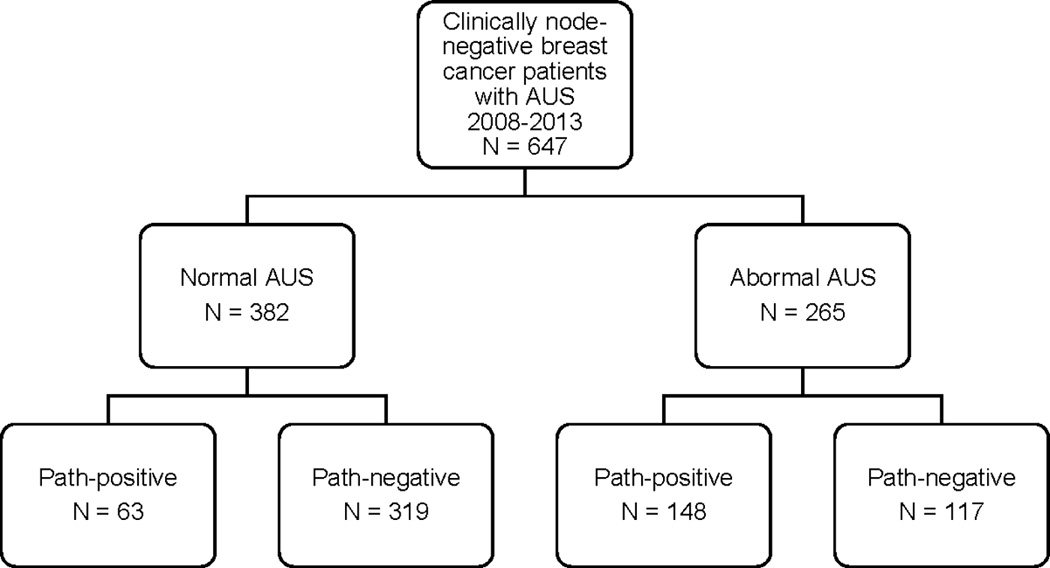
Schematic results of axillary ultrasound (AUS) and final lymph node pathology.
Table 2.
| A: Performance characteristics of axillary
ultrasound (AUS) for detection of micrometastatic or macrometastatic lymph node disease | ||
|---|---|---|
| Positive pathology | Negative pathology | |
| Abnormal AUS | 148 | 117 |
| Normal AUS | 63 | 319 |
| B: Performance characteristics of axillary
ultrasound (AUS) for detection of macrometastatic (> 2 mm) lymph node disease | ||
|---|---|---|
| Positive pathology | Negative pathology | |
| Abnormal AUS | 129 | 136 |
| Normal AUS | 41 | 341 |
Sensitivity 70%, Specificity 73%, Negative Predictive Value 84%, Positive Predictive Value 56%
Sensitivity 76%, Specificity 71%, Negative Predictive Value 89%, Positive Predictive Value 49%
In order to evaluate the potential impact of FN AUS on adjuvant therapy decision making, two medical oncologists performed a blinded review of information abstracted from all FN AUS patients and from matched TN AUS patients. Data provided to the medical oncologists included patient age, menopausal status, Eastern Cooperative Oncology Group (ECOG) performance status, medical co-morbidities, prior cancer history (where applicable), tumor histology, tumor grade, presence or absence of multifocality, biomarker profile, use of neoadjuvant therapy and type used (chemotherapy, anti-HER2 therapy, or endocrine therapy), T stage (both pathologic and clinical), and Oncotype DX score (when available). Patient demographics and tumor characteristics are shown in Supplementary Table 4. Although the FN and TN groups were well matched, tumor histology and tumor size did differ between the two groups.
Based on the data provided, the two medical oncologists made blinded adjuvant treatment recommendations for each patient based on the assumption that the patient was pathologically node-negative. The recommendations of the two medical oncologists were then compared to the actual treatments received. Concordance between the two medical oncologists was also assessed. The recommendations of the two medical oncologists were the same as the actual treatment received in the majority of patients in both the FN and TN groups. This suggests that the small volume of disease identified by SLNB in patients with a FN AUS does not significantly impact medical decision making in the majority of cases (Table 3). Agreement between the actual treatment received and medical oncologist #1 was 65% in the FN group and 73% in the TN group. Agreement between actual treatment received and medical oncologist #2 was 74% in the FN group and 62% in the TN group. Agreement between medical oncologist #1 and medical oncologist #2 was 76% in the FN group and 72% in the TN group.
Table 3.
Concordance between actual treatment and blind review treatment recommendations, Group 1 (False Negative Axillary Ultrasound) and Group 2 (True Negative Axillary Ultrasound)
| Group 1 | Group 2 | p-value | ||
|---|---|---|---|---|
| Overall | Actual and #1 | 65% (40/62) | 73% (44/60) | 0.332 |
| Actual and #2 | 74% (46/62) | 62% (37/60) | 0.175 | |
| #1 and #2 | 76% (47/62) | 72% (43/60) | 0.682 | |
| ER+, Age > 50 | Actual and #1 | 58% (25/43) | 84% (37/44) | 0.009 |
| Actual and #2 | 65% (28/43) | 68% (30/44) | 0.822 | |
| #1 and #2 | 74% (32/43) | 68% (30/44) | 0.637 | |
| HER2+ | Actual and #1 | 55% (6/11) | 50% (3/6) | > 0.99 |
| Actual and #2 | 64% (7/11) | 50% (3/6) | 0.644 | |
| #1 and #2 | 64% (7/11) | 100% (6/6) | 0.237 | |
| TNBC | Actual and #1 | 67% (4/6) | 36.4% (4/11) | 0.335 |
| Actual and #2 | 100% (6/6) | 45.5% (5/11) | 0.043 | |
| #1 and #2 | 67% (4/6) | 82% (9/11) | 0.584 |
ER, estrogen receptor; TNBC, triple negative breast cancer; #1, Medical oncologist #1; #2, Medical oncologist #2
Subgroup analyses were also performed to determine if there is a specific subgroup of breast cancer patients where a FN AUS may be associated with a more significant impact on medical decision making. Three breast cancer subgroups were studied (patients greater than 50 years of age with ER-positive disease, patients with triple negative disease, and patients with HER2-positive disease). In these subgroup analyses, the recommendations of the two medical oncologists were the same as the actual treatment received in the majority of patients in both the FN and TN groups. These results suggest that there is no specific subgroup of patients where a FN AUS may be associated with a more significant impact on medical decision making. Details of the actual treatments received, and the treatments recommended in the blinded review are presented in Supplementary Figure 1.
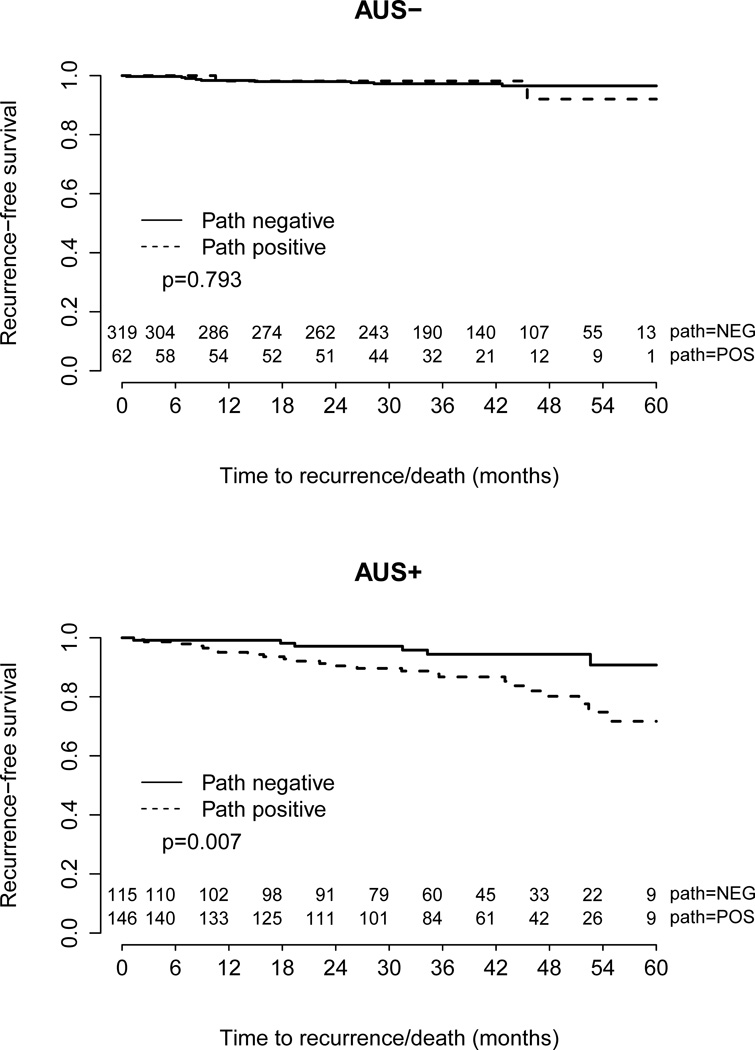
To address the issue of the clinical impact of a FN AUS in an alternative way, we performed survival analyses comparing RFS based on AUS and pathology results. The RFS between FN and TN patients was equivalent (p > 0.05). One possible explanation for this result is that the (typically) small amount of disease missed by AUS does not significantly impact treatment and/or prognosis. The RFS for patients with TP AUS was significantly worse than for patients with a FP AUS (p = 0.007) (Figure 2). One possible explanation for this result is that patients with a TP AUS typically have a clinically significant burden of axillary disease.
Figure 2.
Recurrence-free survival for patients with true negative and false negative axillary ultrasound and for patients with true positive and false positive axillary ultrasound.
DISCUSSION
This study demonstrates that AUS has excellent performance characteristics for identification of clinically significant ALN disease in women with clinical T1–T2, N0 breast cancer. The sensitivity of AUS for detection of any ALN disease was 70%, and the sensitivity for detection of clinically significant disease (> 2.0 mm) was even higher at 76%. These results are similar to, but generally superior to those reported in a meta-analysis by Houssami et al. (median sensitivity 61.4%). They are also similar to the results of our initial experience published in 2008 evaluating AUS with FNA (sensitivity 79%).24, 29 The specificity in the current study is lower than the specificity observed in these previous studies, likely because the current study compares the results of AUS alone to pathology, whereas previous studies compared the results of AUS with percutaneous sampling to pathology. Some of the patients in this study with a positive AUS did undergo percutaneous sampling. However in the post ACOSOG Z0011 era percutaneous sampling is no longer routinely recommended and is of questionable relevance27, 31–33.
The size of missed tumor deposits in FN AUS cases was very small in the current study (median 2.0 mm, mean 3.7 mm), and the performance characteristics of AUS for detection of macrometastatic disease (>2.0 mm) were excellent. This is important because the clinical importance of micrometastatic (≤ 2.0mm) ALN disease remains controversial. In fact, there is strong evidence that micrometastatic disease may have little, if any clinical significance. Krag et al. performed immunohistochemistry on specimens from the NSABP B-32 trial, demonstrating that there was a small, but statistically significant decrease in recurrence-free (RFS), and overall survival (OS) in patients with micrometastatic ALN disease.34 Despite this result, they concluded that the small difference in survival was clinically insignificant. Mittendorf et al. reached a very similar conclusion in their retrospective analysis of specimens from MD Anderson Cancer Center and ACOSOG Z0010.35 Mittendorf et al. found that there was no significant difference in RFS, or OS in patients with pathologic N0 disease compared to patients with micrometastatic disease (Stage 1A vs. 1B). In addition, predictors of tumor biology (tumor grade, ER status) were more informative than detection of micrometastatic ALN disease for predicting prognosis. Current practice guidelines appear to recognize the limited relevance of micrometastatic disease, as the current recommendation for pathologic analysis of SLNs is to perform H&E analysis only, although it is well known that additional sections and IHC will detect micrometastatic disease in a significant percentage of cases.3, 9, 34, 36
In fact, decisions regarding adjuvant systemic therapy for breast cancer patients are complex, and integrate a significant amount of clinical and pathologic information such as patient age, medical history, menopausal status, tumor histology, tumor size, tumor grade, biomarker profile, and ALN status. Although lymph node staging remains important, it is of decreasing importance as a driver of adjuvant therapy decision making. The importance of anatomic ALN staging may be further eroded in the future, as molecular profiling tests capable of better characterizing underlying tumor biology such as Oncotype DX, are increasingly used to better define a patient’s risk of distant recurrence. Several large trials have evaluated the utility of these molecular profiling tests. Paik et al. and Habel et al. evaluated outcomes of ER-positive, node-negative women treated in the context of randomized clinical trials. Patients with a low Oncotype DX recurrence score have a low risk of distant recurrence and receive little benefit from the addition of chemotherapy, whereas patients with a high recurrence score are significantly more likely to experience distant recurrence, and benefit from the addition of adjuvant chemotherapy.15, 37 The Trial Assessing IndividuaLized Options for Treatment (TAILORx) in the United States and the MIcroarray in Node-negative Disease May Avoid Chemotherapy (MINDACT) trial in Europe have enrolled ER-positive, node-negative breast cancer patients and randomized them to either endocrine therapy alone, or chemotherapy and endocrine therapy based on gene expression profiles.12 Similarly, the MINDACT and the Rx for Positive NoDe Endocrine Responsive (RxPONDER) breast cancer trials have enrolled ER-positive, node-positive breast cancer patients and randomized them to either endocrine therapy alone, or chemotherapy and endocrine therapy.38, 39 Although these prospective randomized trials are ongoing, molecular profiling tests are already being used by medical oncologists to help make adjuvant therapy recommendations, highlighting the eroding importance of ALN status for adjuvant therapy decision making.
The results of the blinded review of treatment recommendations in patients with FN AUS results further support the concept that anatomic staging is decreasing in importance for adjuvant treatment decision making. Despite being blinded to pathologic lymph node status, the recommendations of the two medical oncologists were the same as the actual treatment received in the majority of patients in both the FN and TN groups. Some of the variability in adjuvant chemotherapy recommendations between medical oncologist #1 and #2 may be attributable to the delicate balance between increased survival and increased treatment risk that are considered when making treatment recommendations. Of note, there was no significant difference in RFS for patients with a FN AUS compared to a TN AUS, suggesting that if AUS misses axillary disease, it is likely to be clinically insignificant (Figure 2).
One weakness of this study is that it is based on a retrospective analysis of cases identified in a prospectively maintained database. Although all of the data was confirmed based on review of electronic medical records, there was variation in the quality and type of information available in patient records, and information regarding why AUS was performed for some patients but not others was not available. In addition, although the TN AUS patients in the control group were randomly selected and matched in a 1:1 ratio with the FN AUS patients for the blinded review, there were differences in the two groups in tumor size and histology. Finally, radiation oncology decision making may also be influenced by the presence, absence, and/or extent of axillary disease. The impact of false negative AUS on radiation oncology decision making was not assessed.
Of note, the false negative rate of AUS for the detection of clinically relevant disease was 24%. This is higher than the false negative rate of SLNB observed in large randomized trials (10%).4 However, as noted above, SLNB is not therapeutic in patients with clinical T1–T2, N0 breast cancer and in the majority of patients with a false negative AUS medical decision making was not impacted. As such, it remains unclear if the increased false negative rate associated with AUS will significantly impact outcomes.
The current study confirms that the performance characteristics of AUS are excellent, with a NPV of 89% for clinically significant (> 2.0 mm) disease. Given the morbidity and expense of SLNB, we believe that AUS represents a potential alternative to SLNB for staging of the axilla in breast cancer. As such, the current study provides strong rationale for a randomized prospective trial comparing SLNB to no further staging in clinical T1–T2, N0 breast cancer patients with a negative AUS. Two such trials are currently ongoing. In Europe, patients with T1 breast cancer and negative AUS are being randomized to AUS alone or to SLNB in the Sentinel node vs. Observation after axillary UltraSouND (SOUND) trial.40 At WUSM, patients with clinical T1–T2, N0 M0 invasive breast cancer who have a negative AUS are being randomized to either AUS alone or SLNB (https://clinicaltrials.gov/ct2/show/NCT01821768). These studies will provide additional insight into the role of AUS in staging of the axilla in patients with early stage, clinically node-negative breast cancer.
Supplementary Material
Acknowledgments
We would like to thank Enid McIntosh for her assistance with the submission of the manuscript.
This publication was supported by the Longer Life Foundation, the NCI (support for NT and KG, T32 CA009621, support for FA, 1K12 CA167540) and the Washington University Institute of Clinical and Translational Sciences (support for NT KG and FA, UL1 TR000448 from the National Center for Advancing Translational Sciences). Biostatistics services were provided by the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, MO. The Siteman Cancer Center is supported in part by an NCI Cancer Center Support Grant #P30 CA91842.
References
- 1.Giuliano AE, Kirgan DM, Guenther JM, et al. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391–398. doi: 10.1097/00000658-199409000-00015. discussion 398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–7720. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Lyman GH, Temin S, Edge SB, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2014;32:1365–1383. doi: 10.1200/JCO.2013.54.1177. [DOI] [PubMed] [Google Scholar]
- 4.Krag DNA, SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, Weaver DL, Miller BJ, Jalovec LM, Frazier TG, Noyes RD, Robidoux A, Scarth HM, Mammolito DM, McCready DR, Mamounas EP, Costantino JP, Wolmark N. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881–888. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 5.Fleissig A, Fallowfield LJ, Langridge CI, et al. Post-operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat. 2006;95:279–293. doi: 10.1007/s10549-005-9025-7. [DOI] [PubMed] [Google Scholar]
- 6.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006;98:599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 7.Ashikaga T, Krag DN, Land SR, et al. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol. 2010;102:111–118. doi: 10.1002/jso.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25:3657–3663. doi: 10.1200/JCO.2006.07.4062. [DOI] [PubMed] [Google Scholar]
- 9.Wilke LG, McCall LM, Posther KE, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol. 2006;13:491–500. doi: 10.1245/ASO.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881–888. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 11.Land SR, Kopec JA, Julian TB, et al. Patient-reported outcomes in sentinel node-negative adjuvant breast cancer patients receiving sentinel-node biopsy or axillary dissection: National Surgical Adjuvant Breast and Bowel Project phase III protocol B-32. J Clin Oncol. 2010;28:3929–3936. doi: 10.1200/JCO.2010.28.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zujewski JA, Kamin L. Trial assessing individualized options for treatment for breast cancer: the TAILORx trial. Future Oncol. 2008;4:603–610. doi: 10.2217/14796694.4.5.603. [DOI] [PubMed] [Google Scholar]
- 13.Partin JF, Mamounas EP. Impact of the 21-gene recurrence score assay compared with standard clinicopathologic guidelines in adjuvant therapy selection for node-negative, estrogen receptor-positive breast cancer. Ann Surg Oncol. 2011;18:3399–3406. doi: 10.1245/s10434-011-1698-z. [DOI] [PubMed] [Google Scholar]
- 14.McVeigh TP, Hughes LM, Miller N, et al. The impact of Oncotype DX testing on breast cancer management and chemotherapy prescribing patterns in a tertiary referral centre. Eur J Cancer. 2014;50:2763–2770. doi: 10.1016/j.ejca.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 16.Mustonen P, Farin P, Kosunen O. Ultrasonographic detection of metastatic axillary lymph nodes in breast cancer. Ann Chir Gynaecol. 1990;79:15–18. [PubMed] [Google Scholar]
- 17.Tate JJ, Lewis V, Archer T, et al. Ultrasound detection of axillary lymph node metastases in breast cancer. Eur J Surg Oncol. 1989;15:139–141. [PubMed] [Google Scholar]
- 18.Yamashita M, Hovanessian-Larsen L, Sener SF. The role of axillary ultrasound in the detection of metastases from primary breast cancers. Am J Surg. 2013;205:242–244. doi: 10.1016/j.amjsurg.2012.10.009. discussion 244–245. [DOI] [PubMed] [Google Scholar]
- 19.Diepstraten SC, Sever AR, Buckens CF, et al. Value of preoperative ultrasound-guided axillary lymph node biopsy for preventing completion axillary lymph node dissection in breast cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2014;21:51–59. doi: 10.1245/s10434-013-3229-6. [DOI] [PubMed] [Google Scholar]
- 20.Mills P, Sever A, Weeks J, et al. Axillary ultrasound assessment in primary breast cancer: an audit of 653 cases. Breast J. 2010;16:460–463. doi: 10.1111/j.1524-4741.2010.00952.x. [DOI] [PubMed] [Google Scholar]
- 21.Park SH, Kim MJ, Park BW, et al. Impact of preoperative ultrasonography and fine-needle aspiration of axillary lymph nodes on surgical management of primary breast cancer. Ann Surg Oncol. 2011;18:738–744. doi: 10.1245/s10434-010-1347-y. [DOI] [PubMed] [Google Scholar]
- 22.Solon JG, Power C, Al-Azawi D, et al. Ultrasound-guided core biopsy: an effective method of detecting axillary nodal metastases. J Am Coll Surg. 2012;214:12–17. doi: 10.1016/j.jamcollsurg.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 23.Cools-Lartigue J, Meterissian S. Accuracy of axillary ultrasound in the diagnosis of nodal metastasis in invasive breast cancer: a review. World J Surg. 2012;36:46–54. doi: 10.1007/s00268-011-1319-9. [DOI] [PubMed] [Google Scholar]
- 24.Houssami N, Ciatto S, Turner RM, et al. Preoperative ultrasound-guided needle biopsy of axillary nodes in invasive breast cancer: meta-analysis of its accuracy and utility in staging the axilla. Ann Surg. 2011;254:243–251. doi: 10.1097/SLA.0b013e31821f1564. [DOI] [PubMed] [Google Scholar]
- 25.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252:426–432. doi: 10.1097/SLA.0b013e3181f08f32. discussion 432–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hieken TJ. The promise of axillary imaging in individualized surgical management of breast cancer patients: another step forward. Ann Surg Oncol. 2014;21:3369–3371. doi: 10.1245/s10434-014-3853-9. [DOI] [PubMed] [Google Scholar]
- 28.Verheuvel NC, van den Hoven I, Ooms HW, et al. The Role of Ultrasound-Guided Lymph Node Biopsy in Axillary Staging of Invasive Breast Cancer in the Post-ACOSOG Z0011 Trial Era. Ann Surg Oncol. 2014 doi: 10.1245/s10434-014-4071-1. [DOI] [PubMed] [Google Scholar]
- 29.Holwitt DM, Swatske ME, Gillanders WE, et al. Scientific Presentation Award: The combination of axillary ultrasound and ultrasound-guided biopsy is an accurate predictor of axillary stage in clinically node-negative breast cancer patients. Am J Surg. 2008;196:477–482. doi: 10.1016/j.amjsurg.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bedi DG, Krishnamurthy R, Krishnamurthy S, et al. Cortical morphologic features of axillary lymph nodes as a predictor of metastasis in breast cancer: in vitro sonographic study. AJR Am J Roentgenol. 2008;191:646–652. doi: 10.2214/AJR.07.2460. [DOI] [PubMed] [Google Scholar]
- 31.Humphrey KL, Saksena MA, Freer PE, et al. To do or not to do: axillary nodal evaluation after ACOSOG Z0011 Trial. Radiographics. 2014;34:1807–1816. doi: 10.1148/rg.347130141. [DOI] [PubMed] [Google Scholar]
- 32.Boone BA, Huynh C, Spangler ML, et al. Axillary Lymph Node Burden in Invasive Breast Cancer: A Comparison of the Predictive Value of Ultrasound-Guided Needle Biopsy and Sentinel Lymph Node Biopsy. Clin Breast Cancer. 2015 doi: 10.1016/j.clbc.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Verheuvel NC, van den Hoven I, Ooms HW, et al. The role of ultrasound-guided lymph node biopsy in axillary staging of invasive breast cancer in the post-ACOSOG Z0011 trial era. Ann Surg Oncol. 2015;22:409–415. doi: 10.1245/s10434-014-4071-1. [DOI] [PubMed] [Google Scholar]
- 34.Weaver DLA, T, Krag DN, Skelly JM, Anderson SJ, Harlow SP, Julian TB, Mamounas EP, Wolmark N. Effect of occult metastases on survival in node-negative breast cancer. N Engl J Med. 2011;364:412–421. doi: 10.1056/NEJMoa1008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mittendorf EA, Ballman KV, McCall LM, et al. Evaluation of the Stage IB Designation of the American Joint Committee on Cancer Staging System in Breast Cancer. J Clin Oncol. 2015;33:1119–1127. doi: 10.1200/JCO.2014.57.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weaver DL, Krag DN, Manna EA, et al. Detection of occult sentinel lymph node micrometastases by immunohistochemistry in breast cancer. An NSABP protocol B-32 quality assurance study. Cancer. 2006;107:661–667. doi: 10.1002/cncr.22074. [DOI] [PubMed] [Google Scholar]
- 37.Habel LA, Shak S, Jacobs MK, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res. 2006;8:R25. doi: 10.1186/bcr1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramsey SD, Barlow WE, Gonzalez-Angulo AM, et al. Integrating comparative effectiveness design elements and endpoints into a phase III, randomized clinical trial (SWOG S1007) evaluating oncotypeDX-guided management for women with breast cancer involving lymph nodes. Contemp Clin Trials. 2013;34:1–9. doi: 10.1016/j.cct.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rutgers E, Piccart-Gebhart MJ, Bogaerts J, et al. The EORTC 10041/BIG 03-04 MINDACT trial is feasible: results of the pilot phase. Eur J Cancer. 2011;47:2742–2749. doi: 10.1016/j.ejca.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 40.Gentilini O, Veronesi U. Abandoning sentinel lymph node biopsy in early breast cancer? A new trial in progress at the European Institute of Oncology of Milan (SOUND: Sentinel node vs Observation after axillary UltraSouND) Breast. 2012;21:678–681. doi: 10.1016/j.breast.2012.06.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.