Abstract
The development of therapeutic substances to treat diseases of the central nervous system is hampered by the tightness and selectivity of the blood-brain barrier. Moreover, testing of potential drugs is time-consuming and cost-intensive. Here, we established a new microfluidically supported, biochip-based model of the brain endothelial barrier in combination with brain cortical spheroids suitable to detect effects of neuroinflammation upon disruption of the endothelial layer in response to inflammatory signals. Unilateral perfusion of the endothelial cell layer with a cytokine mix comprising tumor necrosis factor, IL-1β, IFNγ, and lipopolysaccharide resulted in a loss of endothelial von Willebrand factor and VE-cadherin expression accompanied with an increased leakage of the endothelial layer and diminished endothelial cell viability. In addition, cytokine treatment caused a loss of neocortex differentiation markers Tbr1, Tbr2, and Pax6 in the cortical spheroids concomitant with reduced cell viability and spheroid integrity. From these observations, we conclude that our endothelial barrier/cortex model is suitable to specifically reflect cytokine-induced effects on barrier integrity and to uncover damage and impairment of cortical tissue development and viability. With all its limitations, the model represents a novel tool to study cross-communication between the brain endothelial barrier and underlying cortical tissue that can be utilized for toxicity and drug screening studies focusing on inflammation and neocortex formation.
INTRODUCTION
The development of suitable drugs for the treatment of central nervous system (CNS) diseases is challenging as the blood-brain barrier (BBB) efficiently prevents passage of nearly all polar substances from the vasculature to the brain tissue. The permeability of the BBB is determined by specialized endothelial cells of the cerebral vasculature (cerebral microvascular endothelial cell, CMEC) that only allow passage of molecules smaller than 400 Da.1 The development of such small compounds represents an essential limitation requiring time-consuming drug-screening and preclinical studies. In consequence, suitable and reliable in vitro models are urgently needed to streamline the drug-screening processes and to curtail cost-intensive animal experimentation. In this context, miniaturized, microfluidically perfused BBB models attracted much attention during recent years.2,3
Mechanotransduction induced by fluidic shear stress was demonstrated to influence endothelial cell differentiation and to regulate expression of endothelial junctional proteins necessary for the maintenance of the endothelial barrier function.4–6 Apical junctional complex proteins determine endothelial polarity and barrier function, thereby regulating diffusion of small nutritional molecules and gases but excluding larger molecules, i.e., potential neurotoxins or microorganisms from the brain extracellular fluid.7 Recently, the immortalized human CMEC (hCMEC)/D3 cell line has been described to express specific cerebral endothelial marker proteins including cell adhesion and tight junction (TJ) proteins as well as CNS relevant transporter systems.8 Due to this favourable characteristics, the cell line has already been used in microfluidically perfused BBB models where it showed a transendothelial electrical resistance (TEER) of 120 Ω cm2 with a high expression of zona occludens-1 (ZO-1), a protein of the tight junctional plaque that is important for the maintenance of BBB integrity.9,10 Moreover, hCMEC/D3 cells were reported to mimic barrier characteristics of the BBB even in the absence of additional cell types, i.e., astrocytes or pericytes.8
In this context, it was shown that tight junction (TJ) protein expression is specifically down-regulated in these cells in response to pro-inflammatory cytokines such as tumor necrosis factor (TNF),10–13 suggesting that hCMEC/D3 cells represent a reliable tool to study inflammation-related modulation of the cerebral microvascular endothelial barrier function.
For a more comprehensive modelling of events during neuroinflammatory diseases, it is essential to combine an endothelial barrier and neural tissue to see how effects on endothelial barrier function affect neural tissue and vice versa. In this proof-of-principle study, we established an integrative biochip-based model including an endothelial barrier composed of human CMECs and cortical tissue spheroids derived from murine embryonic stem cells (ESCs). We demonstrate that our model is able to reflect in vitro the cytokine-mediated disruption of the endothelial barrier in response to stimulation with a pro-inflammatory cytokine mix containing TNF, IL-1β, IFNγ, and lipopolysaccharide (LPS). Stimulation resulted in a diminished expression of endothelial VE-cadherin and ZO-1 indicating disruption of the endothelial barrier. In consequence, diminished expression of neural neocortex differentiation markers such as Tbr1, Tbr2, and Pax6 within cortical spheroids was observed. This demonstrates that our biochip model reflects neuroinflammatory processes at brain endothelial cell layers in association with the cortical tissue in vitro.
MATERIALS AND METHODS
Biochip fabrication
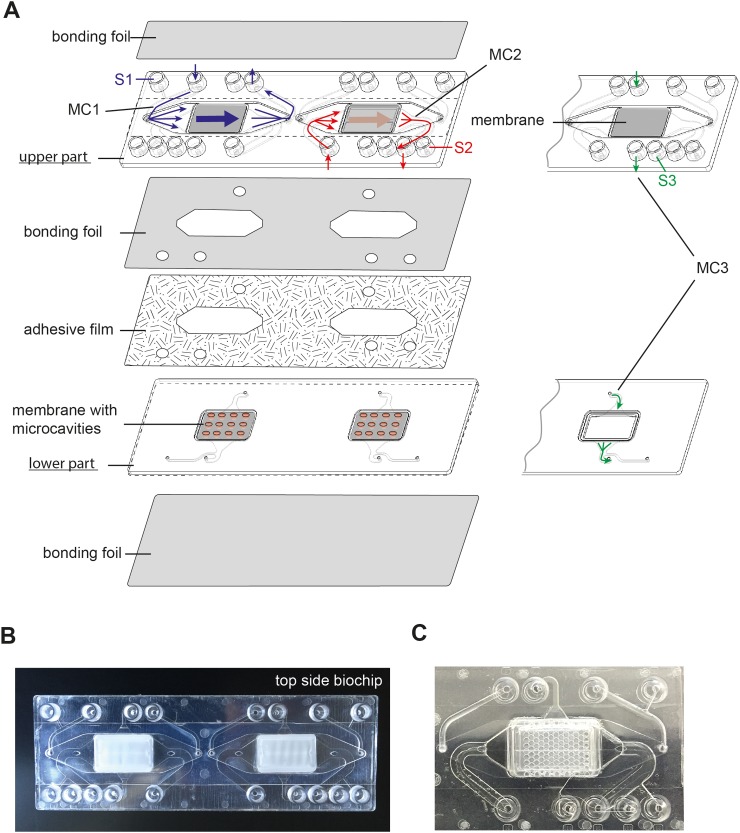
Biochips were made by injection moulding from polystyrene (PS) and manufactured by microfluidic ChipShop GmbH (Jena, Germany) as described previously.6 See supplementary Figure 1 (Ref. 14) for embedded structures of the biochip with indicated lengths and heights. A 12 μm thick Polyethylene terephthalate (PET) membrane (TRAKETCH) with a pore diameter of 8 μm and a pore density of 1 × 105 pores/cm2 (Sabeu, Radeberg, Germany) was integrated in the upper part of the biochip by heat-sealing with the bulk material. A polycarbonate membrane (300 μm, Karlsruhe, Germany) with thermo-formed micro-cavities with a diameter of 800 μm comprising pores with a diameter of 5 μm was heat-sealed in the lower part of the biochip (Figures 1 and 2).
FIG. 1.
Design of the components of the microfluidically supported biochip for a co-culture model of a cerebral microvascular cell layer forming an endothelial barrier and cortical structures. (a) The biochip is composed of an upper and a lower part that are combined with an adhesive film. The biochip includes two cavities. In each cavity, a planar membrane is integrated in the upper part and a membrane with micro-cavities is mounted in the lower part of the biochip. The upper and the lower chambers are sealed by bonding membranes to cover the micro-channels. The bonding foil that seals the bottom of the upper part holds openings to enable medium flow between the upper and the lower part of the biochip. Three micro-channel systems are integrated in the biochip: Micro-channel 1 (MC1) perfuses the apical side of the flat membrane (blue lines). The second micro-channel (MC2) allows medium flow between both membranes (red lines). Micro-channel 3 (MC3) perfuses the basal side of the membrane with microcavities (green lines). Each micro-channel is connected to a lateral channel and a sample port that allows medium sampling during cell perfusion (sample ports S1, S2, and S3). (b) and (c) Photograph of the (b) entire biochip and (c) zoomed section of the biochip with embedded micro-cavity membrane.
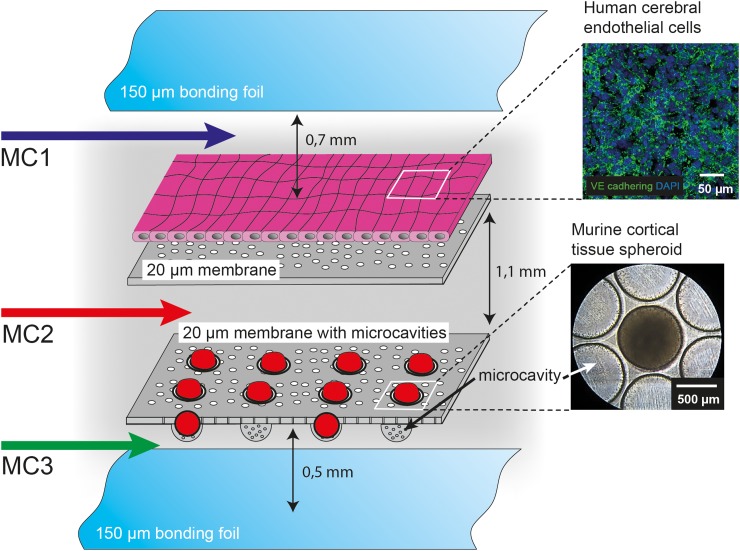
FIG. 2.
Cellular setup in the biochip. On the flat membrane, human cerebral endothelial (hCMEC/D3) cells were cultured to mimic the endothelial barrier within the BBB. Murine cortical spheroids were cultured underneath the endothelial cell layer in the micro-cavities of the lower membrane simulating the cortex. Cortical spheroids were transferred into the biochip via the micro-channel 2 (MC2). HCMEC/D3 cells were perfused via the micro-channel 1 (MC1) at the apical side and spheroids were perfused via the micro-channel 3 (MC3) at the basal side. Spheroids are protected from direct impact of shear stress by the membrane that allows nutrition supply through the pores. Arrows indicate the distance between the membranes as well as the distances between the individual membranes and bonding foil.
Chips and channel structures were sealed on the top and bottom sides with an extruded 125 μm thick PS bonding foil using a low temperature bonding method. The upper and the lower parts of the biochip were assembled by a double-sided adhesive film. Oxygen plasma treatment for hydrophilization of the whole chip surface was performed to support cell adhesion and to reduce air bubble formation in the chips.
Cell culture
HCMEC/D3 cells were purchased from BIOZOL (Eching, Germany). Cells were cultured in complete EndoGRO-MV Basal Medium (endothelial cell medium (ECM)) containing 5% (v/v) fetal bovine serum (FBS), 0.2% (v/v) EndoGRO-LS supplement, 5 ng/ml recombinant human epidermal growth factor, 10 mM L-glutamine, 1 μg/ml hydrocortisone-hemisuccinate, 0.75 U/ml heparin-sulphate, and 50 μg/ml ascorbic acid (all additives were obtained from Merck-Millipore, Darmstadt, Germany).
Cortical spheroids were formed by self-aggregation as described previously.15 Briefly, D3 mouse embryonic stem cells were seeded at a concentration of 1 × 103 cells in 100 μl per well into a 96-well PrimeSurface low adhesion cell culture plate (Sumitomo Bakelite Co., Ltd., Tokyo, Japan). Cells were differentiated in cortical differentiation medium consisting of Glasgow minimal essential medium (GMEM) supplemented with 10% (v/v) knock-out serum replacement (KSR) Non-Essential Amino Acids supplement (NEAA), pyruvate, antibiotics, and mercaptoethanol until day 7. Subsequently, cortical maturation was induced by replacing the cortical differentiation medium with cortical maturation medium (CMM) consisting of DMEM/F-12 with GlutaMAX supplemented with 1% (v/v) of N2 supplement (Thermo Fischer Scientific, Erlangen, Germany) and 100 U/ml penicillin and 100 μg/ml streptomycin (Life Technologies, Karlsruhe, Germany).
For spheroid culture in the biochips, the cell culture medium was equilibrated overnight to culture conditions of 37 °C and 5% CO2 to reduce air bubble formation within the biochip. Gas permeable silicon tubing was used for oxygen equilibration throughout the experiments. Bright field images of cells and spheroids within the biochip were taken with a Leica DM IL LED (light-emitting diode) microscope (Leica, Wetzlar, Germany) equipped with a Canon IXUS 95 IS camera (Canon, Krefeld, Germany). Images were analysed using ImageJ2 software (Fiji; NIH, USA).
BBB/cortex model assembly
The membrane used to grow hCMEC/D3 cells within the biochip was coated with 150 μg/ml collagen A (Biochrom, Berlin, Germany) for 1 h. Subsequently, 1.5 × 105 hCMEC/D3 cells were seeded on the membrane and cultured in ECM medium for 48 h. Subsequently, the culture medium was stepwise adjusted to cortical tissue medium (CTM)/ECM medium as described in Table I.
TABLE I.
Culture of hCMEC/D3 cells and cortical tissue spheroids in stepwise diluted culture medium.
| Composition of cell culture medium for cortical tissue spheroids (CTM %/ECM %) | ||||
| Day 10: 100/0 | Day 11: 90/10 | Day 12: 80/20 | Day 13: 70/30 | Day 14: 50/50 |
| Composition of cell culture medium for hCMEC/D3 (CTM %/ECM %) | ||||
| Day 10: 0/100 | Day 11: 10/90 | Day 12: 20/80 | Day 13: 30/70 | Day 14: 50/50 |
On day 14, the cortical spheroids were transferred into the micro-cavities of the lower membrane. On day 15, the fully assembled brain endothelial layer/cortex model was cultured under perfusion conditions for 24 h. The hCMEC/D3 layer was perfused with 350 μl/min corresponding to 4 dyn/cm2 via micro-channel 1 of the upper part of the biochip (Figure 1). A flow rate of 0.4 μl/min corresponding to a shear stress rated of 0.5 dyn/cm2 was applied in the lower microfluidic channel 3 for perfusion of the cortical spheroids from their basal side. Shear stress rates were calculated as described previously.6
To mimic neuroinflammation, a cytokine mixture consisting of 50 ng/ml human TNF, 10 ng/ml human IL-1β, 10 ng/ml human IFNγ (all from Calbiochem, Darmstadt, Germany), and 100 ng/ml LPS (Sigma-Aldrich, Darmstadt, Germany) was added to the medium and used for the perfusion of the hCMEC/D3 layer for 24 h via micro-channel 1 of the biochip (Figure 1).
Permeability assay
To test the permeability of the endothelial cell layer, 10 mg/ml of 3 kDa fluorescein isothiocyanate (FITC)-dextran (Sigma-Aldrich) was suspended in CTM/ECM medium, injected via micro-channel 1 into the biochip, and incubated for 30 min on top of the hCMEC/D3 cell layer under static conditions. Permeated FITC-dextran was collected from micro-channel 2, and the fluorescence of the labelled dextran was measured with a Mithras LB 940 fluorometer using the Mikrowin 2000 software (Berthold Technologies, Bad Wildbach, Germany).
Viability staining and immunofluorescence analyses
Cell viability of hCMEC/D3 cells and cortical spheroids was assessed with calcein-AM (Life Technologies) and propidium iodide (PI; Sigma-Aldrich) staining. The cells were washed with phosphate buffered saline (PBS), incubated with 2.5 μM calcein-AM and 1 μg/ml PI in PBS for 15 min, washed again with PBS, and subsequently cells were imaged.
For immunofluorescence staining, cortical spheroids were collected, fixed with 2% paraformaldehyde (PFA) (Sigma-Aldrich) for 30 min, embedded, and stored in TissueTek OCT (Sakura, Staufen, Germany) at −80 °C. Samples were cut into 10 μm thick cryo-sections prior to staining. The sections were permeabilized with 0.1% (w/v) Saponin (Fluka, St. Gallen, Switzerland) and blocked with 3% (v/v) normal goat serum (Dianova, Hamburg, Germany) for 30 min. Cortical spheroids were stained with chicken anti-Tbr-1, rabbit anti-Tbr-2 (Merck-Millipore, Darmstadt, Germany), and mouse anti-Pax-6 (Santa Cruz, Heidelberg, Germany) antibodies overnight.
For analysis of cells cultured in the biochips, the cavities of the biochip were opened by cutting the bonding foils with a scalpel. HCMEC/D3 cell layers on PET membranes were fixed with 2% PFA for 5 min with subsequent methanol treatment for 10 min at −20 °C and stained with rabbit anti-human von Willebrand factor (vWf; Dako, Hamburg, Germany), mouse anti-human VE-cadherin, mouse anti-human β-catenin (both BD Biosciences, Heidelberg, Germany), and rabbit anti-human ZO-1 (Life Technologies) overnight. The following secondary antibodies were used: goat anti-chicken AF488 (Abcam, Cambridge, UK), goat anti-mouse Cy3, and goat anti-rabbit AF647 (Dianova, Hamburg, Germany). Nuclei were stained with 4′,6-Diamidine-2′-phenylindole dihydrochloride (DAPI) (Life Technologies, Karlsruhe, Germany). Fluorescence imaging was performed on an Axio Observer Z1 fluorescence microscope equipped with Apotome.2 (Carl Zeiss AG, Jena, Germany). Fluorescence images were analysed with the ImageJ2 software.
Statistics
All results are reported as average means with standard deviation of at least three independent experiments. Statistical significance was calculated with two-tailed, non-paired student's t-test using GraphPad Prism 6.07 software (GraphPad, La Jolla, CA, USA).
RESULTS
Design of the biochip to co-culture brain microvascular endothelial cells and cortical tissue spheroids
Based on our recently described Multi-Organ-Tissue-Flow (MOTiF) biochip design allowing improved endothelial cell culture conditions under flow,6 the biochip comprises an upper part, holding a flat membrane that serves as cell substrate for the culture of hCMEC/D3 cells. This membrane is connected to two micro-channel systems that allow the independent perfusion of the membrane from the apical and basal side as recently described (Figure 1).6 Micro-channels are sealed at the top and bottom of the upper biochip part by a bonding foil and have a height of 0.45 mm for micro-channel 1 (upper channel), 0.7 mm for micro-channel 2 (middle channel), and 0.2 mm for micro-channel 3 (lower channel) (Figures 1 and 2). For co-culture with cortical tissue spheroids, a special membrane with thermo-formed micro-cavities was integrated for immobilization of the spheroids during perfusion of the endothelial layer in the lower part of the biochip (Figure 1).16 Each micro-channel system is connected to a separate sampling port that allows sample collection without interruption of cell culture perfusion with medium (Figure 1).
HCMEC/D3 cells were cultured on the flat membrane of the upper part of the biochip and grown to full confluence to assemble an endothelial barrier (Figure 2). Cortical tissue spheroids were cultured for 14 days in non-adhesive tissue plates to allow cell aggregation. Subsequently, spheroids were transferred into the biochip via micro-channel 2 of the lower part of the biochip and cultured in the micro-cavities of the lower membrane (Figure 2). The shear stress at the endothelial barrier within brain capillaries ranges between 3 and 20 dyn/cm2.17 To enable physiological mechanostimulatory conditions, the hCMEC/D3 cell layer was perfused with 350 μl/min, corresponding to a shear stress rate of 4 dyn/cm2 via the top channel (marked as micro-channel 1 in Figures 1 and 2). The cortical spheroids were perfused from the basal side via micro-channel 3 with a low flow rate of 0.4 μl/min corresponding to a shear stress rate of 0.5 dyn/cm2 (Figures 1 and 2) to provide sufficient medium exchange, but to prevent potential cell stress on the neural tissue spheroids.
Adjustment of cell culture medium and characterization of cell growth
To facilitate a reliable co-culture of hCMEC/D3 cells and cortical tissue spheroids, the cell culture medium for both cell types was adjusted to a 50/50 (v/v) composition of ECM and cortical tissue medium (CTM). Cortical tissue spheroids were adapted to the new cell culture medium by a daily stepwise reduction of the CTM portion in the culture medium and a concomitant increase in ECM. HCMEC/D3 cells vice versa were adapted to the co-culture medium in a similar way but with a reverse medium mix series (see Table I). Before transfer to the biochip, the cells were cultured for additional two days in the adjusted CTM/ECM medium.
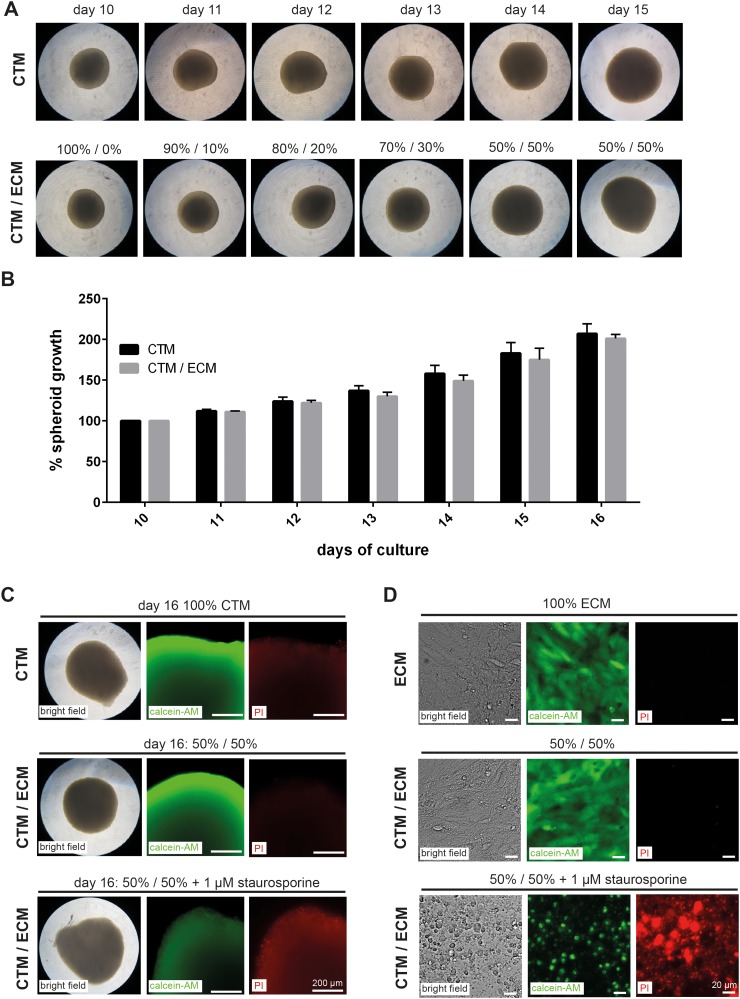
We observed no adverse impact on spheroid growth after transfer and subsequent culture of spheroids to the co-culture medium (Figures 3(a) and 3(b)). Moreover, no effect of medium adjustment was detectable on cortical spheroid viability as analysed by calcein-AM staining. The number of dead cells within the spheroids was also not increased as analysed by propidium iodide (PI) staining (Figure 3(c)). Similarly, we observed no differences in cell viability or cell death in hCMEC/D3 cells cultured in adjusted CTM/ECM compared with cells grown in ECM medium (Figure 3(d)). Treatment of spheroids or hCMEC/D3 cells with 1 μM of the apoptosis inducer staurosporine for 24 h served as control in cell viability staining and cell death assays. Staurosporine treatment induced a disintegration of cortical spheroids and detachment of hCMEC/D3 cells and was associated with a decline in the calcein-AM and an increase in the PI fluorescence signals (Figures 3(c) and 3(d)).
FIG. 3.
Adaption of hCMEC/D3 cells and cortical spheroid growth to CTM/ECM medium. (a) Bright field images of cortical spheroids grown from day 10 to day 15 in ECM and CTM/ECM cell culture medium. (b) Changes in the diameter of spheroids grown in CTM and CTM/ECM medium. The diameter at day 10 was normalized to 100%. The mean of the measurements is shown. Error bars indicate the standard deviation of three independent experiments. (c) and (d) Bright field microscopy images and fluorescence microscopy images of calcein-AM (green) and propidium iodide (PI, red) staining of spheroids at day 16 (c) and hCMEC/D3 cultured in ECM and CTM/ECM medium (d). Treatment with 1 μM staurosporine for 24 h served as control for viability and cell death analyses. (a), (c), and (d) Representative data of three independent experiments performed in 96-well plates are shown.
Detection of typical cortical and endothelial cell marker proteins
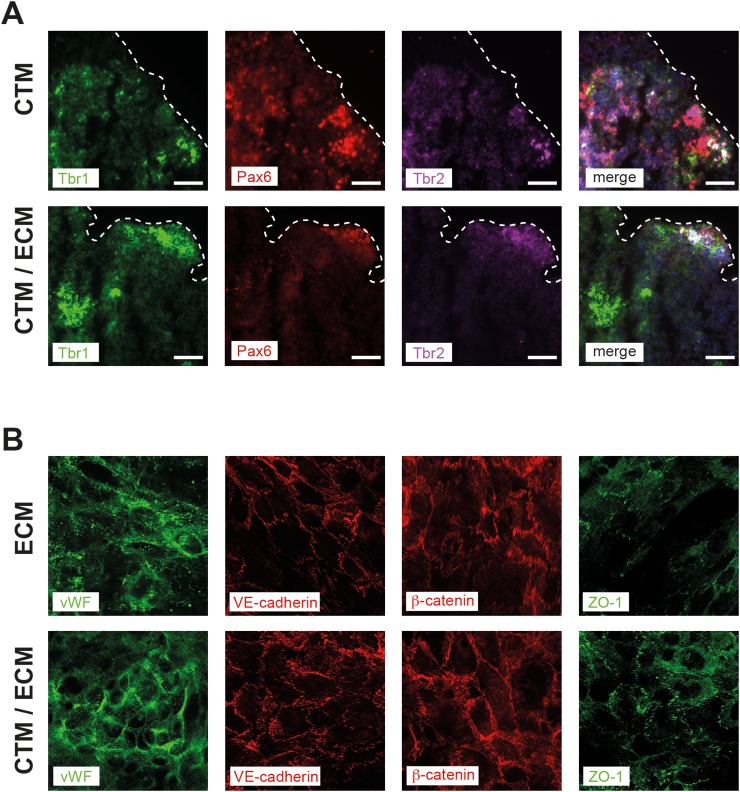
We next checked the expression of Tbr1, Tbr2, and Pax6 representing important regulators of the neocortical development in cortical spheroids cultured in CTM and CTM/ECM medium. At day 11, spheroids were adapted to adjusted CTM/ECM medium as described in Table I and cultured for additional two days in final 50/50 CTM/ECM medium composition. No difference in the expression levels of the indicated neural differentiation markers was observed between spheroids cultured in CTM or CTM/ECM. In both media, spheroids showed a sustained and defined expression of Tbr1, Tbr2, and Pax6 (Figure 4(a)).
FIG. 4.
Expression of cell differentiation markers in cortical spheroids and hCMEC/D3 cells cultured in ECM or CTM/ECM medium. (a) Cortical spheroids at day 16 of spheroid culture were stained for neural differentiation markers Tbr1 (green), Pax6 (red), and Tbr2 (magenta). In the merged image, cell nuclei are stained with DAPI (blue). Dashed lines indicate the borders of the spheroid cross-sections. (b) Expression of endothelial cell marker proteins von Willebrand factor (vWF, green), VE-cadherin (red), β-catenin (red), and ZO-1 (green) expressed in hCMEC/D3 cells. Representative images of three independent experiments are shown. Imaging was performed with fixed cells on membranes cut out from the biochips after perfusion.
In hCMEC/D3 cell layers, we analysed the expression of von Willebrand factor (vWF), a protein that is involved in regulation of coagulation and moreover is a central regulator of permeability and flexibility of the endothelial cell layer within the BBB.18 In addition, we examined the expression of VE-cadherin and β-catenin, proteins required for the maintenance of endothelial barrier integrity and CNS homeostasis,19 as well as the tight junction protein ZO-1. We observed no alterations in the protein expression of these proteins during adaption and subsequent culture of CMEC in CTM/ECM medium compared with ECM medium (Figure 4(b)).
Modulation of endothelial barrier integrity with a pro-inflammatory cytokine mix
As a next step, co-culture of hCMEC/D3 cells and cortical spheroids within the microfluidically supported biochip was established. HCMEC/D3 cells adapted to CTM/ECM medium were seeded at the top membrane and cultured for 2 days to allow full confluence and formation of a tight endothelial barrier. Cortical spheroids were subsequently transferred into the micro-cavities of the lower membrane via micro-channel 2 of the biochip. Inflammation in response to LPS is associated with BBB disruption and the release of the pro-inflammatory cytokines TNF, IL-1β, and IFNγ derived from pericytes.20–23 These cytokines can act synergistically at the individual cell types of the BBB modulating its function during neuroinflammation.22 To simulate a LPS-induced neuroinflammation in our biochip model in the absence of pericytes, a mixture of cytokines was added to the perfusion medium in the micro-channel 1. See supplementary Figure 2 (Ref. 14) for a scheme of cytokine stimulation via micro-channel 2. The final concentration of cytokines in the perfusion medium was 50 ng/ml TNF, 10 ng/ml IL-1β, 10 ng/ml IFNγ, and 100 ng/ml LPS.
The cytokine mix was added only to the medium that perfused the apical side of the endothelial cell layer. In this setting, cortical spheroids should only be affected by the cytokine mix upon preceding disruption of the endothelial barrier.
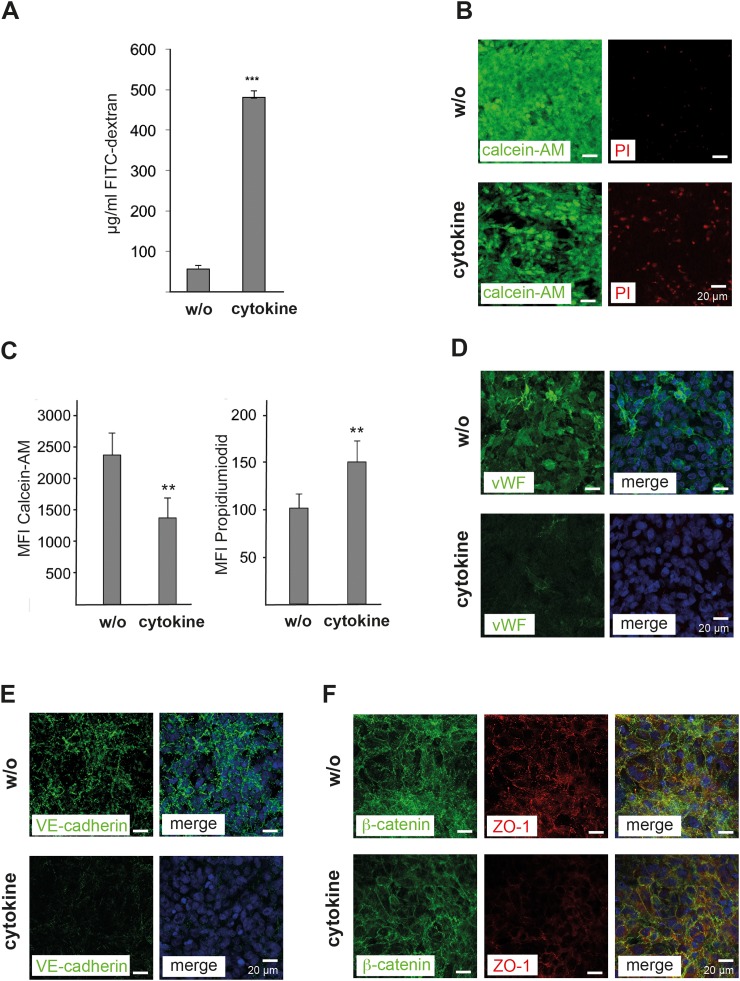
To determine the endothelial cell layer permeability, 3 kDa FITC-dextran was added to the perfusion medium and the intensity of fluorescence signals in the lower chamber of the biochip was assessed. Aliquot sampling was performed via the sample port S2 of the biochip (Figure 1). After 24 h of cytokine-mix perfusion of the endothelial BBB layer and subsequent incubation with 10 mg/ml FITC-dextran for 30 min, we observed a considerable increase in the fluorescence signal indicating a cytokine-induced impairment of the endothelial barrier integrity (Figure 5(a)). The increased permeability was associated with a reduced viability and an increased cell death of hCMEC/D3 cells determined by calcein-AM and PI staining, respectively (Figures 5(b) and 5(c)). We further observed reduced endothelial expression of vWF, VE-cadherin, and ZO-1 in response to cytokine treatment (Figures 5(d)–5(f)). By contrast, the expression of β-catenin was not altered by cytokine stimulation of the endothelial cell layer (Figure 5(f)).
FIG. 5.
Modulation of the endothelial integrity in response to cytokine treatment. (a) Measurement of the permeability of hCMEC/D3 cell layers co-cultured with cortical spheroids in the biochip using 3 kDa FITC-dextran beads. The amount of labelled dextran beads (μg/ml) permeating from the apical to the basal side of the endothelial cell layer was quantified by fluorescence spectrometry. (b) Immunofluorescence microscopic images of hCMEC/D3 cell layers without (w/o) and with cytokine-mix (cytokine) treatment for 24 h. Stainings with calcein-AM (green) and propidium iodide (PI, red) are shown. (c) Quantification of calcein-AM and PI signals in hCMEC/D3 cells. (d)–(f) Fluorescence microscopic images of hCMEC/D3 cells treated as described in (b) and stained for (d) von Willebrand factor (vWF, green), (e) VE-cadherin (green), (f) β-catenin (green), and ZO-1 (red). (d)–(f) In the merged images, cell nuclei were stained with DAPI (blue). (a) and (c) The mean values of three independent measurements are shown; error bars indicate the standard deviation. Statistical significance was calculated using student's t-test (** p < 0.01, *** p < 0.001). (b)–(f) Representative data of three independent experiments are shown.
Disruption of the endothelial barrier is associated with a diminished expression of neural differentiation marker expression in cortical spheroids
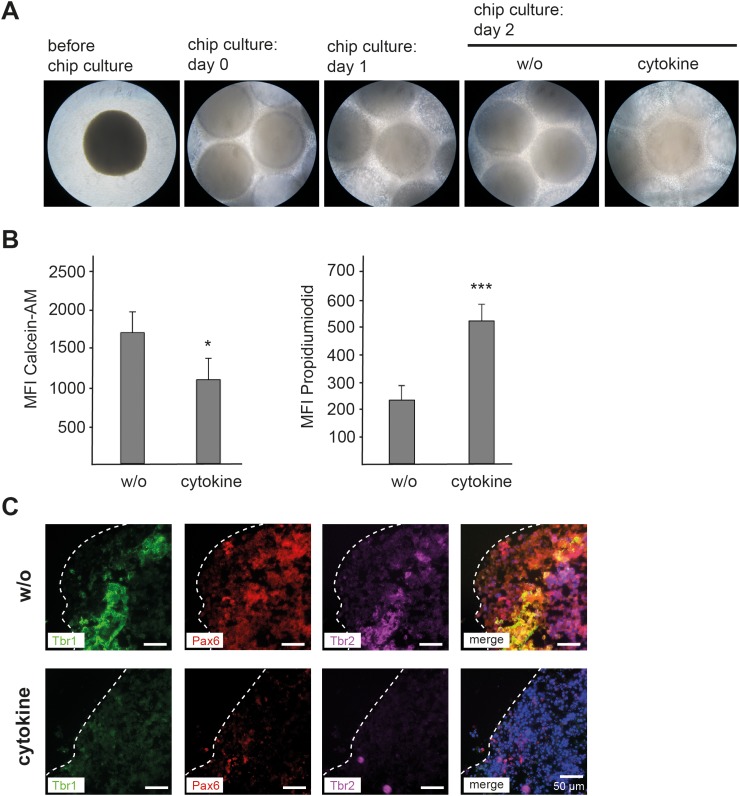
After transfer into the biochip, cortical spheroids remained stable, were viable, and showed no signs of increased cell death (Figure 6(a)). However, in response to cytokine treatment of the endothelial cell layer, signs of spheroid disintegration and an attenuated viability similar to staurosporine treatment were detectable at the levels of reduced density of cortical spheroids in bright field microscopy, as well as a diminished calcein-AM staining and increased PI fluorescence signals (Figure 6(b)). Furthermore, the defined expression of neocortex differentiation regulators Tbr1, Tbr2, and Pax6 was lost upon perfusion of the endothelial barrier with the cytokine mix medium.
FIG. 6.
Impact of endothelial barrier disruption on cortical spheroid growth and expression of neural differentiation markers in response to cytokine mix treatment. (a) Bright field microscopic images of cortical spheroids before transfer into the biochip (before chip culture), and co-culture with an endothelial hCMEC/D3 cell layer immediately after transfer into the biochip (chip culture: day 0), after 24 h culture in the biochip (chip culture: day 1), after 48 h culture in the biochip (chip culture: day 2, w/o), and after 48 h culture in the biochip with 24 h of cytokine mix treatment (chip culture: day 2, cytokine). (b) Fluorescence microscopical analyses of calcein-AM and propidium iodide stainings of spheroids at day 16 of co-culture with hCMEC/D3 cells without (w/o) and 24 h of cytokine mix treatment (cytokine). Mean values of three independent measurements are shown. Error bars indicate standard deviations. Statistical significance was calculated using student's t-test (* p < 0.05, *** p < 0.001). (c) Expression of cell differentiation markers in cortical spheroids co-cultured in the biochip cultured as described in (b). Cortical spheroids at day 16 of spheroid culture were stained for neural differentiation markers Tbr1 (green), Pax6 (red), and Tbr2 (magenta). In the merged image, cell nuclei are stained with DAPI (blue). Dashed lines indicate the borders of the spheroid cross-sections. (a) and (c) Representative data of three independent experiments are shown.
DISCUSSION
The majority of published BBB models so far uses static cell culture approaches.24 Recently, a three-dimensional model of the BBB comprising RBE4 rat brain endothelial cell layers wrapped inside a cylindrical lumen of a hollow collagen gel was reported. This model was shown to be sensitive to mediators of neuroinflammation.25 A similar approach has also been chosen in a study by Herland et al.,26 in which human brain microvascular endothelial cells (hBMECs) were co-cultured with pericytes and astrocytes. Here, the BBB was responsive to stimulation with pro-inflammatory TNF as detected by the release of G-CSF and IL-6 in the supernatant of the culture medium. In addition, a model with human umbilical vein endothelial cells (HUVECs) has been reported revealing an increased endothelial cell layer tightness when HUVECs were co-cultured with astrocytes.27 However, only a few microfluidically supported BBB models with the ability to mimic physiological shear stress conditions were reported so far. Booth and Kim recently described the establishment of the so-called μBBB model consisting of a co-culture of murine CMECs and astrocytes on a permeable membrane. The authors report that the co-culture of both cell types results in a significant increase in TEER values and tightness of the BBB, which was further increased by application of physiological shear stress to the endothelial cell layer.28 Similar results have been reported by Prabhakarpandian et al. for the Sym-BBB model, in which shear stress stimulation and astrocyte-conditioned medium result in an increased tight junction protein expression and enhanced tightness of RBE4 cell layers.29 Recently, a novel microfluidic bioreactor (“NeuroVascular Unit,” NVU) for the co-culture of endothelial cells, astrocytes, and pericytes under flow conditions has been reported. Within this bioreactor, a microenvironment is provided that favours the paracrine signalling between the co-cultured cell types needed for the long-term stable differentiation of the BBB and growing human neurons.30
An important feature of the hCMEC/D3 cell line used in our study, however, is the formation of a physiological barrier already in the absence of glia cells or astrocytes.8,31 We therefore omitted inclusion of additional cell types in the endothelial layer. However, future studies to improve our model should include astrocytes and pericytes. Most of the current microfluidically supported BBB models were established in polydimethylsiloxane (PDMS)-based systems. PDMS is often utilized because of its straightforward application in rapid prototyping.32 However, the use of PDMS comprises some important drawbacks, such as hydrophobic molecules can easily get adsorbed to the chip surface.33,34 Moreover, uncrosslinked free PDMS monomers leach out into the media and affect cellular behaviour.33,34 In addition, PDMS is known to be permeable to gases and water vapour and induces changes in the osmolarity of cell culture media.35 We therefore selected polystyrene as chip bulk material. This polymer is used since decades as a reliable cell culture substrate allows simple surface modifications and does not interfere with bright field or fluorescence microscopy.
Here, we demonstrated that our biochip-based model allows the co-culture of endothelial hCMEC/D3 cell layers and cortical tissue spheroids under physiological perfusion conditions. By stepwise adaption to an adjusted CTM/ECM medium, we were able to culture both cell types in a mutual cell culture medium without affecting the expression levels or distribution of the endothelial or neural cell marker proteins analysed. HCMEC/D3 cell layers expressed central marker proteins known to regulate BBB integrity and flexibility in vivo, such as vWF, VE-cadherin, ZO-1, and β-catenin.4–6,9,10,18,19 Furthermore, this model is able to reflect critical aspects of neuroinflammation, as depicted by its ability to specifically reproduce a cytokine-induced disruption of the endothelial barrier associated with subsequent alterations in neural cell morphology, increased neural cell death, and diminished expression of neural differentiation marker proteins. Our results are in agreement with the findings from studies in mice that analysed the development of the neocortex under inflammatory conditions. Similar to the in vivo model, we also observed a reduced expression of the neocortex development regulators Tbr1,36 Tbr2,37 and Pax638 in response to inflammation and pro-inflammatory cytokines. The data thus show that our in vitro model of an endothelial barrier co-cultured with neural tissue spheroids is able to reflect crucial aspects of neuroinflammation during neocortex formation.
However, we are also aware of the limitations of our model. Our biochip-based system currently lacks the option of direct measurements of TEER generated by the endothelial cell layer as electrodes between the endothelial cell layer and spheroids potentially affect spheroid growth and interfere with spheroid transfer into the biochip. Moreover, the distance between the endothelial barrier and the top of microcavity-embedded neural spheroids is approximately 0.7 mm forming a total media volume of approximately 160 μl between both cellular compartments. These dimensions are considerably larger than in the in vivo situation and thus may affect endocrine and paracrine signalling between the endothelial and neural tissue.
Another limitation of our model is the absence of pericytes and astrocytes. However, the current approach was chosen to have a first proof-of-principle with a simple cellular setup and a sustainable co-culture of different cell types within the biochip. Follow-up studies will be performed to test whether a co-culture of cerebral microvascular endothelial cells together with astrocytes and/or pericytes can be established to bring our model even closer to the physiological conditions. In this context, it is worth to note that human brain microvascular endothelial cells (hBMECs) generated from induced pluripotent stem cells have also been co-cultured with astrocytes, pericytes, and neural cells. In this co-culture model of the BBB, a significant tightness and the expression of tight and adherence junction proteins as well as multidrug-resistance proteins were demonstrated.39,40
CONCLUSIONS
To the best of our knowledge, we here describe for the first time a microfluidically supported biochip that integrates a model of cerebral microvascular endothelial barrier and cortical tissue spheroids. The model resembles physiological shear stress conditions at the endothelial barrier and allows a direct assessment of neuroinflammatory effects on cortical tissue differentiation and viability. It thus represents a novel tool to screen drugs affecting cerebral microvascular endothelial barrier integrity and mediating neuroinflammatory effects. In addition, the new biochip design allows the integration of single cell layers and spheroidal cell aggregates within an integrative microfluidically supported biochip. Moreover, this biochip design is adaptable to other organ-on-a-chip models that rely on integration of an endothelial cell layer resembling the vasculature and spheroid cultures resembling organ specific tissue.
From our data, we conclude that the presented new biochip model is able to mimic an inflammation-related impairment of the cerebral microvascular endothelial barrier concomitantly affecting expression of neural cell differentiation markers and cell viability in response to cytokine treatment. This model thus represents a valuable tool mimicking the cerebral vascular system in its cross-talk with cortical tissue under neuroinflammatory conditions in vitro. However, more work is required to fully characterize its potential in, e.g., toxicological or pharmaceutical screening studies.
ACKNOWLEDGMENTS
We are grateful to the excellent technical assistance of Fatina Siwczak and Margot Voigt at the University Hospital Jena as well as to Birgitta Slawik and Konrad Gulich at the BfR for providing technical support in generation of cortical organoids. The authors acknowledge support of this work by a grant from the German Federal Institute of Risk Assessment (Grant No. 1329-533).
References
- 1. Pardridge W. M., NeuroRx 2, 3 (2005). 10.1602/neurorx.2.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhatia S. N. and Ingber D. E., Microfluidic Organs-on-Chips ( Nature Publishing Group, 2014), pp. 760–772. [DOI] [PubMed] [Google Scholar]
- 3. Bhise N. S., Ribas J., Manoharan V., Zhang Y. S., Polini A., Massa S., Dokmeci M. R., and Khademhosseini A., J. Controlled Release 190, 82 (2014). 10.1016/j.jconrel.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Galbraith C. G., Skalak R., and Chien S., “ Shear stress induces spatial reorganization of the endothelial cell cytoskeleton,” Cell Motil. Cytoskeleton 40(4), 317–330 (1998). [DOI] [PubMed] [Google Scholar]
- 5. Cucullo L., Couraud P.-O. O., Weksler B., Romero I.-A. A., Hossain M., Rapp E., and Janigro D., J. Cereb. Blood Flow Metab. 28, 312 (2008). 10.1038/sj.jcbfm.9600525 [DOI] [PubMed] [Google Scholar]
- 6. Raasch M., Rennert K., Jahn T., Peters S., Henkel T., Huber O., Schulz I., Becker H., Lorkowski S., Funke H., and Mosig A., Biofabrication 7, 15013 (2015). 10.1088/1758-5090/7/1/015013 [DOI] [PubMed] [Google Scholar]
- 7. Persidsky Y., Ramirez S. H., Haorah J., and Kanmogne G. D., J. Neuroimmune Pharmacol. 1, 223 (2006). 10.1007/s11481-006-9025-3 [DOI] [PubMed] [Google Scholar]
- 8. Weksler B., Romero I. A., and Couraud P.-O., The hCMEC/D3 Cell Line as a Model of the Human Blood Brain Barrier ( BioMed Central Ltd., 2013), p. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stevenson B. R., Siliciano J. D., Mooseker M. S., and Goodenough D. A., J. Cell Biol. 103, 755 (1986). 10.1083/jcb.103.3.755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Griep L. M., Wolbers F., De Wagenaar B., Ter Braak P. M., Weksler B. B., Romero I. A., Couraud P. O., Vermes I., Van Der Meer A. D., and Van Den Berg A., Biomed. Microdevices 15, 145 (2013). 10.1007/s10544-012-9699-7 [DOI] [PubMed] [Google Scholar]
- 11. Frankola K. A., Greig N. H., Luo W., and Tweedie D., “ Targeting TNF-α to elucidate and ameliorate neuroinflammation in neurodegenerative diseases,” CNS Neurol Disord Drug Targets 10(3), 391–403 (2011). 10.2174/187152711794653751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mc Guire C., Beyaert R., and van Loo G., “ Death receptor signalling in central nervous system inflammation and demyelination,” Trends Neurosci. 34(12), 619–628 (2011). 10.1016/j.tins.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 13. Park K. M. and Bowers W. J., “ Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction,” Cell Signal. 22(7), 977–983 (2010). 10.1016/j.cellsig.2010.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.See supplementary material at http://dx.doi.org/10.1063/1.4955184E-BIOMGB-10-005604 for structures of the biochip with indicated lengths and heights and a scheme of cytokine stimulation via micro-channel 2 of the biochip.
- 15. Eiraku M. and Sasai Y., Nat. Protoc. 7, 69 (2011). 10.1038/nprot.2011.429 [DOI] [PubMed] [Google Scholar]
- 16. Giselbrecht S., Gietzelt T., Gottwald E., Trautmann C., Truckenmüller R., Weibezahn K. F., and Welle A., Biomed. Microdevices 8, 191 (2006). 10.1007/s10544-006-8174-8 [DOI] [PubMed] [Google Scholar]
- 17. van der Helm M. W., van der Meer A. D., Eijkel J. C. T., van den Berg A., and Segerink L. I., Tissue Barriers 4, e1142493 (2016). 10.1080/21688370.2016.1142493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suidan G. L., Brill A., De Meyer S. F., Voorhees J. R., Cifuni S. M., Cabral J. E., and Wagner D. D., Arterioscler., Thromb., Vasc. Biol. 33, 2112 (2013). 10.1161/ATVBAHA.113.301362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tran K. A., Zhang X., Predescu D., Huang X., Machado R. F., Göthert J. R., Malik A. B., Valyi-Nagy T., and Zhao Y.-Y., Circulation 133(2), 177–186 (2016). 10.1161/CIRCULATIONAHA.115.015982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Edelman D. a., Jiang Y., Tyburski J. G., Wilson R. F., and Steffes C. P., J. Trauma 62, 89 (2007). 10.1097/TA.0b013e31802dd712 [DOI] [PubMed] [Google Scholar]
- 21. Ghosh A., Birngruber T., Sattler W., Kroath T., Ratzer M., Sinner F., and Pieber T. R., PLoS One 9, 3 (2014). 10.1371/journal.pone.0098143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jansson D., Rustenhoven J., Feng S., Hurley D., Oldfield R. L., Bergin P. S., Mee E. W., Faull R. L., and Dragunow M., J. Neuroinflammation 11, 104 (2014). 10.1186/1742-2094-11-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Banks W. A., Gray A. M., Erickson M. A., Salameh T. S., Damodarasamy M., Sheibani N., Meabon J. S., Wing E. E., Morofuji Y., Cook D. G., and Reed M. J., J. Neuroinflammation 12, 223 (2015). 10.1186/s12974-015-0434-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stanimirovic D. B., Bani-Yaghoub M., Perkins M., and Haqqani A. S., Expert Opin. Drug Discovery 10, 141 (2015). 10.1517/17460441.2015.974545 [DOI] [PubMed] [Google Scholar]
- 25. Cho H., Seo J. H., Wong K. H. K., Terasaki Y., Park J., Bong K., Arai K., Lo E. H., and Irimia D., Sci. Rep. 5, 15222 (2015). 10.1038/srep15222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herland A., van der Meer A. D., FitzGerald E. A., Park T.-E., Sleeboom J. J. F., and Ingber D. E., PLoS One 11, e0150360 (2016). 10.1371/journal.pone.0150360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yeon J. H., Na D., Choi K., Ryu S.-W., Choi C., and Park J.-K., Biomed. Microdevices 14, 1141 (2012). 10.1007/s10544-012-9680-5 [DOI] [PubMed] [Google Scholar]
- 28. Booth R. and Kim H., “ Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB),” Lab Chip 12(10), 1784 (2012). 10.1039/C2LC40094D [DOI] [PubMed] [Google Scholar]
- 29. Prabhakarpandian B., Shen M.-C., Nichols J. B., Mills I. R., Sidoryk-Wegrzynowicz M., Aschner M., and Pant K., “ SyM-BBB: A microfluidic blood brain barrier model,” Lab Chip 13(6), 1093–1101 (2013). 10.1039/C2LC41208J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brown J. A., Pensabene V., Markov D. A., Allwardt V., Diana Neely M., Shi M., Britt C. M., Hoilett O. S., Yang Q., Brewer B. M., Samson P. C., McCawley L. J., May J. M., Webb D. J., Li D., Bowman A. B., Reiserer R. S., and Wikswo J. P., Biomicrofluidics 9, 054124 (2015). 10.1063/1.4934713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weksler B. B., Subileau E. A., Perrière N., Charneau P., Holloway K., Leveque M., Tricoire-Leignel H., Nicotra A., Bourdoulous S., Turowski P., Male D. K., Roux F., Greenwood J., Romero I. A., and Couraud P. O., FASEB J. 19, 1872 (2005). 10.1096/fj.04-3458fje [DOI] [PubMed] [Google Scholar]
- 32. Young E. W. and Simmons C. A., “ Macro- and microscale fluid flow systems for endothelial cell biology,” Lab Chip 10(2), 143–160 (2010). 10.1039/B913390A [DOI] [PubMed] [Google Scholar]
- 33. Toepke M. W. and Beebe D. J., “ PDMS absorption of small molecules and consequences in microfluidic applications,” Lab Chip 6(12), 1484–1486 (2006). 10.1039/B612140C [DOI] [PubMed] [Google Scholar]
- 34. Regehr K. J., Domenech M., Koepsel J. T., Carver K. C., Ellison-Zelski S. J., Murphy W. L., Schuler L. A., Alarid E. T., and Beebe D. J., “ Biological implications of polydimethylsiloxane-based microfluidic cell culture,” Lab Chip 9(15), 2132–2139 (2009). 10.1039/B903043C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thuenauer R., Rodriguez-Boulan E., and Römer W., “ Microfluidic approaches for epithelial cell layer culture and characterisation,” Analyst 139, 3206–3218 (2014). 10.1039/C4AN00056K [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carpentier P. A., Haditsch U., Braun A. E., Cantu A. V., Moon H. M., Price R. O., Anderson M. P., Saravanapandian V., Ismail K., Rivera M., Weimann J. M., and Palmer T. D., J. Neurosci. 33, 16874 (2013). 10.1523/JNEUROSCI.4654-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tronnes A. A., Koschnitzky J., Daza R., Hitti J., Ramirez J. M., and Hevner R., Reprod. Sci. 23(6), 771–778 (2016). 10.1177/1933719115618273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Soumiya H., Fukumitsu H., and Furukawa S., J. Neurosci. Res. 89, 1575 (2011). 10.1002/jnr.22704 [DOI] [PubMed] [Google Scholar]
- 39. Lippmann E. S., Azarin S. M., Kay J. E., Nessler R. A., Wilson H. K., Al-Ahmad A., Palecek S. P., and Shusta E. V., “ Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells,” Nat. Biotechnol. 30(8), 783–791 (2012). 10.1038/nbt.2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lippmann E. S., Al-Ahmad A., Azarin S. M., Palecek S. P., and Shusta E. V., Sci. Rep. 4, 4160 (2014). 10.1038/srep04160 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- See supplementary material at http://dx.doi.org/10.1063/1.4955184E-BIOMGB-10-005604 for structures of the biochip with indicated lengths and heights and a scheme of cytokine stimulation via micro-channel 2 of the biochip.