Abstract
Advanced glycation end products (AGEs) play an important factor for pathophysiology of diabetes and its complications. Moringa oleifera is one of the medicinal plants that have anti-hyperglycemic activity. However, anti-glycation property of Moringa oleifera leaf extract on the different types of reducing monosaccharides-induced protein glycation has not been investigated. Therefore, the aim of this study was to examine the protective effect of Moringa oleifera aqueous leaf extract (MOE) on reducing sugars-induced protein glycation and protein oxidation. Total phenolic content of MOE was measured using the Folin–Ciocalteu method. Bovine serum albumin was incubated with 0.5 M of reducing sugars (glucose or fructose) with or without MOE (0.5–2.0 mg/mL) for 1, 2, 3 and 4 weeks. The results found that total phenolic content was 38.56 ± 1.50 mg gallic acid equivalents/g dry extract. The formation of fluorescent and non-fluorescent AGEs [Nε-(carboxymethyl) lysine (CML)] and the level of fructosamine were determined to indicate protein glycation, whereas the level of protein carbonyl content and thiol group were examined for protein oxidation. MOE (0.5–2.0 mg/mL) significantly inhibited the formation of fluorescent, Nε-CML and markedly decreased fructosamine level (P < 0.05). Moreover, MOE significantly prevented protein oxidation manifested by reducing protein carbonyl and the depletion of protein thiol in a dose-dependent manner (P < 0.05). Thus, the findings indicated that polyphenols containing in MOE have high potential for decreasing protein glycation and protein oxidation that may delay or prevent AGE-related diabetic complications.
Keywords: Moringa oleifera, Polyphenol, Glycation, Glucose, Fructose
Background
Advanced glycation end products (AGEs) are a complex of heterogeneous group of molecules that are formed from non-enzymatic glycation of carbonyl group of a reducing sugar with an amino group of proteins, lipids, or nucleic acids (Kaneko et al. 2005). The accumulation of AGEs in various types of tissues causes the alteration of proteins leading to change their characteristics, physiochemical, and biochemical properties (Vanessa et al. 2013). The interaction of AGEs with the receptor of advanced glycation end products evokes oxidative stress and subsequently elicits vascular inflammation and thrombosis (Kang 2003). Studies have also shown that reactive oxygen species (ROS) formed by AGEs cause DNA damage and induction of cell apoptosis (Kang 2003). Glucose and fructose, the most common reducing sugar found in blood circulation react spontaneously with amino groups of proteins to AGEs. Although glucose plays a vital role in the formation of AGEs, it is now known that fructose undergoes protein glycation much faster than glucose (Semchyshyn et al. 2014). Endogenous fructose production by the sorbitol pathway is also considered to contribute the formation of AGEs and consequently accumulate in the tissues (Suarez et al. 1989; Vinson and Howard 1996). There are several clinical studies which demonstrate the link between the long-term consumption of fructose and the development of aging process (Levi and Werman 1998). Therefore, there has been seriously concern regarding the critical role of fructose in the glycation process. Studies on antiglycating agents have recently emerged as the new therapeutic approaches in preventing AGE-related diseases (Adisakwattana et al. 2010). Aminoguanidine (AG) is one of therapeutic agents for use in the prevention of AGE formation by cleavage of AGE-induced chemical cross-links (Brownlee et al. 1986). However, it has shown serious side effects including vascularitis, gastrointestinal disturbances, and anemia (Brownlee et al. 1986). For this reason, the search for alternative prevention of AGE formation has been focused on the natural products.
Polyphenolic compounds are commonly found in vegetables, fruits, spices, and medicinal herbs. Previously, it has been shown that polyphenols play an important role in human health, including reduced risk of chronic and degenerative diseases (Vauzour et al. 2010; Ngamukote et al. 2011; Adisakwattana and Chanathong 2011). Moringa oleifera (Ma-rum) is the most widely cultivated species of a monogeneric family, the Moringaceae which is commonly found in tropical countries such as India, Afghanistan, as well as Thailand. In addition, Many studies have reported the flavonoid contents such as keamferol, quercetin, ferulic acid, gallic acid, rutin, caffeic acid as well as other phenolic compounds in multi-part of Moringa tree (Fahey 2005; Anwar et al. 2007). A number of previous studies have reported pharmacological properties of Moringa oleifera particular in antioxidant property and antidiabetic activity that may provide benefits for diabetic patients (Jaiswal et al. 2009; Chumark et al. 2008; Adisakwattana and Chanathong 2011). However, there are no reports in the literature showing that MOE can inhibit protein glycation induced by different types of reducing monosaccharaides in vitro models. Thus, the aim of present study was to evaluate the inhibitory effects of MOE on glucose- and fructose-induced protein glycation, about which no previous reports exist. Furthermore, the inhibitory effects of MOE on oxidation-dependent damages to bovine serum albumin mediated by glycation were also determined.
Methods
Chemicals
Glucose, fructose, and 2,4-dinitrophenyl hydrazine (DNPH) were purchased from Ajax Finechem (Taren Point, Australia). Catechin, gallic acid, sodium azide, Nitroblue tetrazolium (NBT), aminoguanidine hydrochloride (AG), guanidine hydrochloride, Thioflavin T (ThT), 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), and l-cysteine were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). Trichloroacetic acid (TCA) was purchased from Merck (Darmstadt, Germany). OxiSelectTM CML ELISA kit was purchased from Cell Biolabs (San Diego, CA, USA). All other reagents used were of analytical grade.
Preparation of Moringa oleifera aqueous leaf extract
The leaves of Moringa oleifera were obtained from local areas of Bangkok in Nongkhame district, Thailand. The herbarium number of A014172 (BCU) was authenticated by a Taxonomist at Department of Botany, Faculty of Science, Chulalongkorn University, Thailand. The dried leaves (250 g) were extracted with distilled water twice (3 L) for 3 h at 100 °C. The extraction was filtered through Whatman No. 1 filter paper under the vacuum. The filtrate was further subjected to a spray dryer SD-100 (Eyela world, Tokyo Rikakikai Co., LTD, Japan) to obtain the extract powder. The spray drying conditions, inlet and outlet air temperature was set at 160 and 89–99 °C, respectively.
Determination of total phenolic content
Total phenolic content of Moringa oleifera leaf extract (MOE) was determined by the Folin-Ciocalteu method (Verma et al. 2009). The extract powder was dissolved in distilled water (1.25 mg/mL). The freshly prepared Folin-Ciocalteu reagent was gently mixed with 10 µL of sample. Then, 75 µL of 7.5 % sodium carbonate (Na2CO3) was added and allowed to stand for 30 min at room temperature in the dark. The mixture was measured at 725 nm by a spectrophotometer. Gallic acid (0.025–0.4 mg/mL) was used as a standard and the content of total phenolics was expressed as mg gallic acid equivalents/g dried extract.
Preparation of glycated bovine serum albumin (BSA)
Glycated BSA was performed according to a previously described method (Povichit et al. 2010) with slight modifications. Briefly, BSA (10 mg/mL) was incubated with glucose or fructose (0.5 M) in 0.1 M phosphate buffer (pH 7.4) containing 0.02 % sodium azide (NaN3) with or without (MOE) (0.5–2.0 mg/mL) and aminoguanidine (AG, 1.0 mg/mL) at 37 °C for 4 weeks. Samples were kept at −20 °C until analysis.
Determination of advanced glycation end product (AGE) formation
The fluorescent AGEs, the irreversible products at the end stage of non-enzymatic glycation, were determined by a spectrofluorometer (Wallac 1420 Victor3 V, PerkinElmer, Santa Clara, CA, USA) at excitation and emission wavelengths of 355 nm and 460 nm, respectively (Povichit et al. 2010).
Determination of Nε-(carboxymethyl) lysine (CML)
Non-fluorescent AGEs, Nε-(carboxymethyl) lysine (Nε-CML), is the most abundant product of glycation reaction. Commercially available ELISA kit was used for measurement of Nε-CMLformation (Cell Biolabs, CA, USA).
Determination of fructosamine
The levels of fructosamine was analyzed by nitroblue-tetrazolium (NBT) assay with minor modification (Armbruster 1987). Briefly, 90 µL of 2.5 mM nitrobluetetrazolium (NBT) reagent was added to 10 µL of glycated BSA in carbonate buffer (pH 10.3). After 10 and 15 min of incubation, the mixture was measured at 590 nm. The concentration of fructosamine was calculated by using the different absorption at 10 and 15 min time points compared with the standard 1-deoxy-1-morpholino-fructose (1-DMF) curve.
Determination of protein carbonyl
Protein carbonyl content were determined according to a previously published method with minor modifications (Levine et al. 1990). In brief, 10 mM 2,4-dinitrophenylhydrazine (DNPH) in 2.5 M HCl (400 µL) was added to 100 µL of glycated BSA and incubated in the dark at room temperature for 60 min. Then, 20 % (w/v) trichloroacetic acid (500 µL) was added and kept on ice for 5 min. Protein precipitation was centrifuged and the protein pellet was then washed with 1:1 (v/v) ethanol/ethyl acetate and dissolved in 6 M guanidine hydrochloride. The absorbance was determined at 370 nm. The concentration of protein carbonyl content was calculated using an absorption coefficient of 22,000 M−1cm−1. The results were expressed as nmol carbonyl/mg protein.
Determination of protein thiol groups
The determination of free thiol groups were performed according to Ellman’s assay using 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) (Ellman 1959). Glycated BSA (10 µL) was incubated with 6 mM DTNB in 0.1 M PBS (pH 7.4) for 15 min at room temperature. The absorbance was determined at 410 nm. The free thiol concentration was calculated for the standard curve of l-cysteine (0.3–10 µM) and expressed as nmol/mg protein.
Statistical analysis
Data are expressed as mean ± standard error of mean (SEM) of triplicate determination (n = 3). Differences among groups were analyzed for statistical significance by one-way ANOVA followed by Duncan as post hoc comparison. P value < 0.05 was considered statistically significant.
Results
Phytochemical analysis
In the present study, the content of total phenolic compounds in Moringa oleifera leaf extract (MOE) was 38.56 ± 1.50 mg gallic acid equivalents/g extract.
Effect of Moringa oleifera leaf extract on the different types of reducing monosaccharide-induced fluorescent AGE formation
The formation of fluorescent AGEs in different monosaccharide-induced protein glycation was monitored during 4 weeks of incubation. As shown in Fig. 1, the significant increase in fluorescent intensity in BSA incubated with glucose and fructose was seen during 4 weeks of the incubation. The results demonstrated that the fluorescent AGE formation was increased 3.24-fold in glucose model (Fig. 1A) and 5.76-fold in fructose model (Fig. 1B) whereas MOE (0.5–2.0 mg/mL) inhibited the formation of AGEs in a dose-dependent manner during experimental periods both glucose and fructose models at week 4. The percentage inhibition of AGE formation by MOE (0.5–2.0 mg/mL) ranged from 14.52–40.65 % in glucose-glycated BSA and 45.82–65.43 % in fructose-glycated BSA. However, MOE has less potent in the inhibition of AGE formation when compared with AG at the same concentration (1 mg/mL).
Fig. 1.
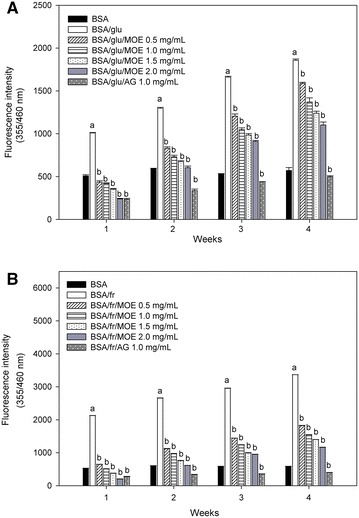
The effect of Moringa oleifera leaf extract (MOE, 0.5–2.0 mg/mL) and aminoguanidine (AG, 1.0 mg/mL) on the formation of fluorescent AGEs in A bovine serum albumin (BSA) incubated with 0.5 M glucose (glu) and B bovine serum albumin (BSA) incubated with 0.5 M fructose (fr) at week 1, 2, 3 and 4 of incubation. The results are expressed as mean ± SEM (n = 3). a P < 0.05 when compared to BSA alone, b P < 0.05 when compared to BSA with glucose (BSA/glu) or BSA with fructose (BSA/fr) at the same week of incubation
Effect of Moringa oleifera leaf extract on the level of Nε-(carboxymethyl) lysine (CML)
In order to examine the formation of non-fluorescent AGEs, the level of Nε-CML was measured at week 4 of incubation. As shown in Fig. 2, it was found that the level of Nε-CML dramatically increased in glucose-glycated BSA (3.10-fold) and fructose-glycated BSA (8.60-fold). Conversely, the addition of MOE to the solution (1.0 mg/mL) inhibited Nε-CML formation about 31.02 % in glucose-glycated BSA and about 66.82 % in fructose-glycated BSA, whereas AG (1.0 mg/mL) inhibited Nε-CML formation about 44.17 % in glucose-glycated BSA and about 72.45 % in fructose-glycated BSA.
Fig. 2.
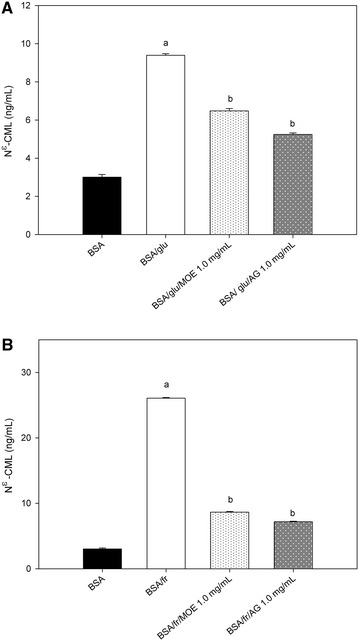
The effect of Moringa oleifera leaf extract (MOE, 1.0 mg/mL) and aminoguanidine (AG, 1.0 mg/mL) on the level of N ε-(carboxymethyl) lysine (CML) in A bovine serum albumin (BSA) incubated with 0.5 M glucose (glu) and B bovine serum albumin (BSA) incubated with 0.5 M fructose (fr) at week 4 of incubation. The results are expressed as mean ± SEM (n = 3). a P < 0.05 when compared to BSA alone, b P < 0.05 when compared to BSA with glucose (BSA/glu) or BSA with fructose (BSA/fr) at the same week of incubation
Effect of Moringa oleifera leaf extract on the level of fructosamine
The effects of MOE on the level of fructosamine are shown in Fig. 3. The level of fructosamine in glucose-glycated BSA and fructose-glycated BSA markedly increased throughout 4 weeks of the experiment. In contrast, the increasing level of fructosamine was attenuated by MOE (0.5–2.0 mg/mL) during 4 weeks of the study. At week 4 of incubation, MOE (0.5–2.0 mg/mL) reduced the level of fructosamine in a concentration-dependent manner in glucose-glycated BSA (71.21–80.30 %) and fructose-glycated BSA (18.96–49.56 %). In addition, the percentage reduction of fructosamine by AG (1 mg/mL) was 42.92 % for glucose-glycated BSA and 45.45 % for fructose-glycated BSA.
Fig. 3.
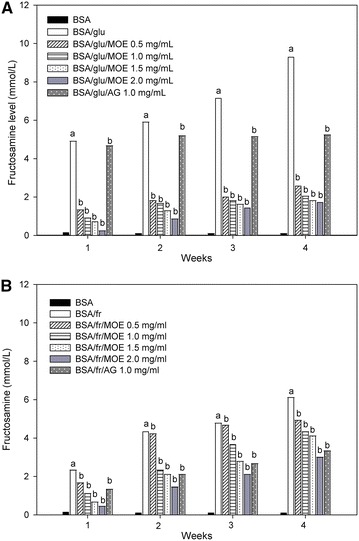
The effect of Moringa oleifera leaf extract (MOE, 0.5–2.0 mg/mL) and aminoguanidine (AG, 1.0 mg/mL) on fructosamine level in A bovine serum albumin (BSA) incubated with 0.5 M glucose (glu) and B bovine serum albumin (BSA) incubated with 0.5 M fructose (fr) at week 1, 2, 3 and 4 of incubation. The results are expressed as mean ± SEM (n = 3). a P < 0.05 when compared to BSA alone, b P < 0.05 when compared to BSA with glucose (BSA/glu) or BSA with fructose (BSA/fr) at the same week of incubation
Effect of Moringa oleifera leaf extract on the level of protein carbonyl content and protein thiol groups
The level of carbonyl content and thiol groups were used for indication of the protein oxidation mediated by glycation process. As shown in Fig. 4, the carbonyl content of glucose-glycated BSA and fructose-glycated BSA significantly increased during the experimental period, whereas MOE (0.5–2.0 mg/mL) significantly suppressed an increase in protein carbonyl content of glucose-glycated BSA and fructose-glycated BSA. When comparing with glucose-glycated BSA and fructose-glycated BSA at week 4, the percentage reduction of carbonyl content by MOE (0.5–2.0 mg/mL) ranged from 51.12 to 76.31 % in glucose-glycated BSA and 58.79 to 77.76 % in fructose-glycated BSA. A significant reduction of protein carbonyl content (91.59 % for glucose-glycated BSA and 88.29 % for fructose-glycated BSA) was observed in the presence of AG (1 mg/mL) at the same week. The effects of MOE on the oxidation of protein thiols are presented in Fig. 5. When BSA was incubated with glucose or fructose, the level of thiol groups had continuously declined throughout the experimental periods. Interestingly, there was a significant increase in the level of thiol groups after addition of MOE (0.5–2.0 mg/mL) as well as AG (1.0 mg/mL). The findings showed that the percentage prevention of depleting thiol group by MOE ranged from 7.57 to 9.77 % in glucose-glycated BSA and 5.73 to 10.32 % in fructose-glycated BSA, whereas AG (1.0 mg/mL) significantly prevented the depletion of protein thiol groups around 9.46 % and 14.09 % in glucose-glycated BSA and fructose-glycated BSA, respectively at the week 4.
Fig. 4.
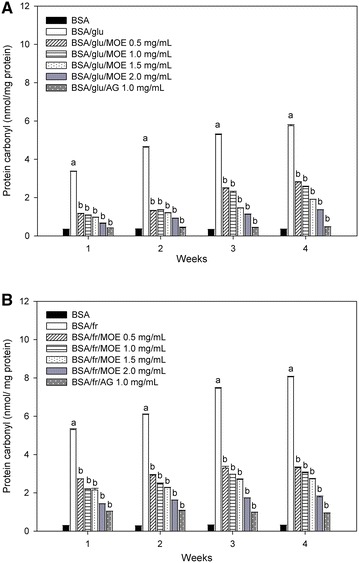
The effect of Moringa oleifera leaf extract (MOE, 0.5–2.0 mg/mL) and aminoguanidine (AG, 1.0 mg/mL) on protein carbonyl content in A bovine serum albumin (BSA) incubated with 0.5 M glucose (glu) and B bovine serum albumin (BSA) incubated with 0.5 M fructose (fr) at week 1, 2, 3 and 4 of incubation. The results are expressed as mean ± SEM (n = 3). a P < 0.05 compared to BSA alone, b P < 0.05 when compared to BSA with glucose (BSA/glu) or BSA with fructose (BSA/fr) at the same week of incubation
Fig. 5.
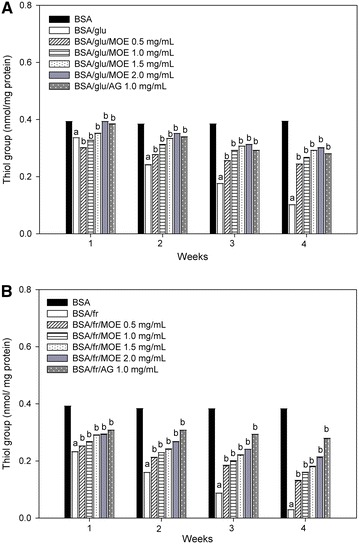
The effects of Moringa oleifera leaf extract (MOE, 0.5–2.0 mg/mL) and aminoguanidine (AG, 1.0 mg/mL) on thiol group in A bovine serum albumin (BSA) incubated with 0.5 M glucose (glu) and B bovine serum albumin (BSA) incubated with 0.5 M fructose (fr) at week 1, 2, 3 and 4 of incubation. The results are expressed as mean ± SEM (n = 3). a P < 0.05 compared to BSA alone, b P < 0.05 when compared to BSA with glucose (BSA/glu) or BSA with fructose (BSA/fr) at the same week of incubation
Discussion
In a present study, MOE was investigated the effect on glucose- and fructose-induced florescent and non-fluorescent AGE formation. The results showed that MOE efficiently inhibited fluorescent and non-fluorescent AGE formation. MOE also reduced the level of fructosamine associated with the reduction of AGE formation in glucose-glycated BSA and fructose-glycated BSA. A significant decrease of protein carbonyl content and oxidation of thiols in BSA were seen when MOE was added to the systems, it markedly suppressed these processes. The blockage of the carbonyl group in reducing sugars, the trapping of reactive oxygen species (ROS) and carbonyls during glycation, and breaking the crosslinking structure in the formed AGEs have recently been revealed as the underlying mechanisms of antiglycating agents (Price et al. 2001). The scavenging free radical generation during glycation process may highlight other mechanisms for the prevention of AGE formation (Rout and Banerjee 2007). The Amadori products can be fragmented and consequently generate superoxide anion to form fluorescent and non-fluorescent AGEs at the early stage of glycation (Peyroux and Sternberg 2006). An excess production of ROS causes oxidative damage to proteins which lead to introduce carbonyl groups on the side chain of proteins and deplete thiol group of protein (Dalle-Donne et al. 2003).
Recent studies have shown that polyphenolic compounds from the edible plants play a vital role to protect monosaccharide-induced protein glycation and oxidation (Adisakwattana et al. 2010). Additionally, there is a strong link between the polyphenolic content in the tested plant extracts and the ability to inhibit AGE formation (Peng et al. 2011; Wu and Yen 2005). Evidence also supports that the inhibitory effect of polyphenols against protein glycation is strongly related to their ability of scavenging free radical derived from the glycoxidation process (Povichit et al. 2010). Our findings indicate that MOE has high content of polyphenolic compounds. The content of total phenolic compounds in Moringa oleifera leaf extract (MOE) was consistent with previous studies that the content of total phenolic compounds in MOE ranged 33.82–45.21 mg gallic acid equivalents/g extract. According to the results obtained, we addressed the hypothesis that polyphenolic compounds in MOE may be a major contributor to inhibit the formation of AGEs. Previous studies have demonstrated that MOE has antioxidant activity against free radicals including DPPH free radical, superoxide, hydroxyl and nitric oxide radical (Siddhuraju and Becker 2003). Therefore, the inhibitory effect of MOE on glycation-induced protein oxidation may be due to its antioxidant properties. However, certain active biological constituents of MOE remain unknown. To prove this hypothesis, separation and characterization of polyphenolic compound in MOE using HPLC–MS are required for further study.
Conclusion
Moringa oleifera aqueous leaf extract effectively inhibits reducing monosaccharide-induced AGE formation, protein oxidation and protein cross-linking in glycation reaction. This finding could be suggested that Moringa oleifera aqueous leaf extract may be used as an antiglycation agent to prevent the progression of diabetic complications.
Authors’ contributions
SN, WS and SA was responsible for conception and design, drafted the manuscript and revised it critically for important intellectual content. WD, KM, and SS contributed to the data analysis and interpretation of the findings. PN conducted the experiments, organized the data analysis, and interpretation of data. All authors contributed to the drafting of the manuscript. In addition, all authors also read and approved the final manuscript.
Acknowledgements
We gratefully acknowledge to Thailand Research Fund (TRF) and Ratchadaphiseksomphot Endowment Fund, Chulalongkorn University and Grant for International Research Integration: Chula Research Scholar, Ratchadaphiseksomphot Endowment Fund for financial support. Weerachat Sompong would like to thank Ratchadaphiseksomphot Fund for Postdoctoral Fellowship, Chulalongkorn University. The authors would like to thank The Faculty of Allied Health Sciences and The Halal Science Center for their instrumental support.
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- AGEs
advanced glycation end products
- Nε-CML
Nε-(carboxymethyl) lysine
- BSA
bovine serum albumin
- AG
aminoguanidine
- MOE
Moringa oleifera leaf extract
Contributor Information
Pornpimon Nunthanawanich, Email: pornpimon.ntwn@gmail.com.
Weerachat Sompong, Email: kradat_pup@hotmail.com.
Sukrit Sirikwanpong, Email: sirikwanpong.s@gmail.com.
Kittana Mäkynen, Email: kittana.chanda@gmail.com.
Sirichai Adisakwattana, Email: adisakwattana_siri@yahoo.com.
Winai Dahlan, Email: winai.hsc@gmail.com.
Sathaporn Ngamukote, Phone: +66-2-218-1116, Email: amppam10@gmail.com.
References
- Adisakwattana S, Chanathong B. Alpha-glucosidase inhibitory activity and lipid-lowering mechanisms of Moringa oleifera leaf extract. Eur Rev Med Pharmacol Sci. 2011;15:803–808. [PubMed] [Google Scholar]
- Adisakwattana S, Jiphimai P, Prutanopajai P, Chanathong B, Sapwarobol S, Ariyapitipan T. Evaluation of α-glucosidase, α-amylase and protein glycation inhibitory activities of edible plants. Int J Food Sci Nutr. 2010;61:295–305. doi: 10.3109/09637480903455963. [DOI] [PubMed] [Google Scholar]
- Anwar F, Latif S, Ashraf M, Gilani AH. Moringa oleifera. A food plant with multiple medicinal uses. Phytother Res. 2007;21:17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- Armbruster DA. Fructosamine: structure, analysis, and clinical usefulness. Clin Chem. 1987;33:2153–2163. [PubMed] [Google Scholar]
- Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986;232:1629–1632. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- Chumark P, Khunawat P, Sanvarinda Y, Phornchirasilp S, Morales NP, Phivthong-Ngam L, et al. The in vitro and ex vivo antioxidant properties, hypolipidaemic and antiatherosclerotic activities of water extract of Moringa oleifera Lam. leaves. J Ethnopharmacol. 2008;116:439–446. doi: 10.1016/j.jep.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329:23–38. doi: 10.1016/S0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fahey JW. Moringa oleifera: a review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Part 1. Trees Life J. 2005;1:5–20. [Google Scholar]
- Jaiswal D, Kumar Rai P, Kumar A, Mehta S, Watal G. Effect of Moringa oleifera Lam leaves aqueous extract therapy on hyperglycemic rats. J Ethnopharmacol. 2009;123:392–396. doi: 10.1016/j.jep.2009.03.036. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Bucciarelli L, Hwang YC, Lee L, Yan SF, Schmidt A, et al. Aldose reductase and AGE-RAGE pathways: key players in myocardial ischemic injury. Ann NY Acad Sci. 2005;1043:702–709. doi: 10.1196/annals.1333.081. [DOI] [PubMed] [Google Scholar]
- Kang JH. Oxidative damage of DNA induced by methylglyoxal in vitro. Toxicol Lett. 2003;145:181–187. doi: 10.1016/S0378-4274(03)00305-9. [DOI] [PubMed] [Google Scholar]
- Levi B, Werman MJ. Long-term fructose consumption accelerates glycation and several age-related variables in male rats. J Nutr. 1998;128:1442–1449. doi: 10.1093/jn/128.9.1442. [DOI] [PubMed] [Google Scholar]
- Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz A-G, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-H. [DOI] [PubMed] [Google Scholar]
- Ngamukote S, Mäkynen K, Thilawech T, Adisakwattana S. Cholesterol-lowering activity of the major polyphenols in grape seed. Molecules. 2011;16(6):5054–5061. doi: 10.3390/molecules16065054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Ma J, Chen F, Wang M. Naturally occurring inhibitors against the formation of advanced glycation end-products. Food Funct. 2011;2:289–301. doi: 10.1039/c1fo10034c. [DOI] [PubMed] [Google Scholar]
- Peyroux J, Sternberg M. Advanced glycation endproducts (AGEs): pharmacological inhibition in diabetes. Pathol Biol. 2006;54:405–419. doi: 10.1016/j.patbio.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Povichit N, Phrutivorapongkul A, Suttajit M, Chaiyasut C, Leelapornpisid P. Phenolic content and in vitro inhibitory effects on oxidation and protein glycation of some Thai medicinal plants. Pak J Pharm Sci. 2010;23:403–408. [PubMed] [Google Scholar]
- Price DL, Rhett PM, Thorpe SR, Baynes JW. Chelating activity of advanced glycation end-product inhibitors. J Biol Chem. 2001;276:48967–48972. doi: 10.1074/jbc.M108196200. [DOI] [PubMed] [Google Scholar]
- Rout S, Banerjee R. Free radical scavenging, anti-glycation and tyrosinase inhibition properties of a polysaccharide fraction isolated from the rind from Punica granatum. Bioresour Technol. 2007;98:3159–3163. doi: 10.1016/j.biortech.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Semchyshyn HM, Miedzobrodzki J, Bayliak MM, Lozinska LM, Homza BV. Fructose compared with glucose is more a potent glycoxidation agent in vitro, but not under carbohydrate-induced stress in vivo: potential role of antioxidant and antiglycation enzymes. Carbohydr Res. 2014;384:61–69. doi: 10.1016/j.carres.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J Agric Food Chem. 2003;51:2144–2155. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- Suarez G, Rajaram R, Oronsky A, Gawinowicz M. Nonenzymatic glycation of bovine serum albumin by fructose (fructation). Comparison with the Maillard reaction initiated by glucose. J Biol Chem. 1989;264:3674–3679. [PubMed] [Google Scholar]
- Vanessa FT, Prioletta A, Zuo P, Folli F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr Pharm Des. 2013;19:5695–5703. doi: 10.2174/1381612811319320005. [DOI] [PubMed] [Google Scholar]
- Vauzour D, Rodriguez-Mateos A, Corona G, Oruna-Concha MJ, Spencer JP. Polyphenols and human health: prevention of disease and mechanisms of action. Nutrients. 2010;2(11):1106–1131. doi: 10.3390/nu2111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma AR, Vijayakumar M, Mathela CS, Rao CV. In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem Toxicol. 2009;47:2196–2201. doi: 10.1016/j.fct.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Vinson JA, Howard TB. Inhibition of protein glycation and advanced glycation end products by ascorbic acid and other vitamins and nutrients. J Nutr Biochem. 1996;7:659–663. doi: 10.1016/S0955-2863(96)00128-3. [DOI] [Google Scholar]
- Wu CH, Yen GC. Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation endproducts. J Agric Food Chem. 2005;53:3167–3173. doi: 10.1021/jf048550u. [DOI] [PubMed] [Google Scholar]


