Abstract
Background
The activation of polymorphonuclear neutrophils (PMNs) plays an important role in sepsis. Previously, we showed that ATP release and feedback via ATP receptors are essential for PMN activation; however, the dynamics remain poorly understood. Two new fluorescent chemosensors, PMAP-1 and MitoAP-1, were developed to detect ATP in the plasma membrane and mitochondria of living cells, respectively. In this study, we aimed to evaluate ATP localization using these chemosensors in PMNs of sepsis patients.
Methods
Live PMNs isolated from 16 sepsis patients and healthy controls (HCs) were stained with these chemosensors and observed by confocal microscopy, and their mean fluorescence intensities (MFIs) were evaluated using flow cytometry. CD11b expression in PMNs was also evaluated.
Results
The MFIs of PMAP-1 and MitoAP-1 and CD11b expression in PMNs from sepsis patients on days 0–1 were significantly higher than those of HCs. The MFI of PMAP-1 and CD11b expression on days 3–4 decreased significantly compared to those observed at days 0–1, whereas MitoAP-1 MFI was maintained at a high level. The PMAP-1 MFI was significantly positively correlated with CD11b expression, white blood cell counts, neutrophil counts, and C-reactive protein levels in patients.
Conclusions
The higher MFIs of PMAP-1 and MitoAP-1 in sepsis patients suggest a pivotal role of ATP for PMN activation. The temporal difference in ATP levels suggests that ATP plays different roles in the mitochondria and on the cell surface. These data should contribute to the understanding of the dynamics of ATP in PMNs and help to develop a novel therapy for sepsis.
Keywords: Polymorphonuclear neutrophils (PMNs), PMAP-1, MitoAP-1, Plasma membrane, Mitochondria, Flow cytometry
Background
ATP is well known as the energy currency of cells and is involved in both intracellular signaling and extracellular signaling. Although polymorphonuclear neutrophils (PMNs) are one of the most important phagocytic cell types in defending against invading pathogens and play a pivotal role in inflammation, their activation can be a double-edged sword. In particular, their pathophysiological processes can damage the host tissue when activated during sepsis, trauma, ischemia–reperfusion injury, and inflammatory diseases [1]. PMNs can migrate to compromised tissues in response to chemotactic gradients. The activation of PMNs is triggered by the recognition of bacterial peptides and innate inflammatory mediators. Although a signal amplification system is crucial for the activation of PMNs, the underlying mechanisms remain obscure [2–4]. We previously found that ATP was released extracellularly by PMNs through pannexin-1 (PANX1) hemichannels and that autocrine feedback through the P2Y2 receptor, one of eight P2Y G protein-coupled receptors, is an essential mechanism for activating PMNs [5, 6]. We also reported that plasma ATP levels were correlated with PMN activation and were significantly higher in mouse models of cecal ligation and puncture (CLP) [7].
In recent decades, much effort has been dedicated to developing fluorescent chemosensors for ATP to study its diverse functions [8, 9]. Consequently, two new fluorescent chemosensors were developed (PMAP-1 and MitoAP-1), which can detect ATP and its derivatives in specific regions of living cells [10]. PMAP-1 and MitoAP-1 are also referred to as 2-2Zn(ΙΙ) and 3-2Zn(ΙΙ), respectively [10]. PMAP-1 can localize to the plasma membrane and detect the extracellular release of ATP. In contrast, MitoAP-1 can spontaneously localize in the mitochondria and detect local ATP.
Sepsis is the major cause of death in critically ill patients and is the predominant cause of multiple organ dysfunction syndrome [11, 12]. In spite of progress in intensive care medicine and strict adherence to established treatment protocols such as the “Surviving Sepsis Campaign” guidelines [13], mortality in sepsis patients remains high. Millions of patients worldwide are afflicted each year, resulting in the death of one in three patients [11]. Multiple organ failure in sepsis is the prevailing cause of death, and mitochondrial dysfunction has been suggested to contribute to the development of organ dysfunction and failure in sepsis [14]. However, the associated pathogenic mechanisms remain unclear.
In this study, we evaluated a new approach to quantify ATP release on the cell surface and ATP production in the mitochondria of PMNs by flow cytometry. As described above, ATP is known to play a key role in the activation of PMNs; thus, we hypothesized that ATP levels on the plasma membrane and in the mitochondria of PMNs could serve as an informative biomarker for sepsis. Here, we report the correlation between ATP levels and the clinical conditions of sepsis patients.
Methods
Patients
After obtaining approval from the ethics committee of Juntendo University, Urayasu Hospital (25–47), 16 sepsis patients admitted to our critical care center between October 2013 and April 2015 were enrolled in the study. All patients met the diagnostic criteria for sepsis determined by the American College of Chest Physicians and Society of Critical Care Medicine [15], and all patients (or their legal representatives) provided informed consent for participation in the research. Patients with malignant disease or chronic administration of steroids were excluded. The patients comprised nine males and seven females, with a mean ± SD age of 67 ± 9 years, a serum C-reactive protein (CRP) level of 27.0 ± 12.1 mg/dL, a white blood cell (WBC) count of 13.3 ± 9.1 × 103/μL, an Acute Physiology and Chronic Health Evaluation (APACHE) II score [16] of 22.1 ± 7.8, a Sequential Organ Failure Assessment (SOFA) score [17] of 7.9 ± 4.6, and a Japanese Association for Acute Medicine (JAAM) disseminated intravascular coagulation (DIC) score [18] of 3.8 ± 1.7. The cause of sepsis was urinary tract infection in five patients; burn in three patients; pneumonia in three patients; and necrotic cholecystitis, cellulitis, gas gangrene, cervical abscess, and peritonitis in one patient each. Two patients died during hospitalization (Table 1). The healthy control (HC) group consisted of six males and three females, with a mean ± SD age of 37 ± 5 years.
Table 1.
Characteristics of sepsis patients
| Patients | Cause of sepsis | APACHE ΙΙ score | SOFA score | JAAM DIC score | WBC (/μL) | CRP (mg/dL) | Mortality |
|---|---|---|---|---|---|---|---|
| 1. | Necrotic cholecystitis | 31 | 15 | 7 | 4600 | 27.9 | Alive |
| 2. | Pneumonia | 18 | 3 | 4 | 18,600 | 48.8 | Alive |
| 3. | Burn | 12 | 2 | 2 | 10,600 | 23.9 | Alive |
| 4. | Pneumonia | 24 | 7 | 3 | 17,900 | 35.8 | Dead |
| 5. | Cellulitis | 11 | 3 | 3 | 19,300 | 54.0 | Alive |
| 6. | Burn | 29 | 12 | 4 | 4100 | 14.2 | Dead |
| 7. | UTI | 36 | 14 | 8 | 13,900 | 17.9 | Alive |
| 8. | UTI | 26 | 11 | 3 | 16,400 | 15.5 | Alive |
| 9. | UTI | 14 | 6 | 3 | 20,700 | 10.4 | Alive |
| 10. | Burn | 29 | 13 | 4 | 4600 | 25.5 | Alive |
| 11. | UTI | 20 | 8 | 6 | 31,900 | 25.8 | Alive |
| 12. | Gas gangrene | 17 | 2 | 2 | 17,100 | 15.6 | Alive |
| 13. | Cervical abscess | 20 | 5 | 3 | 5000 | 29.4 | Alive |
| 14. | UTI | 11 | 4 | 3 | 800 | 29.7 | Alive |
| 15. | Peritonitis | 29 | 9 | 3 | 23,700 | 21.8 | Alive |
| 16. | Pneumonia | 27 | 13 | 3 | 900 | 36.0 | Alive |
APACHE Acute Physiology and Chronic Health Evaluation, SOFA Sequential Organ Failure Assessment, JAAM Japanese Association for Acute Medicine, DIC disseminated intravascular coagulation, WBC white blood cell counts, CRP C-reactive protein, UTI urinary tract infection
Blood samples
A 10-mL heparinized blood sample was collected via the peripheral vein of each healthy volunteer and via arterial lines in sepsis patients. Blood collection for all sepsis patients was performed within 24 h after the patients were diagnosed with sepsis (days 0–1) and at days 3–4 of sepsis.
Isolation of PMNs
Human PMNs were isolated from the heparinized whole blood of healthy volunteers and sepsis patients using Polymorphprep™ centrifugation (Alere Technologies AS, Oslo, Norway). The neutrophil population was >95 % pure, as determined by Wright–Giemsa staining.
PMAP-1 and MitoAP-1
Both PMAP-1 and MitoAP-1 were designed on the basis of a fluorescent ATP probe containing a xanthene fluorophore [10]. PMAP-1 selectively localizes on the plasma membrane via a biocompatible anchor for the membrane, which possesses a hydrophobic oleyl moiety at the end of a long ethylene glycol linker [10]. MitoAP-1 selectively localizes to the mitochondria via a positively charged pyronin ring [10].
Imaging of PMNs with confocal microscopy
Isolated human PMNs (2.0 × 106 cells/mL) were plated on a 35-mm glass-bottomed dish (IWAKI, Tokyo, Japan), coated with 100 μL of poly-l-lysine solution (Sigma-Aldrich, St. Louis, MO, USA), and incubated for 10 min. All incubations were performed under a humidified atmosphere of 5 % CO2 at 37 °C in the dark. Cells were treated with 400 nM (final concentration) of MitoTracker® Green FM (Invitrogen, Carlsbad, CA, USA), followed by incubation with 1 μM MitoAP-1 (final concentration) for 20 min. Cells were incubated with 5 μM PMAP-1 (final concentration) for 10 min. After washing twice with 37 °C Hanks’ balanced salt solution (HBSS, Sigma-Aldrich), stained cells were examined with a TCS SP5 Leica confocal laser-scanning microscope equipped with a Plan-Apochromat 100× oil-immersion objective lens at 37 °C, under a 5 % CO2 humidified atmosphere.
Fluorescence quantification by flow cytometry
Isolated PMNs were washed with HBSS and incubated at 37 °C for 10 min with or without N-formyl-met-leu-phe (fMLP, ScyTek Laboratories, Logan, UT, USA) stimulation before staining. Cells were incubated with 1 μM (final concentration) of MitoAP-1 and allophycocyanin-conjugated anti-CD11b antibodies (BD Biosciences, San Jose, CA, USA) at 37 °C for 20 min, followed by incubation with 5 μM PMAP-1 (final concentration) at 37 °C for 10 min. The cells were placed on ice for 10 min and centrifuged at 1500 rpm for 5 min at 4 °C. After washing twice with HBSS, cells were fixed with fluorescence-activated cell sorting (FACS) sheath fluid (BD Biosciences) and then analyzed by flow cytometry (FACSCalibur, BD Bioscience).
Statistical analysis
The data are expressed as the mean ± SEM unless stated otherwise. Statistical analyses were performed using GraphPad Prism version 6.0 (GraphPad, San Diego, CA, USA). The data were analyzed for significant differences by a paired t test or Wilcoxon signed-rank test for comparisons of paired groups. One-way analysis of variance (ANOVA) and the Kruskal–Wallis test were used for comparisons of three groups. Spearman’s rank correlation was performed as indicated. Differences were considered statistically significant at p < 0.05.
Results
Successful establishment of a staining protocol for human PMNs with MitoAP-1
Imaging studies using PMAP-1 and MitoAP-1 were limited to cultured cells [10] and T cells [19] until Bao et al. [20] first reported imaging studies of human PMNs using PMAP-1. As in their study, the bright green fluorescence emitted by PMAP-1 was predominantly observed on the plasma membrane of PMNs (Fig. 1a). However, there has been no previous report of the successful staining of human PMNs with MitoAP-1. We observed a punctate pattern of bright red fluorescence emitted by MitoAP-1 within the cells. MitoAP-1 fluorescence overlapped well with that of MitoTracker® Green FM, a typical dye used to stain the mitochondria, indicating that MitoAP-1 selectively and spontaneously localized to the mitochondria (Fig. 1b). Thus, we successfully established a method for staining human PMNs with MitoAP-1.
Fig. 1.
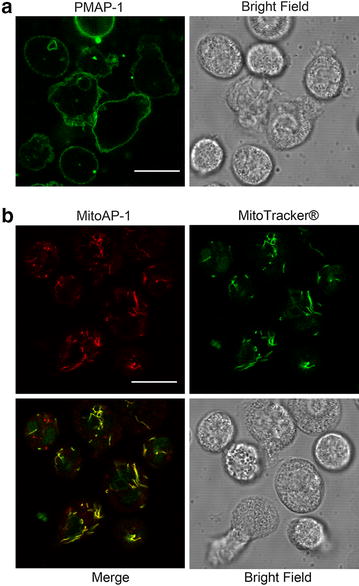
Confocal micrographs of human polymorphonuclear neutrophils (PMNs) stained with PMAP-1 and MitoAP-1. a ATP on the plasma membrane was stained with PMAP-1. The bright green fluorescence observed on the plasma membrane of PMNs resulted from PMAP-1 conjugation to ATP (×100 oil objective, NA 1.4). Scale bar 10 μm. b ATP in the mitochondria was stained with MitoAP-1 (red) and MitoTracker® Green FM (green), and they colocalized well (×100 oil objective, NA 1.4). Scale bar 10 μm
ATP release on the plasma membrane of HC PMNs increased with fMLP stimulation
Stimulation of the formyl peptide receptor was previously found to cause ATP release from PMNs through maxi-anion channels and PANX1 hemichannels [6]. To confirm this finding, the MFI of PMAP-1 on the plasma membrane of HC PMNs was quantified by flow cytometry after stimulation with the indicated concentrations of fMLP (Fig. 2a). The MFI of PMAP-1 following fMLP stimulation increased in a dose-dependent manner. CD11b expression on the plasma membrane of PMNs was also measured as a marker of PMN activation. Cell surface CD11b expression in PMNs stimulated with fMLP also increased in a dose-dependent manner (Fig. 2b).
Fig. 2.
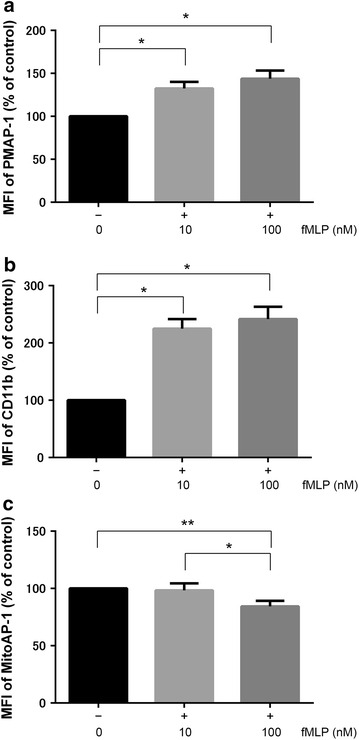
ATP level and CD11b expression after fMLP stimulation in healthy control subjects. a Changes in the mean fluorescence intensity (MFI) of PMAP-1 after stimulation with the indicated fMLP concentrations; MFI values were normalized to those of controls (no fMLP) (n = 8 per group). The data shown are the mean ± SEM, and groups were compared with one-way ANOVA and Tukey’s post hoc test (*p < 0.01). b Changes in CD11b expression of polymorphonuclear neutrophils after fMLP stimulation; expression levels were normalized to those of controls (no fMLP) (n = 8 per group). The data shown are the mean ± SEM, and groups were compared with one-way ANOVA with Tukey’s post hoc test (*p < 0.01). c Changes in MFI of MitoAP-1 after stimulation with the indicated fMLP concentrations; MFI values were normalized to those of controls (no fMLP) (n = 8 per group). The data shown are the mean ± SEM, and groups were compared with one-way ANOVA with Tukey’s post hoc test (*p < 0.01, **p < 0.05)
Mitochondrial ATP levels in the HC group decreased with fMLP stimulation
Mitochondria are often referred to as the powerhouse of the cells, as they generate ATP by oxidative phosphorylation. To our knowledge, changes in mitochondrial ATP levels in PMNs have not been reported. The MFI of MitoAP-1 following fMLP stimulation decreased significantly in a dose-dependent manner (Fig. 2c).
ATP release on the plasma membrane and mitochondrial ATP levels in PMNs were higher in sepsis patients than in HC subjects
The MFIs of PMAP-1 and MitoAP-1 in sepsis patients were evaluated by flow cytometry within 24 h after diagnosis of sepsis (days 0–1). Both values were significantly higher than those of the HC group (Fig. 3a, b). CD11b expression on the plasma membrane was also upregulated in sepsis patients (Fig. 3c). Activated PMNs in sepsis patients appeared to cause a burst of extracellular ATP release and to increase ATP synthesis by oxidative phosphorylation in the mitochondria. The same experiments were conducted at days 3–4 as the patients’ clinical conditions improved, which revealed that the MFIs of PMAP-1 and CD11b expression decreased significantly compared to those at days 0–1 (Fig. 3a, c), whereas a high MitoAP-1 MFI was maintained (Fig. 3b). Namely, the temporal changes of ATP release and CD11b expression on the plasma membrane were similar, but ATP production showed distinct behavior in the mitochondria.
Fig. 3.
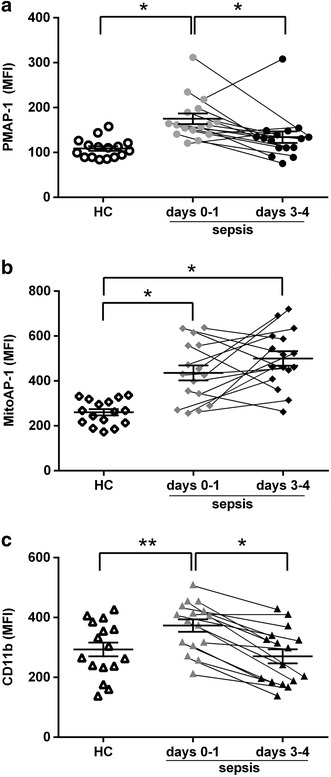
Mean fluorescent intensity (MFI) of PMAP-1, MitoAP-1, and CD11b expression in PMNs in sepsis patients and healthy control (HC) subjects. a MFI of PMAP-1 in sepsis patients (days 0–1 postdiagnosis) was significantly higher than that of HCs and decreased significantly on days 3–4 (mean ± SEM, Kruskal–Wallis test with Dunn’s post hoc test, *p < 0.01). b The MFI of MitoAP-1 in sepsis patients (days 0–1) was significantly higher than that of HCs and did not decrease significantly on days 3–4 (mean ± SEM, Kruskal–Wallis test with Dunn’s post hoc test, *p < 0.01). c CD11b expression in sepsis patients (days 0–1) was significantly higher than that of HCs and decreased significantly on days 3–4 (mean ± SEM, one-way ANOVA with Tukey’s post hoc test, *p < 0.01, **p < 0.05)
ATP release on the plasma membrane correlated with PMN activation
As described above, ATP release and CD11b expression decreased as the clinical conditions of the patients improved. Furthermore, the MFIs of PMAP-1 and CD11b expression showed a significant positive correlation in the sepsis patients (Fig. 4).
Fig. 4.
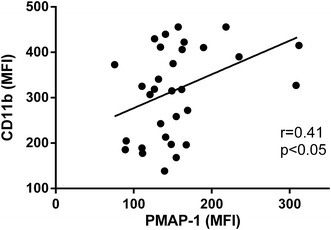
Correlation between the mean fluorescent intensity (MFI) of PMAP-1 and polymorphonuclear neutrophil (PMN) activation (CD11b expression). Comparison of PMAP-1 and CD11b expression on the plasma membrane showed a significant positive correlation in sepsis patients (n = 16, Spearman’s rank correlation coefficient, p < 0.05). The 32 data points shown consist of samples from both days 0–1 and days 3–4 following sepsis diagnosis
ATP release on the plasma membrane correlated with WBC counts, neutrophil counts, and CRP levels
To determine relationships between ATP release on the plasma membrane or ATP production in the mitochondria and clinical conditions (WBC counts, neutrophil counts, CRP levels, SOFA scores, APACHE II scores, and JAAM DIC scores), their correlation coefficients were investigated. The MFI of PMAP-1 was significantly positively correlated with WBC counts, neutrophil counts, and CRP levels (Fig. 5), but not with SOFA scores, APACHE II scores, and JAAM DIC scores. No correlation was found between the MFI of MitoAP-1 of PMNs and any clinical variable.
Fig. 5.
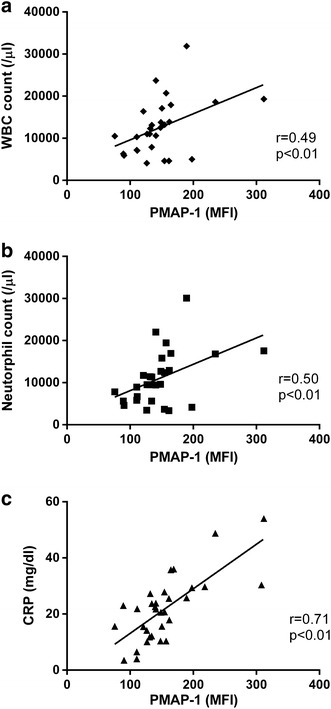
Correlation of PMAP-1 MFI with white blood cell (WBC) counts, neutrophil counts, and C-reactive protein (CRP) levels in sepsis patients. a, b Spearman’s rank correlation coefficient, p < 0.01 (n = 14). Two patients with leukopenia were excluded. The 28 data points shown consist of samples from both days 0–1 and days 3–4 following sepsis diagnosis. c Spearman’s rank correlation coefficient, p < 0.01 (n = 16). The 32 data points shown consist of samples from both days 0–1 and days 3–4 of sepsis
Discussion
To our knowledge, this is the first report of the visualization of mitochondrial ATP in the PMNs of sepsis patients with fluorescent chemosensors. PMNs are the major leukocytes recruited to inflamed sites in response to infection. Although PMNs play a central role in protecting the host from infection, their pathophysiological processes can also cause tissue damage in the host [1, 21]. To develop therapies that can reduce PMN-induced tissue damage, it is crucial to elucidate the mechanisms underlying the activation of PMNs.
ATP, which is well known as the “molecular unit of currency” of intracellular energy transfer, is released extracellularly by physiological stimulation and acts as a signaling molecule. Burnstock et al. [22] first reported that healthy neurons can release cellular ATP, which then serves as an intercellular messenger. Recently, neurons and many other types of cells have been shown to release ATP under physiological stimulation, including PMNs [23–33]. Previously, we showed that ATP release and purinergic signaling are essential for PMN activation. Extracellular ATP is released by PANX1 hemichannels and controls the chemotaxis of PMNs through P2Y2 receptors [5, 6]. The autocrine feedback through P2Y2 receptors is essential for PMN activation [6].
Substantial effort has been dedicated to developing fluorescent chemosensors for ATP to elucidate its diverse physiological functions [8–10, 34, 35]. To date, a few imaging studies using genetically encoded fluorescence ATP probes have been reported for this purpose [34, 35]. These probes are posttranslationally conjugated to a protein anchored to a certain organelle through genetically encoded localization signals. Although successful, these techniques are not convenient, because they require cumbersome and time-consuming processes for protein expression and maturation. Therefore, Kurishita et al. [10] developed two new types of fluorescent chemosensors, PMAP-1 and MitoAP-1, which can spontaneously and selectively localize in a specific cellular region [10]. For example, PMAP-1 localizes to the plasma membrane and can be used to detect the extracellular release of ATP, whereas MitoAP-1 localizes to the mitochondria and senses mitochondrial ATP. Thus, these fluorescent chemosensors are highly suited for investigating the dynamics of ATP in biological events occurring in target cellular regions [10].
The finding that cell surface ATP release and CD11b expression in the HC group increased dose-dependently following fMLP stimulation is consistent with our previous finding that ATP is released by fMLP stimulation via PANX1 hemichannels [6]. In sepsis patients, extracellular ATP release in PMNs was elevated on days 0–1, corroborating our previous work in mouse sepsis models, wherein plasma ATP levels were elevated and contributed to PMN activation [7]. Collectively, these findings indicate that PMNs may be a main source of elevated circulating ATP levels. Furthermore, ATP release on the plasma membrane was correlated with CD11b expression, WBC counts, neutrophil counts, and CRP levels in sepsis patients; thus, ATP release on the plasma membrane may reflect PMN activation and could potentially serve as an activation marker for sepsis. Given that excessive activation of PMNs can damage host organs, inhibition of purinergic signaling could serve as a potential therapeutic strategy for treating sepsis. Previously, we reported that the P2 receptor antagonist suramin reduced PMN activation in a mouse CLP model [7]. Thus, suramin may potentially be used as an effective therapeutic strategy for sepsis patients. In contrast to the complications mediated by an overactive neutrophil compartment, severe systemic inflammation is a risk factor for the development of immune suppression. The circulating neutrophil pool displays a paradoxical phenotype, exhibiting characteristics of both activated and suppressed functionality. Therefore, further investigations are required to resolve this paradox of the role of PMNs in sepsis [36].
Mitochondria play an essential role in the activation of PMNs by producing ATP, which triggers the purinergic signaling processes [20]. During this process, mitochondria generate ATP and release ATP extracellularly following fMLP stimulation. However, in the HC group, the mitochondrial ATP levels in PMNs decreased with fMLP stimulation in a dose-dependent manner, demonstrating that the mitochondrial ATP was consumed in this process in healthy PMNs. Mitochondrial ATP levels in PMNs from sepsis patients were significantly higher than those of the HC group. Although further investigations are required to determine the definite cause for this difference, it is intriguing to speculate that pathways other than fMLP stimulation may be essential for ATP elevation in the mitochondria. Evaluation of mitochondrial function in various cells has been previously reported in the context of sepsis [37, 38]. For example, Adrie et al. [37] demonstrated reduced mitochondrial membrane potential in peripheral blood monocytes from patients with severe sepsis. On the other hand, Belikova et al. [38] showed peripheral blood mononuclear cells from patients with severe sepsis had a significantly higher baseline value of oxygen consumption compared with healthy volunteer cells. This report supports our study that mitochondrial ATP production in PMNs from sepsis patients was increased. Therefore, understanding the interaction among cells is essential to elucidate the mechanism of sepsis. Our method shows potential to clarify the mechanism of sepsis from the point of view of mitochondrial function.
Furthermore, although the PMAP-1 MFI on days 3–4 of sepsis decreased in parallel with CD11b downregulation, the MFI of MitoAP-1 did not change. These findings suggest that ATP serves different functions on the cell surface and in the mitochondria of PMNs in sepsis patients.
Conclusions
In conclusion, we developed a novel approach for quantifying ATP release on the cell surface and mitochondrial ATP production in PMNs with two new types of fluorescent chemosensors, which was applied to the study of sepsis patients. ATP release on the plasma membrane of PMNs was demonstrated to be a potentially valuable biomarker for sepsis. Taken together with our previous studies, our current findings suggest that PMN responses can be regulated by therapeutic strategies that target the purinergic signaling mechanisms of PMNs. Additional studies are needed to determine whether and how these purinergic signaling mechanisms can be targeted in order to best benefit sepsis patients.
Authors’ contributions
KS and YS designed and performed the experiments. KS and YS analyzed the data and prepared the manuscript. YK performed the experiments with FACS. KI supervised and carried out the experiments with confocal microscopy. HN and KI supervised the isolation of PMNs. YK, HS, and IH synthesized PMAP-1 and MitoAP-1 and supervised the usage of PMAP-1 and MitoAP-1. YI and HT conceived the idea, reviewed the manuscript, and supervised the whole project. All authors read and approved the final manuscript.
Acknowledgements
This study was supported in part by the Japan Society for the Promotion of Science (23792086, 25462830, to Y.S.), a Grant-in-Aid (S1311011) from the Foundation of Strategic Research Projects in Private Universities from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan (to K.S.), and Juntendo University Young Investigator Award 2013 (K1310, to K.S).
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- ANOVA
analysis of variance
- APACHE
Acute Physiology and Chronic Health Evaluation
- CLP
cecal ligation and puncture
- CRP
C-reactive protein
- DIC
disseminated intravascular coagulation
- FACS
fluorescence-activated cell sorting
- fMLP
N-formyl-met-leu-phe
- HBSS
Hanks’ balanced salt solution
- HC
healthy controls
- JAAM
Japanese Association for Acute Medicine
- MFI
mean fluorescence intensity
- PMN
polymorphonuclear neutrophil
- SOFA
Sequential Organ Failure Assessment
- WBC
white blood cell
Contributor Information
Koichiro Sueyoshi, Phone: +81-47-353-3111, Email: ksueyoshi@juntendo-urayasu.jp.
Yuka Sumi, Email: ysumi@juntendo-urayasu.jp.
Yoshiaki Inoue, Email: yinoue@juntendo-urayasu.jp.
Yoko Kuroda, Email: y.kuroda@m.jcnnet.jp.
Kumiko Ishii, Email: ishiikum@riken.jp.
Hitoshi Nakayama, Email: nhitoshi@juntendo.ac.jp.
Kazuhisa Iwabuchi, Email: iwabuchi@juntendo.ac.jp.
Yasutaka Kurishita, Email: kurishita.y@gmail.com.
Hajime Shigemitsu, Email: hshigemitsu@sbchem.kyoto-u.ac.jp.
Itaru Hamachi, Email: ihamachi@sbchem.kyoto-u.ac.jp.
Hiroshi Tanaka, Email: htanaka@juntendo-urayasu.jp.
References
- 1.Nussler AK, Wittel UA, Nussler NC, Beger HG. Leukocytes, the Janus cells in inflammatory disease. Langenbecks Arch Surg. 1999;384:222–232. doi: 10.1007/s004230050196. [DOI] [PubMed] [Google Scholar]
- 2.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 3.Van Haastert PJ, Devreotes PN. Chemotaxis: signalling the way forward. Nat Rev Mol Cell Biol. 2004;5:626–634. doi: 10.1038/nrm1435. [DOI] [PubMed] [Google Scholar]
- 4.Zigmond SH. Ability of polymorphonuclear leukocytes to orient in gradients of chemotactic factors. J Cell Biol. 1977;75:606–616. doi: 10.1083/jcb.75.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Yao Y, Sumi Y, Li A, To UK, Elkhal A, et al. Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci Signal. 2010;3:ra45. doi: 10.1126/scisignal.2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sumi Y, Woehrle T, Chen Y, Bao Y, Li X, Yao Y, et al. Plasma ATP is required for neutrophil activation in a mouse sepsis model. Shock. 2014;42:142–147. doi: 10.1097/SHK.0000000000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ojida A, Takashima I, Kohira T, Nonaka H, Hamachi I. Turn-on fluorescence sensing of nucleoside polyphosphates using a xanthene-based Zn(II) complex chemosensor. J Am Chem Soc. 2008;130:12095–12101. doi: 10.1021/ja803262w. [DOI] [PubMed] [Google Scholar]
- 9.Kurishita Y, Kohira T, Ojida A, Hamachi I. Rational design of FRET-based ratiometric chemosensors for in vitro and in cell fluorescence analyses of nucleoside polyphosphates. J Am Chem Soc. 2010;132:13290–13299. doi: 10.1021/ja103615z. [DOI] [PubMed] [Google Scholar]
- 10.Kurishita Y, Kohira T, Ojida A, Hamachi I. Organelle-localizable fluorescent chemosensors for site-specific multicolor imaging of nucleoside polyphosphate dynamics in living cells. J Am Chem Soc. 2012;134:18779–18789. doi: 10.1021/ja308754g. [DOI] [PubMed] [Google Scholar]
- 11.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 12.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 13.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 14.Jeger V, Djafarzadeh S, Jakob SM, Takala J. Mitochondrial function in sepsis. Eur J Clin Invest. 2013;43:532–542. doi: 10.1111/eci.12069. [DOI] [PubMed] [Google Scholar]
- 15.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 16.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 18.Gando S, Iba T, Eguchi Y, Ohtomo Y, Okamoto K, Koseki K, et al. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit Care Med. 2006;34:625–631. doi: 10.1097/01.CCM.0000202209.42491.38. [DOI] [PubMed] [Google Scholar]
- 19.Ledderose C, Bao Y, Lidicky M, Zipperle J, Li L, Strasser K, et al. Mitochondria are gate-keepers of T cell function by producing the ATP that drives purinergic signaling. J Biol Chem. 2014;289:25936–25945. doi: 10.1074/jbc.M114.575308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bao Y, Ledderose C, Seier T, Graf AF, Brix B, Chong E, et al. Mitochondria regulate neutrophil activation by generating ATP for autocrine purinergic signaling. J Biol Chem. 2014;289:26794–26803. doi: 10.1074/jbc.M114.572495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 22.Burnstock G. Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci. 2006;27:166–176. doi: 10.1016/j.tips.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol. 2001;38:900–908. doi: 10.1097/00005344-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Buxton IL, Kaiser RA, Oxhorn BC, Cheek DJ. Evidence supporting the Nucleotide Axis Hypothesis: ATP release and metabolism by coronary endothelium. Am J Physiol Heart Circ Physiol. 2001;281:H1657–H1666. doi: 10.1152/ajpheart.2001.281.4.H1657. [DOI] [PubMed] [Google Scholar]
- 25.Eltzschig HK, Eckle T, Mager A, Kuper N, Karcher C, Weissmuller T, et al. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- 26.Fabre AC, Vantourout P, Champagne E, Terce F, Rolland C, Perret B, et al. Cell surface adenylate kinase activity regulates the F(1)-ATPase/P2Y (13)-mediated HDL endocytosis pathway on human hepatocytes. Cell Mol Life Sci. 2006;63:2829–2837. doi: 10.1007/s00018-006-6325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerasimovskaya EV, Ahmad S, White CW, Jones PL, Carpenter TC, Stenmark KR. Extracellular ATP is an autocrine/paracrine regulator of hypoxia-induced adventitial fibroblast growth. Signaling through extracellular signal-regulated kinase-1/2 and the Egr-1 transcription factor. J Biol Chem. 2002;277:44638–44650. doi: 10.1074/jbc.M203012200. [DOI] [PubMed] [Google Scholar]
- 28.Homolya L, Steinberg TH, Boucher RC. Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarized epithelia. J Cell Biol. 2000;150:1349–1360. doi: 10.1083/jcb.150.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roman RM, Fitz JG. Emerging roles of purinergic signaling in gastrointestinal epithelial secretion and hepatobiliary function. Gastroenterology. 1999;116:964–979. doi: 10.1016/S0016-5085(99)70081-8. [DOI] [PubMed] [Google Scholar]
- 30.Schwiebert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta. 2003;1615:7–32. doi: 10.1016/S0005-2736(03)00210-4. [DOI] [PubMed] [Google Scholar]
- 31.Sorensen CE, Novak I. Visualization of ATP release in pancreatic acini in response to cholinergic stimulus. Use of fluorescent probes and confocal microscopy. J Biol Chem. 2001;276:32925–32932. doi: 10.1074/jbc.M103313200. [DOI] [PubMed] [Google Scholar]
- 32.Sprague RS, Stephenson AH, Ellsworth ML. Red not dead: signaling in and from erythrocytes. Trends Endocrinol Metab. 2007;18:350–355. doi: 10.1016/j.tem.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Yegutkin G, Bodin P, Burnstock G. Effect of shear stress on the release of soluble ecto-enzymes ATPase and 5′-nucleotidase along with endogenous ATP from vascular endothelial cells. Br J Pharmacol. 2000;129:921–926. doi: 10.1038/sj.bjp.0703136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berg J, Hung YP, Yellen G. A genetically encoded fluorescent reporter of ATP:ADP ratio. Nat Methods. 2009;6:161–166. doi: 10.1038/nmeth.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imamura H, Nhat KP, Togawa H, Saito K, Iino R, Kato-Yamada Y, et al. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc Natl Acad Sci USA. 2009;106:15651–15656. doi: 10.1073/pnas.0904764106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pillay J, Ramakers BP, Kamp VM, Loi AL, Lam SW, Hietbrink F, et al. Functional heterogeneity and differential priming of circulating neutrophils in human experimental endotoxemia. J Leukoc Biol. 2010;88:211–220. doi: 10.1189/jlb.1209793. [DOI] [PubMed] [Google Scholar]
- 37.Adrie C, Bachelet M, Vayssier-Taussat M, Russo-Marie F, Bouchaert I, Adib-Conquy M, et al. Mitochondrial membrane potential and apoptosis peripheral blood monocytes in severe human sepsis. Am J Respir Crit Care Med. 2001;164:389–395. doi: 10.1164/ajrccm.164.3.2009088. [DOI] [PubMed] [Google Scholar]
- 38.Belikova I, Lukaszewicz AC, Faivre V, Damoisel C, Singer M, Payen D. Oxygen consumption of human peripheral blood mononuclear cells in severe human sepsis. Crit Care Med. 2007;35:2702–2708. doi: 10.1097/01.CCM.0000295593.25106.C4. [DOI] [PubMed] [Google Scholar]


