Abstract
Integrins are an important family of adhesion molecules that were first discovered two decades ago. Integrins are transmembrane heterodimeric glycoprotein receptors consisting of α and β subunits, and are comprised of an extracellular domain, a transmembrane domain, and a cytoplasmic tail. Therein, integrin cytoplasmic domains may associate directly with numerous cytoskeletal proteins and intracellular signaling molecules, which are crucial for modulating fundamental cell processes and functions including cell adhesion, proliferation, migration, and survival. The purpose of this review is to describe the unique structure of each integrin subunit, primary cytoplasmic association proteins, and transduction signaling pathway of integrins, with an emphasis on their biological functions.
Keywords: Integrin, Structure, Associated proteins, Signal transduction pathway, Biological function, Adhesion
Background
Integrins are heterodimers consisting of two subunits. Hynes discovered there were 18 α and 8 β subunits forming 24 αβ heterodimers by noncovalent bonds (Hynes 2002). An electron microscope result showed that integrins have a globular head and two leg regions (one from α subunits and the other from β subunits) inserted into the plasma membrane, indicating each integrin subunit has an extracellular domain, a transmembrane domain, and a cytoplasmic tail (Srichai and Zent 2010). The α subunits mainly decide the type of ligands, and both α and β subunits are involved in cell signal transduction which are assisted by the contribution of adhesion molecules. The characteristics of integrin function and molecular diversity were initially clarified in 2000 (Zamir et al. 2000).
Based on the unique structure of integrins, including the α- and β-subunits, integrins can bind with extracellular matrix (ECM) proteins such as collagen (CO), laminin (LN), firbronection (FN), vitronectin (VN), and some other cellular receptors (Plow et al. 2000). The discovery of integrins at molecular level occurred in the late 1970s and 1980s, which was followed by further discoveries of integrin adhesion-related proteins, including structural protein members and signaling molecules (Rohrschneider 1980). Among these, the short intracellular cytoplasmic domains of integrins may associate directly with numerous cytoskeletal proteins and intracellular signaling molecules. These associated proteins provide a basis for modulating fundamental cell processes and various biological outcomes including proliferation, migration, cell differentiation, and apoptosis (Schwartz et al. 1995) by regulating signal transduction pathways. In recent years, many researchers have gradually developed a deep understanding of integrins using techniques like gene knockout, overexpression and specific antibodies. Meanwhile, researchers have also realized the crucial roles of integrins and make a greatly improved understanding for their unique structure, biological function, and integrin-mediated signal transduction mechanism in multiple cellular processes.
This review mainly focuses on the thorough understanding of the different subunit structural characteristics, biological functions, and associated proteins in cells.
Integrin structure and distribution
Integrin α subunits
The structures of different α subunits are very similar. The extracellular domains contain 7 homologous repeat domains with 30–40 amino acids, and the interval between these sequences has 20–30 amino acids. Extracellular domains also contain a ‘metal-ion-dependent adhesive site’ (MIDAS) that can bind divalent metal cations (Mg2+ or Ca2+) and is important in ligand binding. The transmembrane domains of integrins are single-spanning structures with 5 common amino acid sequences, ‘GFFKR’, its specific function is regulating integrin affinity by mediating an alpha–beta subunit cytoplasmic tail interaction. Cytoplasm domains of α subunits are generally short.
At least 18 α subunits have been found, including α1–α11, αD, αE, αL, αM, αV, αX, and αIIb. To date, their molecular structures have been studied using X-rays, nuclear magnetic resonance, electron microscopy, and three-dimensional ultrasonography. The components of extracellular domains include I-domain, β-Propeller, Thigh, Calf-1, and Calf-2 (Fig. 1). Nine different α subunits (α1, α2, α10, α11, αD, αL, αE, αM, αX) contain the I-domain structure, which is crucial for ligand binding sites. Several other α subunits (α3, α4, α5, α6, α7, α8, α9, αV, αIIb) contain no I-domain but constitute the ligand binding sites by β-Propeller. This article describes the characteristics of α subunit structures and tissue distributions in detail as shown in Table 1.
Fig. 1.
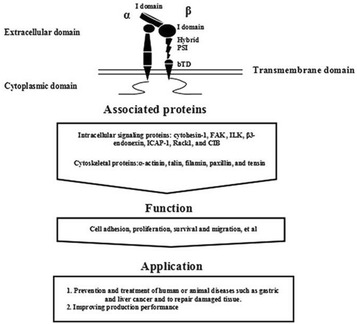
Structure, primary cytoplasmic association proteins and biological functions of integrins
Table 1.
Structural characteristics and tissue distributions of α subunits
| Integrin subunits | Molecular weight (kDa) | Heterodimeric type | Structural characteristics | Tissue distributions | References |
|---|---|---|---|---|---|
| α1 | 210 | α1β1 | Has I-domain structure and heavily N-glycosylated when compared with other α chains | Embryo, liver, muscle, several inflammation tissues and epithelial cells | Isacke and Horton (2000) |
| α2 | 165 | α2β1 | Has I-domain structure and three cation binding sites | Epiderm in proliferation basal layer | Isacke and Horton (2000), Teige et al. (2010) |
| α3 | 130, 25 | α3β1 | No I-domain structure although has seven homologous repeated domains and most membrane proximal domains of α3, have divalent cation binding sites | Histological abnormalities of kidney, lungs, small skin blisters and glomerulus | Isacke and Horton (2000), Romanska et al. (2013) |
| α4 | 150 | α4β1, α4β7 | No I-domain structure, otherwise extracellular portions of α4 have three EF-hand loop-like domains for divalent cations binding | Placenta and heart during embryogenesis, also in ladder smooth muscle cells | Isacke and Horton (2000), Luo et al. (2013) |
| α5 | 135, 25 | α5β1 | No I-domain structure, yet has five potential divalent cation binding sites | Embryo, vascellum, wound healing tissues and epithelial cells | Isacke and Horton (2000), Tartaglia et al. (2013) |
| α6 | 120, 30 | α6β1, α6β4 | Structure is most homologous to integrin α3 | Platelet, basal surface of most epithelial cells, schwann cells, keratinocytes, prostate cancer cells and endothelial cells | Isacke and Horton (2000), Mercurio et al. (2001), Wilschut et al. (2011), Stewart and O’Connor (2015), Berg et al. (2016) |
| α7 | 100, 30 | α7β1 | No I-domain structure, otherwise proteolytically cleaved | Skeletal muscles, smooth muscles, cardiac muscle and nervous system | (Isacke and Horton 2000) |
| α8 | α8β1 | No I-domain structure, yet proteolytically cleaved | Smooth muscles, kidney and epithelial cells | Isacke and Horton (2000), Benoit et al. (2009) | |
| α9 | α9β1 | No I-domain structure, still post-translationally cleaved | Intestinal epithelia, skin, muscles and liver | Isacke and Horton (2000), Hynes (2002) | |
| α10 | 160 | α10β1 | Structure is most homologous to integrin α1 and α2 | Heart and skeletal muscles | Hynes (2002), Isacke and Horton (2000) |
| α11 | α11β1 | The longest integrin α chain with 1166 amino acids and has I-domain structure, however has no GFFKR sequence | Adult uterus, heart and skeletal muscles | Zhang et al. (2002), Velling et al. (1999) | |
| αv | 125, 25 | αvβ1, αvβ3, αvβ5, αvβ6, αvβ8 | No I-domain structure, otherwise proteolytically cleaved | Neural crest cells, muscles, glial cells, epithelia, osteoclasts, and blood vessels during development or angiogenesis | Delannet et al. (1994), Breuss et al. (1995), Drake et al. (1995), Isacke and Horton (2000), Kaneko et al. (2014) |
| αIIb | 125, 22 | αIIbβ3 | No I-domain structure, yet proteolytically cleaved | Human blood platelets and macrophagocyte | Isacke and Horton (2000), Ley et al. (2016) |
| αD | 150 | αDβ2 | Has I-domain structure | Tissue macrophages such as spleen and peripheral blood leucocytes | Isacke and Horton (2000) |
| αL | 180 | αLβ2 | Has I-domain structure and an imperfect MIDAS, with seven repetitive domains in extracellular domains | Leukocyte receptors | Shimaoka et al. (2002), Springer and Sen (2016) |
| αM | 170 | αMβ2 | Has I-domain structure although not proteolytically cleaved, with five exposed loops surrounding MIDAS | Leukocyte receptors | Hee et al. (2007) |
| αX | 150 | αXβ2 | Has I-domain structure yet not proteolytically cleaved, with five exposed loops surrounding MIDAS | Leukocyte receptors | Isacke and Horton (2000) |
| αE | 150, 25 | αLβ7 | Has I-domain structure even proteolytically cleaved | Leukocyte receptors | Isacke and Horton (2000) |
Integrin β subunits
Integrin β subunits have an I-like domain similar to the I-domain in α subunits which is crucial for ligand binding. Other components include a plexin/semaphorin/integrin (PSI) domain, a hybrid domain, four epidermal growth factor (EGF) repeats, and a membrane proximal b tail domain (bTD), shown in Fig. 1. The β subunits also contain a large extracellular domain, a single-spanning transmembrane domain, and a short cytoplasmic tail (except for β4). The cytoplasmic domains lack catalytic activity themselves and are comprised of 60 amino acids (except for β4, which contains 1000 amino acids) (Hogervorst et al. 1990). Its cytoplasmic domains typically have two NP × Y sequences that provide binding sites to many proteins with phosphotyrosine-binding (PTB) domains (Bouaouina et al. 2008) and participate in cellular signal transduction by linking with cytoplasmic signal molecules (Gilcrease 2007). The super-family of integrin β can be divided into β1–β8 and their structural characteristics and tissue distributions are described in Table 2.
Table 2.
Structural characteristics and tissue distributions of β subunits
| Integrin subunits | Molecular weight (kDa) | Heterodimeric type | Structural characteristics | Tissue distributions | References |
|---|---|---|---|---|---|
| β1 | 115 | α1β1, α2β1, α3β1, α4β1, α5β1, α6β1, α7β1, α8β1, α9β1, α10β1, α11β1, αvβ1 | Has 56 residues in four repeat regions and internally disulphide bounded | Widely distributed | Isacke and Horton (2000) |
| β2 | 95 | αDβ2, αLβ2, αMβ2, αXβ2 | Cytoplasmic tail contains eight potential phosphorylation sites | Leucocytes | Isacke and Horton (2000), Takada et al. (2007) |
| β3 | 105 | αvβ3, αIIbβ3 | Its Tyr 773 is potentially phosphorylated | Platelets and macrophages | Coppolino and Dedhar (2000), Isacke and Horton (2000), Mor-Cohen (2016) |
| β4 | 220 | α6β4 | Contains a large cytoplasmic domain approximately 1000 amino acids | Epithelial cells | Mercurio et al. (2001) |
| β5 | 100 | αvβ5 | Neural crest cells, blood vessels and tumors | Memmo and McKeown-Longo (1998), Hu et al. (2014) | |
| β6 | 105 | αvβ6 | Has a small cytoplasmic extension with unique 11 amino acids | Epithelial cells | Bandyopadhyay and Raghavan (2009) |
| β7 | 110 | α4β7, αEβ7 | Has two NPX(Y/F) motifs for potential tyrosine kinase binding | NK cells, B cells, eosinophils, intraepithelial cells, lymphocytes and peripheral cells | Schippers et al. (2012) |
| β8 | 95 | αvβ8 | No interact with cytoskeleton | Kidney, placenta, uterus, ovary and transformed cell lines | Isacke and Horton (2000) |
Integrin-associated proteins
Integrin cytoplasmic domains associate directly with numerous cytoskeletal proteins and intracellular signaling molecules to modulate fundamental cell processes, as is shown in Fig. 1. Both α and β chains can participate in ligand binding specificity, but β chains alone seem to define cytoskeletal interactions.
The ability of integrin cytoplasmic domains may associate directly with several cytoskeletal proteins including α-actinin, talin, filamin, paxillin, and tensin (Reszka et al. 1992; Otey et al. 1993; Lyman et al. 1997; Geiger et al. 2001). Their binding sites and functions to integrins were summarized in Table 3.
Table 3.
Integrin-associated proteins (cytoskeletal proteins)
| Cytoskeletal proteins | Binding sites to integrins | Functions | Roles in diseases | References |
|---|---|---|---|---|
| α-actinin | Central repeat of α-actinin can bind to integrin β1 and activated β2 Membrane-spanning region can bind to integrin β3 Cytoplasmic domain position can bind to integrin αIIb |
Enhancing signaling from matrix adhesion sites and stimulating integrin-mediated cell-to-matrix adhesion | Non-muscle α-actinins playing roles in the development and progression of cancer, such as metastatic breast, colorectal, pancreatic and ovarian cancer etc | Pavalk and LaRoche (1993), Vinogradova et al. (2002), Kikuchi et al. (2008), Barbolina et al. (2008) |
| Talin | Containing an atypical four point one protein, ezrin, radixin and moesin (FERM) domain that binding to integrin cytoplasmic tails | Important for transducing signals, actin network organization, focal adhesion composition, and integrin activation | Over expression leading the progression to metastatic disease such as prostate cancer | Desiniotis and Kyprianou (2011), Kim et al. (2011), Goult et al. (2013), Das et al. (2014), Tan et al. (2015) |
| Filamin | Binding to integrin β tails | Important in integrin signaling transduction and the reorganization of the actin cytoskeleton | Mutation causing congenital anomalies and epileptic seizures | Feng and Walsh (2004), Robertson (2005), Robertson et al. (2006), Kim et al. (2010) |
| Paxillin | Binding to the membrane proximal region of the integrin β1 | Acting as a crucial intermediary in the transduction of signals generated by cell adhesion through integrins | Has an association between Paxillin gene expression and invasive tumor behavior, including lung cancer and breast carcinoma etc | Schaller et al. (1995), Tanaka et al. (1996), Salgia et al. (1999), Madan et al. (2006) |
| Tensin | Containing a PTB domain | Involved in integrin-mediated focal adhesions | Negativity producing unfavorable prognosis in terms of overall survival in breast cancer | Lo et al. (1994), Yang et al. (2016) |
In addition, integrin cytoplasmic domains may also interact directly with several intracellular signaling proteins such as cytohesin-1 (Kolanus et al. 1996), focal adhesion kinase (FAK) (Schaller et al. 1995), integrin-linked kinase (ILK) (Hannigan et al. 1996), β3-endonexin (Shattil et al. 1995), cytoplasmic domain associated protein-1 (ICAP-1) (Chang et al. 1997), receptor for activated protein kinase C (Rack1) (Liliental and Chang 1998), and calcium- and integrin-binding protein (CIB) (Naik et al. 1997) (Table 4).
Table 4.
Integrin-associated proteins (intracellular signaling proteins)
| Intracellular signaling proteins | Binding sites to integrins | Functions | Roles in diseases | References |
|---|---|---|---|---|
| Cytohesin-1 | Sec7 domain binds to cytoplasmic tail of integrin β2 | Affecting the PI3 K-dependent activation of integrin β2 | Regulating human polymorphonuclear neutrophil | Nagel et al. (1998), Azreqa and Bourgoina (2011) |
| FAK | Directly binding to integrin β1 tail | Playing an essential role in integrin-stimulated signaling mechanism | Important for tumor progression in cancer | Sun et al. (2014) |
| ILK | C-terminus of ILK binding to the cytoplasmic tails of integrin β1 and β3 | Regulating actin cytoskeleton by interacting with various actin-binding actin regulatory proteins and mediating the integrin-dependent signaling | Playing an important function to upregulate several types of cancers, as leukemia | Persad and Dedhar (2003), Böttcher et al. (2009) |
| β3-endonexin | Binding to integrin β3 cytoplasmic tail (Asn-IIe-Thr-Tyr (NITY) motif) | Increasing integrins affinity for ligand | Playing roles in proliferative disease, for example atherosclerosis. | Hannigan et al. (1996) |
| ICAP-1 | C-terminal region containing a PTB domain that providing a binding site for integrin β1 | Acting as a messenger that transmits information to the cellular nucleus for controlling gene expression and cell proliferation in a β1-independent manner | Important for body development and pathogenesis | Bouvard et al. (2006), Faurobert et al. (2012) |
| Rack1 | Interacting with the cytoplasmic tails of integrin β1, β2, and β5 | Important in the control of integrin-dependent PKC associated signaling cascades | Serving as a scaffold protein in promoting angiogenesis | Liliental and Chang (1998), Li et al. (2000) |
| CIB | Interacting with integrin | Main function still needing to be tested in a cellular environment | Naik et al. (1997) |
Biological functions and related signaling pathways
Integrins are responsible for sensing many aspects of the cellular microenvironment, including the composition and structure of the ECM and some biochemical signals generated by growth factor or cytokine stimulation. Integrins transmit bidirectional signaling across the plasma membrane by coupling extracellular conformational changes via the unclasping and separation of α and β transmembrane and cytoplasmic domains (Luo and Springer 2006). Inside-out signals regulate integrin affinity for adhesive ligands, outside-in signals depend on ligands that regulate cellular responses to adhesion (Ginsberg et al. 2005). Integrins have no intrinsic catalytic activities, and they transduce intracellular signals via adaptor proteins. Integration of these complex signals contributes to mediate cell biological processes (Parsons et al. 2010).
Integrins in cell adhesion
Integrin-mediated cell adhesion to extracellular matrix components is essential for the organization, maintenance, and repair of numerous tissues (De and Georges-Labouesse 2000). The cell adhesion process is complex and has a series of steps (Friedl and Wolf 2003), including binding to the extracellular matrix, receptor clustering, and the recruitment of cytoskeletal elements. Integrin-mediated cell adhesion occurs via focal adhesions involving the signaling pathway through ILK (serving as a multifunctional adaptor protein that links focal adhesion to the actin cytoskeleton (Hannigan et al. 2005), FAK, phospholipase C (PLC), and the activation of Pho family proteins. Therein, FAK modulates integrin activity (Lawson et al. 2012) and increases tyrosine phosphorylation in response to integrin activation depending on an intact integrin β cytoplasmic tail (Burridge et al. 1992). The Pho family proteins are important as well. Even the exact relationships between GTPase and integrin mediated-signal pathway are not clear, the integrin-dependent regulation of intracellular PH can occur by Pho GTPase, which has necessary effects on cell spreading and cell adhesion (Tominaga and Barber 1998). The signaling molecules involved in integrin-mediated adhesion are the upstream pathways that mediate other cell functions. Therefore, it is easy to see the link between cell adhesion and other integrin-mediated biological functions such as cell proliferation, survival, and migration. This may explain why integrin α5β1, after binding with FN and intracellular cytoskeletal components located in partial adhesion sites, can induce a series of signal transductions affecting cell motility and migration (Su et al. 2005). Kiwanuka et al. (2013) also indicated that α5β1, αVβ1, and αVβ6 integrin formed adhesions to provide points of traction for cell translocation during keratinocyte migration. Therefore, cell adhesion is the precondition of integrin-mediated biological functions.
Integrins in cell proliferation
Proliferation of mammalian cells is regulated by various environmental factors, primarily adhesion to ECM. Integrin-mediated adhesion and soluble factors are crucial to cell proliferation, the loss of cell adhesion leads to cell invasion and apoptosis (Blandin et al. 2016). A related study showed that integrins could be an indispensable player during intestinal tumorigenesis and serve as functional platforms to coordinate intestinal stem cell (ISC) maintenance, differentiation, and proliferation in response to environmental factors (Lin et al. 2013). The α2 and α3 subunits displayed an expression spatial gradient in the crypt and were implicated as cell growth patterns and phenotype modulators required for the process of intestinal epithelial cell differentiation (Zhang et al. 2003). Integrins also interact with growth factor receptors and other factors to regulate cell proliferation. Integrins and growth factor receptors can regulate G1 phase cyclins and related kinases that determine the cell cycle via various cytoplasmic signaling pathways (Moreno-Layseca and Streuli 2013; Eberwein et al. 2015).
There are many indications that not all integrin-mediated cell cycle signaling is the same. Most integrins activate FAK, extracellular regulated kinase (ERK), mitogen-activated protein kinases (MAPKs) and Rho family GTPases on rigid ECMs (Luo et al. 2013; Naci and Aoudjit 2014). However, integrin αvβ3 is selectively associated with enhanced signaling by RTK receptors. It can also activate several other pathways including calcium entry into cells (Schwartz and Denninghoff 1994), NF-ΚB (Scatena et al. 1998), and possibly some others. In addition, some integrins cannot induce similar effects despite their similar abilities at promoting cell adhesion and cytoskeletal organization. Integrins αvβ3, α5β1, and α1β1 interact with caveolin to stimulate Shc phosphorylation and possibly other factors to promote DNA synthesis (Wary et al. 1996). Integrins αvβ3 and α5β1 also activate PI3 K, which are phoaphatidylinositol lipids that modify enzymes implicated as mediators of integrin-mediated cytoskeletal changes and play an important role in cell migration (Cary et al. 1999).
Integrins in cell survival and migration
Cell migration is also vital to various biological phenomena. It is involved in not only normal but also pathological events. For example, cell migration is essential in homeostatic processes such as repairing injured tissues and body immune responses in adults (Steffensen et al. 2001). Cell adhesion receptors are essential for cell migration, and many belong to integrins (Liddington and Bankston 2000). A related report showed that integrin α4β7 had a high expression in mast leukocytes in mucosal inflammation, which promoted the migration of precursor cells to the intestinal tract. Meighan revealed that integrins expressions were up-regulated in migratory cells, and their activities were linked to cellular physiological differentiation (Meighan and Schwarzbauer 2008). During cell migration, integrins must have been recycled or synthesized.
Cell migration involves the localized activation of Rac for the directed protrusion of the cellular membrane only at the leading edges through both the ILK- and FAK-mediated pathways. The intracellular pH and calcium fluxes by integrins also affect cell migration (Schwartz et al. 1989; Marks et al. 1991).
Integrins also play a crucial role in cell survival and protect anchored cells against serum starvation-induced apoptosis. When epithelial and endothelial cell matrix attachment is disrupted, it induces cell apoptosis. Integrin-mediated cell survival is promoted by signaling through the PI3K-AKT, AKT, and ERK pathways (Naci and Aoudjit 2014). If cells are displaced or begin to migrate in an inappropriate environment, they will lose integrin-mediated survival signals (Gilcrease 2007). Signaling through AKT mediates cell survival in adherent epithelial cells by phosphorylating and sequestering BAD, which is a pro-apoptotic Bcl-2 family protein (Cory and Adams 2002). The signaling through the PI3 K-AKT pathway results in the phosphorylation of Bax, which is also a pro-apoptotic Bcl-2 family protein (Gilmore et al. 2000). Integrin-mediated signaling through the ERK pathway down-regulated the pro-apoptotic protein Bim (Reginato et al. 2003).
Conclusions and future prospects
Integrins have gradually become a research hotspot in cell biology, physiology, genetics, and pathology. Expression levels of integrins in cell membranes not only affect cell morphology, proliferation, differentiation, migration, and some macromolecular syntheses but also important in maintaining organization and structural integrity. According to the specific distribution and function of different integrin subunits, immense researchers have applied the unique structural and biological functions of integrins to study the prevention and treatment of human or animal diseases such as gastric cancer, liver cancer, and damaged tissues, et al. Additionally, integrins transmit bidirectional signaling to exert their biological functions, which plays an important role in cellular processes.
However, many questions remain to be elucidated, such as what the exact regulatory mechanisms are and how to determine integrin-mediated cell proliferation, migration, or survival in different cell or tissue types. Therefore, a better understanding of integrin characteristics and influences on human or animal functioning protein may provide a theoretical basis for clarifying the molecular mechanism of metastasis and solving these problems. The study of integrin-mediated signal transduction will also be an important area of research in the future.
Authors’ contributions
LP designed and wrote this manuscript, LP, YZ and ZY was involved in collecting relevant literature. GQ supervised the writing of this review. All authors read and approved the final manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (Nos. 31572415 and 31572439).
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Li Pan, Email: panli0628@126.com.
Yuan Zhao, Email: zhaoyuan@jlau.edu.cn.
Zhijie Yuan, Email: yuanzj1990@126.com.
Guixin Qin, Phone: +86-431-8453-3506, Email: qgx@jlau.edu.cn.
References
- Azreqa MAE, Bourgoina SG. Cytohesin-1 regulates human blood neutrophil adhesion to endothelial cells through β2 integrin activation. Mol Immunol. 2011;48:1408–1416. doi: 10.1016/j.molimm.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A, Raghavan S. Defining the role of integrin alphavbeta6 in cancer. Curr Drug Targets. 2009;10:645–652. doi: 10.2174/138945009788680374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbolina MV, Adley BP, Kelly DL, Fought AJ, Scholtens DM, Shea LD, Stack MS. Motility-related actinin alpha-4 is associated with advanced and metastatic ovarian carcinoma. Lab Invest. 2008;88:602–614. doi: 10.1038/labinvest.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit YD, Lussier C, Beaulieu JF. Integrin α8β1 regulates adhesion, migration and proliferation of human intestinal crypt cells via a predominant RhoA/ROCK dependent mechanism. Gastroenterology. 2009;101:695–708. doi: 10.1042/BC20090060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg K, Lange T, Mittelberger F, Schumacher U, Hahn U. Selection and characterization of an α6β4 integrin blocking DNA aptamer. Mol Ther Nucleic Acids. 2016 doi: 10.1038/mtna.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandin AF, Noulet F, Renner G, Mercier MC, Choulier L, Vauchelles R, Ronde P, Carreiras F, Etienne-Selloum N, Vereb G, Lelong-Rebel I, Martin S, Dontenwill M, Lehmann M. Glioma cell dispersion is driven by α5 integrin-mediated cell–matrix and cell–cell interactions. Cancer Lett. 2016;376:328–338. doi: 10.1016/j.canlet.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Böttcher RT, Lange A, Fässler R. How ILK and kindlins cooperate to orchestrate integrin signaling. Curr Opin Cell Biol. 2009;21:670–675. doi: 10.1016/j.ceb.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Bouaouina M, Lad Y, Calderwood DA. The N-terminal domains of talin cooperate with the phosphotyrosine binding-like domain to activate beta1 and beta3 integrins. J Biol Chem. 2008;283:6118–6125. doi: 10.1074/jbc.M709527200. [DOI] [PubMed] [Google Scholar]
- Bouvard D, Millon-Fremillon A, Dupe-Manet S, Block MR, Albiges-Rizo C. Unraveling ICAP-1 function: toward a new direction? Eur J Cell Biol. 2006;85:275–282. doi: 10.1016/j.ejcb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF, Nishimura SL, Aldape K, Landers DV, Carpenter W. Expression of the β6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci. 1995;108:2241–2251. doi: 10.1242/jcs.108.6.2241. [DOI] [PubMed] [Google Scholar]
- Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and pp 125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary LA, Han DC, Guan JL. Integrin-mediated signal transduction pathways. Histol Histopathol. 1999;14(3):1001–1009. doi: 10.14670/HH-14.1001. [DOI] [PubMed] [Google Scholar]
- Chang DD, Wong C, Smith H, Liu J. ICAP-1, a novel β1 integrin cytoplasmic domain-associated protein, binds to a conserved and functionally important NPXY sequence motif of β1 integrin. J Cell Biol. 1997;138:1149–1157. doi: 10.1083/jcb.138.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppolino MG, Dedhar S. Bidirectional signal transduction by integrin receptors. Int J Biochem Cell Biol. 2000;32(2):171–188. doi: 10.1016/S1357-2725(99)00043-6. [DOI] [PubMed] [Google Scholar]
- Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- Das M, Subbayya Ithychanda S, Qin J, Plow EF. Mechanisms of talin-dependent integrin signaling and crosstalk. Biochim Biophys Acta. 2014;1838:579–588. doi: 10.1016/j.bbamem.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De AA, Georges-Labouesse E. Integrin and ECM functions: roles in vertebrate development. Trends Genet. 2000;16:389–395. doi: 10.1016/S0168-9525(00)02074-6. [DOI] [PubMed] [Google Scholar]
- Delannet M, Martin F, Bossy B, Cheresh DA, Reichardt LF, Duband JL. Specific roles of the αVβ1, αVβ3 and αVβ5 integrins in avian neural crest cell adhesion and migration on vitronectin. Development. 1994;120:2687–2702. doi: 10.1242/dev.120.9.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desiniotis A, Kyprianou N. Significance of talin in cancer progression and metastasis. Int Rev Cell Mol Biol. 2011;289:117–147. doi: 10.1016/B978-0-12-386039-2.00004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CJ, Cheresh DA, Little CD. An antagonist of integrin alpha v beta 3 prevents maturation of blood vessels during embryonic neovascularization. J Cell Sci. 1995;108:2655–2661. doi: 10.1242/jcs.108.7.2655. [DOI] [PubMed] [Google Scholar]
- Eberwein P, Laird D, Schulz S, Reinhard T, Steinberg T, Tomakidi P. Modulation of focal adhesion constituents and their down-stream events by EGF: on the cross-talk of integrins and growth factor receptors. Biochim Biophys Acta. 2015;1853:2183–2198. doi: 10.1016/j.bbamcr.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Faurobert E, Rome C, Boulday G, Lisowska J, Manet S, Malbouyres M, Kéramidas M, Bouvard D, Tournier-Lasserve E, Ruggiero F, Coll JL, Albiges-Rizo C. Defective vascular integrity upon KRIT1/ICAP-1 complex loss in CCM correlates with aberrant beta 1 integrin-dependent extracellular matrix remodeling. Vasc Pharmacol. 2012;56(5):332–333. doi: 10.1016/j.vph.2011.08.080. [DOI] [Google Scholar]
- Feng Y, Walsh CA. The many faces of filamin: a versatile molecular scaffold for cell motility and signaling. Nat Cell Biol. 2004;6(11):1034–1038. doi: 10.1038/ncb1104-1034. [DOI] [PubMed] [Google Scholar]
- Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane extracellular matrix–cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- Gilcrease MZ. Integrin signaling in epithelial cells. Cancer Lett. 2007;247:1–25. doi: 10.1016/j.canlet.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Gilmore AP, Metcalfe AD, Romer LH, Streuli CH. Integrin-mediated survival signals regulate the apoptotic function of Bax through its conformation and subcellular localization. J Cell Biol. 2000;149:431–446. doi: 10.1083/jcb.149.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol. 2005;17:509–516. doi: 10.1016/j.ceb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Goult BT, Xu XP, Gingras AR, Swift M, Patel B, Bate N, Kopp PM, Barsukov IL, Critchley DR, Volkmann N, Hanein D. Structural studies on full-length talin1 reveal a compact auto-inhibited dimer: implications for talin activation. J Struct Biol. 2013;184:21–32. doi: 10.1016/j.jsb.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan GE, Leunghagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, Dedhar S. Regulation of cell adhesion and anchorage-dependent growth by a new β1 integrin-linked protein kinase. Nature. 1996;379:91–96. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- Hannigan G, Troussard AA, Dedhar S. Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat Rev Cancer. 2005;5:51–63. doi: 10.1038/nrc1524. [DOI] [PubMed] [Google Scholar]
- Hee LJ, Jeongsuk C, Sang-Uk N. Critical residues of αX I-domain recognizing fibrinogen central domain. Biochem Biophys Res Commun. 2007;355:1058–1063. doi: 10.1016/j.bbrc.2007.02.082. [DOI] [PubMed] [Google Scholar]
- Hogervorst F, Kuikman I, von dem Borne AEGK, Sonnenberg A. Cloning and sequence analysis of beta-4 cDNA: an integrin subunit that contains a unique 118 kd cytoplasmic domain. Eur Mol Biol Org J. 1990;9:765–770. doi: 10.1002/j.1460-2075.1990.tb08171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JX, Niu MM, Li XY, Lu DY, Cui J, Xu WJ, Li G, Zhan J, Zhang HQ. FERM domain-containing protein FRMD5 regulates cell motility via binding to integrin β5 subunit and ROCK1. FEBS Lett. 2014;588:4348–4356. doi: 10.1016/j.febslet.2014.10.012. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Isacke CM, Horton MA. The adhesion molecule factsbook. 2. London: Academic Press; 2000. pp. 152–211. [Google Scholar]
- Kaneko K, Ito M, Naoe Y, Lacy-Hulbert A, Ikeda K. Integrin αv in the mechanical response of osteoblast lineage cells. Biochem Biophys Res Commun. 2014;447(2):352–357. doi: 10.1016/j.bbrc.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi S, Honda K, Tsuda H, Hiraoka N, Imoto I, Kosuge T, Umaki T, Onozato K, Shitashige M, Yamaguchi U, Ono M, Tsuchida A, Aoki T, Inazawa J, Hirohashi S, Yamada T. Expression and gene amplification of actinin-4 in invasive ductal carcinoma of the pancreas. Clin Cancer Res. 2008;14:5348–5356. doi: 10.1158/1078-0432.CCR-08-0075. [DOI] [PubMed] [Google Scholar]
- Kim H, Nakamura F, Lee W, Hong C, Pérez-Sala D, McCulloch CA. Regulation of cell adhesion to collagen via β1 integrins is dependent on interactions of filamin A with vimentin and protein kinase C epsilon. Exp Cell Res. 2010;316:1829–1844. doi: 10.1016/j.yexcr.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Kim C, Ye F, Ginsberg MH. Regulation of integrin activation. Annu Rev Cell Dev Biol. 2011;27:321–345. doi: 10.1146/annurev-cellbio-100109-104104. [DOI] [PubMed] [Google Scholar]
- Kiwanuka E, Andersson L, Caterson EJ, Junker JP, Gerdin B, Eriksson E. CCN2 promotes keratinocyte adhesion and migration via integrin α5β1. Exp Cell Res. 2013;319(19):2938–2946. doi: 10.1016/j.yexcr.2013.08.021. [DOI] [PubMed] [Google Scholar]
- Kolanus W, Nagel W, Schiller B, Zeitlmann L, Godar S, Stockinger H, Seed B. αLβ2 integrin/LFA-1 binding to ICAM-1 induced by cytohesin-1, a cytoplasmic regulatory molecule. Cell. 1996;86:233–242. doi: 10.1016/S0092-8674(00)80095-1. [DOI] [PubMed] [Google Scholar]
- Lawson C, Lim ST, Uryu S, Chen XL, Calderwood DA, Schlaepfer DD. FAK promotes recruitment of talin to nascent adhesions to control cell motility. J Cell Biol. 2012;196(2):223–232. doi: 10.1083/jcb.201108078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Rivera-Nieves J, Sandborn WJ, Shattil S. Integrin-based therapeutics: biological basis, clinical use and new drugs. Nat Rev Drug Discov. 2016;15(3):1–11. doi: 10.1038/nrd.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Hu Y, Sturm G, Wick G, Xu Q. Ras/Racdependent activation of p38 mitogen-activated protein kinases in smooth muscle cells stimulated by cyclic strain stress. Arterioscler Thromb Vasc Biol. 2000;20:E1–E9. doi: 10.1161/01.ATV.20.3.e1. [DOI] [PubMed] [Google Scholar]
- Liddington RC, Bankston LA. The structural basis of dynamic cell adhesion: heads, tails, and allostery. Exp Cell Res. 2000;261:37–43. doi: 10.1006/excr.2000.5058. [DOI] [PubMed] [Google Scholar]
- Liliental J, Chang DD. Rack1, a receptor for activated protein kinase C, interacts with integrin β subunit. J Biol Chem. 1998;273:2379–2383. doi: 10.1074/jbc.273.4.2379. [DOI] [PubMed] [Google Scholar]
- Lin G, Zhang X, Ren J, Pang Z, Wang C, Xu N, Xi R. Integrin signaling is required for maintenance and proliferation of intestinal stem cells in Drosophila. Dev Biol. 2013;377:177–187. doi: 10.1016/j.ydbio.2013.01.032. [DOI] [PubMed] [Google Scholar]
- Lo SH, An Q, Bao S, Wong W, Liu Y, Janmey PA, Hartwig JA, Chen LB. Molecular cloning of chick cardiac muscle tensin. Full-length cDNA sequence, expression, and characterization. J Biol Chem. 1994;269:22310–22319. [PubMed] [Google Scholar]
- Luo BH, Springer TA. Integrin structures and conformational signaling. Curr Opin Cell Biol. 2006;18:579–586. doi: 10.1016/j.ceb.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo DY, Wazir R, Tian Y, Yue X, Wei TQ, Wang KJ. Integrin αv mediates contractility whereas integrin α4 regulates proliferation of human bladder smooth muscle cells via FAK pathway under physiological stretch. J Urol. 2013;190(4):1421–1429. doi: 10.1016/j.juro.2013.04.027. [DOI] [PubMed] [Google Scholar]
- Lyman S, Gilmore A, Burridge K, Gidwitz S, White GC. Integrin-mediated activation of focal adhesion kinase is independent of focal adhesion formation or integrin activation. J Biol Chem. 1997;272:22538–22547. doi: 10.1074/jbc.272.36.22538. [DOI] [PubMed] [Google Scholar]
- Madan R, Smolkin MB, Cocker R, Fayyad R, Oktay MH. Focal adhesion proteins as markers of malignant transformation and prognostic indicators in breast carcinoma. Hum Pathol. 2006;37:9–15. doi: 10.1016/j.humpath.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Marks PW, Hendey B, Maxfied FR. Attachment to fibronectin or vitronectin makes human neutrophil migration sensitive to alterations in cytosolic free calcium concentration. J Cell Biol. 1991;112:149–158. doi: 10.1083/jcb.112.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meighan CM, Schwarzbauer JE. Temporal and spatial regulation of integrins during development. Curr Opin Cell Biol. 2008;20:520–524. doi: 10.1016/j.ceb.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memmo LM, McKeown-Longo P. The alphavbeta5 integrin functions as an endocytic receptor for vitronectin. J Cell Sci. 1998;111:425–433. doi: 10.1242/jcs.111.4.425. [DOI] [PubMed] [Google Scholar]
- Mercurio AM, Rabinovitz I, Shaw LM. The α6β4 integrin and epithelial cell migration. Curr Opin Cell Biol. 2001;13:541–545. doi: 10.1016/S0955-0674(00)00249-0. [DOI] [PubMed] [Google Scholar]
- Mor-Cohen R. Disulfide bonds as regulators of integrin function in thrombosis and hemostasis. Antioxid Redox Signal. 2016;24(1):16–31. doi: 10.1089/ars.2014.6149. [DOI] [PubMed] [Google Scholar]
- Moreno-Layseca P, Streuli CH. Signalling pathways linking integrins with cell cycle progression. Matrix Biol. 2013;34:144–153. doi: 10.1016/j.matbio.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Naci D, Aoudjit F. Alpha2beta1 integrin promotes T cell survival and migration through the concomitant activation of ERK/Mcl-1 and p38 MAPK pathways. Cell Signal. 2014;26:2008–2015. doi: 10.1016/j.cellsig.2014.05.016. [DOI] [PubMed] [Google Scholar]
- Nagel W, Zeitlmann L, Schilcher P, Geiger C, Kolanus J, Kolanus W. Phosphoinositide 3-OH kinase activates the β2 integrin adhesion pathway and induces membrane recruitment of cytohesin-1. J Biol Chem. 1998;273:14853–14861. doi: 10.1074/jbc.273.24.14853. [DOI] [PubMed] [Google Scholar]
- Naik UP, Patel PM, Parise LV. Identification of a novel calcium binding protein that interacts with the integrin allb cytoplasmic domain. J Biol Chem. 1997;272:4651–4654. doi: 10.1074/jbc.272.8.4651. [DOI] [PubMed] [Google Scholar]
- Otey CA, Vasquez GB, Burridge K, Erickson BW. Mapping of the α-actinin binding site within the β1 integrin cytoplasmic domain. J Biol Chem. 1993;268:21193–21197. [PubMed] [Google Scholar]
- Parsons JT, Slack-Davis JK, Tilghman RW, Iwanicki M, Martin KH. Handbook of cell signaling. 2. London: Academic Press; 2010. Chapter 66-Integrin signaling: cell migration, proliferation, and survival; pp. 491–499. [Google Scholar]
- Pavalk FM, LaRoche SM. Activation of human neutrophils induces an interaction between the integrin beta 2-subunit (CD18) and the actin binding protein alpha-actinin. J Immunol. 1993;151(7):3795–3807. [PubMed] [Google Scholar]
- Persad S, Dedhar S. The role of integrin-linked kinase (ILK) in cancer progression. Cancer Metastasis Rev. 2003;22:375–384. doi: 10.1023/A:1023777013659. [DOI] [PubMed] [Google Scholar]
- Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. J Biol Chem. 2000;275:21785–21788. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J, Muthuswamy SK, Brugge JS. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol. 2003;5:733–740. doi: 10.1038/ncb1026. [DOI] [PubMed] [Google Scholar]
- Reszka AA, Hayashi Y, Horwitz AF. Identification of amino acid sequences in the integrin β1 cytoplasmic domain implicated in cytoskeletal association. J Cell Biol. 1992;117:1321–1330. doi: 10.1083/jcb.117.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SP. Filamin A: phenotypic diversity. Curr Opin Genet Dev. 2005;15:301–307. doi: 10.1016/j.gde.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Robertson SP, Jenkins ZA, Morgan T, Ades L, Aftimos S, Boute O, Fiskerstrand T, Garcia-Minaur S, Grix A, Green A, Der Kaloustian V, Lewkonia R, McInnes B, van Haelst MM, Mancini G, Illes T, Mortier G, Newbury-Ecob R, Nicholson L, Scott CI, Ochman K, Brozek I, Shears DJ, Superti-Furga A, Suri M, Whiteford M, Wilkie AO, Krakow D. Frontometaphyseal dysplasia: mutations in FLNA and phenotypic diversity. Am J Med Genet A. 2006;140:1726–1736. doi: 10.1002/ajmg.a.31322. [DOI] [PubMed] [Google Scholar]
- Rohrschneider LR. Adhesion plaques of Rous sarcoma virus-transformed cells contain the src gene product. Proc Natl Acad Sci USA. 1980;77:3514–3518. doi: 10.1073/pnas.77.6.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanska HM, Potemski P, Collins SI, Williams H, Parmar S, Berditchevski F. Loss of CD151/Tspan24 from the complex with integrin α3β1 in invasive front of the tumour is a negative predictor of disease-free survival in oral squamous cell carcinoma. Oral Oncol. 2013;49:224–229. doi: 10.1016/j.oraloncology.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Salgia R, Li JL, Ewaniuk DS, Wang YB, Sattler M, Chen WC, Richards W, Pisick E, Shapiro GI, Rollins BJ, Chen LB, Griffin JD, Sugarbaker DJ. Expression of the adhesion protein paxillin in lung cancer and its relation to cell motility. Oncogene. 1999;18:66–67. doi: 10.1038/sj.onc.1202273. [DOI] [PubMed] [Google Scholar]
- Scatena M, Almeida M, Chaisson ML, Fausto N, Nicosia RF, Giachelli CM. NF-kB mediates αvβ3 integrin-induced cell survival. J Cell Biol. 1998;141:1083–1093. doi: 10.1083/jcb.141.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Otey CA, Hilderbrand JD, Parsons JT. Focal adhesion kinase and paxillin bind to peptides mimicking β integrin cytoplasmic domains. J Cell Biol. 1995;130:1181–1187. doi: 10.1083/jcb.130.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippers A, Kochut A, Pabst O, Frischmann U, Clahsen T, Tenbrock K, Müller W, Wagner N. β7 integrin controls immunogenic and tolerogenic mucosal B cell responses. Clin Immunol. 2012;144(2):87–97. doi: 10.1016/j.clim.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Denninghoff K. αv integrins mediate the rise in intracellular calcium in endothelial cells on fibronectin even though they play a minor role in adhesion. J Biol Chem. 1994;269(15):11133–11337. [PubMed] [Google Scholar]
- Schwartz MA, Both G, Lechene C. Effect of cell spreading on cytoplasmic pH in normal and transformed fibroblasts. Proc Natl Acad Sci USA. 1989;86:4525–4529. doi: 10.1073/pnas.86.12.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Shattil SJ, O’Toole T, Eigenthaler M, Thon V, Williams M, Babior BM, Ginsberg MH. β3-endonexin, a novel polypeptide that interacts specifically with the cytoplasmic tail of the integrin β3 subunit. J Cell Biol. 1995;131:807–816. doi: 10.1083/jcb.131.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimaoka M, Lu C, Palframan RT, von Andrian UH, McCormack A, Takagi J, Shimaoka M, Takagi J, Springer TA. Conformational regulation of integrin structure and function. Annu Rev Biophys Biomol Struct. 2002;31:485–516. doi: 10.1146/annurev.biophys.31.101101.140922. [DOI] [PubMed] [Google Scholar]
- Springer TA, Sen M. Leukocyte integrin αLβ2 headpiece structures: the αI domain, the pocket for the internal ligand, and concerted movements of its loops. Proc Natl Acad Sci USA. 2016;113(11):2940–2945. doi: 10.1073/pnas.1601379113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srichai MB, Zent R. Integrin structure and function. In: Zent R, Pozzi A, editors. Cell-extracellular matrix interactions in cancer. New York: Springer; 2010. pp. 19–41. [Google Scholar]
- Steffensen B, Häkkinen L, Larjava H. Proteolytic events of wound-healing coordinated interaction among matrix metalloproteinases (MMPs), integrins, and extracellular matrix molecules. Crit Rev Oral Biol Med. 2001;12:373–398. doi: 10.1177/10454411010120050201. [DOI] [PubMed] [Google Scholar]
- Stewart RL, O’Connor KL. Clinical significance of the integrin α6β4 in human malignancies. Lab Invest. 2015;95:976–986. doi: 10.1038/labinvest.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su JM, Wang LY, Ling YL, Zha XL. Role of cell adhesion signal molecules in hepatocellular carcinoma cell apoptosis. World J Gastroenterol. 2005;11(30):4667–4673. doi: 10.3748/wjg.v11.i30.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CC, Zargham R, Shao Q, Gui XY, Marcus V, Lazaris A, Salmand A, Metrakos P, Que XJ, Gao ZH. Association of CD98, integrin β1, integrin β3 and Fak with the progression and liver metastases of colorectal cancer. Pathol Res Pract. 2014;210:668–674. doi: 10.1016/j.prp.2014.06.016. [DOI] [PubMed] [Google Scholar]
- Takada Y, Ye XJ, Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CL, Kwok JCF, Heller JPD, Zhao R, Eva R, Fawcett JW. Full length talin stimulates integrin activation and axon regeneration. Mol Cell Neurosci. 2015;68:1–8. doi: 10.1016/j.mcn.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Yamaguchi R, Sabe H, Sekiguchi K, Healy JM. Paxillin association in vitro with integrin cytoplasmic domain peptides. FEBS Lett. 1996;399:53–58. doi: 10.1016/S0014-5793(96)01280-X. [DOI] [PubMed] [Google Scholar]
- Tartaglia LJ, Bennett A, Plattner AS, Muzyczka N, Ling C, Srivastava A, Agbandje-McKenn M. Molecular cloning, overexpression, and an efficient one-step purification of α5β1 integrin. Protein Expr Purif. 2013;92:21–28. doi: 10.1016/j.pep.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teige I, Bäcklund A, Svensson L, Kvist PH, Petersen HK, Kemp K. Induced keratinocyte hyper-proliferation in α2β1 integrin transgenic mice results in systemic immune cell activation. Int Immunopharmacol. 2010;10:107–114. doi: 10.1016/j.intimp.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Tominaga D, Barber DL. Na–H exchange acts downstream of Rho A to regulate integrin-induced cell adhesion and spreading. Mol Biol Cell. 1998;9:2287–2303. doi: 10.1091/mbc.9.8.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velling T, Kusche-Gullberg M, Sejersen T, Gullberg D. cDNA cloning and chromosomal localization of human α11 integrin. A collagen-binding, I domain-containing β1-associated integrin α-chain present in muscle tissues. J Biol Chem. 1999;274:25735–25742. doi: 10.1074/jbc.274.36.25735. [DOI] [PubMed] [Google Scholar]
- Vinogradova O, Velyvis A, Velyviene A, Hu B, Haas T, Plow E, Qin J. A structural mechanism of integrin alpha(IIb)beta(3) inside-out activation as regulated by its cytoplasmic face. Cell. 2002;110:587–597. doi: 10.1016/S0092-8674(02)00906-6. [DOI] [PubMed] [Google Scholar]
- Wary KK, Maneiro F, Isakoff SJ, Marcantonio EE, Giancotti FG. The adapter protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. doi: 10.1016/S0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- Wilschut KJ, van Tol HTA, Arkesteijn GJA, Haagsman HP, Poelen BAJ. Alpha 6 integrin is important for myogenic stem cell differentiation. Stem Cell Res. 2011;7:112–123. doi: 10.1016/j.scr.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Yang ZY, Yu YY, Yuan JQ, Shen WX, Zheng DY, Chen JZ, Mao C, Tang JL. The prognostic value of phosphatase and tensin homolog negativity in breast cancer: a systematic review and meta-analysis of 32 studies with 4393 patients. Crit Rev Oncol Hema. 2016;101:40–49. doi: 10.1016/j.critrevonc.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Zamir E, Katz M, Posen Y, Erez N, Yamad KM, Katz BZ, Lin S, Lin DC, Bershadsky A, Kam Z, Geiger B. Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat Cell Biol. 2000;2(4):191–196. doi: 10.1038/35008607. [DOI] [PubMed] [Google Scholar]
- Zhang WM, Popova SN, Bergman C, Velling T, Gullberg MK, Gullberg D. Analysis of the human integrin α11 gene (ITGA11) and its promoter. Matrix Biol. 2002;21:513–523. doi: 10.1016/S0945-053X(02)00054-9. [DOI] [PubMed] [Google Scholar]
- Zhang X, Cromwell JW, Kunjummen BD, Yee D, Garcia-Aguilar J. The α2 and α3 integrins are required for morphologic differentiation of an intestinal epithelial cell line. Surgery. 2003;133(4):429–437. doi: 10.1067/msy.2003.107. [DOI] [PubMed] [Google Scholar]


