Abstract
Omega-3 fatty acid products are available as prescription formulations (icosapent ethyl, omega-3-acid ethyl esters, omega-3-acid ethyl esters A, omega-3-carboxylic acids) and dietary supplements (predominantly fish oils). Most dietary supplements and all but one prescription formulation contain mixtures of the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Products containing both EPA and DHA may raise low-density lipoprotein cholesterol (LDL-C). In clinical trials, the EPA-only prescription product, icosapent ethyl, did not raise LDL-C compared with placebo. To correct a common misconception, it is important to note that omega-3 fatty acid dietary supplements are not US FDA–approved over-the-counter drugs and are not required to demonstrate safety and efficacy prior to marketing. Conversely, prescription products are supported by extensive clinical safety and efficacy investigations required for FDA approval and have active and ongoing safety monitoring programs. While omega-3 fatty acid dietary supplements may have a place in the supplementation of diet, they generally contain lower levels of EPA and DHA than prescription products and are not approved or intended to treat disease. Perhaps due to the lack of regulation of dietary supplements, EPA and DHA levels may vary widely within and between brands, and products may also contain unwanted cholesterol or fats or potentially harmful components, including toxins and oxidized fatty acids. Accordingly, omega-3 fatty acid dietary supplements should not be substituted for prescription products. Similarly, prescription products containing DHA and EPA should not be substituted for the EPA-only prescription product, as DHA may raise LDL-C and thereby complicate the management of patients with dyslipidemia.
Key Points
| Omega-3 products are available as dietary supplements and prescription formulations; products containing docosahexaenoic acid (DHA) may have the unwanted effect of raising low-density lipoprotein cholesterol (LDL-C). |
| Dietary supplements are not over-the-counter (OTC) products, and efficacy, quality, and safety of omega-3 dietary supplements are questionable because of a lack of regulation and potential content variability; currently, there are no approved OTC omega-3 fatty acid products. |
| Omega-3 dietary supplements are not appropriate for the treatment of disease and are not therapeutically equivalent to, and should not be substituted for, prescription omega-3 fatty acid products; prescription products containing DHA should not be substituted for icosapent ethyl (highly purified eicosapentaenoic acid [EPA]). |
Introduction
For more than 35 years, omega-3 fatty acids have been thought to provide cardiovascular benefits, beginning with epidemiologic studies linking high omega-3 fatty acid dietary intake with reduced rates of cardiovascular disease in Greenland Inuits [1–3]. Subsequent diet-based studies suggested that increased omega-3 fatty acid consumption reduced cardiovascular mortality in high-risk (but not low-risk) individuals [4]. Stemming from these and other findings, numerous fish oil dietary supplements containing omega-3 fatty acids have become commercially available.
Over the last decade, several prescription omega-3 fatty acid products have been approved by the US FDA based on clinical intervention trials. Dietary supplements of other classes of omega-3 fatty acid products are widely available, but there are no FDA-approved over-the-counter (OTC) omega-3 fatty acid drugs. This article provides an overview of the basic biochemistry and potential cardiovascular benefits of omega-3 fatty acids and compares omega-3 fatty acid prescription products and fish oil dietary supplements, highlighting key considerations for their clinical use.
Overview of Fatty Acid Biochemistry
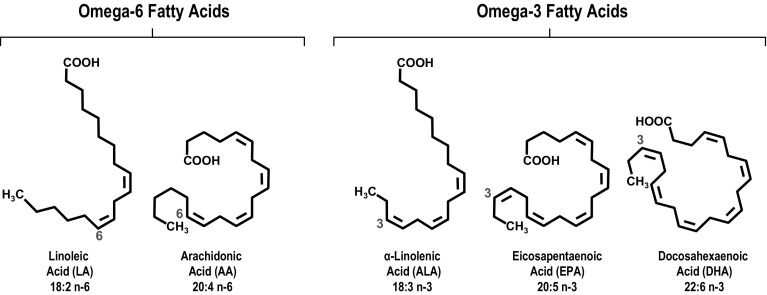
Fatty acids are carboxylic acids; they have a carboxyl group (COOH) at one end, they contain long carbon chains, and most have an even number of carbon atoms. The nomenclature for fatty acids takes into account whether the molecule contains double bonds: saturated fatty acids have no double bonds (thus the carbon atoms are “saturated” with hydrogen), whereas polyunsaturated fatty acids have multiple double bonds. The full name begins with the number of carbon atoms, followed by the number of double bonds. For those with double bonds, the location of the first double bond (counting from the methyl [CH3] end) is also defined in the nomenclature, using “n-x” or “omega-x” [5, 6]. As shown in Fig. 1, polyunsaturated fatty acids that have the first double bond in the third position (counting from the methyl end) belong to the n-3 (omega-3) family and include α-linolenic acid (ALA; 18:3, n-3), eicosapentaenoic acid (EPA; 20:5, n-3), and docosahexaenoic acid (DHA; 22:6, n-3); those that have the first double bond in the sixth position (counting from the methyl end) belong to the n-6 (omega-6) family and include linoleic acid (LA; 18:2, n-6) and arachidonic acid (AA; 20:4, n-6).
Fig. 1.
Structures of omega-3 fatty acids. Both omega-6 and omega-3 fatty acids are polyunsaturated fatty acids, meaning that the hydrocarbon chain contains multiple double bonds. The naming convention is [number of carbon atoms]:[number of double bonds], n- (or ω)-[position of first double bond starting from the methyl end of the chain, shown in red]. Omega-3 fatty acids generally have anti-inflammatory and anti-thrombotic properties, whereas omega-6 fatty acids generally have pro-inflammatory and pro-thrombotic properties
Some fatty acids are considered essential because they are required for good health but cannot be synthesized in sufficient quantities by the body and therefore must be obtained in the diet. ALA and LA are essential fatty acids and precursors of key n-3 and n-6 fatty acids [7, 8]. Certain oils (e.g., flaxseed, canola) are high in ALA, which is a precursor of the longer-chain omega-3 fatty acids, including EPA and DHA (Fig. 1). However, the conversion of ALA into EPA and DHA is not very efficient in humans [8]. Fish are good sources of EPA and DHA because fish consume algae that produce EPA and DHA [8] or are predators of other fish that consume algae. While EPA and DHA are structurally and chemically distinct, DHA can be derived from EPA [7–9]. Other oils (e.g., corn, safflower, sunflower) are high in the omega-6 fatty acid LA, which is the precursor of AA.
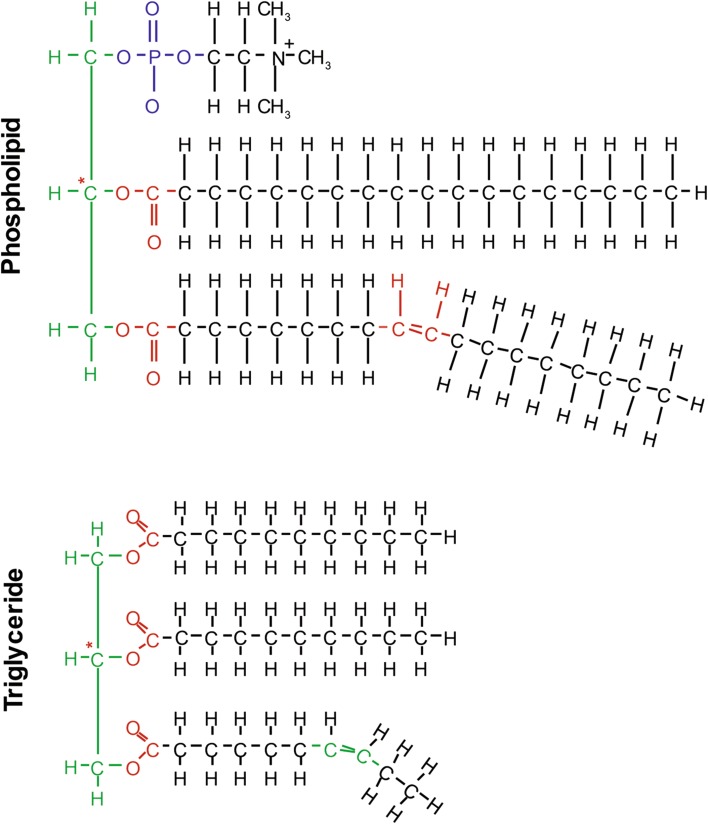
Fatty acids are key components of both triglycerides and membrane phospholipids; triglycerides contain a glycerol backbone that is esterified to three fatty acids. Phospholipids containing a glycerol backbone are known as phosphoglycerides and are similar to triglycerides, but one of the three fatty acids is replaced by a phosphate group (Fig. 2). When fatty acids are not attached to other molecules, they are known as free fatty acids. Omega-3 and omega-6 fatty acids are incorporated into membrane phospholipids, where they alter the physical properties of cellular membranes and serve as precursors for several classes of lipid mediators [7, 8]. The relative amount, not the absolute amount, of these polyunsaturated fatty acids is key, because EPA and DHA compete with AA for the second carbon, or “sn-2,” position in membrane phospholipids [7]. When cells are exposed to inflammatory stimuli, the enzyme phospholipase A2 acts to release the incorporated polyunsaturated fatty acids from the sn-2 position of the phospholipid. The fatty acids are then converted by cyclooxygenase into eicosanoid-signaling molecules [9]. AA is converted into eicosanoids that generally have pro-inflammatory or pro-thrombotic effects, whereas those derived from EPA typically have anti-inflammatory or anti-thrombotic effects [6, 9].
Fig. 2.
Omega-3 fatty acids are components of triglycerides and phospholipids, and may also be found as free fatty acids. Star indicates sn-2 position; phospholipase A2 releases fatty acids from the sn-2 position of membrane phospholipids
Cardiovascular Benefits of Omega-3 Fatty Acids
The omega-3 fatty acids EPA and DHA are generally effective in reducing triglycerides and non-high-density lipoprotein cholesterol (non-HDL-C) in patients with hypertriglyceridemia; these effects are observed with monotherapy and when administered in combination with a statin [10–16]. However, EPA and DHA may have differential effects on low-density lipoprotein cholesterol (LDL-C). DHA may raise LDL-C, whereas EPA has a neutral effect or may slightly lower LDL-C [17, 18]. The effect of DHA on LDL-C may be attributed in part to findings that DHA may downregulate the LDL receptor [19, 20].
The benefit of reducing triglycerides on cardiovascular outcomes remains to be conclusively proven, but emerging evidence from genetic studies suggests that triglycerides and triglyceride-rich lipoproteins play a causal role in coronary atherosclerosis [21–26]. In two independent studies of large US and Danish cohorts, individuals with life-long low triglycerides due to mutations in the apolipoprotein C3 (APOC3) gene had approximately 40 % lower coronary heart disease (CHD) risk than individuals with normal APOC3 alleles [21, 22]. In the Danish cohort, this association was attenuated by adjusting for non-fasting triglycerides, implying that the effect of APOC3 on triglycerides was at least partially responsible for the lowered CHD risk [23]. ApoC-III is a protein coded by the APOC3 gene that is part of the very-low-density lipoprotein complex and inhibits lipases, thus decreasing the uptake of triglyceride-rich lipoprotein particles [23]. More recently, genetic studies have revealed that inactivating mutations in the gene encoding ANGPTL4 (a regulatory factor in triglyceride metabolism) are associated with lower triglyceride levels and lower risk of coronary artery disease [25, 26].
The potential cardiovascular benefits of omega-3 fatty acids are not restricted to their effects on lipids. Omega-3 fatty acids, particularly EPA, have pleiotropic effects at multiple steps involved in atherosclerosis [27, 28]. For example, EPA has beneficial effects on endothelial function [29, 30] by improving the balance between the vasodilator nitric oxide and damaging reactive oxygen species [31]. EPA has been shown to have beneficial effects on monocyte migration and subsequent differentiation into macrophages and foam cells, which are key steps in early atherosclerotic lesion development [32–35]. Atherosclerosis is a chronic inflammatory disease [36–38] and EPA may reduce the components of inflammation via beneficial effects on eicosanoids, as discussed earlier [6, 9], and by altering pro-inflammatory cytokine levels [39, 40]. EPA has also been shown to reduce plaque volume and carotid intima-media thickness, suggesting it may influence plaque formation and progression [41–46]. Finally, the omega-3 fatty acids reduce adenosine diphosphate–induced platelet aggregation, suggesting the potential for limiting thrombus formation following plaque rupture [47].
Prescription Omega-3 Fatty Acid Products
Several omega-3 fatty acid prescription products have been approved by the FDA for use as an adjunct to diet for reducing triglycerides in adults with severe hypertriglyceridemia (triglycerides ≥500 mg/dl) (Table 1). These include products containing both EPA and DHA (Lovaza® [omega-3-acid ethyl esters], GlaxoSmithKline, Research Triangle Park, NC, USA; Omtryg® [omega-3-acid ethyl esters A], Trygg Pharma, Inc, Arlington, VA, USA; Epanova® [omega-3-carboxylic acids], AstraZeneca Pharmaceuticals, Wilmington, DE, USA; and generic Lovaza formulations) [48–52]. Omega-3-acid ethyl esters [48] and omega-3-acid ethyl esters A [49] contain omega-3-acid ethyl esters, whereas the omega-3-carboxylic acids product is a fish oil-derived mixture of polyunsaturated free fatty acids, including omega-3 fatty acids, the most abundant of which are EPA and DHA [13, 50, 53]. There is one FDA-approved EPA-only product: a high-purity formulation containing icosapent ethyl, the ethyl ester of EPA (Vascepa®, Amarin Pharma, Inc, Bedminster, NJ, USA); it does not contain DHA or any other omega-3 fatty acid [54].
Table 1.
US FDA-approved prescription omega-3 fatty acid products indicated as an adjunct to diet to reduce triglyceride levels in adult patients with severe (≥500 mg/dl) hypertriglyceridemia
| Brand name | Generic name | EPA contenta | DHA contenta | Dosing/administration | Adverse reactions in clinical trials |
|---|---|---|---|---|---|
| Vascepa | Icosapent ethyl | 1 g | None | 4 g/day (2 capsules bid) with food | Arthralgia,b oropharyngeal pain |
| Lovazac | Omega-3-acid ethyl esters | ~0.465 g | ~0.375 g | 4 g/day (4 capsules od or 2 capsules bid) with or without food | Eructation,d dyspepsia,d taste perversion,d constipation, GI disorder, vomiting, increased ALT/AST, pruritus, rash |
| Epanovae | Omega-3-carboxylic acids | 0.55 g | 0.2 g | 2 g or 4 g/day (2 or 4 capsules od). In clinical trials, administration took place without regard to meals | Diarrhea,d nausea,d abdominal pain or discomfort,d eructation,d abdominal distension, constipation, vomiting, fatigue, nasopharyngitis, arthralgia, dysgeusia |
| Omtryg | Omega-3-acid ethyl esters A | ~0.465 g | ~0.375 g | 4 g/day (4 capsules od or 2 capsules bid) with food | Eructation,d dyspepsia,d taste perversion,d constipation, GI disorder, vomiting, increased ALT/AST, pruritus, rash |
ALT alanine aminotransferase, AST aspartate aminotransferase, bid twice daily, DHA docosahexaenoic acid, EPA eicosapentaenoic acid, GI gastrointestinal, od once daily
aPer capsule
bIncidence ≥2 % and >placebo
cGeneric formulations of Lovaza are available
dIncidence ≥3 % and >placebo
eEpanova is approved but not available at the time of the writing of this review
The safety and lipid-lowering efficacy of the prescription omega-3 fatty acid products have been evaluated in randomized, blinded, placebo-controlled trials of patients with very high baseline triglycerides (≥500 mg/dl) (Fig. 3) [10–13, 48–50, 54] and patients with residually high triglycerides (≥200 to <500 mg/dl) despite statin therapy (Table 2) [14–16, 48]. In addition to the reductions in triglycerides, non-HDL-C, and total cholesterol observed across these clinical trials, it is notable that increases in LDL-C compared with placebo were observed for trials of products containing DHA and EPA but not for trials of the EPA-only product, icosapent ethyl (Fig. 3; Table 2). Also notable is the finding that higher baseline triglycerides were generally associated with greater triglyceride reductions [12, 18, 55]. Thus, it is expected that the differences in the magnitude of triglyceride reductions observed across clinical trials of prescription omega-3 fatty acid products shown in Table 2 and Fig. 3 are reflections of differences in patient baseline triglycerides. All of the prescription omega-3 fatty acid products have been generally well tolerated in clinical trials and have well-established safety and tolerability profiles. The most common adverse events for the prescription omega-3 fatty acid products are provided in Table 1 [48–52, 54]. Notably, the most common adverse events occurring with prescription products containing DHA and EPA were predominantly gastrointestinal, but not with the EPA-only product, icosapent ethyl (Table 1). Use of products containing both DHA and EPA also require periodic monitoring of LDL-C during therapy due to the potential for increases in this lipid parameter, while treatment with the EPA-only product, icosapent ethyl, has no LDL-C monitoring requirement [48–50, 54].
Fig. 3.

Percentage change from baseline versus placebo in key lipid parameters from clinical trials of prescription omega-3 fatty acid products (4 g/day) in patients with very high triglycerides (≥500 mg/dl) [48–50, 54]. Upper limit for triglycerides was 2000 mg/dl in studies of omega-3-acid ethyl esters, omega-3-carboxylic acids, and icosapent ethyl and 1500 mg in the omega-3-acid ethyl esters A study. Omega-3-acid ethyl esters values are based on pooled data from two studies [10, 11] (6 and 16 weeks’ duration) as reported in the omega-3-acid ethyl esters prescribing information [48]. Icosapent ethyl data are from the MARINE (Multi-Center, Placebo-Controlled, Randomized, Double-Blind, 12-Week Study with an Open-Label Extension) study (12 weeks) [12]. Omega-3-acid ethyl esters A data (12 weeks) have only been published in the product’s prescribing information [49]. Omega-3-carboxylic acids data are from the EVOLVE (Epanova for Lowering Very High Triglycerides) study (12 weeks) [13]. Difference versus placebo: omega-3-acid ethyl esters = omega-3-acid ethyl esters median % change—placebo median % change; icosapent ethyl = median of [icosapent ethyl % change—placebo % change] (Hodges–Lehmann estimate); omega-3-acid ethyl esters A = Hodges–Lehmann median of all pairwise differences from placebo; omega-3-carboxylic acids = median of [omega-3-carboxylic acids % change—placebo % change] (Hodges–Lehmann estimate). HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, TC total cholesterol, TG triglycerides
Table 2.
Effects of omega-3 fatty acids (4 g/day) added to statin therapy on key lipid parameters in patients with high triglyceride levels at baseline (≥200 and <500 mg/dl)a
| Parameter | COMBOS study [14, 48] (8 weeks) | ANCHOR study [15] (12 weeks) | ESPRIT study [16] (6 weeks) | |||
|---|---|---|---|---|---|---|
| Omega-3-acid ethyl estersb + statinc (n = 122) | Placebo + statinc (n = 132) | Icosapent ethyl + statind (n = 226) | Placebo + statind (n = 227) | Omega-3-carboxylic acids + statine (n = 207) | Placebo + statine (n = 211) | |
| TG | ||||||
| Baseline level (mg/dl) | 268 | 271 | 265 | 259 | 287 | 280 |
| % change from baseline | −30 | −6 | −18 | +6 | −21 | −8 |
| % difference vs. placebo | −23f (p < 0.0001) | −22f (p < 0.0001) | NR (p < 0.001) | |||
| Total cholesterol | ||||||
| Baseline level (mg/dl) | 184 | 184 | 167 | 168 | 178 | 174 |
| % change from baseline | −5 | −2 | −3 | +9 | −4 | +0.5 |
| % difference vs. placebo | –3f (p < 0.05) | –12f (p < 0.0001) | NR (p < 0.001) | |||
| Non-HDL-C | ||||||
| Baseline level (mg/dl) | 137 | 141 | 128 | 128 | 139 | 135 |
| % change from baseline | −9 | −2 | −5 | +10 | −7 | −0.9 |
| % difference vs. placebo | −7f (p < 0.0001) | −14f (p < 0.0001) | NR (p < 0.001) | |||
| LDL-C | ||||||
| Baseline level (mg/dl) | 91 | 88 | 82 | 84 | 94 | 92 |
| % change from baseline | +0.7 | −3 | +2 | +9 | +1 | +1 |
| % difference vs. placebo | +4f (p = 0.05) | –6f (p = 0.0067) | NR (NS) | |||
| HDL-C | ||||||
| Baseline level (mg/dl) | 46 | 43 | 37 | 39 | 39 | 39 |
| % change from baseline | +3 | −1 | −1 | +5 | +3 | +2 |
| % difference vs. placebo | +5f (p < 0.05) | −5f (p = 0.0013) | NR (NS) | |||
COMBOS Combination of Prescription Omega-3 with Simvastatin study, ESPRIT Epanova Combined with a Statin in Patients with Hypertriglyceridemia to Reduce Non-HDL Cholesterol study, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, NCEP ATP III National Cholesterol Education Program Adult Treatment Panel III, NR not reported, NS not significant (p > 0.05), TG triglycerides
aAdditional lipid inclusion criteria: COMBOS, mean LDL-C ≤10 % above NCEP ATP III goal; ANCHOR, LDL-C ≥40 and <100 mg/dl on optimized statin therapy, with criteria later expanded to mean of 2 TG-qualifying values ≥185 mg/dl with at least 1 value ≥200 mg/dl and upper limit of LDL-C ≤115 mg/dl; ESPRIT, LDL-C at ≤110 % of NCEP ATP III goal or on maximally tolerated statin dose
bIn 2014, data regarding patients with TG levels 200–499 mg/dl was removed from the prescribing information
cSimvastatin 40 mg/dl
dStable statin therapy with atorvastatin, rosuvastatin, or simvastatin
eStable statin therapy with lovastatin, pravastatin, fluvastatin, simvastatin, atorvastatin, or rosuvastatin
fDifference vs. placebo: omega-3-acid ethyl esters = omega-3-acid ethyl esters median % change—placebo median % change; icosapent ethyl = median of (icosapent ethyl % change—placebo % change) (Hodges–Lehmann estimate)
Omega-3 Fatty Acid Dietary Supplements
Several types of dietary supplements containing omega-3 fatty acids are available, including fish oils, krill oils, algal oils, and plant oils (Table 3). Except for plant sources (e.g., soybeans, walnuts, and canola oil, which contain ALA), all contain a mixture of EPA and DHA, but may also include saturated fats, other lipids, and potentially harmful ingredients [56–62]. These products are widely available in drug and health food stores, with more than 300 “omega-3” products listed in the US National Institutes of Health Dietary Supplement Label Database [63].
Table 3.
Non-prescription omega-3 fatty acid products: dietary supplements
| Products | More than 300 products available |
|---|---|
| Formulations | Soft gels, liquids, powders, gummies |
| Sources | Fish oils, krill oils, algal oils, plant oils |
| Omega-3 fatty acid content and purity | Predominantly DHA + EPA in varying quantities; DHA may raise LDL-C levels May contain inconsistent DHA/EPA levels May contain saturated fat, cholesterol, oxidation products, and/or other contaminants that may have adverse health effects |
| Regulatory requirements | Not required to demonstrate efficacy or safety prior to marketing Should not be substituted for prescription omega-3 fatty acid products Dietary supplements are not OTC drugs; no OTC omega-3 fatty acid or fish oil products are approved or available |
DHA docosahexaenoic acid, EPA eicosapentaenoic acid, LDL-C low-density lipoprotein cholesterol, OTC over the counter
Although the widely available omega-3 fatty acid dietary supplements can be obtained without a prescription, it is important to understand that they are not OTC drugs. It is a common misconception among physicians, pharmacists, and patients that omega-3 fatty acid dietary supplements are FDA-approved OTC drugs. As per the Dietary Supplement Health and Education Act (DSHEA) of 1994, dietary supplements are not considered drugs for purposes of FDA regulation, and therefore are not subject to the same regulatory oversight as prescription or OTC drugs [64]. As such, dietary supplements are not required to demonstrate safety prior to marketing [65]. Moreover, dietary supplements do not require pre-marketing approval from the FDA, and, under the DSHEA of 1994, anything labeled as a dietary supplement is assumed to be safe until proven otherwise. Importantly, it has been suggested that physicians and patients cannot be assured that dietary supplements are safe without sweeping legal and regulatory changes [66]. No OTC omega-3 fatty acid products are currently approved or available in the USA.
There are issues and concerns regarding the content, quality, and purity of omega-3 fatty acid dietary supplements. It is important to recognize that dietary supplements do not always contain what is specified on their label and may differ in EPA and DHA content from batch to batch [62, 67–69]. For example, analytic studies have reported actual EPA and DHA concentrations in dietary supplements sold in the USA ranging from 51 to 124 % (EPA) and from 61 to 153 % (DHA) of the amounts stated on the product labels [62]. More recent studies have confirmed these findings (range 66–184 % [EPA] and 62–184 % [DHA] of the stated label amounts), with 74 % of supplements tested containing less than 100 % of the stated amounts and 16 % of supplements containing less than 80 % of the stated amounts [67]. In a recent study of fish oil supplements marketed in New Zealand, two-thirds of supplements tested contained less than 67 % of the stated amounts of EPA and DHA, with 2 of 32 supplements containing only one-third of the stated label amounts [68]. Supplements have also been reported to meet total EPA + DHA content claimed on the label but not stated levels of individual components (i.e., lower EPA content and higher DHA content than stated on the label) [69].
Regarding additional ingredients found in omega-3 fatty acid dietary supplements, one study specifically testing the contents found that half of the samples tested provided 30–50 % of recommended daily cholesterol intake at a dose of 3.4 g EPA/DHA, and two-thirds of the products provided at least 2.5 g of saturated fats [61]. Some omega-3 fatty acid dietary supplements have also been found to be highly oxidized. Omega-3 fatty acids are highly prone to oxidation because of the double bonds within the fatty acid chain, leaving fewer non-oxidized fatty acids for therapeutic benefit and replacing them with a complex mixture of lipid peroxides and secondary oxidation products [68]. In product testing, only 8 % of fish oil supplements met all international recommendations for levels of oxidation markers, and the safety of these oxidation products has not been well investigated [68, 70]. Testing of 171 Canadian omega-3 fatty acid dietary supplements revealed that 50 % of products failed at least one of the voluntary safety standards recommended for product oxidation [71]. Recent data have demonstrated that oxidation products found in dietary supplements can interfere with the ability of omega-3 fatty acids to inhibit human small, dense LDL oxidation and hence may interfere with their potential biological benefits [72]. A review of the literature found that oxidized lipids can be absorbed and metabolized and alter cholesterol metabolism in multiple models by increasing cholesterol uptake by macrophages, decreasing cholesterol re-uptake in the liver, and increasing total cholesterol levels [73]. Oxidized lipids were also noted to adversely affect markers of oxidative stress and to cause pro-inflammatory effects in animal and human studies, leading the authors of those studies to conclude that oxidized lipids have the potential to increase the risk of atherosclerosis and thrombosis [73]. Taken together, the data on the purity and content of omega-3 fatty acid dietary supplements coupled with the lack of regulation regarding these products shed light upon the fact that omega-3 fatty acid dietary supplements are not being adequately monitored by manufacturers or government agencies.
In addition to all of the content variability and safety issues discussed above, one further aspect hindering the therapeutic use of omega-3 fatty acid dietary supplements is that dietary supplements may require a high pill burden to achieve the dose levels found in prescription products, which in turn may adversely impact treatment adherence [74, 75].
Discussion
The average patient is exposed to numerous sources of information about their healthcare, often only remembering the information that resonates. The concept that a deficiency of a substance can lead to illness or disease harkens back to the discovery of vitamins in the 19th century. Subsequently, this concept has led to purported “miracle” cures and unsubstantiated claims of benefit from thousands of supplement and nutraceutical manufacturers. It is the role of the clinician to review data or lack thereof when discussing supplements with their patients. The indication for use of prescription omega-3 products should be reviewed with the patient along with the product’s benefits, safety, and the difference between the prescription product and dietary supplements.
As their name indicates, dietary supplements are only appropriate to supplement the diet. They contain low doses of omega-3 fatty acids that are inappropriate for treating disease. Further, the doses found in omega-3 fatty acid dietary supplements may not be consistent with those specified on the label. Lack of regulation and product quality control may also be responsible for the noted issues of batch-to-batch variability and presence of contaminating ingredients in omega-3 fatty acid dietary supplements, which may also impact proper dosing and potentially safety. The low doses found in dietary supplements may not be sufficient for addressing cardiovascular risk, and professional organizations have been moving away from recommending these low-dose supplements for cardiovascular protection [76, 77]. However, the triglyceride-lowering efficacy of prescription omega-3 fatty acids has been shown to be dose-dependent [12, 13, 15, 16, 78]. Thus, patients attempting to administer doses of omega-3 fatty acid dietary supplements to achieve the level of omega-3 fatty acids in prescription products—and, potentially, their desired effects—would need to consume a higher number of capsules than with prescription products. For these reasons, it is important for primary care physicians to understand and educate their patients that omega-3 fatty acid dietary supplements are not approved or intended to treat disease and should not be substituted for prescription products. As cost concerns may sometimes lead to inappropriate substitution of omega-3 fatty acid dietary supplements for prescription products, it may be worth noting that patient discount programs exist for some prescription omega-3 fatty acid products. At the same time, it is equally important that prescription products containing both DHA and EPA not be substituted for the purified EPA product icosapent ethyl, given that DHA may raise LDL-C and complicate the treatment of patients with dyslipidemia. Per FDA equivalence codes, pure EPA is not therapeutically equivalent to DHA-containing products [79].
Although statin therapy significantly reduces cardiovascular events and mortality, elevated triglycerides and residual cardiovascular risk remain in patients with dyslipidemia despite well-controlled LDL-C [80]. Thus, there is a need for adjunctive therapy to reduce this risk. In the JELIS study of hypercholesterolemic Japanese patients, major coronary events were significantly reduced in patients treated with EPA plus a statin compared with patients receiving only statin therapy (relative risk reduction of 19 % in the EPA group, p = 0.011) [81]. More recently, clinical trials of ezetimibe (IMPROVE-IT [82]) and proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors (OSLER study [83] and ODYSSEY LONG TERM study [84]) have confirmed or suggested that statin add-on therapy may be effective in further reducing residual cardiovascular risk. These studies emphasize that “lower LDL-C is better,” and that more can be done for statin-treated patients to address residual cardiovascular risk [85].
Given the multiple beneficial effects of omega-3 fatty acids, it is reasonable to consider adding a prescription omega-3 fatty acid to a statin to help address residual cardiovascular risk. However, previous clinical outcome studies with products containing omega-3 fatty acids have produced inconsistent results. Some trials showed significant improvements in clinical outcomes [81, 86, 87], whereas others did not [88–91]. These disappointing results may be explained in part by insufficient doses of omega-3 fatty acids and/or powering of studies, thus emphasizing the potential need for high-purity, prescription-strength dosing and adequately powered studies. Two ongoing clinical outcome trials are addressing these issues and are using high-dose prescription omega-3 fatty acids. REDUCE-IT is comparing icosapent ethyl plus statin versus placebo plus statin in patients aged ≥45 years who have hypertriglyceridemia after receiving statin therapy for at least 4 weeks, and who have established, or a high risk of, cardiovascular disease [92]. STRENGTH is comparing omega-3-carboxylic acids plus statin versus placebo plus statin in adults with LDL-C <100 mg/dl, high triglycerides, and low HDL after at least 4 weeks of statin therapy, and who are at high risk of a future cardiovascular event [93]. The results of these trials will help define the role of prescription omega-3 fatty acid products as add-on therapy to statins in addressing the residual cardiovascular risk in patients receiving statin therapy.
Conclusions
Prescription omega-3 fatty acid products have proven safety and lipid-lowering efficacy and can be prescribed with confidence to patients with elevated triglycerides, including those who are already receiving a statin. In contrast, dietary supplements are not OTC drugs, and therefore are not required to have a proven safety and efficacy profile. Dietary supplements are highly variable in omega-3 fatty acid content and doses between brands, and, for some products, even between batches. They may also contain additional ingredients that could counter potential benefits. Accordingly, they should not be substituted for prescription products. Similarly, prescription products containing DHA and EPA should not be substituted for a purified EPA product, because products that contain DHA may raise LDL-C, thereby confounding treatment of patients with dyslipidemia.
Acknowledgments
Medical writing assistance was provided by Peloton Advantage, Parsippany, NJ, USA, and funded by Amarin Pharma Inc. Medical scientific reference checks and associated assistance were provided by Sephy Philip, RPh, PharmD, and Joy Bronson of Amarin Pharma Inc. and Sumita Chowdhury, MD, MPH, FACC, MBA, a consultant for Amarin Pharma Inc.
Compliance with Ethical Standards
Funding
No external funding was received to prepare this manuscript.
Conflict of interest
Jonathan Fialkow has served on speakers’ bureaus and received honoraria from Amarin, Bristol Myers-Squibb, Pfizer, and Amgen.
References
- 1.Bang HO, Dyerberg J, Nielsen AB. Plasma lipid and lipoprotein pattern in Greenlandic West-coast Eskimos. Lancet. 1971;1(7710):1143–1145. doi: 10.1016/S0140-6736(71)91658-8. [DOI] [PubMed] [Google Scholar]
- 2.Dyerberg J, Bang HO, Stoffersen E, et al. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 1978;2(8081):117–119. doi: 10.1016/S0140-6736(78)91505-2. [DOI] [PubMed] [Google Scholar]
- 3.Bang HO, Dyerberg J, Sinclair HM. The composition of the Eskimo food in north western Greenland. Am J Clin Nutr. 1980;33(12):2657–2661. doi: 10.1093/ajcn/33.12.2657. [DOI] [PubMed] [Google Scholar]
- 4.Marckmann P, Gronbaek M. Fish consumption and coronary heart disease mortality: a systematic review of prospective cohort studies. Eur J Clin Nutr. 1999;53(8):585–590. doi: 10.1038/sj.ejcn.1600832. [DOI] [PubMed] [Google Scholar]
- 5.IUPAC-IUB Commission on Biochemical Nomenclature The nomenclature of lipids: recommendations, 1976. Eur J Biochem. 1977;79:11–21. doi: 10.1111/j.1432-1033.1977.tb11778.x. [DOI] [Google Scholar]
- 6.Adkins Y, Kelley DS. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J Nutr Biochem. 2010;21(9):781–792. doi: 10.1016/j.jnutbio.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Jump DB, Depner CM, Tripathy S. Omega-3 fatty acid supplementation and cardiovascular disease. J Lipid Res. 2012;53(12):2525–2545. doi: 10.1194/jlr.R027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83(6 Suppl):1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 9.Larsson SC, Kumlin M, Ingelman-Sundberg M, et al. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004;79(6):935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 10.Harris WS, Ginsberg HN, Arunakul N, et al. Safety and efficacy of Omacor in severe hypertriglyceridemia. J Cardiovasc Risk. 1997;4(5–6):385–391. [PubMed] [Google Scholar]
- 11.Pownall HJ, Brauchi D, Kilinc C, et al. Correlation of serum triglyceride and its reduction by omega-3 fatty acids with lipid transfer activity and the neutral lipid compositions of high-density and low-density lipoproteins. Atherosclerosis. 1999;143(2):285–297. doi: 10.1016/S0021-9150(98)00301-3. [DOI] [PubMed] [Google Scholar]
- 12.Bays HE, Ballantyne CM, Kastelein JJ, et al. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial) Am J Cardiol. 2011;108(5):682–690. doi: 10.1016/j.amjcard.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Kastelein JJP, Maki KC, Susekov A, et al. Omega-3 free fatty acids for the treatment of severe hypertriglyceridemia: the EpanoVa fOr Lowering Very high triglyceridEs (EVOLVE) trial. J Clin Lipidol. 2014;8(1):94–108. doi: 10.1016/j.jacl.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Davidson MH, Stein EA, Bays HE, et al. Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: an 8-week, randomized, double-blind, placebo-controlled study. Clin Ther. 2007;29(7):1354–1367. doi: 10.1016/j.clinthera.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Ballantyne CM, Bays HE, Kastelein JJ, et al. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study) Am J Cardiol. 2012;110(7):984–992. doi: 10.1016/j.amjcard.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 16.Maki KC, Orloff DG, Nicholls SJ, et al. A highly bioavailable omega-3 free fatty acid formulation improves the cardiovascular risk profile in high-risk, statin-treated patients with residual hypertriglyceridemia (the ESPRIT trial) Clin Ther. 2013;35(9):1400–1411. doi: 10.1016/j.clinthera.2013.07.420. [DOI] [PubMed] [Google Scholar]
- 17.Wei MY, Jacobson TA. Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: a systematic review and meta-analysis. Curr Atheroscler Rep. 2011;13(6):474–483. doi: 10.1007/s11883-011-0210-3. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson TA, Glickstein SB, Rowe JD, et al. Effects of eicosapentaenoic acid and docosahexaenoic acid on low-density lipoprotein cholesterol and other lipids: a review. J Clin Lipidol. 2012;6(1):5–18. doi: 10.1016/j.jacl.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Ishida T, Ohta M, Nakakuki M, et al. Distinct regulation of plasma LDL cholesterol by eicosapentaenoic acid and docosahexaenoic acid in high fat diet-fed hamsters: participation of cholesterol ester transfer protein and LDL receptor. Prostaglandins Leukot Essent Fatty Acids. 2013;88:281–288. doi: 10.1016/j.plefa.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Dawson K, Zhao L, Adkins Y, et al. Modulation of blood cell gene expression by DHA supplementation in hypertriglyceridemic men. J Nutr Biochem. 2012;23(6):616–621. doi: 10.1016/j.jnutbio.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG, et al. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371(1):32–41. doi: 10.1056/NEJMoa1308027. [DOI] [PubMed] [Google Scholar]
- 22.TG and HDL Working Group of the Exome Sequencing Project NHLaBI. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371(1):22–31. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khetarpal SA, Rader DJ. Triglyceride-rich lipoproteins and coronary artery disease risk: new insights from human genetics. Arterioscler Thromb Vasc Biol. 2015;35(2):e3–e9. doi: 10.1161/ATVBAHA.114.305172. [DOI] [PubMed] [Google Scholar]
- 24.Libby P. Triglycerides on the rise: should we swap seats on the seesaw? Eur Heart J. 2015;36(13):774–776. doi: 10.1093/eurheartj/ehu500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dewey FE, Gusarova V, O’Dushlaine C, et al. Inactivating variants in ANGPTL4 and risk of coronary artery disease. N Engl J Med. 2016;374(12):1123–1133. doi: 10.1056/NEJMoa1510926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myocardial Infarction Genetics and CARDIoGRAM Exome Consortia Investigators Coding variation in ANGPTL4, LPL, and SVEP1 and the risk of coronary disease. N Engl J Med. 2016;374(12):1134–1144. doi: 10.1056/NEJMoa1507652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borow KM, Nelson JR, Mason RP. Biologic plausibility, cellular effects, and molecular mechanisms of eicosapentaenoic acid (EPA) in atherosclerosis. Atherosclerosis. 2015;242(1):357–366. doi: 10.1016/j.atherosclerosis.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 28.Endo J, Arita M. Cardioprotective mechanism of omega-3 polyunsaturated fatty acids. J Cardiol. 2016;67(1):22–27. doi: 10.1016/j.jjcc.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki J, Miwa T, Odawara M. Administration of highly purified eicosapentaenoic acid to statin-treated diabetic patients further improves vascular function. Endocr J. 2012;59(4):297–304. doi: 10.1507/endocrj.EJ11-0394. [DOI] [PubMed] [Google Scholar]
- 30.Toyama K, Nishioka T, Isshiki A, et al. Eicosapentaenoic acid combined with optimal statin therapy improves endothelial dysfunction in patients with coronary artery disease. Cardiovasc Drugs Ther. 2014;28(1):53–59. doi: 10.1007/s10557-013-6496-3. [DOI] [PubMed] [Google Scholar]
- 31.Mason RP, Jacob RF, Corbalan JJ, et al. Combination eicosapentaenoic acid and statin treatment reversed endothelial dysfunction in HUVECs exposed to oxidized LDL [abstract 160] J Clin Lipidol. 2014;8(3):342–343. doi: 10.1016/j.jacl.2014.02.074. [DOI] [Google Scholar]
- 32.Grenon SM, Aguado-Zuniga J, Hatton JP, et al. Effects of fatty acids on endothelial cells: inflammation and monocyte adhesion. J Surg Res. 2012;177(1):e35–e43. doi: 10.1016/j.jss.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thies F, Garry JM, Yaqoob P, et al. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet. 2003;361(9356):477–485. doi: 10.1016/S0140-6736(03)12468-3. [DOI] [PubMed] [Google Scholar]
- 34.Nishio R, Shinke T, Otake H, et al. Stabilizing effect of combined eicosapentaenoic acid and statin therapy on coronary thin-cap fibroatheroma. Atherosclerosis. 2014;234(1):114–119. doi: 10.1016/j.atherosclerosis.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 35.Cawood AL, Ding R, Napper FL, et al. Eicosapentaenoic acid (EPA) from highly concentrated n-3 fatty acid ethyl esters is incorporated into advanced atherosclerotic plaques and higher plaque EPA is associated with decreased plaque inflammation and increased stability. Atherosclerosis. 2010;212(1):252–259. doi: 10.1016/j.atherosclerosis.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 36.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 37.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 38.Klingenberg R, Hansson GK. Treating inflammation in atherosclerotic cardiovascular disease: emerging therapies. Eur Heart J. 2009;30(23):2838–2844. doi: 10.1093/eurheartj/ehp477. [DOI] [PubMed] [Google Scholar]
- 39.Dangardt F, Osika W, Chen Y, et al. Omega-3 fatty acid supplementation improves vascular function and reduces inflammation in obese adolescents. Atherosclerosis. 2010;212(2):580–585. doi: 10.1016/j.atherosclerosis.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 40.Satoh-Asahara N, Shimatsu A, Sasaki Y, et al. Highly purified eicosapentaenoic acid increases interleukin-10 levels of peripheral blood monocytes in obese patients with dyslipidemia. Diabetes Care. 2012;35(12):2631–2639. doi: 10.2337/dc12-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niki T, Wakatsuki T, Bando M, et al. Effects of additional eicosapentaenoic acid to statin therapy on inflammatory cytokines and the tissue characterization of coronary plaque assessed by integrated backscatter intravascular ultrasound systems [abstract 14434] Circulation. 2012;126(21 Suppl.):14434. [Google Scholar]
- 42.Wakita Y, Wakida Y, Itou T, et al. High-purity-eicosapentaenoic-acid in addition to a strong statin makes regression of coronary plaque in patients with angina-pectoris and impaired glucose tolerance [abstract] Circ J. 2013;77(Suppl. 1):I-2678. [Google Scholar]
- 43.Shintani Y, Kawasaki T. The impact of a pure-EPA omega-3 fatty acid on coronary plaque stabilization: a plaque component analysis with 64-slice multi-detector row computed tomography [abstract] J Am Coll Cardiol. 2012;59(13):E1731. doi: 10.1016/S0735-1097(12)61732-X. [DOI] [Google Scholar]
- 44.Mita T, Watada H, Ogihara T, et al. Eicosapentaenoic acid reduces the progression of carotid intima-media thickness in patients with type 2 diabetes. Atherosclerosis. 2007;191(1):162–167. doi: 10.1016/j.atherosclerosis.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 45.Katoh A, Ikeda H. Daily intake of eicosapentaenoic acid inhibits the progression of carotid intimal-media thickness in patients with dyslipidemia [in Japanese] Ther Res. 2011;32(6):863–868. [Google Scholar]
- 46.Ando K, Watanabe T, Daidoji H, et al. Combination therapy of eicosapentaenoic acid and pitavastatin for coronary plaque regression evaluated by integrated backscatter intravascular ultrasonography: a randomized controlled trial [abstract 12007] Circulation. 2015;132:A12007. [Google Scholar]
- 47.Gajos G, Rostoff P, Undas A, et al. Effects of polyunsaturated omega-3 fatty acids on responsiveness to dual antiplatelet therapy in patients undergoing percutaneous coronary intervention: the OMEGA-PCI (OMEGA-3 Fatty Acids After PCI to Modify Responsiveness to Dual Antiplatelet Therapy) study. J Am Coll Cardiol. 2010;55(16):1671–1678. doi: 10.1016/j.jacc.2009.11.080. [DOI] [PubMed] [Google Scholar]
- 48.Lovaza [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2014.
- 49.Omtryg [package insert]. Arlington: Trygg Pharma, Inc.; 2014.
- 50.Epanova [package insert]. Wilmington: AstraZeneca Pharmaceuticals LP; 2014.
- 51.Omega-3 acid ethyl esters [package insert]. Spring Valley: Par Pharmaceuticals Companies, Inc.; 2014.
- 52.Omega-3 acid ethyl esters [package insert]. Sellersville: Teva Pharmaceuticals; 2014.
- 53.Offman E, Marenco T, Ferber S, et al. Steady-state bioavailability of prescription omega-3 on a low-fat diet is significantly improved with a free fatty acid formulation compared with an ethyl ester formulation: the ECLIPSE II study. Vasc Health Risk Manag. 2013;9:563–573. doi: 10.2147/VHRM.S50464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vascepa [package insert]. Bedminster: Amarin Pharma Inc.; 2015.
- 55.Nieman K, Dicklin M, Bell M, et al. Relationship between baseline triglyceride concentration and triglyceride reduction with 4 g/d long-chain omega-3 acid ethyl esters [abstract 1035.6]. FASEB J. 2014;28(1 Suppl.):1035.6.
- 56.Tur JA, Bibiloni MM, Sureda A, et al. Dietary sources of omega 3 fatty acids: public health risks and benefits. Br J Nutr. 2012;107(Suppl. 2):S23–S52. doi: 10.1017/S0007114512001456. [DOI] [PubMed] [Google Scholar]
- 57.Bunea R, El Farrah K, Deutsch L. Evaluation of the effects of Neptune Krill Oil on the clinical course of hyperlipidemia. Altern Med Rev. 2004;9(4):420–428. [PubMed] [Google Scholar]
- 58.Bernstein AM, Ding EL, Willett WC, et al. A meta-analysis shows that docosahexaenoic acid from algal oil reduces serum triglycerides and increases HDL-cholesterol and LDL-cholesterol in persons without coronary heart disease. J Nutr. 2012;142(1):99–104. doi: 10.3945/jn.111.148973. [DOI] [PubMed] [Google Scholar]
- 59.Pan A, Chen M, Chowdhury R, et al. alpha-Linolenic acid and risk of cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr. 2012;96(6):1262–1273. doi: 10.3945/ajcn.112.044040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weitz D, Weintraub H, Fisher E, et al. Fish oil for the treatment of cardiovascular disease. Cardiol Rev. 2010;18(5):258–263. doi: 10.1097/CRD.0b013e3181ea0de0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Truong P, Johnson C, Gabriel D. Variability of cholesterol and saturated fat content in dietary supplements [abstract P72] Circulation. 2007;115(8):e238. [Google Scholar]
- 62.Shim SM, Santerre CR, Burgess JR, et al. Omega-3 fatty acids and total polychlorinated biphenyls in 26 dietary supplements. J Food Sci. 2003;68(8):2436–2440. doi: 10.1111/j.1365-2621.2003.tb07042.x. [DOI] [Google Scholar]
- 63.Dietary Supplement Label Database: Omega-3 results. National Institutes of Health. Available at: http://www.dsld.nlm.nih.gov/dsld/rptQSearch.jsp?item=omega-3&db=adsld. Accessed 17 Mar 2016.
- 64.Regulatory information: Dietary Supplement Health and Education Act of 1994. Food and Drug Administration website. Available at: http://health.gov/dietsupp/ch1.htm. Accessed 17 Mar 2016.
- 65.Lopez JAG, Ito MK. PLA chapter update: prescription fish oil and Blue Cross of Idaho. LipidSpin. 2010;8(3):32–34. [Google Scholar]
- 66.Cohen PA. Hazards of hindsight–monitoring the safety of nutritional supplements. N Engl J Med. 2014;370(14):1277–1280. doi: 10.1056/NEJMp1315559. [DOI] [PubMed] [Google Scholar]
- 67.Kleiner AC, Cladis DP, Santerre CR. A comparison of actual versus stated label amounts of EPA and DHA in commercial omega-3 dietary supplements in the United States. J Sci Food Agric. 2015;95(6):1260–1267. doi: 10.1002/jsfa.6816. [DOI] [PubMed] [Google Scholar]
- 68.Albert BB, Derraik JG, Cameron-Smith D, et al. Fish oil supplements in New Zealand are highly oxidised and do not meet label content of n-3 PUFA. Sci Rep. 2015;5:7928. doi: 10.1038/srep07928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ritter JC, Budge SM, Jovica F. Quality analysis of commercial fish oil preparations. J Sci Food Agric. 2013;93(8):1935–1939. doi: 10.1002/jsfa.5994. [DOI] [PubMed] [Google Scholar]
- 70.Halvorsen BL, Blomhoff R. Determination of lipid oxidation products in vegetable oils and marine omega-3 supplements. Food Nutr Res. 2011;55. doi:10.3402/fnr.v55i0.5792. [DOI] [PMC free article] [PubMed]
- 71.Jackowski SA, Alvi AZ, Mirajkar A, et al. Oxidation levels of North American over-the-counter n-3 (omega-3) supplements and the influence of supplement formulation and delivery form on evaluating oxidative safety. J Nutr Sci. 2015;4:e30. doi: 10.1017/jns.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mason R, Sherratt S. Analysis of omega-3 fatty acid dietary supplements with respect to content: are they appropriate for patients? [abstract E21]. J Manag Care Spec Pharm. 2015;21(10 Suppl. a):S34.
- 73.Turner R, McLean CH, Silvers KM. Are the health benefits of fish oils limited by products of oxidation? Nutr Res Rev. 2006;19(1):53–62. doi: 10.1079/NRR2006117. [DOI] [PubMed] [Google Scholar]
- 74.Zargar A, Ito MK. Long chain omega-3 dietary supplements: a review of the National Library of Medicine Herbal Supplement Database. Metab Syndr Relat Disord. 2011;9(4):255–271. doi: 10.1089/met.2011.0004. [DOI] [PubMed] [Google Scholar]
- 75.Bradberry JC, Hilleman DE. Overview of omega-3 fatty acid therapies. P&T. 2013;38(11):681–691. [PMC free article] [PubMed] [Google Scholar]
- 76.An International Atherosclerosis Society position paper global recommendations for the management of dyslipidemia–full report. J Clin Lipidol. 2014;8(1):29–60. doi: 10.1016/j.jacl.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 77.Standards of medical care in diabetes 2015. Diabetes Care. 2015;38(Suppl. 1):S1–S93. doi: 10.2337/dc15-S003. [DOI] [PubMed] [Google Scholar]
- 78.Tatsuno I, Saito Y, Kudou K, et al. Efficacy and safety of TAK-085 compared with eicosapentaenoic acid in Japanese subjects with hypertriglyceridemia undergoing lifestyle modification: the Omega-3 fatty acids randomized double-blind (ORD) study. J Clin Lipidol. 2013;7(3):199–207. doi: 10.1016/j.jacl.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 79.Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. U.S. Food and Drug Administration. Available at: http://www.accessdata.fda.gov/scripts/cder/ob/. Accessed 17 Mar 2016.
- 80.Fruchart JC, Davignon J, Hermans MP, et al. Residual macrovascular risk in 2013: what have we learned? Cardiovasc Diabetol. 2014;13(1):26. doi: 10.1186/1475-2840-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369(9567):1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 82.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 83.Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500–1509. doi: 10.1056/NEJMoa1500858. [DOI] [PubMed] [Google Scholar]
- 84.Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489–1499. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 85.Jarcho JA, Keaney JF., Jr Proof that lower is better - LDL cholesterol and IMPROVE-IT. N Engl J Med. 2015;372(25):2448–2450. doi: 10.1056/NEJMe1507041. [DOI] [PubMed] [Google Scholar]
- 86.GISSI Prevenzione Investigators Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354(9177):447–455. doi: 10.1016/S0140-6736(99)07072-5. [DOI] [PubMed] [Google Scholar]
- 87.GISSI-HF Investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9645):1223–30. [DOI] [PubMed]
- 88.ORIGIN Trial Investigators n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367(4):309–318. doi: 10.1056/NEJMoa1203859. [DOI] [PubMed] [Google Scholar]
- 89.The Risk and Prevention Study Collaborative Group. n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med. 2013;368:1800–8. [DOI] [PubMed]
- 90.Kromhout D, Giltay EJ, Geleijnse JM. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363(21):2015–2026. doi: 10.1056/NEJMoa1003603. [DOI] [PubMed] [Google Scholar]
- 91.Rauch B, Schiele R, Schneider S, et al. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010;122(21):2152–2159. doi: 10.1161/CIRCULATIONAHA.110.948562. [DOI] [PubMed] [Google Scholar]
- 92.A study of AMR101 to evaluate its ability to reduce cardiovascular events in high risk patients with hypertriglyceridemia and on statin (REDUCE-IT). ClinicalTrials.gov. Available at: http://clinicaltrials.gov/show/NCT01492361. Accessed 17 Mar 2016.
- 93.Outcomes Study to Assess STatin Residual Risk Reduction With EpaNova in HiGh CV Risk PatienTs With Hypertriglyceridemia (STRENGTH). Available at: http://clinicaltrials.gov/ct2/show/NCT02104817?term=strength+and+omega-3&rank=3. Accessed 17 Mar 2016.