Abstract
Objective:
Serotonin reuptake inhibitors are frequently used in first-line treatments for patients with obsessive-compulsive disorder. Nevertheless, many of these patients do not respond well to initial therapy. The hypothesis of glutamatergic dysfunction in specific brain regions has been proposed in the pathophysiology of obsessive-compulsive disorder. This study was designed to evaluate the possible efficacy of lamotrigine, a glutamatergic agent in Serotonin reuptake inhibitors-resistant patients with obsessive-compulsive disorder.
Method:
This study was a 12-week, double blind, randomized, placebo-controlled trial of adjunctive fixed-doses of lamotrigine (100 mg) to Serotonin reuptake inhibitors therapy in obsessive-compulsive disorder. Eligible subjects who had a total Y-BOCS of 21 or above were randomly assigned to receive adjunctive treatment with either lamotrigine (n = 26), or placebo (n = 27). Response to lamotrigine was defined as clinical improvement (>25% decrease in the total Y-BOCS score), which was administered at weeks 0, 8 and 12.
Results:
At the endpoint (week 12), significant differences were observed in obsession, compulsion, and total Y-BOCS scores comparing lamotrigine to placebo (P = 0.01, 0.005 and 0.007 respectively). The mean reduction in obsession, compulsion and total scores in lamotrigine group was about 4.15, 4.50 and 8.73, respectively. Similarly, the mean reductions in the placebo group were 2.52, 2.56 and 5.07. Effect sizes for efficacy measureswerecalculatedbyCohen’sd, and it was calculated as 0.54 for the total YBOCS.
Conclusion:
Our findings provide evidence that this augmentation is well tolerated and may be an effective strategy for patients with refractory obsessive-compulsive disorder.
Keywords: Drug Augmentation, Lamotrigine, Obsessive-Compulsive Disorder, Serotonin Reuptake Inhibitors, Treatment-Resistant
Obsessive-compulsive disorder (OCD) is a debilitating and frustrating disorder. Early onset in adolescence period, chronic course and significant comorbidity with other psychiatric disorders are the main clinical characteristics of this disorder (1). It affects about 1–3% of the general population. OCD is not a prevalent disorder, but it has a significant impact on the quality of life, interpersonal relationships and daily activities of the affected patients (2). The main symptoms of OCD are time-wasting obsessions and compulsions (3). Obsessions include repetitive, intrusive and uncontrollable thoughts, impulses or images; and compulsions include repetitive and stereotyped behaviors or mental acts. The patients do compulsions to reduce the tensions, which are induced by obsessive thoughts (3, 4).
Serotonin reuptake inhibitors (SRIs) and Clomipramine are the first-line medical treatments of patients with OCD. Nevertheless, many patients do not respond well to these agents (5, 6). Strategies for incomplete responders are switching to another SRI, augmentation with Haloperidol or newer antipsychotics, monotherapy with serotonin- norepinephrine reuptake inhibitors (SNRIs) and monoamine oxidase inhibitors (MAOIs) (3, 7 and 8). However, therapies aimed at modulating serotonergic and dopaminergic neurotransmitters are accompanied by inadequate response in many cases. Nearly 25% of patients with OCD acquire little or no benefit from the mentioned treatment strategies and continue to experience significant obsessive-compulsive symptoms (9, 10).
Many authors have anticipated that abnormalities in glutamate neurotransmission, especially in the corticostriato-thalamo-cortical circuitry (CSTC), may contribute to OCD. The metabolism of projection neurons of globus pallidus, striatum, orbitofrontal cortex, and some other parts of the CSTC circuitry that are mainly glutamatergic, increases in patients with OCD. The indicator of increased firing of these projection neurons are excessive striatal glutamate measures in MRS studies (11, 12) and increased glutamate levels in the CSF of patients with OCD (13). The glutamate levels and the mentioned hyperactive parts of the brain become normal after successful treatment (12, 14 and 15).
The hypothesis of glutamatergic dysfunction in specific parts of the brain has been proposed in the pathophysiology of OCD. Accordingly, refractory symptoms of these patients may respond to new augmentation strategies aimed at normalizing glutamatergic neurotransmission dysfunction (11,14). The findings of these studies have persuaded many researchers in testing the efficacy of glutamate modulating agents in unresponsive-to-treatment patients with OCD (13, 16,17, 18 and 19).
Evidence supports the efficacy of Riluzole (10, 21), Memantine (18, 22, 23, 24, 25 and 26), N-acetylcysteine (21, 27), D-cycloserine (28, 29) and other glutamate-modulating agents (30) in the treatment of patients with OCD. Lamotrigine is a member of sodium channel blocking class of antiepileptic drugs, which is well tolerated. Lamotrigine has been used for the maintenance treatment of bipolar disorder particularly preventing depressive episodes (31). The neuronal membrane is stabilized by lamotrigine blocking effect on voltage-sensitive sodium channels (32). Lamotrigine inhibits the release of excitatory neurotransmitters, decreases glutamate transmission, and reduces calcium influx. The intracellular mechanisms of resting transmitter release are modified by lamotrigine (15, 32).
There are only a few case reports, case studies and open label trials and only one placebo controlled study on Lamotrigine augmentation of SRI-resistant patients with OCD (5, 33 and 34).
Due to the small sample size and the short-term follow-up, and the absence of a placebo group, there are some disagreements about the efficacy of this glutamatergic agent in patients with OCD. This study was designed to evaluate the possible efficacy of lamotrigine in a group of SRIs-resistant patients with OCD.
Materials and Method
This was a 12-week, double-blind, randomized, placebo-controlled trial of adjunctive fixed doses of Lamotrigine to treatment regimen of SRIs-resistant/unresponsive-to-treatment patients with OCD that was carried out at two outpatient psychiatry clinics in Rasht, the capital of Guilan province, in the north of Iran from May 2014 to Feb 2016.
The participants were unresponsive-to-SRIs patients with OCD. We defined unresponsive-to-treatment as persistent obsessive-compulsive symptoms despite two adequate trials, at least 12 weeks for each trial, with two different SRIs (300 mg Fuvoxamine, 80 mg Fluoxetine, 80 mg Citalopram or 200 mg Sertraline). The inclusion criteria were a total score of 21 or above in Y-BOCS (Yale-Brawn obsessive-compulsive scale) (16), and stable SRIs dosages for at least three months before the study. Patients with any other major psychiatric disorder, mental retardation, significant concurrent medical illnesses, organic brain disorder, history of substance and alcohol abuse and dependency, pregnant or lactating women, and those patients who had received concomitant psychotherapy or any other psychiatric medications were excluded from the study.
The cases were selected from two private psychiatry clinics in Rasht. Two board certified psychiatrists interviewed the patients, using the Persian version of structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Axis I disorders (35). All of the participants met the diagnosis of OCD according to DSMIV-TR diagnostic criteria. In addition, they were defined as unresponsive-to-SRIs by clinical interview, and the two abovementioned psychiatrists reviewed their medical records. An expert professor of clinical psychology reevaluated the patients and carried out Y-BOCS. After the baseline interview and evaluation by psychiatrists and a clinical psychologist, patients fulfilling inclusion criteria were considered for an augmentation trial of a 12-week, double blind, randomized, placebo-controlled trial of adjunctive fixed-doses (100 mg) of Lamotrigine. A psychiatry resident randomly assigned the patients to receive adjunctive treatment with either Lamotrigine or placebo under double-blind conditions and allocated them to parallel groups, which was determined by pre-randomized codes generated by a computer. She kept the randomization list secure during the study.
The psychiatrists and the clinical psychologist, who assessed and treated the patients, were unaware of the patient assignment list until the end of the study. Patients were not notified about their medications. To ensure blindness, the psychiatry resident had no role in the treatment or data collection. Lamotrigine and placebo were dispensed in identical pills and both groups took equal numbers.
According to treatment guidelines, routine follow-ups and clinical monitoring were enough. Plasma levels of Lamotrigine were not assessed. The patients were educated and warned about any possible medication side effects. In addition, they received instruction papers and a mobile phone number for necessary situations. The side effects were easily diagnosed and controlled. The prescribed dose of Lamotrigine in this study was one- half of the recommended dose in bipolar disorder and the drug was titrated up slowly. Lamotrigine increased from 25 mg/day to 100 mg/day during the first four weeks, in increments of 25 mg/week. This dosage was maintained unchanged to the end of the study.
Treatment response was measured by the change from baseline to final value in 12th week of the study. Response to Lamotrigine was defined as clinical improvement in the obsessive–compulsive scores (>25% decrease in the total Y-BOCS score). The psychologist administered clinical rating. The patients attended five visits: Initial screening and interview, eligibility and randomization (week 0), and three further visits in weeks 4, 8 and 12. Y-BOCS was administered in weeks 0, 8 and 12. In each session, psychiatrists visited the patients to perform a complete physical examination, evaluate drug tolerance and address any unwanted adverse effects. We recorded and classified data in terms of onset, duration, severity, action taken and outcomes. Laboratory tests, including hematology, clinical chemistry, and urine analysis were performed on admission and at the end of adjunctive treatment. Blood pressure and heart rate were measured, and skin examination was done in all study visits.
The Y-BOCS was used as a primary efficacy measure. The Yale-Brown obsessive-compulsive scale is an acceptable clinical checklist that is designed to rate the severity and type of symptoms of patients with OCD. This rating scale is designed for use as a semi-structured interview. The rater should evaluate the items in the checklist and use the questions provided. The type of obsessions or compulsions does not influence it. Although Y-BOCS rely on the patient’s report, the final rating is based on the clinical judgment of the rater. Y-BOCS is a 10-item scale, each item rating from zero (no symptoms) to four (extreme symptoms).
The total range is from 0 to 40 with separate subtotal ranges for severity of obsessions and compulsions. Inter-rater reliability for the total Yale-Brown scale score and each of the 10 individual items is excellent. Y-BOCS has a high degree of internal consistency among all item scores demonstrated with Cranach’s α coefficient. Findings suggest that the Yale-Brown scale is a reliable instrument for measuring the severity of illness in patients with obsessive-compulsive disorder with a range of severity and types of obsessive-compulsive symptoms (35).
Prior to the beginning of the study, sample size was calculated to detect a 25% difference in improvement between Lamotrigine and placebo. Under the assumption of a significant level of 0.05 with a power of 0.80, a minimal sample size of 50 with 25 cases in each group was determined. Estimating a dropout rate of 25%, we decided to recruit 30 participants for each group.
Data obtained from the study underwent check and quality control, and descriptive and inferential statistical analysis. A per-protocol analysis with last-observation-carried-forward (LOCF) was performed. Continuous data were expressed by mean ± SD. The comparison between the groups at baseline and at the end of weeks was performed using the Mann– Whitney U test for two independent samples. The within-group differences in efficacy ratings between baseline and final test were analyzed by the Wilcoxon rank sum test. To measure the magnitude of the treatment’s effect, effect size was provided using Cohen’s d statistic, which gives a measure of the standardized differences in the mean values of change in scores between medications. Non-continuous data were expressed by percentages, and the comparison between the two groups was performed using the Chi-Square test. The statistical analysis was performed with SPSS 16.0 software (SPSS Inc, Chicago, IL, USA).
After a full explanation of the protocol design, all patients provided written informed consent. This trial was approved by the Ethics Committee of the Guilan University of Medical Sciences (Ethics Approval Code No: 1930452601) and registered in the Iranian Registry of Clinical Trials (www.irct.ir; identifier: IRCT2014031717045N1). All phases of this study were performed according to the declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects.
Results
Sixty patients with OCD, 17 men and 43 women, aged 16–60 years, fulfilled inclusion criteria and entered the study. Baseline characteristics, subtypes and daily dosages of SRIs and duration of disease are detailed in Table 1.
Table 1.
Demographic and Clinical Characteristics of the Patients
| Lamotrigine | Placebo | |||
|---|---|---|---|---|
| Patients (completers) | 30 (26) | 30 (27) | ||
| Sex No (%) | ||||
| Male | 7 (23.3) | 10 (33.3) | ||
| Female | 23 (76.7) | 20 (66.7) | ||
| Age No (%) | ||||
| <31 | 9 (30) | 7 (23.3) | ||
| 31–40 | 13 (43.3) | 12 (40) | ||
| >40 | 8 (26.7) | 11 (36.7) | ||
| Duration of illness No (%) | ||||
| 1–3 yrs. | 4 (13.3) | 6 (20) | ||
| 3–5 yrs. | 8 (26.7) | 4 (13.3) | ||
| >5 yrs. | 18 (60) | 20 (66.7) | ||
| Baseline YBOCS Scores | 26.83 | 28.6 | ||
Mann- Whitney U Test: Non-significant
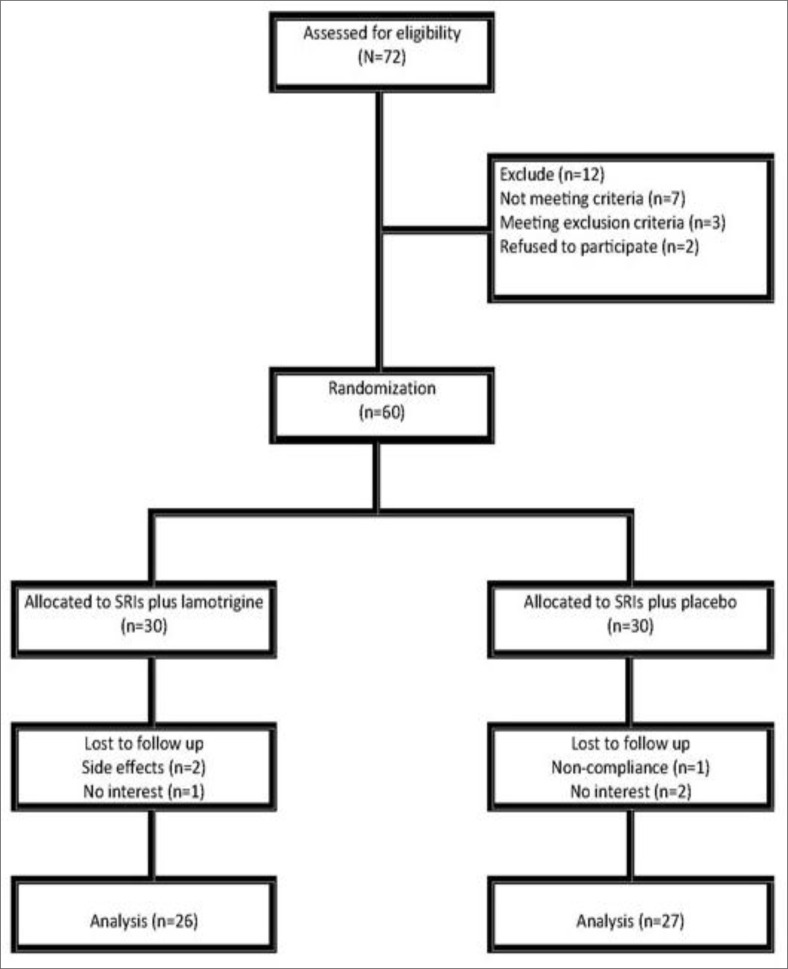
The completion rate was 88%, and 53 patients completed the study. Discontinuation rates in Lamotrigine and placebo group were 13% and 10%, respectively. There were seven dropouts, four in the Lamotrigine group and three in the placebo group. In the Lamotrigine group, one dropout was due to non-compliance, and the other three were because of side effects (development of a skin rash); in the placebo group, one was due to non-compliance with multiple visits and two due to a subjectively assessed lack of efficacy. The diagram is shown in Chart 1.
Chart 1.
Consort Flow Chart of Participants through Each Phase in the Study
Table 2 demonstrates the baseline and final values of the different variables in the Lamotrigine and placebo groups (LOCF). At the baseline (week 0), there were no significant differences in Y-BOCS between the two groups (P = 0.07). At the endpoint (week 12), significant differences were observed in obsession, compulsion, and total Y-BOCS scores between the Lamotrigine to placebo groups (P= 0.01, 0.005 and 0.007, respectively).
Table 2.
Clinical Changes in Patients with OCD Receiving Lamotrigine versus Placebo at Baseline and Week 12 (LOCF)
| Lamotrigine | Placebo | Mann- Whitney p | ||||
|---|---|---|---|---|---|---|
| Baseline | Week12† | Baseline | Week12 ‡ | Baseline | Week12 | |
| YBOCS | ||||||
| Obsession Mean±SD | 13.17±2.2 | 9±3.6 | 13.9±2.2 | 11.3 ±3.1 | 0.202 | 0.019 |
| Compulsion Mean±SD | 13.60±2.7 | 9.31±3.5 | 14.7±2.5 | 12.19 ±3.4 | 0.103 | 0.005 |
| Total score Mean± SD | 26.83±4.0 | 18.31±6.6 | 28.60±3.8 | 23.48 ±5.8 | 0.108 | 0.007 |
Observed cases (Lamotrigine group n = 26) Wilcoxon Signed Ranks Test Z = −4.29/ p ≤ 0.0001, Z = −4.05 p ≤ 0.0001, Z= −2.38/p ≤ 0.0001.
Observed cases (Placebo group n = 27) Wilcoxon Signed Ranks Test Z = −3.69/ p ≤ 0.0001, Z = −3.72 p ≤ 0.0001, Z = −4.20/ p ≤0.0001.
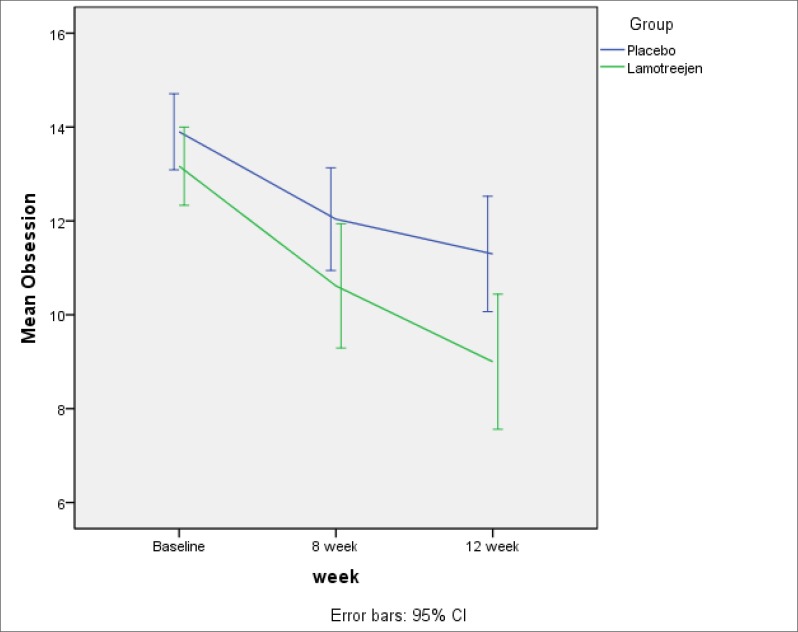
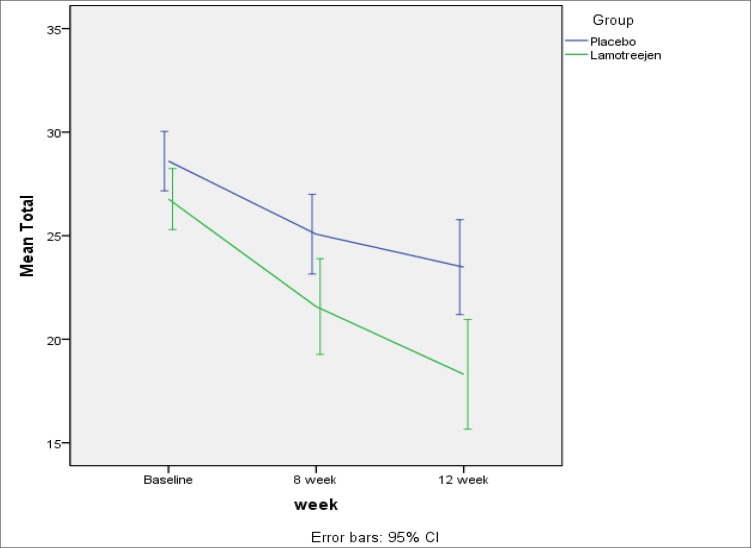
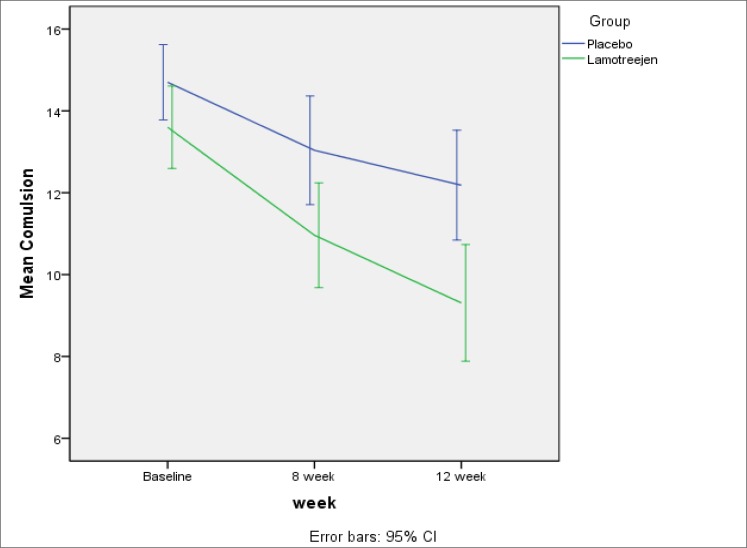
The mean reductions in obsession, compulsion and total scores in the Lamotrigine group were about 4.15, 4.50 and 8.73, respectively. Similarly, the mean reductions in the placebo group were 2.52, 2.56 and 5.07, which is more noticeable in Figures 1 to 3. Cohen’s d calculated effect sizes for efficacy measures (Table 3).
Figure 1.
Reduction in Obsession Scores was Significantly more than Placebo
Figure 3.
Reduction in the Total Scores was Significantly more than Placebo
Table 3.
Significance of Change during the Study Period and Effect Sizes for Efficacy Measures
| Lamotrigine | Placebo | P* | Cohen’s d | |
|---|---|---|---|---|
| YBOCS | ||||
| Obsession Mean±SD | −4.15± 2.9 | −2.52± 2.9 | 0.045 | 0.5 |
| Compulsion Mean±SD | −4.50± 3.9 | −2.56± 2.7 | 0.038 | 0.45 |
| Total Score Mean±SD | −8.73± 6.1 | −5.07± 4.3 | 0.015 | 0.54 |
Mann- Whitney U Test
Figure 2.
Reduction in Compulsion Scores was Significantly more than Placebo
Lamotrigine was well tolerated and no serious adverse effects were recorded. Only one patient in the lamotrigine group developed expanding erythematous rash over her chest, shoulders and back in the third week after starting the drug, which was completely resolved after holding Lamotrigine and supportive treatments. She was excluded from the study. Two other cases also developed much milder rashes over their bodies between weeks 8–10. In these cases, Lamotrigine therapy was suspended and they were excluded from the study after consulting a dermatologist. The most common adverse effects of Lamotrigine were headache (n = 5, 16%) and skin rash (n = 3, 10%). In the placebo group, the most common adverse effects reported by patients were constipation (n = 6, 20%) and weight gain (n = 1, 3.3%).
Discussion
With respect to the positive evidence supporting the role of glutamatergic system in OCD, there is only one placebo-controlled study (with 20 patients in each group) and only one open label study and some case reports which have studied the augmentation therapy with Lamotrigine in SRIs-resistant patients. Considering the positive findings of these trials, we hypothesized that Lamotrigine is effective for treating OCD patients resistant to SRIs (5, 36).
This study is the second double-blind placebo controlled study that added Lamotrigine to the treatment regimen of SRIs-resistant patients. The results of this study demonstrated that augmentation with Lamotrigine is effective and superior to placebo in reducing obsessive and compulsive symptoms in SRIs-resistant patients with OCD. At the end of the 12th week of the study, significant reductions were observed in obsessive and compulsive scores in the Lamotrigine group.
In an unblended randomize trial, Poyurovski and colleagues (36) added 200 mg Lamotrigine to the drug regimen of a group of schizophrenic and schizoaffective patients with obsessive-compulsive symptoms (Y-BOCS>16). The clinical response considered reduction in YBOCS>35% from baseline assessment. They found that Lamotrigine was effective in reducing obsessive-compulsive symptoms (P = 0.033) and depressive symptoms (P = 0.013) of the patients without any significant reduction of their psychotic symptoms. Improvement of depressive symptoms was positively correlated with improvement of obsessive-compulsive symptoms. However, the questions about the role of Lamotrigine in depressive symptoms and its impact on the obsessive-compulsive symptoms remained unanswered. The dosage of Lamotrigine was in the range of the recommended dosage for treating affective disorders. The effectiveness of lamotrigine in depressive disorders has been reported in a number of studies (37). Arrojo-Romero and colleagues reported the positive response to Lamotrigine in two resistant-to-treatment cases with OCD (34). Considering Bruno and colleagues’ study (5) that found no response to placebo, they concluded that positive placebo response in moderate-to-server OCD cases are not likely and may be related to Lamotrigine augmentation effect. The cases in Bruno’s study were only unresponsive to SRIs, but the cases who were presented in the mentioned case report were sever treatment-resistant patients with a long-lasting OCD and lack of response to multiple treatments with different classes of drugs.
Despite Bruno and colleagues’ study (5), which claimed that placebo had no effect on Y-BOCS scores, some studies have reported positive placebo response in placebo-controlled studies of patients with OCD (21, 38). Grant and colleagues (21) examined the efficacy of 100 mg Riluzole, as an adjunctive therapy for moderate-to-severe treatment-resistant children and adolescents with OCD in a 12-week, double blind, and placebo-controlled study. All patients showed significant reductions in Y-BOCS scores but no significant difference was detected on Y-BOCS scores reduction between placebo and Riluzole group.
Multitude of mechanisms contribute to placebo effects such as expectations, conditioning, learning, memory, motivation, somatic focus, reward, anxiety reduction and meaning, and improvement of symptoms in positive emotional states is likely (39); similarly, expectation might work in patients who might hope to get better, although they have said that there would be no response. Although Bruno and colleagues’ (5) study showed effectiveness of adding Lamotrigine to SRIs in treatment-resistant patients with OCD, it did not report any positive responses in the placebo group. The effect sizes reported by them were higher than ours (0.54 Vs 2.4 for total score, 0.5 Vs 2.2 for obsessions and 0.45 Vs 1.6 for compulsions).
We observed five cases of headache and three cases of skin rashes (10%) in the Lamotrigine group and six cases of constipation and one case of weight gain with placebo. The overall incidence of Lamotrigine-induced skin rash is 9.98% from prospective studies, 7.19% from retrospective studies, and 2.09% from post-marketing reports (40). Despite the slow increasing of Lamotrigine dosage, significant rashes appeared in one case. It seems that developing skin rashes is the most important adverse event in this study. Although the prevalence of skin rash is nearly equal to the mentioned Meta-analysis (40), the average dosage of Lamotrigine was lower in our study. On the other hand, although slow titration reduces the risk of developing skin rashes, it increases the waiting time for clinical responses to appear and might reduce the patients’ adherence to treatment. Complications seen in this study are somehow comparable to those in Bruno and colleagues’ (5). They reported four cases of sedation, two cases of fatigue, two cases of headache and one case of skin rash. In the placebo group, they reported three cases of nausea, two cases of headache and two cases of sedation. We did not find any unintended effects in either group. It can be claimed that Lamotrigine is safe and well tolerated, but major concerns persist about developing skin rashes, which calls for further investigations.
It is not possible to generalize the results of this study to all patients. First, we selected a group of moderate-to-severe patients with OCD. It is possible that higher response rates would have been achieved if we had selected less severe types of patients with OCD. Second, we did not classify the patients according to their phenomenological pattern (e.g., taboo, ordering). Subgroups of OCD may show different clinical responses to treatments. Third, comorbidity with other psychiatric disorders is common in patients with OCD (41), which was not considered in this study. Fourth, the treatment-resistant patients who had not responded effectively to any other treatment strategies were not included.
Although most adult studies on bipolar disorder with Lamotrigine generally recommend doses between 200 and 400mg/day, the question that remainsis, ‘how to recommend 100 mg Lamotrigine for these patients?’ We selected 100 mg Lamotrigine because the previous double-blinded placebo-controlled study had chosen this dose for augmentation and it was claimed that 100 mg was significantly effective in reducing obsessive-compulsive symptoms. Therefore, we had no reason to choose a higher dose of Lamotrigine. In addition, the clinical response to Serotoninergic Antidopaminergic drugs (SADs) augmentation with SRIs in patients with OCD can be achieved by smaller doses of these drugs. Besides trying to impose fewer side effects, we decided to prescribe the minimum dosage that may be effective.
Despite Bruno and colleagues’ (5) study that considered positive response equal or more than 30% in Y-BOCS score, we considered 25%. It was somehow due to our clinical judgments that 25% reduction of symptoms would be clinically acceptable. In addition, we considered Y-BOCS (21 vs. 16) in comparison to the previous study to examine our hypothesis with more severe cases of patients with OCD.
Patients who received Lamotrigine as an augmenting agent revealed a wide spectrum of clinical responses. This phenomenon can somehow be explained by multi-neurotransmitter system involvement in OCD, meaning that response rate in a special patient may be related to synergistic power of glutamatergic agent, which can result in different clinical responses. Being able to anticipate clinical response to a special treatment regimen in an individual patient is challenging in OCD management, which if possible, can result in choosing the precise treatment regimen.
Limitations
This study had some limitations. First, the duration of this study was short. Second, the dose of Lamotrigine that was used in this study was only 100 mg. It is possible that further benefits in patients will be achieved with higher doses of Lamotrigine or longer duration of treatment. Although the duration of the study was compatible to similar studies (42, 43), it seems to be insufficient to observe more effects of Lamotrigine. Third, we only used one method to measure the primary outcome. Finally, the clinical response to SRIs-treatment augmentation with SADs in patients with OCD can be achieved approximately in week 12 of the treatment (44), but the placebo effect may become apparent sooner, although it weakens as time passes. Thus, the longer follow-up the patients receive, the greater effect size would be achieved.
We excluded obsessive-compulsive patients who had some common comorbidities such as generalized anxiety disorder, depression, bipolar disorder, and other OCD spectrum disorders (45). Lamotrigine is one of the first line FDA approved treatments for treating bipolar disorder (46). Clinical studies have reported that about 10–15% of patients with OCD have comorbid bipolar disorders (45). There are an increasing number of studies investigating efficacy of Lamotrigine in unipolar depression, anxiety disorders and schizophrenia (47, 48). We implied that if further controlled studies prove Lamotrigine efficacy in OCD, it might take a significant role in the treatment of OCD and its challenging comorbidities.
Conclusion
Our findings, similar to the previous double-blind placebo-controlled study that added Lamotrigine to ongoing treatment with SRIs, provide evidence that this augmentation therapy is well tolerated and may be an effective strategy; although the effect sizes between the two studies were different. It seems that further double-blind placebo-controlled studies, with a larger sample size, longer duration of follow-up and variable dosages of Lamotrigine are necessary.
Acknowledgments
This project was supported by Guilan University of Medical Sciences (GUMS), and was part of Dr. Aram’s thesis toward graduation to receive her degree in Psychiatry, and no grants was received from any resources. We would like to express our thanks to M. Latifi for editing, S. Moghaddam for proofreading, and the personnel of Dr. Khalkhali’s clinic, and Dr. Mohaghegh’s pharmacy for their cooperation.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1. Subramaniam M, Abdin E, Vaingankar JA, Chong SA. Obsessive—compulsive disorder: prevalence, correlates, help-seeking and quality of life in a multiracial Asian population. Soc Psychiatry Psychiatr Epidemiol 2012; 47: 2035– 2043. [DOI] [PubMed] [Google Scholar]
- 2. Subramaniam M, Soh P, Vaingankar JA, Picco L, Chong SA. Quality of life in obsessive-compulsive disorder: impact of the disorder and of treatment. CNS Drugs 2013; 27: 367– 383. [DOI] [PubMed] [Google Scholar]
- 3. Seibell PJ, Hollander E. Management of obsessive-compulsive Disorder. F1000Prime Rep. 2014; 1: 6: 68– 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fornaro M. Switching from serotonin reuptake inhibitors to agomelatine in patients with refractory obsessive-compulsive disorder: a 3 month follow-up case series. Ann Gen Psychiatry 2011; 10: 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bruno A, Mico U, Pandolfo G, Mallamace D, Abenavoli E, Di Nardo F, et al. Lamotrigine augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a double-blind, placebo-controlled study. J Psychopharmacol 2012; 26: 1456– 1462. [DOI] [PubMed] [Google Scholar]
- 6. Dold M, Aigner M, Lanzenberger R, Kasper S. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol 2013; 16: 557– 574. [DOI] [PubMed] [Google Scholar]
- 7. Abudy A, Juven-Wetzler A, Zohar J. Pharmacological management of treatment-resistant obsessive-compulsive disorder. CNS Drugs 2011; 25: 585– 596. [DOI] [PubMed] [Google Scholar]
- 8. Bandelow B. The medical treatment of obsessive-compulsive disorder and anxiety. CNS Spectr 2008; 13: 37– 46. [DOI] [PubMed] [Google Scholar]
- 9. Bloch MH, Landeros-Weisenberger A, Kelmendi B, Coric V, Bracken MB, Leckman JF. A systematic review: antipsychotic augmentation with treatment refractory obsessive-compulsive disorder. Mol Psychiatry 2006; 11: 622– 632. [DOI] [PubMed] [Google Scholar]
- 10. Coric V, Taskiran S, Pittenger C, Wasylink S, Mathalon DH, Valentine G, et al. Riluzole augmentation in treatment-resistant obsessive-compulsive disorder: an open-label trial. Biol Psychiatry 2005; 58: 424– 428. [DOI] [PubMed] [Google Scholar]
- 11. Naaijen J, Lythgoe DJ, Amiri H, Buitelaar JK, Glennon JC. Fronto-striatal glutamatergic compounds in compulsive and impulsive syndromes: a review of magnetic resonance spectroscopy studies. Neurosci Biobehav Rev 2015; 52: 74– 88. [DOI] [PubMed] [Google Scholar]
- 12. Wu K, Hanna GL, Rosenberg DR, Arnold PD. The role of glutamate signaling in the pathogenesis and treatment of obsessive-compulsive disorder. Pharmacol Biochem Behav 2012; 100: 726– 735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bhattacharyya S, Chakraborty K. Glutamatergic dysfunction—newer targets for anti-obsessional drugs. Recent Pat CNS Drug Discov 2007; 2: 47– 55. [DOI] [PubMed] [Google Scholar]
- 14. Pittenger C, Krystal JH, Coric V. Glutamate-modulating drugs as novel pharmacotherapeutic agents in the treatment of obsessive-compulsive disorder. NeuroRx 2006; 3: 69– 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pittenger C, Bloch MH, Williams K. Glutamate abnormalities in obsessive compulsive disorder: neurobiology, pathophysiology, and treatment. Pharmacol Ther 2011; 132: 314– 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goodman WK, Grice DE, Lapidus KA, Coffey BJ. Obsessive-compulsive disorder. Psychiatric Clinics of North America 2014; 37: 257– 267. [DOI] [PubMed] [Google Scholar]
- 17. Nakamae T. [ Novel treatment strategies for refractory patients with obsessive-compulsive disorder]. Seishin shinkeigaku zasshi = Psychiatria et neurologia Japonica 2013; 115: 997– 1003. [PubMed] [Google Scholar]
- 18. Grados MA, Specht MW, Sung HM, Fortune D. Glutamate drugs and pharmacogenetics of OCD: a pathway-based exploratory approach. Expert Opin Drug Discov 2013; 8: 1515– 1527. [DOI] [PubMed] [Google Scholar]
- 19. Fineberg NA, Brown A, Reghunandanan S, Pampaloni I. Evidence-based pharmacotherapy of obsessive-compulsive disorder. Int J Neuropsychopharmacol 2012; 15: 1173 – 1191. [DOI] [PubMed] [Google Scholar]
- 20. Grant P, Lougee L, Hirschtritt M, Swedo SE. An open-label trial of riluzole, a glutamate antagonist, in children with treatment-resistant obsessive-compulsive disorder. J Child Adolesc Psychopharmacol 2007; 17: 761– 767. [DOI] [PubMed] [Google Scholar]
- 21. Grant PJ, Joseph LA, Farmer CA, Luckenbaugh DA, Lougee LC, Zarate CA, Jr, et al. 12-week, placebo-controlled trial of add-on riluzole in the treatment of childhood-onset obsessive-compulsive disorder. Neuropsychopharmacology 2014; 39: 1453– 1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bakhla AK, Verma V, Soren S, Sarkhel S, Chaudhury S. An open-label trial of memantine in treatment-resistant obsessive-compulsive disorder. Ind Psychiatry J 2013; 22: 149– 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hosenbocus S, Chahal R. Memantine: a review of possible uses in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry 2013; 22: 166– 171. [PMC free article] [PubMed] [Google Scholar]
- 24. Haghighi M, Jahangard L, Mohammad-Beigi H, Bajoghli H, Hafezian H, Rahimi A, et al. In a double-blind, randomized and placebo-controlled trial, adjuvant memantine improved symptoms in inpatients suffering from refractory obsessive-compulsive disorders (OCD). Psychopharmacology (Berl) 2013; 228: 633– 640. [DOI] [PubMed] [Google Scholar]
- 25. Ghaleiha A, Entezari N, Modabbernia A, Najand B, Askari N, et al. Memantine addon in moderate to severe obsessive-compulsive disorder: randomized double-blind placebo-controlled study. J Psychiatr Res. 2013; 47: 175– 180. [DOI] [PubMed] [Google Scholar]
- 26. Stewart SE, Jenike EA, Hezel DM, Stack DE, Dodman NH, Shuster L, et al. A single-blinded case-control study of memantine in severe obsessive-compulsive disorder. J Clin Psychopharmacol 2010; 30: 34– 39. [DOI] [PubMed] [Google Scholar]
- 27. Egashira N, Shirakawa A, Abe M, Niki T, Mishima K, Iwasaki K, et al. N-acetyl-L-cysteine inhibits marble-burying behavior in mice. J Pharmacol Sci 2012; 119: 97– 101. [DOI] [PubMed] [Google Scholar]
- 28. Xia J, Du Y, Han J, Liu G, Wang X. D-cycloserine augmentation in behavioral therapy for obsessive-compulsive disorder: a meta-analysis. Drug Des Devel Ther 2015; 9: 2101– 2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chasson GS, Buhlmann U, Tolin DF, Rao SR, Reese HE, Rowley T, et al. Need for speed: evaluating slopes of OCD recovery in behavior therapy enhanced with d-cycloserine. Behav Res Ther 2010; 48: 675– 679. [DOI] [PubMed] [Google Scholar]
- 30. Berlin HA, Koran LM, Jenike MA, Shapira NA, Chaplin W, Pallanti S, et al. Double-blind, placebo-controlled trial of topiramate augmentation in treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry 2011; 72: 716– 721. [DOI] [PubMed] [Google Scholar]
- 31. Reid JG, Gitlin MJ, Altshuler LL. Lamotrigine in psychiatric disorders. J Clin Psychiatry. 2013; 74: 675– 684. [DOI] [PubMed] [Google Scholar]
- 32. Italiano D, Perucca E. Clinical pharmacokinetics of new-generation antiepileptic drugs at the extremes of age: an update. Clin Pharmacokinet 2013; 52: 627– 645. [DOI] [PubMed] [Google Scholar]
- 33. Kumar TC, Khanna S. Lamotrigine augmentation of serotonin re-uptake inhibitors in obsessive-compulsive disorder. Aust N Z J Psychiatry 2000; 34: 527– 528. [DOI] [PubMed] [Google Scholar]
- 34. Arrojo-Romero M, Tajes Alonso M, de Leon J. Lamotrigine augmentation of serotonin reuptake inhibitors in severe and long-term treatment-resistant obsessive-compulsive disorder. Case Rep Psychiatry 2013; 2013: 612459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, et al. The Yale-Brown Obsessive Compulsive Scale II. Validity. Arch Gen Psychiatry 1989; 46: 1012– 6. [DOI] [PubMed] [Google Scholar]
- 36. Poyurovsky M, Glick I, Koran LM. Lamotrigine augmentation in schizophrenia and schizoaffective patients with obsessive-compulsive symptoms. J Psychopharmacol 2010; 24: 861– 866. [DOI] [PubMed] [Google Scholar]
- 37. Zavodnick AD, Ali R. Lamotrigine in the treatment of unipolar depression with and without comorbidities: a literature review. Psychiatr Q 2012; 83: 371– 383. [DOI] [PubMed] [Google Scholar]
- 38. Afshar H, Akuchekian SH, ahaky B, Zarean E. Topiramate augmentation in refractory obsessive-compulsive disorder: A randomized, double-blind, placebo-controlled trial. J Res Med Sci 2014; 19: 976– 981. [PMC free article] [PubMed] [Google Scholar]
- 39. Damien F, Ted K, Franklin M, Fabrizio B. Placebo Effects: Biological, Clinical and Ethical Advances. Lancet 2010; 375: 686– 695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang XQ, Xiong J, Xu WH, Yu SY, Huang XS, Zhang JT, et al. Risk of a lamotrigine-related skin rash: current meta-analysis and postmarketing cohort analysis. Seizure 2015; 25: 52– 61. [DOI] [PubMed] [Google Scholar]
- 41. Pallanti S, Grassi G. Pharmacologic treatment of obsessive-compulsive disorder comorbidity. Expert Opin Pharmacother 2014; 15: 2543– 2552. [DOI] [PubMed] [Google Scholar]
- 42. Woody E, Szechtman H. Motivation, time course, and heterogeneity in obsessive-compulsive disorder. Psychological Review 2005; 112: 658– 661. [DOI] [PubMed] [Google Scholar]
- 43. Watson HJ, Rees CS. Meta-analysis of randomized, controlled treatment trials for pediatric obsessive-compulsive disorder. J Child Psychol Psychiatry 2008; 49: 489– 498. [DOI] [PubMed] [Google Scholar]
- 44. Bandelow B. The medical treatment of obsessive-compulsive disorder and anxiety. CNS Spectr 2008; 13: 37– 46. [DOI] [PubMed] [Google Scholar]
- 45. Amerio A, Stubbs B, Odone A, Tonna M, Marchesi C, Ghaemi SN. The prevalence and predictors of comorbid bipolar disorder and obsessive-compulsive disorder: A systematic review and meta-analysis. J Affect Disord 2015; 186: 99– 109. [DOI] [PubMed] [Google Scholar]
- 46. Geddes JR, Calabrese JR, Goodwin GM. Lamotrigine for treatment of bipolar depression: independent meta-analysis and meta-regression of individual patient data from five randomised trials. Br J Psychiatry 2009; 194: 4– 9. [DOI] [PubMed] [Google Scholar]
- 47. Jeong JH, Lee JG, Kim MD, Sohn I, Shim SH, Wang HR, et al. Korean Medication Algorithm for Bipolar Disorder 2014: comparisons with other treatment guidelines. Neuropsychiatr Dis Treat 2015; 11: 1561– 1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang HR, Woo YS, Bahk WM. Anticonvulsants to treat post-traumatic stress disorder. Hum Psychopharmacol 2014; 29: 427– 433. [DOI] [PubMed] [Google Scholar]