Abstract
Purpose
Fenretinide and tamoxifen have additive antitumor effects preclinically. We performed a randomized, placebo-controlled, double-blind adjuvant trial in breast cancer patients treated for five years with tamoxifen, with or without fenretinide.
Patients and methods
Between October 1995 and October 1999, 426 postmenopausal women with hormone receptor-positive breast cancer were randomized. Patients were monitored for efficacy and toxicity.
Results
Four hundred nineteen patients were evaluable. The study was terminated early due to slow accrual. There were no significant differences between treatment groups in DFS, TTR or survival. More patients stopped treatment early on the fenretinide arm than on placebo (p=.02). Grade 3/4 toxicities, including visual problems and musculoskeletal complaints were more common in patients receiving fenretinide (p=.007). A Night Blindness Questionnaire was used to monitor nyctalopia, which was slightly, but not significantly, more common on fenretinide.
Conclusions
In this underpowered study, no significant difference was observed in efficacy between treatment groups. This trial provides important toxicity information about fenretinide, a retinoid which has been used in the prevention setting, because it is the only placebo-controlled, double-blind randomized study ever performed.
Introduction
Adjuvant tamoxifen significantly reduces the risk of breast cancer recurrence.1 It is orally administered and well tolerated.
Retinoids are natural and synthetic analogues of vitamin A. Their properties, including inhibition of cellular proliferation and growth factor synthesis, induction of differentiation, and cytostatic activity, have stimulated interest in using these compounds for the treatment of malignancies. Retinamides are synthetic retinoids which have been modified to increase target organ specificity, augment anticarcinogenic activity and reduce toxicity.2
Fenretinide, a semisynthetic retinamide, has significant antitumor activity in rat carcinogenesis models. In human and animal models, fenretinide accumulates in breast tissue and has high activity and low toxicity when compared to other retinoids.3 High-dose fenretinide significantly reduces the incidence of carcinogen-induced rat mammary cancers4 and induces remission of established mammary cancers.5
Furthermore, fenretinide suppresses recurrence of mammary cancer after primary tumor removal5 and inhibits progression of ductal hyperplastic lesions and ductal carcinoma in situ.7 A favorable interaction between tamoxifen and fenretinide has been demonstrated in preclinical models of carcinogen-induced rat mammary cancer.8
Fenretinide may have anti-tumor activity in humans also. In a randomized phase III trial of fenretinide vs. no further treatment after surgical resection, fenretinide reduced the occurrence of ipsilateral and contralateral breast cancer in premenopausal women.9
The chemopreventive activities of some retinoids, including fenretinide, are enhanced by hormonal maneuvers such as oophorectomy,10,11 the addition of bromocryptine12 or tamoxifen.8 Retinoids, specifically all-trans-retinoic acid, when combined with tamoxifen in a phase I/II trial in patients with potentially hormone responsive advanced breast cancer demonstrated responses in patients who had previously shown disease progression when treated with tamoxifen alone.13 A phase I/II trial of tamoxifen with or without fenretinide in women with hormone receptor-positive, previously untreated, metastatic breast cancer showed improvement or stabilization of disease in 12/15 patients and was well-tolerated.14
Preclinical studies suggest that the mechanism of action of fenretinide is through apoptosis and/or inhibition of insulin growth factor (IGF).15,16,17,18,19,20,21,22 Higher circulating insulin-like growth factor-I levels are associated with greater risk of developing subsequent breast cancer in premenopausal women.23 Both tamoxifen and fenretinide decrease plasma IGF-1 levels.24,25 Hence, the long term effects of fenretinide were studied by measuring levels of IGF-I and other biomarkers in women randomized to either fenretinide or no treatment. There was an association between decreased IGF-I levels in premenopausal women and reduction of second breast cancers.26
Tamoxifen and fenretinide are attractive agents for adjuvant treatment of breast cancer because of preclinical studies supporting additive benefit of the combination, oral route of administration, tolerability in phase II clinical trials, and the known efficacy of adjuvant tamoxifen. However, fenretinide has never been subjected to a placebo-controlled trial. The purpose of this study was to determine whether treatment with tamoxifen/fenretinide could improve disease-free survival (DFS) compared to tamoxifen/placebo. Secondary objectives included evaluations of overall survival (OS), time to recurrence (TTR) and toxicity. Postmenopausal women were chosen because of the unclear benefit of chemotherapy in addition to tamoxifen at that time the trial was initiated, between 1995 and 1999. Also, during this time, tamoxifen was the standard anti-hormonal treatment for postmenopausal breast cancer. It was assumed that older postmenopausal women would find this trial appealing due to the ease of administration of the study drug.
Materials and Methods
Patients
Eligible women were postmenopausal and had adenocarcinoma of the breast that was estrogen receptor (ER) positive or progesterone receptor (PgR) positive. Eligibility criteria included either pathologic T2 or T3 or pathologic N1 or N2, without evidence of M1.27 A sentinel lymph node biopsy was acceptable to establish nodal positivity. Axillary dissection was required only in clinically node positive patients. Tumors with focal microscopic dermal invasion or microscopic focal dermal lymphatic involvement were allowed. Synchronous invasive bilateral breast cancers were allowed. Both invasive primaries must have been positive for hormone receptors and at least one must have had positive nodes or primary tumor > 2 cm. Patients must have undergone surgical treatment for their breast cancer within 12 weeks prior to randomization. They were required to be ≥ 65 years, or under the age of 65, postmenopausal, and ineligible/inappropriate for or declined other active node positive adjuvant studies. Postmenopausal was defined as natural menopause (one year without menses), surgical menopause (bilateral oophorectomy), hysterectomy with one or both ovaries left behind and age > 60, or age ≤ 65 with postmenopausal FSH. If they were < 60 without surgical menopause and taking hormonal replacement therapy (HRT), then HRT must have been discontinued with FSH in the postmenopausal range at least 2 weeks after discontinuation. Eligible subjects should have had a life expectancy from the standpoint of non-cancer illness equal to or exceeding 7 years (patients > 85 years old generally did not meet this criterion), adequate hematologic, renal and hepatic function and written informed consent.
In May 1998, the ECOG Data Monitoring Committee (DMC) activated protocol changes based on safety information to simplify the study and improve accrual. The requirement for axillary dissection in patients who were clinically node negative was dropped, and clinically or pathologically node negative patients were allowed to enroll if their primary tumors were > 2 cm in diameter. The original protocol required the completion of a Night Blindness Questionnaire (NBQ) prior to entry in the study (appendix 1). Patients who answered TRUE to any of the questions had to have a negative electroretinography (ERG) exam to be eligible for entry. The NBQ and ERG requirements were dropped as were annual eye examinations. The original protocol had excluded patients with serum cholesterol above 350 mg% or with triglycerides above 210 mg%. These requirements were also removed.
Exclusion criteria included clinical or pathological T4 and clinical N2 tumors,27 preoperative arm edema, prior hormonal or chemotherapy for breast cancer (except for < 1 month of tamoxifen prior to randomization), previous or concurrent malignancy (except inactive non-melanoma skin cancer, in situ cervical cancer, or a disease-free interval of > 10 years from previous cancers), extensive signs of macular degeneration (as reported in a baseline eye examination by an ophthalmologist performed within 1 year prior to study entry), prior history of invasive breast cancer, (prior non-invasive breast cancer was allowed) and consumption of vitamin A supplements while on study.
Treatment
Patients were randomized to receive either 5 years tamoxifen (20 mg/d) and fenretinide or tamoxifen/placebo. Fenretinide/placebo was taken as 4 capsules (100mg of fenretinide per capsule) every night. There was a 3 day fenretinide/placebo holiday each month to minimize nyctalopia.
Lumpectomy patients were required to receive radiation therapy (RT) according to the accepted standards of medical care, and mastectomy patients could receive RT at the discretion of the investigator. Therapy with fenretinide/placebo was started within 2 weeks of completiing RT or within 2 weeks of study entry for those patients not receiving RT. The start of fenretinide/placebo could not be delayed > 10 weeks after randomization. Tamoxifen was started after randomization, even if the start of fenretinide/placebo was delayed for RT.
Statistical Methods
The primary endpoint of the study was DFS, defined as the time from randomization to the earliest of breast cancer recurrence, new primary breast cancer, or death without recurrence. Patients disease-free and alive were censored at the date last known to be alive. Secondary endpoints included overall survival (the time from randomization to death from any cause), and time to recurrence (TTR). TTR was the time from randomization to either breast cancer recurrence or a new primary breast cancer. For patients without a breast cancer event, TTR was censored at the date last reported to be disease-free.
This was a double blind study. Randomization was performed using permuted blocks within strata with dynamic balancing within main institutions and their affiliate networks.28 The stratification factors were age (< 70 vs. ≥ 70), node dissection (yes vs. no), involved nodes (1-3 vs. 4+ vs. no dissection vs. no involved nodes), and nodes removed (< 6 or no dissection vs. > 6). The node dissection factor was added with the protocol changes in May 1998, and prior to this, the involved nodes factor only included the levels 1-3 vs. 4+.
This study was designed to be able to detect a 33% reduction in the recurrence hazard from the addition of fenretinide to tamoxifen alone. Allowing for the dilution of this difference in the DFS endpoint from deaths without recurrence and making a modest allowance for noncompliance, the original design required entering 1,500 patients, with the final analysis planned for when 509 DFS events had occurred, to give 82% power. The revised design, activated in May 1998, targeted the same relative reduction in recurrence rates and made the same allowances for deaths without recurrence and noncompliance, but the baseline event rates were adjusted to reflect the inclusion of some node-negative patients. To give > 81% power for a one-sided 2.5% logrank test, this design required 1,565 patients to provide a total of 550 events among eligible patients for the final analysis within a reasonable follow-up period, allowing up to 5% of the patients to be ineligible.
The distributions of censored event times were estimated using the Kaplan and Meier method.29 Comparisons of event times used the log rank test.30 The Cox proportional hazards model was used to estimate hazard ratios.31 Fisher's exact test was used to compare proportions. Two-sided p-values are reported. The term ‘significant’ is used if two-sided p-values are <0.05. The primary analysis was an intention-to-treat analysis of the eligible subset of patients. An intention to treat analysis of all randomized patients was also performed, and gave similar results.
In October 1999, the DMC recommended that accrual be terminated because of slow accrual and compliance problems. Fenretinide/placebo therapy was discontinued and study treatment was unblinded. Tamoxifen treatment was continued for 5 years.
Results
Patients
The study was opened on October 24, 1995, and closed prematurely on November 1, 1999. 426 patients were entered. The analysis for this report reflects data in the ECOG database as of December 20, 2003. The median follow-up of living patients was 5.1 years.
Of the 426 patients enrolled, seven were ineligible, leaving 419 cases for primary analysis. Two-hundred and six patients were randomized to tamoxifen/fenretinide and 213 to tamoxifen/placebo. Twenty-three randomized patients did not start fenretinide/placebo therapy. They were excluded in the toxicity analysis, but included in other analyses by their assigned treatment groups. Patient characteristics (Table 1) were well-balanced between the two treatment arms. Twenty-seven percent of the patients were <65 years old at the time of entry, 53% had primary tumors ≤ 2 cm and 94% were lymph node-positive. Of these, 68 had breast cancer recurrences or new primary breast cancers. Sixty-eight patients died, 27 without recurrence reported. Ninety-five events were available for comparison of DFS.
Table 1.
Patient Characteristics for Eligible Patients. Entries in %.
| Tam+Fenretinide | Tam+Placebo | |
|---|---|---|
| Factor | (n=206) | (n=213) |
|
| ||
| Race: White | 93.2 | 91.5 |
| Black | 3.9 | 5.2 |
| Hispanic | 1.5 | 1.4 |
| Other | 1.5 | 1.9 |
| Age: 44-64 | 28.2 | 26.3 |
| 65-69 | 21.4 | 22.1 |
| 70-74 | 27.7 | 28.6 |
| 75-86 | 22.8 | 23.0 |
| Prior HRT | 40.3 | 44.8 |
| Bilateral Breast Cancer | 1.5 | 1.9 |
| Primary Surgery: Mastectomy | 67.0 | 69.0 |
| Lumpectomy | 33.0 | 31.0 |
| Sentinel Node Biopsy | 3.9 | 6.6 |
| No Axillary Sampling | 0.0 | 0.9 |
| ER: Pos | 97.6 | 96.2 |
| PgR: Pos | 81.1 | 87.1 |
| Tumor Size: ≤2cm | 52.9 | 53.3 |
| 2.1-5.0cm | 44.7 | 44.3 |
| >5cm | 2.4 | 2.4 |
| # Nodes Examined: No dissec. | 0.0 | 0.9 |
| 1-10 | 26.7 | 25.8 |
| >10 | 73.3 | 73.2 |
| # Positive Nodes: No dissec. | 0.0 | 0.9 |
| 0 | 4.4 | 6.6 |
| 1-3 | 78.2 | 77.0 |
| 4+ | 17.5 | 15.5 |
1 case race unknown, 1 case missing tumor size, one case missing prior hormone therapy, 3 cases missing PgR
Duration of Treatment and Compliance
Fenretinide/placebo treatment was terminated for all patients as of November 1, 1999. Because of the early study termination, all patients received less fenretinide/placebo than had been planned. For 5 patients, the duration of fenretinide/placebo was not reported. For the other 414 patients, the median of the duration of treatment was 1.17 years (range 0-3.97 years) and 1.40 years (0-3.73 years) for those assigned to fenretinide and placebo respectively.
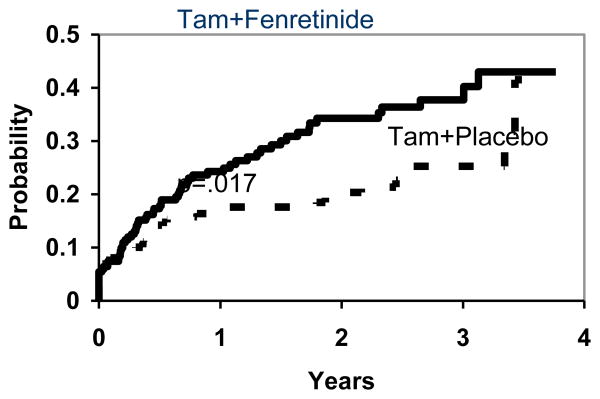
The cumulative rate of early termination of treatment was estimated. Patients who terminated treatment because of relapse or death were considered to have completed their assigned protocol therapy, and so are not counted as terminating early. The reasons for early termination are summarized in Table 2. Figure 1 shows that the estimated proportions of patients terminating treatment early was 38% and 25% by 3 years for those assigned to fenretinide and placebo respectively. Non-compliance rates were much higher than had been assumed in the design of this study.
Table 2.
Reasons for Stopping Fenretinide/Placebo Treatment. Entries in %.
| Tam+Fenretinide | Tam+Placebo | |
|---|---|---|
| Reason | (n=206) | (n=213) |
|
| ||
| Never Started | 5.3 | 5.6 |
| Relapse | 4.9 | 6.1 |
| Death | 0.5 | 0.5 |
| Patient Withdrew | 6.3 | 3.8 |
| Toxicity (Patient Refused) | 5.8 | 5.2 |
| Toxicity (MD Stopped RX) | 6.8 | 1.4 |
| Other Disease | 1.9 | 0.5 |
| Other | 2.4 | 1.4 |
| Unknown | 3.9 | 3.8 |
| Still On Rx at Study Termination | 62.1 | 71.8 |
Figure 1. Estimated Proportion Terminating Fenretinide/Placebo Treatment Early for Reasons Other than Recurrence or Death prior to Early Termination of the Study.
DFS, Survival and TTR
The primary endpoint of this study was DFS. There were 51 events on the tamoxifen/fenretinide arm and 44 events on the tamoxifen/placebo arm. The hazard ratio for DFS is 1.21 (95% CI 0.81-1.81). Table 3 gives the type of first DFS event, with all first events in the opposite breast considered ‘new primary breast cancers’ and those in the ipsilateral breast regarded as local recurrences. Hormone receptor status of the events was not recorded.
Table 3.
Type of DFS Event for Eligible Patients
| Tam+Fenretinide | Tam+Placebo | Combined | |
|---|---|---|---|
| Type | (n=206) | (n=213) | (n=419) |
|
| |||
| New Primary Breast Cancer | 7 | 2 | 9 |
| Recurrence, Local-Regional Only | 11 | 7 | 18 |
| Recurrence, Distant Involvement | 18 | 21 | 39 |
| Recurrence, Sites Unknown | 2 | 0 | 2 |
| Death Without Recurrence | 13 | 14 | 27 |
|
| |||
| Total | 51 | 44 | 95 |
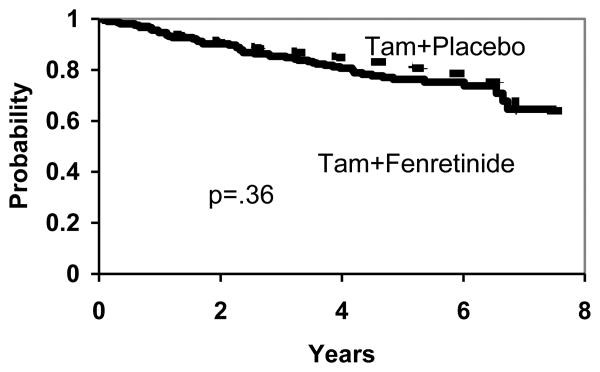
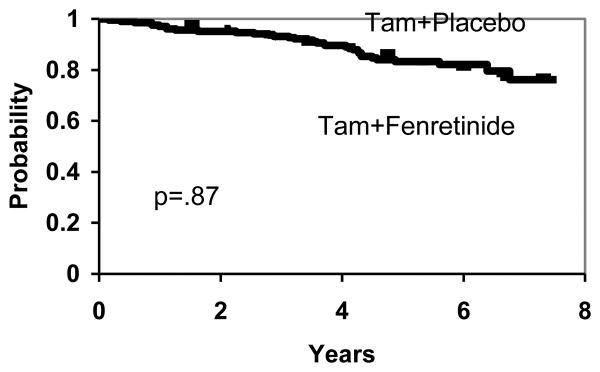
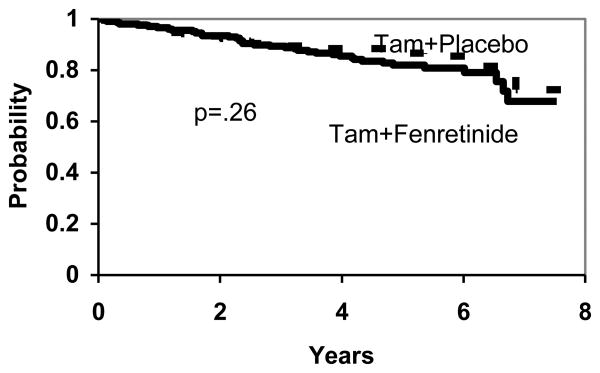
There are no significant differences between the two treatment arms in DFS (p=.36), (Figure 2), overall survival (p=.87), (Figure 3) and TTR (p=.26), (Figure 4). To the extent that any differences are present, outcomes are slightly worse with fenretinide, including new breast cancers.
Figure 2. DFS by Treatment.
Figure 3. Overall Survival by Treatment.
Figure 4. TTR by Treatment.
Toxicity
Degree of toxicity by treatment is given in Table 4. Patients assigned to tamoxifen/fenretinide had significantly more visual problems (p=.0001). Visual disturbances were reported in 27% of patients in the fenretinide group versus 11.8% in the control group. In the fenretinide group, 3.5% of the patients reported grade 3 visual toxicity versus none in the placebo group. A 3 day drug holiday was given monthly to minimize nyctalopia, but this did not eliminate symptoms. Conjunctivitis occurred in 2.5% of patients treated with fenretinide compared to 0.5% of patients given placebo. Burning eyes were reported in 5.0% of patients taking fenretinide vs. 3.5% of patients taking placebo. There was also a suggestion of higher rates of taste changes (p=.06) and neuro-motor toxicities (p=.08) in fenretinide-treated patients.
Table 4. Toxicity by Treatment (%).
| Type of Toxicity | Tam+Fenretinide | Tam+Placebo | ||||||
|---|---|---|---|---|---|---|---|---|
| Grade | Grade | |||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Vision problems | 15.0 | 8.5 | 3.5 | - | 7.4 | 4.4 | - | - |
| Burning eyes | 4.0 | 1.0 | - | - | 2.5 | 1.0 | - | - |
| Epigastric distress | 5.0 | 1.0 | - | - | 2.5 | 1.5 | - | - |
| Conjunctivitis | 1.5 | 1.0 | - | - | 0.5 | - | - | - |
| Hypertension | 3.5 | 1.5 | 3.5 | - | 6.9 | 1.0 | 3.9 | - |
| Non-Neuro dizziness | 1.5 | - | - | - | 3.9 | - | - | - |
| Musculoskeletal pain | 21.0 | 8.0 | 3.0 | 0.5 | 19.2 | 6.4 | - | - |
| Neuro-motor | 9.5 | 2.5 | 1.5 | - | 5.9 | 1.0 | 1.0 | - |
Comparing rates of grade 3/4 toxicities, only visual problems (p=.007) and musculoskeletal pain (p=.007) were significantly higher in the tamoxifen/fenretinide arm. No toxicities were significantly higher on tamoxifen and placebo. Four cases were coded as possible lethal toxicities. One patient on the placebo arm died of a heart attack. In the fenretinide arm, two patients died of CVA and one of cardiac-respiratory failure. Deaths without recurrence are summarized in Table 5 and second primary cancers for sites other than breast in Table 6.
Table 5.
Deaths Without Recurrence for Eligible Patients.
| Cause | Tam+Fenretinide | Tam+Placebo |
|---|---|---|
|
| ||
| Stroke/Cerebral Vascular Accident | 4 | 1 |
| Cardiac Arrest | 2 | 1 |
| Pancreatic Cancer | 1 | 3 |
| Lung Cancer | 1 | 2 |
| Myeloma | 1 | 0 |
| Pneumonia | 1 | 0 |
| Pulmonary Embolus | 0 | 1 |
| Pancreatitis | 1 | 0 |
| Alzheimer's Disease | 0 | 1 |
| Unknown | 2 | 5 |
|
| ||
| Total | 13 | 14 |
Table 6. Second Cancers (sites other than breast).
| Site | Tam+Fenretinide | Tam+Placebo |
|---|---|---|
|
| ||
| Colorectal | 6 | 3 |
| Pancreas | 1 | 3 |
| NHL | 1 | 1 |
| Myeloma | 1 | 1 |
| Lung | 2 | 2 |
| Melanoma | 1 | 1 |
| Other Skin | 4 | 8 |
| Ovarian | 1 | 1 |
| Endometrium | 0 | 2 |
| Renal | 0 | 1 |
| Bladder | 1 | 0 |
| Cervix | 0 | 1 |
| Other Gyn | 0 | 1 |
Night Blindness Questionnaire (NBQ)
An NBQ was used to screen patients for symptoms of nyctalopia (see appendix). The questionnaire was to be administered before study entry, and then every 4-6 months thereafter. If patients entered TRUE to any questions at baseline, then normal results on electroretinography (ERG) were required for eligibility. ERG was also required at any follow-up administration where a patient answered TRUE to a question to which she had answered FALSE at baseline. Initially, the NBQ had 6 items, however, it was shortened to 4 items in June 1996. All NBQ and ERG requirements were dropped in May 1998, based in part on the fact that no significant problems with nyctalopia had been observed.
Of the 262 cases with NBQ data, 6 did not start fenretinide/placebo therapy and 4 were ineligible. These cases were excluded from the following analyses, leaving 252 cases for analysis. From these, there were a total of 992 NBQs completed. As questions 1 and 3 were eventually excluded, only questions 2, 4, 5 and 6 are considered here. Of the 992 NBQs, 864 (87%) had responses to all 4 questions. Most of the missing responses (124) were for question 4. For the purposes of this analysis, missing responses are treated the same as FALSE responses. Overall, for the 992 questionnaires, question 2 was answered TRUE for 94/987 (9.5%), question 4 for 77/868 (8.9%), question 5 for 55/992 (5.5%), and question 6 for 53/991 (5.3%).
Five cases do not have a documented date that fenretinide/placebo ended. All follow-up questionnaires for these cases are analyzed as being during treatment. Fifteen of the follow-up NBQs from 14 patients were completed after fenretinide/placebo treatment was stopped. These NBQs are discussed separately below.
The results are summarized in Table 7 for the 977 NBQs performed prior to stopping treatment. A “change from baseline” means a TRUE answer to at least one question on a follow-up NBQ that had not had a TRUE response on the baseline NBQ. At baseline, 15/122 patients (12%) on tamoxifen/fenretinide and 11/130 patients (8%) on tamoxifen/placebo had TRUE responses to at least 1 question. After the baseline evaluations, the proportion of TRUE responses is slightly higher on the fenretinide arm, but the differences in the proportion of positive responses and in the proportion of cases with changes from baseline are not significantly different between the two arms in any of the time intervals. There were a total of 215 cases with follow-up NBQs at some point during treatment. Of these, 53 (25%) had changes from baseline, with 31/106 (29%) having changes on fenretinide and 22/109 (20%) on placebo. The difference in these overall rates was also not significant (p=.15).
Table 7. Summary of Night Blindness Questionnaire Results.
| Follow-up Interval (Months) | Tam+Fenretinide | Tam+Placebo | ||||||
|---|---|---|---|---|---|---|---|---|
| # Cases | # NBQs | # (%) Cases Pos. | # (%) Cases CFB | # Cases | # NBQs | # (%) Cases Pos. | # (%) Cases CFB | |
| Baseline | 122 | 122 | 15 (12) | -- | 130 | 130 | 11 (8) | -- |
| 2-5 | 88 | 90 | 17 (19) | 16 (18) | 97 | 99 | 16 (16) | 10 (10) |
| 6-9 | 82 | 85 | 15 (18) | 12 (15) | 80 | 82 | 12 (15) | 8 (10) |
| 10-13 | 64 | 69 | 14 (22) | 13 (20) | 57 | 60 | 6 (11) | 6 (11) |
| 14-17 | 48 | 52 | 9 (19) | 8 (17) | 44 | 49 | 4 (9) | 3 (7) |
| 18-21 | 34 | 35 | 8 (24) | 7 (21) | 36 | 37 | 5 (14) | 5 (14) |
| 22-25 | 19 | 20 | 4 (21) | 3 (16) | 18 | 20 | 4 (22) | 4 (22) |
| > 25 | 11 | 18 | 3 (27) | 3 (27) | 8 | 9 | 1 (12) | 1 (12) |
A case is positive (Pos.) if the response to any of the 4 questions is TRUE. CFB = Change From Baseline. A case has a CFB if a response of TRUE is given at a follow-up NBQ to a question that had not been answered TRUE at baseline.
There were also 15 NBQs from 14 cases (9 fenretinide, 5 placebo) which were completed after stopping treatment. The 5 placebo cases had no TRUE responses, while 3/9 cases who had received fenretinide had TRUE responses, 2 of which were changes from baseline.
Prior to May 1998, the protocol required that an ERG exam be performed when follow-up NBQ responses were a change from baseline. There were 104 NBQs which showed such changes, 83 of these were done prior to May 1998. Of these 83, ERG results are available for 23, while it was confirmed that ERG was not performed for 57, and the information is missing for 3. These 23 ERG exams were performed on 14 patients (8 fenretinide, 6 placebo). The 13 ERG exams on the 8 fenretinide patients were all normal. Three of the 10 evaluations on the 6 placebo patients were abnormal.
Discussion
The goal of this study was to evaluate the efficacy and safety of adjuvant therapy with fenretinide when combined with tamoxifen in postmenopausal patients with hormone receptor-positive breast cancer. Despite promising preclinical data, there were no differences in DFS, overall survival or TTR between the groups receiving fenretinide or placebo.
Limitations of this study include poor treatment compliance and early study termination due to slow accrual. The lack of compliance to an oral treatment regimen is notable in this population of women who were mainly elderly. Some patients had difficulty swallowing the fenretinide/placebo tablets.
With 95 DFS events, this study had approximately 80% power to detect a reduction of about 45% in the hazard rate between fenretinide and placebo. Given the limited period that most patients received fenretinide treatment and the rate of deaths from causes unrelated to breast cancer, it is highly unlikely that there would have been such a large difference in DFS between the assigned treatment groups. It is not surprising, then, that the results are not significant. It is therefore not possible to conclude that fenretinide does not have therapeutic benefit.
An interaction between hormonal status of our patients and fenretinide may have contributed to the results. Only postmenopausal women were enrolled. In another study of 5 years of fenretinide, a retrospective subset analysis showed a benefit in the subgroup of premenopausal patients with surgically resected stage I breast cancer or ductal carcinoma in situ, with reductions in the incidence of ipsilateral and contralateral breast cancer.9 In that trial, the preventative effects of fenretinide were associated with circulating levels of estrogen and progesterone. That trial showed that fenretinide decreased the levels of circulating insulin-like growth factor-I (IGF-1) in premenopausal women, whereas postmenopausal women did not have the same benefit.26 A large prospective study in premenopausal women showed that higher circulating insulin-like growth factor-I levels were associated with a higher risk of developing subsequent breast cancer.23 A randomized double blind 2 × 2 trial of low dose tamoxifen and fenretinide in premenopausal women with 5 year Gail risk ≥ 1.3, intraepithelial neoplasia or pT1mic/pT1a breast cancers showed that the combination of tamoxifen and fenretinide vs. placebo lowered IGF-1 levels but showed no reduction in breast cancer events. Both single agents did show a numerical reduction in the annual odds of breast cancer events.32 IGF-1 levels were not checked in our trial.
As this was the first randomized trial of fenretinide vs. placebo in postmenopausal women, the side effects of fenretinide could be adequately assessed. While there were some differences in side effect profiles between the fenretinide and placebo groups, treatment with the combination of tamoxifen/fenretinide was generally well tolerated. Of the side effects, only visual problems and musculoskeletal pain were significantly different between groups. The clinical side effects described by the patients taking fenretinide were generally mild and most of the patients recovered spontaneously. Only a minority of the patients discontinued treatment due to adverse effects.
Visual complaints, especially diminished dark adaptation, is an expected adverse effect of fenretinide due to reduction in plasma retinol levels.33,34 This trial used an NBQ composed of 6 questions to screen patients. However, 2 of the questions were dropped early in the study, and of the NBQs completed, only a minority had answers to those 2 questions. Also, the original protocol required that electroretinography (ERG) be done for any positive answers to the questions. The NBQ and the requirement for ERG were eventually dropped when it was realized that there were no significant problems with nyctalopia. Also, despite the ERG requirements, only a small number were actually performed. Interestingly, all of those done on the fenretinide patients were normal. These results are consistent with the safety analysis from the clinical trial using fenretinide to prevent contralateral breast cancer, in which the positive predictive value of the questionnaire was only 8% for an abnormal ERG.34 Therefore, in our study, the NBQ may have been cumbersome for the patients to use, without providing much useful data on follow-up ophthalmologic examinations.
In conclusion, our trial did not show a beneficial effect of adjuvant therapy with the combination of fenretinide and tamoxifen in postmenopausal women. Toxicities of fenretinide were discovered and compliance to a simple, outpatient oral regimen was suboptimal in this mostly elderly cohort.
Acknowledgments
This study was conducted by the Eastern Cooperative Oncology Group (Robert L. Comis, M.D., Chair) and supported in part by Public Health Service Grants CA23318, CA66636, CA21115, CA47559, CA07968, CA04919, CA25224, andCA37404 and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Appendix 1: Night Blindness Questionnaire
Initially, the NBQ consisted of the 6 questions below. Patients could answer TRUE or FALSE to each. In 1996, items 1 and 3 were dropped.
I see better in normal daylight than in the evening.
I don't adapt easily when going from a bright area to a dim one (for example, when driving into a tunnel during the daylight or going into a movie theater when a film is being shown).
I see poorly in the dark (for example, when getting up at night to go to the bathroom or looking for a light switch in the basement).
I have difficulty driving at night.
When I am in dim light, I can't distinguish the outlines of objects well.
When I pass from a dimly lit room or place to a strongly lit one, I am dazzled (for example, when leaving a tunnel ro leaving a subway station during daylight).
Footnotes
-
-Cobleigh M, Gray R, Graham M, et al: Fenretinide (FEN) vs. placebo in postmenopausal breast cancer patients receiving adjuvant tamoxifen (TAM), an Eastern Cooperative Oncology Group Phase III Intergroup Trial (EB193, INT-0151). Proc Am Soc Clin Oncol, 2000 (abstr)
References
- 1.Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 2.Cobleigh ML. Breast cancer and fenretinide, an analogue of vitamin A. Leukemia. 1994;8(suppl 3):S59–63. [PubMed] [Google Scholar]
- 3.Mehta RG, Moon RC, Hawthorne M, et al. Distribution of fenretinide in the mammary gland of breast cancer patients. Eur J Cancer. 1991;27:138–141. doi: 10.1016/0277-5379(91)90471-o. [DOI] [PubMed] [Google Scholar]
- 4.Moon RC, Thompson HJ, Becci PJ, et al. N-(4-hydroxyphenyl) retinamide, a new retinoid for prevention of breast cancer in the rat. Cancer Res. 1979;39:1339–1346. [PubMed] [Google Scholar]
- 5.Dowlatshahi K, Mehta R, Thomas C, et al. Therapeutic effect of N-(4-hydroxyphenyl) retinamide on N-methyl-N-nitrosurea-induced rat mammary cancer. Cancer Lett. 1989;47:187–192. doi: 10.1016/0304-3835(89)90089-x. [DOI] [PubMed] [Google Scholar]
- 6.Moon RC, Pritchard JF, Mehta RG, et al. Suppression of rat mammary cancer development by N-(4-hydroxyphenyl) retinamide (4-HPR) following surgical removal of first palpable tumor. Carcinogenesis. 1989;10:1645–1649. doi: 10.1093/carcin/10.9.1645. [DOI] [PubMed] [Google Scholar]
- 7.Green A, Shilkaitis A, Christov K. 4-(hydroxyphenyl) retinamide selectively inhibits the development and progression of ductal hyperplastic lesions and carcinoma in situ in mammary gland. Carcinogenesis. 1999;20:1535–1540. doi: 10.1093/carcin/20.8.1535. [DOI] [PubMed] [Google Scholar]
- 8.Ratko TA, Detrisac CJ, Dinger NM, et al. Chemopreventive efficacy of combined retinoid and tamoxifen treatment following surgical excision of a primary mammary cancer in female rats. Cancer Res. 1989;49:4472–4476. [PubMed] [Google Scholar]
- 9.Veronesi U, De Palo G, Marubini E, et al. Randomized trial of fenretinide to prevent secondary breast malignancy in women with early breast cancer. J Natl Cancer Inst. 1999;91:1847–1856. doi: 10.1093/jnci/91.21.1847. [DOI] [PubMed] [Google Scholar]
- 10.McCormick DL, Mehta RG, Thompson CA, et al. Enhanced inhibition of mammary carcinogenesis by combined treatment with 4-HPR and ovariectomy. Cancer Res. 1982;42:508–512. [PubMed] [Google Scholar]
- 11.McCormick DL, Sowell ZL, Thompson CA, et al. Inhibition by retinoid and ovariectomy of additional primary malignancies in rats following surgical removal of the first mammary cancer. Cancer. 1983;51:594–599. doi: 10.1002/1097-0142(19830215)51:4<594::aid-cncr2820510407>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.Welsch C, Brown C, Goodrich-Smith, et al. Synergistic effect of chronic prolactin suppression and retinoid treatment in the prophylaxis of N-methyl-N-nitrosurea-induced mammary tumorigenesis in female Sprague-Dawley rats. Cancer Res. 1980;40:3095–3098. [PubMed] [Google Scholar]
- 13.Budd GT, Adamson PC, Gupta M, et al. Phase I/II trial of all-trans-retinoic acid and tamoxifen in patients with advanced breast cancer. Clin Cancer Res. 1998;4:6635–6642. [PubMed] [Google Scholar]
- 14.Cobleigh MA, Dowlatshahi K, Deutsch TA, et al. Phase I/II Trial of tamoxifen with or without fenretinide, an analog of vitamin A, in women with metastatic breast cancer. J Clin Oncol. 1993;11:474–477. doi: 10.1200/JCO.1993.11.3.474. [DOI] [PubMed] [Google Scholar]
- 15.Lotan J. Retinoids and apoptosis: implications for cancer chemoprevention and therapy. J Natl Cancer Inst. 1995;87:1655–1657. doi: 10.1093/jnci/87.22.1655. [DOI] [PubMed] [Google Scholar]
- 16.Seewaldt VL, Johnson BS, Parker MB, et al. Expression of the retinoic acid receptor β mediates retinoic acid-induced growth arrest and apoptosis in breast cancer cells. Cell Growth Differ. 6:1077–1088. [PubMed] [Google Scholar]
- 17.Wang TT, Phang JM. Effect of N-(4-hydroxyphenyl) retinamide on apoptosis in human breast cancer cells. Cancer Lett. 1996;107:65–71. doi: 10.1016/0304-3835(96)04344-3. [DOI] [PubMed] [Google Scholar]
- 18.Sun SY, Li W, Yue P, et al. Mediation of N-(4-hydroxyphenyl) retinamide induced apoptosis in human cancer cells by different mechanisms. Cancer Res. 1999;59:2493–2498. [PubMed] [Google Scholar]
- 19.Srivastava RK, Srivastava AR, Cho-Chug YS, et al. Synergistic effects of retinoic acid and 8-chloro-adenosine3′,5′-cyclic monophosphate on the regulation of retinoic acid receptor β and apoptosis: involvement of mitochondria. Clin Cancer Res. 1999;5:1892–1904. [PubMed] [Google Scholar]
- 20.Oridate N, Lotan D, Xu XC, et al. Differential induction of apoptosis by all-trans-retinoic acid and N-(4-hydroxyphenyl) retinamide in human head and neck squamous cell carcinoma cell lines. Clin Cancer Res. 1996;2:855–863. [PubMed] [Google Scholar]
- 21.Poot M, Hosier S, Swisshelm K. Distinct patterns of mitochondrial changes precede induction of apoptosis by all-trans-retinoic acid and N-(4-hydroxyphenyl) retinamide in MCF7 breast cancer cells. Exp Cell Res. 2002;279:128–140. doi: 10.1006/excr.2002.5582. [DOI] [PubMed] [Google Scholar]
- 22.Favoni R, de Cupis A, Bruno S, et al. Modulation of the insulin-like growth factor-I system by N-(4-hydroxyphenyl)-retinamide in human breast cancer cell lines. Br J Cancer. 1998;77:2138–2147. doi: 10.1038/bjc.1998.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hankinson SE, Willet WC, Colditz GA, et al. Circulating concentrations of insulin-like growth factor I and risk of breast cancer. Lancet. 1998;351:1393–1396. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 24.Torrisi R, Parodi S, Fontana V, et al. Effect of fenretinide on plasma IGF-I and iGFBP-3 in early breast cancer patients. Int J Cancer. 1998;76:787–790. doi: 10.1002/(sici)1097-0215(19980610)76:6<787::aid-ijc3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 25.Decensi A, Veronesi U, Miceli R, et al. Relationships between plasma insulin-like growth factor-I and insulin-like growth factor binding protein-3 and second breast cancer risk in a prevention trial of fenretinide. Clin Cancer Res. 2003;9:4722–4729. [PubMed] [Google Scholar]
- 26.Decensi A, Johansson H, Miceli R, et al. Long-term effects of fenretinide, a retinoic acid derivative, on the insulin-like growth factor system in women with early breast cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:1047–1053. [PubMed] [Google Scholar]
- 27.American Joint Committee on Cancer. AJCC cancer staging manual. Philadelphia, PA: Lippincott-Raven; 1997. [Google Scholar]
- 28.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27:365–375. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan EL, Meier P. Nonparametric estimation of incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 30.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 31.Cox DR. Regression models and life tables. Journal of the Royal Statistical Society, Series B. 1972;34:187–220. [Google Scholar]
- 32.Decensi A, Robertson C, Guerrieri-Gonzaga A, et al. Randomized double-blind 2 × 2 trial of low-dose Tamoxifen and Fenretinide for breast cancer prevention in high-risk premenopausal women. J Clin Oncol. 2009;27:3749–3756. doi: 10.1200/JCO.2008.19.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mariani L, Formelli F, De Palo G, et al. Chemoprevention of breast cancer with fenretinide (4-HPR): Study of long term visual and ophthalmologic tolerability. Tumori. 1996;82:444–449. doi: 10.1177/030089169608200506. [DOI] [PubMed] [Google Scholar]
- 34.Camerini T, Mariani L, De Palo G, et al. Safety of the synthetic retinoid fenretinide: Long-term results from a controlled clinical trial for the prevention of contralateral breast cancer. J Clin Oncol. 2001;19:1664–1670. doi: 10.1200/JCO.2001.19.6.1664. [DOI] [PubMed] [Google Scholar]