Abstract
Major questions remain about how sex hormones influence human brain development and cognition. Studies in humans and animals suggest a strong impact of androgen on the structure and function of the medial temporal lobe (MTL) and striatum. Using voxel-based morphometry (DARTEL), we compared MTL and striatal structures in 13 [mean age (±s.d.) 12.7 ± 3.2 yr, mean bone age 14.8 ± 3.2 yr] boys with familial male precocious puberty (FMPP), characterized by early excess androgen secretion, and 39 healthy age-matched boys (mean age 14.3 ± 2.5 yr). The FMPP group showed significantly larger grey-matter volume (GMV) in parahippocampal and fusiform gyri as well as putamen relative to controls. By comparison, larger GMV for controls relative to patients was only apparent in the precentral gyrus. Exploratory regression analyses that examined the impact of age on the current findings revealed a significant increase of GMV in the putamen with age in patients suffering from excess androgen but not in controls. Finally, current levels of free testosterone were obtained in the patient group. Analyses revealed a significant negative association indicating that FMPP boys with low levels of bioavailable testosterone exhibited high GMV in the bilateral striatum. The findings suggest a critical influence of androgen on human brain development and are discussed in relation to male-dominant psychiatric childhood disorders.
Keywords: ADHD, brain development, FMPP, precocious puberty, sex steroid
Introduction
Major questions remain about the role of androgen in human brain development. Studies in animals have shown that early exposure to steroid hormones during sensitive periods of development leads to permanent, organizational changes in the structure of the nervous system (Phoenix et al. 1959; Romeo, 2003; Schulz et al. 2009). Later, exposure to gonadal steroid hormones can have activational effects, i.e. transient changes that facilitate certain behaviours such as reproduction (Sisk & Foster, 2004). Recently, extensive studies by Paus and colleagues (Paus et al. 2010; Perrin et al. 2008) and Peper et al. (2009) in healthy adolescents have reported an impact of gonadal steroids such as testosterone on brain development. Testosterone plays a role in emotional processing (Derntl et al. 2009; Mueller et al. 2009), depression (Amore et al. 2009), anxiety (Fernandez-Guasti & Martinez-Mota, 2005), aggression (Olweus et al. 1980), cognition (Maki et al. 2007; Moffat et al. 2002) and addictive behaviors (Kuhn et al. 2010). In parallel, androgens have also been implicated in neurodevelopmental disorders that predominate in boys, such as autism spectrum disorders (Baron-Cohen, 2002), conduct disorder (van Goozen et al. 1998) or Tourette’s syndrome (Peterson et al. 1992). A better understanding of the role of androgens on the development of brain structures, and on emotion and cognition, may help to clarify underlying mechanisms of aberrant behaviours emerging early in life.
Several lines of evidence have suggested a critical impact of androgens on medial temporal lobe (MTL) structures and striatum. Regarding the MTL, sex steroid receptors are abundant in the amygdala and hippocampus (Abdelgadir et al. 1999; Beyenburg et al. 2000; Simerly et al. 1990). Functional neuroimaging studies have demonstrated that testosterone treatment in older adults alters neural activation in the ventromedial temporal cortex concurrent with decreases in verbal memory (Maki et al. 2007). Patients with congenital adrenal hyperplasia (CAH), a disorder of both androgen excess and cortisol deficiency (Merke & Bornstein, 2005), exhibit impaired emotional memory, particularly for negative visual stimuli (Maheu et al. 2008) and show perturbed emotional face processing (Mazzone et al. in press). Consistent with these data, structural imaging studies in endocrinological disorders of androgen dysfunction indicate perturbations in MTL structures (Itti et al. 2006; Merke et al. 2003). For example, male youths and adults with chronic testosterone deficit have been documented to exhibit reductions in temporal lobe volume (Giedd et al. 2007; Itti et al. 2006). Finally, much research in patients with (medial) temporal lobe epilepsy has suggested a tight connection among changes within MTL structures, testosterone level, sex prevalence rates of temporal lobe epilepsy, mood, and emotion processing (Funayama et al. 2001; Herzog, 1999; Kanner, 2008; Mejias-Aponte et al. 2002).
Regarding striatal function, administration of testosterone prior to metamphetamine challenge increased striatal dopamine depletion in male mice (Lewis & Dluzen, 2008). Administration of testosterone has also been linked to increased levels of concentration of dopamine in the striatum (Thiblin et al. 1999). In humans, dopamine release in the striatum of healthy volunteers differs between sex (Munro et al. 2006), and adult males with known basal ganglia deficits exhibit reduced levels of testosterone relative to healthy males (Markianos et al. 2005). Finally, in youths with male-dominant neurodevelopmental disorders, striatal dysfunction has been noted (Casey et al. 1997; Ernst et al. 1999; Teicher et al. 2000). These dysfunctions are consistent with studies that document structural perturbations in grey-matter volume (GMV) of this region, specifically the putamen, in attention deficit hyperactivity disorder (ADHD; Wang et al. 2007), pervasive developmental disorder (Langen et al. 2009; McAlonan et al. 2008), and Tourette’s syndrome (Ludolph et al. 2006).
Experimental hormonal manipulations during development in child and adolescent populations are prohibited by ethical considerations. By contrast, natural models of steroid dysfunction in humans may help in understanding the impact of androgen on brain development and cognition. An ideal example of such an endocrinological model is familial male precocious puberty (FMPP), or testotoxicosis, an extremely rare (prevalence rate up to 9 in 1 million, Orphanet), male-limited, genetic disorder. FMPP is characterized by isolated dysfunction of androgen secretion caused by an activating mutation of the luteinizing hormone (LH) receptor gene that results in continuous unregulated secretion of testosterone from the testicular Leydig cells (Latronico et al. 1995; Shenker et al. 1993). Boys with FMPP typically present very early signs of hyperandrogenism, including skeletal advancement, pubic hair, phallic enlargement, acne, and growth acceleration, with puberty starting around age 2–3 yr (Leschek, 2004). Treatment consists of anti-androgen medication that helps to restore growth velocity and bone maturation to normal prepubertal levels (Almeida et al. 2008). However, because of advanced growth acceleration and bone maturation, bone age rather than chronological age is commonly used in FMPP as an estimate of physiological maturation. Treatment is usually maintained throughout childhood and is often discontinued in adolescence (ages 11–13 yr) when hormonal levels in FMPP match typical levels (Leschek, 2004). At that time, pubertal development resumes and progresses in a normal fashion.
In a recent study of psychiatric diagnoses in endocrine disorders, ADHD was present in 44% of a sample of 18 FMPP patients (Mueller et al. in press). Such high incidence of ADHD is consistent with previous studies that reported symptoms of hyperactivity in isolated cases of FMPP (Reiter & Norjavaara, 2005; Weissenberger et al. 2001). Furthermore, motivated by evidence of the impact of testosterone on limbic MTL structures, we examined the ability of boys with FMPP to regulate emotion and found that they responded faster to fearful faces than unaffected peers (Mueller et al. 2009). This finding is consistent with recent pharmacological work also showing quicker response times to fearful faces during testosterone administration in healthy human adults (van Honk et al. 2005).
To our knowledge, no information on brain morphology in FMPP has been published at the present time. The aim of the current work is to address this question using voxel-based morphometry (VBM). VBM, a fully-automated technique, has become increasingly popular to examine GMV on a voxel-wise basis (Ashburner & Friston, 2000). It has been used successfully in developmental samples (Neufang et al. 2009; Peper et al. 2009) as well as a variety of disorders including, but not limited to, conduct disorder (De Brito et al. 2009), ADHD (Brieber et al. 2007), bipolar disorder (Dickstein et al. 2005), and autism (McAlonan et al. 2008). We hypothesized that excessive androgen levels early in life would be associated with perturbed GMV in structures of the MTL and striatum, based on (1) animal research on the organizational role of androgens on brain development (Romeo, 2003; Sisk et al. 2003) and the hippocampus (Hebbard et al. 2003; Isgor & Sengelaub, 2003); (2) previous findings of perturbed hippocampal function during an emotional processing task in patients with FMPP (Mueller et al. 2009); (3) a high rate of disruptive behavioural disorders in FMPP (Mueller et al. in press) that are associated with striatal dysfunction (Brieber et al. 2007; Casey et al. 1997; Ernst et al. 1999; McAlonan et al. 2008); and (4) a large body of literature on the involvement of androgens in temporal lobe epilepsy (Herzog, 1999; Kanner, 2008).
Methods
Subjects
Thirteen boys with FMPP [mean chronological age (±s.d.) 12.7 ± 3.2 yr, mean bone age 14.8 ± 3.2 yr, age at diagnosis ~4 yr) and 39 healthy boys (14.3 ± 2.5 yr) completed the study. Independent t tests revealed no significant differences between groups on age (t50 = 1.87, n.s.), bone age (t50 = −0.44, n.s.), Intelligence Quotient (IQ) (t50 = −1.92, n.s.) (Wechsler, 1999), or Tanner stage (t41 = 1.38, n.s.) (Marshall & Tanner, 1970) (Table 1). However, given that age influences the structural development of the brain (Gogtay et al. 2004), the effect of chronological age was controlled by using it as a covariate of nuisance in all analyses. The Edinburgh Inventory (Oldfield, 1971) was used to determine handedness. Of the FMPP group, one subject was left-handed and two were ambidextrous. Of the controls, two subjects were left-handed and two were ambidextrous (, n.s.). FMPP patients were recruited from an ongoing study of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. Control subjects were recruited by advertisement in local newspapers. The Institutional Review Board of the NIMH approved the study. Parents signed consent forms and adolescents signed assent forms after a detailed explanation of the study.
Table 1.
Demographic information for the patient and control groups
| Subject | FMPP (n = 13) Mean (s.d.) |
Control (n = 39) Mean (s.d.) |
|---|---|---|
| Age (yr) | 12.7 (3.2) | 14.3 (2.5) |
| Bone age (yr)a | 14.7 (3.2) | – |
| Age range (yr) | 9–19 | 9–19 |
| Age of diagnosis | – | |
| Sex | Male | Male |
| Intelligence Quotient | 109 (14.0) | 116 (10.4) |
| Aggression rating (LHA) | 8.4 (5.3) | – |
| Anxiety rating (SCARED) | 13.3 (6.8) | – |
| Tanner stageb | 3.9 (1.1) | 3.4 (1.0) |
| Testosterone total (ng/dl) | 379 (168) | – |
| Testosterone free (ng/dl) | 43 (41) | – |
| Height (cm) | 157.9 (19.0) | – |
| Weight (kg) | 57.6 (28.3) | – |
| Body mass index | 21.8 (5.7) | – |
FMPP, Familial male precocious puberty; LHA, Lifetime History of Aggression scale; SCARED, Screen for Child Anxiety Related Emotional Disorders.
Note that chronological age equals bone age in healthy youth.
Information for Tanner stage of nine control subjects is missing.
All subjects completed a physical, neurological and psychiatric assessment. Psychiatric status was assessed via a standardized, structured psychiatric interview (Kiddie Schedule for Affective Disorders and Schizophrenia – Present and Lifetime version, K-SADS-PL; Kaufman et al. 1997). Two children with FMPP met criteria for current ADHD, and one for oppositional defiant disorder (ODD) and ADHD. All other children were free of psychiatric disorders. The full-scale IQ scores were prorated based on the Vocabulary and Block Design subtests of the Wechsler Intelligence Scales for Children (Wechsler, 1999). All patients with FMPP had received treatment for their condition in the form of a combination of spironolactone (an anti-androgen) and testolactone (an aromatase inhibitor). Five of them were still on medication at the time of the present study. As it is standard practice to discontinue treatment around the time of typical puberty onset (11–13 yr), the remaining eight adolescents were not on treatment at the time of testing.
Materials
Hormonal assays
As part of the endocrine work-up of the FMPP patients, blood samples were taken at 08:00 hours, prior to medication in the cases of the treated patients, and within 1 or 2 d of the magnetic resonance imaging (MRI) study. Testosterone was measured with high-performance liquid chromatography/tandem mass spectrometry (nmol/l = 0.0347 × ng/dl) (Mayo Medical Laboratories, USA). No blood samples were taken from the healthy control group.
fMRI data acquisition
A whole brain, high-resolution T1-weighted anatomical image was acquired on a 3-T General Electric Signa Scanner (USA). Head movement was restricted using foam padding. For reasons independent of the present work, the MRI data were collected under two acquisition sequences. A first set of subjects (n = 23) were scanned using a magnetization-prepared rapid-acquisition gradient echo (MPRAGE) sequence consisting of 124 1.2-mm axial slices (no-gap), field of view (FOV) = 220 mm, matrix = 256 × 192 units, time to inversion (TI) = 725 ms, flip angle 6 degrees, and voxel size 0.86 mm × 1.15 mm × 1.2 mm. A second set of subjects (n = 29) were scanned using a fast spoiled gradient-recalled (FSPGR) imaging sequence consisting of 124 1.2-mm axial slices (no-gap), FOV = 240 mm, matrix = 256 × 256 units, flip angle 15 degrees and voxel size 0.94 mm × 0.94 mm × 1.2 mm. To avoid the potential confound of using two different acquisition sequences, the control and FMPP groups were equally distributed on both scan sequences (, n.s.). In addition, all results were controlled for scan sequence using this variable as a covariate of nuisance in MRI analyses.
fMRI processing
Using the Statistical Parametric Mapping (SPM) approach (Friston et al. 2007), VBM (Ashburner & Friston, 2000) was performed with SPM5 software (Wellcome Department of Imaging Neuroscience, University College of London, UK), Matlab 7 (The Mathworks Inc., USA), and the diffeomorphic anatomical registration using exponentiated Lie algebra (DARTEL) toolbox for VBM analysis. Following Ashburner (2007), DARTEL was implemented in its standard version. Prior to processing, data were examined for scanner artefacts and gross anatomical abnormalities. Subsequently, the image origin was set at the anterior commissure (AC). Then, structural imaging data were segmented into white-matter, grey-matter and CSF tissue classes. Data were warped to an isotropic voxel size of 1.5 mm × 1.5 mm × 1.5 mm. A study-specific brain template was created from all individual rigidly aligned tissue-class images of each subject. Subsequently, data were modulated by using Jacobian-transformed tissue probability maps. The modulation step was utilized to obtain ‘volume’ differences rather than ‘concentration’ differences in grey matter (Ashburner & Friston, 2000). Data were then checked for homogeneity across the sample and smoothed with a standard 8-mm isotropic full-width half-maximum (FWHM) Gaussian kernel. Finally, data were spatially normalized to the Montreal Neurologic Institute (MNI) coordinate reference system. These smoothed, modulated, normalized images were used for the statistical analysis.
VBM analysis
Based on our a-priori hypotheses, the primary statistical analyses were region-of-interest (ROI) based. A Gaussian random-field threshold was used to determine significance of statistical comparisons. Activation had to survive the small-volume correction (SVC) Gaussian random-field threshold (α = 0.05) within pre-specified ROIs. Two large ROIs were used: the first ROI consisted of the MTL and included the amygdala, hippocampus, parahippocampal gyrus and fusiform gyrus. The second ROI consisted of the striatum and included the caudate and putamen. MNI coordinates are reported for significant results within these ROIs. Anatomical ROIs were selected using the Wake Forest University (WFU) PickAtlas (Maldjian et al. 2003, 2004). In addition, in order to examine potential changes in other regions outside of the a priori ROIs, whole-brain analyses were conducted using a threshold of p < 0.001, uncorrected. The data were subjected to a 2 (group) × 2 (scan sequence) analysis of covariance with chronological age as covariate. In addition, the relatively wide age range of our sample (9–19 yr) allowed us to explore the effect of age × group on brain volume in ROIs using regression analyses. Finally, given that testosterone levels and anxiety and aggression ratings were also available for the patient group, additional correlations between GMV and these variables were conducted in that group only and corrected for multiple comparisons. The potential impact of medication status (on vs. off) or comorbid psychiatric conditions was examined using independent t tests.
Results
Patient characteristics
FMPP patients reported mean subjective anxiety ratings of 13.3 (s.d. = 6.8) and mean aggression ratings of 8.4 (s.d. = 5.3). Their height (157.9 cm, s.d. = 19.0), weight (57.6 kg, s.d. = 28.3), and body mass index (21.8, s.d. = 5.7) were all within the normal developmental range (Growth Charts, National Center for Health Statistics, USA). Mean total testosterone levels (379 ng/dl, s.d. = 168) were also well within the normal range for both the adjusted age (reference range 7–1200 ng/dl) and the Tanner stage (Tanner stages 3 and 4, reference range 26–1200 ng/dl). By contrast, free circulating levels of testosterone (43 ng/dl, s.d. = 41) were slightly above the norm (reference range 9–30 ng/dl).
ROI analyses
MTL
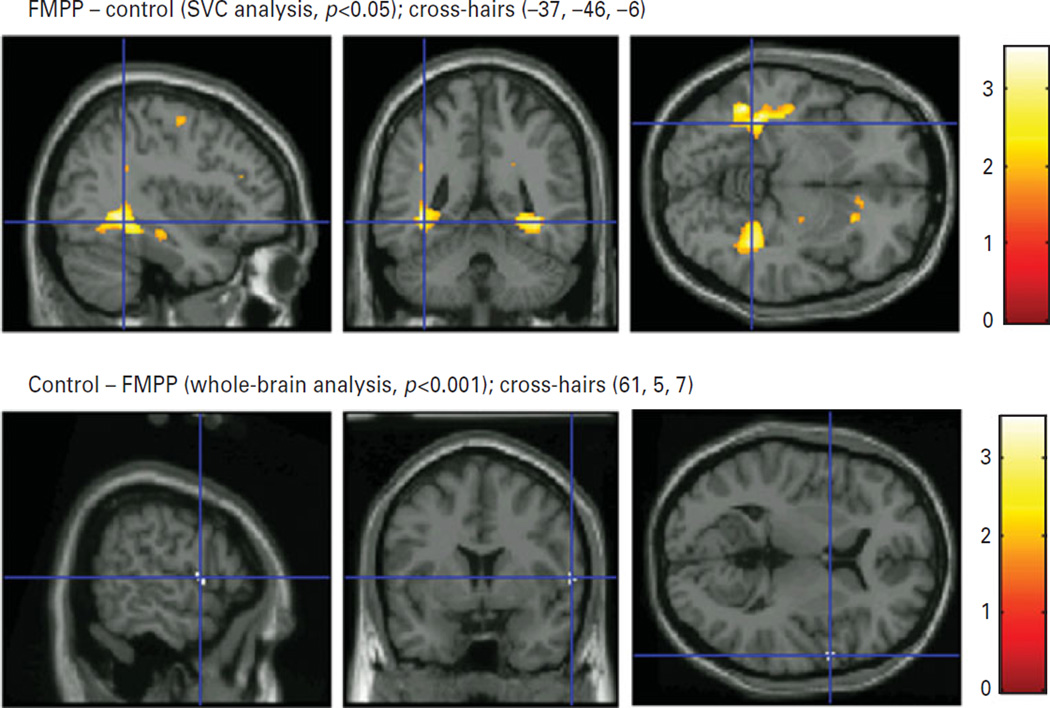
Significantly increased GMV for males with FMPP relative to healthy control males were found in a number of regions within the MTL, including the left (−37, −46, −6) and right (37, −47, −6) parahippocampus in Brodmann area (BA) 19, the left (−32, −20, −25) and right (33, −23, −23) parahippocampus in BA 36, and the left (−34, −38, −11) and right (−38, −9, 37) fusiform gyrus in BA 37 (Fig. 1 upper panel, Table 2). Group differences in bilateral amygdala or hippocampus volumes were not significant.
Fig. 1.
Upper panel. Increased grey-matter volume in familial male precocious puberty (FMPP) vs. controls for the parahippocampal and fusiform gyri. Lower panel. Decreased grey-matter volume in FMPP vs. controls for the precentral gyrus overlaid on a high-resolution anatomical T1 image in MNI space, provided by SPM. The figure displays sagittal (left), coronal (middle) and axial (right) slices.
Table 2.
Regions of group differences in grey-matter volume
| ROI analysis (SVC, p < 0.05) |
Brodmann area |
Cluster size k |
T value |
Equivalent Z value |
p (uncorr.) |
MNI coordinates x, y, z (mm) |
|---|---|---|---|---|---|---|
| FMPP – control | ||||||
| L parahippocampus | 19 | 293 | 2.90 | 2.76 | 0.003 | −37, −46, −6 |
| L parahippocampus | 36 | 2.70 | 2.59 | 0.005 | −32, −20, −25 | |
| L fusiform | 37 | 2.49 | 2.41 | 0.008 | −34, −38, −11 | |
| R parahippocampus | 19 | 215 | 3.18 | 3.01 | 0.001 | 37, −47, −6 |
| R parahippocampus | 36 | 6 | 1.78 | 1.75 | 0.04 | 33, −23, −23 |
| R fusiform | 37 | 2.69 | 2.58 | 0.005 | 37, −38, −9 | |
| Hippocampus (bilateral) | n.s. | |||||
| Amygdala (bilateral) | n.s. | |||||
| L putamen | 23 | 2.10 | 2.05 | 0.02 | −21, 14, 8 | |
| R putamen | 25 | 2.30 | 2.23 | 0.01 | 22, 16, 7 | |
| Caudate (bilateral) | n.s. | |||||
| Control – FMPP none | ||||||
| Whole brain (p < 0.001 uncorr.) | ||||||
| FMPP – control none | ||||||
| Control – FMPP | ||||||
| R precentral gyrus | 44 | 25 | 3.42 | 3.22 | 0.001 | 61, 5, 7 |
Group differences (FMPP vs. controls) for the small-volume corrected (SVC) region-of-interest (ROI) analyses (p < 0.05, corrected) are displayed in the upper half and group differences for the additional whole-brain analyses at p < 0.001 uncorrected are displayed in the lower half. x, y, z values represent Montreal Neurological Institute (MNI) coordinates in mm. The table also lists Brodmann areas, number of voxels of the significant cluster (cluster size), T and equivalent Z values, and the p value before correction. Regional peak loci within each significant cluster are reported.
Striatum
Within the striatum, a significantly greater volume in the left (−21, 14, 8) and right (22, 16, 7) putamen (Table 2) was observed in the FMPP group compared to the control group.
Whole-brain analysis
The whole-brain analysis revealed significantly smaller GMV in the right precentral gyrus in BA 44 for males with FMPP compared to controls (61, 5, 7) (Fig. 1 lower panel, Table 2). There were no other regional volume differences between FMPP and controls.
Exploratory age effect analyses
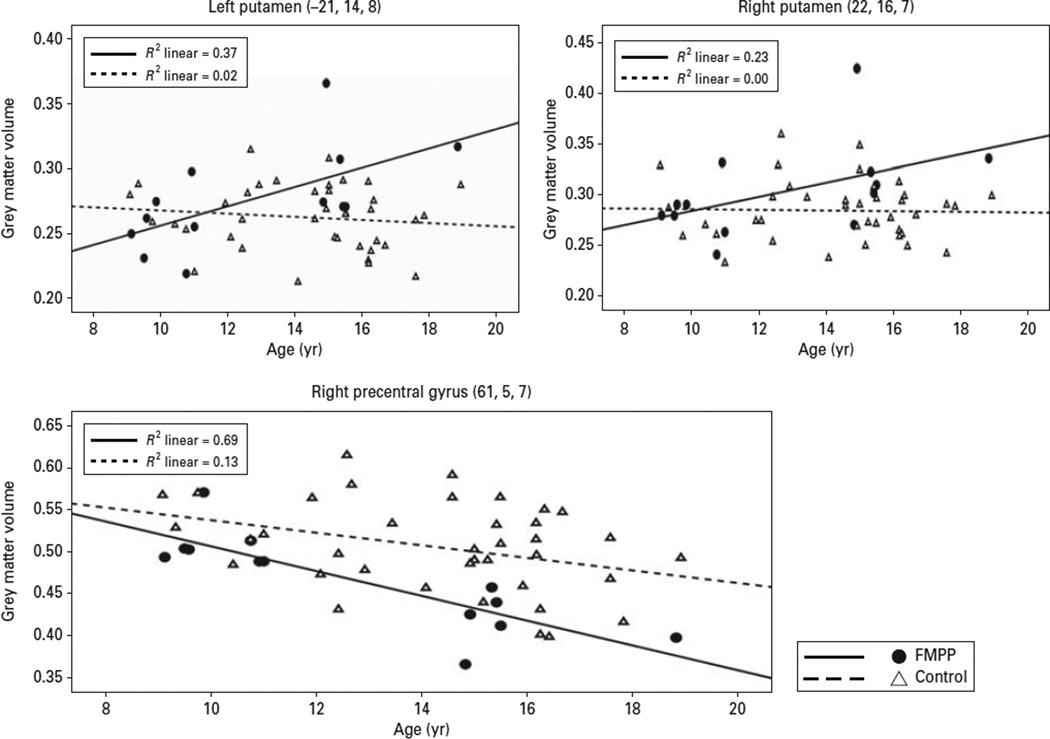
The regression analysis of age × group was conducted on the extracted significant peak voxels found in the a-priori ROI analyses using SPSS software (SPSS Inc., USA) (Table 2). A significant age × group interaction in the left putamen (β = 2.48, p = 0.006) showed significant increase in volume with age for the FMPP but no age-related effect (non-significant decrease) in controls (Fig. 2). A similar interaction between age and group was observed in the right putamen, although it was only marginally significant (β = 1.77, p = 0.052). By comparison, there was no significant influence of age as a main effect or in interaction with group in the fusiform gyrus or parahippocampus.
Fig. 2.
Upper panels. Regression analyses of the left and right putamen and the significant group × age interaction. The scatterplot shows that grey-matter volume increases with age in the familial male-precocious puberty (FMPP) group, but remains constant in the controls. Lower panel shows the main effect of age, revealing grey-matter loss with age for both groups in the right PFC.
A similar regression analysis was conducted on whole-brain VBM. This analysis revealed reduced GMV with age in the right precentral gyrus in both groups (β = 1.08, p = 0.02). This effect was not further qualified by a group effect or a group × age interaction (Fig. 2, lower panel).
GMV associations with current free testosterone levels, aggression, and anxiety
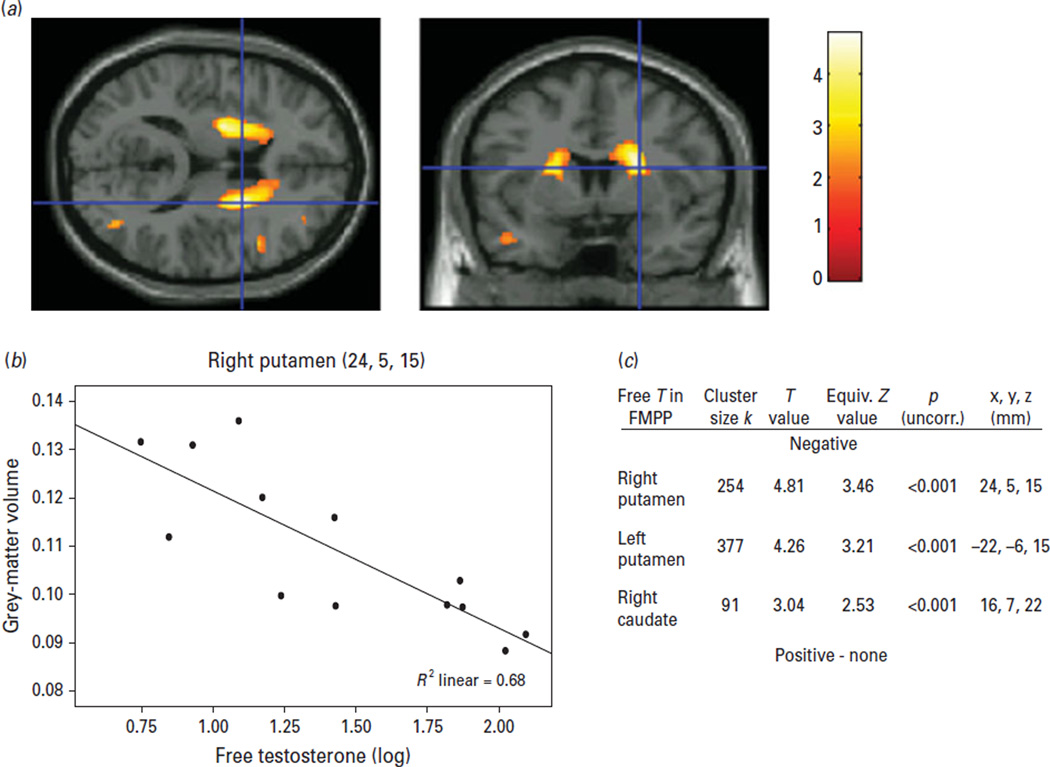
Given that free circulating testosterone levels were slightly elevated relative to the norm in patients, the potential impact of this measure on the findings in MTL and striatum was examined in FMPP. Strikingly, testosterone levels showed a significant (p < 0.05, corrected) bilateral negative correlation with GMV in the left (−22, −6, 15) and right (24, 5, 15) putamen as well as the right caudate (16, 7, 22) (Fig. 3). In other words, the higher the testosterone levels were, the smaller were these regional GMVs. No significant correlations were found between GMV and reported levels of aggression or subjective ratings of anxiety.
Fig. 3.
(a) Axial and coronal slice illustrating the significant correlation between free circulating testosterone and grey-matter volume (p < 0.05, corrected). Cross-hairs are positioned at MNI coordinates x = 24 mm, y = 5 mm, z = 15 mm. (b) Scatterplot with the mean regression line and individual data-points for the same coordinates as above in the right putamen. (c) Table with cluster size of significant correlation, T and Z values, p value before correction and MNI coordinates.
Exploratory associations with medication or comorbid illness
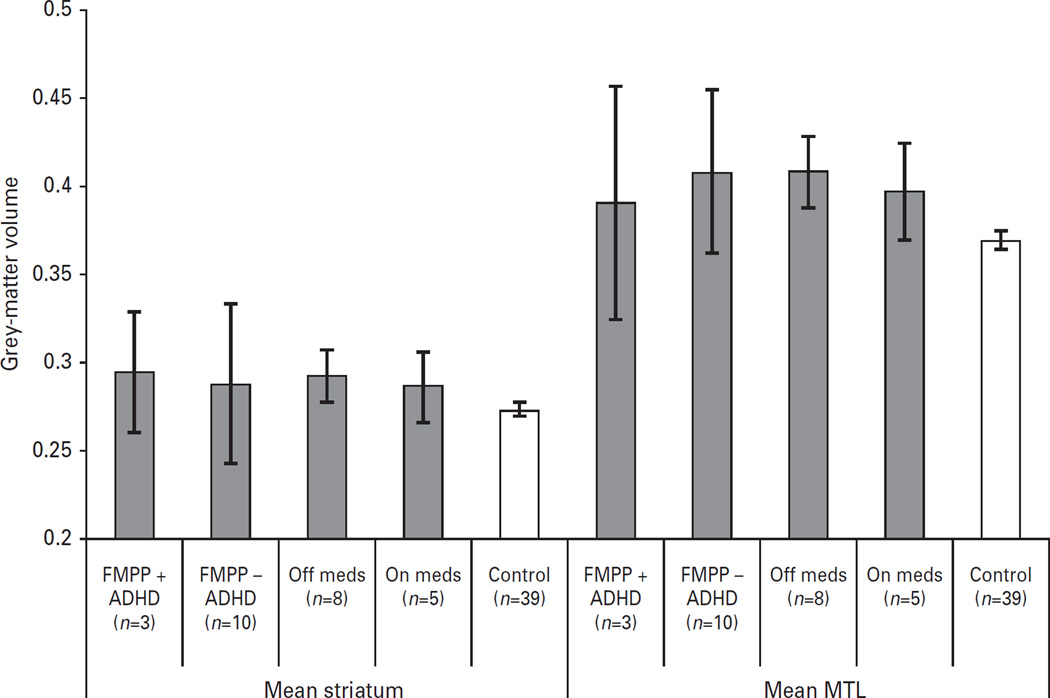
To examine the potential effect of current anti-androgen and aromatase inhibitor medication status, we compared the patients off medication (n = 8) to those on medication (n = 5) on our a-priori ROIs, i.e. the MTL and striatum. Similarly, to examine whether comorbid ADHD might have impacted the results, we compared patients with ADHD (n = 3) to those without ADHD (n = 10), and found no differences between the groups (all p values > 0.45). In addition, given the small sample size for comparison, GMV differences were explored visually and by means of effect sizes (Cohen’s d). The ADHD subgroup mean was within the mean of the patient sample and appeared to exert little effect (d = 0.17 for striatum, d = 0.32 for MTL) (Fig. 4). However, when the region within the precentral gyrus (pars opercularis) was compared between youths with vs. youths without ADHD, effect sizes were moderate (d = 0.47).
Fig. 4.
Mean volumes of striatum and medial temporal lobe in the control group and the familial male precocious puberty (FMPP) group are presented segregated along two factors : the presence (n = 3) or absence (n = 10) of ADHD, and the medication status (n = 5 on, and n = 8 off). This graph indicates that the group differences between FMPP and controls were not driven by diagnostic or medication status. Error bars denote s.e.m.
Discussion
The present work examined the effects of early androgen excess on brain GMV in boys suffering from an extremely rare genetic disorder of early testosterone hypersecretion (Shenker et al. 1993). Based on human and animal studies indicating an impact of androgens on MTL and striatal development and function, we hypothesized GMV perturbations in these regions. In line with expectations, findings revealed significant group differences in regional GMV within the MTL and striatum. Specifically, GMV of bilateral parahippocampus, bilateral fusiform gyrus, and bilateral putamen were larger in the FMPP group than in healthy controls. In contrast, GMV of the amygdala or hippocampus did not differ between groups. Abnormalities within the parahippocampus and fusiform gyrus suggest that the cognitive and affective functions associated with these structures might be impaired in the FMPP group, or, more generally, in early androgen dysfunction. Evidence is linking the parahippocampal gyrus with spatial navigation (Aguirre et al. 1996) and memory (Eichenbaum & Lipton, 2008); and the fusiform gyrus with face identification (Kanwisher et al. 1997) and social processes (Schultz et al. 2003). Based on the tight connections of parahippocampus and fusiform gyrus with the amygdala, these structures may also play a role in threat processes. Consistent with this hypothesis, we recently reported in a fMRI study that patients with FMPP processed fearful faces faster than controls concurrent with activations in MTL (Mueller et al. 2009) on an emotion discrimination task used to probe symptoms of anxiety disorders (e.g. Masten et al. 2008). Despite little information on the cognitive and affective characteristics of FMPP, the present findings can be contrasted with previous reports in other disorders of steroid abnormalities to gain a more comprehensive picture of the impact of sex steroids on abnormal brain development. For example, the finding of deviant facial threat discrimination in FMPP by Mueller et al. (2009) is in line with reports indicating abnormal attentional (Ernst et al. 2007) or memory (Maheu et al. 2008; Mazzone et al. in press) processing of negative faces in patients with congenital adrenal hyperplasia. Moreover, relative to unaffected controls, reduced mean temporal lobe volumes have been documented in youths with androgen deficiency due to Klinefelter’s syndrome (Giedd et al. 2007) and, although not significant, higher mean temporal lobe volumes have been documented in males with androgen excess (Merke et al. 2003). The current findings are consistent with these prior data.
The second main finding of the present study is the larger GMV of the putamen in patients with FMPP compared to controls. The hypothesized perturbation of striatal volume in FMPP was based on the heightened incidence rate of hyperactivity symptoms in patients with FMPP (Mueller et al. in press; Reiter & Norjavaara, 2005; Weissenberger et al. 2001), together with strong evidence linking ADHD with dopaminergic dysfunction (Casey et al. 1997; Ernst et al. 1999), as well as functional (Teicher et al. 2000) and structural (McAlonan et al. 2008; Wang et al. 2007) striatal abnormalities. In animal studies, testosterone has been shown to affect dopamine activity in the mesolimbic system (Hernandez et al. 1994), and the midbrain (Johnson et al. 2010), and to influence the development of dopamine neurons in the frontal cortex (Kritzer et al. 2007). Of note, enhanced GMV of the putamen in the FMPP group was not due to the three FMPP participants who carried a diagnosis of ADHD, suggesting that striatal perturbation represents a risk factor for ADHD rather than a direct causal contributor to ADHD. Moreover, our exploratory regression analyses revealed that the putamen, but not the MTL, showed an increase in GMV with age in FMPP patients but not controls, suggesting a differential effect of early sex steroid perturbation on the ontogeny of the striatal or dopamine system.
A striking negative correlation between GMV and bioavailable testosterone was found in the striatum in patients with FMPP. Although these results may appear contradictory to the main finding of increased GMV in FMPP patients relative to controls, we speculate that this finding may in fact mitigate a limitation of the study, i.e. the difficulty in distinguishing between organizational and activational effects. It is conceivable that the observed increased GMV in the patients represents an effect of early and/or childhood androgen exposure. With the onset of ‘normal’ puberty during adolescence when hormonal levels in FMPP are starting to match those of healthy adults, the effect by which hormones impact brain development could change. In fact, a recent study in healthy adults (Witte et al. 2010) has reported similar negative correlations of GMV with progesterone and testosterone levels in temporal and frontal lobes, respectively.
An alternative explanation could be that this effect is not directly related to testosterone but to other hormones in the cascade process of hormone-conversion or hormone-unrelated processes. For example, studies in rodents have shown an increase of circulating testosterone and striatal deficits after administration of an aromatase inhibitor (Mogami et al. 2008). Given that androgens are converted into oestrogens by aromatase, an intriguing possibility could be that some of the current effects may be oestrogen-related. Interestingly, in a small sample of healthy youth, Neufang et al. (2009) found positive correlations of GMV with oestrogen in the parahippocampal gyrus in females, while males showed positive correlations between testosterone and GMV in the parietal cortex and the diencephalon. More research is needed to clarify this possiblity.
Finally, whole-brain analyses provided additional, not predicted, leads to the study of sex steroid effects on brain development. In contrast to striatal and MTL structures, GMV in the precentral gyrus was smaller in FMPP patients than in controls. In fact, this region was the only area which seemed to be affected by the presence of ADHD in the FMPP sample. Thus, one possible interpretation could be an influence of comorbid ADHD on this region. Previous studies have documented reduced GMV in this same inferior region of the precentral gyrus in ADHD youths (Batty et al. 2010). The general GMV decrease with age in this region that did not differ between groups is further consistent with similar parallel reductions of prefrontal GMV in adolescent ADHD and healthy controls (Castellanos et al. 2002). This effect, although premature for interpretation, is important as it evidences that our main findings of increased regional GMV in FMPP are not part of a general effect, but rather are regionally specific to our a-priori ROIs. Moreover, consistent with prior data, these findings may indicate a sensitivity to comorbid psychopathology.
The findings of the current study may provide some clues on the mechanisms underlying psychopathology that predominates in males, such as externalizing disorders (Hicks et al. 2007) or pervasive developmental disorder (Fombonne, 2002). Indeed, some preliminary findings of abnormal GMV or grey-matter concentration have been reported in striatum and MTL in children with ADHD (Brieber et al. 2007; Castellanos et al. 2002; Wang et al. 2007), Asperger’s syndrome and high-functioning autism (McAlonan et al. 2008) or Tourette’s syndrome (Ludolph et al. 2006). The present findings may be important in suggesting a contribution of early androgen abnormalities to these pathologies.
A number of limitations need to be discussed. Most importantly, the relatively small sample size of the FMPP group restricted the statistical power of this study. However, the unique pathology of this group, characterized by a highly defined, clear-cut abnormality restricted to male androgen function, represents a fundamental stepping-stone to understanding the role of testosterone in development. The limitation of statistical power in this study warrants caution in interpreting negative findings. Although limited, this sample size represents the recruitment of patients among 1.5 million adolescents based on the FMPP estimated prevalence-rate of up to 9 in 1 million (Orphanet). Second, like most MRI studies, the data are correlational in nature and thus limited in determining the directionality of the effect. Third, as mentioned earlier, this study does not inform on the temporal trajectory of the impact of androgen abnormalities on brain development and does not allow distinguishing between the contribution of organizational vs. activational effects. In the current study, the average age of diagnosis for patients was 4 yr. Patients received treatment with spironolactone, an anti-androgen, which blocks the androgen receptor, and an aromatase inhibitor, which blocks the conversion of androgens to oestrogens. Because spironolactone blocks the androgen receptor, testosterone levels remain elevated in these patients (i.e. elevated free testosterone levels). Spironolactone does not efficiently cross the blood–brain barrier. Therefore, patients with FMPP have peripheral blockade of the effects of androgens (slowing of puberty) but testosterone levels remain elevated, and the effect of high testosterone on the brain is likely to remain. Relatedly, it cannot be ruled out that mutated LH receptors are exerting direct effects on the brain. Thus, in our patients, the effect of testosterone probably occurred throughout prepubertal development suggesting early and continuing impact of sex steroids. However, the negative correlation between GMV and bioavailable testosterone may indicate differences of early and childhood vs. later adolescent/adult effects. These data are consistent with findings from animal studies which also suggest several critical periods throughout development (Sisk et al. 2003). Animal studies or longitudinal studies in FMPP are needed to clarify these findings. Fourth, we could not fully examine the potential impact of medication on the current findings given the small sample size. However, the means of GMV changes of patients on medication were within the group mean of the patients without medication, suggesting little impact of current pharmacological treatment.
In summary, this is the first study, to our knowledge, that examines volumetric brain changes in FMPP, a gonadotropin-independent disorder of androgen excess. The study offers preliminary evidence for a critical role of early androgen on grey matter during brain development, particularly on structures that can affect motor (e.g. hyperactivity), cognitive (e.g. spatial orientation, memory) and affective (e.g. aggression, anxiety) functions. These findings may shed light on the pathophysiology of male-dominant psychiatric disorders with an onset in childhood. Finally, the present work may aid in the development of hypotheses that can be examined in animal models in the future.
Acknowledgments
This research was supported by the Intramural Research Programs of the National Institute of Mental Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institutes of Health Clinical Center.
We thank the three anonymous reviewers for their helpful and constructive comments on earlier versions of the manuscript. D.P.M. is a Commissioned Officer in the US Public Health Service. The views expressed in this article do not necessarily represent the views of the National Institute of Mental Health, National Institutes of Health, or the United States Government.
Statement of Interest
D.P.M. received research funds from Phoqus Pharmaceuticals during 2007–2008.
References
- Abdelgadir SE, Roselli CE, Choate JV, Resko JA. Androgen receptor messenger ribonucleic acid in brains and pituitaries of male rhesus monkeys: studies on distribution, hormonal control, and relationship to luteinizing hormone secretion. Biology of Reproduction. 1999;60:1251–1256. doi: 10.1095/biolreprod60.5.1251. [DOI] [PubMed] [Google Scholar]
- Aguirre GK, Detre JA, Alsop DC, D’Esposito M. The parahippocampus subserves topographical learning in man. Cerebral Cortex. 1996;6:823–829. doi: 10.1093/cercor/6.6.823. [DOI] [PubMed] [Google Scholar]
- Almeida MQ, Brito VN, Lins TS, Guerra-Junior G, et al. Long-term treatment of familial male-limited precocious puberty (testotoxicosis) with cyproterone acetate or ketoconazole. Clinical Endocrinology (Oxford) 2008;69:93–98. doi: 10.1111/j.1365-2265.2007.03160.x. [DOI] [PubMed] [Google Scholar]
- Amore M, Scarlatti F, Quarta AL, Tagariello P. Partial androgen deficiency, depression and testosterone treatment in aging men. Aging Clinical Experimental Research. 2009;21:1–8. doi: 10.1007/BF03324891. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry – the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. The extreme male brain theory of autism. Trends in Cognitive Sciences. 2002;6:248–254. doi: 10.1016/s1364-6613(02)01904-6. [DOI] [PubMed] [Google Scholar]
- Batty MJ, Liddle EB, Pitiot A, Toro R, et al. Cortical gray matter in attention-deficit/hyperactivity disorder : a structural magnetic resonance imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:229–238. doi: 10.1016/j.jaac.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyenburg S, Watzka M, Clusmann H, Blumcke I, et al. Androgen receptor mRNA expression in the human hippocampus. Neuroscience Letters. 2000;294:25–28. doi: 10.1016/s0304-3940(00)01542-1. [DOI] [PubMed] [Google Scholar]
- Brieber S, Neufang S, Bruning N, Kamp-Becker I, et al. Structural brain abnormalities in adolescents with autism spectrum disorder and patients with attention deficit/hyperactivity disorder. Journal Child Psychology and Psychiatry. 2007;48:1251–1258. doi: 10.1111/j.1469-7610.2007.01799.x. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Castellanos FX, Giedd JN, Marsh WL, et al. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. Journal of the American Medical Association. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- De Brito SA, Mechelli A, Wilke M, Laurens KR, et al. Size matters : increased grey matter in boys with conduct problems and callous-unemotional traits. Brain. 2009;132:843–852. doi: 10.1093/brain/awp011. [DOI] [PubMed] [Google Scholar]
- Derntl B, Windischberger C, Robinson S, Kryspin-Exner I, et al. Amygdala activity to fear and anger in healthy young males is associated with testosterone. Psychoneuroendocrinology. 2009;34:687–693. doi: 10.1016/j.psyneuen.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Milham MP, Nugent AC, Drevets WC, et al. Frontotemporal alterations in pediatric bipolar disorder : results of a voxel-based morphometry study. Archives of General Psychiatry. 2005;62:734–741. doi: 10.1001/archpsyc.62.7.734. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Lipton PA. Towards a functional organization of the medial temporal lobe memory system: role of the parahippocampal and medial entorhinal cortical areas. Hippocampus. 2008;18:1314–1324. doi: 10.1002/hipo.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Maheu FS, Schroth E, Hardin J, et al. Amygdala function in adolescents with congenital adrenal hyperplasia: a model for the study of early steroid abnormalities. Neuropsychologia. 2007;45:2104–2113. doi: 10.1016/j.neuropsychologia.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Zametkin A, Matochik JA, Pascualvaca D, et al. High midbrain DOPA decarboxylase activity in children in ADHD. American Journal of Psychiatry. 1999;156:1209–1215. doi: 10.1176/ajp.156.8.1209. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Martinez-Mota L. Anxiolytic-like actions of testosterone in the burying behavior test : role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrinology. 2005;30:762–770. doi: 10.1016/j.psyneuen.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Fombonne E. Epidemiological trends in rates of autism. Molecular Psychiatry. 2002;7(Suppl. 2):S4–S6. doi: 10.1038/sj.mp.4001162. [DOI] [PubMed] [Google Scholar]
- Friston K, Ashburner J, Kiebel S, Nichols T, et al., editors. Statistical Parametric Mapping: The Analysis of Functional Brain Images. London: Academic Press; 2007. [Google Scholar]
- Funayama ES, Grillon C, Davis M, Phelps EA. A double dissociation in the affective modulation of startle in humans: effects of unilateral temporal lobectomy. Journal of Cognitive Neuroscience. 2001;13:721–729. doi: 10.1162/08989290152541395. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Wallace GL, Lenroot RK, et al. XXY (Klinefelter Syndrome): a pediatric quantitative brain magnetic resonance imaging case-control study. Pediatrics. 2007;119:232–240. doi: 10.1542/peds.2005-2969. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbard PC, King RR, Malsbury CW, Harley CW. Two organizational effects of pubertal testosterone in male rats : transient social memory and a shift away from long-term potentiation following a tetanus in hippocampal CA1. Experimental Neurology. 2003;182:470–475. doi: 10.1016/s0014-4886(03)00119-5. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Gonzalez L, Murzi E, Paez X, et al. Testosterone modulates mesolimbic dopaminergic activity in male rats. Neuroscience Letters. 1994;171:172–174. doi: 10.1016/0304-3940(94)90632-7. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Psychoneuroendocrine aspects of temporolimbic epilepsy. Part I. Brain, reproductive steroids, and emotions. Psychosomatics. 1999;40:95–101. doi: 10.1016/S0033-3182(99)71254-5. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Blonigen DM, Kramer MD, Krueger RF, et al. Gender differences and developmental change in externalizing disorders from late adolescence to early adulthood: a longitudinal twin study. Journal of Abnormal Psychology. 2007;116:433–447. doi: 10.1037/0021-843X.116.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgor C, Sengelaub DR. Effects of neonatal gonadal steroids on adult CA3 pyramidal neuron dendritic morphology and spatial memory in rats. Journal of Neurobiology. 2003;55:179–190. doi: 10.1002/neu.10200. [DOI] [PubMed] [Google Scholar]
- Itti E, Gaw Gonzalo IT, Pawlikowska-Haddal A, Boone KB, et al. The structural brain correlates of cognitive deficits in adults with Klinefelter’s syndrome. Journal of Clinical Endocrinology and Metabolism. 2006;91:1423–1427. doi: 10.1210/jc.2005-1596. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Day A, Ho CC, David Walker Q, et al. Androgen decreases dopamine neurone survival in rat midbrain. Journal of Neuroendocrinology. 2010;22:238–247. doi: 10.1111/j.1365-2826.2010.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner AM. Mood disorder and epilepsy: a neurobiologic perspective of their relationship. Dialogues in Clinical Neuroscience. 2008;10:39–45. doi: 10.31887/DCNS.2008.10.1/amkanner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area, a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL) : initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Brewer A, Montalmant F, Davenport M, et al. Effects of gonadectomy on performance in operant tasks measuring prefrontal cortical function in adult male rats. Hormones and Behavior. 2007;51:183–194. doi: 10.1016/j.yhbeh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Kuhn C, Johnson M, Thomae A, Luo B, et al. The emergence of gonadal hormone influences on dopaminergic function during puberty. Hormones and Behavior. 2010;58:122–137. doi: 10.1016/j.yhbeh.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen M, Schnack HG, Nederveen H, Bos D, et al. Changes in the developmental trajectories of striatum in autism. Biological Psychiatry. 2009;66:327–333. doi: 10.1016/j.biopsych.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Latronico AC, Anasti J, Arnhold IJ, Mendonca BB, et al. A novel mutation of the luteinizing hormone receptor gene causing male gonadotropin-independent precocious puberty. Journal of Clinical Endocrinology and Metabolism. 1995;80:2490–2494. doi: 10.1210/jcem.80.8.7629248. [DOI] [PubMed] [Google Scholar]
- Leschek EW. Familial male-limited precocious puberty. The Endocrinologist. 2004;14:148–151. [Google Scholar]
- Lewis C, Dluzen DE. Testosterone enhances dopamine depletion by methamphetamine in male, but not female, mice. Neuroscience Letters. 2008;448:130–133. doi: 10.1016/j.neulet.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Ludolph AG, Juengling FD, Libal G, Ludolph AC, et al. Grey-matter abnormalities in boys with Tourette syndrome: magnetic resonance imaging study using optimised voxel-based morphometry. British Journal of Psychiatry. 2006;188:484–485. doi: 10.1192/bjp.bp.105.008813. [DOI] [PubMed] [Google Scholar]
- Maheu FS, Merke DP, Schroth EA, Keil MF, et al. Steroid abnormalities and the developing brain, declarative memory for emotionally arousing and neutral material in children with congenital adrenal hyperplasia. Psychoneuroendocrinology. 2008;33:238–245. doi: 10.1016/j.psyneuen.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Ernst M, London ED, Mordecai KL, et al. Intramuscular testosterone treatment in elderly men: evidence of memory decline and altered brain function. Journal of Clinical Endocrinology and Metabolism. 2007;92:4107–4114. doi: 10.1210/jc.2006-1805. [DOI] [PubMed] [Google Scholar]
- Maldjian J, Laurienti P, Burdette J. Precentral gyrus discrepancy in electronic versions of the talairach atlas. Neuroimage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Maldjian J, Laurienti P, Burdette J, Kraft RA. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Markianos M, Panas M, Kalfakis N, Vassilopoulos D. Plasma testosterone in male patients with Huntington’s disease : relations to severity of illness and dementia. Annals of Neurology. 2005;57:520–525. doi: 10.1002/ana.20428. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Archives of disease in childhood. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Guyer AE, Hodgdon HB, McClure EB, et al. Recognition of facial emotions among maltreated children with high rates of post-traumatic stress disorder. Child Abuse and Neglect. 2008;32:139–153. doi: 10.1016/j.chiabu.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone L, Mueller SC, Maheu FS, Pine DS, et al. Emotional memory in early steroid abnormalities. An fMRI study of adolescents with congenital adrenal hyperplasia. Developmental Neuropsychology. doi: 10.1080/87565641.2010.549866. (in press) [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Suckling J, Wong N, Cheung V, et al. Distinct patterns of grey matter abnormality in high-functioning autism and Asperger’s syndrome. Journal of Child Psychology and Psychiatry. 2008;49:1287–1295. doi: 10.1111/j.1469-7610.2008.01933.x. [DOI] [PubMed] [Google Scholar]
- Mejias-Aponte CA, Jimenez-Rivera CA, Segarra AC. Sex differences in models of temporal lobe epilepsy : role of testosterone. Brain Research. 2002;944:210–218. doi: 10.1016/s0006-8993(02)02691-4. [DOI] [PubMed] [Google Scholar]
- Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet. 2005;365:2125–2136. doi: 10.1016/S0140-6736(05)66736-0. [DOI] [PubMed] [Google Scholar]
- Merke DP, Fields JD, Keil MF, Vaituzis AC, et al. Children with classic congenital adrenal hyperplasia have decreased amygdala volume: potential prenatal and postnatal hormonal effects. Journal of Clinical Endocrinology and Metabolism. 2003;88:1760–1765. doi: 10.1210/jc.2002-021730. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Blackman MR, et al. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. Journal of Clinical Endocrinology and Metabolism. 2002;87:5001–5007. doi: 10.1210/jc.2002-020419. [DOI] [PubMed] [Google Scholar]
- Mogami M, Hayashi Y, Masuda T, Kohiro K, et al. Altered striatal vulnerability to 3-nitropropionic acid in rats due to sex hormone levels during late phase of brain development. Neuroscience Letters. 2008;436:321–325. doi: 10.1016/j.neulet.2008.03.055. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Mandell D, Leschek EW, Pine DS, et al. Early hyperandrogenism affects the development of hippocampal function : preliminary evidence from a fMR study of boys with Familial Male Precocious Puberty. Journal of Child and Adolescent Psychopharmacology. 2009;19:41–50. doi: 10.1089/cap.2008.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SC, Ng P, Sinaii N, Lescheck EW, et al. Psychiatric characterisation of children with genetic causes of hyperandrogenism. European Journal of Endocrinology. doi: 10.1530/EJE-10-0693. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Wong DF, Oswald LM, et al. Sex differences in striatal dopamine release in healthy adults. Biological Psychiatry. 2006;59:966–974. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Neufang S, Specht K, Hausmann M, Gunturkun O, et al. Sex differences and the impact of steroid hormones on the developing human brain. Cerebral Cortex. 2009;19:464–473. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olweus D, Mattsson A, Schalling D, Low H. Testosterone, aggression, physical, and personality dimensions in normal adolescent males. Psychosomatic Medicine. 1980;42:253–269. doi: 10.1097/00006842-198003000-00003. [DOI] [PubMed] [Google Scholar]
- Paus T, Nawaz-Khan I, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Susman E, Veillette S, Pausova Z. Sexual dimorphism in the adolescent brain : role of testosterone and receptor in global and local volumes of grey and white matter. Hormones and Behavior. 2010;57:63–57. doi: 10.1016/j.yhbeh.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Schnack HG, van Baal GC, et al. Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology. 2009;34:332–342. doi: 10.1016/j.psyneuen.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Herve P-Y, Leonard G, Perron M, et al. Growth of white matter in the adolescent brain : role of testosterone and androgen receptor. Journal of Neuroscience. 2008;28:9519–9524. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Leckman JF, Scahill L, Naftolin F, et al. Steroid hormones and CNS sexual dimorphisms modulate symptom expression in Tourette’s syndrome. Psychoneuroendocrinology. 1992;17:553–563. doi: 10.1016/0306-4530(92)90015-y. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Reiter EO, Norjavaara E. Testotoxicosis : current viewpoint. Pediatric Endocrinology Reviews. 2005;3:77–86. [PubMed] [Google Scholar]
- Romeo RD. Puberty, a period of both organizational and activational effects of steroid hormones on neurobehavioural development. Journal of Neuroendocrinology. 2003;15:1185–1192. doi: 10.1111/j.1365-2826.2003.01106.x. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Grelotti DJ, Klin A, Kleinman J, et al. The role of the fusiform face area in social cognition : implications for the pathobiology of autism. Philosophical Transactions of the Royal Society of London, Series B. Biological Sciences. 2003;358:415–427. doi: 10.1098/rstb.2002.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Zehr JL, Salas-Ramirez KY, Sisk CL. Testosterone programs adult social behavior before and during, but not after, adolescence. Endocrinology. 2009;150:3690–3698. doi: 10.1210/en.2008-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker A, Laue L, Kosugi S, Merendino JJ, Jr, et al. A constitutively activating mutation of the luteinizing hormone receptor in familial male precocious puberty. Nature. 1993;365:652–654. doi: 10.1038/365652a0. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain : an in situ hybridization study. Journal of Computational Neurology. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nature Neuroscience. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Schulz KM, Zehr JL. Puberty: a finishing school for male social behavior. Annals of the New York Academy of Sciences. 2003;1007:189–198. doi: 10.1196/annals.1286.019. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Polcari A, Glod CA, et al. Functional deficits in basal ganglia of children with attention-deficit/hyperactivity disorder shown with functional magnetic resonance imaging relaxometry. Nature Medicine. 2000;6:470–473. doi: 10.1038/74737. [DOI] [PubMed] [Google Scholar]
- Thiblin I, Finn A, Ross SB, Stenfors C. Increased dopaminergic and 5-hydroxytryptaminergic activities in male rat brain following long-term treatment with anabolic androgenic steroids. British Journal of Pharmacology. 1999;126:1301–1306. doi: 10.1038/sj.bjp.0702412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Goozen SH, Matthys W, Cohen-Kettenis PT, Thijssen JH, et al. Adrenal androgens and aggression in conduct disorder prepubertal boys and normal controls. Biological Psychiatry. 1998;43:156–158. doi: 10.1016/S0006-3223(98)00360-6. [DOI] [PubMed] [Google Scholar]
- van Honk J, Peper JS, Schutter DJ. Testosterone reduces unconscious fear but not consciously experienced anxiety : implications for the disorders of fear and anxiety. Biological Psychiatry. 2005;58:218–225. doi: 10.1016/j.biopsych.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Wang J, Jiang T, Cao Q, Wang Y. Characterizing anatomic differences in boys with attention-deficit/hyperactivity disorder with the use of deformation-based morphometry. AJNR American Journal of Neuroradiology. 2007;28:543–547. [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- Weissenberger AA, Leschek EW, Zametkin AJ. Case study, sexual hyperactivity treated with psychostimulants in familial male precocious puberty. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:373–376. doi: 10.1097/00004583-200103000-00018. [DOI] [PubMed] [Google Scholar]
- Witte AV, Savli M, Holik A, Kasper S, et al. Regional sex differences in grey matter volume are associated with sex hormones in the young adult human brain. NeuroImage. 2010;49:1205–1212. doi: 10.1016/j.neuroimage.2009.09.046. [DOI] [PubMed] [Google Scholar]