Abstract
Purpose
A central question for the HIV cure field is to determine new ways to target clinically relevant, latently and actively replicating HIV-infected cells beyond resting memory CD4+ T cells, particularly in anatomical areas of low drug penetrability.
Recent findings
HIV eradication strategies being positioned for targeting HIV for extinction in the CD4 T cell compartment may also show promise in non-CD4 T cells reservoirs. Furthermore, several exciting novel therapeutic approaches specifically focused on HIV clearance from non-CD4 T cells populations are being developed.
Summary
Although reservoir validity in these non-CD4 T cells continues to remain debated, this review will highlight recent advances and make an argument as to their clinical relevancy as we progress towards an HIV cure.
Keywords: HIV, Reservoirs, Eradication
Introduction
Despite the unquestionable success of either prolonged or early institution of combined antiretroviral therapy (cART), a cure for Human immunodeficiency virus (HIV) remains elusive. With the exception of rare cases such as Timothy Brown, aka the Berlin patient, cessation of cART invariably leads to HIV reemergence1-3. Most studies have designed HIV eradication strategies focused primarily on targeting resting memory CD4+ T cells4. Although latently infected resting memory CD4+ T cells represent the predominate reservoir, non-CD4+ T cells reservoirs in blood such as monocytes and other T cells, as well as tissue macrophages in the lung5,6, adipose tissue7, gut associated lymphoid tissue (GALT), genital tract, semen, bone marrow, and central nervous system (CNS) cells including both microglia and astrocytes in brain must not be discounted and instead given attention as targets for elimination. Indeed, comparison of virus archived in resting CD4+ T cells during cART to virus rebounding following cART cessation indicates the presence of another, non-lymphocyte pool of persistent virus8.
Given recent data demonstrating HIV replication persists in lymphoid tissue with low cART penetration, ongoing low-level HIV replication may continue and contribute to the viral reservoir in both CD4 T cells and non-CD4 T cells9. There is a need to not only identify and characterize non-CD4+ T cells in blood and tissues, but also to consider strategies targeted specifically for these populations if we are to effectively ensure no viral rebound after ART cessation.
Advances in Targeting non-CD4+ T cells sources of HIV persistence: Relevance of Location
There are a variety of attributes that make monocyte and tissue macrophages, including microglia, candidates for contributing to the HIV reservoir, both as carriers and replenishers of the viral reservoir. The macrophage reservoir half-life has historically been underdetermined, yet in the presence of cART, macrophages from SIV infected rhesus macaques can sustain viremia for several months10-12. Furthermore, myeloid cells are relatively more resistant to apoptosis induced by HIV infection13, and virus produced by macrophages may be more infectious than virus originating from CD4+ T cells14. Monocytes and macrophages disseminate into most tissues in the body and therefore have the widest capacity for mediating HIV spread. These sites can be difficult to access with conventional ART. Indeed, a well-discussed site is the CNS. Post-mortem brain tissue analysis has revealed that viral DNA is present in 3% to 19% of astrocytes15 despite astrocyte infection being both relatively infrequent and unproductive16. Moreover, our group has used next generation in situ hybridization RNAScope to identify HIV vRNA in cerebellum macrophages of in an infected individual who died with no detectable viral load17. Therefore, it is of the utmost importance to consider the contribution of non CD4+ T cells as reservoirs and sources of HIV viremia.
Another unique non-CD4+ T cell population has recently been demonstrated by DeMaster et al as being both CD4 negative and CD8 negative T cells that are positive for Gag expression with integrated proviral DNA18. DeMaster et al show that these CD4/CD8 “double negative” T cells do not display activation markers and therefore are a quiescent population18. The double negative T cell phenotype was also observed in peripheral blood of cART treated HIV-infected patients18. Although Gag+ cells may be short lived, their low-level capability of producing replication competent virus may contribute new virus to replenish the reservoir, although the longevity of these cells has yet to be extensively studied and may prove to be an under-appreciated cellular reservoir for HIV.
i. Peripheral blood: Monocytes
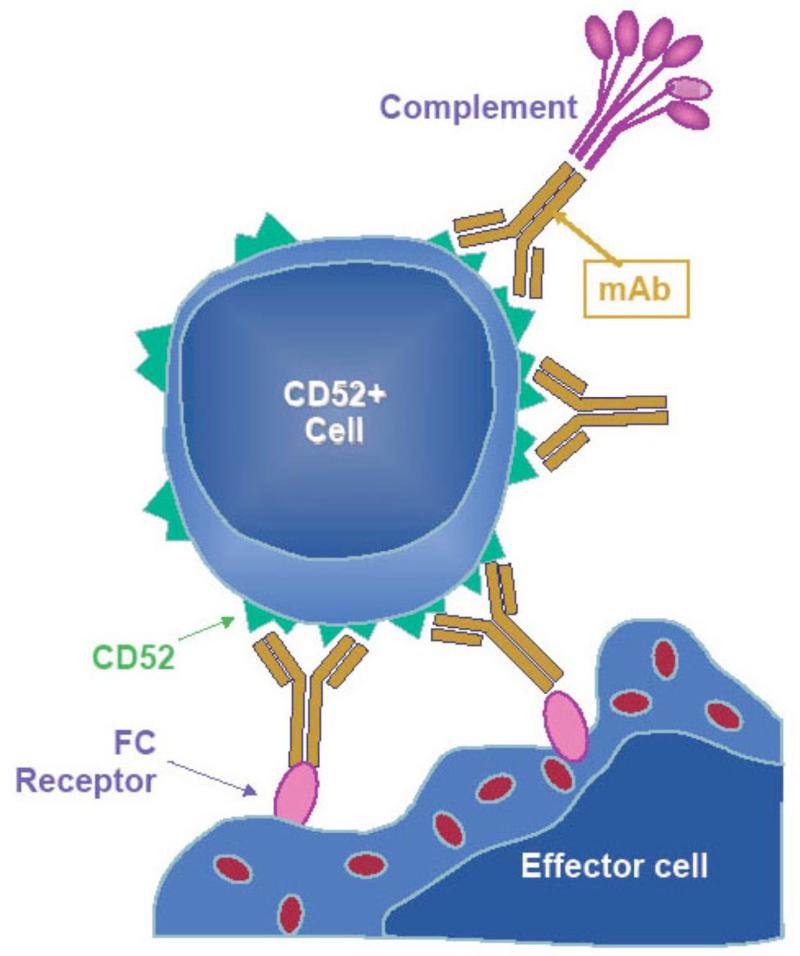
A new study has shown that the CD52 receptor is retained on HIV infected immune cells19. The authors further demonstrate that targeting CD52 in vitro with a monoclonal antibody (alemtuzamab) induced the depletion of HIV-infected immune cells including peripheral monocytes. This was achieved by rendering infected cells sensitive to complement-mediated lysis, a key event induced by alemtuzamab that may improve its effectiveness in vivo (Figure 1).
Figure 1. Mechanism of action of alemtuzumab.
Alemtuzumab lyses not only lymphocytes but monocytes and other non-CD4 T cells presumably via complement fixation, antibody-dependent cell-mediated cytotoxicity (ADCC), and by induction of apoptosis. Adapted with permission from Journal of Virus Eradication 2016; 2: 12–18.
ii. Central Nervous System (CNS): Perivascular Macrophages, Microglia and Astrocytes
Perivascular macrophages have a half-life of ~3 months20 while microglia have a half-life of months-years to years-lifetime21. Therefore, both CNS perivascular macrophages and microglia need to be considered possible long-lived HIV reservoirs, particularly because parenchymal microglia have been shown to be two-thirds of the infected cells in the brains of patients with encephalitis22. Thus, the HIV reservoir in the CNS could be the largest obstacle to achieving complete HIV elimination. Recently, McFarren et al have demonstrated that 213Bi-2556 mAb, a radio-labeled monoclonal antibody to HIV gp41, crossed an in vitro human blood brain barrier and killed significantly more transmigrated HIV-infected PBMCs and monocytes in comparison to an irrelevant 213Bi-1418 mAb or uninfected cells23. Although promising, cytotoxic bystander effects need to be characterized as we may require curative measures that avoid depleting minimally-replenished cells of the brain.
The neutrophil chemotaxis recruiter, interleukin-8 (IL-8) has been shown in vitro in monocyte-derived macrophages (MDM) to not only enhance CCL2-mediated monocyte migration but also enhance HIV replication in both macrophages and T cells24,25. Curative measures that intervene by modulating cell chemotaxis could avoid potentially detrimental cell depletion approaches. Interestingly, IL-8 also is correlated with 2-LTR circle formations, a marker of nuclear import of viral DNA or enhanced infectivity, again in MDM and primary human microglia26.
Methods to target infected macrophages that are in the CNS could develop from the immunotargeting drug liposomal alendronate, which can reduce circulating and disseminated macrophage frequency. Burwitz et al have demonstrated that liposomal alendronate safely and effectively depletes peripheral and tissue macrophages in nonhuman primates followed by an increase in bone marrow macrophage precursor frequency27. Afergan et al used liposomal delivery to target brain monocytes for transport of encapsulated serotonin by negatively charge nano-sized liposomes across the blood brain barrier28. The use of the liposomal alendronate system could provide a more sophisticated approach if used in conjunction with cART to increase drug concentration in tissues with poor cART penetrance. For example, to deliver anatomically relevant cART to the CNS, liposomal alendronate could be paired with the recent advent of cART regimens that have effective CNS-penetration, known as neurocART.
Beyond parenchymal macrophages and microglia, astrocytes are the other predominant cell type that comes into contact with virus-infected cells migrating from the blood to the CNS. The protein kinase C (PKC) activator Bryostatin could become a possible adjunct of CNS targeted HIV-1 elimination therapy. Bryostatin has been shown to have anti-HIV-1 activity by downregulating CXCR4 receptors or indirectly inhibiting HIV-1 Tat function by dephosphorylating a kinase necessary for RNA polymerase-II functional initiation. Furthermore, Bryostatin has been shown to have moderate HIV latency reversing agent (LRA) activity in astrocyte cell lines in vitro and in cultured primary astrocytes by inducing HIV-1 expression through NF-kB activation29.
iii. Macrophages in Adipose Tissue
Adipose tissue is comprised of adipocytes and stromal vasculature. In macaques infected with SIV, adipose density was elevated as well as enhanced immune-activated profiles. Damouche et al demonstrate that HIV-1 DNA was detected in both CD4+ T cells and sorted CD206+ CD14+ macrophages in adipose tissue samples from ART-treated HIV-infected donors7. A phage system that displays a visceral adipose tissue-specific peptide ligand (TDA1) has been developed by Lee et al, which could form the homing moiety basis for a delivery system to target adipose macrophages30.
iv. Lymph Nodes; Follicular dendritic cells (FDC)
are located in the B cell follicle and recently have been shown to harbor infectious HIV virions within a non-degradative cycling compartment31. Heesters and colleagues further demonstrate that virion uptake by FDC appears to be regulated and retained through the complement receptor CD21. Thus exploiting CD21 with targeted reagents such as a soluble CD21-Ig could provide adjunctive therapy to cART or broadly neutralizing antibodies for the elimination of this reservoir compartment.
Current Eradication Approaches Redirected towards Non CD4+ T cell Reservoirs
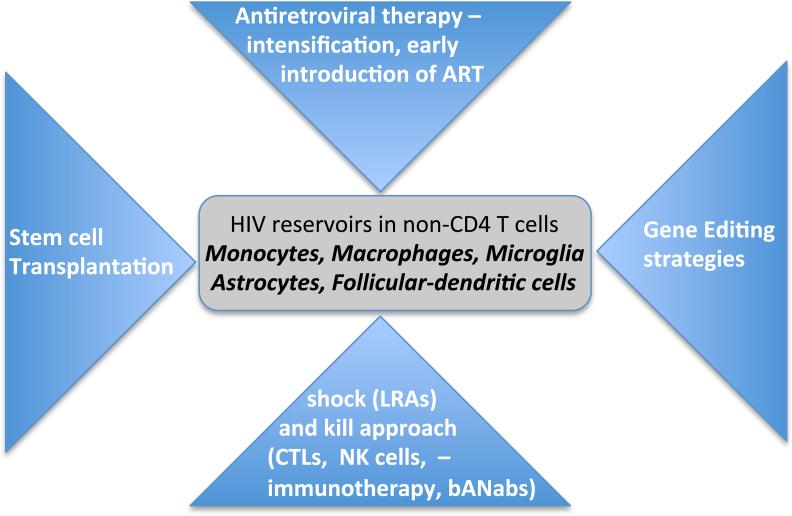
Several insights have been gained from HIV cure strategies targeting the CD4 T cell reservoir compartment, which could be redirected to other cellular HIV viral sanctuaries (Figure. 2).
Figure 2. Current HIV eradication approaches: Focused on non-CD4+ T cell reservoirs.
Overview of cure strategies that are being actively considered for CD4 T cell HIV clearance of relevance to non-CD4 T cell populations
i Limiting the viral reservoir size by early ART
Very early introduction of cART soon after infection is hypothesized to achieve HIV remission. This has been observed in principle in adults in the Visconti cohort1, which is comprised of HIV controllers who are able to spontaneously control HIV replication following cART cession. This phenomenon was also transiently observed in the infant referred to as “the Mississippi baby” who received cART 30 hours after birth. Ongoing studies evaluating early initiation of cART in several cohorts will provide further data on such an approach32-34. However, viral rebound did occur in the Mississippi baby suggesting that early cART intervention alone may not be sufficient as a curative strategy. To what extent the non-CD4 T cell reservoir compartments contributed to viral recrudescence is unclear. Current studies by our group are assessing the myeloid reservoir soon after infection and determining the efficacy of early mega cART interventions in those captured in the early acute stages of infection.
ii. ART intensification
The inability of current antiretroviral drugs to sufficiently suppress virus within circulating monocytes and tissue macrophages including the CNS is an area needing continued investigation. Several in vitro studies have demonstrated varying efficacies of existing cART drugs against HIV infection in macrophages35. Both Maraviroc and Raltegravir have been tested under cART intensification. We have shown that intensification with Maraviroc for 24 weeks in HIV-infected cART treated participants leads to a reduction in monocyte HIV DNA36. Lafeuillade et al showed that from 10 PBMC and gut biopsy samples of HIV-infected patients on either Truvada® or Kaletra® with further intensified antiretroviral therapy by taking Maraviroc and Raltegravir showed no appreciable proviral DNA reservoir reduction in blood or gut biopsies, except in a few patient cases where proviral DNA in gut biopsies showed a modest reduction37. More data from such intensification studies may be useful to develop a systematic clinical intervention sequence of ART therapeutics designed for each reservoir compartment.
iii. Shock and Kill approach
Drugs that are already in clinical trials which target the HIV-1 reservoir exploit a strategy known as ‘shock and kill’ whereby the integrated pro-viral DNA is kicked into transcriptional activity by a latency reversing agent; the virally active cells will subsequently be eliminated with the continuation of antiretroviral therapy during this ‘kill’ phase. Although histone deacetylase inhibitors (HDACi) decrease HIV release from macrophages, the HDAC inhibitors may not alter the initial susceptibility of macrophages to HIV infection38. Churchill et al show that in human primary astrocyte cell lines transfected with patient-derived HIV-1 LTR, treatment with HDACi panobinostat, trichostatin A, vorinostat, and entinostat activated HIV LTRs39.
Simian immunodeficiency virus (SIV)-specific CD8+ T cells (CTL) can efficiently kill SIV-infected CD4+ T cells but not SIV-infected macrophages40. Nef downregulates MHC-I production and enhances CD8+ T cell evasion. Rainho et al show that CTL mediated killing of CD4+ T cells and monocyte derived macrophages infected with SIV nef variants was more efficient when targeting CD4+ T cells than macrophages41 suggesting alternate mechanisms for macrophage resistance to CTL killing41. Finding strategies to enhance CTL activity against infected macrophages are therefore important. Pegu et al have shown that a bi-specific immunomodulatory protein that stimulates CD8+ T-cell effector function thereby initiating latent-infected cell lysis through recognition of Env42 may be retargeted towards HIV infected macrophages. Studies evaluating immunotherapies targeting negative checkpoint receptors43-45 to improve CTL activity as well as harnessing NK cells46 are needed and may serve as alternate strategies to overcome myeloid cell resistance to CTL killing.
iv. Stem Cell Transplantation
The successful homozygous CCR5 delta32 stem-cell transplant into an HIV-1 infected individual with acute myeloid leukemia resulted in an apparent cure whereby Timothy Brown, the ‘Berlin Patient’, could stop antiretroviral treatment without subsequent viremic rebound47. The exact mechanisms for the apparent curative approach remain undefined. In multiple samplings of non-CD4 T cell populations no virus was detected using several different assays48. However, overcoming the HIV reservoir in macrophages by stem cell transplantation is still unresolved. In the case of the ‘Berlin Patient’ prior administration of gemtuzumab (anti-CD33 mAb) may have targeted myeloid cells for depletion. It is unclear whether or not lack of myeloid-targeted depletion contributed to the incomplete reservoir elimination and viral reemergence in other human2 and non-human49 stem cell transplantation cases tested thereafter. Stem cell transplantation remains an understudied, yet highly multifaceted approach to understanding and clearing the latent viral reservoir.
v. Gene Therapy
The CRISPR-Cas9 system is an alternate approach that uses guide RNA instead of custom proteins to home in on target DNA. Zhang et al have reported using a dCas9-synergistic activation mediator (dCas9-SAM) system to reactivate HIV-1 in both CD4+ T cell and microglial cell lines50. The possible advantage of a dCas9-SAM system is that it may minimally affect localized HIV-negative cells. Zhang et al report finding two MS2-mediated single guide RNA that direct the dCas9-SAM system to potently target specific HIV-infected cells50. However, the most promising results are presented by Hu et al where in latently infected microglial, promonocytes, and T cell lines they developed a Cas9/guide RNA system to eradicate the HIV-1 genome and immunize target cells against HIV-1 reactivation51. However, studies using patient-derived myeloid cells have not been reported yet. Studies by the Churchill group and others show CNS viral strains are genotypically distinct from those found in the peripheral blood; in particular the long terminal repeat, also known as the viral promoter region is varied52,53. Therefore, a Cas9 gene therapy system may need to be tailored to each compartmentalized viral reservoir.
vi. Permanent HIV Suppression
Activation of positive transcriptional elongation factor b (p-TEFb) has recently been established as the mechanism by which the LRAs, vorinostat and panobinostat work to activate latent HIV from CD4+ T cells54. However, because p-TEFb is required for RNA polymerase II activation and therefore viral production, p-TEFb could also be a possible target for permanent HIV-1 suppression. Heat shock protein 90 (Hsp90) inhibitors can block triggering the NF-kB pathway and thus suppress HIV-1 transcription55. In addition PIM-1 inhibitors can block HIV-1 reactivation making it a possible permanent suppression therapeutic56. Whether these novel approaches can be extended to non-CD4 T cell reservoirs is untested and should be evaluated further as alternative strategies.
Conclusion and Future Direction
The breadth of possible reservoirs considered here makes the prospect of HIV elimination seem daunting if not impossible; it may be tempting to limit our consideration to only one reservoir at a time, to address only the clinically relevant first—that once we find moderate success with the most clinically relevant reservoir, our success could translate to other cell types. However, the evidence presented here suggests a unique solution may be required for each cell type, and delivery mechanisms or targeting strategies will need to be tissue-specific tailored. New therapeutic strategies that place emphasis on targeting non-CD4+ T cell reservoir contributions to the HIV-1 reservoir will be imperative on the road to HIV eradication.
SUMMARY.
Cessation of antiretroviral therapy invariably leads to HIV reemergence
HIV eradication strategies being positioned for targeting HIV for extinction in the CD4 T cell reservoir may also show promise in other cellular HIV viral sanctuaries.
Several exciting novel therapeutic approaches specifically and broadly focused on HIV clearance from non-CD4 T cells populations such as, monocytes, macrophages, astrocytes and follicular dendritic cells are being developed.
Acknowledgements
The author thanks Elizabeth Laws for her contributions to this review.
Financial support and sponsorship: LCN receives financial support from the National Institutes of Mental Health grant #MH104141. JBS receives financial support from the National Institute of Allergy and Infectious Diseases grants R01AI117802 and R21AI112433.
Footnotes
Conflicts of interest: The authors have no conflicts of interest.
REFERENCES
- 1.Saez-Cirion A, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9:e1003211. doi: 10.1371/journal.ppat.1003211. doi:10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henrich TJ, et al. Long-term reduction in peripheral blood HIV type 1 reservoirs following reduced-intensity conditioning allogeneic stem cell transplantation. J Infect Dis. 2013;207:1694–1702. doi: 10.1093/infdis/jit086. doi:10.1093/infdis/jit086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Persaud D, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013;369:1828–1835. doi: 10.1056/NEJMoa1302976. doi:10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siliciano JD, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9:727–728. doi: 10.1038/nm880. doi:10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 5.Jambo KC, et al. Small alveolar macrophages are infected preferentially by HIV and exhibit impaired phagocytic function. Mucosal Immunol. 2014;7:1116–1126. doi: 10.1038/mi.2013.127. doi:10.1038/mi.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6*.Cai Y, et al. Preferential Destruction of Interstitial Macrophages over Alveolar Macrophages as a Cause of Pulmonary Disease in Simian Immunodeficiency Virus-Infected Rhesus Macaques. J Immunol. 2015;195:4884–4891. doi: 10.4049/jimmunol.1501194. doi:10.4049/jimmunol.1501194. [This study demonstrates the presence of SIV in alveolar macrophages.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7*.Damouche A, et al. Adipose Tissue Is a Neglected Viral Reservoir and an Inflammatory Site during Chronic HIV and SIV Infection. PLoS Pathog. 2015;11:e1005153. doi: 10.1371/journal.ppat.1005153. doi:10.1371/journal.ppat.1005153. [Highlights the role of adipose resident lymphocytes and macrophages as viral reservoirs.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chun TW, et al. Relationship between pre-existing viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active anti-retroviral therapy. Nat Med. 2000;6:757–761. doi: 10.1038/77481. doi:10.1038/77481. [DOI] [PubMed] [Google Scholar]
- 9*.Lorenzo-Redondo R, et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature. 2016;530:51–56. doi: 10.1038/nature16933. doi:10.1038/nature16933. [Demonstration that minimal cART penetrance in tissues facilitates ongoing viral replication.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowe S, Zhu T, Muller WA. The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. J Leukoc Biol. 2003;74:635–641. doi: 10.1189/jlb.0503204. doi:10.1189/jlb.0503204. [DOI] [PubMed] [Google Scholar]
- 11.Igarashi T, et al. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): Implications for HIV-1 infections of humans. Proc Natl Acad Sci U S A. 2001;98:658–663. doi: 10.1073/pnas.021551798. doi:10.1073/pnas.021551798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Micci L, et al. CD4 depletion in SIV-infected macaques results in macrophage and microglia infection with rapid turnover of infected cells. PLoS Pathog. 2014;10:e1004467. doi: 10.1371/journal.ppat.1004467. doi:10.1371/journal.ppat.1004467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar A, Abbas W, Herbein G. HIV-1 latency in monocytes/macrophages. Viruses. 2014;6:1837–1860. doi: 10.3390/v6041837. doi:10.3390/v6041837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaskill PJ, Zandonatti M, Gilmartin T, Head SR, Fox HS. Macrophage-derived simian immunodeficiency virus exhibits enhanced infectivity by comparison with T-cell-derived virus. J Virol. 2008;82:1615–1621. doi: 10.1128/JVI.01757-07. doi:10.1128/JVI.01757-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Churchill MJ, et al. Extensive astrocyte infection is prominent in human immunodeficiency virus- associated dementia. Ann Neurol. 2009;66:253–258. doi: 10.1002/ana.21697. doi:10.1002/ana.21697. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. doi:10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 17*.Lamers SL, et al. The meningeal lymphatic system: a route for HIV brain migration? J Neurovirol. 2015 doi: 10.1007/s13365-015-0399-y. doi:10.1007/s13365-015-0399-y. [This study documents the presence of HIV RNA in macrophages/microglia in the brain of an HIV subject who died while on suppressive ART.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeMaster LK, et al. A subset of CD4/CD8 double negative T cells expresses HIV proteins in patients on ART. J Virol. 2015 doi: 10.1128/JVI.01913-15. doi:10.1128/JVI.01913-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19*.Ruxrungtham KSS. Buranapraditkun S and Krause W. Alemtuzumab-induced elimination of HIV-1-infected immune cells. Journal of Virus Eradication. 2016;2 doi: 10.1016/S2055-6640(20)30694-4. [Presents evidence of targeting several HIV infected cell types by targeted monoclonal Ab to CD52.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koppensteiner H, Brack-Werner R, Schindler M. Macrophages and their relevance in Human Immunodeficiency Virus Type I infection. Retrovirology. 2012;9:82. doi: 10.1186/1742-4690-9-82. doi:10.1186/1742-4690-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soulet D, Rivest S. Bone-marrow-derived microglia: myth or reality? Curr Opin Pharmacol. 2008;8:508–518. doi: 10.1016/j.coph.2008.04.002. doi:10.1016/j.coph.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Beschorner R, et al. CD14 expression by activated parenchymal microglia/macrophages and infiltrating monocytes following human traumatic brain injury. Acta Neuropathol. 2002;103:541–549. doi: 10.1007/s00401-001-0503-7. doi:10.1007/s00401-001-0503-7. [DOI] [PubMed] [Google Scholar]
- 23*.McFarren A, et al. A fully human antibody to gp41 selectively eliminates HIV-infected cells that transmigrated across a model human blood brain barrier. AIDS. 2016;30:563–572. doi: 10.1097/QAD.0000000000000968. doi:10.1097/QAD.0000000000000968. [This study reveals novel effects of an gp41mAb as a therapeutic for targeting HIV infected cells in the CNS.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng JC, et al. HIV-1-infected and/or immune-activated macrophages regulate astrocyte CXCL8 production through IL-1beta and TNF-alpha: involvement of mitogen-activated protein kinases and protein kinase R. J Neuroimmunol. 2008;200:100–110. doi: 10.1016/j.jneuroim.2008.06.015. doi:10.1016/j.jneuroim.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gouwy M, et al. Synergy between coproduced CC and CXC chemokines in monocyte chemotaxis through receptor-mediated events. Mol Pharmacol. 2008;74:485–495. doi: 10.1124/mol.108.045146. doi:10.1124/mol.108.045146. [DOI] [PubMed] [Google Scholar]
- 26.Mamik MK, Ghorpade A. Chemokine CXCL8 promotes HIV-1 replication in human monocyte-derived macrophages and primary microglia via nuclear factor-kappaB pathway. PLoS One. 2014;9:e92145. doi: 10.1371/journal.pone.0092145. doi:10.1371/journal.pone.0092145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burwitz BJ, et al. Technical advance: liposomal alendronate depletes monocytes and macrophages in the nonhuman primate model of human disease. J Leukoc Biol. 2014;96:491–501. doi: 10.1189/jlb.5TA0713-373R. doi:10.1189/jlb.5TA0713-373R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Afergan E, et al. Delivery of serotonin to the brain by monocytes following phagocytosis of liposomes. J Control Release. 2008;132:84–90. doi: 10.1016/j.jconrel.2008.08.017. doi:10.1016/j.jconrel.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 29.Diaz L, et al. Bryostatin activates HIV-1 latent expression in human astrocytes through a PKC and NF-kB-dependent mechanism. Sci Rep. 2015;5:12442. doi: 10.1038/srep12442. doi:10.1038/srep12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee NK, et al. Identification of a novel peptide ligand targeting visceral adipose tissue via transdermal route by in vivo phage display. J Drug Target. 2011;19:805–813. doi: 10.3109/1061186X.2011.572974. doi:10.3109/1061186X.2011.572974. [DOI] [PubMed] [Google Scholar]
- 31.Heesters BA, et al. Follicular Dendritic Cells Retain Infectious HIV in Cycling Endosomes. PLoS Pathog. 2015;11:e1005285. doi: 10.1371/journal.ppat.1005285. doi:10.1371/journal.ppat.1005285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuetz A, et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog. 2014;10:e1004543. doi: 10.1371/journal.ppat.1004543. doi:10.1371/journal.ppat.1004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ananworanich J, Dube K, Chomont N. How does the timing of antiretroviral therapy initiation in acute infection affect HIV reservoirs? Curr Opin HIV AIDS. 2015;10:18–28. doi: 10.1097/COH.0000000000000122. doi:10.1097/COH.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ananworanich J, McSteen B, Robb ML. Broadly neutralizing antibody and the HIV reservoir in acute HIV infection: a strategy toward HIV remission? Curr Opin HIV AIDS. 2015;10:198–206. doi: 10.1097/COH.0000000000000144. doi:10.1097/COH.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Gavegnano C, Schinazi RF. Antiretroviral therapy in macrophages: implication for HIV eradication. Antivir Chem Chemother. 2009;20:63–78. doi: 10.3851/IMP1374. doi:10.3851/IMP1374. [An excellent review of antiviral agent pharmacology in macrophages.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ndhlovu LC, et al. Treatment intensification with maraviroc (CCR5 antagonist) leads to declines in CD16-expressing monocytes in cART-suppressed chronic HIV-infected subjects and is associated with improvements in neurocognitive test performance: implications for HIV-associated neurocognitive disease (HAND). J Neurovirol. 2014;20:571–582. doi: 10.1007/s13365-014-0279-x. doi:10.1007/s13365-014-0279-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lafeuillade A, et al. Failure of combined antiretroviral therapy intensification with maraviroc and raltegravir in chronically HIV-1 infected patients to reduce the viral reservoir: the IntensHIV randomized trial. AIDS Res Ther. 2014;11:33. doi: 10.1186/1742-6405-11-33. doi:10.1186/1742-6405-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Campbell GR, Bruckman RS, Chu YL, Spector SA. Autophagy induction by histone deacetylase inhibitors inhibits HIV type 1. J Biol Chem. 2015;290:5028–5040. doi: 10.1074/jbc.M114.605428. doi:10.1074/jbc.M114.605428. [Highlights the effects of HDACi on HIV infected macrophages.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu HK, et al. Ex vivo response to histone deacetylase (HDAC) inhibitors of the HIV long terminal repeat (LTR) derived from HIV-infected patients on antiretroviral therapy. PLoS One. 2014;9:e113341. doi: 10.1371/journal.pone.0113341. doi:10.1371/journal.pone.0113341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vojnov L, et al. The majority of freshly sorted simian immunodeficiency virus (SIV)-specific CD8(+) T cells cannot suppress viral replication in SIV-infected macrophages. J Virol. 2012;86:4682–4687. doi: 10.1128/JVI.06324-11. doi:10.1128/JVI.06324-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rainho JN, et al. Nef Is Dispensable for Resistance of Simian Immunodeficiency Virus-Infected Macrophages to CD8+ T Cell Killing. J Virol. 2015;89:10625–10636. doi: 10.1128/JVI.01699-15. doi:10.1128/JVI.01699-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pegu A, et al. Activation and lysis of human CD4 cells latently infected with HIV-1. Nat Commun. 2015;6:8447. doi: 10.1038/ncomms9447. doi:10.1038/ncomms9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chew GM, et al. TIGIT Marks Exhausted T Cells, Correlates with Disease Progression, and Serves as a Target for Immune Restoration in HIV and SIV Infection. PLoS Pathog. 2016;12:e1005349. doi: 10.1371/journal.ppat.1005349. doi:10.1371/journal.ppat.1005349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujita T, et al. Expansion of dysfunctional Tim-3-expressing effector memory CD8+ T cells during simian immunodeficiency virus infection in rhesus macaques. J Immunol. 2014;193:5576–5583. doi: 10.4049/jimmunol.1400961. doi:10.4049/jimmunol.1400961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bui JK, Mellors JW. Reversal of T-cell exhaustion as a strategy to improve immune control of HIV-1. AIDS. 2015;29:1911–1915. doi: 10.1097/QAD.0000000000000788. doi:10.1097/QAD.0000000000000788. [DOI] [PubMed] [Google Scholar]
- 46.Tomescu C, Mavilio D, Montaner LJ. Lysis of HIV-1-infected autologous CD4+ primary T cells by interferon-alpha-activated NK cells requires NKp46 and NKG2D. AIDS. 2015;29:1767–1773. doi: 10.1097/QAD.0000000000000777. doi:10.1097/QAD.0000000000000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allers K, et al. Evidence for the cure of HIV infection by CCR5Delta32/Delta32 stem cell transplantation. Blood. 2011;117:2791–2799. doi: 10.1182/blood-2010-09-309591. doi:10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- 48.Yukl SA, et al. Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient. PLoS Pathog. 2013;9:e1003347. doi: 10.1371/journal.ppat.1003347. doi:10.1371/journal.ppat.1003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mavigner M, et al. Persistence of virus reservoirs in ART-treated SHIV-infected rhesus macaques after autologous hematopoietic stem cell transplant. PLoS Pathog. 2014;10:e1004406. doi: 10.1371/journal.ppat.1004406. doi:10.1371/journal.ppat.1004406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y, et al. CRISPR/gRNA-directed synergistic activation mediator (SAM) induces specific, persistent and robust reactivation of the HIV-1 latent reservoirs. Sci Rep. 2015;5:16277. doi: 10.1038/srep16277. doi:10.1038/srep16277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu W, et al. RNA-directed gene editing specifically eradicates latent and prevents new HIV-1 infection. Proc Natl Acad Sci U S A. 2014;111:11461–11466. doi: 10.1073/pnas.1405186111. doi:10.1073/pnas.1405186111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gray LR, et al. CD4 and MHC class 1 down-modulation activities of nef alleles from brain- and lymphoid tissue-derived primary HIV-1 isolates. J Neurovirol. 2011;17:82–91. doi: 10.1007/s13365-010-0001-6. doi:10.1007/s13365-010-0001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gray LR, et al. Reduced basal transcriptional activity of central nervous system-derived HIV type 1 long terminal repeats. AIDS Res Hum Retroviruses. 2013;29:365–370. doi: 10.1089/aid.2012.0138. doi:10.1089/AID.2012.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jamaluddin MS, Hu PW, Danels YJ, Siwak ES, Rice AP. The Broad Spectrum Histone Deacetylase Inhibitors Vorinostat and Panobinostat Activate Latent HIV in CD4+ T cells in part through Phosphorylation of the T-Loop of the CDK9 Subunit of P-TEFb. AIDS Res Hum Retroviruses. 2016 doi: 10.1089/aid.2015.0347. doi:10.1089/AID.2015.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anderson I, et al. Heat shock protein 90 controls HIV-1 reactivation from latency. Proc Natl Acad Sci U S A. 2014;111:E1528–1537. doi: 10.1073/pnas.1320178111. doi:10.1073/pnas.1320178111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duverger A, et al. Kinase control of latent HIV-1 infection: PIM-1 kinase as a major contributor to HIV-1 reactivation. J Virol. 2014;88:364–376. doi: 10.1128/JVI.02682-13. doi:10.1128/JVI.02682-13. [DOI] [PMC free article] [PubMed] [Google Scholar]