Abstract
In the U.S., more than 400,000 individuals with end-stage renal disease (ESRD) require hemodialysis (HD) for renal replacement therapy. ESRD patients experience a high burden of morbidity, mortality, resource utilization, and poor quality of life (QOL). Under current care models ESRD patients receive fragmented care from multiple providers at multiple locations. The Patient-Centered Medical Home (PCMH) is a team approach, providing coordinated care across the healthcare continuum. While this model has shown some early benefits for complex chronic diseases such as diabetes, it has not been applied to HD patients. This study is a non-randomized quasi-experimental intervention trial implementing a Patient-Centered Medical Home for Kidney Disease (PCMH-KD). The PCMH-KD extends the existing dialysis care team (comprised of a nephrologist, dialysis nurse, dialysis technician, social worker, and dietitian) by adding a general internist, pharmacist, nurse coordinator, and a community health worker, all of whom will see the patients together, and separately, as needed. The primary goal is to implement a comprehensive, multidisciplinary care team to improve care coordination, quality of life, and healthcare use for HD patients. Approximately 240 patients will be recruited from two sites; a non-profit university-affiliated dialysis center and an independent for-profit dialysis center. Outcomes include: i) patient-reported outcomes, including QOL and satisfaction; ii) clinical outcomes, including blood pressure and diet; iii) healthcare use, including emergency room visits and hospitalizations; and iv) staff perceptions. Given the significant burden that patients with ESRD on HD experience, enhanced care coordination provides an opportunity to reduce this burden and improve QOL.
Keywords: clinical trial, medical home, quality of life
1. Introduction
Over 400,000 people in the U.S. receive hemodialysis for end-stage renal disease (ESRD).1 Despite the relatively low prevalence of ESRD, healthcare expenditures for these patients are disproportionately high,1, 2 and individuals experience high morbidity, mortality, and poor quality of life (QOL).1,3,4,5,6 In 2012, Medicare expenditures for ESRD totaled $28.6 billion.1 Patients with ESRD on hemodialysis spend 12 days in inpatient care yearly on average and have mortality rates exceeding 150 deaths per 1000 patient-years.1 Additionally, ESRD patients’ self-reported QOL is consistently below that of the general population.5
While some of the excess morbidity, mortality, and poor QOL associated with ESRD are due to ESRD itself and associated comorbid illnesses,3 the effects of these illnesses may be amplified by fragmented healthcare delivery. ESRD patients undergo dialysis treatments for 3–5 hours thrice weekly. This rigorous schedule creates difficulty in managing co-morbid illnesses so patients often have increased complications as well as the use of emergency healthcare. Improved care coordination between primary care physicians and nephrologists could potentially help alleviate this problem.7
An attempt to address the gaps in care coordination has been tested through the Patient-Centered Medical Homes (PCMH) model. PCMH uses a team approach to provide comprehensive care for patients, has been implemented for patients with chronic complex illnesses such as diabetes, and has been found to reduce hospitalizations, emergency room visits, and healthcare costs.8,9 For example, among patients with chronic kidney disease not yet requiring dialysis, use of a multidisciplinary care team, a key element of PCMH, reduced the rate of kidney function decline.10 However, to date, the implementation of a PCMH has not been tested among U.S. hemodialysis patients.11 Although the current U.S. dialysis care team is multidisciplinary in its inclusion of a nephrologist, nurse, dietician, and social worker,12 it lacks integration with primary care.7 The current model also does not include other professionals, such as pharmacists, who have been recognized to improve care for other chronic illnesses.13 Moreover, the current care model does not include nonprofessional team members such as community health workers (CHW) functioning as health promoters, who are individuals without a formal medical background but who receive specialized training as peer educators and liaisons between patients and healthcare professionals to assist in providing culturally sensitive care. In several studies of PCMH for chronic diseases other than ESRD, CHWs have been shown to improve clinical outcomes and reduce care costs.14,15,16
The purpose of this study is to examine a comprehensive, multidisciplinary care model in ESRD patients within the hemodialysis setting. We will implement a Patient-Centered Medical Home for Kidney Disease (PCMH-KD) and expect to enroll approximately 240 in-center hemodialysis patients. We expect that the PCMH-KD will enhance dialysis care by adding a primary care physician, nurse coordinator, pharmacist, and CHW to the care team. This manuscript describes the design and methodology of the trial. The primary goal of the project is to evaluate the effectiveness of the PCMH-KD model compared to the current care model for improving patient- and caregiver-reported outcomes, clinical outcomes, avoidable healthcare utilization and staff perceptions.
2. Materials and Methods
2.1. Hypotheses
This study will test whether, compared to the current standard care model for ESRD patients on hemodialysis, implementation of the PCMH-KD care model will:
Improve patient quality of life
Improve patient knowledge about hemodialysis
Increase patient access to care for conditions other than ESRD
Improve care coordination
Improve medication adherence
Improve compliance with diet and fluid restrictions
Reduce emergency care use and hospitalizations
2.2. Study Design
This study is a non-randomized quasi-experimental intervention trial of implementation of a PCMH-KD over two years at two dialysis units. Patients under care at each site will be observed in the current care model for six months prior to the implementation of the PCMH-KD model. During this observation period, patient reported outcomes, clinical outcomes, healthcare use, and staff perceptions will be measured and will provide the basis for comparison with the PCMH-KD measures throughout the 18-month intervention period. Study procedures have been approved by the University of Illinois at Chicago Institutional Review Board.
2.3. Participants, Setting, and Recruitment
Eligible participants will be English- or Spanish-speaking adults (≥18 years) with ESRD receiving maintenance hemodialysis treatments at two dialysis units in Chicago: 1) University of Illinois Hospital and Health Sciences System Dialysis Center (UIHS-D), and 2) Fresenius Medical Care Chicago Westside Dialysis Center (FMC). UIHS-D is a non-profit, university-affiliated dialysis unit, and FMC is an independent for-profit center owned and operated by Fresenius Medical Care, Inc. Nephrologists from the University of Illinois at Chicago comprise the medical staff at both the UIHS-D and FMC units. Approximately 200 patients currently receive hemodialysis care at the two sites. All patients at each dialysis unit will be offered the intervention but may decline to participate. The patient population at the participating dialysis centers is reflective of the population in the centers’ surrounding service area and has a higher proportion of minority and low-income patients than the national average, as well as a high burden of comorbid illness (Table 1). Only patients receiving in-center hemodialysis (and not peritoneal dialysis) were included in this study due to the significant differences in dialysis care delivery and management between the two types of dialysis.
Table 1.
Dialysis patient characteristics
| UIHS-D (N=138) |
FMC (N=78) |
USRDS Dialysis Cohort (N= 103,874) |
|
|---|---|---|---|
| Mean age in years | 55 | 50 | NA |
| Race/Ethnicity (%) | |||
| White | 4 | 3 | 66 |
| Black | 63 | 40 | 29 |
| Hispanic | 30 | 56 | Hispanic/non-Hispanic 14/86 |
| Other | 3 | 1 | 6 |
| Length of Dialysis (%) | |||
| ≤180 days since initiation | 25 | 31 | NA |
| >180 days since initiation | 75 | 69 | NA |
| Co-morbidities (%) | |||
| Diabetes | 72 | 28 | 45 |
| Hypertension | 59 | 48 | 29 |
| Other | 54 | 24 | 26 |
Prior to enrollment in the study, patients at both dialysis units will be provided with an informational, IRB-approved pamphlet about the study during one of their regularly scheduled dialysis treatments. After patients have reviewed information about the study, a research staff member will approach patients individually during their dialysis treatments to answer any additional questions, and if the patient is willing to participate, obtain informed consent. Patients who do not wish to participate in the study are not eligible to schedule individual visits with the general internists or CHWs who are part of the study intervention team.
2.4. Intervention
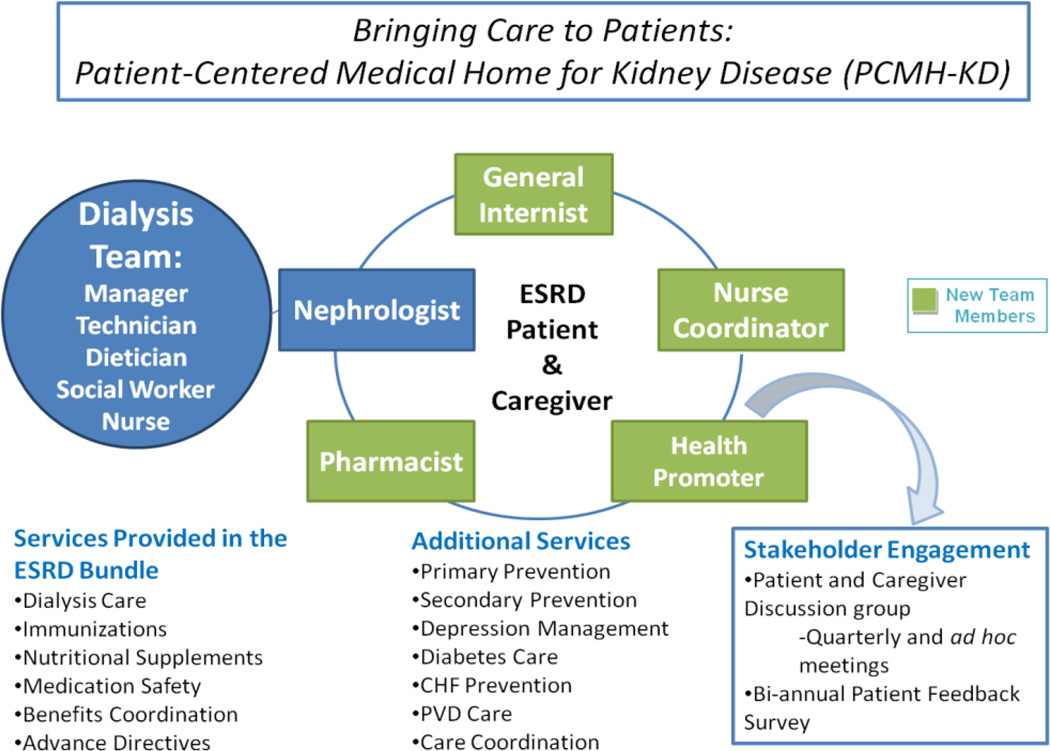
The PCMH-KD intervention expands the existing care team of the dialysis unit (comprised of a nephrologist, dialysis nurse/nurse manager, dialysis technician, social worker, and dietitian) to include a nurse coordinator, general internist, pharmacist, and CHW, all of whom will see the patient during dialysis treatments and separately as needed (Figure 1). The qualifications and responsibilities of the new team members are summarized in Table 2. UIHS-D already employs a pharmacist who assists with medication assessment, dosing, and safety monitoring, and a vascular access nurse who assists with evaluation and scheduling for new vascular access. Neither a pharmacist nor a vascular access nurse is presently available at the Fresenius site. This is a common distinction between university-affiliated centers and freestanding centers, which will make our evaluation more relevant to subsequent implementation nationally.
Figure 1.
Patient-Centered Medical Home for Kidney Disease (PCMH-KD) model of care
Table 2.
Additional care providers in the PCMH-KD model: qualifications and duties
| Team Member |
Qualifications | Duties |
|---|---|---|
|
General Internist |
Board certified or eligible in internal medicine; training and/or experience with ESRD and dialysis |
Primary care for comorbid conditions; preventive care, including age-appropriate cancer screening; coordinate subspecialty care |
|
Nurse Coordinator |
Masters or BSN- level nurse; training and/or experience with ESRD and dialysis |
Care coordination; monitor episodic inpatient care; deliver patient education; coordinate with surgery and radiology for assessment, planning, and completion of vascular access procedures; monitor vascular access sites |
| Pharmacist | PharmD and RPh | Medication assessment, dosing, and safety monitoring; support medication compliance; immunizations; identify community resources for medication delivery |
|
Community Health Workers (CHW) |
Bilingual in English and Spanish (preferred); trained in medical terminology |
Liaison between community, patient/family, and care team; bridge barriers of acculturation, language, and literacy; coordinate scheduling for transportation and other support to enable patient compliance |
Under the current model of care, a nephrologist and dialysis nurse conduct weekly visits with patients in the dialysis unit, and the dietician and social worker assess the patients on a monthly basis. In addition, the nephrologist, dialysis nurse, dietician, and social worker meet monthly at an interdisciplinary meeting to review the patients’ monthly laboratory test results and clinical care plans. In the PCMH-KD intervention, all members of the multidisciplinary team including the pharmacist, nurse coordinator, general internist, and CHW will round weekly on patients within the hemodialysis unit as the patients receive their regularly scheduled hemodialysis treatments, and they will also attend the monthly interdisciplinary meetings. As described below, each care team member in the intervention will also be available to see patients on a more frequent basis as dictated by patient complexity and care needs.
For patients who elect to participate in the intervention and who do not already have a primary provider who they see on a regular basis, the general internist will serve as their primary care provider. Following an initial comprehensive evaluation, the general internist will provide primary care within the framework of the patients’ regularly scheduled dialysis treatments, based on an individual patient’s need or desire to see the general internist. The internist will also be available for consultation for acute general medical care before, during, or after patients’ regular dialysis treatments, or if the patient prefers, during non-dialysis days. The nurse coordinator will be the clinical case manager for patients receiving dialysis at the participating centers. The nurse coordinator will coordinate communication between the team and care providers at other facilities when dialysis patients are hospitalized or require specialty care that affects or is affected by dialysis to ensure cohesive care transitions for both the patient and the providers. The nurse coordinator will also coordinate with surgery and radiology providers to facilitate vascular access placement and to monitor vascular access complications. The pharmacist will work with the nephrologist and internist in supervising medication management and will be responsible for appropriate drug dosing and monitoring for drug interactions and adverse events. Additionally, the pharmacist will coordinate care with other team members to improve medication adherence, oversee indicated immunizations, and reduce polypharmacy. CHWs will serve as patient navigators and will assist the care team in contextualizing care to the patient’s background and community. Patient navigation will include assistance with scheduling and transportation, identification of and referral to community resources, and provide assistance with patient education and dietary and medication adherence.
2.5. Data Collection and Outcomes
Outcomes to be assessed during this intervention are divided into four categories: patient-reported outcomes, clinical outcomes, healthcare use, and staff perceptions. Table 3 contains a summary of outcomes to be examined in this intervention.
Table 3.
PCMH-KD intervention outcome measures
| Measure | Instrument |
|---|---|
| Patient-Reported Outcomes | |
| Primary care coordination | Primary Care Assessment Survey |
| Quality of life | Kidney Disease Quality of Life-36 (KDQOL-36) |
| Management of chronic disease | Self-Efficacy for Managing Chronic Disease |
| Knowledge of hemodialysis | Chronic Hemodialysis Knowledge Survey |
| Depression Screening | Patient Health Questionnaire-9 |
| Medication compliance | Morisky Medication Adherence |
| Patient satisfaction with dialysis care | Consumer Assessment of Healthcare Providers and System: In-center Hemodialysis Survey |
| Fluid and dietary compliance | Renal Adherence Attitudes Questionnaire |
| Renal Adherence Behavior Questionnaire | |
| Comorbidities | Patient intake form |
| Dialysis history | Patient intake form |
| Clinical Outcomes | |
| Primary disease prevention | Receipt of age-appropriate cancer screenings |
| Receipt of recommended vaccinations | |
| Bone densitometry | |
| Periodontal disease | |
| Secondary disease prevention | Hemoglobin A1c measurement |
| Receipt of aspirin and/or beta-blockers | |
| Permanent vascular access | Type of access: fistula, graft, or catheter |
| Dialysis adequacy | Kt/V |
| Interdialytic weight gain (kg) | |
| Pre-and post-dialysis weight (kg) | |
| Urea reduction ratio | |
| Nutrition status | Serum albumin |
| Anemia management | Serum hemoglobin |
| Serum ferritin | |
| Serum transferrin saturation | |
| Bone and mineral metabolism and electrolytes | Serum parathyroid hormone (PTH) |
| Serum phosphorus | |
| Serum calcium | |
| Serum potassium | |
| Other | Pre- and post-dialysis systolic and diastolic blood pressure |
| Blood stream infections | |
| Treatment compliance: missed dialysis treatments or failure to complete entire dialysis treatment | |
| Current medications | |
| Status on transplant list (yes/no) | |
| Health Care Use | |
| Health care utilization | Number of inpatient hospital admissions |
| Number of readmissions to hospital within 30 days of discharge | |
| Number of avoidable hospitalizations | |
| Number of emergency room visits | |
| Staff Perceptions | |
| Dialysis Team Cohesiveness | Healthcare Team Vitality Instrument |
2.5.1. Procedures and data collection
The period of data collection will last a maximum of 18 months for any subject, but may be less due to withdrawal or changes in eligibility/status over time. After subjects consent to participate, they will be administered a baseline interview, and a maximum of 3 follow-up interviews at 6 months, 12 months, and 18 months (Table 4). Each interview will take approximately 60–90 minutes to complete and will be conducted in the dialysis center before, during or after a patient’s dialysis appointments. A trained member of the research staff will administer the questionnaires and record responses using a tablet computer. If patients prefer to complete the surveys at a time not associated with their dialysis appointments, a separate interview time will be scheduled. All questionnaires are available in Spanish and English, and the interview will be conducted in the participant's preferred language. Patients will be notified that if at any point during the interview they become fatigued and no longer wish to continue, the interview will be stopped and a time will be scheduled to complete the remainder of the interview. Participants will be compensated for their time and effort.
Table 4.
Interview schedule for PCMH-KD study participants
| Consent | Baseline | 6 month | 12 month | 18 month | |
|---|---|---|---|---|---|
| Informed consent | X | ||||
| Medical record consent | X | ||||
| Contact information | X | ||||
| Intake form | X | ||||
| Patient update | X | X | X | ||
| PCAS | X | X | |||
| KDQOL-36 | X | X | X | X | |
| SEMCD | X | X | X | X | |
| CHeKS | X | X | X | X | |
| PHQ-9 | X | X | X | X | |
| Morisky | X | X | X | X | |
| CAHPS-ICH | X | X | X | X | |
| RAAQ | X | X | X | X | |
| RABQ | X | X | X | X |
2.5.2. Measures
Both patient-reported outcomes, i.e. subjective measures and objective measures will be assessed. The primary subjective outcome is quality of life. The primary objective outcome is healthcare utilization. We have divided the outcomes into four content areas as reflected in Table 3 and described below.
2.5.2.1. Patient reported outcomes
Patient-reported outcomes as summarized in Table 3 will be obtained at baseline and at intervals as outlined in Table 4. Data regarding comorbidities and dialysis history will be reported by patients at baseline or abstracted from the patient medical record and recorded on a patient intake form by a member of the research team upon patient enrollment in the study. The remainder of the patient-reported outcomes will be obtained using structured interviews of the patient by a member of the research team. Primary care coordination will be assessed using a modified version of the Primary Care Assessment Survey.17 Our version includes Q1 (if there is a doctor who the patient considers to be his or her primary physician) and questions for the following 5 scales: Longitudinal Continuity, Knowledge of Patient, Integration, Communication, and Interpersonal Treatment. Quality of life (QOL) will be measured using the Kidney Disease Quality of Life-36 (KDQOL-36) survey, a kidney-disease-specific quality of life instrument that assesses both general mental and physical health QOL domains as well as kidney-disease and dialysis-related domains of QOL.18 Patient confidence in self-management of chronic disease will be assessed using the Self-Efficacy for Managing Chronic Disease instrument, a 6-item survey.19 The Chronic Hemodialysis Knowledge Survey, a 23-item questionnaire regarding aspects of dialysis care, will be used to determine patient knowledge of hemodialysis.20 For depression screening, the Patient-Health Questionnaire-9 will be used; it has been validated as a screening tool for depression.21 Medication compliance will be evaluated with the Morisky Medication Adherence instrument, an 8-item survey which has been shown to be reliably associated with blood pressure control.22 Patient satisfaction with dialysis will be determined using the Consumer Assessment of Healthcare Providers and System survey.23 Compliance with dietary and fluid restrictions prescribed for ESRD patients will be measured using the Renal Adherence Attitudes Questionnaire (RAAQ) and the Renal Adherence Behavior Questionnaire (RABQ) which assess attitudes toward adherence to dietary restrictions as well as self-reported adherence behaviors.24
2.5.2.2. Clinical outcomes and health care use
Clinical outcomes (Table 3) will also be obtained at baseline. Primary disease prevention parameters such as receipt of age-appropriate cancer screening, bone densitometry, and receipt of recommended vaccinations will be assessed every 6 months using abstraction from the medical record by research personnel. Any clinical outcome endpoint that has occurred since the last event assessment will be recorded. Laboratory parameters such as those related to anemia management, nutrition status, bone and mineral metabolism, urea reduction ratio, and kt/V (a dimensionless quantity used to assess dialysis adequacy) are measured monthly as part of routine dialysis care. Clinical events such as blood stream infections, treatment compliance, transplant status, and type of dialysis access in use will be assessed every 3 months. Average interdialytic weight gain, pre- and post-dialysis weight, and pre- and post-dialysis systolic and diastolic blood pressure will be recorded for the first week of each calendar month.
Information regarding health care utilization will also be included as a category of study outcomes. Events including inpatient hospitalization, hospital readmission within 30 days of discharge, avoidable hospitalization, and emergency room visits will be abstracted from the medical record by study personnel. Starting from baseline, these events will be assessed every 6 months.
2.5.3. Staff component of the intervention
Staff perception of care will also be included as an outcome. The Healthcare Team Vitality Instrument, a questionnaire to assess the perception of nurses and other members of the healthcare team regarding the function of interdisciplinary teams, will be used for this purpose.25 The instrument will be administered to staff at baseline and every 6 months during the period of intervention. All clinicians and staff (i.e. doctors, nurses, dialysis technicians, support and customer service staff, dieticians, social workers, nurse/clinic manager) who are currently employees of either the UIHS-D or FMC will be eligible to participate in this portion of the study. Staff will be consented to participate in the study. The survey will take less than five minutes to complete and will be administered by trained research personnel either in person or over the telephone.
2.5.4. Patient engagement
Patient and stakeholder discussion groups will be convened at both dialysis units to guide implementation of the intervention in an iterative process. A total of 6–10 patients per clinic will be invited by the project coordinator to participate in a single 90–120 minute discussion group, with the option to participate in ongoing discussion groups held every 3 months for the remainder of the first two years of the study. Patients will be given the option to bring an eligible family member/caregiver to participate in the meeting. Over time, additional volunteers may be sought to ensure 10–15 participants per session.
In implementing this stakeholder discussion group, we expect that patients and their family member’s input will help the research team plan, implement, and evaluate the new care model. In particular, we will seek feedback on when to schedule availability of the primary care physician, pharmacist and CHWs in relation to dialysis treatment. We will also discuss particular aspects of kidney disease management to gain insight into patient and family member educational needs and interests. This research may help inform critical decisions that healthcare systems face when structuring care delivery models for the patients who rely on them.
2.6. Sample Size
In contrast to a typical clinical trial, this study involves no randomization of patients or clinics: the focus is on improving outcomes and measuring change within each patient and within each clinic. One clinic handles 138 patients, the other 78, for a total of at most 216 patients available at a given occasion, and with turnover, conservatively estimate approximately 243 patients available over the course of the study. As the nature of a dialysis center is that new patients may enter and exit the center at different points over time, we used a periodization approach to take into account this patient turnover in our sample size calculations. Additionally, this accounts for the fact that patients’ responses are highly correlated within themselves, but that departing patients and the ones who arrive to take their places in a clinic are not correlated.
We employ the method of Rochon26,27,28 to calculate power and incorporate a cross-time correlation of outcomes rho = 0.70, which is appropriate for chronic disease. Using our primary outcome measure, Kidney Disease Quality of Life (KDQOL), we note that typical ESRD patients score about 48.6 points on the KDQOL scale (SD ≈ 11.3).2,4,29,30 With a goal to raise this figure by 10% to 53.5, which can be clinically meaningful, we calculated power based on a comparison of KDQOL score after 12 months of exposure to the intervention to baseline score (or score at entry for those who entered later). Using SAS macro GEESIZE version 3.1,26 under a Gaussian GEE model for repeated measures, 77 patients total are required to test this hypothesis with power 0.80 at the alpha = 0.05 two-sided level of significance. Assuming a mild degree of clustering within clinics (intraclass rho = 0.01) and conservatively using the larger clinic size, the design effect is 1.37, yielding a required sample for 0.80 power of 1.37 × 77 = 106, well below the estimated 243 relevant patients available. Even if we then conservatively correct for 10% patient loss (106/.90 = 118), ample statistical power remains assured. To ensure that we have sufficient power to detect a smaller effect on KDQOL than we anticipate, we repeated the power analysis for a 7% improvement in the score rather than a 10% improvement. In this pessimistic scenario, 143 patients are required to test for a 7% improvement at power 0.80 and two-sided alpha = 0.05. Applying the design effect yields 1.37 × 143 = 196, and correcting for 10% patient loss yields 196/.9 = 218, which also lies below the 243 relevant patients available.
2.7. Data management and analysis plan
2.7.1. Data management
All patient-reported outcomes will be collected via questionnaires administered orally, in-person by a trained member of the research staff. Responses will be entered directly in Research Electronic Data Capture (REDCap), a secure, web-based application for designing and integrating data capture and data management. REDCap includes multiple features for ensuring data quality, such as data type validation (integer, date, etc.), range checks, and missing data notifications. REDCap was developed specifically around HIPAA security guidelines, and all web-based information transmission is encrypted.
All data will be imported into SAS for final data cleaning and analysis. We will run standard checks for outliers and other errors common to complex data entry and processing.
2.7.2. Analysis Plan
The primary analysis will examine longitudinal data to evaluate the impact of the intervention on the outcomes relative to usual care during the pre-intervention period. In addition, descriptive analyses will be carried out to characterize patient subgroups. In particular, we will describe our study population by ethnicity (African American, Hispanic and non-African Americans/non-Hispanics), socioeconomic status (income level and employment status), and age (age less than 65 years versus older). Clinically relevant patient subgroups will also be described including length of dialysis (less than 180 days or beyond) and comorbidities (diabetes, hypertension, congestive heart failure, chronic lung disease, and comorbidity level) across sites. We will also use sensitivity analysis to characterize and address outliers and influential cases. We will also measure and describe the intensity and fidelity of the PCMH-KD model utilized by patients so we can understand which elements are most often used and valued by patients.
3. Discussion
Although the absolute number of ESRD patients receiving maintenance hemodialysis in the United States is a relatively small proportion of the general population, this group represents a disproportionately large proportion of healthcare resource utilization and also suffers exceptionally high morbidity, mortality, and poor quality of life. The PCMH-KD intervention described here is a multidisciplinary comprehensive team approach that is designed to address the well-recognized lack of coordinated care that these patients experience due to the complex nature of their disease and, often, additional co-morbid conditions.7,11 This intervention is particularly timely as increased attention within healthcare in general is focused on both improving patient-centered outcomes and reducing inpatient and emergency room use by enhanced care coordination and reduced fragmentation of care. Additionally, the Centers for Medicare and Medicaid Services (CMS) announced a Comprehensive ESRD Care Initiative this year seeking applications to form ESRD Seamless Care Organizations (ESCOs) among dialysis providers, creating a system in which providers in an ESCO would be responsible for all aspects of a dialysis patient’s care, not just that related to ESRD.31
The potential cost saving of these new care models is important, yet requisite sample sizes and observation periods needed to make these calculations are often lacking in studies due to financial constraints of study budgets or funding agency restrictions as in the case of PCORI. Although our study will not provide a formal cost evaluation it will provide insight about the short-term impacts of the intervention on health care resource use relative to the additional investment of new care providers. It will provide an assessment of the value added by the expanded health care team on patients’ QOL and health care resource use. Further research is needed to address the full economic impact of new models of care, especially the impact on the patient centered costs.
This novel intervention will be implemented in both an academic and private/for-profit care setting. These varied care settings will highlight which aspects of the care intervention are most relevant to a given clinical practice setting, thereby informing development of care models particular to a given setting. It may become apparent, for example, that certain additional providers will function in different capacities depending on the setting.
The CMS has also begun to recognize the importance of assessing patient-reported outcomes as part of routine ESRD care.12 Measurement of QOL using the KDQOL-36 instrument is now mandated for hemodialysis patients.12 In addition, the Renal Network has recently created the Patient Representative Program, in which dialysis units are asked to identify a Patient Representative among their dialysis patient population to identify opportunities to improve patient-centered care and to improve patient engagement in their dialysis care.32 Our intervention emphasizes the patient as the key figure of the healthcare team. The patient engagement aspect of our intervention may therefore inform evolution of the Patient Representative Program.
Our study does have some limitations. Our study employs a health system intervention using a pre-post quasi-experimental design. Lacking features present in a randomized double blind controlled trial, (i.e., subject randomization or blinding of subjects and providers) our design introduces some potential bias. However, inclusion of two dialysis centers with our conservative approach for power calculation and inclusion of both subjective and objective outcome measures, use of sensitivity analysis for outlier evaluation and process evaluation to assess the fidelity of the intervention, we are confident we have adequately addressed these potential biases and that our study will have sufficient internal and external validity to provide insight about the effects of the PCMH model on care of this medically complex patient population. Our results may provide information upon which to base a future multisite randomized controlled trial.
While the implementation of the PCMH model has been shown to be cost-saving in at least one study of chronic disease management in children,33 few studies have addressed the economic impact of PCMH models. Although our study is not powered to explicitly examine costs, our study is powered to examine the impact on healthcare use, specifically inpatient admissions and emergency room visits.
An increased emphasis on both reducing unnecessary healthcare utilization and improving care from the patient perspective has created an urgent need for novel healthcare interventions that address these issues. Our novel PCMH-KD intervention is based on models of care that have been implemented with success in other complex chronic illnesses. The knowledge gained from this study will provide critical insights about the dynamics of the PCMH model for chronic disease management generally, and for hemodialysis patients specifically. Findings from this study will help to generate solutions to improve patient reported QOL and health care resource use of those with ESRD in the US.
Acknowledgments
This work is supported by funding from the Patient-Centered Outcomes Research Institute (PCORI), contract #IH-12-11-5420. The content is solely the responsibility of the authors and does not necessarily reflect the views of PCORI. Additional support for this project was provided by the Office of the Vice President for Health Affairs at the University of Illinois at Chicago.
References
- 1.United States Renal Data System, 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2014. [Google Scholar]
- 2.Hynes DM, Stroupe KT, Fischer MJ, et al. Comparing VA and private sector healthcare costs for end-stage renal disease. Med Care. 2012;50(2):161–170. doi: 10.1097/MLR.0b013e31822dcf15. [DOI] [PubMed] [Google Scholar]
- 3.Longenecker JC, Coresh J, Powe NR, et al. Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: the CHOICE Study. J Am Soc Nephrol JASN. 2002;13(7):1918–1927. doi: 10.1097/01.asn.0000019641.41496.1e. [DOI] [PubMed] [Google Scholar]
- 4.Mapes DL, Lopes AA, Satayathum S, et al. Health-related quality of life as a predictor of mortality and hospitalization: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Kidney Int. 2003;64(1):339–349. doi: 10.1046/j.1523-1755.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 5.Lopes AA, Bragg-Gresham JL, Satayathum S, et al. Health-related quality of life and associated outcomes among hemodialysis patients of different ethnicities in the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis Off J Natl Kidney Found. 2003;41(3):605–615. doi: 10.1053/ajkd.2003.50122. [DOI] [PubMed] [Google Scholar]
- 6.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. Association among SF36 quality of life measures and nutrition, hospitalization, and mortality in hemodialysis. J Am Soc Nephrol JASN. 2001;12(12):2797–2806. doi: 10.1681/ASN.V12122797. [DOI] [PubMed] [Google Scholar]
- 7.Zimmerman DL, Selick A, Singh R, Mendelssohn DC. Attitudes of Canadian nephrologists, family physicians and patients with kidney failure toward primary care delivery for chronic dialysis patients. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2003;18(2):305–309. doi: 10.1093/ndt/18.2.305. [DOI] [PubMed] [Google Scholar]
- 8.Sweeney L, Halpert A, Waranoff J. Patient-centered management of complex patients can reduce costs without shortening life. Am J Manag Care. 2007;13(2):84–92. [PubMed] [Google Scholar]
- 9.Hussey PS, Schneider EC, Rudin RS, Fox DS, Lai J, Pollack CE. Continuity and the costs of care for chronic disease. JAMA Intern Med. 2014;174(5):742–748. doi: 10.1001/jamainternmed.2014.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bayliss EA, Bhardwaja B, Ross C, Beck A, Lanese DM. Multidisciplinary team care may slow the rate of decline in renal function. Clin J Am Soc Nephrol CJASN. 2011;6(4):704–710. doi: 10.2215/CJN.06610810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anvari E, Mojazi Amiri H, Aristimuno P, Chazot C, Nugent K. Comprehensive and personalized care of the hemodialysis patient in tassin, france: a model for the patient-centered medical home for subspecialty patients. ISRN Nephrol. 2013;2013:792732. doi: 10.5402/2013/792732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Department of Health and Human Services, Centers for Medicare and Medicaid Services. Conditions for Coverage for ESRD Facilities, final rule (42 CFR Part 494.80) Fed Reg 20393. 2008 [Google Scholar]
- 13.Carter BL, Bosworth HB, Green BB. The hypertension team: the role of the pharmacist, nurse, and teamwork in hypertension therapy. J Clin Hypertens Greenwich Conn. 2012;14(1):51–65. doi: 10.1111/j.1751-7176.2011.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Philis-Tsimikas A, Walker C, Rivard L, et al. Improvement in diabetes care of underinsured patients enrolled in project dulce: a community-based, culturally appropriate, nurse case management and peer education diabetes care model. Diabetes Care. 2004;27(1):110–115. doi: 10.2337/diacare.27.1.110. [DOI] [PubMed] [Google Scholar]
- 15.Beckham S, Kaahaaina D, Voloch K-A, Washburn A. A community-based asthma management program: effects on resource utilization and quality of life. Hawaii Med J. 2004;63(4):121–126. [PubMed] [Google Scholar]
- 16.Norris SL, Chowdhury FM, Van Le K, et al. Effectiveness of community health workers in the care of persons with diabetes. Diabet Med J Br Diabet Assoc. 2006;23(5):544–556. doi: 10.1111/j.1464-5491.2006.01845.x. [DOI] [PubMed] [Google Scholar]
- 17.Safran DG, Kosinski M, Tarlov AR, et al. The Primary Care Assessment Survey: tests of data quality and measurement performance. Med Care. 1998;36(5):728–739. doi: 10.1097/00005650-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 1994;3(5):329–338. doi: 10.1007/BF00451725. [DOI] [PubMed] [Google Scholar]
- 19.Lorig K, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program for patients with chronic disease. 2001;4(6):256–262. [PubMed] [Google Scholar]
- 20.Cavanaugh KL, Wingard RL, Hakim RM, Elasy TA, Ikizler TA. Patient dialysis knowledge is associated with permanent arteriovenous access use in chronic hemodialysis. Clin J Am Soc Nephrol CJASN. 2009;4(5):950–956. doi: 10.2215/CJN.04580908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens Greenwich Conn. 2008;10(5):348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Paddison CAM, Elliott MN, Haviland AM, et al. Experiences of care among Medicare beneficiaries with ESRD: Medicare Consumer Assessment of Healthcare Providers and Systems (CAHPS) survey results. Am J Kidney Dis Off J Natl Kidney Found. 2013;61(3):440–449. doi: 10.1053/j.ajkd.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Rushe H, McGee HM. Assessing adherence to dietary recommendations for hemodialysis patients: the Renal Adherence Attitudes Questionnaire (RAAQ) and the Renal Adherence Behaviour Questionnaire (RABQ) J Psychosom Res. 1998;45(2):149–157. doi: 10.1016/s0022-3999(97)00228-6. [DOI] [PubMed] [Google Scholar]
- 25.Upenieks VV, Lee EA, Flanagan ME, Doebbeling BN. Healthcare Team Vitality Instrument (HTVI): developing a tool assessing healthcare team functioning. J Adv Nurs. 2010;66(1):168–176. doi: 10.1111/j.1365-2648.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 26.Rochon J. Application of GEE procedures for sample size calculations in repeated measures experiments. Stat Med. 1998;17(14):1643–1658. doi: 10.1002/(sici)1097-0258(19980730)17:14<1643::aid-sim869>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Dahmen G, Rochon J, König IR, Ziegler A. Sample size calculations for controlled clinical trials using generalized estimating equations (GEE) Methods Inf Med. 2004;43(5):451–456. [PubMed] [Google Scholar]
- 28.Dahmen G, Ziegler A. Independence estimating equations for controlled clinical trials with small sample sizes--interval estimation. Methods Inf Med. 2006;45(4):430–434. [PubMed] [Google Scholar]
- 29.Saban KL, Bryant FB, Reda DJ, Stroupe KT, Hynes DM. Measurement invariance of the kidney disease and quality of life instrument (KDQOL-SF) across veterans and non-veterans. Health Qual Life Outcomes. 2010;8:120. doi: 10.1186/1477-7525-8-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wingard RL, Pupim LB, Krishnan M, Shintani A, Ikizler TA, Hakim RM. Early intervention improves mortality and hospitalization rates in incident hemodialysis patients: RightStart program. Clin J Am Soc Nephrol CJASN. 2007;2(6):1170–1175. doi: 10.2215/CJN.04261206. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Medicare and Medicaid Services. Comprehensive ESRD Care Initiative. http://innovation.cms.gov/initiatives/comprehensive-esrd-care/
- 32.The Renal Network. Network Patient Representative Program. http://therenalnetwork.org/services/Patient_Rep_Program.html. [Google Scholar]
- 33.Mosquera RA, Avritscher EBC, Samuels CL, et al. Effect of an enhanced medical home on serious illness and cost of care among high-risk children with chronic illness: a randomized clinical trial. JAMA. 2014;312(24):2640–2648. doi: 10.1001/jama.2014.16419. [DOI] [PubMed] [Google Scholar]