Summary
Aeroterrestrial green algae are exposed to desiccation in their natural habitat, but their actual volume changes have not been investigated. Here, we measure the relative volume reduction (RVRED) in Klebsormidium crenulatum and Zygnema sp. under different preset relative air humidities (RH). A new chamber allows monitoring RH during light microscopic observation of the desiccation process. The RHs were set in the range of ∼4 % to ∼95% in 10 steps. RVRED caused by the desiccation process was determined after full acclimation to the respective RHs. In K. crenulatum, RVRED (mean ± SE) was 46.4 ± 1.9%, in Zygnema sp. RVRED was only 34.3 ± 2.4% at the highest RH (∼95%) tested. This indicates a more pronounced water loss at higher RHs in K. crenulatum versus Zygnema sp. By contrast, at the lowest RH (∼4%) tested, RVRED ranged from 75.9 ± 2.7% in K. crenulatum to 83.9 ± 2.2% in Zygnema sp. The final volume reduction is therefore more drastic in Zygnema sp. These data contribute to our understanding of the desiccation process in streptophytic green algae, which are considered the closest ancestors of land plants.
Keywords: Desiccation, hydraulic parameter, light microscopy, Streptophyta, water loss
Lay description
Green algae living on the surface of soil are potentially exposed to drying. When they lose water, the volume of the alga is changed, but currently no measurements of these changes are available. We here investigate two green algae from different classes. The algae are exposed to defined relative air humidities in a specially constructed microscopic chamber, and the volume changes are measured. A distinct behaviour of the two investigated algae gives new insights in the lifestyle of terrestrial algae, the closest relatives of land plants.
Introduction
Green algae have the ability to perform photosynthesis and their major distribution is in aquatic habitats making them rather an ecological unit than a taxonomic entity. In fact, according to recent phylogenetic research, green algae can be divided in two main lineages (Leliaert et al., 2012): (1) Chlorophyta that contains the majority of described green algal species and (2) Streptophyta that includes the Charophytes, a paraphyletic group of freshwater and terrestrial green algae, as well as all land plants, the Embryophytes (Leliaert et al., 2012). Among both lineages, several groups with terrestrial representatives occur. In case of the Chlorophyta, the classes Ulvophyceae, Trebouxiophyceae and Chlorophyceae include some taxa living in terrestrial habitats, e.g. the Trentepohliales of the Ulvophyceae (Leliaert et al., 2012), the members of Trebouxiophyceae (‘lichen algae group’) and sparse genera in the Chlorophyceae, e.g. Fritschiella (Holzinger & Karsten, 2013). In the streptophyte lineage, terrestrial members can be found in the classes Klebsormidiophyceae, Zygnematophyceae and Coleochaetopyhceae (Graham et al., 2012; Holzinger & Karsten, 2013; Herburger & Holzinger, 2015; Mikhailyuk et al., 2015). The closest relatives to land plants can be found among charophyte green algae (Wodniok et al., 2011; Timme et al., 2012; Delwiche & Cooper, 2015). Their genome holds primary factors for plant terrestrial adaptation (Hori et al., 2014), and transcriptional changes upon severe desiccation stress demonstrated a land plant like defence reaction in Klebsormidium (Holzinger et al., 2014). Raffinose family oligosaccharides have been found to be upregulated upon desiccation stress likely contributing to the osmotic potential (Holzinger et al., 2014). Increasing knowledge on cell wall properties of these algal groups has become available recently, demonstrating the close relationship to land plants (Sørensen et al., 2011; Mikhailyuk et al., 2014; Herburger & Holzinger, 2015). However, green algae are poikilhydric organisms that do not have cuticles or similar structures to protect from water evaporation (Delaux et al., 2013).
Although the hydraulic parameters (osmotic potential at full turgor and relative water content at the turgor loss point) in pokilohydric plants of the embryophytes, ferns, mosses and lichens have been well investigated (see Table 1), only little information is available in aeroterrestrial green algae (Holzinger & Karsten, 2013). This lack of data may be related to the difficulty of determining the volume or weight of terrestrial green algal samples due to their small size, mostly ranging between 5 and 25 μm in diameter (Rindi et al., 2011; Herburger et al., 2015). By contrast, the osmotic potential has been determined in Klebsormidium and Zygnema (Kaplan et al., 2012, 2013). In these studies, the water potential at the turgor loss point (ΨTLP) was determined by means of the ‘incipient plasmolysis technique’. For plasmolysis fully turgescent plant cells, like the cell filaments of the investigated green algae, were exposed to osmotically active solutions with increasing concentrations and the value where 50% of the cells plasmolyzed determined microscopically. At the equilibrium point, the cells have the same osmolarity as the external solution, which allowed determining the osmotic potential. In K. crenulatum a ΨTLP of ‐2.09 MPa whereas in Klebsormidium nitens a ΨTLP of ‐1.67 MPa was found (Kaplan et al., 2012). In the same way, ΨTLP values of ‐1.67 and ‐0.8 MPa were determined in different strains of Zygnema sp. (Kaplan et al., 2013).
Table 1.
Osmotic potential at full turgor (Ψosat) and relative water content at the turgor loss point = incipient plasmolysis (RWCTVP) of different poikilohydric species of angiosperms, ferns, mosses and lichens. The references, from which the data were obtained, are also listed
| Species name | Ψosat (‐MPa) | RWCTVP (%) | References |
|---|---|---|---|
| Angiosperms | |||
| Dicotyledons | |||
| Myrothamnus flabellifolia | 1.92 | 90 | Beckett (1997) |
| Monocotyledons | |||
| Xerophyta scabrida | 0.55 | ― | Tuba et al. (1994) |
| Xerophyta viscosa | 1.41 | 81 | Beckett (1997) |
| Ferns | |||
| Gymnopteris hispida | 1.3 | ― | Walter & Stadelmann (1974) |
| Cheilanthes lindheimeri | 2.4 | ― | ―‖― |
| Trichomanes melanotrichum | 1.77 | 78 | Beckett (1997) |
| Mosses | |||
| Dumortiera hirsuta | 0.38 | 85 | Proctor et al. (1998) |
| Marchantia polymorpha | 0.38 | 60 | ―‖― |
| Conocephalum conicum | 0.54 | 45 | ―‖― |
| Dumortiera hirsuta | 0.59 | 94 | Beckett (1997) |
| Hookeria lucens | 0.95 | 70 | Proctor et al. (1998) |
| Sphagnum girgensohnii | 0.98 | 36 | Hájek & Beckett (2008) |
| Atrichum androgynum | 1.05 | 77 | ―‖― |
| Sphagnum cuspidatum | 1.08 | 62 | ―‖― |
| Sphagnum tenellum | 1.09 | 41 | ―‖― |
| Sphagnum magellanicum | 1.12 | 61 | ―‖― |
| Mnium hornum | 1.21 | 70 | Proctor et al. (1998) |
| Neckera crispa | 1.27 | 65 | ―‖― |
| Sphagnum fuscum | 1.31 | 61 | Hájek & Beckett (2008) |
| Rhytidiadelphus loreus | 1.34 | 70 | Proctor et al. (1998) |
| Tortula ruralis | 1.36 | 75 | ―‖― |
| Antitrichia curtipendula | 1.47 | 65 | ―‖― |
| Andreaea alpina | 1.59 | 70 | ―‖― |
| Anomodon viticulosus | 1.65 | 65 | ―‖― |
| Frullania tamarisci | 1.78 | 60 | ―‖― |
| Porella capensis | 1.89 | 71 | Beckett (1997) |
| Homalothecium lutescens | 2.08 | 70 | Proctor et al. (1998) |
| Polytrichum commune | 2.09 | 75 | ―‖― |
| Plagiomnium rhynchophorum | 2.37 | 55 | Beckett (1997) |
| Lichens | |||
| Peltigera leucophlebia | 0.62 | 70 | Nardini et al. (2013) |
| Peltigera canina | 1.00 | 59 | Beckett (1995) |
| Lobaria scrobiculata | 1.43 | 54 | ―‖― |
| Cladonia convoluta | 1.52 | 60 | Proctor et al. (1998) |
| Peltigera rufescens | 1.55 | 60 | Nardini et al. (2013) |
| Sticta limbata | 1.61 | 51 | Beckett (1995) |
| Ramalina celastri | 1.95 | 44 | ―‖― |
| Roccella hypomecha | 2.19 | 42 | Beckett (1997) |
| Usnea undulata | 2.19 | 46 | Beckett (1995) |
| Pseudocyphellaria aurata | 2.23 | 49 | ―‖― |
| Parmotrema tinctorum | 2.52 | 47 | ―‖― |
| Roccella montagnei | 2.79 | 43 | ―‖― |
In this study, we developed and tested a special chamber that allows microscopic observations at several preset relative air humidity (RH) levels. To illustrate the capacities of this chamber, we analysed the desiccation‐induced volume changes of the cytoplasm in streptophyte green algae. We hypothesized that green algae from distinct classes (Klebsormidiophyceae, Zygnematophycae) show different desiccation patterns. We measured the relative volume reduction (RVRED) of the protoplast at different preset RH levels under controlled conditions. This allowed us to get information on the water loss at different RHs and to enhance our understanding of desiccation processes in the studied organisms.
Materials and methods
Plant material
Two strains from different charophyte classes were selected for the experiments, as contrasting desiccation behaviour was expected from previous studies (Kaplan et al., 2012, 2013).
(1) Klebsormidium crenulatum (Kützing) Lokhorst (Lokhorst & Star, 1985) was previously isolated from an alpine soil sample (Obergurgl, Tyrol, Austria, 46° 50’ 99.8’’ N, 11° 00’ 90.3’’ E; 2,350 m a.s.l.; Karsten et al., 2010; Culture Collection of Algae Göttingen, SAG 2415). K. crenulatum was cultivated in liquid‐modified Bold's Basal Medium (BBM; Starr & Zeikus, 1993) at a light dark regime of 16–8 h at 20°C during light and 15°C during dark phase (Kaplan et al., 2012).
(2) Zygnema sp. (C.Agardh) ‘Saalach’ (SAG 2419), previously collected from the sandy littoral zone of the River Saalach near the City of Salzburg (Austria, 47°47′ 8.70″ N, 12°56′ 42.66″ O; 440 m a.s.l.; Herburger et al., 2015). Zygnema sp. was cultivated in conventional BBM (Bischoff & Bold, 1963) at the same light‐dark regime and temperature as described above.
Test chamber
A test chamber that allowed: (1) maintaining a constant RH for desiccation experiments and (2) microscopic observation of the samples was constructed (Fig. 1). The test chamber was two‐storied, where the ‘upper storey’ consisted of a 120 × 120 mm square transparent polycarbonate Petri dish (Nr. 18252, Gammarad, Bologna, Italy). There, the salt solution or silica gel and in a separated space (created by a 10 mm plastic delimitation) the air humidity sensor (mini data logger PCE‐MSR145W‐THPA, PCE Instruments, Meschede, Germany) were placed. This ‘upper storey’ had a circular hole, where a smaller, circular Petri dish (60 mm Ø, Nr. 628160, Greiner Labortechnik, Kremsmünster, Austria) was attached as the ‘lower storey’ which fitted into the mechanical stage of the microscope. In this ‘lower storey’, the algal samples were directly placed for observation. To separate the sample from the salt solution or silica gel, a plastic ring (9 mm high, made from the lid of round Petri dish), was attached around the hole of the upper Petri dish. The test chamber was covered by a square lid with a rubber seal (140 × 140 mm, 4 mm thick, transparent acrylic glass). Two motorized mini fans (MagLev Motor Blower, Spec. No. D01000940G‐00, 17 × 17 × 3 mm, Sunonwealth Electric Machine Industry, Kaohsiung City, Taiwan) were attached to the lid, and ensured a constant ventilation of the chamber.
Figure 1.
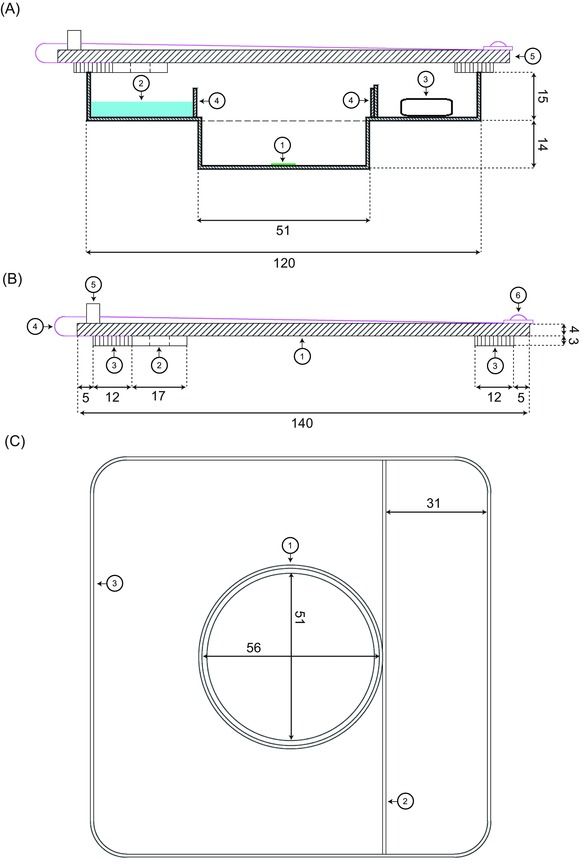
Two‐storied test chamber. (A) Two‐dimensional, cross‐sectional view of the test chamber during a desiccation experiment. Bottom storey with: (1) algal sample; top storey with (2) salt solution; (3) air humidity and temperature sensor; (4) plastic ring of 9 mm height, preventing the contact of the salt solution with the investigated specimen; (5) Perspex lid. (B) Detailed description of the Perspex lid: (1) square panel of Perspex, forming the central body of the lid; (2) cross‐section of one of the two motorized mini fans; (3) sponge rubber seal; (4) power supply cable of one of the two motorized mini fans; (5) power adapter; (6) screw fixing the power supply cable. (C) Top view of the test chamber: (1) plastic ring, (4) in (A); (2) linear plastic delimitation of 10 mm height, preventing a contact of the salt solution with the sensor; (3) outer margin of the petri dish. All dimensions in millimetre.
Experimental setting of the RH
The precise setting of the RH inside the hermetically sealed test chamber was achieved by different nonsaturated lithium chloride (LiCl) solutions (≥98%, Sigma‐Aldrich, Vienna, Austria) according to Hay et al. (2008), see Table 2. Alternatively, a saturated K2CO3 solution (≥99.0%, Sigma‐Aldrich) was used. The lowest RH generated by saturated LiCl solution is 11.2% at 20°C (Hay et al., 2008). Therefore, silica gel (with moisture indicator, Sigma‐Aldrich) was used to generate RH values of 3.7–4.2% (Table 2).
Table 2.
Salt solutions and silica gel used to generate different RH levels in the test chamber; slightly different values were measured after equilibration of the test organisms Klebsormidium crenulatum and Zygnema sp
| Material | Quantity (g/100 mL) | RH (%) K. crenulatum | RH (%) Zygnema sp. |
|---|---|---|---|
| Lithium chloride (LiCl) | 4.8 | 95.4 | 95.8 |
| 9.4 | 89.5 | 91.7 | |
| 13.0 | 85.3 | 85.3 | |
| 17.1 | 78.3 | 78.4 | |
| 24.1 | 65.5 | 66.4 | |
| 30.0 | 56.9 | 56.4 | |
| 43.5 | 31.8 | 32.3 | |
| 64.1 | 15.9 | 15.6 | |
| Potassium carbonate (K2CO3) | 112 | 48.1 | 49.5 |
| Silica gel | ― | 3.7 | 4.2 |
Experimental procedure
The test organisms (K. crenulatum, Zygnema sp.) were placed in a volume of 20 μL culture medium in the ‘lower storey’ of the test chamber. In the ‘upper storey’, the respective salt solution or silica gel was filled. The cell filaments were spread and microscopic images were taken immediately. The cells were then allowed to completely equilibrate for up to 12 h at 20°C to the respective RH before 10–15 random images were taken. The measurements of the diameters at individual protoplasts were performed only on filaments that were found solitary after desiccation, where the protoplasts as well as the cell lumen diameter (CLD) were clearly visible. In K. crenulatum, an average of 22 protoplasts per RH step and in Zygnema sp. an average of 16 protoplasts per RH step were measured.
Determination of the RVRED
The desiccation process was monitored by an inverted Zeiss Axiovert 200M microscope (20x, NA = 0.50; 40x, NA = 0.75; Carl Zeiss AG, Oberkochen, Germany). To determine the protoplast volume, the CLD and the diameter of the desiccated protoplast (PDd) were measured in individual cells and the reduction of the protoplast volume was quantified (Zeiss AxioVision 4.7.1 software).
In this way, assuming a cylindrical shape, the protoplast volume at saturation (Vs in Eq. (1)) and after desiccation (Vd in Eq. (2)) was calculated by the following equations (for abbreviations, see Fig. 1):
| (1) |
| (2) |
The RVRED (%) of the PDd related to the protoplast volume at the initial, saturated state was determined according to Eq. (3).
| (3) |
RVRED were then plotted against RH values of both studied species.
Statistical evaluation
Significant differences between mean values of RVRED [%] for each investigated genus (K. crenulatum, Zygnema sp.) were calculated with one‐way ANOVA followed by Games–Howell's post hoc test; p < 0.01) using SPSS software. Significant differences of RVRED [%] between the two genera at a certain RH level were calculated by Student's t‐test (p < 0.05) by SPSS software.
Results
Test chamber
We first tested our newly constructed test chamber for functionality at different RH. The chamber was filled with the respective LiCl solutions of different concentrations or the saturated K2CO3 solution or with silica gel to generate different levels of RH (Table 2). At high levels of RH (>80%), the RH inside the test chamber reached the saturation point within 3–4 h (Fig. 3), over silica gel, the saturation point was achieved in less than 3 h (Fig. 3). The recorded RH differed only slightly between experiments (Table 2).
Figure 3.
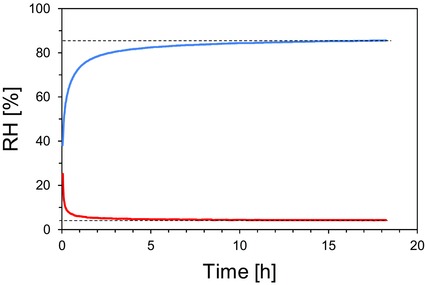
Exemplary presentation of time courses of the RH inside the test chamber using a 13 g/100 mL LiCl solution (blue line) and silica gel (red line). The target moisture content of 85.6% and 4.2%, respectively, is indicated by horizontal dotted lines.
Measurements of volume reduction
After transfer of algal suspensions into the test chamber, an initial phase was observed, where only culture medium evaporated and the cells did not show any change in volume or shape. Then, the desiccation process started, in which the algae lost water for about ∼15–20 min until they reached an equilibrium state. The image series in Figure 4 shows cell filaments of K. crenulatum (78.3% RH, 90–140 min after start of the experiment; Fig. 4A) and Zygnema sp. (56.4% RH, 155–190 min after start of experiment; Fig. 4B) during the desiccation phase.
Figure 4.
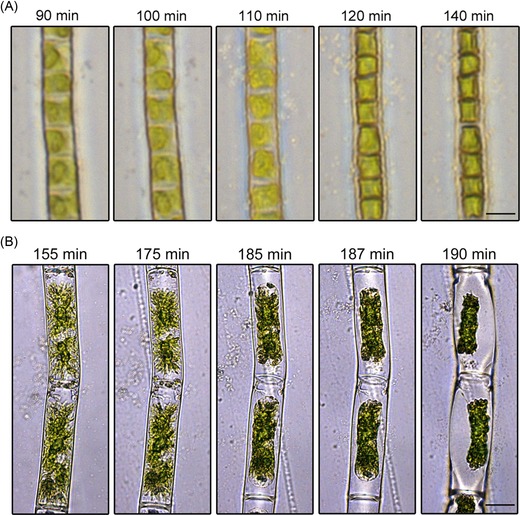
Light microscopic image series of the desiccation process in (A) Klebsormidium crenulatum at 78.3% RH, Scale bar 5 μm and (B) in Zygnema sp. at 56.4% RH, time in minutes (min) after the start of the desiccation experiment. Scale bar 20 μm.
The diameter of the protoplasts experienced a drastic reduction during the desiccation process, whereas the length of the protoplasts usually did not change and the protoplasts remained attached to the cross‐walls (Figs. 2 and 4A). Only in Zygnema sp., protoplasts occasionally were detached from the cross‐walls, which lead to shrinkage in length during the desiccation process (Fig. 4B). As in these cases, severe damage was expected; these cells were not included for volume calculations. In Zygnema sp., the strongest shrinkage of the protoplasts was observed in the central area and therefore a mean value was used to calculate the volume reduction (see Figs. 5A and B). In some cases, the PDd even disintegrated into portions, resembling the chloroplast centres and pyrenoids (see Figs. 5C–E); such protoplasts were not used for measurements.
Figure 2.
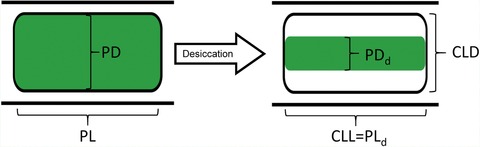
Schematic drawing of the change of protoplast shape during the desiccation process. The parameters, which were used for determining the protoplast volume before (volume in the saturated state, Vs) and after (volume in the desiccated state, Vd) desiccation, are depicted.
Abbreviations: PD = protoplast diameter; PL = protoplast length; CLD = cell lumen diameter after desiccation; CLL = cell lumen length after desiccation; PDd = protoplast diameter after desiccation; PLd = protoplast length after desiccation.
Figure 5.
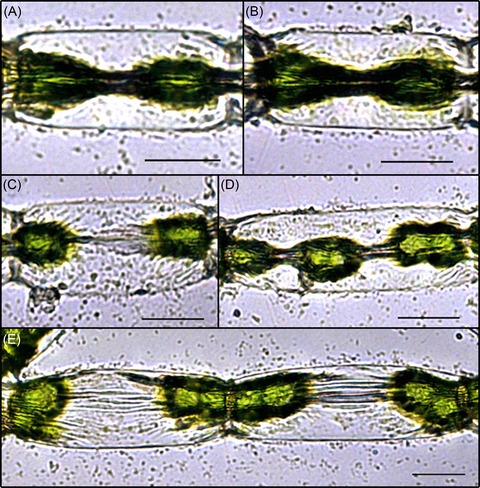
Light microscopic images of Zygnema sp. cells after 3 h 20 min desiccation at 56.4% RH. Images (A) and (B) represent examples of ‘hourglass’ shape. Images (C)–(E) show cases where the protoplast of Zygnema sp. disintegrated as an effect of the desiccation. Scale bars 20 μm.
Calculations of the RVRED were performed by measuring the protoplast diameter after a constant level of RH inside the test chamber was achieved and cells did not show further changes in protoplast volume or shape. In K. crenulatum, the RVRED values (mean ± SE) ranged from 46.4 ± 1.9% at 95.4% RH to 75.9 ± 2.7% at 3.7% RH (Fig. 6). In Zygnema sp., the RVRED values of 34.3 ± 2.4% at the highest RH was significantly higher (p < 0.01) when compared to K. crenulatum. At the lowest RH the value of 83.9 ± 2.2% was significantly (p < 0.05) lower in comparison to K. crenulatum. The development of volume reductions differed between the two genera. Although in K. crenulatum a drastic water loss was observed already at highest RH tested, in Zygnema sp. the initial water loss was less pronounced. However, in Zygnema sp. the water loss at RH values <∼80% was more drastic indicated by higher RVRED values.
Figure 6.
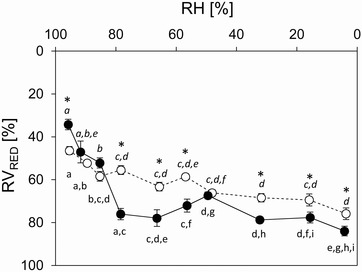
Relative volume reduction (RVRED [%]; mean values ± SE) in Klebsormidium crenulatum (open symbols) and Zygnema sp. (solid symbols) determined at certain RH levels. Significant differences between mean values of RVRED [%] for each investigated genus (K. crenulatum, Zygnema sp.) are indicated by different letters (normal font: Zygnema sp., italics: K. crenulatum; one‐way ANOVA followed by Games–Howell's post hoc test; p < 0.01). Significant differences of RVRED [%] between the two genera at a certain RH level are indicated by asterisks (Student's t‐test; p < 0.05).
Discussion
In this study, a new test chamber allowed the monitoring of desiccation effects at certain preset RH during microscopic observation. This device was used with two green algae, K. crenulatum and Zygnema sp., for which the RVRED was experimentally determined. A distinct desiccation behaviour was found by quantitative analysis of the RVRED at different RHs. The RVRED values at the highest RH tested (∼95% RH) indicated a stronger water loss in K. crenulatum. By contrast, at lower RHs Zygnema sp. showed a stronger water loss. There are also qualitative differences in the shape of the retracted chloroplasts between the two studied green algal genera.
In general, these observations correlate well with physiological parameters, e.g. reduction of the effective quantum yield as a consequence of desiccation in K. crenulatum (Karsten et al., 2010) and Zygnema sp. (Herburger et al., 2015). Herburger and Holzinger (2015) investigated these two genera in a parallel setup and monitored the desiccation and recovery effects of individual filaments by imagining PAM. In both genera, desiccation at ambient air (RH ∼65%) leads to a rapid decrease of the effective quantum yield of photosystem II (YII). Upon rehydration for 180 min, the Y II is re‐established, in Klebsormidum cenulatum to nearly the initial value and in Zygnema sp. to only half of the initial value (Herburger & Holzinger, 2015). In this study, we found clear differences between the two examined genera concerning the range of the RVRED‐RH relationship. Most important, RVRED values below ∼80% RH are considerably higher in Zygnema sp. than K. crenulatum. This means that the protoplasts in Zygnema sp. experience a clearly stronger reduction in volume under similar RH levels. This correlates well with the observations by Kaplan et al. (2012, 2013), and may be attributed to the higher vacuolization status of young Zygnema sp. protoplasts (Kaplan et al., 2013; Herburger & Holzinger, 2015). Moreover, this could also explain the weaker recovery of YII in Zygnema sp. (Herburger & Holzinger, 2015). In Zygnema sp., older cultures form preakinetes, which are in general more stress tolerant (e.g. Pichrtová et al., 2014), and likely have different osmotic potentials (e.g. Fuller, 2013; Kaplan et al., 2013). In this study, we used young cultures, to exclude this phenomenon. However, the measurement of the Zygnema sp. protoplasts was more difficult to perform due to the shape of the desiccated cytoplasm.
In both genera, only individual cells from solitary filaments were measured. Bundles of filaments showed a slower desiccation process and so a prolonged moistness was observed. This is a well‐described strategy for filamentous, soil crust inhabiting organisms to enhance the water holding capacities in nature (e.g. Holzinger & Karsten, 2014). In the experiment, however this leads to complications with the measurements (overlapping of filaments, protoplast shape) and was therefore avoided. Moreover, we used for the calculation only cells where the protoplast remained attached to the cross‐walls, assuring that the cells were not damaged too badly had the potential to recover (Herburger & Holzinger, 2015).
Summarizing the results of this study, here we introduce a functional test chamber suitable to follow the desiccation process during microscopic observation. We demonstrated that the test chamber could create and maintain a constant RH during the desiccation experiments. These conditions allowed to subsequently calculate the volume reduction of the test organism in situ. The more rapid water loss observed in K. crenulatum might in nature be compensated by their habitat in soil crusts, giving protection against desiccation (Karsten & Holzinger, 2014). By contrast, Zygnema sp. collected from the sandy litoral zone of a river (Herburger et al., 2015) might be naturally exposed more frequently to higher RHs, which they are capable to tolerate. The stronger water loss at lower RHs resulted in severe damage, and it has been shown that particularly young vegetative Zygnema sp. cells cannot recover from desiccation over silica gel, whereas they tolerate desiccation at ∼83% RH with good recovery success according to measurements of the effective quantum yield (Pichrtová et al., 2014). We are aware that comparing genera after investigating only one strain per genus might lead to misinterpretations; therefore, future studies should include more strains.
The chamber presented here will allow monitoring desiccation kinetics under defined RHs. Repeated desiccation and rehydration cycles could be created in order to study possible legacy effects. Future studies should elucidate which osmotic compounds are responsible for water holding capacities that enable aeroterrestrial green algae to withstand desiccation. Additionally, this chamber might also be valuable for further studies measuring the photosynthetic activity of single cells during desiccation, which is possible with new‐generation chlorophyll fluorimeters or the observation of other physiological responses employing fluorescent probes, e.g. H2DCF‐DA to detect the formation of reactive oxygen species during the desiccation process.
Acknowledgement
This study was supported by the Austrian Science Fund (FWF) grants P24242‐B16 and I 1951‐B16 to AH.
References
- Beckett, R.P. (1995) Some aspects of the water relations of lichens from habitats of contrasting water status studied using thermocouple psychrometry. Ann. Bot. 76, 211–217. [Google Scholar]
- Beckett, R.P. (1997) Pressure–volume analysis of a range of poikilohydric plants implies the existence of negative turgor in vegetative cells. Ann. Bot. 79, 145–152. [Google Scholar]
- Bischoff, H.W. & Bold, H.C. (1963) Phycological studies IV. Some soil algae from enchanted rock & related algal species. Univ. of Texas Publ. 6318, 1–95. [Google Scholar]
- Delaux, P.‐M. , Nanda, A.K. , Mathé, C. , Sejalon‐Delmas, N. & Dunand, C. (2013) Molecular and biochemical aspects of plant terrestrialization. Persp. Plant Ecol. Evol. 14, 49–59. [Google Scholar]
- Delwiche, C.F. & Cooper, E.D. (2015) The evolutionary origin of a terrestrial flora. Curr. Biol. 25(19), R899–R910. [DOI] [PubMed] [Google Scholar]
- Fuller, C.L. (2013) Examining morphological and physiological changes in Zygnema irregulare during a desiccation and recovery period. Master's Thesis, California State University. [Google Scholar]
- Graham, L.E. , Arancibia‐Avila, P. , Taylor, W.A. , Strother, P.K. & Cook, M.E. (2012) Aeroterrestrial Coleochaete (Streptophyta, Coleochaetales) models early plant adaptation to land. Am. J. Bot. 99, 130–144. [DOI] [PubMed] [Google Scholar]
- Hájek, T. & Beckett, R.P. (2008) Effect of water content components on desiccation and recovery in Sphagnum mosses. Ann. Bot. 101, 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay, F.R. , Adams, J. , Manger, K. & Probert, R. (2008) The use of non‐saturated lithium chloride solutions for experimental control of seed water content. Seed Sci. Technol. 36, 737–746. [Google Scholar]
- Herburger, K. & Holzinger, A. (2015) Localization and quantification of callose in the streptophyte green algae Zygnema and Klebsormidium: correlation with desiccation tolerance. Plant Cell Physiol. 56, 2259–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herburger, K. , Lewis, L.A. & Holzinger, A. (2015) Photosynthetic efficiency, desiccation tolerance and ultrastructure in two phylogenetically distinct strains of alpine Zygnema sp. (Zygnematophyceae, Streptophyta): role of pre‐akinete formation. Protoplasma 252, 571–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzinger, A. & Karsten, U. (2013) Desiccation stress and tolerance in green algae: consequences for ultrastructure, physiological and molecular mechanisms. Front. Plant. Sci. 4, 327. doi: 10.3389/fpls.2013.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzinger, A. , Kaplan, F. , Blaas, K. , Zechmann, B. , Komsic‐Buchmann, K. & Becker, B. (2014) Transcriptomics of desiccation tolerance in the streptophyte green alga Klebsormidium reveal a land plant‐like defense reaction. PLoS ONE 9, e110630. doi:10.1371/journal.pone.0110630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori, K. , Maruyama, F. , Fujisawa, T. , et al (2014) Klebsormidium flaccidum genome reveals primary factors for plant terrestrial adaptation. Nat. Commun. 5, 3978. doi:10.1038/ncomms4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, F. , Lewis, L.A. , Wastian, J. & Holzinger, A. (2012) Plasmolysis effects and osmotic potential of two phylogenetically distinct alpine strains of Klebsormidium (Streptophyta). Protoplasma 249, 789–804. [DOI] [PubMed] [Google Scholar]
- Kaplan, F. , Lewis, L.A. , Herburger, K. & Holzinger, A. (2013) Osmotic stress in Arctic and Antarctic strains of the green alga Zygnema (Zygnematales, Streptophyta): effects on photosynthesis and ultrastructure. Micron 44, 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten, U. & Holzinger, A. (2014) Green algae in alpine biological soil crust communities: acclimation strategies against ultraviolet radiation and dehydration. Biodivers. Conserv. 23, 1845–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten, U. , Lütz, C. & Holzinger, A. (2010) Ecophysiological performance of the aeroterrestrial green alga Klebsormidium crenulatum (Charophyceae, Streptophyta) isolated from an alpine soil crust with an emphasis on desiccation stress. J. Phycol. 46, 1187–1197. [DOI] [PubMed] [Google Scholar]
- Leliaert, F. , Smith, D.R. , Moreau, H. , Herron, M.D. , Verbruggen, H. , Delwiche, C.F. & De Clerck, O. (2012) Phylogeny and molecular evolution of the green algae. Crit. Rev. Plant Sci. 31, 1–46. [Google Scholar]
- Lokhorst, G.M. & Star, W. (1985) Ultrastructure of mitosis and cytokinesis in Klebsormidium mucosum nov. comb., formerly Ulothrix verrucosa (Chlorophyta). J. Phycol. 21, 466–476. [Google Scholar]
- Mikhailyuk, T. , Holzinger, A. , Massalski, A. & Karsten, U. (2014) Morphological and ultrastructural aspects of Interfilum and Klebsormidium (Klebsormidiales, Streptophyta) with special reference to cell division and thallus formation. Eur. J. Phycol. 49, 395–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailyuk, T. , Glaser, K. , Holzinger, A. & Karsten, U. (2015) Biodiversity of Klebsormidium (Streptophyta) from alpine biological soil crusts (Alps, Tyrol, Austria and Italy), J. Phycol. 51, 750–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardini, A. , Marchetto, A. & Tretiach, M. (2013) Water relation parameters of six Peltigera species correlate with their habitat preferences. Fungal Ecol. 6, 397–407. [Google Scholar]
- Pichrtová, M. , Kulichová, J. & Holzinger, A. (2014) Nitrogen limitation and slow drying induce desiccation tolerance in conjugating green algae (Zygnematophyceae) from polar habitats. PLOS one 9(11), e113137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor, M.C. , Nagy, Z. , Csintalan, Z. & Takács, Z. (1998) Water‐content components in bryophytes: analysis of pressure‐volume relationships. J. Exp. Bot. 49, 1845–1854. [Google Scholar]
- Rindi, F. , Mikhailyuk, T.I. , Sluiman, H.J. , Friedl, T. & López‐Bautista, J.M. (2011) Phylogenetic relationships in Interfilum and Klebsormidium (Klebsormidiophyceae, Streptophyta). Mol. Phylogenet. Evol. 58(2), 218–231. [DOI] [PubMed] [Google Scholar]
- Sørensen, I. , Pettolino, F.A. , Bacic, A. , Ralph, J. , Lu, F. & O'Neill, M.A. , et al. (2011) The charophycean green algae provide insights into the early origins of plant cell walls. Plant J. 68, 201–221. [DOI] [PubMed] [Google Scholar]
- Starr, R.C. & Zeikus, J.A. (1993) UTEX—the culture collection of algae at the University of Texas at Austin 1993 list of cultures. J. Phycol. 29(s2), 1–106. [Google Scholar]
- Timme, R.E. , Bachvaroff, T.R. & Delwiche, C.F. (2012) Broad phylogenomic sampling and the sister lineage of land plants. PLoS One 7, e29696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuba, Z. , Lichtenthaler, H.K. , Csintalan, Z. , Nagy, Z. & Szente, K. (1994) Reconstitution of chlorophylls and photosynthetic CO2 assimilation upon rehydration of the desiccated poikilochlorophyllous plant Xerophyta scabrida (Pax) Th. Dur. et Schinz. Planta 192, 414–420. [Google Scholar]
- Walter, H. & Stadelmann, E. (1974) A new approach to the water relations of desert plants. Desert Biol. 2, 213–310. [Google Scholar]
- Wodniok, S. , Brinkmann, H. , Glöckner, G. , Heidel, A.J. , Philippe, H. , Melkonian, M. & Becker, B. (2011).Origin of land plants: do conjugating green algae hold the key? BMC Evol. Biol. 11, 104. doi: 10.1186/1471-2148-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]


