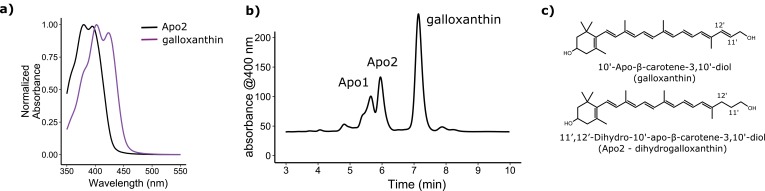
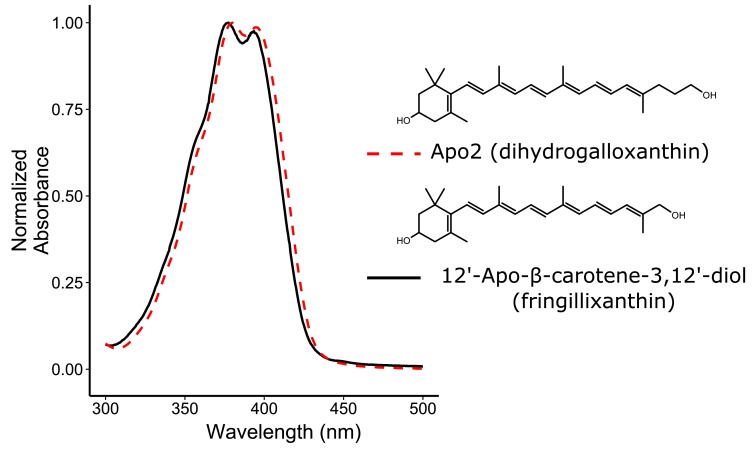
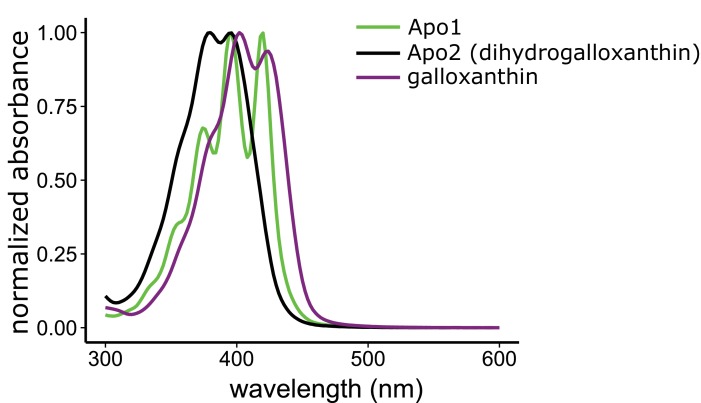
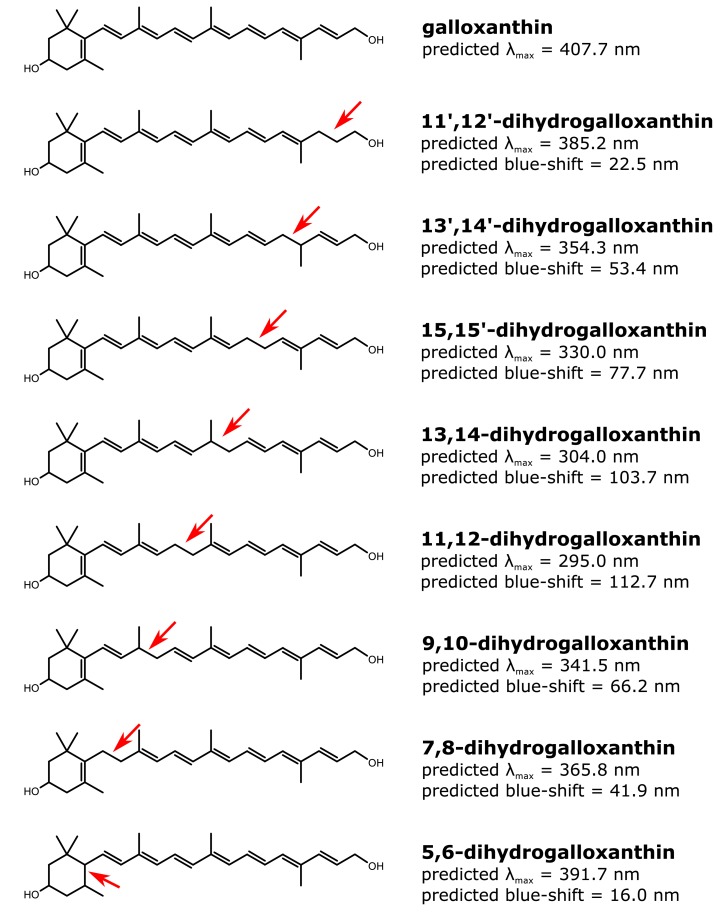
Figure 2. The avian retina contains multiple apocarotenoids that absorb different portions of the light spectrum.
(a) The absorbance spectrum of the major apocarotenoid pigments in the chicken C-type oil droplets. (b) A representative HPLC chromatogram of the apocarotenoids in whole retina extracts of the chicken retina. Apo1 has an absorbance spectrum similar to galloxanthin with more pronounced fine structure, suggesting an ε-ring configuration (Figure 2—figure supplement 2). This pigment is not a major component of the C-type oil droplets (Toomey et al., 2015). (c) The proposed chemical structure of dihydrogalloxanthin and known structure of galloxanthin with the positon of the 11’,12’ double bond indicated.