Abstract
Background
Cannabidiol, a therapeutic with potential serotonin (5-hydroxytryptamine; 5-HT) 5-HT1A receptor agonist activity, is the second most prevalent cannabinoid in Cannabis after Δ9-THC. The extent to which cannabidiol modifies the effects of Δ9-THC has not been firmly established, especially with respect to abuse-related effects in rhesus monkeys where previously antagonistic interactions have been reported for some behavioral outcomes.
Methods
Cannabidiol and the 5-HT1A receptor agonist (±)-8-hydroxy-2-(dipropylamino)tetralin hydrobromide (8-OH-DPAT) were tested in two separate discrimination assays in rhesus monkeys. One group (n=6) discriminated Δ9-tetrahydrocannabinol (Δ9-THC; 0.1 mg/kg i.v.); a second group (n=6) discriminated the cannabinoid antagonist rimonabant (1 mg/kg i.v.) while receiving Δ9-THC daily (1 mg/kg/12 h s.c.). Responding was maintained under a fixed ratio 5 schedule of stimulus-shock termination.
Results
Both training drugs dose-dependently increased the percentage of responses on the respective drug-associated levers. Cannabidiol (up to 17.8 mg/kg) and 8-OH-DPAT (up to 0.178 mg/kg) did not substitute for either training drug; however, both significantly increased the potency of Δ9-THC to produce discriminative stimulus effects. Moreover, 8-OH-DPAT significantly attenuated the discriminative stimulus effects of rimonabant, whereas cannabidiol did not modify the rimonabant discriminative stimulus.
Conclusions
These results, which are consistent with cannabidiol lacking CB1 receptor agonist or antagonist activity in vivo, demonstrate enhancement of the effects of Δ9-THC by cannabidiol, albeit at cannabidiol amounts larger than those in Cannabis or cannabidiol-based therapeutics (nabiximols). In addition to showing that cannabidiol and a 5-HT1A receptor agonist have overlapping behavioral effects, the current results suggest that 5-HT1A agonism enhances the CB1 receptor-mediated effects of Δ9-THC.
Keywords: cannabinoid, rhesus monkey, drug discrimination, cannabidiol, 8-OH-DPAT, serotonin, 5-HT1A, rimonabant, dependence
1. INTRODUCTION
Among the numerous phytocannabinoids in Cannabis sativa (Hill et al., 2012), Δ9-tetrahydrocannabinol (Δ9-THC) has been the most widely studied due to its prominent psychopharmacological effects. There is increasing recognition of the contribution of other phytocannabinoids to the in vivo effects of cannabis, as well as interest in isolating phytocannabinoids for drug-like and potential therapeutic effects. Cannabidiol is the second most prevalent phytocannabinoid in Cannabis after Δ9-THC and there is increasing evidence to suggest that cannabidiol has anti-inflammatory, anticonvulsant, antiemetic, and antipsychotic activity (Campos et al., 2012; Leo et al., 2016). Most recently, cannabidiol was approved in a formulation with Δ9-THC under the generic name nabiximols for the treatment of spasticity and neuropathic pain associated with multiple sclerosis.
Δ9-THC is a cannabinoid receptor agonist, whereas the mechanism of action of cannabidiol remains unclear. Cannabidiol lacks significant binding affinity for the prototypical cannabinoid receptors CB1 or CB2 (Mechoulam et al., 2002). While its mechanism of action remains poorly understood, cannabidiol exhibits a diverse pharmacology that includes activity at serotonin (5-hydroxytryptamine; 5-HT) 5-HT1A receptors, G protein-coupled receptors (GPR) 55, transient receptor potential of the ankyrin type 1 (TRPA1), vanilloid type 1 (TRPV1) and vanilloid type 2 (TRPV2) channels, inhibition of synaptic uptake of norepinephrine, GABA, adenosine, and dopamine, and stimulation of α3 and α1 glycine receptors (Leo et al., 2016).
One goal of the current study was to examine cannabidiol for its capacity to modify the abuse-related effects of Δ9-THC in two separate drug discrimination assays in rhesus monkeys. The first was a Δ9-THC discrimination assay (McMahon, 2006b) and the second was a rimonabant discrimination assay in Δ9-THC treated monkeys sensitive to cannabinoid antagonism (Stewart and McMahon, 2010). Whereas cannabidiol was not expected to substitute for Δ9-THC based on published studies using pigeons (Järbe et al., 1977) and rats (Vann et al., 2008), cannabidiol was expected to modify the effects of Δ9-THC. There are multiple reports of cannabidiol and Δ9-THC interacting to modify each other’s behavioral effects with the type of outcome, including enhancement (Karniol and Carlini, 1973; Takahashi and Karniol, 1975) and antagonism (Karniol and Carlini, 1973; Borgen and Davis, 1974; Karniol et al., 1974), depending on the endpoint of interest. In particular, antagonism was previously reported in rhesus monkeys performing operant conditioning and cognitive-based tasks (Brady and Balster, 1980; Wright et al., 2013), suggesting that cannabidiol could also attenuate the discriminative stimulus effects of Δ9-THC in rhesus monkeys, evidenced in the current study by not only attenuation of the Δ9-THC discriminative stimulus, but also substitution of cannabidiol for rimonabant. A second goal was to compare the effects of cannabidiol to the 5-HT1A receptor agonist (±)-8-hydroxy-2-(dipropylamino)tetralin hydrobromide (8-OH-DPAT); this was undertaken because 5-HT1A agonism appears to be one of the mechanisms by which cannabidiol could produce behavioral effects (Russo et al., 2005).
2. MATERIALS AND METHODS
2.1. Subjects
The Δ9-THC discrimination assay was conducted in six adult rhesus monkeys (Macaca mulatta) including two females and four males. The rimonabant discrimination assay was conducted in three adult females and three adult males. When monkeys were not in operant conditioning chambers they were housed individually on a 14-h light/10-h dark schedule. Body weights ranged from 5.6 kg to 10.1 kg and the diet consisted of fresh fruit, peanuts, and primate chow (High Protein Monkey Diet, Harlan Teklad, Madison, WI). Water was continuously available in the home cage. Monkeys had previously received non-cannabinoids and cannabinoids as described (Stewart and McMahon, 2010; Hruba et al., 2012). The experiments reported here were approved by the Institutional Animal Care and Use Committee of The University of Texas Health Science Center at San Antonio, and were conducted according to the “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council, 2011).
2.2. Surgery
A chronic indwelling catheter (heparin coated polyurethane, od = 1.68 mm, id = 1.02 mm, Instech Solomon, Plymouth Meeting, PA) was inserted into a femoral or subclavian vein while monkeys were anesthetized with ketamine (10 mg/kg i.m.) and isoflurane (1.5–3.0% inhaled via facemask). The catheter was secured in the vessel with suture silk (coated vicryl, Ethicon Inc., Somerville, New Jersey), which was also used to ligate the section of the vessel adjacent to the catheter insertion. The end of the catheter distal to the vessel was attached to a vascular access port (Mida-cbas-c50, Instech Solomon) located s.c. in the mid-scapular region of the back.
2.3. Apparatus
Monkeys were seated in chairs (Model R001, Primate Products, Miami, FL). Feet were secured in shoes containing brass electrodes to which a brief electric stimulus (3 mA, 250 ms) could be delivered from an a/c generator. Discrimination training and test sessions were performed by placing monkeys in operant conditioning chambers ventilated with blower fans. White noise was present for the duration of experimental sessions. Within the chamber was a stainless steel panel containing a left and right lever and a disc above each lever that could be illuminated red. Experimental events were controlled and recorded by an interface (MedAssociates, St. Albans, VT), a computer, and Med-PC software (MedAssociates).
2.4. Drug discrimination procedures
One group of monkeys discriminated Δ9-THC (0.1 mg/kg i.v.) from a vehicle consisting of a mixture of absolute ethanol, Emulphor-620, and saline in a proportion of 1:1:18. The second group of monkeys discriminated rimonabant (1 mg/kg i.v.) from the same vehicle; for monkeys discriminating rimonabant, Δ9-THC (1 mg/kg s.c.) was administered twice daily at 0600 and 1800 h and experimental sessions commenced at 1200 h. Both groups responded under a fixed ratio 5 (FR5) schedule of stimulus-shock termination. The experimental sessions were divided into multiple, consecutive cycles. For the studies with cannabidiol in combination with the training drugs, each cycle began with a 15-min timeout during which lights were not illuminated and responses on the levers resulted in no programmed consequence. The timeout was followed by a 5-min schedule of stimulus-shock termination signaled by the illumination of two red lights, one above each lever. Five consecutive responses within 40 s (Δ9-THC discrimination) or 10 s (rimonabant discrimination) on the correct lever extinguished the red lights, prevented delivery of an electric stimulus, and initiated a 30-s timeout. Otherwise, an electric stimulus was delivered. Incorrect responses reset the response requirement on the correct lever. Determination of correct levers varied among monkeys, e.g., the right lever was associated with vehicle and the left lever was associated with the training dose, and remained the same for that monkey for the duration of the study. For studies with 8-OH-DPAT, the timeout at the beginning of each cycle was shortened to 5 min to accommodate the relatively short duration of action of 8-OH-DPAT evidenced in pilot experiments measuring disruption of operant responding; however, all other experimental parameters remained the same.
Training sessions consisted of administration of the training dose of the training drug (Δ9-THC or rimonabant) or vehicle at the beginning of a cycle. Administration of the training dose at the beginning of a cycle was followed by 0–2 cycles during which vehicle was administered; however, the drug lever was designated as correct during every cycle following administration of the training dose during training sessions. A cycle in which the training dose was administered was preceded by 0–3 cycles, and for these preceding cycles vehicle was administered and the vehicle lever was designated correct. Training sessions in which only vehicle was administered for each cycle consisted of 3–6 cycles and the vehicle lever was designated correct throughout. Monkeys had previously satisfied the criteria for testing, defined as at least 80% of the total responses occurring on the correct lever and fewer than 5 responses occurring on the incorrect lever prior to satisfying the first FR of the cycle on the correct lever for all cycles for 5 consecutive or 6 of 7 training sessions. Tests were conducted after performance for consecutive training sessions, including both vehicle and drug training sessions, satisfied the test criteria.
Five consecutive responses on either lever postponed the shock schedule during test sessions. The control dose-response functions for each training drug were determined by administering vehicle in the first cycle followed by cumulative i.v. doses increasing by 0.5 log unit in subsequent cycles; doses larger than the rimonabant training dose were incremented in 0.25 log unit (i.e., 1.78 and 3.2 mg/kg). To examine the effects of cannabidiol and 8-OH-DPAT, a dose was administered at the beginning of the first cycle followed by cumulative doses of the training drug (Δ9-THC or rimonabant) in subsequent cycles. Cannabidiol was studied from 0.1 mg/kg up to 17.8 mg/kg because 32 mg/kg of cannabidiol produced a convulsion. 8-OH-DPAT was studied at 0.0178, 0.056, and 0.178 mg/kg. The effects of each test drug in combination with a training drug were examined using a within-subjects design (e.g., each monkey served as its own control) in four monkeys, except for cannabidiol tests in the Δ9-THC discrimination assay which included six monkeys. Two monkeys discriminating rimonabant contributed to tests with both cannabidiol and 8-OH-DPAT; of the four remaining monkeys in the rimonabant discrimination assay, two were used for tests with cannabidiol and the other two were included in the tests with 8-OH-DPAT. Control dose-response data for each training drug were calculated separately for the cycle durations of 20 min (cannabidiol) and 10 min (8-OH-DPAT). The control dose-response tests were conducted non-systematically in close temporal proximity to the tests with the various doses of cannabidiol and 8-OH-DPAT.
2.5. Drugs
Rimonabant, cannabidiol, and Δ9-THC (200 mg/ml in absolute ethanol; The Research Technology Branch of the National Institute on Drug Abuse, Rockville, MD) were dissolved in the vehicle mixture consisting of absolute ethanol, Emulphor-620 (Rhodia Inc., Cranbury, NJ), and physiological saline; each of these drugs was administered i.v. for cumulative dose-response tests. Δ9-THC was administered s.c. for daily treatment in the group of monkeys discriminating rimonabant. (±)-8-Hydroxy-2-(dipropylamino)tetralin hydrobromide (8-OH-DPAT; Sigma Chemical Co., Saint Louis, MO) was dissolved in the same vehicle and administered s.c. Drugs were administered in a volume of 0.1–1 ml/kg. Doses were expressed as the weight of the forms listed above in milligrams per kilogram of body weight.
2.6. Data analyses
Discrimination data are expressed as a percentage; the percentage was calculated by dividing the total number responses on the drug lever by the total number of responses on both the drug and vehicle levers, for each test cycle, and multiplying each result by 100. Rate of responding on both levers (i.e., drug and vehicle) is calculated as responses per s excluding responses during timeouts. Rate of responding during a test is expressed as the percentage of the control response rate for individual animals. The control response rate is defined as the average response rate for all cycles during the five previous vehicle training sessions excluding any training sessions in which the test criteria are not satisfied. Discrimination and response rate data are averaged among subjects (± S.E.M.) and plotted as a function of dose.
The analysis of the discrimination dose-response data for each training drug includes the smallest dose tested, which is 0.0032 mg/kg for Δ9-THC and 0.1 mg/kg for rimonabant, up to the largest dose tested as determined per individual monkey. The largest dose tested is the smallest dose producing greater than 80% drug-appropriate responding, decreasing response to less than 20% of the control response rate, or up to 0.32 mg/kg for Δ9-THC or 3.2 mg/kg for rimonabant, whichever occurs first. Discrimination data are not included when the associated response rate is less than 20% of the control as determined per individual monkey. The individual dose-response data for each training drug in combination with various doses of a test drug are analyzed with GraphPad Prism version 6.00 (GraphPad Software, La Jolla, CA) using non-linear regression and the following equation Y=100/(1+10ˆ((LogED-X)*slope)) with LogED calculated as LogED50Control + log(ED50Ratio). The analysis included the common slope shared by the dose-response function of the training drug alone and in combination with the various doses of test drug. ED50Control is the dose of training drug estimated to produce 50% drug-appropriate responding in the absence of test drug and ED50Ratio is the ratio of the ED50 value of the training drug calculated in the presence of a dose of test drug divided by the ED50 value of the training drug alone. A significant shift in the training drug dose-response function is evidenced by a ratio value with 95% confidence limits that do not include 1.
3. RESULTS
3.1. The effects of Δ9-THC alone and in combination with cannabidiol or 8-OH-DPAT
The absolute rate of responding averaged over 5 vehicle training sessions for each respective monkey discriminating Δ9-THC was 0.96, 1.06, 1.36, 1.43, 1.46, and 1.76 responses per s. Vehicle administered prior to determination of the control Δ9-THC dose-response function resulted in 0% responses on the drug-lever (Fig. 1 top, circle above VEH). Δ9-THC dose-dependently increased responding on the drug-lever with the training dose resulting in 97% drug-lever responding; the ED50 value (95% confidence limits) was 0.034 (0.018–0.068) mg/kg (Table 1 left column). Cannabidiol produced no more than 33% of the responses on the Δ9-THC lever and this occurred at 17.8 mg/kg (Fig. 1 top left, triangle over VEH). Cannabidiol doses ranging from 0.1 to 3.2 mg/kg did not significantly modify the discriminative stimulus effects of Δ9-THC (see Fig. 1, diamonds for 3.2 mg/kg of cannabidiol; ineffective doses less than 3.2 mg/kg not shown). In contrast, 10 and 17.8 mg/kg of cannabidiol significantly increased the potency of Δ9-THC to produce discriminative stimulus effects (Fig. 1 top left, squares and triangles, respectively), as evidenced by dose ratios (95% confidence limits) of 0.39 (0.0077–0.78) and 0.23 (0.0030–0.46) (Table 1 left column). These represent 2.6- and 4.2-fold leftward shifts of the Δ9-THC dose-response function. Response rate was not systematically altered following any dose of Δ9-THC and cannabidiol, alone or in combination (Fig. 1 bottom left).
Fig. 1.
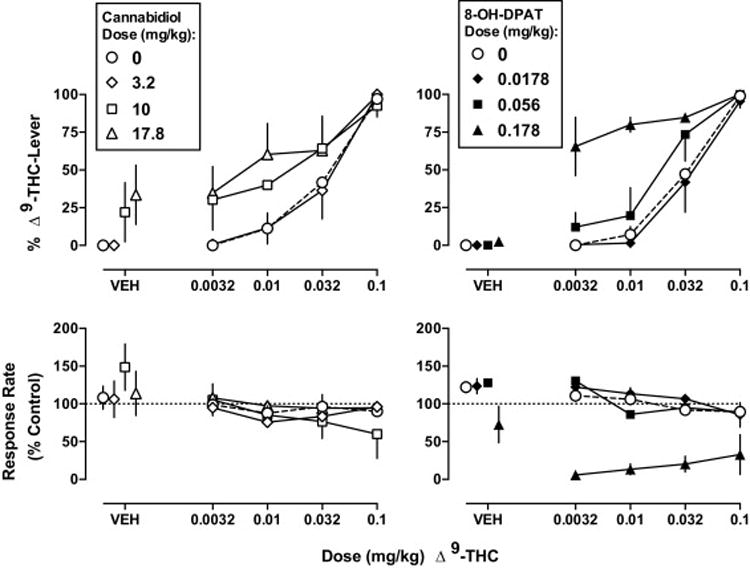
Effects of cannabidiol (left) and 8-OH-DPAT (right) following s.c. administration alone and in combination with Δ9-THC in rhesus monkeys discriminating Δ9-THC (0.1 mg/kg i.v.). Abscissae: vehicle (VEH) or dose of Δ9-THC in milligram per kilogram of body weight. Ordinates: mean (± S.E.M.) percentage of responding on the Δ9-THC lever (top) and mean (± S.E.M.) response rate expressed as a percentage of the VEH control rate (bottom). The dashed line is the dose-response function for Δ9-THC alone. Mean data for cannabidiol are n=6, except for discrimination data at 10 and 17.8 mg/kg cannabidiol, which are 5 out of the 6 monkeys tested. Mean data for 8-OH-DPAT are n=4, except for discrimination data at 0.178 mg/kg 8-OH-DPAT, which are 2 out of the 4 monkeys tested.
Table 1.
ED50 values, dose ratios, and 95% confidence limits (95% CL) for Δ9-THC alone, rimonabant alone, and the respective training drugs in combination with various doses of cannabidiol or 8-OH-DPAT.
| ED50 (95% CL) | Dose ratio (95% CL) | |
|---|---|---|
| Δ9-THC Control | 0.034 (0.018–0.068) | |
| + Cannabidiol (3.2 mg/kg) | 0.037 (0.017–0.083) | 1.0 (0.01–2.2) |
| + Cannabidiol (10 mg/kg) | 0.013 (0.0068–0.027) | 0.39 (0.0077–0.78)* |
| + Cannabidiol (17.8 mg/kg) | 0.0080 (0.0039–0.016) | 0.23 (0.0030–0.46)* |
| Δ9-THC Control | 0.032 (0.023–0.045) | |
| + 8-OH-DPAT (0.0178 mg/kg) | 0.036 (0.026–0.051) | 1.1 (0.58–1.7) |
| + 8-OH-DPAT (0.056 mg/kg) | 0.019 (0.013–0.028) | 0.60 (0.29–0.90)* |
| + 8-OH-DPAT (0.178 mg/kg) | 0.0025 | 0.078 (0.028–0.13)* |
| Rimonabant Control | 0.38 (0.26–0.57) | |
| + Cannabidiol (10 mg/kg) | 0.61 (0.41–0.93) | 1.6 (0.67–2.5) |
| + Cannabidiol (17.8 mg/kg) | 0.49 (0.33–0.76) | 1.3 (0.55–2.0) |
| Rimonabant Control | 0.16 (0.12–0.25) | |
| + 8-OH-DPAT (0.056 mg/kg) | 0.34 (0.27–0.46) | 2.1 (1.1–3.0)* |
significantly different versus control (p<0.05)
The Δ9-THC control dose-response function determined in conjunction with the 8-OH-DPAT tests was strikingly similar to that determined for cannabidiol tests, with vehicle producing 0% drug-lever responding and the training dose (0.1 mg/kg) producing 99% drug-lever responding (Fig. 1 top right, circles). 8-OH-DPAT produced a maximum of 3% responding on the Δ9-THC lever at 0.178 mg/kg (Fig. 1 top right, triangle above VEH). 8-OH-DPAT at a dose of 0.0178 mg/kg did not significantly modify the Δ9-THC discrimination dose-response function (Fig. 1 top right, diamonds). In contrast, 0.056 and 0.178 mg/kg of 8-OH-DPAT significantly increased the ED50 value of Δ9-THC (Fig. 1, squares and triangles, respectively), as evidenced by dose ratios (95% confidence limits) of 0.60 (0.29–0.90) and 0.078 (0.028–0.13), respectively (Table 1 right column). These represent 1.7- and 13-fold leftward shifts of the Δ9-THC dose-response function. The largest dose of 8-OH-DPAT (0.178 mg/kg), when tested alone and in combination with Δ9-THC, markedly decreased response rate with two of four monkeys responding less than 20% of their individual control response rate (Fig. 1 bottom right, triangles).
3.2. The effects of rimonabant alone and in combination with cannabidiol or 8-OH-DPAT in monkeys receiving Δ9-THC (1 mg/kg/12 h)
The absolute rate of responding averaged over 5 vehicle training sessions for each respective Δ9-THC treated monkey discriminating rimonabant was 0.85, 1.12, 1.21, 1.31, 1.41, and 1.72 responses per s. Administration of vehicle produced 0% responding on the rimonabant-associated lever (Fig. 2 top, circles above VEH). Rimonabant dose-dependently increased drug-appropriate responding with the training dose (1 mg/kg) producing 100% responses on the drug-lever. Cannabidiol (10 and 17.8 mg/kg) alone produced no more than 7% of responses on the rimonabant lever. Cannabidiol did not significantly modify the rimonabant dose-response function for producing discriminative stimulus effects in Δ9-THC treated monkeys (Fig. 2 top left, square and triangles, respectively). Up to 17.8 mg/kg cannabidiol did not result in marked reductions in response rate (Fig. 2 bottom left).
Fig. 2.
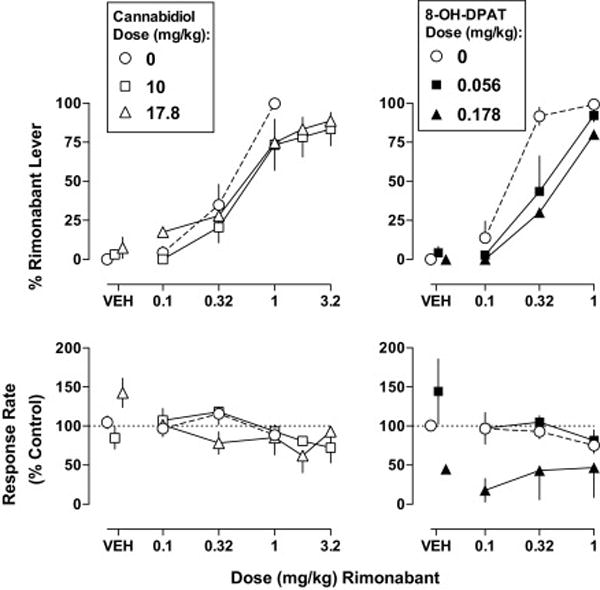
Effects of cannabidiol (left) and 8-OH-DPAT (right) following s.c. administration alone and in combination with rimonabant in rhesus monkeys discriminating rimonabant (1 mg/kg i.v.) while receiving 1 mg/kg/12 h Δ9-THC s.c. Abscissae: vehicle (VEH) or dose of rimonabant in milligram per kilogram of body weight. Ordinates: mean (± S.E.M.) percentage of responding on the rimonabant lever (top) and mean (± S.E.M.) response rate expressed as a percentage of the VEH control rate (bottom). The dashed line is the dose-response function for rimonabant alone. Mean data for cannabidiol are n=4, except for data at 17.8 mg/kg cannabidiol in combination with 3.2 mg/kg rimonabant, which is one monkey. Mean data for 8-OH-DPAT are n=4, except for discrimination data at 0.178 mg/kg 8-OH-DPAT, which is 1 out of the 4 monkeys tested.
Determination of the control rimonabant dose-response function during shortened cycles (i.e., from 20 min for tests with cannabidiol to 10 min for tests with 8-OH-DPAT) resulted in increased potency. The ED50 value (95% confidence limits) of rimonabant to produce discriminative stimulus effects in these tests was 0.16 (0.12–0.25) mg/kg (Table 1). 8-OH-DPAT at a dose of 0.056 mg/kg produced 4% of responses on the rimonabant lever (Fig. 2 top right, square above VEH). This dose of 8-OH-DPAT significantly attenuated the discriminative stimulus effects of rimonabant in Δ9-THC treated monkeys, as evidenced by a dose ratio (95% confidence limits) of 2.1 (1.1–3.1). A larger dose of 8-OH-DPAT (0.178 mg/kg) decreased responding to less than 20% of the control response rate in 3 out of 4 monkeys tested (Fig. 2 bottom right).
4. DISCUSSION
Cannabidiol did not substitute for a Δ9-THC discriminative stimulus and did not substitute for a rimonabant discriminative stimulus in Δ9-THC treated monkeys up to the safest dose that could be studied. This result provides strong evidence that cannabidiol does not bind appreciably to CB1 receptors in primates. Cannabidiol significantly enhanced the potency of Δ9-THC to produce discriminative stimulus effects, albeit at doses exceeding amounts obtained from Cannabis or the currently approved therapeutic nabiximols at prescribed doses. The 5-HT1A agonist 8-OH-DPAT enhanced the effects of Δ9-THC, although the greatest enhancement occurred at a dose of 8-OH-DPAT that disrupted responding in a subset of monkeys. The enhancement by 8-OH-DPAT was evidenced by not only a leftward shift of the Δ9-THC discrimination response-response function, but also a rightward shift of the rimonabant discrimination dose-response function in Δ9-THC treated monkeys. The same type of rightward shift in the rimonabant dose-response function is produced by increasing doses of CB1 receptor agonists including Δ9-THC (Ginsburg et al., 2012). Collectively, these results suggest that 5-HT1A agonism can enhance the CB1 receptor mediated in vivo effects of Δ9-THC, and further suggest that cannabidiol and 5-HT1A receptor agonists have overlapping behavioral effects.
Nabiximols, an oromucosal spray containing equal amounts of cannabidiol and Δ9-THC, is approved for the treatment of neuropathic pain and spasticity in patients diagnosed with multiple sclerosis. Oral Δ9-THC, prescribed under the generic name dronabinol, is an older therapeutic that has limited availability due to concerns over abuse liability. There are similar concerns over the abuse liability of cannabidiol mixed with Δ9-THC, and questions about the extent to which cannabidiol modifies the abuse liability of Δ9-THC. The results of clinical studies suggest that the abuse liability of nabiximols is not different from those of either Cannabis or Δ9-THC (Schoedel et al., 2011). The current study had the advantage of varying cannabidiol dose across a broad range including a dose equal to the training dose of Δ9-THC (0.1 mg/kg) up to a dose producing a convulsion (32 mg/kg). The current study firmly demonstrates that cannabidiol exerts negligible CB1 receptor agonist or antagonist activity across this dose range. In addition, at doses equal to or as large as 32 times greater than Δ9-THC, cannabidiol did not modify the discriminative stimulus effects of Δ9-THC. Only at a dose 100 times greater than Δ9-THC did cannabidiol enhance the potency of Δ9-THC to produce discriminative stimulus effects. Collectively, the current results suggest that cannabidiol carries negligible risk of increasing the subjective effects of Δ9-THC, and further suggest that cannabidiol is unlikely to attenuate the subjective effects of Δ9-THC. To the extent that subjective effects predict abuse liability, these results suggest that cannabidiol has minimal impact on the abuse liability of Δ9-THC. Because Δ9-THC is not unanimously self-administered in non-humans among published studies (Tanda, 2016), drug discrimination will continue to provide critical insight into mechanisms underlying the abuse-related effects of cannabinoids.
The presence and nature of the interaction between cannabidiol and Δ9-THC appears to vary for different effects because previous studies have reported antagonism of some of the effects of Δ9-THC by cannabidiol in rhesus monkeys. Cannabidiol administered in equal amounts with Δ9-THC attenuated some of the disruptive effects of Δ9-THC on some types of learning and motor behavior, but did not alter all of the effects of Δ9-THC (Wright et al., 2013). In another study cannabidiol also attenuated the effects of Δ9-THC on responding under a fixed interval schedule in rhesus monkeys (Brady and Balster, 1980), and in that study antagonism was obtained at a dose of cannabidiol 100 times larger than Δ9-THC (30 and 0.3 mg/kg, respectively). The interaction in the current study was also obtained at a dose of cannabidiol 100 times greater than the Δ9-THC dose, but the direction of the interaction was opposite to that reported previously for rate-decreasing effects. That cannabidiol enhanced the discriminative stimulus effects of Δ9-THC in the current study and attenuated the rate-decreasing effects of Δ9-THC in the previous study at comparable doses in the same species suggests that altered pharmacokinetics cannot explain every type of interaction. Instead, the type of interaction that occurs between cannabidiol and Δ9-THC appears to vary for different behavioral effects, which could reflect differences in the pharmacological mechanisms that mediate the discriminative stimulus effects of Δ9-THC versus its rate-decreasing effects, as demonstrated previously for cannabinoids under experimental conditions similar to those of the current study (Rodriguez and McMahon, 2014).
Multiple mechanisms are implicated in the pharmacological effects of cannabidiol; 5-HT1A receptor agonism appears to be predominant among those mechanisms. Cannabidiol has been reported to displace [3H]8-OH-DPAT binding from cloned human 5-HT1A receptors and to exert 5-HT1A receptor agonist activity as evidenced by [35S]GTPgammaS binding and cyclic AMP production in vitro (Russo et al., 2005). In pre-clinical studies, serotonin 5-HT1A receptors appear to mediate many of the in vivo effects of cannabidiol including anti-anxiety effects (Campos and Guimarães, 2008), anti-emetic effects (Rock et al., 2012), and antinociceptive effects (Ward et al., 2014). The current results demonstrate that the 5-HT1A receptor agonist 8-OH-DPAT and cannabidiol exert qualitatively similar interactions with Δ9-THC in rhesus monkeys. However, whereas 8-OH-DPAT attenuated the effects of rimonabant in Δ9-THC treated monkeys, cannabidiol did not. Both discrimination assays are mediated by CB1 receptor activity (McMahon, 2006a; 2006b). Aside from the opposing effects on cannabinoid signaling of the training drugs, the discrimination assays also differ with respect to Δ9-THC treatment, with the rimonabant discrimination being associated with more (i.e., daily) Δ9-THC treatment than the Δ9-THC discrimination assay. A parsimonious explanation of the rimonabant discrimination in Δ9-THC treated monkeys is that the rimonabant training condition represents antagonism or decreased effectiveness of Δ9-THC, whereas the vehicle training condition represents the presence of Δ9-THC. That cannabidiol enhanced the effects of Δ9-THC in the Δ9-THC discrimination assay but not the rimonabant discrimination assay might reflect chronic Δ9-THC induced loss of 5-HT1A receptor function. However, in a previous study chronic treatment with the cannabinoid agonist WIN-55212,2 increased 5-HT1A expression and enhanced sensitivity to the effects of a relatively small dose of 8-OH-DPAT that was intended to be selective for presynaptic 5-HT1A autoreceptors (Moranta et al., 2009). If 5-HT1A receptor agonism is the pharmacological mechanism by which cannabidiol exerts behavioral effects, however, then Δ9-THC induced changes in 5-HT1A receptor function would have been expected to impact sensitivity to the behavioral effects of both cannabidiol and 8-OH-DPAT.
In summary, cannabidiol enhanced the discriminative stimulus effects of Δ9-THC but only at doses larger than those that would be obtained from Cannabis or nabiximols use. To the extent that the current results provide insight into abuse-related effects, then the potential use of nabiximols as a treatment for cannabinoid dependence and withdrawal, as tested in some clinical studies (Allsop et al., 2014), would seem to carry negligible risk of further increasing Δ9-THC abuse liability. On the other hand, if the rimonabant discrimination assay in Δ9-THC treated monkeys reflects Δ9-THC withdrawal as proposed (McMahon and Stewart, 2010), then the failure of cannabidiol to modify the rimonabant discriminative stimulus suggests cannabidiol alone has limited utility as a drug therapy for cannabinoid dependence. Enhancement of the effects of Δ9-THC and attenuation of the effects of rimonabant by 8-OH-DPAT implicates a role for 5-HT1A receptor agonism in modifying the effects of cannabinoids. While the 5-HT1A agonist buspirone has been reported to decrease cannabis craving, the inability of buspirone to reduce cannabis use and its potential to increase use in some individuals (McRae-Clark et al., 2015) suggests that buspirone and other potential 5-HT1A receptor based therapeutics should be prescribed with caution in Cannabis users.
Highlights.
Cannabidiol lacks CB1 receptor agonist or antagonist activity in primates
Cannabidiol amounts larger than those in Cannabis or nabiximols enhances Δ9-THC
Cannabidiol and a 5-HT1A receptor agonist have overlapping behavioral effects
5-HT1A agonism enhances the CB1 receptor-mediated effects of Δ9-THC.
Acknowledgments
None
Role of Funding Source Nothing declared
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors Lance R. McMahon, PhD
Conflict of Interest No conflict declared
References
- Allsop DJ, Copeland J, Lintzeris N, Dunlop AJ, Montebello M, Sadler C, Rivas GR, Holland RM, Muhleisen P, Norberg MM, Booth J, McGregor IS. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA Psychiatry. 2014;71:281–291. doi: 10.1001/jamapsychiatry.2013.3947. [DOI] [PubMed] [Google Scholar]
- Brady KT, Balster RL. The effects of Δ9-tetrahydrocannabinol alone and in combination with cannabidiol on fixed-interval performance in rhesus monkeys. Psychopharmacology. 1980;72:21–26. doi: 10.1007/BF00433803. [DOI] [PubMed] [Google Scholar]
- Borgen LA, Davis WM. Cannabidiol interaction with Δ9-tetrahydrocannabinol. Res Commun Chem Pathol Pharmacol. 1974;7:663–670. [PubMed] [Google Scholar]
- Campos AC, Guimarães FS. Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology. 2008;199:223–230. doi: 10.1007/s00213-008-1168-x. [DOI] [PubMed] [Google Scholar]
- Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimarães FS. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos Trans R Soc Lond B Biol Sci. 2012;367:3364–3378. doi: 10.1098/rstb.2011.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg BC, Schulze DR, Hruba L, McMahon LR. JWH-018 and JWH-073: Δ9-tetrahydrocannabinol-like discriminative stimulus effects in monkeys. J Pharmacol Exp Ther. 2012;340:37–45. doi: 10.1124/jpet.111.187757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AJ, Williams CM, Whalley BJ, Stephens GJ. Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacol Ther. 2012;133:79–97. doi: 10.1016/j.pharmthera.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Hruba L, Ginsburg BC, McMahon LR. Apparent inverse relationship between cannabinoid agonist efficacy and tolerance/cross-tolerance produced by Δ9-tetrahydrocannabinol treatment in rhesus monkeys. J Pharmacol Exp Ther. 2012;342:843–849. doi: 10.1124/jpet.112.196444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. Guide for the Care and Use of Laboratory Animals. 8th. Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council; Washington, DC: 2011. [Google Scholar]
- Järbe TU, Henriksson BG, Ohlin GC. Δ9-THC as a discriminative cue in pigeons: effects of delta8-THC, CBD, and CBN. Arch Int Pharmacodyn Ther. 1977;228:68–72. [PubMed] [Google Scholar]
- Karniol IG, Carlini EA. Pharmacological interaction between cannabidiol and Δ9-tetrahydrocannabinol. Psychopharmacologia. 1973;33:53–70. doi: 10.1007/BF00428793. [DOI] [PubMed] [Google Scholar]
- Karniol IG, Shirakawa I, Kasinski N, Pfeferman A, Carlini EA. Cannabidiol interferes with the effects of Δ9-tetrahydrocannabinol in man. Eur J Pharmacol. 1974;28:172–177. doi: 10.1016/0014-2999(74)90129-0. [DOI] [PubMed] [Google Scholar]
- Leo A, Russo E, Elia M. Cannabidiol and epilepsy: rationale and therapeutic potential. Pharmacol Res. 2016;107:85–92. doi: 10.1016/j.phrs.2016.03.005. [DOI] [PubMed] [Google Scholar]
- McMahon LR. Discriminative stimulus effects of the cannabinoid CB1 antagonist SR 141716A in rhesus monkeys pretreated with Δ9-tetrahydrocannabinol. Psychopharmacology. 2006a;188:306–314. doi: 10.1007/s00213-006-0500-6. [DOI] [PubMed] [Google Scholar]
- McMahon LR. Characterization of cannabinoid agonists and apparent pA2 analysis of cannabinoid antagonists in rhesus monkeys discriminating Δ9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2006b;319:1211–1218. doi: 10.1124/jpet.106.107110. [DOI] [PubMed] [Google Scholar]
- McRae-Clark AL, Baker NL, Gray KM, Killeen TK, Wagner AM, Brady KT, DeVane CL, Norton J. Buspirone treatment of cannabis dependence: a randomized, placebo-controlled trial. Drug Alcohol Depend. 2015;156:29–37. doi: 10.1016/j.drugalcdep.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Parker LA, Gallily R. Cannabidiol: an overview of some pharmacological aspects. J Clin Pharmacol. 2002;42:11S–19S. doi: 10.1002/j.1552-4604.2002.tb05998.x. [DOI] [PubMed] [Google Scholar]
- Moranta D, Esteban S, García-Sevilla JA. Chronic treatment and withdrawal of the cannabinoid agonist WIN 55,212-2 modulate the sensitivity of presynaptic receptors involved in the regulation of monoamine syntheses in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:61–72. doi: 10.1007/s00210-008-0337-0. [DOI] [PubMed] [Google Scholar]
- Rock EM, Bolognini D, Limebeer CL, Cascio MG, Anavi-Goffer S, Fletcher PJ, Mechoulam R, Pertwee RG, Parker LA. Cannabidiol, a non-psychotropic component of cannabis, attenuates vomiting and nausea-like behaviour via indirect agonism of 5-HT1A somatodendritic autoreceptors in the dorsal raphe nucleus. Br J Pharmacol. 2012;165:2620–2634. doi: 10.1111/j.1476-5381.2011.01621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JS, McMahon LR. JWH-018 in rhesus monkeys: differential antagonism of discriminative stimulus, rate-decreasing, and hypothermic effects. Eur J Pharmacol. 2014;740:151–159. doi: 10.1016/j.ejphar.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1A receptors. Neurochem Res. 2005;30:1037–1043. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- Schoedel KA, Chen N, Hilliard A, White L, Stott C, Russo E, Wright S, Guy G, Romach MK, Sellers EM. A randomized, double-blind, placebo-controlled, crossover study to evaluate the subjective abuse potential and cognitive effects of nabiximols oromucosal spray in subjects with a history of recreational cannabis use. Hum Psychopharmacol. 2011;26:224–236. doi: 10.1002/hup.1196. [DOI] [PubMed] [Google Scholar]
- Stewart JL, McMahon LR. Rimonabant-induced Δ9-tetrahydrocannabinol withdrawal in rhesus monkeys: discriminative stimulus effects and other withdrawal signs. J Pharmacol Exp Ther. 2010;334:347–356. doi: 10.1124/jpet.110.168435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi RN, Karniol IG. Pharmacologic interaction between cannabinol and Δ9-tetrahydrocannabinol. Psychopharmacologia. 1975;41:277–284. doi: 10.1007/BF00428937. [DOI] [PubMed] [Google Scholar]
- Tanda G. Preclinical studies on the reinforcing effects of cannabinoids. A tribute to the scientific research of Dr Steve Goldberg. Psychopharmacology. 2016 doi: 10.1007/s00213-016-4244-7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann RE, Gamage TF, Warner JA, Marshall EM, Taylor NL, Martin BR, Wiley JL. Divergent effects of cannabidiol on the discriminative stimulus and place conditioning effects of Δ9-tetrahydrocannabinol. Drug Alcohol Depend. 2008;94:191–198. doi: 10.1016/j.drugalcdep.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SJ, McAllister SD, Kawamura R, Murase R, Neelakantan H, Walker EA. Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT1A receptors without diminishing nervous system function or chemotherapy efficacy. Br J Pharmacol. 2014;171:636–645. doi: 10.1111/bph.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ, Vandewater SA, Taffe MA. Cannabidiol attenuates deficits of visuospatial associative memory induced by Δ9-tetrahydrocannabinol. Br J Pharmacol. 2013;170:1365–1373. doi: 10.1111/bph.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]


