Abstract
Study Design
Retrospective analysis of prospectively collected registry data.
Objective
This study aimed to compare the clinical and radiologic outcomes between comparative cohorts of patients having anterior lumbar interbody fusion (ALIF) and patients having lateral lumbar interbody fusion (LLIF).
Methods
Ninety consecutive patients were treated by a single surgeon with either ALIF (n = 50) or LLIF (n = 40). Inclusion criteria were patients age 45 to 70 years with degenerative disk disease or grade 1 to 2 spondylolisthesis and single-level pathology from L1 to S1. Patient-reported outcome measures included pain (visual analog scale), disability (Oswestry Disability Index [ODI]), and quality of life (Short Form 36 physical component score [PCS] and mental component scores [MCS]). Assessment of fusion and measurement of lordosis and posterior disk height were performed on computed tomography scans.
Results
At 24 months, patients having ALIF had significant improvements in back (64%) and leg (65%) pain and ODI (60%), PCS (44%), and MCS (26%; p < 0.05) scores. Patients having LLIF had significant improvements in back (56%) and leg (57%) pain and ODI (52%), PCS (48%), and MCS (12%; p < 0.05) scores. Fourteen complications occurred in the ALIF group, and in the LLIF group, there were 17 complications (p > 0.05). The fusion rate was 100% for ALIF and 95% for LLIF (p = 0.1948). ALIF added ∼6 degrees of lordosis and 3 mm of height, primarily measured at L5–S1, and LLIF added ∼3 degrees of lordosis and 2 mm of height between L1 to L5. Mean follow-up was 34.1 months.
Conclusions
In comparative cohorts of patients having ALIF and patients having LLIF at 24 months postoperatively, there were no significant differences in clinical outcomes, complication rates, or fusion rates.
Keywords: anterior, comparison, complications, fusion, lateral, lordosis
Introduction
Lumbar interbody fusion is an accepted surgical technique to address severe degenerative disk disease, radiculopathy, and deformity. Anterior fusion techniques such as ALIF (anterior lumbar interbody fusion) and LLIF (lateral lumbar interbody fusion) are an alternative to traditional posterior methods (posterior and transforaminal lumbar interbody fusion) for achieving interbody fusion.1 2 3 4 5 6 Both ALIF and LLIF can be used as stand-alone procedures (without posterior fixation),7 8 9 or they can be supplemented with transpedicular screws and rods by open or percutaneous insertion.10 11
These anterior techniques are attractive as a means to obtain fusion because they permit surgeons to insert larger-footprint interbody devices, which contain more graft material than posteriorly inserted cages and allow for placement across the cortical bone of the ring apophysis to correct alignment and resist subsidence.12 13 In addition, the wider access to the intervertebral disk space allows for optimal end plate preparation to enhance prospects of interbody fusion. The downsides of these anterior techniques are the individual risk profiles attached to ALIF and LLIF, both in the learning curve and in established practice.1 2 14
The aim of this study was to compare the clinical and radiologic outcomes between comparative cohorts of patients having ALIF and patients having LLIF.
Methods
A total of 90 consecutive patients treated with either ALIF or LLIF by a single surgeon (G.M.M.) from August 2009 to August 2013 were retrospectively analyzed using data collected through a prospective registry. This study did not require Institutional Review Board approval in Australia as the registry data was collected in a private practice.
Inclusion and Exclusion Criteria
Inclusion criteria were patients who underwent a single-level ALIF or LLIF, were age 45 to 70 years, had evidence of degenerative disk disease or grade 1 to 2 spondylolisthesis, and had received recombinant human bone morphogenetic protein-2 (rhBMP-2; Infuse, Medtronic, Inc., Memphis, Tennessee, United States) within the cage. Indications for ALIF were L4–L5 or L5–S1 pathology with body mass index ≤ 30. Indications for LLIF were L1–L2, L2–L3, L3–L4, or L4–L5 pathology with body mass index ≤ 40. Patients were excluded from the analysis if they were current smokers, had prior lumbar fusion surgery, and/or had previous complex/extensive retroperitoneal surgery or abdominal/pelvic radiotherapy.
Patient Characteristics
Baseline patient information included basic demographics as well as the primary diagnosis for surgery. Treatment information included procedure, levels treated, choice of graft materials, and requirement for supplemental posterior instrumentation.
Surgical Technique
The surgical techniques for ALIF and LLIF have previously been described.2 3 15 For the ALIF procedure, with a vascular surgeon, a right lower Rutherford-Morrison transverse incision with a right-sided retroperitoneal approach was used to access the L5–S1 level. A midline lower abdominal incision with a left-sided retroperitoneal approach was used to access the L4–L5 level. For the LLIF procedure, without a vascular surgeon, a 90-degree off-midline retroperitoneal approach to the anterior lumbar spine through the fibers of the psoas muscle was used to access the lateral border of the target disk space.
Interbody Cages and Graft Material
All patients having ALIF received a single separate impacted PEEK (polyetheretherketone) cage (Perimeter, Medtronic, Inc.). The Perimeter cages were 24 mm in length, 30 mm in width and 8, 10, 12, 14, or 16 mm in height, with a lordotic angle of 8 or 12 degrees. All patients having LLIF were fitted with a 10-degree lordotic intervertebral PEEK cage (CoRoent, NuVasive, Inc., San Diego, California, United States). The CoRoent cages were 45, 50, 55, or 60 mm in length; 18 or 22 mm in width; and 8, 10, or 12 mm in height.
A stand-alone LLIF comprised the PEEK cage only without any supplemental fixation (Fig. 1), whereas stand-alone ALIF included the placement of an anterior titanium buttress plate (Pyramid, Medtronic, Inc.) to supplementally fixate the cage (Fig. 2). The anterior plate was triangular with three bone screws at L5–S1 and rectangular with four screws at L4–L5.
Fig. 1.
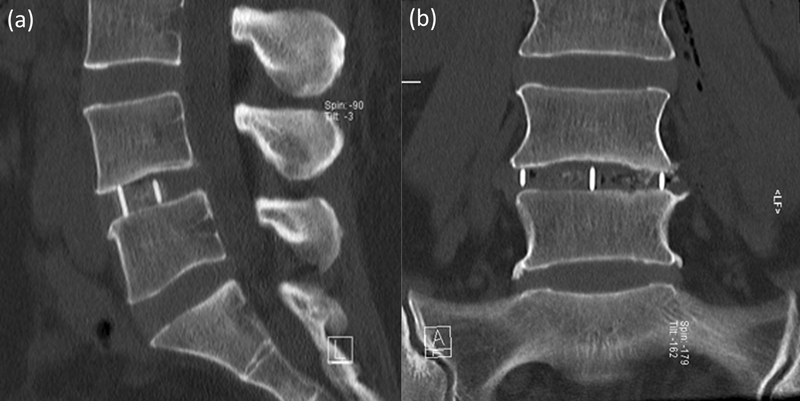
Lateral lumbar interbody fusion stand-alone construct at L4–L5. (a) Sagittal, (b) coronal.
Fig. 2.
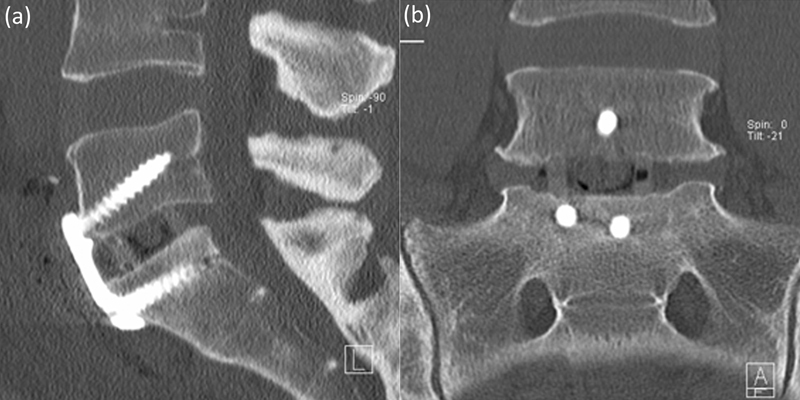
Anterior lumbar interbody fusion stand-alone construct at L5–S1. (a) Sagittal, (b) coronal.
All ALIF and LLIF cages were filled with rhBMP-2 applied to an absorbable collagen sponge (Medtronic, Inc.). The Infuse was prepared at a fixed concentration of 1.5 mg/cc, with the absorbable collagen sponge trimmed to the required cage volume. The Infuse dose was volume-dependent (i.e., internal graft volume of the cage equaled Infuse volume in cubic centimeters).16 No Infuse was placed outside the cage.
The indication for supplemental posterior percutaneous pedicle screw/rod fixation followed guidelines (ALIF) and a treatment algorithm (LLIF) previously published by the authors.2 15 Posterior instrumentation was indicated for: (1) patients having ALIF with reduced bone density and pars defects; (2) patients having LLIF with reduced bone density, facet arthropathy, instability, and pars defects. All patients with posterior instrumentation received percutaneous pedicle screws.
Complications
Complications were identified during hospitalization by an independent physician (F.Y.C.) and postoperatively, until last follow-up, by a research assistant (R.M.P.). The perioperative complications were classified as minor and major as per our previous publications on ALIF and LLIF.2 14
Clinical Outcomes
Patient-reported outcome measures included back and leg pain (visual analogue scale), disability (Oswestry Disability Index [ODI]), and quality of life (Short Form 36 physical component score [PCS] and mental component score [MCS]). Clinical outcomes of the patients having ALIF and patients having LLIF were compared using the minimum clinically important difference (MCID). To be identified as receiving clinical benefit from the surgical procedure, patients had to meet the defined thresholds for MCID: improvement of 1.2 points in back pain, 1.6 in leg pain, 12.8 in ODI, and 4.9 in PCS.17
Radiologic Outcomes
High-definition low-dose computed tomography (CT) scans (Somatom Definition Flash, Siemens AG, Erlangen, Germany) were performed preoperatively and 2 days postoperatively to assess instrumentation, and then at 6, 12, and 24 months until confirmation of solid interbody fusion on coronal and sagittal views was achieved. All postoperative scans were focused at the operative level, rather than a full lumbar CT. CT scans were obtained according to this protocol as part of our standard clinical practice. However, due to concerns regarding cancer risk from radiation exposure, once solid interbody fusion was confirmed, no further scans were performed.18 Fusion was defined as the presence of bridging interbody trabecular bone (Figs. 3 and 4).19 An independent radiologist (C.M.B.) from within the treating institution reported the CT scans.
Fig. 3.
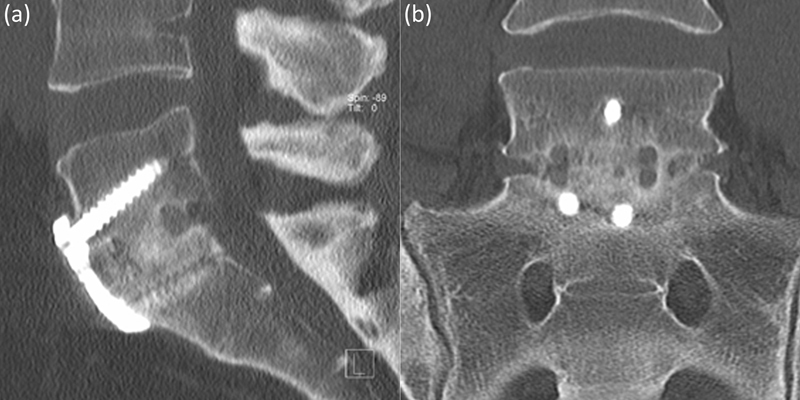
Anterior lumbar interbody fusion solid interbody fusion at L5–S1. (a) Sagittal, (b) coronal.
Fig. 4.
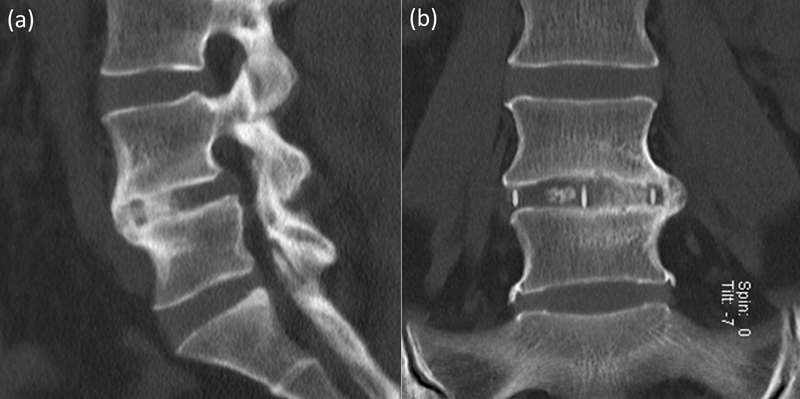
Lateral lumbar interbody fusion solid interbody fusion at L4–L5. (a) Sagittal, (b) coronal.
Segmental lordosis was assessed by measuring the angle between the cranial end plate of the superior vertebra and the cranial end plate of the inferior vertebra. Posterior disk height was measured between the posterior vertebral margins of the caudal and cranial end plates around the disk.15 Lordosis and posterior disk height were measured preoperatively, postoperatively, and at time of fusion. All measurements were obtained digitally using Inteleviewer software (Interad Medical Systems Inc., Quebec, Canada).
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics (Version 21.0, IBM Corp., Armonk, New York, United States) and included paired t tests, independent samples t tests, and Fisher exact tests with statistical significance measured at p < 0.05.
Results
Patient Demographics and Treatment
A total of 90 patients met the inclusion criteria and were included in the study. Fifty patients were included in the ALIF cohort, with a mean age of 55.7 years (range 45 to 70), and 27 (54%) were women. The primary diagnoses were degenerative disk disease in 37 patients (74%) and grade 1 to 2 spondylolisthesis in 13 (26%). Forty patients were included in the LLIF cohort, with a mean age of 58.5 years (range 45 to 70), and 22 (55%) were women. The primary diagnoses were degenerative disk disease in 30 patients (75%) and grade 1 to 2 spondylolisthesis in 10 (25%). There were no significant differences in age, sex, or primary diagnosis between the ALIF and LLIF groups (p > 0.05). There was a significant difference between the number of patients having ALIF and patients having LLIF who had supplemental posterior instrumentation (p = 0.0032), with 9 (18%) patients in the ALIF group and 19 (48%) patients in the LLIF group. There was also a significant difference in the level treated, with ALIF at L5–S1 in 84% of cases and LLIF at L4–L5 in 60% of cases (p < 0.0001). The mean internal volume of the ALIF cage was 2.5 cc and for the LLIF cage was 3.2 cc, corresponding to an Infuse dose of 3.8 and 4.8 mg, respectively (p < 0.0001). The demographic and treatment information for the ALIF and LLIF groups is provided in Table 1.
Table 1. Patient demographic and treatment information for ALIF and LLIF.
| Characteristic | ALIF (n = 50) | LLIF (n = 40) | Significance (p value) |
|---|---|---|---|
| Mean age, y (SD) (range) | 55.7 (6.9) (45–70) | 58.5 (6.8) (45–70) | 0.0594 |
| Sex (% women) | 27 (54) | 22 (55) | >0.9999 |
| BMI, mean (SD) (range) | 25.7 (2.8) (18.8–30.4) | 28.3 (5.2) (18.8–37.9) | 0.0157 |
| Comorbidities | |||
| Prior lumbar spine surgery (%) | 4 (8) | 15 (38) | 0.0013 |
| Laminectomy (% surgery) | 1 (25) | 11 (73) | |
| Microdiskectomy (% surgery) | 3 (75) | 4 (27) | |
| Primary diagnosis | >0.9999 | ||
| Degenerative disk disease (%) | 37 (74) | 30 (75) | |
| Spondylolisthesis (%) | 13 (26) | 10 (25) | |
| Level treated | <0.0001 | ||
| L1–L2 (%) | 0 | 1 (3) | |
| L2–L3 (%) | 0 | 6 (15) | |
| L3–L4 (%) | 0 | 9 (23) | |
| L4-L5 (%) | 8 (16) | 24 (60) | |
| L5–S1 (%) | 42 (84) | 0 | |
| Fixation type | 0.0032 | ||
| Stand-alonea (%) | 41 (82) | 21 (53) | |
| Transpedicular bilateral fixation (%) | 9 (18) | 19 (48) |
Abbreviations: ALIF, anterior lumbar interbody fusion; BMI, body mass index; LLIF, lateral lumbar interbody fusion; SD, standard deviation.
ALIF stand-alone = cage + plate.
One patient who had LLIF was lost to clinical follow-up at 12 months. The patient's son reported the patient had become morbidly obese and was now agoraphobic and unable to leave the house.
Clinical Outcomes
The mean follow-up was 34.1 months (range 24 to 60 months). For the ALIF group, mean back and leg pain improved from 6.5 to 2.3 and 5.5 to 1.9, representing improvements of 64 and 65%, respectively. ODI improved from 49.9 to 20.2 (60%), with PCS and MCS improving 44% (32.0 to 46.1) and 26% (40.4 to 50.9), respectively. For the LLIF group, mean back and leg pain improved from 6.5 to 2.7 and 6.0 to 2.0, representing improvements of 56 and 57%, respectively. ODI improved from 53.0 to 25.6 (52%), with PCS and MCS improving 48% (29.4 to 43.3) and 12% (45.9 to 51.4), respectively. All clinical results for both the ALIF and LLIF groups were significantly improved from baseline (p < 0.0001). A summary of the clinical results for the ALIF and LLIF groups is provided in Table 2.
Table 2. Summary of clinical results for ALIF and LLIF.
| ALIF | LLIF | ||||
|---|---|---|---|---|---|
| Preop (mean ± SD) | 24 mo (mean ± SD) | Preop (mean ± SD) | 24 mo (mean ± SD) | Significance (p value)a | |
| VAS (back) | 6.5 ± 2.3 | 2.3 ± 2.1 | 6.5 ± 2.5 | 2.7 ± 2.5 | 0.4191 |
| VAS (leg) | 5.5 ± 2.5 | 1.9 ± 2.5 | 6.0 ± 2.6 | 2.0 ± 2.5 | 0.4298 |
| ODI | 49.9 ± 16.0 | 20.2 ± 18.7 | 53.0 ± 13.2 | 25.6 ± 17.6 | 0.1877 |
| SF-36 PCS | 32.0 ± 8.0 | 46.1 ± 9.8 | 29.4 ± 8.2 | 43.3 ± 9.6 | >0.9999 |
| SF-36 MCS | 40.4 ± 12.4 | 50.9 ± 11.4 | 45.9 ± 9.7 | 51.4 ± 11.2 | N/Ab |
Abbreviations: ALIF, anterior lumbar interbody fusion; LLIF, lateral lumbar interbody fusion; MCS, mental component score; N/A, not applicable; ODI, Oswestry Disability Index; PCS, physical component score; preop, preoperative; SD, standard deviation; SF-36, Short Form 36; VAS, visual analogue scale.
Comparison based on the number of patients who met the defined thresholds for minimum clinically important difference.17
MCS does not have a minimum clinically important difference.
The MCID criteria were used to compare the number of patients in the ALIF and LLIF groups who met the threshold for clinical benefit from the surgical procedure.17 There were no statistically significant differences (p > 0.05) in the number of patients who met the MCID between the ALIF and LLIF groups (Table 2).
Complications
The complications of ALIF and LLIF were divided into minor and major (Table 3). A total of 14 complications occurred in the ALIF group, 9 minor and 5 major. The most common complications in the ALIF cohort were dysesthesia (sensory changes) and ileus and radiculopathy (new-onset motor deficit), 3 instances of each. There were no significant vascular access injuries (defined as blood loss > 150 mL from a single major vessel injury) or retrograde ejaculation in our series. All male patients were queried regarding the possible presence of retrograde ejaculation using our previously published sexual dysfunction and retrograde ejaculation screening questionnaire.2 A total of 17 complications occurred in the LLIF group, 8 minor and 9 major. The most common complication in this group was radiculopathy in 6 cases. Of note, 1 patient sustained a bowel injury, which has been previously described.14 There was no significant difference between the number of minor (p > 0.9999) and major (p = 0.1445) complications experienced by the patients having ALIF and patients having LLIF.
Table 3. Complications of ALIF and LLIF.
| Complication | ALIF | LLIF | Significance (p value) |
|---|---|---|---|
| Minor | |||
| Dysesthesia (sensory changes) | 3 | 4 | |
| Atelectasis | 1 | 0 | |
| Ileus | 3 | 0 | |
| Superficial wound infection | 1 | 3 | |
| Urinary tract infection | 0 | 0 | |
| Sympathetic chain injury | 0 | 0 | |
| Hematoma | 1 | 1 | |
| Wound hernia/paresis | 0 | 0 | |
| Total | 9 | 8 | >0.9999 |
| Major | |||
| Radiculopathy (motor deficit) | 3 | 6 | |
| Retrograde ejaculation | 0 | 0 | |
| Pneumonia | 2 | 2 | |
| Deep wound infection | 0 | 0 | |
| Bowel injury | 0 | 1 | |
| Major vascular injury | 0 | 0 | |
| Deep vein thrombosis | 0 | 0 | |
| Death | 0 | 0 | |
| Total | 5 | 9 | 0.1445 |
Abbreviations: ALIF, anterior lumbar interbody fusion; LLIF, lateral lumbar interbody fusion.
Interbody Fusion
Interbody fusion rates for the ALIF group progressed from 66% at 6 months to 96% at 12 months and 100% at 24 months. For the LLIF group, fusion rates progressed from 45% at 6 months to 85% at 12 months and 95% at 24 months. There was no significant difference in interbody fusion rates between ALIF and LLIF at 6, 12, or 24 months (p > 0.05); see Table 4.
Table 4. Fusion rates at postoperative 6, 12, and 24 months for ALIF and LLIF.
| ALIF % solid fusion (n fused/n total) | LLIF % solid fusion (n fused/n total) | Significance (p value) | |
|---|---|---|---|
| 6 mo | 66 (33/50) | 45 (18/40) | 0.0559 |
| 12 mo | 96 (48/50) | 85 (34/40) | 0.1356 |
| 24 mo | 100 (50/50) | 95 (38/40) | 0.1948 |
Abbreviations: ALIF, anterior lumbar interbody fusion; LLIF, lateral lumbar interbody fusion.
Segmental Lordosis and Posterior Disk Height
The segmental lordosis and posterior disk heights of the ALIF and LLIF groups are provided in Table 5. For the ALIF group, mean segmental lordosis increased from 14.6 to 21.0 degrees (p = 0.0001) and posterior disk height increased from 3.2 to 6.6 mm (p < 0.0001) preoperatively to time of fusion (range 6 to 24 months). For the LLIF group, segmental lordosis increased from 9.9 to 12.3 degrees (p = 0.0034) and posterior disk height increased from 4.1 to 5.7 mm (p = 0.0028) preoperatively to fusion. Overall, at time of fusion ALIF added ∼6 degrees of lordosis and 3 mm of height, primarily measured at L5–S1, and LLIF added ∼3 degrees of lordosis and 2 mm of height between L1 to L5.
Table 5. Preop, postop, and time of fusion segmental lordosis and posterior disk height for ALIF and LLIF.
| ALIF | LLIF | |||
|---|---|---|---|---|
| Mean segmental angle (degrees) | Mean posterior disk height (mm) | Mean segmental angle (degrees) | Mean posterior disk height (mm) | |
| Preop | 14.6 ± 3.6 | 3.2 ± 1.1 | 9.9 ± 4.2 | 4.1 ± 1.7 |
| Postop | 21.9 ± 4.7 | 7.2 ± 1.3 | 12.6 ± 5.0 | 6.4 ± 1.6 |
| Fusiona | 21.0 ± 4.3 | 6.6 ± 1.2 | 12.3 ± 4.6 | 5.7 ± 1.6 |
| p (preop and postop) | <0.0001 | <0.0001 | 0.0001 | <0.0001 |
| p (preop and fusion) | 0.0001 | <0.0001 | 0.0034 | 0.0028 |
| p (postop and fusion) | 0.3318 | 0.0154 | 0.7802 | 0.0494 |
Abbreviations: ALIF, anterior lumbar interbody fusion; LLIF, lateral lumbar interbody fusion; preop, preoperative; postop, postoperative.
Time of fusion (range 6 to 24 months).
To compare the two techniques, the segmental lordosis and posterior disk height at L4–L5 were compared (Table 6). There was no significant difference in the preoperative measurements or in postoperative and fusion posterior disk height (p > 0.05); however, ALIF gained significantly more postoperative and fusion segmental lordosis at L4–L5 (p < 0.05). These results are based on small number of patients having ALIF (8/50) and as a result should be interpreted with caution.
Table 6. Preop, postop, and time of fusion segmental lordosis and posterior disk height at L4–L5 for ALIF and LLIF.
| ALIF (n = 8) | LLIF (n = 24) | Comparison (p value) | ||||
|---|---|---|---|---|---|---|
| Mean segmental angle (degrees) | Mean posterior disk height (mm) | Mean segmental angle (degrees) | Mean posterior disk height (mm) | Segmental angle | Posterior disk height | |
| Preop | 9.7 ± 2.4 | 3.2 ± 1.1 | 10.6 ± 4.7 | 4.2 ± 1.5 | 0.6005 | 0.1071 |
| Postop | 18.9 ± 3.4 | 7.5 ± 1.0 | 15.2 ± 4.2 | 6.5 ± 1.6 | 0.0478 | 0.1300 |
| Fusiona | 18.0 ± 3.4 | 7.0 ± 0.8 | 14.2 ± 4.2 | 5.7 ± 1.7 | 0.0422 | 0.0557 |
Abbreviations: ALIF, anterior lumbar interbody fusion; LLIF, lateral lumbar interbody fusion; preop, preoperative; postop, postoperative.
Time of fusion (range 6 to 24 months).
Cost
The cost of an ALIF (with vascular surgeon) was A$10,296 for a stand-alone and A$17,422 for instrumented versions. LLIF cost A$6,800 for stand-alone and A$13,926 for instrumented versions (Table 7).20 21
Table 7. Cost of a single level ALIF and LLIF20 21 .
| Item | Cost (A$) | |
|---|---|---|
| ALIF | Vascular surgeon | 696 |
| Perimeter cagea | 3,600 | |
| Pyramid platea | 2,200 | |
| Pyramid screwsa | 600 (3 screws) | |
| Small Infusea | 3,200 | |
| LLIF | CoRoent cageb | 3,600 |
| Small Infusea | 3,200 | |
| Posterior instrumentation | Polyaxial screws | 5,560 (4 screws) |
| Prelordosed rods | 886 (2 rods) | |
| Blockers | 680 (4 blockers) | |
| Total | ||
| Stand-alone ALIF | 10,296 | |
| Stand-alone LLIF | 6,800 | |
| Instrumented ALIF | 17,422 | |
| Instrumented LLIF | 13,926 |
Abbreviations: ALIF, anterior lumbar interbody fusion; LLIF, lateral lumbar interbody fusion.
Medtronic, Inc., Memphis, Tennessee, United States.
NuVasive, Inc., San Diego, California, United States.
Discussion
ALIF and LLIF have much in common as an alternative to posterior techniques for interbody fusion. They are biomechanically attractive as they permit surgeons to insert larger-footprint interbody devices,12 22 23 24 which provide greater intrinsic stability and contain more graft material than posteriorly inserted cages.25 The wider access to the intervertebral disk space allows for superior end plate preparation to enhance interbody fusion.26 27 Additionally, ALIF and LLIF avoid injury to the paraspinal muscles that cause posterior “fusion disease.”28 The anterior approach retains all posterior-stabilizing structures, reduces adjacent segment disease from denervation or injury to the adjacent facet joints and muscles, and avoids both epidural scarring and perineural fibrosis.29
Although both ALIF and LLIF are minimally invasive “anterior” techniques, there are differences. The open technique of ALIF permits excellent visualization of the intervertebral end plates, allowing direct inspection and determination if preparation is satisfactory. In contrast, LLIF relies on feel and sound of the curettes and rasps, with relatively little direct visualization of the disk space. During ALIF, surgeons can also remove the central and foraminal components of a herniated disk prolapse, which is more difficult on the contralateral side in LLIF.
In LLIF, the intervertebral cage is inserted through the lateral annulus and spans both apophyseal cortical rims. This placement provides optimal end plate support and imparts substantial biomechanical stability.12 23 24 ALIF requires resection of the anterior longitudinal ligament (ALL) to insert a cage that sits centrally. This central location, although not having the biomechanical strength of spanning the apophyseal cortical rim, may have an advantage for interbody fusion. Because the cage sits within the concavity of the end plates (particularly at L4–L5), it provides better contact between graft material and the prepared central bony end plates, enhancing graft incorporation.22 30 31 However, convexly shaped implants in LLIF also allow for more direct end plate contact in the cancellous midsections.
ALIF cages are commonly reinforced with an anterior plate (separate or integrated with the cage) to replace the resected ALL.2 32 33 ALIF is usually performed without additional posterior fixation, and LLIF is more frequently supplemented with posterior pedicle screws. In our cohort, the indication for posterior fixation followed our previously published guidelines (ALIF) and treatment algorithm (LLIF).2 15 Supplemental posterior fixation with bilateral pedicle screws is preferred because it provides the optimal biomechanically supportive long-term construct.24
Another notable difference is the use of an access or vascular surgeon in ALIF.2 34 The vascular structures needing to be retracted in ALIF are not a factor in LLIF, which utilizes blunt dissection through the retroperitoneal space and passage through the lateral psoas muscle under EMG guidance.
In our comparative cohort of patients having ALIF and patients having LLIF, there was no significant difference in age, sex, or primary diagnosis (p > 0.05). There was a significant difference in the level treated, with ALIF primarily at L5–S1 (84%) and LLIF at L4–L5 (60%; p < 0.0001). There was also a significant difference in the number of patients receiving supplemental posterior instrumentation, with 18% (9/50) of patients having ALIF versus 48% (19/40) of patients having LLIF (p = 0.0032), though all patients having ALIF received anterior plating as supplemental internal fixation.
Although ALIF and LLIF have differing risk profiles,2 14 we found no significant difference between the number of minor (p > 0.9999) and major (p = 0.1445) complications experienced by the patients having ALIF and the patients having LLIF.
In our study, we found no significant difference between the clinical outcomes of patients having ALIF and patients having LLIF (p > 0.05). These results are supported by the 2-year findings of Smith et al,35 who compared economic as well as clinical differences in ALIF and LLIF outcomes. Although these authors found significantly lower complications, treatment characteristics, and overall cost in LLIF compared with the ALIF group, at 2 years postoperatively, the clinical improvements between the two groups were nearly identical.
In our series, there was a nonsignificant trend toward earlier fusion in patients having ALIF compared with patients having LLIF at 6 months (66% versus 45%; p = 0.0559); however, by 24 months the fusion rates were similar (100% versus 95%; p = 0.1948). This result may be an artifact of supplemental internal fixation, which was used in all ALIF cases (anterior plating with or without posterior fixation); over half of the patients having LLIF were treated with stand-alone constructs. The more rigid construct in the patients having ALIF may have resulted in the higher fusion rates observed at each time point.
Despite a significantly larger Infuse dose in LLIF (4.8 mg) compared with ALIF (3.8 mg), we did not observe higher fusion rates in LLIF.
At the time of fusion, we found ALIF added ∼6 degrees of segmental lordosis and 3 mm of posterior disk height at L4 to S1, and LLIF added ∼3 degrees of lordosis and 2 mm of height at L1 to L5. L5–S1 is the most naturally lordotic disk (24 degrees),36 hence to compare the two techniques we performed a subgroup analysis at L4–L5 of the lordosis and disk height. Between the groups in this scenario, there were no significant differences in preoperative, postoperative, or fusion posterior disk heights. There was, however, a significant difference in segmental lordosis between preoperative versus postoperative and fusion angles (p < 0.05), favoring ALIF. More segmental lordosis was gained in ALIF at L4–L5 despite the majority of cases receiving an 8-degree lordotic cage (5/8 patients), compared with LLIF where all cages were 10 degrees. However, with resection of the ALL in the ALIF group and not in the LLIF group, it is likely that segmental lordosis was greater in the ALIF group due to an anterior release. We did not compare the difference between the segmental lordosis of the stand-alone and instrumented cases as we have previously shown that the addition of posterior percutaneous fixation adds approximately only 1 degree of lordosis,15 which is presumably because of intact posterior elements. The recently introduced hyperlordotic cages for both ALIF and LLIF (when combined with anterior column release) improve segmental lordotic correction for both techniques.37 38
Cost is a consideration when choosing the approach. An ALIF requires the use of a vascular surgeon (A$696) as well as an anterior plate and screws (A$2,800), which adds A$3,496 to the cost of the procedure. Hence, a stand-alone ALIF (A$10,296) is more expensive than a stand-alone LLIF (A$6,800). Posterior instrumentation adds A$7,126 to the cost of the procedure, which results in a total cost of A$17,422 for an instrumented ALIF and A$13,926 for an instrumented LLIF. However, in our cohort posterior instrumentation was more commonly used in LLIF procedures (48 versus 18%), which means that a stand-alone ALIF (with vascular surgeon) was more cost effective than an instrumented LLIF (Table 7).20 21
Both ALIF and LLIF have evolved as alternative approaches to posterior lumbar interbody fusion and are superior in terms of being less invasive and having lower blood loss and higher fusion rates.39 40 We have shown that ALIF and LLIF have similar outcomes, fusion rates, and complication rates, corroborating the earlier work by Smith et al.35 Therefore, we believe for appropriate pathology at L5–S1 ALIF remains the treatment of choice, whereas LLIF is probably ideal for L3–L4 and above. At L4–L5, surgeons can confidently choose either ALIF or LLIF, whichever is preferred, and receive similar clinical and radiologic outcomes.
The strengths of this report were that the study cohorts were similarly matched in terms of pathology and biologics used. CT was used in radiologic follow-up and was reviewed by an independent radiologist, thus increasing the accuracy of long-term fusion results. In addition, there was a consistent surgical technique from a single surgeon and surgeries took place over the same time period, allowing a comparison of these two techniques to be made in parallel rather than in series.
Despite these similarities between the groups, the primary limitation of the current study is the relative heterogeneity between the groups. The patients were largely treated at different surgical levels, L4–L5 versus L5–S1; these levels have different pathologic manifestations and correction concerns that could confound findings. The small number of patients having L4–L5 ALIF makes comparison of segmental lordosis and posterior disk height results difficult. In addition, the use of supplemental internal fixation was significantly different between the groups; all of the patients having ALIF received anterior plating with or without posterior fixation, and over half of the patients having LLIF were treated with stand-alone constructs. Thus fusion results could be confounded by choice of fixation rather than an effect of the surgery. This study was not a randomized controlled trial, which contributed to the heterogeneity between groups. Finally, although the size of the groups compared here is large relative to much of the published literature, when attempting to perform subgroup analyses, the small sample sizes challenge statistical power.
Conclusion
In comparative cohorts of patients having ALIF and patients having LLIF at postoperative 24 months, there were no significant differences in clinical outcomes, complication rates, or fusion rates, which reinforce the similarity of the procedures and reassure surgeons that they can confidently choose either ALIF or LLIF, as the pathology dictates.
Footnotes
Disclosures Gregory M. Malham, Travel expenses: Medtronic, NuVasive, Stryker Rhiannon M. Parker, none Carl M. Blecher, none Fiona Y. Chow, none Kevin A. Seex, none
References
- 1.Than K D, Wang A C, Rahman S U. et al. Complication avoidance and management in anterior lumbar interbody fusion. Neurosurg Focus. 2011;31(4):E6. doi: 10.3171/2011.7.FOCUS11141. [DOI] [PubMed] [Google Scholar]
- 2.Malham G M, Parker R M, Ellis N J, Blecher C M, Chow F Y, Claydon M H. Anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2: a prospective study of complications. J Neurosurg Spine. 2014;21(6):851–860. doi: 10.3171/2014.8.SPINE13524. [DOI] [PubMed] [Google Scholar]
- 3.Ozgur B M, Aryan H E, Pimenta L, Taylor W R. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6(4):435–443. doi: 10.1016/j.spinee.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Marchi L, Oliveira L, Amaral R. et al. Lateral interbody fusion for treatment of discogenic low back pain: minimally invasive surgical techniques. Adv Orthop. 2012;2012:282068. doi: 10.1155/2012/282068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rihn J A, Patel R, Makda J. et al. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J. 2009;9(8):623–629. doi: 10.1016/j.spinee.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Okuda S, Miyauchi A, Oda T, Haku T, Yamamoto T, Iwasaki M. Surgical complications of posterior lumbar interbody fusion with total facetectomy in 251 patients. J Neurosurg Spine. 2006;4(4):304–309. doi: 10.3171/spi.2006.4.4.304. [DOI] [PubMed] [Google Scholar]
- 7.Aryan H E Lu D C Acosta F L Jr Ames C P Stand-alone anterior lumbar discectomy and fusion with plate: initial experience Surg Neurol 20076817–13., discussion 13 [DOI] [PubMed] [Google Scholar]
- 8.Beaubien B P, Freeman A L, Turner J L. et al. Evaluation of a lumbar intervertebral spacer with integrated screws as a stand-alone fixation device. J Spinal Disord Tech. 2010;23(5):351–358. doi: 10.1097/BSD.0b013e3181b15d00. [DOI] [PubMed] [Google Scholar]
- 9.Marchi L, Abdala N, Oliveira L, Amaral R, Coutinho E, Pimenta L. Stand-alone lateral interbody fusion for the treatment of low-grade degenerative spondylolisthesis. ScientificWorldJournal. 2012;2012:456346. doi: 10.1100/2012/456346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavlov P W Meijers H van Limbeek J et al. Good outcome and restoration of lordosis after anterior lumbar interbody fusion with additional posterior fixation Spine (Phila Pa 1976) 200429171893–1899., discussion 1900 [DOI] [PubMed] [Google Scholar]
- 11.Anderson D G, Sayadipour A, Shelby K, Albert T J, Vaccaro A R, Weinstein M S. Anterior interbody arthrodesis with percutaneous posterior pedicle fixation for degenerative conditions of the lumbar spine. Eur Spine J. 2011;20(8):1323–1330. doi: 10.1007/s00586-011-1782-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pimenta L, Turner A WL, Dooley Z A, Parikh R D, Peterson M D. Biomechanics of lateral interbody spacers: going wider for going stiffer. ScientificWorldJournal. 2012;2012:381814. doi: 10.1100/2012/381814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le T V, Baaj A A, Dakwar E. et al. Subsidence of polyetheretherketone intervertebral cages in minimally invasive lateral retroperitoneal transpsoas lumbar interbody fusion. Spine (Phila Pa 1976) 2012;37(14):1268–1273. doi: 10.1097/BRS.0b013e3182458b2f. [DOI] [PubMed] [Google Scholar]
- 14.Malham G M, Ellis N J, Parker R M, Seex K A. Clinical outcome and fusion rates after the first 30 extreme lateral interbody fusions. ScientificWorldJournal. 2012;2012:246989. doi: 10.1100/2012/246989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malham G M Ellis N J Parker R M et al. Maintenance of segmental lordosis and disc height in standalone and instrumented extreme lateral interbody fusion (XLIF) J Spinal Disord Tech 2014; March 24 (Epub ahead of print); doi: 10.1097/BSD.0b013e3182aa4c94 [DOI] [PubMed] [Google Scholar]
- 16.Boden S D, Kang J, Sandhu H, Heller J G. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine (Phila Pa 1976) 2002;27(23):2662–2673. doi: 10.1097/00007632-200212010-00005. [DOI] [PubMed] [Google Scholar]
- 17.Copay A G, Glassman S D, Subach B R, Berven S, Schuler T C, Carreon L Y. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J. 2008;8(6):968–974. doi: 10.1016/j.spinee.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Richards P J, George J, Metelko M, Brown M. Spine computed tomography doses and cancer induction. Spine (Phila Pa 1976) 2010;35(4):430–433. doi: 10.1097/BRS.0b013e3181cdde47. [DOI] [PubMed] [Google Scholar]
- 19.Williams A L, Gornet M F, Burkus J K. CT evaluation of lumbar interbody fusion: current concepts. AJNR Am J Neuroradiol. 2005;26(8):2057–2066. [PMC free article] [PubMed] [Google Scholar]
- 20.Australian Government Department of Health Private Health Insurance Prostheses List, Prostheses List—Part A, February 2015 Available at: http://www.health.gov.au/internet/main/publishing.nsf/Content/prostheses-list-pdf.htm. Accessed August 19, 2015
- 21.Australian Government Department of Health Medicare Benefits Schedule, Complete MBS, July 2015 Available at: http://www.health.gov.au/internet/mbsonline/publishing.nsf/Content/Downloads-2015-07. Accessed August 19, 2015
- 22.Closkey R F, Parsons J R, Lee C K, Blacksin M F, Zimmerman M C. Mechanics of interbody spinal fusion. Analysis of critical bone graft area. Spine (Phila Pa 1976) 1993;18(8):1011–1015. doi: 10.1097/00007632-199306150-00010. [DOI] [PubMed] [Google Scholar]
- 23.Grant J P, Oxland T R, Dvorak M F. Mapping the structural properties of the lumbosacral vertebral endplates. Spine (Phila Pa 1976) 2001;26(8):889–896. doi: 10.1097/00007632-200104150-00012. [DOI] [PubMed] [Google Scholar]
- 24.Cappuccino A Cornwall G B Turner A W et al. Biomechanical analysis and review of lateral lumbar fusion constructs Spine (Phila Pa 1976) 201035(26, Suppl):S361–S367. [DOI] [PubMed] [Google Scholar]
- 25.Matsumura A, Taneichi H, Suda K, Kajino T, Moridaira H, Kaneda K. Comparative study of radiographic disc height changes using two different interbody devices for transforaminal lumbar interbody fusion: open box vs. fenestrated tube interbody cage. Spine (Phila Pa 1976) 2006;31(23):E871–E876. doi: 10.1097/01.brs.0000244593.86975.27. [DOI] [PubMed] [Google Scholar]
- 26.Burkus J K, Gornet M F, Schuler T C, Kleeman T J, Zdeblick T A. Six-year outcomes of anterior lumbar interbody arthrodesis with use of interbody fusion cages and recombinant human bone morphogenetic protein-2. J Bone Joint Surg Am. 2009;91(5):1181–1189. doi: 10.2106/JBJS.G.01485. [DOI] [PubMed] [Google Scholar]
- 27.Rodgers W B, Gerber E J, Patterson J R. Fusion after minimally disruptive anterior lumbar interbody fusion: analysis of extreme lateral interbody fusion by computed tomography. SAS J. 2010;4(2):63–66. doi: 10.1016/j.esas.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zdeblick T A A prospective randomized study of the surgical treatment of L5–S1 degenerative disc disease Paper presented at: Tenth Annual Meeting of the North American Spine Society; October 20, 1995; Washington, DC
- 29.Burkus J K, Gornet M F, Dickman C A, Zdeblick T A. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15(5):337–349. doi: 10.1097/00024720-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Steffen T, Tsantrizos A, Aebi M. Effect of implant design and endplate preparation on the compressive strength of interbody fusion constructs. Spine (Phila Pa 1976) 2000;25(9):1077–1084. doi: 10.1097/00007632-200005010-00007. [DOI] [PubMed] [Google Scholar]
- 31.Lowe T G, Hashim S, Wilson L A. et al. A biomechanical study of regional endplate strength and cage morphology as it relates to structural interbody support. Spine (Phila Pa 1976) 2004;29(21):2389–2394. doi: 10.1097/01.brs.0000143623.18098.e5. [DOI] [PubMed] [Google Scholar]
- 32.Cain C M, Schleicher P, Gerlach R, Pflugmacher R, Scholz M, Kandziora F. A new stand-alone anterior lumbar interbody fusion device: biomechanical comparison with established fixation techniques. Spine (Phila Pa 1976) 2005;30(23):2631–2636. doi: 10.1097/01.brs.0000187897.25889.54. [DOI] [PubMed] [Google Scholar]
- 33.Kornblum M B, Turner A WL, Cornwall G B, Zatushevsky M A, Phillips F M. Biomechanical evaluation of stand-alone lumbar polyether-ether-ketone interbody cage with integrated screws. Spine J. 2013;13(1):77–84. doi: 10.1016/j.spinee.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Jarrett C D, Heller J G, Tsai L. Anterior exposure of the lumbar spine with and without an “access surgeon”: morbidity analysis of 265 consecutive cases. J Spinal Disord Tech. 2009;22(8):559–564. doi: 10.1097/BSD.0b013e318192e326. [DOI] [PubMed] [Google Scholar]
- 35.Smith W D, Christian G, Serrano S, Malone K T. A comparison of perioperative charges and outcome between open and mini-open approaches for anterior lumbar discectomy and fusion. J Clin Neurosci. 2012;19(5):673–680. doi: 10.1016/j.jocn.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Le T V, Vivas A C, Dakwar E, Baaj A A, Uribe J S. The effect of the retroperitoneal transpsoas minimally invasive lateral interbody fusion on segmental and regional lumbar lordosis. ScientificWorldJournal. 2012;2012:516706. doi: 10.1100/2012/516706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bach K, Ahmadian A, Deukmedjian A, Uribe J S. Minimally invasive surgical techniques in adult degenerative spinal deformity: a systematic review. Clin Orthop Relat Res. 2014;472(6):1749–1761. doi: 10.1007/s11999-013-3441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uribe J S, Smith D A, Dakwar E. et al. Lordosis restoration after anterior longitudinal ligament release and placement of lateral hyperlordotic interbody cages during the minimally invasive lateral transpsoas approach: a radiographic study in cadavers. J Neurosurg Spine. 2012;17(5):476–485. doi: 10.3171/2012.8.SPINE111121. [DOI] [PubMed] [Google Scholar]
- 39.Lucio J C, Vanconia R B, Deluzio K J, Lehmen J A, Rodgers J A, Rodgers W. Economics of less invasive spinal surgery: an analysis of hospital cost differences between open and minimally invasive instrumented spinal fusion procedures during the perioperative period. Risk Manag Healthc Policy. 2012;5:65–74. doi: 10.2147/RMHP.S30974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodgers W B Gerber E J Rodgers J A Lumbar fusion in octogenarians: the promise of minimally invasive surgery Spine (Phila Pa 1976) 201035(26, Suppl):S355–S360. [DOI] [PubMed] [Google Scholar]


