Abstract
Study Design
Retrospective review.
Objective
Intraoperative motor evoked potential (MEP) monitoring in spine surgery may assist surgeons in taking corrective measures to prevent neurologic deficits. The efficacy of monitoring MEPs intraoperatively in patients with myelopathy from nondegenerative causes has not been quantified. We compared the sensitivity and specificity of intraoperative MEP monitoring in patients with myelopathy caused by nondegenerative processes to patients with degenerative cervicothoracic spondylotic myelopathy (CSM).
Methods
We retrospectively reviewed our myelopathy surgical cases during a 1-year period to identify patients with degenerative CSM and CSM of nondegenerative causes and collected data on intraoperative MEP changes and postoperative new deficits. Categorical variables were analyzed by Fisher exact test. Receiver operator curves assessed intraoperative MEP monitoring performance in the two groups.
Results
In all, 144 patients were identified: 102 had degenerative CSM and 42 had CSM of nondegenerative causes (24 extra-axial tumors, 12 infectious processes, 5 traumatic fractures, and 1 rheumatoid arthritis). For degenerative CSM, there were 11 intraoperative MEP alerts and 7 new deficits (p < 0.001). The corresponding sensitivity was 71% and the specificity was 94%. In the nondegenerative group, there were 11 intraoperative MEP alerts and 3 deficits, which was not significant (p > 0.99). The sensitivity (33%) and specificity (74%) were lower. Among patients with degenerative CSM, the model performed well for predicting postoperative deficits (area under the curve [AUC] 0.826), which appeared better than the nondegenerative group, although it did not reach statistical significance (AUC 0.538, p = 0.16).
Conclusions
Based on this large retrospective analysis, intraoperative MEP monitoring in surgery for nondegenerative CSM cases appears to be less sensitive to cord injury and less predictive of postoperative deficits when compared with degenerative CSM cases.
Keywords: MEP, neuromonitoring, myelopathy, tumor, infection
Introduction
Intraoperative neurophysiologic monitoring (IONM) is a rapidly advancing modality commonly used in spinal surgery.1 2 3 Although almost universally employed during spinal deformity surgery, the indications for monitoring decompressive procedures are more controversial.4 Some argue against the use of IONM in cervical decompression altogether.4
The sensitivity of combined sensory and motor modality neurophysiologic monitoring may approach 100%, although sensitivities as low as 43% have been reported.5 Somatosensory evoked potentials, although advantageous because they can be monitored continuously, rely on signal averaging over time, and signal decreases may significantly lag behind transcranial motor evoked potential (MEP) changes.6 Monitoring MEP may provide earlier detection of neurologic injury and is associated with high sensitivity.7 However, MEPs cannot be monitored continuously, may induce patient movement, and are adversely affected by a variety of factors such as inhaled anesthetic agents.8
We recently reported high sensitivity of MEP changes in predicting postoperative deficits in patients undergoing surgery for degenerative cervical and thoracic myelopathy. However, the performance varies based on risk factors such as patient comorbidities, age, and preoperative neurologic function.9 Few studies have focused on the use of IONM in patients with myelopathy due to nondegenerative causes. In this study, we analyzed a group of consecutive patients with cervical and cervicothoracic myelopathy secondary to both degenerative and nondegenerative causes who were treated surgically by the authors at a single center (University of California, San Francisco) over the course of 1 year.
Materials and Methods
Data Collection
We routinely use IONM on all cervical spine surgeries at our institution. The study was approved by the University of California, San Francisco Institutional Review Board (11-07069). Cases eligible for this study were identified by first screening our IONM database for all the operations performed in the University of California, San Francisco Department of Neurological Surgery from January 1 to December 31, 2011. The hospital records and operative reports of all patients who underwent spine procedures were examined. All patients with either degenerative cervical spondylotic myelopathy (CSM) or myelopathy of nondegenerative causes who underwent cervical or cervicothoracic decompressive operations with IONM during that time were included in our study. Patients with intramedullary spinal cord tumors were excluded. All patients consented for IONM as part of the surgical informed consent process. The patient demographics were obtained from the hospital and clinic charts. Pre- and postoperative neurologic function data was extracted from chart documentation of the neurosurgeon's objective examination, including the Medical Research Committee–validated motor scale, sensory disturbance, and evidence of hyperreflexia or pathologic reflexes. To capture the postoperative neurologic deficits, the hospital chart was reviewed until the day of discharge. In addition, the outpatient clinic charts were also reviewed for an average of 6 months. The postoperative neurologic deficit was defined as new or worsening motor weakness on the Medical Research Committee scale after surgery. The IONM alerts were extracted from the IONM report. A significant MEP alert was defined as an abrupt decrease in peak-to-peak amplitude > 50% for more than three successive trials over a 1- to 3-minute period.
Recording of Intraoperative Motor Evoked Potential Changes
Transcranial MEPs were generated by multipulse transcranial electrical stimulation (0 to 800 V, 50- to 75-μs pulse duration, 0 to 9 pulses at 1 to 3 ms) delivered to electrodes placed over the motor cortical regions at C3 and C4 using a Cadwell (Kennewick, Washington, United States) TCS-4 constant voltage stimulator. In addition, the electromyography responses were recorded from needle electrodes placed bilaterally in the deltoid (axillary nerve, C5–C6), biceps (musculocutaneous nerve, C5–C7), triceps (radial nerve, C6–C8), and thenar and hypothenar eminences of the hand (C8–TI). The waveforms were recorded on a commercially available Cadwell (model Elite or Cascade) neurophysiology workstation.
Statistical Analysis
A true-positive result was defined as presence of an intraoperative neurophysiologic MEP alert during surgery followed by a neurologic deficit in the postoperative period. Conversely, a true-negative result was defined as the absence of MEP alerts during surgery and the lack of a new neurologic deficit after surgery. A false-positive result was defined as the presence of a persistent nonreversible MEP alert during surgery that was not followed by a neurologic deficit. A false-negative result was defined as the absence of MEP alerts during the operation followed by a new neurologic deficit. The sensitivity was calculated as true-positives / true-positives + false-negatives. The specificity was calculated as true-negatives / true-negatives + false-positives. The associations between IONM of MEP changes and new postoperative neurologic deficits were analyzed by Fisher exact test. Receiver operating characteristic curves were generated to assess the predictive value of IONM of MEP alerts in patients with degenerative CSM and CSM of nondegenerative pathology. The ability of IONM of MEPs to discriminate new postoperative deficits was determined by calculating the area under the receiver operating characteristic curve (AUC) for patients with CSM and patients with CSM of nondegenerative pathology. All analyses were performed using PASW 18.0 Statistics software (SPSS, Inc., Chicago, Illinois, United States), and p values less than 0.05 were considered statistically significant.
Results
Patient Demographics
During the study period, 144 patients underwent surgery for cervical and cervicothoracic myelopathy. The mean age was 63 years (range 29 to 92) and 48 were women (33%). Myelopathy was caused by degenerative spondylosis in 102 patients (71%) and nondegenerative pathology in 42 (29%). In the nondegenerative group, extramedullary tumors caused myelopathy in the majority of the cases, followed by infection, trauma, and inflammatory processes. Of the tumors, 8 were schwannomas (Fig. 1), 8 were metastatic lesions, 4 were meningiomas, 2 were hemangiomas, 1 was a chordoma, and 1 was a neurofibroma. We did not include any intramedullary spinal cord tumors.
Fig. 1.
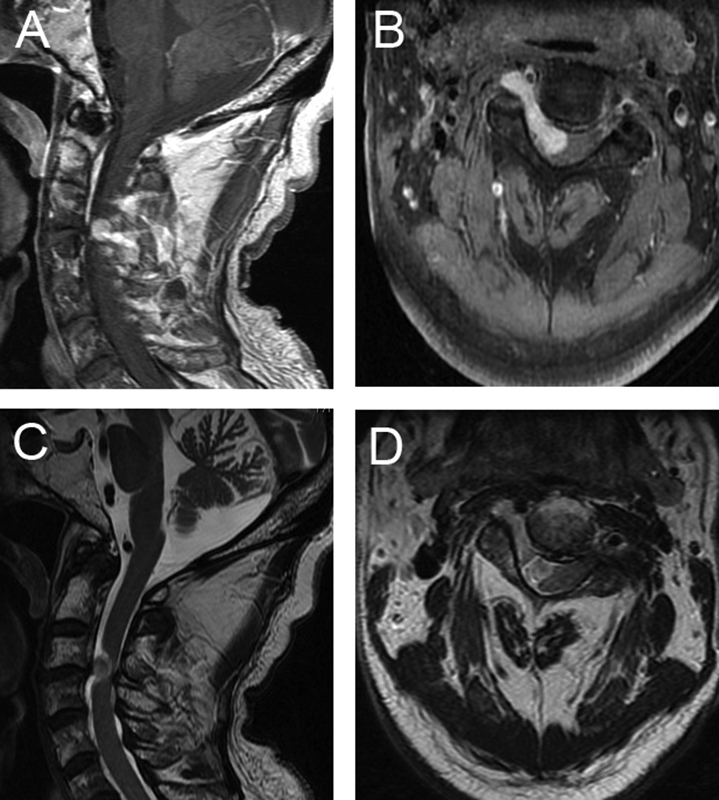
Preoperative imaging in a patient with cervical myelopathy of nondegenerative pathology. An 80-year-old man presented with 6-month history of bilateral upper extremity weakness and numbness. (A, B) T1-weighted magnetic resonance imaging with contrast revealed an enhancing lesion extending from the neural foramen into the spinal canal. (C, D) T2-weighted magnetic resonance imaging was notable for significant cord compression and the absence of hyperintensity in the spinal cord. The patient was taken to the operating room for resection of this lesion, and pathology confirmed the diagnosis of schwannoma.
The degenerative CSM and nondegenerative cervical myelopathy groups were similar with respect to age and preoperative T2-weighted magnetic resonance imaging cord signal abnormality (Table 1). There were significantly more women and more preoperative motor deficits in the nondegenerative group. There were significantly fewer cervical lesions in the nondegenerative group. There were significantly fewer anterior operations and fewer instrumented operations in the nondegenerative group.
Table 1. Clinical, pathologic, and surgical characteristics of the 144 patients in the study population.
| Degenerative CSM (n = 102), n (%) | CSM from nondegenerative causes (n = 42), n (%) | p Value | |
|---|---|---|---|
| Age (y), mean | 64 | 60 | 0.13 |
| Sex | |||
| Female | 28 (28) | 20 (48) | 0.03 |
| Pathology | |||
| Tumor | – | 24 (57) | – |
| Infection | 12 (29) | ||
| Trauma | 5 (12) | ||
| Inflammatory | 1 (2) | ||
| Level | |||
| Cervical | 96 (94) | 19 (45) | <0.001 |
| Cervicothoracic | 1 (1) | 3 (7) | |
| Thoracic | 5 (5) | 20 (48) | |
| Abnormal T2 signal | |||
| Yes | 48 (51) | 22 (54) | 0.85 |
| Preoperative motor deficit | |||
| Yes | 57 (56) | 33 (83) | 0.002 |
| Surgical approach | |||
| Anterior | 25 (25) | 0 (0) | 0.001 |
| Posterior | 72 (71) | 37 (88) | |
| Anterior + posterior | 5 (5) | 5 (12) | |
| Instrumentation | |||
| Yes | 97 (95) | 27 (64) | <0.001 |
Abbreviation: CSM, cervicothoracic spondylotic myelopathy.
Intraoperative Motor Evoked Potential Changes and New Neurologic Deficits
The discrimination of IONM of MEP alerts was assessed to determine their ability to identify patients who incur new postoperative deficits. Among the patients with degenerative CSM, the model performed well for predicting postoperative deficits with an AUC of 0.826 (Table 2). This result was better than the prediction in patients with CMS of nondegenerative causes, although it did not reach statistical significance (AUC 0.538, p = 0.16, Table 3).
Table 2. Association between intraoperative MEP alerts and new postoperative neurologic deficits in patients with cervicothoracic spondylotic myelopathy.
| MEP alert (n = 11), n (%) | No alert (n = 91), n (%) | |
|---|---|---|
| New motor deficit | ||
| Yes | 5 (45) | 2 (2) |
| No | 6 (55) | 89 (98) |
Abbreviation: MEP, motor evoked potential.
Note: p < 0.001; sensitivity 71%; specificity 94%.
Table 3. Association between intraoperative MEP alerts and new postoperative neurologic deficits in patients with nondegenerative causes of myelopathy.
| MEP alert (n = 11), n (%) | No alert (n = 31), n (%) | |
|---|---|---|
| New motor deficit | ||
| Yes | 1 (9) | 2 (6) |
| No | 10 (91) | 29 (94) |
Abbreviation: MEP, motor evoked potential.
Note: p > 0.99; sensitivity 33%; specificity 74%.
Intraoperative Motor Evoked Potential Monitoring Performance in Subgroups of Pathologies
The numbers of patients in each subgroup were too small to statistically analyze. We compared the differences in the performance measures to assess for trends. Within the tumor subgroup, IONM of MEP changes was associated with a sensitivity of 0% and a specificity of 61% (Fig. 2). IONM of MEP changes appeared to perform slightly better in the infection subgroup (sensitivity 50%, specificity 100%). As mentioned above, there were significantly more thoracic lesions in the nondegenerative group. To address this discrepancy, we analyzed the cervical cases. There were 96 cervical-only cases in the degenerative group and 19 in the nondegenerative group. Within this subgroup, the observed trend persisted. In the degenerative group, MEP alerts were associated with new neurologic deficits (p < 0.001) (Table 4), although they were not associated with new deficits in the nondegenerative group (p > 0.05) (Table 5).
Fig. 2.
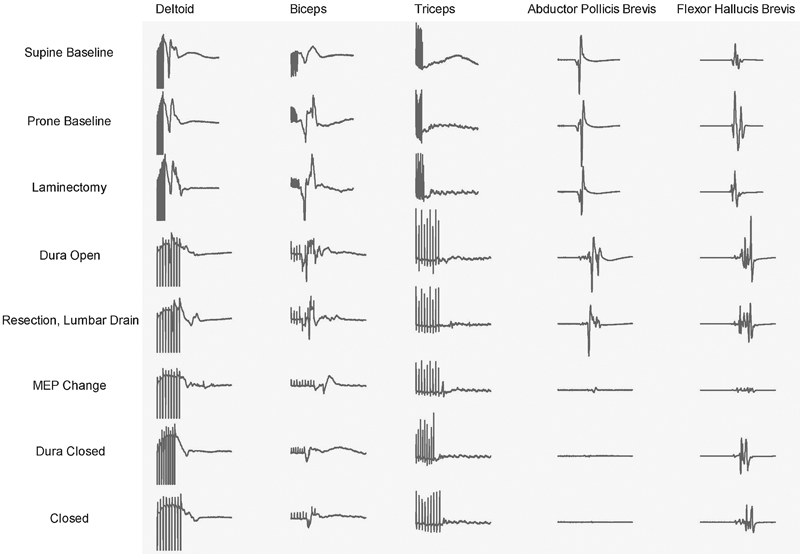
Representative intraoperative neurophysiologic motor evoked potential (MEP) monitoring recordings obtained from the patient described in Fig. 1. During tumor resection, there was loss of MEP signal in the biceps, abductor pollicis brevis, and flexor hallucis brevis. The abductor pollicis brevis did not recover despite intraoperative measures. Nevertheless, the patient awoke with no new neurologic deficits.
Table 4. Intraoperative MEP alerts and new postoperative neurologic deficits in patients with degenerative lesions in the cervical region.
| MEP alert (n = 10), n (%) | No alert (n = 86), n (%) | |
|---|---|---|
| New motor deficit | ||
| Yes | 5 (50) | 1 (1) |
| No | 5 (50) | 85 (99) |
Abbreviation: MEP, motor evoked potential.
Note: p < 0.001.
Table 5. Intraoperative MEP alerts and new postoperative neurologic deficits in patients with nondegenerative lesions in the cervical region.
| MEP alert (n = 5), n (%) | No alert (n = 14), n (%) | |
|---|---|---|
| New motor deficit | ||
| Yes | 0 (0) | 2 (14) |
| No | 5 (100) | 12 (86) |
Abbreviation: MEP, motor evoked potential monitoring.
Note: p > 0.99.
Discussion
We recently reported high sensitivity and specificity of IONM of MEP alerts in predicting new postoperative deficits in patients undergoing surgery for degenerative CSM.9 In the current study, we were interested in examining the association between persistent intraoperative MEP alerts and new postoperative deficits in patients with myelopathy undergoing surgery for compressive, nondegenerative extra-axial lesions. Our data suggests that IONM of MEPs predicts postoperative deficits better in surgery for degenerative compared with nondegenerative myelopathy.
Bias may have been introduced into our study due to the differences in a few preoperative variables between our two study groups, which may have been secondary to a more rapid onset of spinal cord compression (weeks or even days) in nondegenerative pathologies compared with months or years in degenerative conditions. Specifically, more patients had preoperative motor deficits in the nondegenerative cervical myelopathy group. In our prior study of patients with degenerative CSM, we demonstrated significantly increased sensitivity of intraoperative MEP alerts in patients with preoperative motor deficits.9 Therefore, it is unlikely that this difference affected the results because increased sensitivity would then be expected in a group of patients with increased rates of preoperative deficits. Likewise, there were higher numbers of women, thoracic lesions, posterior-only operations, and noninstrumented operations in the nondegenerative cervical myelopathy group. These differences were not shown to affect the sensitivity of intraoperative MEP alerts in our previous study, and we do not suspect that these differences introduced significant bias in this study. The thoracic spinal cord is more susceptible to vascular compromise, therefore we chose to perform a subgroup analysis on the cervical cases to see if the trends persisted. In the cervical-only group, MEP alerts remained associated with new deficits in the degenerative group but not the nondegenerative group. Our study is limited by the retrospective nature of the data as well as a small sample size. Future larger studies could potentially be amenable to further subgroup analysis to detect the differences between extramedullary tumor, infection, trauma, and inflammatory processes.
IONM of MEPs is frequently used in spinal deformity surgery. Studies have demonstrated high sensitivity and specificity for detecting neurologic deficit,10 11 which may be due to lack of preoperative neurologic deficit and cord compression and the defined period of intraoperative time where the risk for neural injury is high in these cases.12 IONM of MEPs can be focused during the time of maximal manipulation of neural elements, such as during osteotomy closure. Likewise, IONM of MEPs is associated with very high sensitivity and specificity for spinal cord tumor surgery.13 One study demonstrated improved outcomes if IONM was used in conjunction with the tumor resection.14 If a change is detected, specific maneuvers can be performed by the surgeon, the neurophysiologist, and the anesthesiologist with the goal of eliciting a return to baseline of IONM of MEP.6 15 We have recently reported an intraoperative checklist to standardize the rapid response of the entire operating room team to a neuromonitoring alert.8
In contrast, during decompression for cervical myelopathy, the risk to the spinal cord and nerve roots exists throughout the entire decompression procedure and not during a defined short period. IONM can be useful in cervical myelopathy cases, but it appears to be more sensitive and predictive of postoperative deficits in patients with degenerative CSM compared with those with nondegenerative cervical myelopathies. The causes of this difference in sensitivity and PPV of IONM are debatable. Patients with nondegenerative myelopathies often have a more focal lesion causing the cord compression compared with those with degenerative CSM. In addition, degenerative CSM often occurs over a period of time, allowing the spinal cord to adapt slowly to the compressive agents, whereas patients with nondenegerative myelopathy may have more acute cord compression. How these differences affect IONM are not clear at this time, which warrants further study in the future.
Conclusion
We found that IONM of MEPs may be associated with lower performance (decreased sensitivity) in decompressive surgery for patients with myelopathy secondary to nondegenerative, such as extra-axial tumor, infection, trauma, and inflammatory processes, when compared with patients with degenerative CSM. The etiology of the difference in efficacy of MEP monitoring between cases with degenerative CSM and CSM of nondegenerative causes is not understood, and further study on this topic is needed. Surgeons using IONM of MEPs for cases with nondegenerative cervical myelopathy should be aware that the sensitivity and PPV of the modality may be limited in these cases. The value of IONM of MEPs arising from reversible alerts that may have prevented occurrence of permanent postoperative neurologic deficit by altering the surgical procedure could not be assessed in this study and may represent the major justification for IONM of spinal cord function.
Footnotes
Disclosures Aaron J. Clark, none Michael Safaee, none Dean Chou, Consulting: Orthofix, Globus, Medtronic, Depuy Philip R. Weinstein, none Annette M. Molinaro, none John P. Clark III, none Praveen V. Mummaneni, Honoraria: AOSpine, Depuy, Globus; Grant: NREF; Stock: Spinicity
References
- 1.Epstein N E, Danto J, Nardi D. Evaluation of intraoperative somatosensory-evoked potential monitoring during 100 cervical operations. Spine (Phila Pa 1976) 1993;18(6):737–747. doi: 10.1097/00007632-199305000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Gokaslan Z L, Samudrala S, Deletis V, Wildrick D M, Cooper P R. Intraoperative monitoring of spinal cord function using motor evoked potentials via transcutaneous epidural electrode during anterior cervical spinal surgery. J Spinal Disord. 1997;10(4):299–303. [PubMed] [Google Scholar]
- 3.Papastefanou S L, Henderson L M, Smith N J, Hamilton A, Webb J K. Surface electrode somatosensory-evoked potentials in spinal surgery: implications for indications and practice. Spine (Phila Pa 1976) 2000;25(19):2467–2472. doi: 10.1097/00007632-200010010-00008. [DOI] [PubMed] [Google Scholar]
- 4.Traynelis V C, Abode-Iyamah K O, Leick K M, Bender S M, Greenlee J D. Cervical decompression and reconstruction without intraoperative neurophysiological monitoring. J Neurosurg Spine. 2012;16(2):107–113. doi: 10.3171/2011.10.SPINE11199. [DOI] [PubMed] [Google Scholar]
- 5.Xu R, Ritzl E K, Sait M. et al. A role for motor and somatosensory evoked potentials during anterior cervical discectomy and fusion for patients without myelopathy: analysis of 57 consecutive cases. Surg Neurol Int. 2011;2:133. doi: 10.4103/2152-7806.85606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz D M, Auerbach J D, Dormans J P. et al. Neurophysiological detection of impending spinal cord injury during scoliosis surgery. J Bone Joint Surg Am. 2007;89(11):2440–2449. doi: 10.2106/JBJS.F.01476. [DOI] [PubMed] [Google Scholar]
- 7.Hilibrand A S, Schwartz D M, Sethuraman V, Vaccaro A R, Albert T J. Comparison of transcranial electric motor and somatosensory evoked potential monitoring during cervical spine surgery. J Bone Joint Surg Am. 2004;86-A(6):1248–1253. doi: 10.2106/00004623-200406000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Ziewacz J E, Berven S H, Mummaneni V P. et al. The design, development, and implementation of a checklist for intraoperative neuromonitoring changes. Neurosurg Focus. 2012;33(5):E11. doi: 10.3171/2012.9.FOCUS12263. [DOI] [PubMed] [Google Scholar]
- 9.Clark A J, Ziewacz J E, Safaee M. et al. Intraoperative neuromonitoring with MEPs and prediction of postoperative neurological deficits in patients undergoing surgery for cervical and cervicothoracic myelopathy. Neurosurg Focus. 2013;35(1):E7. doi: 10.3171/2013.4.FOCUS13121. [DOI] [PubMed] [Google Scholar]
- 10.Langeloo D D, Lelivelt A, Louis Journée H, Slappendel R, de Kleuver M. Transcranial electrical motor-evoked potential monitoring during surgery for spinal deformity: a study of 145 patients. Spine (Phila Pa 1976) 2003;28(10):1043–1050. doi: 10.1097/01.BRS.0000061995.75709.78. [DOI] [PubMed] [Google Scholar]
- 11.Quraishi N A, Lewis S J, Kelleher M O, Sarjeant R, Rampersaud Y R, Fehlings M G. Intraoperative multimodality monitoring in adult spinal deformity: analysis of a prospective series of one hundred two cases with independent evaluation. Spine (Phila Pa 1976) 2009;34(14):1504–1512. doi: 10.1097/BRS.0b013e3181a87b66. [DOI] [PubMed] [Google Scholar]
- 12.Skinner S A, Holdefer R N. Intraoperative neuromonitoring alerts that reverse with intervention: treatment paradox and what to do about it. J Clin Neurophysiol. 2014;31(2):118–126. doi: 10.1097/WNP.0000000000000030. [DOI] [PubMed] [Google Scholar]
- 13.Kothbauer K F, Deletis V, Epstein F J. Motor-evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical and neurophysiological data in a series of 100 consecutive procedures. Neurosurg Focus. 1998;4(5):e1. doi: 10.3171/foc.1998.4.5.4. [DOI] [PubMed] [Google Scholar]
- 14.Sala F Palandri G Basso E et al. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: a historical control study Neurosurgery 20065861129–1143., discussion 1129–1143 [DOI] [PubMed] [Google Scholar]
- 15.Lieberman J A, Lyon R, Feiner J, Hu S S, Berven S H. The efficacy of motor evoked potentials in fixed sagittal imbalance deformity correction surgery. Spine (Phila Pa 1976) 2008;33(13):E414–E424. doi: 10.1097/BRS.0b013e318175c292. [DOI] [PubMed] [Google Scholar]


