In an earlier publication, we discussed the importance of loss to follow-up, how it should be calculated, and how many patients can be lost to follow-up without mistrusting the results.1 We argued that incomplete follow-up could bias the results when the dropout rates are different between study groups or when the patients who drop out are different from those who do not drop out. Simply put, the patients lost to follow-up often have a different prognosis than those who complete the study. We discussed that properly calculating the loss to follow-up can only be done by determining the right denominator. That includes all those randomly assigned in a randomized controlled trial and all who had the procedure during a prespecified time in a cohort study. A good rule of thumb is that <5% loss leads to little bias, and >20% poses serious threats to validity. However, even less than 20% loss to follow-up can be a problem.
Now that we understand the significant impact that loss to follow-up can have on our study results, we want to shift our focus to strategies to limit loss to follow-up. Unfortunately for retrospective studies where the data has already been collected, the toothpaste is out of the tube; nothing can be done to improve patient participation in the study. However, with this study design it is worth documenting the flow of the patients through each stage of the study: enrollment, assignment, follow-up, and analysis. A diagram is strongly recommended (Fig. 1).
Fig. 1.
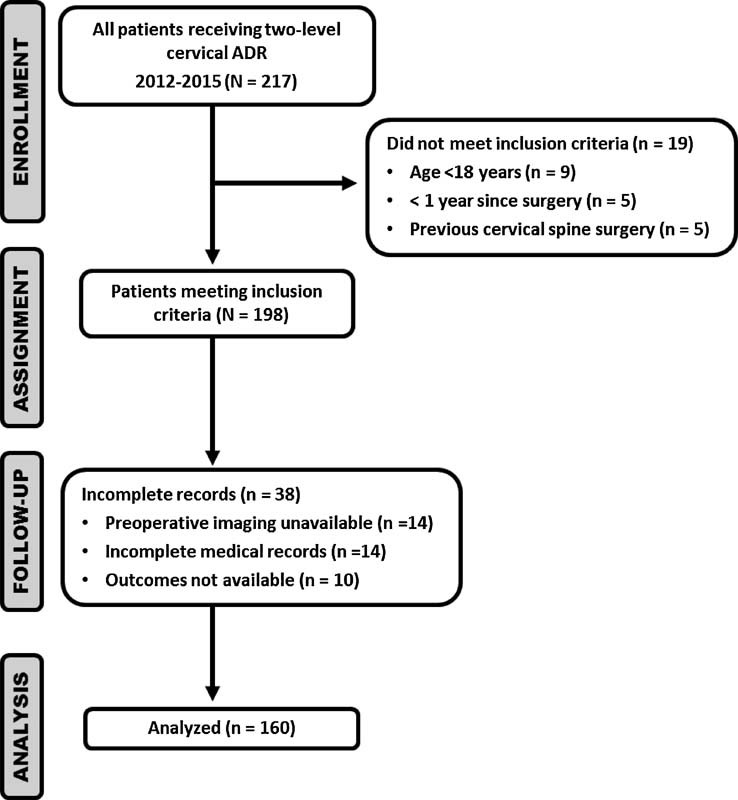
Example of participant flow in hypothetical retrospective case series evaluating the incidence of adjacent segment degeneration in two-level cervical artificial disk replacement (ADR). Enrollment includes the number of participants who were screened for eligibility, were found to be eligible or not eligible, declined to be enrolled, and were enrolled in the study. Assignment shows the number of participants assigned to a study condition. Follow-up denotes the number of participants who completed the follow-up or did not complete the follow-up (i.e., were lost to follow-up), by study condition. Analysis shows the number of participants included in or excluded from the main analysis, by study condition.
However, if you are involved in prospective data collection for randomized trials, longitudinal prospective cohort studies, or prospective registries, there are several safeguards and strategies that you should consider implementing from the beginning of the project. These will be discussed in this edition of Science in Spine.
It is tempting to get excited about enrollment and then let your guard down during the follow-up phase. The problem is that it does not matter how many participants are enrolled if you cannot get them to the finish line. So the planning starts up front and the process continues with a diligent effort to maintain very high retention throughout the course of the study until the very last enrolled subject has finished his or her final follow-up. This result is difficult to accomplish without personnel and a commitment of resources. Every prospective study requiring follow-up beyond the perioperative period necessitates a project coordinator with experience in following participants over time, which can rarely be done effectively by clinician investigators who are busy with the day-to-day clinical responsibilities. The number of sites involved (if it is a multisite study) and the number of participants anticipated for enrollment will dictate what level of commitment you need from this project coordinator. Sometimes 25% effort is enough but often it requires 50% or more, even up to 100%, in larger multisite trials. This person needs to have strong organizational and communication skills. Ideally, the coordinator will have spent time on the “front lines” recruiting and following participants so that he or she can speak and problem solve the challenges that the study coordinators face in retaining subjects. An alternative is a central methods center that employs more than one coordinator part-time who is versed in the retention strategies. Once this person(s) is identified, and a comprehensive follow-up strategy is established, then flexibility and ingenuity are paramount as the study progresses because no study is alike. There will always be obstacles and nuances that may impact retention. Good project coordinators can respond to these challenges and enlist their clinical investigators and study coordinators along the way to help problem solve.
The following sections provide a fairly self-explanatory checklist of strategies that have been employed by many successful clinical trialists. These are divided into a prestudy planning phase and a study execution phase, although there is distinct overlap in many of these. All need to be planned for and considered for Institutional Review Board (IRB) approval.
Prestudy Planning Phase
During the study planning and protocol development phase, avoid an inordinate amount of study measures that require time by both study staff and participants. Study burden may be the single greatest obstacle to study compliance and retention.
In the exclusion criteria, include factors that may lead to difficulty in contacting patients or compliance with follow-up. Listing them here is beyond the scope of this article but include the obvious ones such as a willingness to participate over the length of the study, lack of a stable address and/or back-up addresses and contact information, dementia, and others.
During the protocol and IRB phase, plan for the collection of baseline information that will facilitate tracking subjects (e.g., addresses, phone numbers, and email addresses not only for the participant but also for possible contacts such as next of kin or close friends).
Provide educational material for each subject that includes the importance of attending every study visit. It is not uncommon for a participant to think, “I'm better now so I don't need to keep participating.” It is even possible for those not doing well to go somewhere else for care and therefore stop their study visits. Participants need to be educated that their study visits, though they may coincide with a clinical visit, are independent of their clinical care and critical to attend.
Include in the study participant educational material contact information for the study coordinator and/or investigator in case participants have questions and encourage them to notify you if they are changing their address and/or any contact information.
Consider electronic data capture through a study Web site that can double as a study resource for both investigators and participants and can also be the portal for efficient data collection with built-in reminders for follow-up appointments.
If possible with respect to the study measures, allow for telephone interviews in lieu of an in-person visit especially for patients who live far away or have difficulty with transportation. Telephone interviews work well with patient-reported outcomes but obviously not for study measurements requiring laboratory tests, X-rays, or magnetic resonance imaging scans.
Select study coordinators who are skilled in establishing rapport with their participants and who have experience with following patients over a period of time.
Set the bar very high with the study coordinators. The goal of retention should be 100%.
Present examples to study coordinators on the pitfalls and biases that result with loss to follow-up. Understanding the ramifications of losing a participant will better inform and motivate those who are doing the heavy labor of study retention.
Create a comprehensive tracking system that is standardized across study coordinators and sites so that all investigators are using the same methods and can communicate efficiently and effectively. These should be constructed during the study planning phase and coordinators should be briefed on how to use them. A central Web site for maintaining these is ideal so that the project coordinator has oversight capabilities and it will ease the communication.
Consider payment for successful follow-ups, which can apply to the sites recruiting and following the subjects. This practice can also apply to the participants. Discussion of payment strategies and regulatory issues is beyond the scope of this article but it certainly provides an incentive for maintaining high retention rates.
Study Execution Phase
It all starts with the rapport that the study coordinator or investigator establishes with the patient. The better participants get along with this person and feel that their contribution is of the utmost value to the research, the more likely they will remain compliant.
Send out quarterly newsletters for the study staff that include follow-up rates by site. The newsletters should not embarrass or single out those not doing well. It is all in the presentation. However, it can go a long way in creating some internal accountability and even friendly competition to see who can achieve the highest retention rates.
Send out a quarterly newsletter to study participants providing interesting tidbits about the study. The newsletter reminds the participants that they are involved in something very important, helps keep them engaged, and reminds them they will have future follow-up appointments.
Hold study coordinator meetings at least monthly and include retention rates in the agenda. The meetings should not be a forum for pointing the finger but rather an opportunity for the study coordinators to share their challenges and their successful strategies. This practice undoubtedly will help the other coordinators in their setting.
Build in multiple follow-up appointments along the course of the study, which not only provides more study data but keeps the participants engaged. For example, if the primary outcome is 12 months after surgery, not having a follow-up for 12 months is a recipe for a quick voyage to “The Land of the Lost.” Ideally, follow-up at least every 3 months will keep the participants engaged. If in-person follow-ups are not always necessary, at least telephone follow-ups to ask about complications or other patient-reported outcomes are a good idea.
At each study visit, encourage the participants to make their appointment for their next visit even if it is months in the future.
Make periodic reminder phone calls or send reminder letters or e-mails. Study coordinators can use the established study participant tracking system (item 11 in previous list) for these reminders and should have some administrative time built into their schedule to send reminders on a routine basis.
Send birthday and holiday cards to recipients, which is a nice gesture that will continue to reinforce to the participants that they are important to the study team and the success of the study.
Make multiple attempts to reach nonresponders. Attempts should be planned during the protocol and IRB phase to include telephone calls, letters, and e-mail communication. Do not give up too easily unless the participant requests to withdraw. One additional strategy is to have the physician investigator make a phone call to patients to remind them of the study's importance. These phone calls are not meant to be coercion, and you must have IRB approval, but sometimes hearing from “the doctor” will motivate patients to maintain participation when they may be considering dropping out.
These are just some example strategies that we have found to produce retention rates above 90%. Getting new participants to consent and enroll in a study is paramount; however, if the participants end up in “The Land of the Lost,” then the efforts to recruit and enroll could be useless. We hope this article both acknowledges the challenges of retention and also provides encouragement and strategies for retention success in your next prospective clinical study.
Reference
- 1.Dettori J R. Loss to follow-up. Evid Based Spine Care J. 2011;2(1):7–10. doi: 10.1055/s-0030-1267080. [DOI] [PMC free article] [PubMed] [Google Scholar]


