Abstract
Study Design
Review of the literature.
Objective
Surgery and cement augmentation procedures are effective palliative treatment of symptomatic spinal metastases. Our objective is to systematically review the literature to describe the survival, prognostic factors, and clinical outcomes of surgery and cement augmentation procedures for breast cancer metastases to the spine.
Methods
We performed a literature review using PubMed to identify articles that reported outcomes and/or prognostic factors of the breast cancer patient population with spinal metastases treated with any surgical technique since 1990.
Results
The median postoperative survival for metastatic breast cancer was 21.7 months (8.2 to 36 months), the mean rate of any pain improvement was 92.9% (76 to 100%), the mean rate of neurologic improvement was 63.8% (53 to 100%), the mean rate of neurologic decline was 4.1% (0 to 8%), and the local tumor control rate was 92.6% (89 to 100%). Kyphoplasty studies reported a high rate of pain control in selected patients. Negative prognostic variables included hormonal (estrogen and progesterone) and human epidermal growth factor receptor 2 (HER2) receptor refractory tumor status, high degree of axillary lymph node involvement, and short disease-free interval (DFI). All other clinical or prognostic parameters were of low or insufficient strength.
Conclusion
With respect to clinical outcomes, surgery consistently yielded neurologic improvements in patients presenting with a deficit with a minimal risk of worsening; however, negative prognostic factors associated with shorter survival following surgery include estrogen receptor/progesterone receptor negativity, HER2 negativity, and a short DFI.
Keywords: breast cancer, surgery, tumor, kyphoplasty, vertebroplasty, survival, spine, metastasis
Introduction
The prevalence of breast cancer has risen in the past few decades within the Western World.1 In North America and Western Europe, breast cancer is the most common malignancy and the second most common cause of cancer-related death for women.2 3 The American Cancer Society estimates that over 230,000 new cases of invasive breast cancer were diagnosed in 2013.3 Fortunately, advances in systemic therapies, radiotherapy indications, and surgical techniques have prolonged patient survival with more recent major gains occurring in the management of patients who develop metastatic disease. However, as a result, the frequency of long-term sequelae has increased; 10 to 30% of patients with primary malignancies are expected to develop spinal epidural metastases later in life, and there will be increased demand for aggressive therapies including surgery to optimize patient outcomes.1 4 5
The ideal management of spinal metastases involves the collaboration of numerous specialties including spine surgery, surgical oncology, medical oncology, radiation oncology, interventional radiology, pain specialists, and rehabilitation.6 7 Though management strategies have become more aggressive and have led to improved clinical outcomes,4 8 treatments for spinal metastases remain palliative. At a minimum, therapies should involve radiotherapy and pharmacotherapy, such as chemotherapy/hormonal therapy, bisphosphonates, steroids, and analgesics as necessary. Surgery may be indicated for a variety of reasons, including one or more of the following: mechanical instability, tumors progressing while on radiation or failed radiation, medically intractable pain, and functionally significant or progressive neurologic dysfunction. However, in general, surgery may be relatively contraindicated for candidates with limited life expectancy (less than 3 months) or poor health status.1 8 9 Numerous open surgical techniques are used, including anterior, posterior, or anteroposterior decompression, followed by complete or partial tumor resection and stabilization. Less invasive operative procedures include percutaneous stabilization, cement augmentation, and mini–open decompression, which are indicated with intractable pain resulting from vertebral body deformity or minimal epidural cord compression causing neurologic deficit.10 11 Within the past decade, the latter set of techniques have become increasingly attractive due to their ability to achieve the surgical goal while minimizing morbidity.12
To date, most recommendations for treatment options have been based on studies looking at metastatic spine disease from a heterogeneous cohort of primary malignancies. There is limited information on the management of metastatic spine disease from breast cancer alone, and thus this study was intended to provide more specific recommendations for those patients with spinal metastases from breast cancer.
In this study, a systematic review of the clinical outcomes of the aforementioned operative techniques restricted to patients with breast cancer with spinal metastases was performed. The overall objectives of this article are to answer the following clinical questions: (1) What is the postoperative survival, rate of change in neurologic status, local tumor control rate, and pain improvement rate for operative treatment? (2) Are there any clinical, radiographic, or histologic variables in the surgical literature that may help prognosticate which patients will perform better or worse with surgery?
Methods
Electronic Literature Search
A systematic review of the literature was performed using PubMed, as well as a review of the bibliographies of eligible articles. The broad search query was designed to include the breast cancer patient population with spinal metastases treated with any surgical technique since 1990. Additionally, a prognostic variable search specific to patients with metastatic breast cancer was conducted with emphasis on the metastatic-free interval (the duration between diagnosis of primary disease and the first metastasis) to supplement the limited prognostic variables provided by the surgical studies. A summary of the search strings as well as the inclusion and exclusion criteria are provided in Table 1. The designation of “decompression” or “decompressive” is meant to include all surgical procedures in which removal of compressive pathology from the neural elements was indicated (whether anterior or posterior), and the indications for the “decompression” included neurologic deficit, pain, and local tumor control.
Table 1. Selection criteria.
| Study type | Key search string(s) | Inclusion | Exclusion |
|---|---|---|---|
| Surgical | “Breast spine metastatic surgery” | • Publication date: 1990 or later • Language: English or with a complete English translation • Articles describing operative techniques used to treat spinal metastases in patients with breast cancer • Fully published, peer-reviewed, retrospective or prospective studies including randomized controlled trials, nonrandomized trials, cohort studies, case control studies, and case series |
• Articles that did not provide clinical outcomes and statistics specific to the patients with breast spinal metastases • Articles that lumped all breast spinal metastases cohort outcomes with that of other primary tumor types • Study size: <6 patients in the breast spinal metastases cohort with respect to surgical techniques • Articles that did not specify the operative procedure used |
| Cement augmentation procedure | “Kyphoplasty breast cancer”; “vertebroplasty breast cancer” | • Articles that did not provide clinical outcomes and statistics specific to the patients with breast spinal metastases • Articles that lumped all breast spinal metastases cohort outcomes with that of other primary tumor types • Study size: <2 patients with breast cancer related spinal metastases • Articles that did not specify the operative procedure used |
|
| Supplementary prognostic variable studies | “Metastatic free interval prognostic breast” | • Publication date: 2000 or later • Language: English or with a complete English translation • Articles evaluating statistical significance of prognostic variables in metastatic breast cancer patients • Fully published, peer-reviewed, retrospective or prospective studies including randomized controlled trials, nonrandomized trials, cohort studies, case control studies, and case series • MFI or DFI exclusively defined as time between diagnosis of primary disease and first metastasis |
• Study size: ≤300 patients • Studies not investigating MFI/DFI prognostic variable as defined in the inclusion criteria |
Abbreviations: DFI, disease-free interval; MFI, metastatic-free interval.
Data Extraction
The following data regarding operative techniques was extracted: patient population (the number of patients with spinal metastases and the percent of patients with breast cancer who comprise the entire study population), survival information (postoperative survival time and/or postoperative survival rate), change in neurologic function (the percent of breast cancer cohort with preoperative neurologic deficit, and the percent of breast cancer cohort with identical or worse postoperative neurologic deficit), local tumor control rate (the percent, evaluated at a mean or median follow-up ≥ 12 months), and change in pain (the percent of breast cancer cohort with preoperative pain, and the percent of breast cancer cohort with identical or worse postoperative pain). The percent of patients experiencing improvements in pain or neurologic deficit that existed preoperatively was also calculated.
Study Eligibility and Quality Assessment
All potentially eligible studies were determined by two reviewers. A third reviewer resolved instances of disagreement. After finalizing the series of studies to be analyzed, two reviewers extracted data to answer the inquiries posed in the objectives. A third reviewer confirmed these results. The overall body of evidence was based on the Grades of Recommendation Assessment, Development and Evaluation (GRADE) Working Group and recommendations of the Agency for Healthcare Research and Quality.13 14 15 16 17
The final overall strength of the literature was determined by the reviewers' confidence that the effect size closely matched the true effect and was stable. The data extracted from the relevant studies was then presented to the AOSpine Tumor Knowledge Forum, a spinal oncology expert group including 20 neurosurgeons, orthopedic surgeons, radiation oncologists, and medical oncologists. Expert opinion distilled using the modified Delphi approach allowed for clinical recommendations and consensus statements to be made.
All panelists were provided with full publications that included the extracted data, a summary of the GRADE working group article, as well as the body of evidence for each recommendation prior to the scheduled meeting. Each recommendation was presented to the panelists, and then grades were assigned according to the criteria set forth below.
The following grades were assigned: high, moderate, low, or insufficient. “High” was assigned to a body of evidence in which a majority of studies were class of evidence I or II, and there was confidence that the true effect was close to the estimated effect. “Low” was assigned to a body of evidence in which a majority of studies were class III or IV, and the true effect may have been significantly different than the estimated effect. “Insufficient” was assigned if there was very little confidence in the estimated result or no evidence or too little evidence to estimate an effect. The overall strength could be downgraded if results were inconsistent, evidence was indirect, effect estimates were imprecise, or there were no a priori subgroup analyses. In contrast, the overall strength could be upgraded if there was a large magnitude of effect or a dose–response gradient.18
Results
Study Selection
The query “breast spine metastatic surgery” yielded 308 results, and “kyphoplasty breast cancer” and “vertebroplasty breast cancer” yielded 22 and 45 results, respectively. Percutaneous vertebroplasty (PVP) and kyphoplasty studies had a less restrictive patient population criterion due to the lack of literature providing breast cancer-specific information. Ultimately, 19 operative studies were included in this review based on the eligibility criteria (15 surgical, 4 cement augmentation procedures). However, the surgical cohort studied by Sciubba et al1 and Shehadi et al19 was identical, yielding a total of 14 unique surgical populations.
The literature was organized based on the type of operative procedure. PVP and kyphoplasty studies were described separately as well. All operative literature is indexed chronologically in Table 2. Additionally, Tables 3 and 4 describe the postoperative outcomes with respect to surgical and cement augmentation techniques, respectively. Four of the surgical studies found provided some degree of analysis on the prognostic variables. In addition to these, six nonsurgical studies were found analyzing prognostic variables in large populations of patients with metastatic breast cancer (Table 5).
Table 2. Clinical outcomes for breast cancer patients with spinal metastases treated with operative procedures.
| Study | Design and procedure | Outcomes | Level of evidence |
|---|---|---|---|
| Hammerberg, 199242 | • Retrospective • 56 consecutive patients operated on 1980–1988 for spinal metastases • Techniques: anterior decompression + reconstruction, bilateral posterolateral decompression + fixation, or combined anterior-posterior approach + stabilization • PP: n = 21; 37% • Mean age: 58 y (29–83)a |
SI: 19 mo (mean); 67% at 1 y NC: — LTC: — PC: — |
IV |
| Kocialkowski et al, 199243 | • Retrospective • Series of 70 patients operated on 1985–1989 for extradural metastases • Techniques: anterior, posterior, or anteroposterior decompression with or without stabilization • PP: n = 17; 24% • Mean age: 56 y (37–75) |
SI: 8.2 mo (mean); 75% at ∼2 mo, 50% at ∼6.5 mo, 25% at 11 mo NC: 82%; 29%b LTC: – PC: 88%; 12% |
IV |
| Jonsson et al, 199444 | • Retrospective • 51 consecutive patients operated on 1982–1991 for lesions of the cervical spine • Technique: anterior resection followed by cervical stabilization using screws, bone cement, and plates (posterior or anteroposterior stabilization) • PP: n = 19; 37% • Mean age: 55 y (38–79) |
SI: 13 mo (mean); 47% at 1 y NC: 0%; 0%c LTC: 100% PC: 53%; 0% |
IV |
| Bauer et al, 199545 | • Prospective • Series of 153 patients operated on 1986–1994 for extremity metastases; series of 88 patients operated on for spinal metastases • Techniques: predominantly posterior decompression + stabilization, also anterior procedures • PP: n = 14; 6% • Median age: 63 y (23–85)a |
SI: 48% at 1 y NC: – LTC: – PC: – |
IV |
| Sioutos et al, 199546 | • Retrospective • 109 consecutive patient operated on 1980–1994 for thoracic metastases and cord compression • Techniques: anterior transthoracic or posterolateral resection + instrumentation, laminectomy, or combined vertebrectomy and laminectomy + instrumentation • PP: n = 19; 17% • Mean age: 59 y (31–80) |
SI: 22.5 mo (mean), 13.5 mo (median) NC: – LTC: – PC: – |
IV |
| Jonsson et al, 199647 | • Prospective • 51 patients operated on 1991–1992 for thoracic or lumbar metastases • Techniques: laminectomy, reduction, or epidural tumor resection with pedicle screw instrumentation • PP: n = 8; 16% • Median age: 57 y (44–66) |
SI: 10 mo (median); 38% at 1 y NC: 63%; 0%c LTC: – PC: 100%; 13% |
IV |
| Onimus et al, 199648 | • Retrospective • Consecutive series of 100 patients operated on for lumbar or thoracic metastases between 1987 and 1992 • Techniques: anterior tumoral resection, cord decompression, and reconstruction using methyl methacrylate; posterior laminectomy and stabilization; combined approaches • PP: n = 18; 18% • Mean age: 60 y (–)a |
SI: 12 mo (mean) NC: 28%; 11% LTC: – PC: 39%; 0% (morphinic data) |
IV |
| Gokaslan et al, 199849 | • Retrospective • Series of 72 patients operated on 1994–1997 for thoracic spinal metastases • Techniques: anterior transthoracic vertebrectomy, decompression, methyl methacrylate reconstruction, and fixation using plates and screws • PP: n = 10; 14% • Median age: 56 y (19–78)a |
SI: 63% at ∼17 mo NC: – LTC: – PC: – |
IV |
| Sundaresan et al, 200234 | • Retrospective • Series of 80 patients operated on 1986–1997 treated for solitary metastases • Techniques: en bloc or intralesional resection using posterior, anterior, or anteroposterior approaches, followed by stabilization • PP: n = 18; 23% • Mean age: 56 y (22–81)a |
SI: 36 mo (median); 22% at 5 y NC: – LTC: – PC: – |
IV |
| Chen et al, 200450 | • Retrospective • 70 consecutive patients operated on 1980–2001 for spinal metastases • Techniques: posterior decompression + resection + bilateral instrumented stabilization (minimum of 4 fixation points) • PP: n = 13; 19% • Mean age: 58 y (24–75)a |
SI: 18 mo (mean) NC: – LTC: – PC: – |
IV |
| Sciubba et al, 20071 | • Retrospective • 87 patients operated on 1993–2001 for spinal metastases • Techniques: anterior, anterolateral, posterior, posterolateral, posterior bipedicular, or combined anterior-posterior resection followed by stabilization; 78% vertebrectomy and 22% laminectomy • PP: n = 87; 100% • Median age: 53 y (35–84) |
SI: 21 mo (median); 62% at 1 y; 44% at 2 y; 33% at 3 y; 27% at 4 y; 24% at 5 y NC: 40%; – LTC: 89% PC: – |
IV |
| Shehadi et al, 200719 | • Retrospective • 87 patients operated on 1993–2001 for spinal metastases secondary to breast cancer • Techniques: anterior, anterolateral, posterior, posterolateral, posterior bipedicular, or combined anterior-posterior resection followed by stabilization • PP: n = 87; 100% • Median age: 53 y (35–84) |
SI: 21 mo (median); 62% at 1 y; 44% at 2 y; 33% at 3 y; 24% at 5 y NC: 40%; 27% LTC: 89% PC: – |
IV |
| Chen et al, 200910 | • Retrospective • 31 patient operated on 2003–2005 for spinal metastases causing vertebral body collapse • Technique: percutaneous vertebroplasty • PP: n = 7; 23% • Mean age: 58 y (40–73) |
SI: 86% at 6 mo, 14% at 1 y NC: – LTC: – PC: 100%; 0% |
IV |
| Gerszten et al, 200920 | • Prospective • 11 patient operated on for pain secondary to compression fractures from metastatic spinal tumors • Techniques: transpedicular coblation corpectomy combined with closed fracture reduction and fixation involving kyphoplasty followed by spinal radiosurgery • PP: n = 2; 18% • Median age: 58 y (38–87)a |
SI: – NC: – LTC: 100% PC: 100%; 0% |
IV |
| Lee et al, 200951 | • Retrospective • 19 patients operated on 2004–2008 for spinal metastases • Technique: percutaneous vertebroplasty • PP: n = 8; 42% • Mean age: 69 y (60–86) |
SI: – NC: – LTC: – PC: 100%; 0% |
IV |
| Sun et al, 201052 | • Retrospective • 10 patients operated on 2003–2008 for C2 osteolytic metastases • Technique: percutaneous vertebroplasty using anterolateral or posterolateral (1 patient) access • PP: n = 2; 20% • Median age: 62 y (41–82)a |
SI: – NC: - LTC: – PC: 100%; 0% |
IV |
| Tancioni et al, 201121 | • Retrospective • 23 consecutive patients operated on 2004–2009 with symptomatic MESCC • Techniques: (1) minimal resection (palliative surgery) + instrumented fixation; curettage (subtotal tumorectomy) + stabilization; total tumorectomy + stabilization with anterior, posterior, or combined approaches • PP: n = 23; 100% • Median age: 55 y (29–70) |
SI: 36 mo (median); 70% at 1 y, 60% at 2 y, 42% at 3 y, 34% at 4 and 5 y NC: 66%; 0% LTC: 100%; PC: 100%; 0% |
IV |
| Walcott et al, 201122 | • Retrospective • Series of 15 patients operated on 2001–2009 for metastatic breast tumor causing cord compression • Techniques: laminectomy with or without fusion, transpedicular or anterior corpectomy + fusion, occipital cervical fusion • PP: n = 15; 100% • Mean age: 60 y (39–81) |
SI: 33.7 mo (median) NC: 64; 29d LTC: – PC: – |
IV |
| Zadnik et al, 20144 | • Retrospective • 43 patients operated on 2002–2011 for spinal metastases secondary to breast cancer • Techniques: anterior, posterior, or combined approach resection + stabilization • PP: n = 43; 100% • Median age: 56 y (27–91) |
SI: 26.8 mo (median)e; 66% at 1 y; 25% at 3 y; 7% at 4 y; 4% at 5 y NC: 23; – LTC: – PC: – |
IV |
Abbreviations: LTC, local tumor control rate (percent; evaluated at a mean or median follow-up ≥ 12 months); MESCC, metastases with symptomatic epidural spinal cord compression; NC, neurologic function change (percent of breast cohort with preoperative neurologic deficit; percent of breast cohort with identical or worse postoperative neurologic deficit, typically determined by change in Frankel scale); PC, pain change (percent of breast cohort with preoperative pain; percent of breast cohort with identical or worse postoperative pain); PP, patient population (number of patients with breast spinal metastases; percent of entire study population); SI, survival information (postoperative survival time or postoperative survival rate, %).
Data applies to the general study population, not specifically to the breast metastases cohort.
Based on ambulatory ability; 9 of 17 (53%) regained the ability to walk.
Evaluated using Brice-McKissock (1965) classification.
Evaluated using American Spinal Injury Association scale.
Median survival for single (posterior/anterior) approach was 29.6 months, median survival for combined approach was 23.2 months.
Table 3. Surgical results for metastatic spine disease secondary to breast cancer.
| Approach and author(s) | Year | Patients (n) | Postoperative survival (mo), mean or median | % Pain improveda b | % Neurologic improvementa c | % Neurologic declined | Local tumor control rate (%)e |
|---|---|---|---|---|---|---|---|
| Posterior approach | |||||||
| Jonsson et al47 | 1996 | 8 | 10 | 87 | 100 | 0 | – |
| Chen et al50 | 2004 | 13 | 18 | – | – | – | – |
| Weighted mean | 15 | 87 | 100 | 0 | – | ||
| Total patients | 21 | ||||||
| Anterior approach | |||||||
| Jonsson et al44 | 1994 | 19 | 13 | 100 | – | 0 | 100 |
| Gokaslan et al49 | 1998 | 10 | – | – | – | – | – |
| Weighted mean | 13 | 100 | – | 0 | 100 | ||
| Total patients | 29 | ||||||
| Mixed approach | |||||||
| Hammerberg42 | 1992 | 21 | 19 | – | – | – | |
| Kocialkowski et al43 | 1992 | 17 | 8.2 | 76 | 64 | 0 | – |
| Bauer et al45 | 1995 | 14 | – | – | – | – | – |
| Sioutos et al46 | 1995 | 19 | 13.5 | – | – | – | – |
| Onimus et al48 | 1996 | 18 | 12 | 100 | 60 | – | – |
| Sundaresan et al34 | 2002 | 18 | 36 | – | – | – | – |
| Shehadi et al19 | 2007 | 87 | 21 | – | 53 | 8 | 89 |
| Tancioni et al21 | 2011 | 23 | 36 | 96.1 | 100 | 0 | 100 |
| Walcott et al22 | 2011 | 15 | 33.7 | – | 56 | 0 | – |
| Zadnik et al4 | 2014 | 43 | 26.8 | – | – | – | – |
| Weighted mean | 22.9 | 91.4 | 62 | 4.9 | 91.3 | ||
| Total patients | 275 | ||||||
| All surgical studies | |||||||
| Weighted mean | 21.7 | 92.9 | 63.8 | 4.1 | 92.6 | ||
| Total patients | 325 | ||||||
Only considers patient population with preoperative pain/deficits.
Patients used to calculate mean pain improved: 85 (5 studies).
Patients used to calculate mean neurologic improvement: 168 (6 studies).
Patients used to calculate mean neurologic decline: 169 (6 studies).
Patients used to calculate mean local tumor control: 129 (3 studies).
Table 4. Treatment results for metastatic spine disease secondary to breast cancer: vertebroplasty and kyphoplasty (n = 19 patients).
| Author(s) | Year | Technique | Patients (n) | % Pain improvedb | Local tumor control rate (%)b |
|---|---|---|---|---|---|
| Lee et al51 | 2009 | Vertebroplasty | 8 | 100 | – |
| Chen et al10 | 2009 | Vertebroplasty | 7 | 100 | – |
| Sun et al52 | 2010 | Vertebroplasty | 2 | 100 | – |
| Gerszten et al20 | 2009 | Kyphoplastya | 2 | 100 | 100 |
Preceded by transpedicular coblation corpectomy and followed by spinal radiosurgery.
Weighted mean = 100.
Table 5. Significant negative prognostic variables for patients with metastatic breast cancer.
| Studies and authors | Year | Patients (n) | Level of evidence | Outcome measured (dependent variable) | Significant negative prognostic variables |
|---|---|---|---|---|---|
| Spine surgery studies | |||||
| Sciubba et al1 | 2007 | 87 | IV | Postoperative survival for spinal metastasis | Univariate analysis: (1) ER negativity (2) Cervical metastasis (relative to other levels of the spine) (3) PR negativity |
| Multivariate analysis: (1) ER negativity (2) Cervical metastasis | |||||
| Tancioni et al21 | 2011 | 23 | IV | Postoperative survival for spinal metastasis | • Presence of other skeletal metastases |
| Walcott et al22 | 2011 | 15 | IV | Postoperative survival for spinal metastasis | • Patients who do not improve neurologic status postoperative (univariate analysis) • Surgical complications (univariate analysis) |
| Zadnik et al4 | 2014 | 47 | IV | Postoperative survival for spinal metastasis | • Single-modality postoperative adjuvant therapy (compared with dual therapy: radiation and chemotherapy) |
| Total | 172 | ||||
| Nonsurgical studies | |||||
| Solomayer et al28 | 2000 | 648 | IV | Survival period after first metastasis | Univariate analysis: (1) ER/PR negativity, DFI (<24 mo vs. >24 mo); viscera as location of first metastases (soft tissue vs. viscera vs. bone) (2) Longer S-phase fraction (<5% vs. ≥5%) and tumor size |
| Largillier et al24 | 2008 | 1,038 | IV | Survival period after first metastasis | • Old age (≥50 y) at initial diagnosis • ER/PR negativity • Shorter DFI (<24 mo) • Brain or multiple metastases |
| Dawood et al23 | 2010 | 2,881 | IV | Survival period after first metastasis | • Old age at diagnosis of metastasis (≥50 y) • Black race (white vs. black) • HR negativity • Greater ALN invasion • Higher grade disease • Visceral metastases • Shorter DFI • Prior chemotherapy • HER2 negativity |
| Puente et al27 | 2010 | 2,322 | IV | Survival period after first metastasis | • Old age at diagnosis • Advanced stage of disease at diagnosis • High histologic grade • HR negativity • Greater ALN invasion • Administration of neo- and/or adjuvant chemotherapy (especially anthracyclines) • Visceral or lung metastasis • Greater number of metastases • Less aggressive chemotherapy regimen for metastatic disease • Resistance to first-line treatment |
| Planchat et al26 | 2011 | 511 | IV | Survival period after first metastasis | • Old age at initial diagnosis • Greater SBR • Greater ALN invasion • Shorter DFI (only for univariate analysis) |
| Lobbezoo et al25 | 2013 | 815 | IV | Survival period after first metastasis | • Triple negative receptor status • Old age (≥50 y) at initial diagnosis • Shorter DFI (<24 mo vs. ≥24 mo) • Brain as first metastatic site • Multiple metastases • Adjuvant endocrine therapy |
| Total | 8,215 | ||||
Abbreviations: ALN, axillary lymph node; DFI, disease-free interval, the time from primary tumor diagnosis until first metastasis; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HR, hormone receptors (i.e., ERs and PRs); PR, progesterone receptor; SBR, Scarff–Bloom–Richardson grade.
Note: Numbering indicates a ranked list (in order of decreasing statistical significance); bolded items indicate worst survival for a given variable.
Operative Result Summaries
In all, 19 operative studies with level IV evidence were found suitable for analysis and included 344 patients. There were 325 patients in the surgical group with the following breakdown by decompression: 21 posterior, 29 anterior, and 275 mixed (patients who had a combined approach and/or the patient cohort included both types of single approaches). Additionally, the PVP and kyphoplasty literature consisted of 19 total patients (Table 3).
Surgery yielded the following mean postoperative results: a median survival of 21.7 months (8.2 to 36 months), a 92.9% rate of pain improvement (76 to 100%), a 63.8% rate of neurologic improvement (53 to 100%), a 4.1% rate of neurologic decline (0 to 8%), and a 92.6% rate of local tumor control (89 to 100%). Moreover, PVP and kyphoplasty studies reported a 100% rate of pain control. This data is summarized in Tables 3 and 4, and postoperative survival for surgical studies is trended in Fig. 1. However, the reported statistics should be taken with a caveat. For example, many mean surgical postoperative outcomes are based on a fraction of the 14 surgical studies (Table 3). In addition, the total number of patients comprising the cement augmentation procedures (19) is likely not large enough to broadly generalize a rate of pain improvement (Table 4). Finally, the kyphoplasty study was actually a combination of transpedicular coblation corpectomy (i.e., using Cavity SpineWand [ArthroCare Corp., Austin, Texas, United States] to circumferentially ablate and debulk tumor under fluoroscopy) combined with kyphoplasty, followed by spinal radiosurgery at a mean of 14 days later.20
Fig. 1.
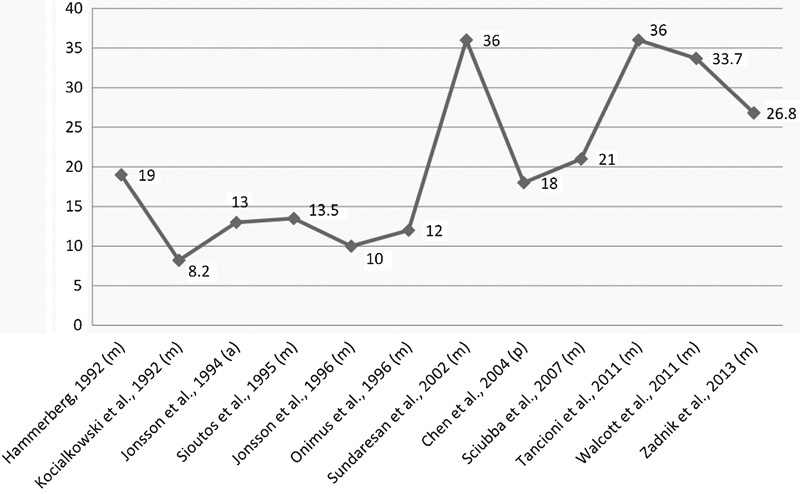
Median or mean postoperative survival for metastatic breast cancer patients in months. Abbreviations: a, anterior decompression; m, mixed decompression (combined or including both single approaches); p, posterior decompression.
Prognostic Variables
Of the spine surgery studies, only 4 unique studies (172 total patients) analyzed prognostic factors of postoperative survival. Sciubba et al determined that estrogen receptor (ER) positivity of the tumor conferred a positive prognostic value (p = 0.001) and found a trend for poorer survival in patients with cervical lesions (p = 0.006).1 Tancioni et al found that worse survival was associated in the presence of other skeletal metastases.21 Walcott et al found on univariate analysis that patients with improved American Spinal Injury Association scores and patients who did not have surgical complications had a statistically significant longer survival (p < 0.005).22 Finally, Zadnik et al found that dual therapy (chemotherapy and radiotherapy) was associated with significantly higher survival when compared with single-modality postoperative adjuvant therapy (p = 0.042).4 Age ≥ 65 years, preoperative functional status, location of metastasis, presence of visceral metastases, and spinal instability did not have a significant impact on the survival. The postoperative results were in agreement with those reported by Sciubba et al in that visceral metastases did not impact survival1; however, cervical lesions in this 2013 study were not associated with decreased survival (Table 5).
An additional six nonsurgical studies (8,215 total patients) analyzing prognostic variables in the survival of patients with metastatic breast cancer were found; ER/progesterone receptor (PR)/human epidermal growth factor receptor 2 (HER2) receptor negativity, shorter disease-free interval (DFI), visceral metastasis, a greater degree of axillary lymph node invasion, and old age at initial diagnosis (≥50 years) were found to be recurring variables portending poor survival (Table 5).23 24 25 26 27 28
Study Quality and Overall Strength of Literature
All 19 operative publications and 6 prognostic variable studies were case series without control groups. Hence, all publications had a baseline class of evidence of level IV. Based on the class of evidence and the quality and consistency of data, the overall strength of findings is moderate to insufficient as outlined with each summary of findings (Table 6).
Table 6. Strength of findings in patients with metastatic breast cancer.
| Finding | Summary | Modification | Strength of evidence |
|---|---|---|---|
| Survival | Hormone- and HER2-naïve patients have a statistically and temporally significant survival advantage over resistant receptor patients. | Upgrade: large effect (source: nonsurgical studies, Sciubba et al1 study) | Moderate |
| DFI and a greater degree of axillary lymph node invasion have a statistically and temporally significant negative impact on survival. | Upgrade: large effect (source: large nonsurgical studies) | Moderate | |
| Single-modality postoperative adjuvant therapy (compared with dual therapy: radiation and chemotherapy) has a statistically and temporally significant negative impact on survival. | Source: Zadnik et al4 only | Low | |
| Visceral metastasis, surgical complications, presence of other skeletal metastasis, presence of cervical metastasis, and age have a statistically and temporally significant negative impact on survival. | Downgrade: inconsistent results across studies, for age different cutoffs are used (Sciubba et al1 vs. Zadnik et al4 vs. nonsurgical results) | Insufficient | |
| Pain outcome | Surgery provides pain relief in over 75% of cases with preoperative pain. | Based on 85 patients (see Table 3) | Low |
| Cement augmentation procedures provide a high rate of pain relief (>90%). | Downgrade: small sample size (19 patients) | Insufficient | |
| Neurologic outcome | Surgery improves neurologic function in over 50% of cases with preoperative deficit. | Upgrade: large effect (based on 168 patients) | Moderate |
| Surgery treatment has ∼5% risk of neurologic deterioration. | Upgrade: large effect (based on 169 patients) | Moderate | |
| Local tumor control | Surgical resection results in local tumor control rates of >90% for up to 12 mo. | Based on 129 patients | Low |
Note: High indicates majority of articles level I or II; low indicates majority of articles level III or IV. Upgrade means large effect or gradient response; downgrade means inconsistence, imprecision of effect, indirect evidence, publication bias.
Due to large, consistent effects, neurologic outcome improvement was upgraded. With respect to prognostic variables, receptor status (HER2 and ER/PR), DFI, and axillary lymph node invasion were upgraded. Based on inconsistent results, the following prognostic variables were downgraded: visceral metastasis, surgical complications, presence of other skeletal metastasis, presence of cervical metastasis, and old age at initial diagnosis. Due to a limited patient population (19 patients), pain relief data for cement augmentation procedures was downgraded (Table 6).
Consensus Statement
Although there is a paucity of breast-specific literature, the data extracted from the relevant studies and systematic review allowed for clinical recommendations to be made.
There is moderate strength of evidence that surgery can provide improvement in most patients (i.e., >50%), with a low risk of diminished neurologic function (i.e., <5%). Moreover, there is a moderate strength of evidence suggesting the significantly negative impact of hormonally and HER2 refractory tumor status, high degree of axillary lymph node involvement, and short DFI on survival. Experts agree that for these patients, less invasive options should be considered. With respect to DFI, formal guidelines have not been established, but studies suggest that a DFI less than 24 months is concerning for a shortened survival period after diagnosis of metastases.29 Furthermore, although only a small population size could be analyzed for the clinical efficacy of vertebral augmentation procedures, based on mixed-histology studies, experts feel that PVP and kyphoplasty are excellent options for the palliation of pain in patients with favorable anatomy.
Discussion
After the 2005 Patchell trial, direct decompressive surgery plus radiation became the preferred treatment modality for selected single-level spinal metastases with symptomatic epidural spinal cord compression, given the gains in functional status and a suggested improvement in survival.30 31 32 Less invasive operative techniques, such as PVP and kyphoplasty, have demonstrated effective pain relief for metastatic vertebral lesions. Minimally invasive techniques can be utilized in patients with limited life expectancy, tumor-related malnourishment, and/or diminished immune system function who are precluded from surgery.33 Moreover, percutaneous cement augmentation procedures are appropriate for those with intractable pain secondary to a vertebral body deformity. Cement augmentation is contraindicated for those with epidural compression, neurologic deficits, or instability amenable only to open fixation.
Until recently, sizeable studies specific to surgical outcomes for patients with breast cancer spinal metastases have not been conducted. To our knowledge, the first and largest exclusive cohort, consisting of 87 patients, was that reported by Sciubba et al1 and Shehadi et al in 2007.19 In 2013, Zadnik et al studied a cohort of 43 patients.4 Aside from these studies, all other published articles since 1990 have not included more than 23 patients with metastatic breast cancer, highlighting the need for more large-scale studies to provide statistically powered conclusions with respect to operative outcomes. Heterogeneity in surgical instrumentation, patient characteristics (e.g., solitary versus multiple metastases), and adjuvant therapies were other confounding factors.
As mentioned previously, our overall surgical results after reviewing 325 patients were the following: 21.7-month survival, 92.9% rate of pain improvement, 63.8% rate of neurologic improvement, 4.1% rate of neurologic decline, and 92.6% rate of local tumor control. Our reported pain improvement is slightly superior to those reported by two literature reviews of mixed-pathology symptomatic metastatic epidural spinal cord compression, but within a 10% range from the surgical procedures resulting in the poorest pain outcomes. Moreover, our reported neurologic improvement was remarkably similar to laminectomy and radiotherapy plus posterior stabilization as reported by Witham et al (64%) and Kaloostian et al (62%),8 32 but somewhat inferior to those of purely anterior procedures (75 and 68%).8 32
Interestingly, with regard to postsurgical survival, a prolonged survival beginning with the 36-month median survival reported by Sundaresan et al in 2002 was observed (Fig. 1).34 Prior to this report, breast cancer survival ranged from 8.2 to 19 months, and studies from 2002 onward reported a mean or median survival of 18 to 36 months. One operative factor influencing outcomes after this point may have been the acceptance of more aggressive and complete tumor resection; Sundaresan et al noted that toward the end of their study period, aggressive en bloc resection was gaining recognition in the spine community.34 However, we agree with Zadnik et al who attributed the later improvements in breast cancer survival to the United States Food and Drug Administration (FDA) approval of trastuzumab and tamoxifen in 1998, dose-dense chemotherapy regimens in 2003, docetaxel and gemcitabine in 2004, as well as the increase in surgical experience, expertise, and techniques.4 Most recently in 2013, the FDA approved the HER2-targeting combination of trastuzumab, pertuzumab, and docetaxel as a first-line treatment for metastatic breast cancer, which will likely further improve patient survival.35
The cement augmentation studies found yielded excellent pain control rates. Although cement augmentation procedures are very frequently performed for metastatic disease of any origin, there were only 17 cases of PVP and 2 cases for ablation and kyphoplasty that met our criteria in the literature, possibly reflecting a selection bias. Whether polymethyl methacrylate injections are especially beneficial to patients with breast cancer is a point of contention, though some studies claim that methyl methacrylate monomer has a specific cytotoxicity to breast cancer cells.36 However, it is likely that pain relief achieved via cement augmentation procedures is not a chemical mechanism (i.e., breast cancer cell-specific cytotoxicity), as suggested by a matched case–control vertebroplasty study comparing the effects of toxic polymethyl methacrylate to that of nontoxic calcium phosphate.37 Moreover, the study reported by Gerszten et al was not a true kyphoplasty study, as it tested a novel paradigm that combines tumor resection, kyphoplasty, and radiosurgery.20 Overall, it is likely that the clinical outcomes achieved by cement augmentation procedures for breast cancer are similar to that of other tumor pathologies.
With respect to our second objective, identifying prognostic factors that predict patient survival, we came across a dearth of surgical literature analyzing such variables. Specifically, understanding the role that each prognostic factor plays in the postoperative survival, rate of change in neurologic status, local tumor control, and pain for those patients who have already been selected for surgery is critical. In the context of the surgical decision-making process, prognostic variables can be used to decide on the invasiveness of the operation based on the life expectancy of the patient. Across the surgical studies, certain negative prognostic factors were not consistently found to be statistically significant or were simply unable to be evaluated, likely resulting from a small sample size and bias for selecting a healthier subset of surgical candidates.1 4 For example, Sciubba et al did not find visceral metastasis and multiple metastases to be a significant predictive factor, despite confirmation in previous surgical spinal metastasis studies.38 39 Moreover, Zadnik et al were not able to analyze the influence of ER negativity due to the overwhelming majority of ER-positive patients.4 Commonly recurring significant negative prognostic variables found in the nonsurgical literature search, such as shorter DFI and axillary lymph node involvement, were found to be insignificant in two surgical studies1 22 and one surgical study,1 respectively, possibly due to small patient populations in the surgical studies.
Despite this discrepancy, we suggest that, in conjunction with surgical prognostic scoring systems, DFI be further investigated as a readily available, multifactorial prognostic tool in patients with metastatic spine disease, as it is one of the strongest indicators of disease aggressiveness.24 40 Studies have already calculated the survival based on varying DFIs for patients with breast cancer (Table 5).23 24 25 26 41 For example, in 2010, Dawood et al found that DFIs of <6 months, ≥6 months to <2 years, ≥2 years to <5 years, ≥5 years were linked to median metastatic survivals (i.e., time from first distant metastasis to death) of 17.4 months, 17.3 months, 30.4 months, and 47.4 months, respectively (p < 0.0001) in 2,881 patients.23 To account for subtler differences in estimated survival, which may be relevant when selecting an operative treatment, a greater degree of DFI stratification may be necessary when studying a large surgical cohort. Perhaps this metric can eventually be combined with established surgical scoring systems to optimize treatment selection.
Conclusions
A systematic literature review of operative techniques to treat metastatic spine disease secondary to breast cancer generated 19 class IV case series, only 3 of which were prospective. The body of evidence is graded as moderate to insufficient, which provides further rationale for multicenter prospective clinical studies to be performed to provide stronger evidence from which to make clinical recommendations.
This review asked two main questions with the results outlined above and summarized below:
-
What is the postoperative survival, rate of change in neurologic status, local tumor control rate, and pain improvement rate for a given operative treatment?
Based on the available surgical literature, the median postoperative survival was 21.7 months (8.2 to 36 months), the mean rate of pain improvement was 92.9% (76 to 100%), the mean rate of neurologic improvement was 63.8% (53 to 100%), the mean rate neurologic decline was 4.1% (0 to 8%), and the mean rate local tumor control rate was 92.6% (89 to 100%). Increased survival from 2002 onward was likely due to FDA approval of new chemotherapies.
Are there any clinical, radiographic, or histologic variables in the surgical literature that may help prognosticate which patients will perform better or worse with surgery?
There was moderate strength of evidence that negative receptor status (HER2 and ER/PR), short DFI (<24 months), and a high degree of axillary lymph node invasion corresponded with poor survival. Moreover, there was a low strength of evidence that single-modality postoperative adjuvant therapy (compared with dual therapy of radiation and chemotherapy) had a statistically and temporally significant negative impact on the survival. Finally, based on inconsistent results, there was insufficient strength of evidence for the following negative prognostic variables: visceral metastasis, surgical complications, presence of other skeletal metastasis, presence of cervical metastasis, and old age at initial diagnosis.
Footnotes
Disclosures Daniel M. Sciubba, Consulting: Depuy-Synthes, Medtronic, Nuvasive, Stryker C. Rory Goodwin, Fellowship support: UNCF Merck Postdoctoral Fellowship; Grant: Burroughs Wellcome Fund and NREF Alp Yurter, none Derek Ju, none Ziya L. Gokaslan, Stock ownership: US Spine, Spinal Kinetics; Consulting, speaking, and teaching: AO Foundation; Research support: DePuy, NREF, AOSpine, AO North America Charles Fisher, Consulting: Medtronic, Nuvasive; Royalties: Medtronic; Research support: OREF, AOSpine, Medtronic Laurence D. Rhines, Consulting: Stryker, Globus Michael G. Fehlings, none Daryl R. Fourney, Travel expenses: AOSpine International; Grants: Asubio Pharmaceuticals, Canadian Institutes of Health Research, Spinal Cord Injury Solutions Network, Saskatchewan Health Research Foundation, AOSpine North America; Ownership, Proven Care Pathways, LLC (no payments received) Ehud Mendel, none Ilya Laufer, Consulting: SpineWave, DePuy/Synthes Chetan Bettegowda, none Shreyaskumar R. Patel, Consulting: Novartis, GSK, EMD Serono, Janssen, CytRx Y. Raja Rampersaud, Consulting: Medtronic Arjun Sahgal, Speaking and/or teaching arrangements: Medtronic Jeremy Reynolds, Support for travel to meetings: Nuvasive; Speaking and/or teaching arrangements: Globus, Medtronic; Fellowship support: DePuy/Synthes Dean Chou, Consulting: Orthofix, Medtronic, DePuy, Globus Michael H. Weber, none Michelle J. Clarke, none
References
- 1.Sciubba D M, Gokaslan Z L, Suk I. et al. Positive and negative prognostic variables for patients undergoing spine surgery for metastatic breast disease. Eur Spine J. 2007;16(10):1659–1667. doi: 10.1007/s00586-007-0380-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esteva F J, Valero V, Pusztai L, Boehnke-Michaud L, Buzdar A U, Hortobagyi G N. Chemotherapy of metastatic breast cancer: what to expect in 2001 and beyond. Oncologist. 2001;6(2):133–146. doi: 10.1634/theoncologist.6-2-133. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society . Atlanta, GA: American Cancer Society; 2014. Cancer Facts & Figures 2014. [Google Scholar]
- 4.Zadnik P L, Hwang L, Ju D G. et al. Prolonged survival following aggressive treatment for metastatic breast cancer in the spine. Clin Exp Metastasis. 2014;31(1):47–55. doi: 10.1007/s10585-013-9608-3. [DOI] [PubMed] [Google Scholar]
- 5.Eleraky M, Papanastassiou I, Vrionis F D. Management of metastatic spine disease. Curr Opin Support Palliat Care. 2010;4(3):182–188. doi: 10.1097/SPC.0b013e32833d2fdd. [DOI] [PubMed] [Google Scholar]
- 6.Sciubba D M, Gokaslan Z L. Diagnosis and management of metastatic spine disease. Surg Oncol. 2006;15(3):141–151. doi: 10.1016/j.suronc.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Sciubba D M, Petteys R J, Dekutoski M B. et al. Diagnosis and management of metastatic spine disease. A review. J Neurosurg Spine. 2010;13(1):94–108. doi: 10.3171/2010.3.SPINE09202. [DOI] [PubMed] [Google Scholar]
- 8.Kaloostian P E, Yurter A, Zadnik P L, Sciubba D M, Gokaslan Z L. Current paradigms for metastatic spinal disease: an evidence-based review. Ann Surg Oncol. 2014;21(1):248–262. doi: 10.1245/s10434-013-3324-8. [DOI] [PubMed] [Google Scholar]
- 9.Landreneau F E, Landreneau R J, Keenan R J, Ferson P F. Diagnosis and management of spinal metastases from breast cancer. J Neurooncol. 1995;23(2):121–134. doi: 10.1007/BF01053417. [DOI] [PubMed] [Google Scholar]
- 10.Chen K Y, Ma H I, Chiang Y H. Percutaneous transpedicular vertebroplasty with polymethyl methacrylate for pathological fracture of the spine. J Clin Neurosci. 2009;16(10):1300–1304. doi: 10.1016/j.jocn.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Laufer I, Sciubba D M, Madera M. et al. Surgical management of metastatic spinal tumors. Cancer Contr. 2012;19(2):122–128. doi: 10.1177/107327481201900206. [DOI] [PubMed] [Google Scholar]
- 12.Yimin Y, Zhiwei R, Wei M, Jha R. Current status of percutaneous vertebroplasty and percutaneous kyphoplasty—a review. Med Sci Monit. 2013;19:826–836. doi: 10.12659/MSM.889479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atkins D, Best D, Briss P A. et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balshem H, Helfand M, Schünemann H J. et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Owens D K, Lohr K N, Atkins D. et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions—Agency for Healthcare Research and Quality and the effective health-care program. J Clin Epidemiol. 2010;63(5):513–523. doi: 10.1016/j.jclinepi.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Methods Guide for Effectiveness and Comparative Effectiveness Reviews. AHRQ Publication No. 10(14)-EHC063-EF Rockville, MD: Agency for Healthcare Research and Quality; January 2014. Available at: www.effectivehealthcare.ahrq.gov [PubMed]
- 17.West S, King V, Carey T S. et al. Systems to rate the strength of scientific evidence. Evid Rep Technol Assess (Summ) 2002;2002(47):1–11. [PMC free article] [PubMed] [Google Scholar]
- 18.Traynelis V C, Arnold P M, Fourney D R. et al. Alternative procedures for the treatment of cervical spondylotic myelopathy: arthroplasty, oblique corpectomy, skip laminectomy: evaluation of comparative effectiveness and safety. Spine (Phila Pa 1976) 2013;38(22) 01:S210–S231. doi: 10.1097/BRS.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 19.Shehadi J A, Sciubba D M, Suk I. et al. Surgical treatment strategies and outcome in patients with breast cancer metastatic to the spine: a review of 87 patients. Eur Spine J. 2007;16(8):1179–1192. doi: 10.1007/s00586-007-0357-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerszten P C, Monaco E A III. Complete percutaneous treatment of vertebral body tumors causing spinal canal compromise using a transpedicular cavitation, cement augmentation, and radiosurgical technique. Neurosurg Focus. 2009;27(6):E9. doi: 10.3171/2009.9.FOCUS09184. [DOI] [PubMed] [Google Scholar]
- 21.Tancioni F, Navarria P, Mancosu P. et al. Surgery followed by radiotherapy for the treatment of metastatic epidural spinal cord compression from breast cancer. Spine (Phila Pa 1976) 2011;36(20):E1352–E1359. doi: 10.1097/BRS.0b013e318207a222. [DOI] [PubMed] [Google Scholar]
- 22.Walcott B P, Cvetanovich G L, Barnard Z R, Nahed B V, Kahle K T, Curry W T. Surgical treatment and outcomes of metastatic breast cancer to the spine. J Clin Neurosci. 2011;18(10):1336–1339. doi: 10.1016/j.jocn.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 23.Dawood S, Broglio K, Ensor J, Hortobagyi G N, Giordano S H. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol. 2010;21(11):2169–2174. doi: 10.1093/annonc/mdq220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Largillier R, Ferrero J M, Doyen J. et al. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol. 2008;19(12):2012–2019. doi: 10.1093/annonc/mdn424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lobbezoo D J, van Kampen R J, Voogd A C. et al. Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Cancer Res Treat. 2013;141(3):507–514. doi: 10.1007/s10549-013-2711-y. [DOI] [PubMed] [Google Scholar]
- 26.Planchat E, Durando X, Abrial C. et al. Prognostic value of initial tumor parameters after metastatic relapse. Cancer Invest. 2011;29(9):635–643. doi: 10.3109/07357907.2011.621911. [DOI] [PubMed] [Google Scholar]
- 27.Puente J, López-Tarruella S, Ruiz A. et al. Practical prognostic index for patients with metastatic recurrent breast cancer: retrospective analysis of 2,322 patients from the GEICAM Spanish El Alamo Register. Breast Cancer Res Treat. 2010;122(2):591–600. doi: 10.1007/s10549-009-0687-4. [DOI] [PubMed] [Google Scholar]
- 28.Solomayer E F, Diel I J, Meyberg G C, Gollan C, Bastert G. Metastatic breast cancer: clinical course, prognosis and therapy related to the first site of metastasis. Breast Cancer Res Treat. 2000;59(3):271–278. doi: 10.1023/a:1006308619659. [DOI] [PubMed] [Google Scholar]
- 29.Khanfir A, Lahiani F, Bouzguenda R, Ayedi I, Daoud J, Frikha M. Prognostic factors and survival in metastatic breast cancer: a single institution experience. Rep Pract Oncol Radiother. 2013;18(3):127–132. doi: 10.1016/j.rpor.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartels R H, van der Linden Y M, van der Graaf W T. Spinal extradural metastasis: review of current treatment options. CA Cancer J Clin. 2008;58(4):245–259. doi: 10.3322/CA.2007.0016. [DOI] [PubMed] [Google Scholar]
- 31.Patchell R A, Tibbs P A, Regine W F. et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 32.Witham T F Khavkin Y A Gallia G L Wolinsky J P Gokaslan Z L Surgery insight: current management of epidural spinal cord compression from metastatic spine disease Nat Clin Pract Neurol 20062287–94., quiz 116 [DOI] [PubMed] [Google Scholar]
- 33.Molina C A, Gokaslan Z L, Sciubba D M. A systematic review of the current role of minimally invasive spine surgery in the management of metastatic spine disease. Int J Surg Oncol. 2011;2011:598148. doi: 10.1155/2011/598148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sundaresan N, Rothman A, Manhart K, Kelliher K. Surgery for solitary metastases of the spine: rationale and results of treatment. Spine (Phila Pa 1976) 2002;27(16):1802–1806. doi: 10.1097/00007632-200208150-00021. [DOI] [PubMed] [Google Scholar]
- 35.Jelovac D, Emens L A. HER2-directed therapy for metastatic breast cancer. Oncology (Williston Park) 2013;27(3):166–175. [PubMed] [Google Scholar]
- 36.Yang H L, Sun Z Y, Wu G Z, Chen K W, Gu Y, Qian Z L. Do vertebroplasty and kyphoplasty have an antitumoral effect? Med Hypotheses. 2011;76(1):145–146. doi: 10.1016/j.mehy.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 37.Nakano M, Hirano N, Ishihara H, Kawaguchi Y, Watanabe H, Matsuura K. Calcium phosphate cement-based vertebroplasty compared with conservative treatment for osteoporotic compression fractures: a matched case-control study. J Neurosurg Spine. 2006;4(2):110–117. doi: 10.3171/spi.2006.4.2.110. [DOI] [PubMed] [Google Scholar]
- 38.Ogihara S, Seichi A, Hozumi T. et al. Prognostic factors for patients with spinal metastases from lung cancer. Spine (Phila Pa 1976) 2006;31(14):1585–1590. doi: 10.1097/01.brs.0000222146.91398.c9. [DOI] [PubMed] [Google Scholar]
- 39.Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 2005;30(19):2186–2191. doi: 10.1097/01.brs.0000180401.06919.a5. [DOI] [PubMed] [Google Scholar]
- 40.Insa A, Lluch A, Prosper F, Marugan I, Martinez-Agullo A, Garcia-Conde J. Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat. 1999;56(1):67–78. doi: 10.1023/a:1006285726561. [DOI] [PubMed] [Google Scholar]
- 41.Chang J, Clark G M, Allred D C, Mohsin S, Chamness G, Elledge R M. Survival of patients with metastatic breast carcinoma: importance of prognostic markers of the primary tumor. Cancer. 2003;97(3):545–553. doi: 10.1002/cncr.11083. [DOI] [PubMed] [Google Scholar]
- 42.Hammerberg K W. Surgical treatment of metastatic spine disease. Spine (Phila Pa 1976) 1992;17(10):1148–1153. doi: 10.1097/00007632-199210000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Kocialkowski A, Webb J K. Metastatic spinal tumours: survival after surgery. Eur Spine J. 1992;1(1):43–48. doi: 10.1007/BF00302142. [DOI] [PubMed] [Google Scholar]
- 44.Jonsson B, Jonsson H Jr, Karlstrom G, Sjostrom L. Surgery of cervical spine metastases: a retrospective study. Eur Spine J. 1994;3(2):76–83. doi: 10.1007/BF02221444. [DOI] [PubMed] [Google Scholar]
- 45.Bauer H C, Wedin R. Survival after surgery for spinal and extremity metastases. Prognostication in 241 patients. Acta Orthop Scand. 1995;66(2):143–146. doi: 10.3109/17453679508995508. [DOI] [PubMed] [Google Scholar]
- 46.Sioutos P J, Arbit E, Meshulam C F, Galicich J H. Spinal metastases from solid tumors: analysis of factors affecting survival. Cancer. 1995;76(8):1453–1459. doi: 10.1002/1097-0142(19951015)76:8<1453::aid-cncr2820760824>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 47.Johnson B, Sjostrom L, Olerud C, Andreasson I, Bring J, Rauschning W. Outcome after limited posterior surgery for thoracic and lumbar spine metastases. Eur Spine J. 1996;5(1):36–44. doi: 10.1007/BF00307825. [DOI] [PubMed] [Google Scholar]
- 48.Onimus M, Papin P, Gangloff S. Results of surgical treatment of spinal thoracic and lumbar metastases. Eur Spine J. 1996;5(6):407–411. doi: 10.1007/BF00301969. [DOI] [PubMed] [Google Scholar]
- 49.Gokaslan Z L, York J E, Walsh G L. et al. Transthoracic vertebrectomy for metastatic spinal tumors. J Neurosurg. 1998;89(4):599–609. doi: 10.3171/jns.1998.89.4.0599. [DOI] [PubMed] [Google Scholar]
- 50.Chen L H, Niu C C, Fu T S, Lai P L, Wong C B, Chen W J. Posterior decompression and stabilization for metastatic spine diseases. Chang Gung Med J. 2004;27(12):903–910. [PubMed] [Google Scholar]
- 51.Lee B, Franklin I, Lewis J S. et al. The efficacy of percutaneous vertebroplasty for vertebral metastases associated with solid malignancies. Eur J Cancer. 2009;45(9):1597–1602. doi: 10.1016/j.ejca.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 52.Sun G, Jin P, Li M. et al. Percutaneous vertebroplasty for treatment of osteolytic metastases of the C2 vertebral body using anterolateral and posterolateral approach. Technol Cancer Res Treat. 2010;9(4):417–422. doi: 10.1177/153303461000900411. [DOI] [PubMed] [Google Scholar]


