Abstract
Study Design
Retrospective matched cohort analysis.
Objective
The majority of the literature on venous thromboembolism (VTE) after spine surgery is limited to studies of thoracolumbar surgery. Less is known regarding the incidence of VTE and associated risk factors following cervical spine surgery.
Methods
A total of 5,405 patients at our institution underwent cervical diskectomy, laminectomy, corpectomy, laminoplasty, or fusion between 1995 and 2012; 85 of the 5,405 patients (1.57%) suffered either a DVT (55) or pulmonary embolus (51) within 30 days postoperatively. The cases were matched 1:2 to controls based on age, sex, and date of surgery. Data regarding multiple perioperative factors, demographics, and comorbidities was collected.
Results
Several risk factors were identified for VTE. Significant medical comorbidities included chronic venous insufficiency (odds ratio [OR] = 3.40), atrial fibrillation (OR = 2.69), obesity (OR = 2.67), and ischemic heart disease (OR = 2.18). Staged surgery (OR = 28.0), paralysis (OR = 19.0), combined approach (OR = 7.46), surgery for infection (OR = 18.5), surgery for trauma (OR = 11.1), comorbid traumatic injuries (OR > 10), oncologic procedures (OR = 5.2), use of iliac crest autograft (OR = 4.16), two or more surgical levels (OR = 3.48), blood loss > 300 mL (OR = 1.66), and length of stay 5 days or greater (OR = 3.47) were all found to be risk factors for VTE (p < 0.05) in univariate analysis. Multivariate analysis found staged surgery (OR = 35.7), paralysis (OR = 7.86), and nonelective surgery (OR = 6.29) to be independent risk factors for VTE.
Conclusions
Although the incidence of VTE following cervical spine surgery is low, we identified several risk factors that may be predictive. More aggressive approaches to prophylaxis and surveillance in certain patient populations may be warranted.
Keywords: cervical spine surgery, venous thromboembolism, pulmonary embolus, deep vein thrombosis
Introduction
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolus (PE), is a common postoperative complication. Although many thromboembolic events are clinically silent, symptomatic VTE can have fatal consequences. A high incidence of VTE has been well demonstrated in hip and knee arthroplasty.1 The incidence of DVT has been as high as 40% in some studies.1 2 Less is known about the incidence of VTE in spinal surgery.3 4 However, recent studies have shown that the incidence of VTE following spinal surgery may be higher than previously reported.4 Although the overall prevalence of symptomatic VTE is considerably lower when compared with major lower extremity surgery, fatal PE is a well-known cause of postoperative mortality following spine surgery, as high as 2% in certain populations.5 For this reason, it is critical to determine the incidence of VTE in patients who undergo spine surgery as well as risk factors.
Several studies have examined the incidence of VTE in thoracolumbar procedures including various subgroups such as thoracolumbar trauma, deformity correction, and oncologic procedures.6 7 However, the number of studies looking specifically at VTE in cervical spine surgery is limited. The goal of this study was to examine the 30-day incidence of and risk factors for VTE including PE and DVT at a single institution between 1995 and 2012. We hypothesize that multiple risk factors will be identified including various patient comorbidities and procedure-related risk factors.
Methods
After Institutional Review Board approval, an institutional database at a level I trauma center was queried to create a list of 5,405 patients 18 years or older who underwent cervical spine procedures based on Current Procedural Terminology (CPT) codes for cervical diskectomy, corpectomy, laminectomy, laminoplasty, and anterior or posterior arthrodesis. (See online supplementary material for CPT codes.) This group of patients was cross-referenced for lower extremity DVT or acute PE based on International Statistical Classification of Disease and Related Health Problems 9th Revision (ICD-9-CM) codes. (See online supplementary material for ICD-9-CM codes.) The charts of the patients identified were reviewed to determine whether the VTE event occurred within 30 days postoperatively. Patients who suffered a VTE outside of the 30-day postoperative period or for whom the cervical surgery was not the primary procedure were excluded from the study.
The prevalence of various comorbidities were identified between the VTE and non-VTE patients including chronic venous insufficiency, obesity (body mass index of 30 or greater), atrial fibrillation, ischemic heart disease, malignancy, hypertension, peripheral vascular disease, diabetes, and tobacco use based on ICD-9-CM codes. Given the size of the patient sample, a matched cohort was created to analyze other potential risk factors for VTE. The VTE cases were matched 1:2 with the non-VTE controls. Multiple potential risk factors were reviewed in the patient records including demographics, length of stay, surgical indications (elective, traumatic, oncologic, or infectious), surgical approach (anterior, posterior, or both), number of surgical levels, comorbid traumatic injuries (head, abdominal, or lower extremity trauma), estimated blood loss, use of iliac crest autograft, paralysis (diagnosis of complete or incomplete quadriplegia), and staged surgery (defined as two planned cervical surgeries within 7 days). The medical record was also reviewed for use of perioperative VTE prophylaxis including inferior vena cava (IVC) filters, warfarin, and low-molecular-weight heparin.
The data was summarized and reported as mean (standard deviation) for continuous variables and count (percentage) for categorical data. The analysis focused on comparing patients who experienced a VTE within 30 days following surgery (cases) to a set of matched patients who did not experience a VTE within 30 days of surgery (controls). The controls were matched to cases in a 2:1 ratio on age (±5 years), sex, and year of surgery (±2 years). The association of potential risk factors with the outcome of VTE was evaluated using conditional logistic regression. Potential risk factors included patient-specific characteristics and surgical factors. Both univariate and multivariable models were generated. All statistical tests were two-sided, and p values less than 0.05 were considered significant. All analysis was conducted using SAS version 9.2 (SAS Institute Inc., Cary, North Carolina, United States).
Results
Of the 5,405 patients who underwent cervical spine surgery in our cohort, 85 (1.57%) suffered a VTE event. Of those, 55 (1.02%) suffered a DVT and 51 (0.94%) suffered a PE. There was one postoperative mortality secondary to PE (0.02%). All DVT diagnoses were made using DVT ultrasound. All PE diagnoses were made using a spiral computed tomography scan protocol. Patients were not routinely screened for VTE and all diagnostic tests were performed based on clinical suspicion. The frequency of medical comorbidities between the VTE and non-VTE groups is demonstrated in Fig. 1. Univariate analysis of patient comorbidities demonstrated chronic venous insufficiency (odds ratio [OR] 3.40), atrial fibrillation (OR 2.69), obesity (OR 2.67), and ischemic heart disease (OR 2.21) as significant risk factors for VTE (Table 1). Of the 85 cases of VTE identified, 3 were excluded from the matched cohort analysis secondary to insufficient data in the medical record. Thus 82 cases of VTE were matched 2:1 based on age and sex to a control group of 164 cases without VTE.
Fig. 1.
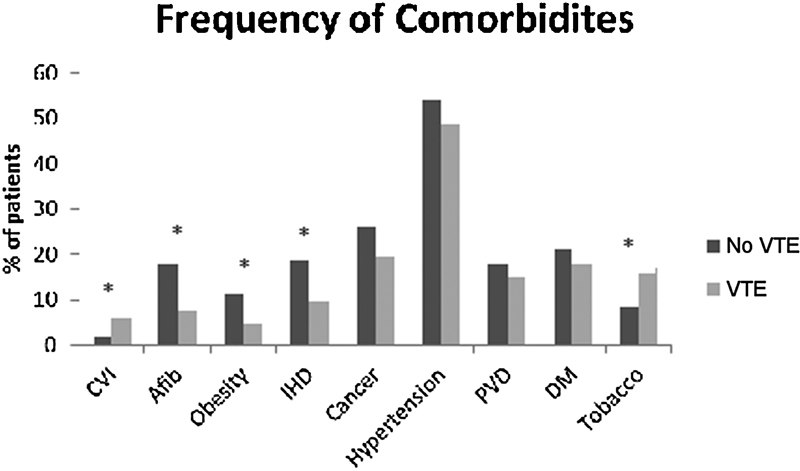
Frequency of comorbidities in the VTE and non-VTE groups. *Significant difference. Abbreviations: Afib, atrial fibrillation; CVI, chronic venous insufficiency; DM, diabetes mellitus; IHD, ischemic heart disease; PVD, peripheral vascular disease; VTE, venous thromboembolism.
Table 1. Patient comorbidities predictive of VTE after cervical spine surgery.
| Risk factor | Odds ratio | 95% CI | p Value |
|---|---|---|---|
| Chronic venous insufficiency | 3.40a | 1.35–8.58 | 0.01 |
| Atrial fibrillation | 2.69a | 1.53–4.79 | <0.001 |
| Obesity | 2.67a | 1.36–5.23 | 0.004 |
| Ischemic heart disease | 2.18a | 1.26–3.79 | 0.006 |
| Cancer | 1.45 | 0.89–2.37 | 0.137 |
| Hypertension | 1.28 | 0.82–1.93 | 0.298 |
| Peripheral vascular disease | 1.25 | 0.684–2.107 | 0.525 |
| Diabetes | 1.24 | 0.73–2.1 | 0.418 |
| Tobacco use | 0.45a | 0.204–0.965 | 0.04 |
Abbreviations: CI, confidence interval; VTE, venous thromboembolism.
Significant result.
Summaries of the patient demographics and perioperative characteristics are found in Table 2. The average age of the cohort was 58.6 ± 16.9 years with a range from 21 to 89 years old. The majority of the patients were men (68.3%). The most common surgical indication was elective in both the VTE patients (39.0%) and controls (79.3%). However, a high percentage (34.1%) of the VTE cases were found in patients treated for cervical trauma. A posterior surgical approach was the most common (70.3%) in the cohort with an increased percentage of combined approach in the VTE cohort (23.2%) relative to the controls (3%). The overall incidence of comorbid traumatic injuries was low (5.3%) but higher in the VTE cohort (13.4%). The VTE cases had higher estimated blood loss (average > 450 mL). They also more frequently involved four or more levels (46.3% versus 28.7%) and use of iliac crest autograft (39% versus 14%). A higher percentage of VTE patients were paraplegic at the time of surgery (23.1%) and underwent staged surgery (17.1%).
Table 2. Patient demographics for matched cohort analysis.
| Variable | Case (n = 82) | Control (n = 164) |
|---|---|---|
| Age (y) | 58.6 ± 16.9 | 58.5 ± 16.9 |
| Male gender, n (%) | 56 (68.3%) | 112 (68.3%) |
| Length of stay (d) | 18.0 ± 16.6 | 4.6 ± 3.6 |
| Surgical indication, n (%) | ||
| Elective | 32 (39.0%) | 130 (79.3%) |
| Trauma | 28 (34.1%) | 17 (10.4%) |
| Oncology | 12 (14.6%) | 14 (8.5%) |
| Infection | 10 (12.2%) | 3 (1.8%) |
| Surgical approach, n (%) | ||
| Posterior | 50 (61.0%) | 123 (75.0%) |
| Anterior | 13 (15.9%) | 36 (22.0%) |
| Combined | 19 (23.2%) | 5 (3.0%) |
| Comorbid trauma | 11 (13.4%) | 2 (1.2%) |
| Estimated blood loss (mL) | 812.3 ± 948.9 | 339.2 ± 444.0 |
| Iliac crest autograft, n (%) | 32 (39.0%) | 23 (14.0%) |
| Number of levels, n (%) | ||
| 1 | 3 (3.7%) | 20 (12.2%) |
| 2–3 | 41 (50.0%) | 97 (59.1%) |
| 4+ | 38 (46.3%) | 47 (28.7%) |
| Paralysis, n (%) | 19 (23.2%) | 2 (1.2%) |
| Staged surgery, n (%) | 14 (17.1%) | 1 (0.6%) |
Per our institution's protocol, sequential compression devices were started in all patients postoperatively. A form of additional VTE prophylaxis was started in 22 patients (25.9%) in the VTE group (prior to the VTE event) and 8 patients (4.9%) in the control group. Decisions regarding additional prophylaxis are made on a case-by-case basis by the treating teams based on assessed VTE risk. Prophylactic subcutaneous heparin was started in 5 patients (6.1%) in the VTE group compared with 4 patients (2.4%) in the control group. Prophylactic IVC filter was placed by interventional radiology in 14 patients (16.5%) in the VTE group compared with 1 patient (0.6%) in the control group. Warfarin was restarted for atrial fibrillation in 3 patients (3.5%) in the VTE group compared with 3 patients (1.8%) in the control group. In the VTE group following the VTE event, 6 patients (7.1%) were placed on subcutaneous heparin and 7 patients (8.2%) were placed on Coumadin. IVC filters were placed in 2 patients (2.4%) following an acute PE. Despite prophylactic IVC filter placement in 14 patients in the VTE group, 4 patients developed an acute PE. All 4 of these patients had a spinal cord injury. Of the 2 patients who had an IVC filter placed following a PE, 1 had a superior vena cava filter placed as well.
Univariate analysis of the risk factors revealed several significant predictors (Table 3). Staged surgery (OR 28.0) and paralysis (OR 19.0) were highly predictive of VTE. Nonelective surgical indications including infection (OR 18.5), trauma (OR 11.1), and oncologic procedures (OR 5.2) were all significant risk factors as well. In addition, comorbid traumatic injuries (OR >10) and length of stay greater than 5 days (OR 3.47) were correlated with VTE events. With regards to surgical characteristics, combined anterior-posterior approach (OR 7.46 in comparison to posterior alone), use of iliac crest autograft (OR 4.16), 4 or more operative levels (OR 4.84, compared with 1 operative level only), and estimated blood loss greater than 300 mL (OR 1.66) were all predictive of VTE. Estimated blood loss and length of stay were not included in the multivariate analysis secondary to insufficient data and an overlapping time period with the 30-day outcome window. Following multivariate analysis, three independent risk factors were identified for VTE: nonelective surgery (OR 6.29), paralysis (OR 7.86), and staged surgery (OR 35.7) (Table 4).
Table 3. Univariate analysis: risk factor for VTE after cervical spine surgery.
| Risk factor | Odds ratio | 95% CI | p Value |
|---|---|---|---|
| Staged surgery | 28.0a | 3.68–213 | 0.001 |
| Paralysis | 19.0a | 4.43–81.6 | <0.001 |
| Surgical indication | <0.001 | ||
| Infection versus elective | 18.5a | 3.39–101 | <0.001 |
| Trauma versus elective | 11.2a | 4.23–29.5 | 0.002 |
| Oncology versus elective | 5.2a | 1.79–15.1 | |
| Comorbid trauma | >10a | 9.0–∞b | <0.001 |
| Surgical approach | |||
| Combined versus posterior | 7.46a | 2.74–20.3 | <0.001 |
| Anterior versus posterior | 0.93 | 0.45–1.90 | 0.831 |
| Iliac crest autograft | 4.16a | 2.11–8.21 | <0.001 |
| Number of levels | |||
| 2–3 versus 1 | 2.7 | 0.75–9.49 | 0.129 |
| 4+ versus 1 | 4.84a | 1.35–17.4 | 0.016 |
| Estimated blood loss (300 mL or greater) | 1.66a | 1.65–1.66 | <0.001 |
| Length of stay (5 d or greater) | 3.47a | 3.15–3.81 | <0.001 |
Abbreviations: CI, confidence interval; VTE, venous thromboembolism.
Significant result.
Confidence interval generated using a profile likelihood approach.
Table 4. Multivariate analysis: risk factors for VTE after cervical spine surgery.
| Risk factor | Odds ratio | 95% CI | p Value |
|---|---|---|---|
| Staged surgery | 35.7a | 2.7–472 | 0.007 |
| Paralysis | 7.86a | 1.55–39.8 | 0.013 |
| Nonelective surgery | 6.29a | 2.51–15.8 | <0.001 |
Abbreviations: CI, confidence interval; VTE, venous thromboembolism.
Significant result.
Discussion
This study describes the 30-day VTE rate in a large series of 5,405 consecutive patients who underwent cervical spine surgery at a single institution between 1995 and 2012. Our VTE rate of 1.57% with a 1.02% DVT rate and 0.94% PE rate are consistent with reports in the literature. A recent review of thromboembolic disease in spine surgery highlighted the limited amount of literature on the subject. Glotzbecker et al utilized a systematic review to address the incidence of DVT or PE after spinal surgery.4 A total of 25 studies satisfied the inclusion criteria. Due to the diversity of the studies included, a formal meta-analysis could not be performed. However, the authors were able to determine that the overall VTE risk ranged from 0.3 to 31%.4 There are significant limitations in this study secondary to the heterogeneity of included studies, some of which involved select high-risk populations, did not report PE rates, looked exclusively at thoracolumbar surgery, or utilized screening surveillance methods.7 8 9 10 11 Other studies have reported DVT and PE rates of 3.7 and 2.2%, respectively.12 PE rates have been reported as high as 12%.5
With regards to cervical surgery, Epstein prospectively compared DVT rates in a limited group of patients undergoing anterior corpectomy (1%) versus multilevel anterior corpectomy and posterior fusion (7%).13 A recent review of the Nationwide Inpatient Sample (NIS) demonstrated a DVT rate of 0.27 to 1.34% and a PE rate of 0.10 to 0.63% in patients undergoing surgery for degenerative conditions of the cervical spine.14 This rate is consistent with the overall VTE rates reported in this study. Although this study shows a significant rate of VTE following cervical spine surgery, the NIS database is limited in that it provides no follow-up after discharge, is based entirely on ICD-9 codes, and is unable to determine specific patient- and procedure-related risk factors found in review of patient's medical records.
Although the overall incidence of VTE was low, several patient comorbidities were identified as risk factors for VTE following cervical spine surgery. Chronic venous insufficiency was identified as a significant risk factor for VTE, likely secondary to global venous dysfunction, which causes increased stasis of blood in the lower extremity veins.15 16 Obesity was also shown to be a risk factor for VTE, which is consistent with previous studies looking at lumbar spine surgery.17 Ischemic heart disease was also a predictor for VTE, which is expected because these patients are likely more prone to thrombosis and have additional medical comorbidities that increase their VTE risk.18 Interestingly, atrial fibrillation was also identified as a risk factor despite many of these patients being on chronic anticoagulation therapy. This finding is likely reflective as well of a patient population with a higher number of medical comorbidities but may also represent a temporary procoagulant state that occurs following resumption of warfarin.19 Smoking was found to be slightly protective in our study but is likely a clinically insignificant finding given previous evidence showing an increased risk for VTE in this patient population.20 21 22 Although these patient comorbidities identified as risk factors are probably unmodifiable, they may help guide risk stratification and identify patients who may benefit from increased surveillance or chemical prophylaxis.
Performing a matched cohort analysis allowed us to identify additional risk factors related to the surgical approach, surgical indication, and operative details. In general, we found the greater magnitude and morbidity of the procedure, the higher the risk for postoperative VTE. Thus, combined anterior-posterior surgery, use of iliac crest autograft, increased number of surgical levels, and increased estimated blood loss were all found to be significant predictors of VTE in the univariate analysis. Such surgeries would be anticipated to have increased operative times, longer periods of recumbency, and slower postoperative mobilization, increasing the risk of VTE.6 11 Similarly, comorbid traumatic injuries may lead to additional surgeries and more prolonged immobilization, increasing the risk of VTE.23 Increased length of stay was also identified as a risk factor for VTE. Certainly, caution should be applied in interpreting this result because increased length of stay may often be secondary to VTE rather than causative. Regardless, increased length of stay in the VTE cohort has important financial implications as it may raise hospital costs as much as twofold.14
Three independent risk factors for VTE were identified in this study. Staged surgery had a markedly increased risk (OR 35.7). Of the 15 cases in this cohort that underwent staged surgery, 6 operations were for traumatic injury, 4 were for oncologic resection, 2 were for infection, and 1 each for the treatment of cervical myelopathy, pseudarthrosis, and deformity. Of these 15 cases, all but 3 were done with a combined anterior-posterior surgical approach. This finding is interesting because it suggests that although a combined anterior-posterior approach is correlated with postoperative VTE, the added morbidity of staging the surgery is the significant independent risk factor. Previous studies have demonstrated higher VTE rates with combined approaches and staged surgery.6 7 8 9 Although only 1/15 patients who underwent staged surgery received prophylactic IVC filter placement, previous evidence suggests these patients may benefit from routine prophylaxis.7 24 Paralysis (OR 7.86) was also found to be an independent risk factor. Previous studies have demonstrated an increased rate of VTE in spinal cord injury patients with DVT rates as high as 38.6%.25 26 Nonelective surgery (OR 6.29) including traumatic, oncologic, and infectious indications was a predictor of VTE. Higher rates of VTE have been described for patients undergoing surgery for spinal trauma, even after excluding spinal cord injury.4 Malignancy is associated with a prothrombotic state that leads to an increase in VTE events.27
Although this study identifies several risk factors for VTE in patients undergoing cervical spine surgery, further studies are needed to determine which patients would benefit from screening surveillance, chemical VTE prophylaxis, or IVC filters. The benefits of intermittent pneumatic compression (IPC) devices, which were used in all patients in this cohort, have been well demonstrated in the literature. The effectiveness of IPC has been shown following both lumbar and cervical spine surgery for VTE prophylaxis.2 13 Epstein demonstrated that the 1 to 2% PE rate following anterior cervical surgery (with IPC prophylaxis) in their cohort was similar to rates reported following low-dose heparin prophylaxis regimens without the associated hemorrhage risk.13 Although spinal epidural hematoma is always a concern following the initiation of chemical VTE prophylaxis,28 29 30 there may be a role for more aggressive initiation of chemoprophylaxis in certain patient populations with close neurologic monitoring.31 Despite chemoprophylaxis in one series, no difference in VTE rate was seen, although these results may be subject to selection bias given the study's retrospective nature.31 A randomized trial by Kurtoglu et al showed no difference in VTE rates or mortality with postoperative chemical prophylaxis although other prospective studies have demonstrated a reduced VTE rate following chemical prophylaxis.32 33 The North American Spine Society clinical practice guidelines for antithrombotic therapies in spine surgery suggest that chemoprophylaxis may be warranted in complex cases, combined anterior-posterior approaches, spinal cord injury, or malignancy.34 IVC filters offer an alternative that may be effective in preventing PE in spinal surgery patients contraindicated for chemical prophylaxis, such as those undergoing staged surgery. Although previous studies have demonstrated the effectiveness of IVC filters, there are significant risks associated with implantation and removal.5 33 In addition, this study found four patients who developed acute PE despite prophylactic IVC filter placement. This outcome has been described in previous reports and can occur in the setting of an unrecognized upper extremity DVT, collateral venous channels, or a clot forming on the proximal portion of the filter.24 35 36 Regardless, there is little consensus regarding postoperative VTE prophylaxis for spinal surgery as evidenced by a recent study showing that the 63% of surgeons base their decisions for VTE prophylaxis on personal experience.37
This study does have several limitations. The VTE rate may be subject to underreporting. Given the large cohort of patients, ICD-9-CM codes were used to identify patients who suffered a VTE event, which may lead to errors related to miscoding. In addition, surveillance methods are not standardized, possibly leading to underdiagnosis of VTE events. Given the retrospective nature of the study, patients may have been lost to follow-up; this rate is estimated to be 6% in this cohort. In addition, the follow-up was limited to 30 days, which would miss patients with more delayed presentations of VTE. Although this study makes several observations regarding VTE prophylaxis, no clear recommendations can be made regarding prophylaxis or screening based on this data.
In conclusion, in a review of 5,405 cervical spine cases at a single institution, a VTE rate of 1.57% was established, with a DVT rate of 1.02% and PE rate of 0.94%. Several patient comorbidities were identified as risk factors including chronic venous insufficiency, atrial fibrillation, obesity, and ischemic heart disease. After a matched cohort analysis, several additional risk factors were identified including combined anterior-posterior approach, comorbid traumatic injuries, use of iliac crest autograft, number of surgical levels, increased blood loss, and increased length of stay. Three independent risk factors for VTE were identified including staged surgery, nonelective surgery, and paralysis. Clearly, there is a subset of patients at an increased risk for postoperative VTE following cervical spine surgery. Further studies are needed to determine if certain subsets may benefit from more aggressive VTE screening or prophylaxis.
Footnotes
Disclosures Arjun S. Sebastian, none Bradford L. Currier, Royalties: DePuy Spine, Stryker Spine, Zimmer Spine; Stockholder: Tenex, Spinology; Board member: Lumbar Spine Research Society; Advisory board: Zimmer Spine; Institutional fellowship support: AOSpine North America Michelle J. Clarke, none Dirk Larson, none Paul M. Huddleston III, Consulting: DePuy Synthes Spine; Fellowship support: AOSpine Ahmad Nassr, Grants: Cervical Spine Society, OREF, AOSpine North America; Fellowship support: AOSpine North America
Supplementary Material
References
- 1.Geerts W H Pineo G F Heit J A et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy Chest 2004126(3, Suppl):338S–400S. [DOI] [PubMed] [Google Scholar]
- 2.Epstein N E. Efficacy of pneumatic compression stocking prophylaxis in the prevention of deep venous thrombosis and pulmonary embolism following 139 lumbar laminectomies with instrumented fusions. J Spinal Disord Tech. 2006;19(1):28–31. doi: 10.1097/01.bsd.0000173454.71657.02. [DOI] [PubMed] [Google Scholar]
- 3.Catre M G. Anticoagulation in spinal surgery. A critical review of the literature. Can J Surg. 1997;40(6):413–419. [PMC free article] [PubMed] [Google Scholar]
- 4.Glotzbecker M P, Bono C M, Wood K B, Harris M B. Thromboembolic disease in spinal surgery: a systematic review. Spine (Phila Pa 1976) 2009;34(3):291–303. doi: 10.1097/BRS.0b013e318195601d. [DOI] [PubMed] [Google Scholar]
- 5.Rosner M K, Kuklo T R, Tawk R, Moquin R, Ondra S L. Prophylactic placement of an inferior vena cava filter in high-risk patients undergoing spinal reconstruction. Neurosurg Focus. 2004;17(4):E6. doi: 10.3171/foc.2004.17.4.6. [DOI] [PubMed] [Google Scholar]
- 6.Dearborn J T, Hu S S, Tribus C B, Bradford D S. Thromboembolic complications after major thoracolumbar spine surgery. Spine (Phila Pa 1976) 1999;24(14):1471–1476. doi: 10.1097/00007632-199907150-00013. [DOI] [PubMed] [Google Scholar]
- 7.Leon L, Rodriguez H, Tawk R G, Ondra S L, Labropoulos N, Morasch M D. The prophylactic use of inferior vena cava filters in patients undergoing high-risk spinal surgery. Ann Vasc Surg. 2005;19(3):442–447. doi: 10.1007/s10016-005-0025-1. [DOI] [PubMed] [Google Scholar]
- 8.Ferree B A, Stern P J, Jolson R S, Roberts J M V, Kahn A III. Deep venous thrombosis after spinal surgery. Spine (Phila Pa 1976) 1993;18(3):315–319. doi: 10.1097/00007632-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Ferree B A, Wright A M. Deep venous thrombosis following posterior lumbar spinal surgery. Spine (Phila Pa 1976) 1993;18(8):1079–1082. doi: 10.1097/00007632-199306150-00019. [DOI] [PubMed] [Google Scholar]
- 10.Oda T, Fuji T, Kato Y, Fujita S, Kanemitsu N. Deep venous thrombosis after posterior spinal surgery. Spine (Phila Pa 1976) 2000;25(22):2962–2967. doi: 10.1097/00007632-200011150-00019. [DOI] [PubMed] [Google Scholar]
- 11.Rokito S E Schwartz M C Neuwirth M G Deep vein thrombosis after major reconstructive spinal surgery Spine (Phila Pa 1976) 1996217853–858., discussion 859 [DOI] [PubMed] [Google Scholar]
- 12.Agnelli G. Prevention of venous thromboembolism in surgical patients. Circulation. 2004;110(24) 01:IV4–IV12. doi: 10.1161/01.CIR.0000150639.98514.6c. [DOI] [PubMed] [Google Scholar]
- 13.Epstein N E. Intermittent pneumatic compression stocking prophylaxis against deep venous thrombosis in anterior cervical spinal surgery: a prospective efficacy study in 200 patients and literature review. Spine (Phila Pa 1976) 2005;30(22):2538–2543. doi: 10.1097/01.brs.0000186318.80139.40. [DOI] [PubMed] [Google Scholar]
- 14.Oglesby M, Fineberg S J, Patel A A, Pelton M A, Singh K. The incidence and mortality of thromboembolic events in cervical spine surgery. Spine (Phila Pa 1976) 2013;38(9):E521–E527. doi: 10.1097/BRS.0b013e3182897839. [DOI] [PubMed] [Google Scholar]
- 15.Kanth A M, Khan S U, Gasparis A, Labropoulos N. The distribution and extent of reflux and obstruction in patients with active venous ulceration. Phlebology. 2015;30(5):350–356. doi: 10.1177/0268355514530277. [DOI] [PubMed] [Google Scholar]
- 16.Obermayer A, Garzon K. Identifying the source of superficial reflux in venous leg ulcers using duplex ultrasound. J Vasc Surg. 2010;52(5):1255–1261. doi: 10.1016/j.jvs.2010.06.073. [DOI] [PubMed] [Google Scholar]
- 17.Marquez-Lara A, Nandyala S V, Sankaranarayanan S, Noureldin M, Singh K. Body mass index as a predictor of complications and mortality after lumbar spine surgery. Spine (Phila Pa 1976) 2014;39(10):798–804. doi: 10.1097/BRS.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 18.Sørensen H T, Horvath-Puho E, Lash T L. et al. Heart disease may be a risk factor for pulmonary embolism without peripheral deep venous thrombosis. Circulation. 2011;124(13):1435–1441. doi: 10.1161/CIRCULATIONAHA.111.025627. [DOI] [PubMed] [Google Scholar]
- 19.Ansell J Hirsh J Dalen J et al. Managing oral anticoagulant therapy Chest 2001119(1, Suppl):22S–38S. [DOI] [PubMed] [Google Scholar]
- 20.Goldhaber S Z, Grodstein F, Stampfer M J. et al. A prospective study of risk factors for pulmonary embolism in women. JAMA. 1997;277(8):642–645. [PubMed] [Google Scholar]
- 21.Hansson P O, Eriksson H, Welin L, Svärdsudd K, Wilhelmsen L. Smoking and abdominal obesity: risk factors for venous thromboembolism among middle-aged men: “the study of men born in 1913.”. Arch Intern Med. 1999;159(16):1886–1890. doi: 10.1001/archinte.159.16.1886. [DOI] [PubMed] [Google Scholar]
- 22.Powell J T. Vascular damage from smoking: disease mechanisms at the arterial wall. Vasc Med. 1998;3(1):21–28. doi: 10.1177/1358836X9800300105. [DOI] [PubMed] [Google Scholar]
- 23.Ho K M, Burrell M, Rao S, Baker R. Incidence and risk factors for fatal pulmonary embolism after major trauma: a nested cohort study. Br J Anaesth. 2010;105(5):596–602. doi: 10.1093/bja/aeq254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McClendon J Jr, Oʼshaughnessy B A, Smith T R. et al. Comprehensive assessment of prophylactic preoperative inferior vena cava filters for major spinal reconstruction in adults. Spine (Phila Pa 1976) 2012;37(13):1122–1129. doi: 10.1097/BRS.0b013e31824abde2. [DOI] [PubMed] [Google Scholar]
- 25.Chung S B Lee S H Kim E S Eoh W Incidence of deep vein thrombosis after spinal cord injury: a prospective study in 37 consecutive patients with traumatic or nontraumatic spinal cord injury treated by mechanical prophylaxis J Trauma 2011714867–870., discussion 870–871 [DOI] [PubMed] [Google Scholar]
- 26.Furlan J C, Fehlings M G. Role of screening tests for deep venous thrombosis in asymptomatic adults with acute spinal cord injury: an evidence-based analysis. Spine (Phila Pa 1976) 2007;32(17):1908–1916. doi: 10.1097/BRS.0b013e31811ec26a. [DOI] [PubMed] [Google Scholar]
- 27.Lin P P, Graham D, Hann L E, Boland P J, Healey J H. Deep venous thrombosis after orthopedic surgery in adult cancer patients. J Surg Oncol. 1998;68(1):41–47. doi: 10.1002/(sici)1096-9098(199805)68:1<41::aid-jso9>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 28.Awad J N, Kebaish K M, Donigan J, Cohen D B, Kostuik J P. Analysis of the risk factors for the development of post-operative spinal epidural haematoma. J Bone Joint Surg Br. 2005;87(9):1248–1252. doi: 10.1302/0301-620X.87B9.16518. [DOI] [PubMed] [Google Scholar]
- 29.Kou J, Fischgrund J, Biddinger A, Herkowitz H. Risk factors for spinal epidural hematoma after spinal surgery. Spine (Phila Pa 1976) 2002;27(15):1670–1673. doi: 10.1097/00007632-200208010-00016. [DOI] [PubMed] [Google Scholar]
- 30.Yi S, Yoon D H, Kim K N, Kim S H, Shin H C. Postoperative spinal epidural hematoma: risk factor and clinical outcome. Yonsei Med J. 2006;47(3):326–332. doi: 10.3349/ymj.2006.47.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunningham J E, Swamy G, Thomas K C. Does preoperative DVT chemoprophylaxis in spinal surgery affect the incidence of thromboembolic complications and spinal epidural hematomas? J Spinal Disord Tech. 2011;24(4):E31–E34. doi: 10.1097/BSD.0b013e3181f605ea. [DOI] [PubMed] [Google Scholar]
- 32.Kurtoglu M, Yanar H, Bilsel Y. et al. Venous thromboembolism prophylaxis after head and spinal trauma: intermittent pneumatic compression devices versus low molecular weight heparin. World J Surg. 2004;28(8):807–811. doi: 10.1007/s00268-004-7295-6. [DOI] [PubMed] [Google Scholar]
- 33.Papadimitriou K, Amin A G, Kretzer R M. et al. Thromboembolic events and spinal surgery. J Clin Neurosci. 2012;19(12):1617–1621. doi: 10.1016/j.jocn.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 34.Bono C M, Watters W C III, Heggeness M H. et al. An evidence-based clinical guideline for the use of antithrombotic therapies in spine surgery. Spine J. 2009;9(12):1046–1051. doi: 10.1016/j.spinee.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Cherian J, Gertner E. Recurrent pulmonary embolism despite inferior vena cava filter placement in patients with the antiphospholipid syndrome. J Clin Rheumatol. 2005;11(1):56–58. doi: 10.1097/01.rhu.0000152150.01274.1b. [DOI] [PubMed] [Google Scholar]
- 36.Urban M K, Jules-Elysee K, MacKenzie C R. Pulmonary embolism after IVC filter. HSS J. 2008;4(1):74–75. doi: 10.1007/s11420-007-9071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glotzbecker M P, Bono C M, Harris M B, Brick G, Heary R F, Wood K B. Surgeon practices regarding postoperative thromboembolic prophylaxis after high-risk spinal surgery. Spine (Phila Pa 1976) 2008;33(26):2915–2921. doi: 10.1097/BRS.0b013e318190702a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


