Abstract
Despite the evidence for a significant contribution of brainstem serotonergic (5HT) systems to the control of spinal cord “pain” transmission neurons, attention has turned recently to the influence of nonserotonergic neurons, including the facilitatory and inhibitory controls that originate from so-called “on” and “off” cells of the rostroventral medulla (RVM). Unclear, however, is the extent to which these latter circuits interact with or are influenced by the serotonergic cell groups. To address this question we selectively targeted expression of a transneuronal tracer, wheat germ agglutinin (WGA), in the 5HT neurons so as to study the interplay between the 5HT and non-5HT systems. In addition to confirming the direct medullary 5HT projection to the spinal cord we also observed large numbers of non-5HT neurons, in the medullary nucleus reticularis gigantocellularis and magnocellularis, that were WGA-immunoreactive, i.e., were transneuronally labeled from 5HT neurons. Fluoro-Gold injections into the spinal cord established that these reticular neurons are not only postsynaptic to the 5HT neurons of the medulla, but that most are also at the origin of descending, bulbospinal pathways. By contrast, we found no evidence that neurons of the midbrain periaqueductal gray that project to the RVM are postsynaptic to midbrain or medullary 5HT neurons. Finally, we found very few examples of WGA-immunoreactive noradrenergic neurons, which suggests that there is considerable independence of the monoaminergic bulbospinal pathways. Our results indicate that 5HT neurons influence “pain” processing at the spinal cord level both directly and indirectly via feedforward connections with multiple non-5HT descending control pathways.
Indexing terms: WGA, pain, serotonin, brainstem, analgesia, RVM
Until recently, studies of the bulbospinal systems that originate from the rostral ventral medulla (RVM) emphasized their contribution to inhibitory control of “pain” transmission. Most important, opioid injection into or electrical stimulation of the midbrain periaqueductal gray (PAG) activates these descending pathways and concurrently produces a profound antinociceptive action, including inhibition of the firing of dorsal horn nociresponsive neurons and behavioral analgesia (Basbaum and Fields, 1984). Although neurochemically distinct pathways arise from the RVM, considerable evidence pointed to the contribution of medullary serotonergic neurons. For example, morphine evokes the release of serotonin at the level of the spinal cord and RVM (Matos et al., 1992; Taylor and Basbaum, 2003) and serotonin depletion using pharmacological or ablative procedures blocks the analgesia induced by systemic administration of morphine (Vogt, 1974; Proudfit and Anderson, 1975; Yaksh et al., 1977).
In agreement with these findings, Zhao et al. (2007a,b) reported that inflammatory pain is enhanced and opioid analgesia is severely compromised in mice lacking serotonergic neurons. Interestingly, these mice were less sensitive to mechanical stimuli, indicating that there are bidirectional serotonergic controls upon nociceptive processing. Comparable conclusions were made from studies in the rat (Porreca et al., 2002; Suzuki et al., 2004b). For example, although intrathecal injection of relatively non-selective 5HT receptor antagonists reduces the analgesia induced by chemical or electrical stimulation of the RVM (Hammond and Yaksh, 1984; Jensen and Yaksh, 1984; Barbaro et al., 1985), studies using more selective antagonists demonstrated that descending 5HT systems can exert both inhibitory or facilitatory actions on nociceptive processing via the 5HT1A and 1B/D receptors or the 5HT1A, 2A, and 3 receptors, respectively (Green et al., 2000; Zeitz et al., 2002; Sasaki et al., 2006).
Electrophysiological classification of RVM neurons into so-called “on” (i.e., pain facilitatory) and “off” (pain inhibitory) cell groups surprisingly did not include 5HT neurons (Potrebic et al., 1994; Mason, 1997; Gao and Mason, 2000). Rather, serotonergic neurons constitute a heterogeneous population, with slow, regular discharge patterns and variable responses to noxious stimuli and to opioid agonists (Gao and Mason, 2001; Zhang et al., 2006). Indeed, Gao et al. (1998) concluded that neither 5HT nor activity of serotonergic cells is required for the analgesia evoked by opioids. Taken together, these results suggest that there is a serotonergic regulation of pain control mechanisms, but that these controls can occur in the absence of 5HT (Jacobs and Azmitia, 1992). If anything, these findings argue against a direct involvement of 5HT neurons in pain control. Hence the question: how independent is the 5HT system from the circuits through which “on” and “off” cells regulate spinal cord “pain” transmission neurons?
In the present study we used a genetic transneuronal tracing system (Braz et al., 2002, 2005) to examine some of the pain-relevant neuronal networks engaged by 5HT neurons in the mouse. These studies utilize a transgenic mouse, referred to as the ZW mouse, in which expression of the anterograde/transneuronal tracer wheat germ agglutinin (WGA) can be induced, after Cre recombination, in defined subpopulations of neurons of the central nervous system (CNS). Here, we selectively triggered expression of WGA in 5HT neurons of the brainstem raphe. Using this approach we could not only map the projections of 5HT neurons, but we also identified many of the neurons that lie downstream of the 5HT populations of brainstem raphe neurons. We provide evidence that in the mouse parallel, but interconnected serotonergic and nonserotonergic descending pathways arise from the 5HT neurons of the rostral ventral medulla (RVM) and that the 5HT neurons are critical integrators of these outputs. We also provide new information on the interconnections of the RVM and the midbrain periaqueductal gray.
MATERIALS AND METHODS
Animals
All experiments were reviewed and approved by the Institutional Care and Animal Use Committee at the University of California San Francisco. We generated double transgenic ePet-ZW mice in which transneuronal anterograde transport of the tracer WGA can be triggered in brainstem and midbrain neurons that express serotonin. These mice were generated by crossing our ZW line (Braz et al., 2002) with mice that express Cre recombinase under the control of the ePet-1 promoter (Scott et al., 2005). To determine the Cre expression pattern in the ePet-Cre mouse, we crossed the ePet-Cre mice with the ROSA26 Cre reporter mice (Soriano, 1999) in which expression of β-galactosidase (β-gal) is induced in neurons where Cre recombination occurs.
Fluorogold injections
Four-week-old ePet-ZW animals were anesthetized by intraperitoneal injection of ketamine (60 mg/kg)/xylazine (8 mg/kg) and placed in a stereotaxic instrument. Following incision of the skin we used a dental drill to make a small midline burr hole over the cerebellum. To target the dorsal raphe and nucleus raphe magnus we inserted a micropipette, attached to a manual microinjector (Sutter Instrument, Novato, CA), to a depth of 3 or 6 mm, respectively, below the skull. We made a single injection of FluoroGold (1 μL of a 2% solution). The micropipette was kept in place for an additional 2 minutes, then withdrawn. Once injections were complete the scalp was sutured and the mice were maintained under a warming lamp until they recovered from the anesthesia, after which they were returned to standard housing.
Immunohistochemistry
Antibodies used were polyclonal rabbit anti-WGA (1: 50,000 for fluorescence or 1:200,000 for DAB, Sigma, St. Louis, MO, #T4144), mouse anti-tyrosine hydroxylase (1: 5000, RBI, Natick, MA, #T-186), rat anti-5HT (1:500, Protos Biotech, New York, NY, #NT 101) and rabbit anti-β-gal (1:10,000, Cappell, Malvern, PA, #55976). The antibody characteristics are described in the manufacturer’s information sheets. Anti-WGA antibodies were raised in rabbit using purified WGA as the immunogen. Identity and purity of the specific antibody was established by immunoelectrophoresis. Our own studies have demonstrated that the anti-WGA shows no immunostaining in wildtype mice (i.e., in mice that do not express the WGA transgene). Anti-TH antibodies were raised using rat TH as the immunogen. This antibody recognizes an epitope present in the N-terminal region (between amino acids 9–16) of both rodent (≈60 kD) and human (62–68 kD) TH. In Western blots of PC-12 rat pheochromocytoma cells the anti-TH antibody detects a single band at 60 kD. Anti-5HT antibodies were raised in rats using serotonin conjugated to hemocyanin as immunogen. The patterns of 5HT and TH-immunoreactivity that we observed with these antisera are very comparable to those reported in many other studies of the distribution of 5HT and TH in the mouse and rat brain (Dahlström and Fuxe, 1964; Beitz, 1982; Vander-Horst and Ulfhake, 2006). Anti-β-gal antibodies were produced by hyperimmunizing rabbits with the enzyme β-gal from Escherichia coli. Our studies have established that there is no β-gal immunoreactivity in wildtype mice (i.e., in mice that do not express the lacZ transgene).
Because the detection of WGA in postsynaptic neurons is time-dependent (the tracer has to be expressed, accumulated in vesicles, transported, released, and taken up by postsynaptic neurons), we studied ePet-ZW animals at different ages. Thus, to localize the WGA we anesthetized ePet-ZW mice at 3, 6, and 11 weeks of age (Nembutal; 100 mg/kg) and then perfused them transcardially with 10 mL of saline (0.9% NaCl) followed by 30 mL of 10% formaldehyde in phosphate buffer (PB) 0.1 M, pH 7.4, at room temperature (RT). Tissues were dissected out, postfixed in the same solution for 3 hours, and cryoprotected in 30% sucrose phosphate-buffered saline (PBS) overnight at 4°C. Twenty (spinal cord) and 40 μm (brain and brainstem) cryostat sections were preincubated for 30 minutes at RT in PBS containing 0.5% Triton X-100 and 10% normal goat serum (NPBST) and then immunostained overnight at RT in the same buffer containing the polyclonal anti-WGA antibody. After washing in NPBST, sections were incubated for 1 hour with an Alexa-conjugated antirabbit IgG secondary antibody (1:700), rinsed in NPBST, mounted in Fluoromount-G (Southern Biotechnology, Birmingham, AL) and coverslipped. Sections were viewed with a Nikon Eclipse fluorescence microscope and images were collected with a Spot Camera. Brightness and contrast were adjusted using Adobe Photoshop, v. 6.0 (San Jose, CA). Magenta-green copies of Figures 2, 5, 7, 8, and 10 are available as supplementary figures.
RESULTS
WGA expression in 5HT neurons (first-order neurons)
In this study we induced expression of the transgene by crossing ZW mice with mice in which Cre recombinase is driven off of the ePet-1 promoter (ePet-Cre), a transcription factor that defines 5HT neurons (Scott et al., 2005). In double transgenic ePet-ZW mice, Cre-mediated excision of the floxed-LacZ cDNA results in WGA induction in 5HT neurons only. To identify the neurons that synthesize the tracer (referred to as first-order neurons), we double-labeled sections for WGA and 5HT in ePet-ZW mice. As expected, serotonergic neurons in all raphe nuclei of the brainstem contain WGA-positive neurons (Fig. 1). Double-labeled neurons were particularly numerous in the dorsal and median raphe. Most were intensely labeled, consistent with these being first-order neurons that express the transgene.
Fig. 1.
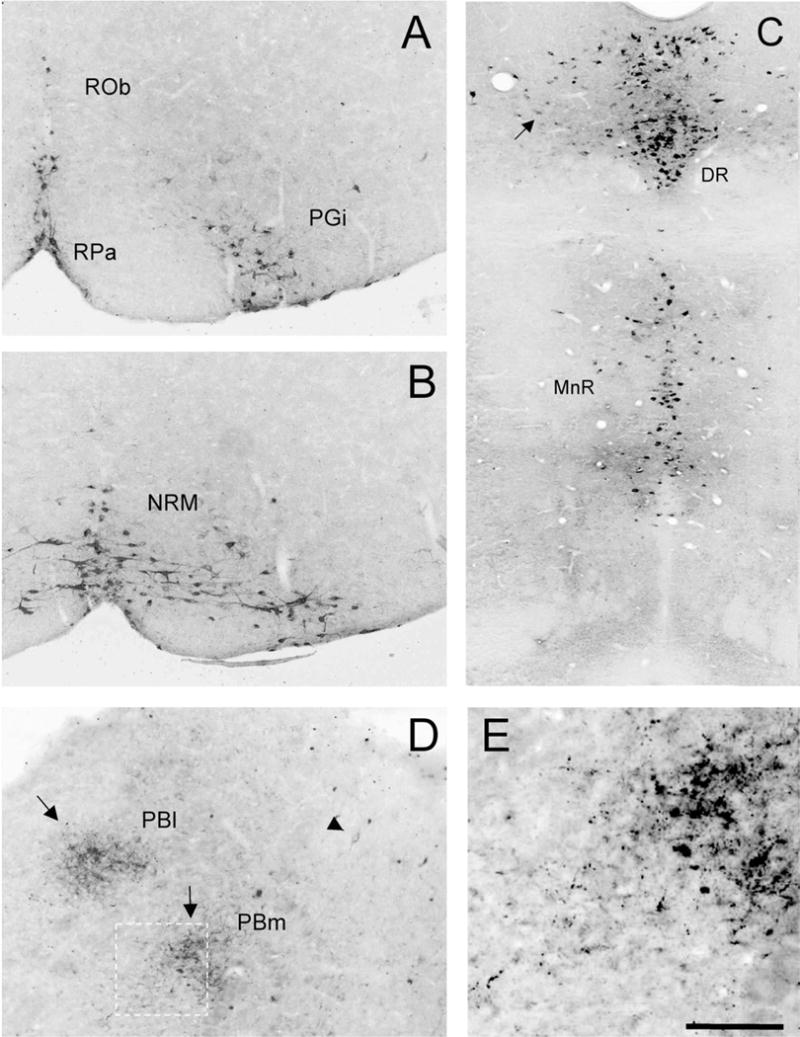
WGA expression in 5HT-immunoreactive raphe neurons in ePet-ZW mice. Cre-mediated excision of the floxed lacZ cDNA initiates WGA expression in 5HT-expressing neurons of the brainstem and midbrain. All raphe nuclei contain the WGA tracer (black): (A) raphe obscurus (Rob), raphe pallidus (RPa), and nucleus reticularis paragigantocellularis (PGi); (B) Raphe magnus (NRM); (C) Midbrain dorsal (DR) and median raphe (MnR). There is dense accumulation of transported WGA in presumptive terminals (arrows) in the lateral (Pbl) and medial (Pbm) parabrachial nuclei (D,E). E: Higher magnification of the boxed area in D. Arrowhead in D points to a transneuronally labeled neuron in the PB. Scale bars = 100 μm in A,B,D,E; 200 μm in C.
As there are very few studies of 5HT neuronal distribution in the mouse (see VanderHorst and Ulfhake, 2006), we begin by describing the overall pattern of WGA labeling that we observed. At the level of the PAG, WGA-positive neurons were most extensively distributed in the dorsal raphe (DR). In addition to the dense dorsal and ventral clusters of neurons along the midline (corresponding to area B7 of Dahlstrom and Fuxe, 1964), we observed scattered WGA-immunoreactive neurons laterally (Fig. 1C, arrow). Ventral to the DR, the WGA-positive neurons were concentrated in the median raphe (MnR or area B8; Fig. 1C) and laterally, in the reticular formation (area B9; data not shown), in a region extending from the rostral border of the trigeminal motor nucleus to the caudal pole of the red nucleus. At pontine levels the WGA neurons were also concentrated near the midline, in the caudal DR (area B6; data not shown), and more ventrally in the raphe pontis (area B5). At medullary levels the WGA-immunoreactive neurons were present in area B3, corresponding to the nucleus raphe magnus (NRM), and in its lateral extension, the nucleus reticularis paragigantocellularis (PGi; Fig. 1A,B), as well as in area B1 (the raphe pallidus; RPa, Fig. 1A). Finally, we observed extensive labeling in neurons of the most caudal raphe obscurus (ROb), i.e., B2 (Fig. 1A).
In addition to neuronal cell bodies, we observed labeled fibers throughout the CNS. These likely arose from anterograde transport of WGA in the 5HT neurons, i.e., in the neurons that expressed the transgene. Although we expected that longer survival times (11 vs. 3 weeks) would reveal more extensive WGA patterns, this was not the case. The labeling of terminals appeared as intensely immunoreactive dots, which presumably correspond to synaptic terminals or boutons en passant. These were particularly notable in the septum, the hippocampus, and in the nucleus accumbens (data not shown). We also found abundant terminals in the parabrachial (Fig. 1D,E) and trigeminal nuclei of the brainstem. Finally, there was extensive axonal labeling in the white and gray matter of the spinal cord.
Postsynaptic targets of 5HT neurons (second- and higher-order neurons)
Not all 5HT neurons immunostained for WGA, presumably because there is mosaic expression of the transgene (Braz et al., 2002). More important, perhaps, we detected many WGA-immunoreactive raphe neurons that did not immunostain for 5HT (arrows in Fig. 2). Because the WGA tracer is synthesized only in 5HT neurons, (i.e., where the Cre-recombination event occurred), its detection in a non-5HT neuron can only have resulted from transneuronal transfer of the WGA from the 5HT (first order) neuron to the second order, non-5HT neuron. WGA-immunoreactive, non-5HT neurons are thus postsynaptic to the 5HT neurons. The majority of these WGA-non-5HT neurons were found at the level of, but mostly lateral to, the 5HT neurons of the DR and MnR. Other single-labeled WGA neurons intermingled with 5HT neurons located in the region of the lateral lemniscus of the rostral pons and caudal midbrain (B9). From this pattern of labeling we conclude that the tracer was transneuronally transferred from 5HT (first-order neuron, primary site of expression) to non-5HT neurons (after release from first-order neurons) both within the midbrain raphe nuclei and beyond them.
Fig. 2.
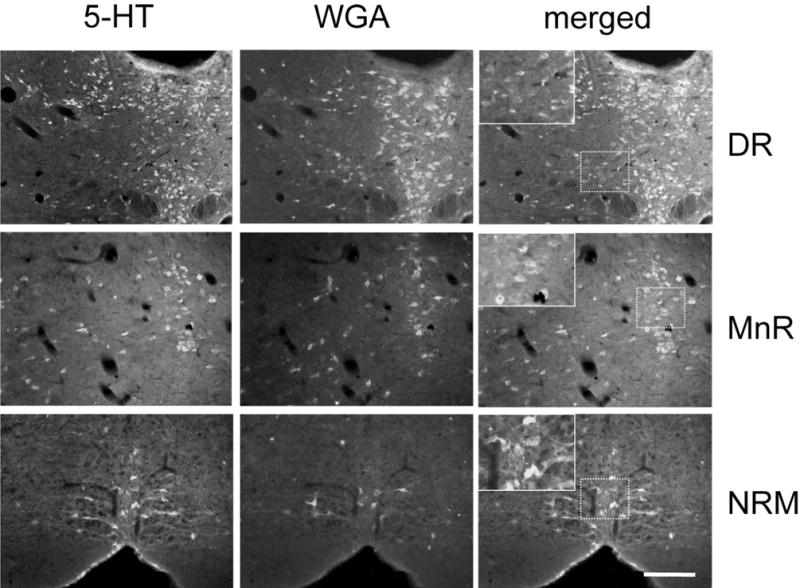
Intermingled populations of 5HT and non-5HT raphe neurons contain the WGA tracer in ePet-ZW mice. Double labeling for serotonin (5HT, green, column 1) and WGA (red, column 2) illustrates the transneuronal transfer of WGA from 5HT to non-5HT neurons in the dorsal (DR), and median raphe (MnR) and in the nucleus raphe magnus (NRM). Inset: high magnification of the boxed area showing single-labeled neurons that correspond to neurons that are postsynaptic to 5HT neurons. A magenta-green version of this figure is available as a supplementary figure online. Scale bar = 100 μm.
The medullary raphe nuclei, in particular, the nucleus raphe magnus of the RVM, also contained a mixed population of fusiform 5HT- and non-5HT WGA-positive neurons (Fig. 2). These were located along the midline and in the PGi. In addition, we found many single-labeled WGA-immunoreactive neurons outside the borders of the NRM. Using nomenclature that we adopted in our earlier tracing studies in cat and rat (Basbaum et al., 1978; Basbaum and Fields, 1979) these correspond to the more dorsally located nucleus reticularis gigantocellularis (RGc, Fig. 3B) and the nucleus reticularis magnocellularis (RMc, Fig. 3A), which is located ventral to RGc. Single-labeled, WGA-immunoreactive neurons were found in a region ≈ 1,400μm in length, extending from the caudal NRM to the caudal pole of the trigeminal motor nucleus (Fig. 4). We also consistently detected transneuronally labeled neurons in the nucleus of the trapezoid body (data not shown).
Fig. 3.
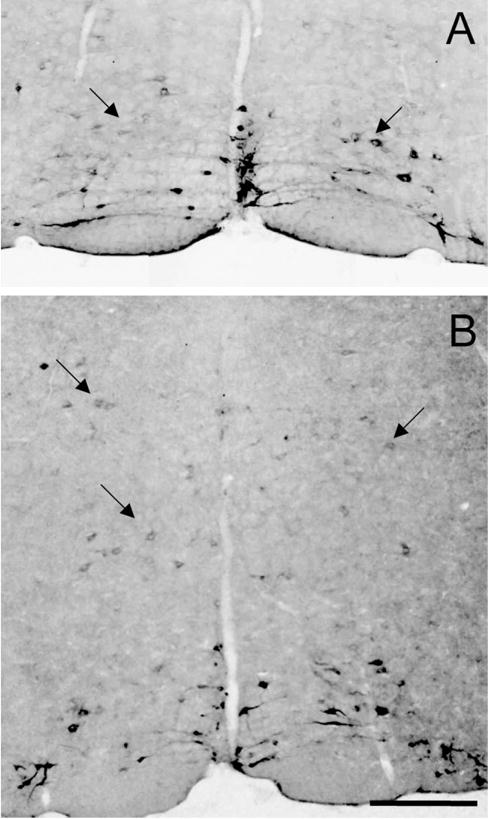
Transneuronal transfer of WGA to medullary reticular neurons in ePet-ZW mice. Outside the boundaries of the midline raphe nuclei many non-5HT neurons contain the WGA tracer (black). These were located dorsal and lateral to the raphe magnus, in nucleus reticularis magnocellularis (A) and in the nucleus reticularis gigantocellularis (B). Scale bar 100 μm.
Fig. 4.
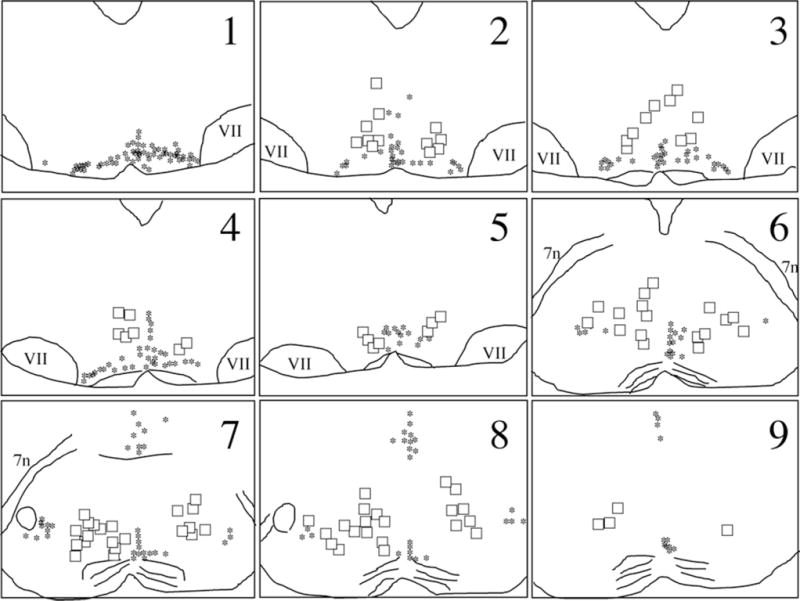
Distribution of postsynaptic, WGA-labeled neurons in nucleus reticularis magnocellularis and nucleus reticularis gigantocellularis. Single WGA-labeled neurons (black asterisks) were detected throughout the brainstem, in a region, ≈ 1,400 μm in length, extending from the caudal raphe magnus (1) to the caudal pole of the trigeminal motor nucleus (9). The neurons were located lateral and dorsal to the 5HT/WGA double-labeled neurons (squares) of the raphe magnus. VII: seventh nucleus; 7n: seventh nerve.
To conclude that the pattern of labeling in these brainstem areas indeed resulted from transneuronal transfer of the lectin from 5HT neurons, it is essential to show that synthesis of the WGA only occurs in serotonergic neurons, i.e., after Cre-recombination. To this end we crossed the ePet-Cre mice with the ROSA26 Cre reporter mouse (Soriano, 1999), in which the enzyme β-gal is expressed in neurons only after Cre recombination. In these mice we only found β-gal immunostaining in 5HT-immunoreactive neurons (red and green, respectively, Fig. 5). Note that not all 5HT neurons express β-gal, presumably because there is some mosaicism in the ROSA26 Cre reporter mouse. Furthermore, and in agreement with Scott et al. (2005), there was absolutely no β-gal immunoreactivity in the forebrain, which shows that there is no ectopic expression of Cre in the ePet-Cre animals. Based on this analysis, we conclude that any WGA immunoreactivity detected in non-5HT neurons in the ePet-ZW mice resulted from transneuronal transfer of the lectin after its synthesis in and transport by the 5HT neurons.
Fig. 5.
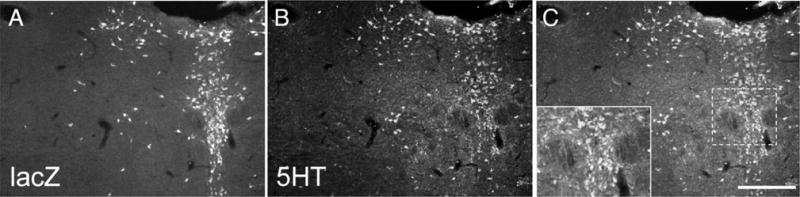
Cre recombinase expression pattern in ePet-Cre mice. To determine whether or not the Cre recombinase is expressed exclusively in 5HT neurons we crossed the ePet-Cre mice with the ROSA26 Cre reporter mice, in which expression of β-galactosidase (β-gal) is induced in Cre-expressing neurons. Double labeling for β-gal (red in A) and 5HT (green in B) illustrates that all β-gal-positive neurons are 5HT-immunoreactive (yellow in C). Inset: high magnification of the boxed area showing double-labeled neurons. Note that not all 5HT neurons express β-gal, presumably because there is some mosaicism in the ROSA26 Cre reporter mouse. A magenta-green version of this figure is available as a supplementary figure online. Scale bar = 100 μm.
Tyramide signal amplification (TSA)
Clearly the ability to detect transneuronal label depends on the amount of tracer that is synthesized and transported as well as the sensitivity of the detection method. In fact, what was a rather restricted distribution of transneuronal label changed considerably when we used a TSA amplification method to enhance the WGA labeling. For example, we found transneuronally labeled-neuronal cell bodies in the nucleus accumbens, hippocampus, and parabrachial nuclei (data not shown). TSA amplification also significantly increased the terminal labeling at all levels of the spinal cord (Fig. 6), in white matter, and in all laminae of the gray matter. Importantly, hemisection of the spinal cord at midthoracic levels not only eliminated the labeling ipsilateral and caudal to the lesion, but also resulted in buildup of WGA immunoreactivity just proximal to the lesion (data not shown). The latter experiment established that the labeling indeed arose from transport of the tracer, presumably from neurons of the RVM. Surprisingly and somewhat disappointingly, we found very limited cell body labeling in the spinal cord. We presume that dilution of the tracer after its transneuronal transport reduces the likelihood of its detection in postsynaptic (spinal cord) neurons of the 5HT circuitry.
Fig. 6.
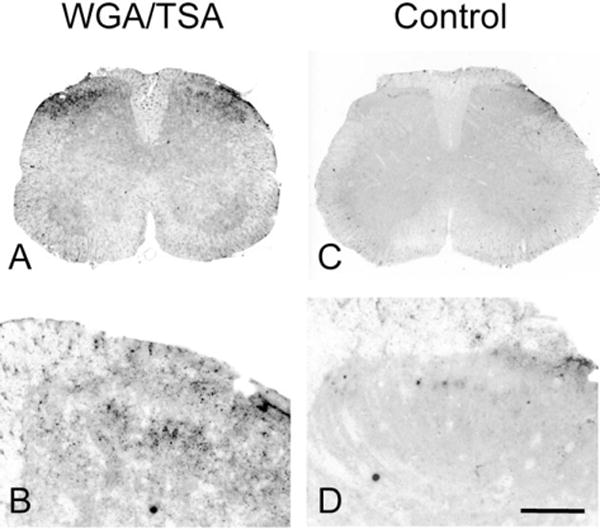
Transneuronal transport of WGA in the spinal cord of ePet-ZW mice. Tyramide signal amplification of immunoreactive WGA labeling reveals the transport of the WGA tracer to terminals in superficial laminae of the dorsal horn of the spinal cord. There is no labeling in control experiments, where the primary antibody was omitted (column 2). Scale bars = 500 μm in A,C; 150 μm in B,D.
Targets of the transneuronally labeled neurons
Previous studies demonstrated that many neurons of the RGc and RMc project to the spinal cord, to the ventral and dorsal horn, respectively (Basbaum et al., 1978). Other reticular neurons project rostrally (Vertes, 1991) and, of course, there are many local circuit neurons (for review, see Fields et al., 1991). To identify the target of the medullary neurons that we found to be postsynaptic to the 5HT neurons, we injected FluoroGold (FG) into the cervical spinal cord of ePet-ZW animals. As our objective was to maximize the number of retrogradely labeled medullary neurons, we did not attempt to make selective injections into the dorsal or ventral horns (Fig. 7A).
Fig. 7.
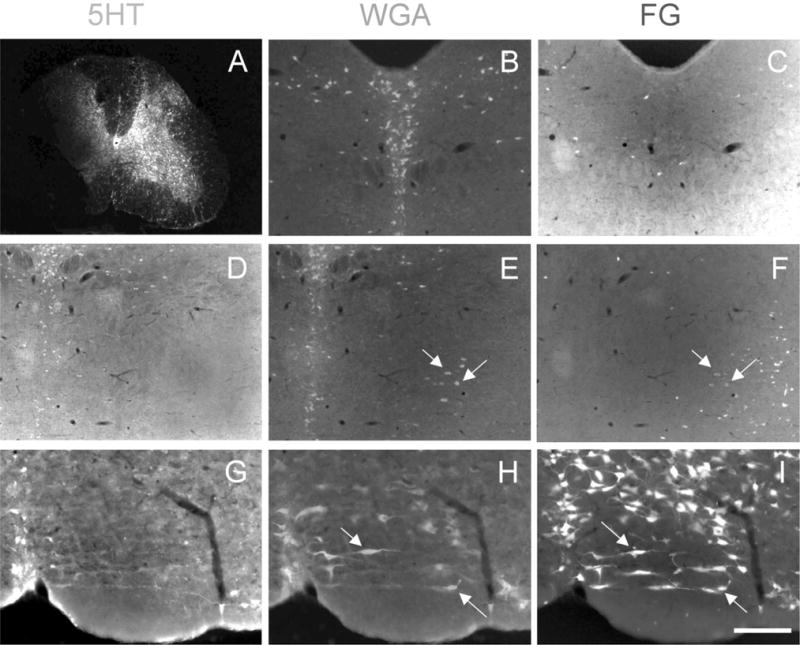
Non-5HT neurons that project to the spinal cord are postsynaptic to medullary 5HT neurons. FluoroGold injection in the spinal cord of ePet-ZW mice (A) retrogradely labels a heterogeneous population of 5HT (green) and non-5HT/WGA (red) neurons throughout the brainstem (FG-positive neurons are white). 5HT neurons in the midbrain dorsal raphe (B,C) and median raphe (D–F) do not project to the spinal cord. In contrast, the medullary raphe nuclei (notably the raphe magnus; G–I) contain large numbers of 5HT neurons that project to the spinal cord (G–I). Arrows point to single-labeled WGA neurons that project to the spinal cord, but are not 5HT. These lie downstream of the “primary” 5HT neurons. A magentagreen version of this figure is available as a supplementary figure online. Scale bars = 600 μm in A; 100 μm in B–I.
Figure 7 illustrates the results obtained in one of the four animals that we studied. As expected, we recorded large numbers of retrogradely labeled neurons throughout the brainstem, with the densest labeling within or near the RVM. The majority of FG-labeled neurons were found ipsilateral to the injection site. More important, we found a large overlap in the distribution of spinal cord-projecting (FG-positive) and WGA-immunoreactive neurons, indicating that many of the neurons that are targeted by the 5HT neurons project to the spinal cord. These cells were found in all medullary raphe nuclei, in the RVM at the level of the VIIth nucleus, as well as in the more dorsally located RGc. Not surprisingly, all of these regions, but especially the PGi, contained neurons triple-labeled for FG, WGA, and 5-HT (Fig. 7G–I). These are presumably first-order 5HT projection neurons that contain the ZW transgene. It is, however, possible that some of these 5HT neurons, whether or not they express the transgene, took up the WGA after its transneuronal transport from other 5HT neurons. This would represent 5HT–5HT neuronal interconnections. Even though the extent of such connections is difficult to estimate, it has been reported that 5HT neurons receive high densities of 5HT appositions (Potrebic et al., 1995). Finally, we found that a very large number of non-5HT neurons in the RGc and RMc were WGA-immunoreactive and contained the FG retrograde tracer (Fig. 8).
Fig. 8.
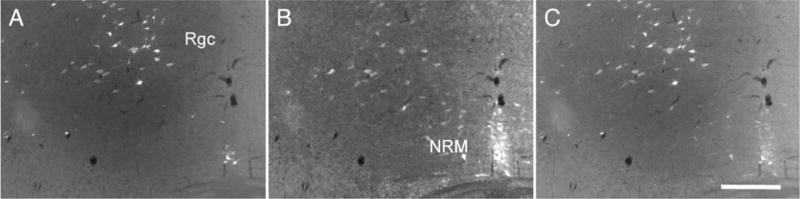
Serotonin inputs to neurons of the nucleus reticularis gigantocellularis that project to the spinal cord. FluoroGold injections in the spinal cord of ePet-ZW mice retrogradely label large numbers of neurons (white in A) in the nucleus reticularis gigantocellularis (RGc). Double labeling for WGA (red in B) shows that most RGc neurons (80%) that receive 5HT inputs project to the spinal cord. A magenta-green version of this figure is available as a supplementary figure online. Scale bar = 100 μm.
The pattern of labeling in the medulla and midbrain differed considerably after injection of FG into the spinal cord. As previously reported, compared to the medulla, there are many fewer PAG neurons that project directly to the spinal cord. This is especially true for the midline DR. Furthermore, we never found overlap of FG and WGA labeling in this region (blue and red neurons, respectively, in Fig. 7B,C) and only occasionally observed double-labeled cells in the region of the MnR. This indicates that few if any 5HT neurons of the DR or MnR project directly to the spinal cord. However, we did find a small number of FG-WGA-immunoreactive, but 5HT-negative neurons lateral to the midbrain raphe nuclei, indicating that the 5HT neurons of the DR and MnR likely target midbrain neurons that project to the spinal cord (green neurons in Fig. 7D–F).
What is the source of the input to spinally projecting neurons of the medulla?
As noted above, the RVM is a major relay for the antinociceptive controls exerted by neurons of the midbrain PAG (Basbaum and Fields, 1984; Hermann et al., 1997; Fields and Basbaum, 1999; Mason, 2001). However, the contribution of the 5HT-containing neurons of DR in this circuit is still unclear. This is of particular interest as some of the earliest studies of midbrain antinociceptive controls emphasized the critical contribution of the serotonergic neurons of the dorsal raphe (Guilbaud et al., 1973). Given that we found that many of the non-5HT spinally projecting neurons of the medulla lie downstream of 5HT neurons, we next addressed the source of this 5HT input. Although it has been established that there is a limited dorsal raphe 5HT projection to the medulla (Beitz, 1982), here we could also address the possibility that these 5HT neurons indirectly regulate medullary neurons through their connections within the PAG.
In these studies we injected FG into the RVM and recorded the distribution of retrogradely labeled and WGA-immunoreactive cells in the PAG. Consistent with previous studies, we found that FG injections into the RVM retrogradely labeled many neurons throughout the brainstem (Abols and Basbaum, 1981), and as expected, many of these were concentrated in the ventrolateral PAG (Fig. 9). However, very few were double-labeled with WGA, suggesting that there is neither a significant direct 5HT projection from the midbrain raphe nuclei to the RVM, nor a significant indirect projection, i.e., the major ventrolateral PAG projection to the RVM appears not to be regulated by 5HT neurons of the dorsal or median raphe.
Fig. 9.
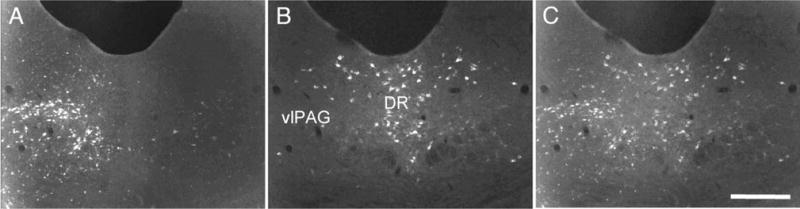
The PAG-RVM pathway does not include 5HT neurons. FluoroGold injections in the RVM retrogradely labeled large numbers of neurons (white in A) in the ventrolateral periaqueductal gray (vlPAG). However, double-labeling for WGA (green in B) shows that none of these receive direct or indirect inputs from 5HT (i.e., primary) neurons. Scale bar = 100 μm.
Interaction between brainstem 5HT and noradrenergic cell groups
As there are parallel noradrenergic (for a review, see Pertovaara, 2006) and serotonergic antinociceptive controls that originate in the brainstem, it is of interest to study the interactions between these different monoaminergic systems. Although anatomical studies reported that there are reciprocal connections between RVM and NA cell groups (Clark and Proudfit, 1991; Kwiat and Basbaum, 1992; Tanaka et al., 1996), here we asked if NA neurons lie downstream of the 5HT population. We used tyrosine hydroxylase (TH) as a marker for noradrenergic neurons and detected clusters of TH-immunoreactive neurons at all levels of the brainstem and caudal midbrain. As previously reported, we found that NA- and 5HT-containing neurons constitute distinct populations that never overlap. Moreover, as illustrated in Figure 10 (from the A10 cell group located within the PAG), we found that WGA-positive neurons rarely costained for TH, even in areas where we observed dense clusters of both TH- and WGA-immunoreactive neurons.
Fig. 10.
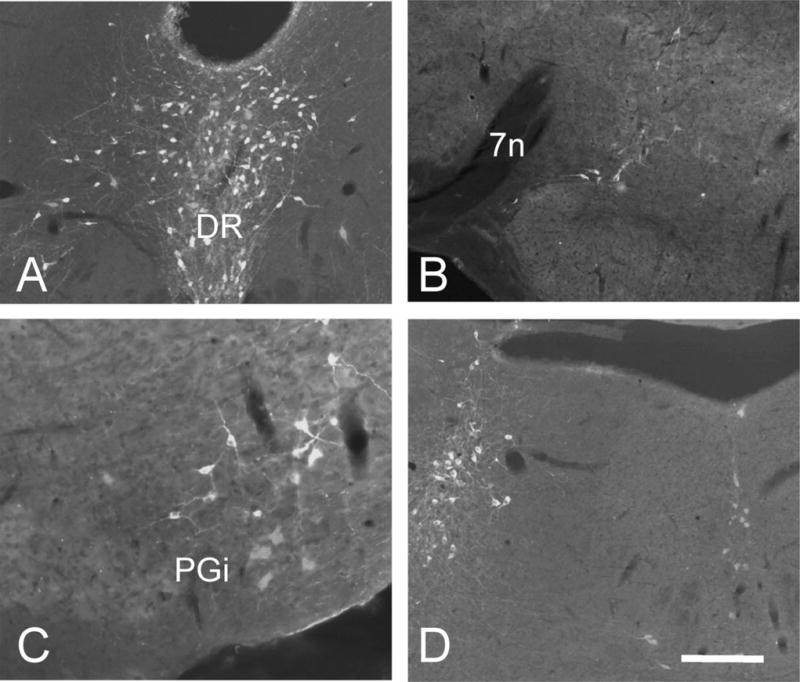
Brainstem noradrenergic neurons do not receive 5HT inputs in ePet-ZW mice. Double labeling for WGA (red) and tyrosine hydroxylase (green) illustrate that noradrenaline- and serotonin-containing neurons constitute distinct populations. Moreover, the lack of WGA labeling in TH-positive neurons indicates that NA-immunoreactive neurons are not postsynaptic to 5HT (primary) neurons. A: rostral PAG; A10. B: A5 cell group. C: A1 cell group. D: locus coeruleus (A6) and subcoeruleus (A7). 7n, seventh nerve; PGi, nucleus reticularis paragigantocellularis; DR, dorsal raphe. A magenta-green version of this figure is available as a supplementary figure online. Scale bar = 100 μm.
DISCUSSION
This genetic transneuronal tracing study provides a comprehensive analysis of the brainstem circuits engaged by the 5HT population of raphe neurons in the rostral ventral medulla and midbrain periaqueductal gray of the mouse. We conclude that there is a major 5HT input to interneurons within the medullary nucleus raphe magnus and midbrain dorsal raphe and to spinally projecting neurons within the NRM and adjacent medullary reticular formation. We did not identify the neurochemistry of the target neurons; for the most part they were neither serotonin- nor noradrenaline-containing. Without electrophysiological characterization of the RVM neurons it is, of course, impossible to identify the “on” or “off” nature of the WGA-labeled neurons, but it is likely that at least some correspond to one of these two cell types. For this reason we suggest that the connections that we identified subserve a feedforward regulation of the “on” and “off” cell pain control network, and that the regulation is triggered via collaterals of the neurons that are at the origin of the descending serotonergic pathway.
Local connections made by serotonergic neurons in the medulla and midbrain
The 5HT system has been extensively studied in rats but there is only limited information in mice (Vander-Horst and Ulfhake, 2006). This is likely due to the difficulty of performing tracing experiments in mice because of their small size. Although the overall organization of neuronal populations in the CNS is similar in rats and mice, the relative location, size, and/or connectivity of cell groups may differ. Here, using a genetic transneuronal tracing system (Braz et al., 2002), we analyzed in the mouse the brainstem circuits in which the 5HT neuronal population participates. Given that brainstem 5HT neurons influence cognitive and neuroendocrine functions, and have been implicated in the regulation of sleep–wake states and pain, our results likely bear on diverse behaviors. This is particularly true for the 5HT neurons of the midbrain dorsal raphe.
On the other hand, the majority of studies of the medullary raphe have emphasized the contribution of 5HT neurons to the inhibitory controls exerted upon spinal cord “pain” transmission neurons. The development of enhanced inflammatory pain in mice with a genetic deletion of all 5HT neurons is consistent with that conclusion (Zhao et al., 2007a). On the other hand, the same mice showed decreased mechanical sensitivity, indicative of reduced facilitatory controls. The latter result is consistent with recent reports of a descending facilitatory pathway that is, at least in part, mediated via descending 5HT axons that act upon spinal 5HT3 receptors. Indeed, activation of 5HT3 receptors enhances the excitability of spinal cord neurons (Richardson et al., 1985; Sufka et al., 1992; Green et al., 2000) and contributes to persistent pain states (Zeitz et al., 2002; Suzuki et al., 2004a). Bidirectional control is also generated through the action of the “on” and “off” cells of the RVM (for reviews, see Fields et al., 1991; Fields, 2004), which respectively facilitate and reduce nociceptive transmission at the level of the spinal cord.
Given that the 5HT-mediated descending control is distinct from the control exerted by the non-5HT neurons, it was clearly of interest to determine the extent to which these systems are interconnected. Traditional anatomical tracer techniques, however, are not suited to addressing this question. These techniques are limited by the fact that the brainstem raphe groups are not homogeneous, but rather consist of both serotonergic and nonserotonergic neurons (Menétrey and Basbaum, 1987; Jones et al., 1992). As a result, the traditional tracers cannot selectively define circuits that arise from the 5HT neurons. In contrast, by genetically targeting expression of the WGA tracer to 5HT neurons, we could selectively study the output of this cell group. More important, because WGA is a transneuronal tracer, we were able to label unambiguously the postsynaptic neurons that receive 5HT inputs, something that cannot be achieved with conventional tracing methods.
We recognize that WGA, when injected into the brain, will transport in the retrograde as well as the anterograde direction. However, we have previously demonstrated that following its synthesis by CNS neurons, WGA is transported and transneuronally transferred exclusively in an anterograde manner (Braz et al., 2002). As we found that the WGA that is synthesized in 5HT neurons is transneuronally transported to large numbers of nonserotonergic neurons, both within and outside of the midline raphe, we conclude that these non-5HT neurons are located postsynaptic to the 5HT neurons. It is, of course, impossible to conclude that the function of these neurons is pain-related. However, many of these postsynaptic neurons project to the spinal cord, where they may regulate the activity of pain-relevant circuits. This is particularly true for the RMc neurons, which we previously demonstrated target the dorsal horn (Basbaum et al., 1978). Our results suggest, therefore, that RVM serotonergic neurons influence spinal cord nociceptive processing both directly, via a 5HT projection to the dorsal horn (Basbaum et al., 1982; VanderHorst and Ulfhake, 2006) and indirectly, via a polysynaptic pathway involving the spinally projecting, non-5HT cell populations. To what extent the latter control circuit arises from “on” and “off” cells cannot be determined from our analysis; however, Potrebic et al. (1995) reported that 5HT neurons and “off” cells receive the highest density of 5HT appositions. Thus, it is likely that at least some of the WGA-immunoreactive neurons that were transneuronally labeled include off cells.
Given that the “off” cell exerts inhibitory controls on spinal cord “pain” transmission neurons, it is reasonable to propose that 5HT inputs enhance those controls, which is consistent, of course, with the early views about descending 5HT controls. It is equally possible, however, that this input underlies an inhibitory control of the “off” cell output, which might contribute to the 5HT-mediated facilitation noted above. In fact, electrophysiological studies have provided some evidence that 5HT exerts inhibitory effects on RVM cells, some of which had properties comparable to the “off” cells that are defined in vivo (Pan et al., 1993). Furthermore, iontophoresis of 5HT in the RVM reduces the spontaneous activity of “off” cells in the anesthetized rat (Hentall et al., 1993), an effect that could involve activation of 5HT1A receptors (Wang and Lovick, 1992).
Medullary nucleus reticularis gigantocellularis
We found extensive transneuronal labeling of large, and more dorsally located, neurons of the nucleus reticularis gigantocellularis. In contrast to the majority of the “on” and “off” neurons, which are located in the RVM and which project to the spinal cord via the dorsal part of the lateral funiculus (DLF), RGc neurons project to intermediate gray matter and ventral horns, via ventral pathways. These connections likely provide for a 5HT-mediated feedforward regulation of motor circuits, including the reflex responses to noxious stimulation, rather than the rostral transmission of nociceptive information by dorsal horn nociresponsive neurons. Based on the effects of spinal administration of 5HT antagonists, Zhuo and Gebhart (1991, 1992) concluded that the RGc is at the origin of a serotonergic descending pathway that exerts both facilitatory and inhibitory effects on spinal nociceptive processing. As RGc does not contain serotonergic neurons, it is likely that the effects that they observed involved RGc connections with spinally projecting 5HT neurons. This suggests that there are reciprocal connections between 5HT and RGc neurons and is consistent with our previous suggestion (Basbaum and Fields, 1984) and that of others (Cervero and Wolstencroft, 1984) that RGc relays nociceptive inputs that activate the descending control systems that originate in the PAG.
Even though most RGc neurons project to the spinal cord, ≈20% of the transneuronally labeled RGc neurons were not retrogradely labeled after spinal cord injection of FluoroGold. This subset of RGc neurons is, therefore, likely a component of the spinoreticulothalamic pathway that transmits nociceptive messages from the spinal cord to the brainstem and thalamus (Torvik and Brodal, 1957; Blomqvist and Berkley, 1982; Peschanski and Besson, 1984). Interestingly, although we retrogradely labeled some RGc neurons after FG injection into the PAG, none of these were WGA-positive. In other words, unlike the organization that we discovered from the medullospinal projections, the direct RVM serotonergic projection to the PAG (Beitz et al., 1986) is not paralleled by an indirect pathway from serotonergic neurons to RGc neurons and from these neurons to the PAG. Our results also indicate that there is a significant heterogeneity of the ascending reticular neurons. Some are regulated by 5HT neurons, but other RGc neurons, including a population that projects to the PAG, are not. Whether the neurons with descending axons also have an ascending collateral or whether descending and ascending fibers originate from different RGc neurons is unclear.
Parallelism of the 5HT and noradrenergic output
Several studies have reported interactions between brainstem 5HT neurons and other brainstem networks (for a review, see Millan, 2002). For example, in a study using saporin-conjugated toxins to ablate the 5HT neurons, Nattie et al. (2004) suggested that brainstem 5HT-and neurokinin-1 receptor-expressing cells, which constitute distinct populations of neurons, are part of a unitary chemoreceptive circuit. In contrast, and although there is considerable evidence that descending control systems arise from 5HT and noradrenergic (NA) brainstem cell groups (Barbaro et al., 1985; Hammond et al., 1980), we did not find evidence for a direct 5HT input to the NA neurons. This result is consistent with a report of Clark and Proudfit (1991) who found that, although neurons located in the NRM or PGi send dense axonal projections to the A7 NA cell group (subcoeruleus), these connections arose mainly from non-5HT neurons. The lack of transneuronal transfer of WGA to noradrenergic neurons (even in areas containing large populations of both TH- and WGA-positive neurons) suggests that there are limited synaptic contacts between 5HT and NA neurons. Based on the present results, we conclude that the segregation of the 5HT and NA systems is not unique to the subcoeruleus, but holds for all of the NA-containing cell groups of the brainstem.
Output of the midbrain serotonergic neurons
It is of interest that the earliest studies of stimulation-produced analgesia pointed to the midline dorsal raphe, rather than the PAG as the critical target for generating analgesia (Mayer et al., 1971; Akil and Mayer, 1972; Guilbaud et al., 1973; Liebeskind et al., 1973; Basbaum et al., 1977). In fact, because putative serotonin antagonists blocked the analgesia and inhibition of the firing of spinal cord “pain” responsive neurons, the Besson group concluded that the 5HT neurons of the DR were key to initiating the descending controls (Guilbaud et al., 1973; Liebeskind et al., 1973). Although subsequent studies emphasized the contribution of the ventrolateral PAG (Fardin et al., 1984), the extent to which neurons in that region are regulated via the DR has not been addressed. We found that very few WGA-positive PAG neurons were retrogradely labeled when FG was injected in the RVM, although, as expected, we found considerable label of WGA-negative neurons. This indicates that 5HT neurons of the DR do not make a significant contribution, either directly or indirectly, to the ventrolateral PAG controls that are exerted via the RVM.
Technical considerations
There are some inherent limitations of this novel tract-tracing method that should be mentioned. First, because of the mosaicism of the ZW line (Braz et al., 2002), not all 5HT neurons carry the transgene and thus express the WGA tracer after recombination. Second, because there is dilution of the tracer after its transneuronal transport, low levels of WGA in postsynaptic neurons may be missed. This may limit the number of neurons identified in a particular circuit. As a result, we likely underestimate the number of systems that are influenced by the 5HT neurons. Highly divergent inputs may also be missed because there is limited transneuronal transfer of the tracer. This last point may underlie our failure to detect transneuronal labeling in some of the expected targets of 5HT neurons, notably in the spinal cord. The descending 5HT projection may be too divergent to allow for efficient detection of the WGA tracer in spinal neurons. On the other hand, a particularly powerful advantage of this genetic transneuronal tracing procedure is that despite there being dilution of the tracer after it crosses several synapses, convergence of inputs to a higher-order cell effectively “reconcentrates” the tracer so that it is readily detected (Braz et al., 2005).
It is also of interest that not all 5HT terminals in the spinal cord make traditional synaptic contacts. Indeed most 5HT varicosities establish nonsynaptic contacts with dorsal horn neurons (Marlier et al., 1991; Ridet et al., 1993). Even though some 5HT neurons clearly act via conventional synapses (Jankowska et al., 1995; Maxwell and Jankowska, 1996), there appears to be a predominant nonsynaptic “volume transmission” mechanism that operates (Fuxe and Agnati, 1991). Conceivably, such nonsynaptic interactions are not readily detected using the WGA transneuronal transport approach.
CONCLUSIONS
Our results underscore the complexity of the output that arises from what is a heterogeneous population of RVM neurons. We conclude that there are not only significant descending serotonergic projections, but also that the bidirectional controls that arise from “on” and “off” cells are regulated by collaterals of these 5HT neurons. On the other hand, we found no evidence for a comparable 5HT-mediated feedforward regulation of the PAG neurons that are at the origin of the PAG-RVM descending control network.
Supplementary Material
Acknowledgments
We thank Drs. Michael Scott and Evan Deneris at Case Western University for providing the ePet1-Cre mice.
Grant sponsor: National Institutes of Health (NIH); Grant numbers: NS14627 and 48499.
Footnotes
This article includes Supplementary Material available via the Internet at http://www.interscience.wiley.com/jpages/0021-9967/suppmat.
LITERATURE CITED
- Abols IA, Basbaum AI. Afferent connections of the rostral medulla of the cat: a neural substrate for midbrain–medullary interactions in the modulation of pain. J Comp Neurol. 1981;201:285–297. doi: 10.1002/cne.902010211. [DOI] [PubMed] [Google Scholar]
- Akil H, Mayer DJ. Antagonism of stimulation-produced analgesia by p-CPA, a serotonin synthesis inhibitor. Brain Res. 1972;44:692–697. doi: 10.1016/0006-8993(72)90338-1. [DOI] [PubMed] [Google Scholar]
- Barbaro NM, Hammond DL, Fields HL. Effects of intrathecally administered methysergide and yohimbine on microstimulation-produced antinociception in the rat. Brain Res. 1985;343:223–229. doi: 10.1016/0006-8993(85)90738-3. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. The origin of descending pathways in the dorsolateral funiculus of the spinal cord of the cat and rat: further studies on the anatomy of pain modulation. J Comp Neurol. 1979;187:513–531. doi: 10.1002/cne.901870304. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Marley NJ, O’Keefe J, Clanton CH. Reversal of morphine and stimulus-produced analgesia by subtotal spinal cord lesions. Pain. 1977;3:43–56. doi: 10.1016/0304-3959(77)90034-3. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Clanton CH, Fields HL. Three bulbospinal pathways from the rostral medulla of the cat: an autoradiographic study of pain modulating systems. J Comp Neurol. 1978;178:209–224. doi: 10.1002/cne.901780203. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Glazer EJ, Lord BA. Simultaneous ultrastructural localization of tritiated serotonin and immunoreactive peptides. J Histochem Cytochem. 1982;30:780–784. doi: 10.1177/30.8.7119422. [DOI] [PubMed] [Google Scholar]
- Beitz AJ. The sites of origin of brain stem neurotensin and serotonin projections to the rodent nucleus raphe magnus. J Neurosci. 1982;2:829–842. doi: 10.1523/JNEUROSCI.02-07-00829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitz AJ, Clements JR, Mullett MA, Ecklund LJ. Differential origin of brainstem serotonergic projections to the midbrain periaqueductal gray and superior colliculus of the rat. J Comp Neurol. 1986;250:498–509. doi: 10.1002/cne.902500408. [DOI] [PubMed] [Google Scholar]
- Blomqvist W, Ma W, Berkley KJ. Spinal input to the parabrachial nucleus in the cat. Brain Res. 1989;480:29–36. doi: 10.1016/0006-8993(89)91563-1. [DOI] [PubMed] [Google Scholar]
- Braz JM, Rico B, Basbaum AI. Transneuronal tracing of diverse CNS circuits by Cre-mediated induction of wheat germ agglutinin in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:15148–15153. doi: 10.1073/pnas.222546999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47:787–793. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Cervero F, Wolstencroft JH. A positive feedback loop between spinal cord nociceptive pathways and antinociceptive areas of the cat’s brain stem. Pain. 1984;20:125–138. doi: 10.1016/0304-3959(84)90094-0. [DOI] [PubMed] [Google Scholar]
- Clark FM, Proudfit HK. Projections of neurons in the ventromedial medulla to pontine catecholamine cell groups involved in the modulation of nociception. Brain Res. 1991;540:105–115. doi: 10.1016/0006-8993(91)90496-i. [DOI] [PubMed] [Google Scholar]
- Dahlström A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand. 1964;62:1–54. [PubMed] [Google Scholar]
- Fardin V, Oliveras JL, Besson JM. Projections from the periaqueductal gray matter to the B3 cellular area (nucleus raphe magnus and nucleus reticularis paragigantocellularis) as revealed by the retrograde transport of horseradish peroxidase in the rat. J Comp Neurol. 1984;223:483–500. doi: 10.1002/cne.902230403. [DOI] [PubMed] [Google Scholar]
- Fields H. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- Fields HL, Basbaum AI. Central nervous system mechanisms of pain modulation. In: Wall PD, Melzack R, editors. Textbook of pain. 4th. Edinburgh: Churchill Livingston; 1999. pp. 309–329. [Google Scholar]
- Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219–245. doi: 10.1146/annurev.ne.14.030191.001251. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Agnati LF. Two principal modes of electrochemical communication in the brain: volume versus wiring transmission. Adv Neurosci. 1991;1:1–9. [Google Scholar]
- Gao K, Mason P. Serotonergic raphe magnus cells that respond to noxious tail heat are not ON or OFF cells. J Neurophysiol. 2000;84:1719–1725. doi: 10.1152/jn.2000.84.4.1719. [DOI] [PubMed] [Google Scholar]
- Gao K, Mason P. Physiological and anatomic evidence for functional subclasses of serotonergic raphe magnus cells. J Comp Neurol. 2001;439:426–439. doi: 10.1002/cne.1360. [DOI] [PubMed] [Google Scholar]
- Green GM, Scarth J, Dickenson A. An excitatory role for 5-HT in spinal inflammatory nociceptive transmission; state-dependent actions via dorsal horn 5-HT(3) receptors in the anaesthetized rat. Pain. 2000;89:81–88. doi: 10.1016/S0304-3959(00)00346-8. [DOI] [PubMed] [Google Scholar]
- Guilbaud G, Besson JM, Oliveras JL, Liebeskind JC. Suppression by LSD of the inhibitory effect exerted by dorsal raphe stimulation on certain spinal cord interneurons in the cat. Brain Res. 1973;61:417–422. doi: 10.1016/0006-8993(73)90549-0. [DOI] [PubMed] [Google Scholar]
- Hammond DL, Yaksh TL. Antagonism of stimulation-produced antinociception by intrathecal administration of methysergide or phentolamine. Brain Res. 1984;298:329–337. doi: 10.1016/0006-8993(84)91432-x. [DOI] [PubMed] [Google Scholar]
- Hammond DL, Levy RA, Proudfit HK. Hypoalgesia following microinjection of noradrenergic antagonists in the nucleus raphe magnus. Pain. 1980;9:85–101. doi: 10.1016/0304-3959(80)90031-7. [DOI] [PubMed] [Google Scholar]
- Hentall ID, Andresen MJ, Taguchi K. Serotonergic, cholinergic and nociceptive inhibition or excitation of raphe magnus neurons in barbiturate-anesthetized rats. Neuroscience. 1993;5:303–310. doi: 10.1016/0306-4522(93)90158-c. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars alpha demonstrated by iontophoretic application of choleratoxin (subunit b) J Chem Neuroanat. 1997;13:1–21. doi: 10.1016/s0891-0618(97)00019-7. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Maxwell DJ, Dolk S, Krutki P, Belichenko PV, Dahlström A. Contacts between serotonergic fibres and dorsal horn spinocerebellar tract neurons in the cat and rat: a confocal microscopic study. Neuroscience. 1995;67:477–487. doi: 10.1016/0306-4522(95)00059-r. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Yaksh TL. Spinal monoamine and opiate systems partly mediate the antinociceptive effects produced by glutamate at brainstem sites. Brain Res. 1984;321:287–297. doi: 10.1016/0006-8993(84)90181-1. [DOI] [PubMed] [Google Scholar]
- Jones SL, Light AR. Serotonergic medullary raphespinal projection to the lumbar spinal cord in the rat: a retrograde immunohistochemical study. J Comp Neurol. 1992;322:599–610. doi: 10.1002/cne.903220413. [DOI] [PubMed] [Google Scholar]
- Kwiat GC, Basbaum AI. The origin of brainstem noradrenergic and serotonergic projections to the spinal cord dorsal horn in the rat. Somatosens Motor Res. 1992;9:157–173. doi: 10.3109/08990229209144768. [DOI] [PubMed] [Google Scholar]
- Liebeskind JC, Guibaud G, Besson JM, Oliveras JL. Analgesia from electrical stimulation of the periaqueductal gray matter in the cat: behavioral observations and inhibitory effects on spinal cord interneurons. Brain Res. 1973;50:441–446. doi: 10.1016/0006-8993(73)90748-8. [DOI] [PubMed] [Google Scholar]
- Marlier L, Sandillon F, Poulat P, Rajaofetra N, Geffard M, Privat A. Serotonergic innervation of the dorsal horn of rat spinal cord: light and electron microscopic immunocytochemical study. J Neurocytol. 1991;20:310–322. doi: 10.1007/BF01235548. [DOI] [PubMed] [Google Scholar]
- Mason P. Physiological identification of pontomedullary serotonergic neurons in the rat. J Neurophysiol. 1997;77:1087–1098. doi: 10.1152/jn.1997.77.3.1087. [DOI] [PubMed] [Google Scholar]
- Mason P. Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Annu Rev Neurosci. 2001;24:737–777. doi: 10.1146/annurev.neuro.24.1.737. [DOI] [PubMed] [Google Scholar]
- Matos FF, Rollema H, Brown JL, Basbaum AI. Do opioids evoke the release of serotonin in the spinal cord? An in vivo microdialysis study of the regulation of extracellular serotonin in the rat. Pain. 1992;48:439–447. doi: 10.1016/0304-3959(92)90097-U. [DOI] [PubMed] [Google Scholar]
- Mayer DJ, Wolfle TL, Akil H, Carder B, Liebeskind JC. Analgesia from electrical stimulation in the brainstem of the rat. Science. 1971;174:1351–1354. doi: 10.1126/science.174.4016.1351. [DOI] [PubMed] [Google Scholar]
- Maxwell DJ, Jankowska E. Synaptic relationships between serotonin-immunoreactive axons and dorsal horn spinocerebellar tract cells in the cat spinal cord. Neuroscience. 1996;70:247–253. doi: 10.1016/0306-4522(95)00377-u. [DOI] [PubMed] [Google Scholar]
- Menétrey D, Basbaum AI. The distribution of substance P-, enkephalin-, and dynorphin-immunoreactive neurons in the medulla of the rat and their contribution to bulbospinal pathways. Neuroscience. 1987;23:173–187. doi: 10.1016/0306-4522(87)90281-8. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A, Richerson G, Lappi DA. Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. J Physiol. 2004;556:235–253. doi: 10.1113/jphysiol.2003.059766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan ZZ, Wessendorf MW, Williams JT. Modulation by serotonin of the neurons in rat nucleus raphe magnus in vitro. Neuroscience. 1993;54:421–429. doi: 10.1016/0306-4522(93)90263-f. [DOI] [PubMed] [Google Scholar]
- Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80:53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Peschanski M, Besson JM. A spino-reticulo-thalamic pathway in the rat: an anatomical study with reference to pain transmission. Neuroscience. 1984;12:165–178. doi: 10.1016/0306-4522(84)90145-3. [DOI] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Potrebic SB, Fields HL, Mason P. Serotonin immunoreactivity is contained in one physiological cell class in the rat rostral ventromedial medulla. J Neurosci. 1994;14:1655–1665. doi: 10.1523/JNEUROSCI.14-03-01655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potrebic SB, Mason P, Fields HL. The density and distribution of serotonergic appositions onto identified neurons in the rat rostral ventromedial medulla. J Neurosci. 1995;15:3273–3283. doi: 10.1523/JNEUROSCI.15-05-03273.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfit HK, Anderson EG. Morphine analgesia: blockade by raphe magnus lesions. Brain Res. 1975;98:612–618. doi: 10.1016/0006-8993(75)90380-7. [DOI] [PubMed] [Google Scholar]
- Richardson BP, Engel G, Donatsch P, Stadler PA. Identification of serotonin M-receptor subtypes and their specific blockade by a new class of drugs. Nature. 1985;316:126–123. doi: 10.1038/316126a0. [DOI] [PubMed] [Google Scholar]
- Ridet JL, Rajaofetra N, Teilhac JR, Geffard M, Privat A. Evidence for nonsynaptic serotonergic and noradrenergic innervation of the rat dorsal horn and possible involvement of neuron-glia interactions. Neuroscience. 1993;52:143–157. doi: 10.1016/0306-4522(93)90189-m. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Obata H, Kawahara K, Saito S, Goto F. Peripheral 5-HT2A receptor antagonism attenuates primary thermal hyperalgesia and secondary mechanical allodynia after thermal injury in rats. Pain. 2006;122:130–136. doi: 10.1016/j.pain.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S, Jiang W, Conlon RA, Strowbridge BW, Deneris ES. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci U S A. 2005;102:16472–16477. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Sufka KJ, Schomburg FM, Giordano J. Receptor mediation of 5-HT-induced inflammation and nociception in rats. Pharmacol Biochem Behav. 1992;41:53–56. doi: 10.1016/0091-3057(92)90058-n. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Rahman W, Hunt SP, Dickenson AH. Descending facilitatory control of mechanically evoked responses is enhanced in deep dorsal horn neurones following peripheral nerve injury. Brain Res. 2004a;1019:68–76. doi: 10.1016/j.brainres.2004.05.108. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004b;25:613–617. doi: 10.1016/j.tips.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Matsumoto Y, Murakami T, Hisa Y, Ibata Y. The origins of catecholaminergic innervation in the rostral ventromedial medulla oblongata of the rat. Neurosci Lett. 1996;207:53–56. doi: 10.1016/0304-3940(96)12487-3. [DOI] [PubMed] [Google Scholar]
- Taylor BK, Basbaum AI. Systemic morphine-induced release of serotonin in the rostroventral medulla is not mimicked by morphine microinjection into the periaqueductal gray. J Neurochem. 2003;86:1129–1141. doi: 10.1046/j.1471-4159.2003.01907.x. [DOI] [PubMed] [Google Scholar]
- Torvik A, Brodal A. The origin of reticulospinal fibers in the cat; an experimental study. Anat Rec. 1957;128:113–137. doi: 10.1002/ar.1091280110. [DOI] [PubMed] [Google Scholar]
- VanderHorst VG, Ulfhake B. The organization of the brainstem and spinal cord of the mouse: relationships between monoaminergic, cholinergic, and spinal projection systems. J Chem Neuroanat. 2006;31:2–36. doi: 10.1016/j.jchemneu.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Vogt M. The effect of lowering the 5-hydroxytryptamine content of the rat spinal cord on analgesia produced by morphine. J Physiol. 1974;236:483–498. doi: 10.1113/jphysiol.1974.sp010448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WH, Lovick TA. Inhibitory serotonergic effects on rostral ventrolateral medullary neurons. Pflugers Arch. 1992;422:93–97. doi: 10.1007/BF00370407. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Plant RL, Rudy TA. Studies on the antagonism by raphe lesions of the antinociceptive action of systemic morphine. Eur J Pharmacol. 1977;41:399–408. doi: 10.1016/0014-2999(77)90260-6. [DOI] [PubMed] [Google Scholar]
- Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, Bonhaus DW, Stucky CL, Julius D, Basbaum AI. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci. 2002;22:1010–1019. doi: 10.1523/JNEUROSCI.22-03-01010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Sykes KT, Buhler AV, Hammond DL. Electrophysiological heterogeneity of spinally projecting serotonergic and nonserotonergic neurons in the rostral ventromedial medulla. J Neurophysiol. 2006;95:1853–1856. doi: 10.1152/jn.00883.2005. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Chiechio S, Sun YG, Zhang KH, Zhao CS, Scott M, Johnson RL, Deneris ES, Renner KJ, Gereau RW, 4th, Chen ZF. Mice lacking central serotonergic neurons show enhanced inflammatory pain and enhanced analgesic response to antidepressant drugs. J Neurosci. 2007a;27:6045–6053. doi: 10.1523/JNEUROSCI.1623-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ, Gao YJ, Sun YG, Zhao CS, Gereau RW, 4th, Chen ZF. Central serotonergic neurons are differentially required for opioid analgesia but not for morphine tolerance or morphine reward. Proc Natl Acad Sci U S A. 2007b;104:14519–14524. doi: 10.1073/pnas.0705740104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo M, Gebhart GF. Spinal serotonin receptors mediate descending facilitation of a nociceptive reflex from the nuclei reticularis gigantocellularis and gigantocellularis pars alpha in the rat. Brain Res. 1991;550:35–48. doi: 10.1016/0006-8993(91)90402-h. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Gebhart GF. Characterization of descending facilitation and inhibition of spinal nociceptive transmission from the nuclei reticularis gigantocellularis and gigantocellularis pars alpha in the rat. J Neurophysiol. 1992;67:1599–1614. doi: 10.1152/jn.1992.67.6.1599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


