Summary
Monoclonal antibodies initiated the unprecedented breakthroughs in cancer immunotherapy and are rapidly evolving with multiple therapeutic platforms. One next generation strategy engineers multivalent proteins that ligate single-chain variable fragments targeting cellular effectors, tumor-associated antigens and cytokines. These developing therapeutics target and regulate cellular effector bioactivity and significantly improve clinical outcomes.
In this issue of Clinical Cancer Research, Vallera and colleagues (1) report the generation of a trispecific killer engager (TriKE) that incorporates single-chain variable fragments (scFVs) against CD16 and CD33. This engineered protein crosslinks NK and myeloid tumor cells while also stably incorporating IL-15 to induce the expansion, activation and survival of NK cells. The preclinical therapeutic potential of this drug was documented using immune deficient mice bearing an acute myelogenous leukemia cell line (HL-60) resulting in the improved survival of NK cells and prolonged survival of the tumor bearing hosts. In these studies the therapeutic activity of engineered CD16×CD33 scFVs with or without IL-15 were compared. Both, therapeutics demonstrated improved control of tumor burden relative to no-drug control mice, while long term activity was significantly improved with the engineered therapeutic incorporating IL-15 relative to the drug without IL-15.
Tumor-specific monoclonal antibodies (mAbs) provide effective cancer immunotherapy for patients with a variety of malignancies (2). The first therapeutic antibody, Rituximab was approved by the US Food and Drug Administration (FDA) in 1997. These first-generation mAbs have achieved significant success in the treatment of neoplasia and since Rituximab’s approval numerous monoclonals have been approved. Next-generation monoclonal variants have been engineered and are undergoing clinical development with encouraging results suggesting increased potency and an improved safety profile, resulting in an exploding focus on engineered mAb development (3). Indeed, two such engineered antibodies have received regulatory approval and more than 30 are in clinical development. These engineered proteins include bispecific Abs that simultaneously target tumor cell antigens and retarget NK or T-cells to the tumor cells. NK cells, have traditionally been considered innate immune cells and initial therapeutic strategies focused on NK cells utilizing IL-2 administration to expand their numbers, an approach that received regulatory approval associated with both a significant clinical response (around 15%) and toxicity (4). Thus, therapeutic strategies to target NK cells to tumors without systemic expansion are expected to reduce toxicity with the retention of therapeutic activity.
Mechanistically, mAbs utilize a variety of strategies to mediate antitumor activity (2). One such mechanism, antibody dependent cellular cytotoxicity (ADCC), involves an interaction between the antibody Fc region and Fc receptors on immune cells including NK cells, macrophages, and neutrophils resulting in the lysis of infectious agents and infected and malignant cells. Despite their noteworthy clinical achievements, therapeutic mAbs are still not approved for the majority of human cancers, primarily due to lack of suitable ‘tumor cell specific’ antigens as well as challenges with mAb pharmacology (5). A second confounder to the routine clinical use of mAbs is that standard of care interventions, chemotherapy, and radiation can be cytotoxic to NK cells hampering their ability to mediate ADCC.
In recent years, bispecific mAbs and engineered molecules have been used to redirect NK cells to tumor cells (6) with a mechanism of action dependent on ADCC. The CD16 specific scFVs have advantages over monospecific mAbs as they result in stronger NK cell ADCC and improved pharmacodynamic characteristics (7). A second approach to improving their immunotherapeutic activity has been the combination of bispecific mAbs with cytokines, such as IL-2 or IL-15 (Figure 1), that have the potential to improve the efficacy of mAb therapy through their ability to activate and expand NK cells in vivo, to upregulate NK cell-activating receptors, and override inhibitory interactions mediated by self-MHC class I molecules that can suppress NK cell ADCC. The current focus is on IL-15, which has a critical role in NK cell developmental homeostasis, proliferation, survival, and activation (8) in the absence of the toxicities associated with systemic IL-2 administration. One additional advantage of IL-15 over IL-2 therapy is that it does not engage Treg suppressor (9).
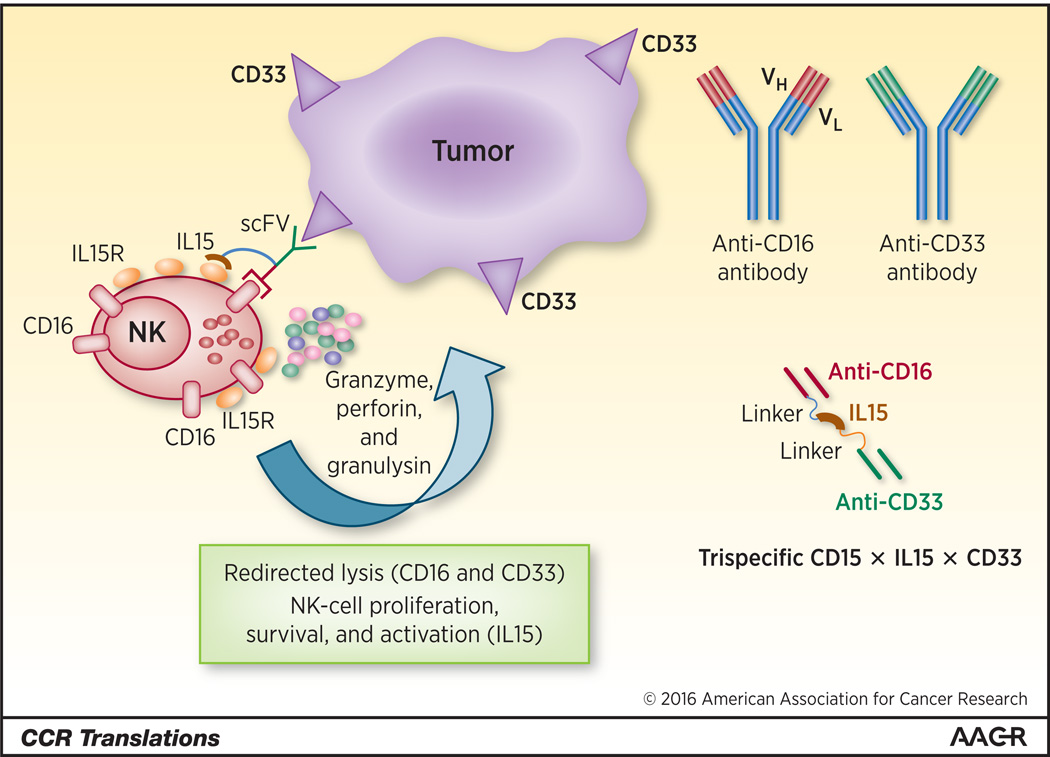
Figure 1.
Trispecific engineered proteins can enhance an immunologic synapse by a variable single-chain fragments (scFV) to a tumor-associated antigen (CD33) linked to a targeting and activating scFV (CD16) for NK cells combined with a second linker to IL-15. The addition of IL-15 can prolong NK cell survival, increase proliferation and activation resulting in NK cell secretion of cytotoxic mediators (left). These engineered proteins are generated by a linker between a scFV targeting a specific tumor associated antigen (CD33) and a CD16 scFVs that ligates NK cells. These are combined with a second linker to a cytokine (IL15) (right).
In the article by Vallera and colleagues (1), it is reported that the trispecific CD16 × IL-15 × CD33 recombinant molecule increased ADCC toxicity in vitro and in vivo as compared to the bispecific antibody without IL-15. Further, this trispecific molecule was demonstrated to expand and sustain NK cells viability in vivo using a xenograft model. This preclinical model of therapeutic activity supports the potential utility of TriKEs to increase the selectivity of NK cell therapy not only for myeloid malignancies, but indirectly also for solid tumors if appropriate tumor antigens are targeted. The mechanism by which these recombinant molecules act is not fully understood, although their ability to bring NK cells into close proximity with tumor cells is critical. As part of this interaction, an immune lytic synapse forms between the NK effector cells and tumor targets, resulting in accelerated tumor cell killing.
Similar to bispecific antibodies, immunocytokines are mAb/scFV and cytokine fusion proteins. These engineered proteins can use full mAbs or scFVs linked to cytokines (10). Following tumor cell binding, immunocytokines and their associated pro-inflammatory cytokines can mediate multiple bioactivities. For example, IL-2, IL-12, and TNF can stimulate leukocytic infiltration into a tumor mass, which may result in therapeutic activity (11). As such, bispecific and trispecific mAbs can be directed against tumor cell antigens, non-tumor associated targets found in the tumor extracellular matrix such as endothelial cells, effector cells such as NK cells and T-cells, and may include a variety of cytokines to manipulate cellular effector or target effector cells to tumor cells or all of the above.
The success of engineered molecules critically relies on the identification of functional tumor-associated antigens and their ligation by scFVs to effector cells and immunoregulatory cytokines. Challenges to the success of this therapy strategy include impediments similar to chemotherapy, whereby the downregulation of the targeted tumor antigens allows the tumor to become refractory to intervention. Another challenge to the success of engineered multivalent proteins is cellular targeting by the cytokine as opposed to targeting by scFVs to tumor cells. The scFVs are expected to mediate tumor targeting; however, in at least one study the cytokine moiety was shown to govern biodistribution, resulting in the unexpected loss of tumor targeting (12). Similar challenges may be associated with abnormal cellular biodistribution following ligation, based on capillary arrest in non-tumor organs, such as the lungs, liver, or spleen. Depending on the characteristics of the engineered fusion product, as well as the linked cytokine, the mononuclear phagocyte system may regulate biodistribution of an engineered molecule bound to a target cell(s). Therefore, careful evaluation of the biodistribution and pharmacokinetic of engineered molecules is critical to assess the influence of cytokine affinity, receptor distribution, size, and avidity on the effectiveness of engineered protein/effector cell accumulation. Despite these challenges, based on the results from the studies by Vallera and colleagues, the future is bright for trispecific engineered therapeutics.
Acknowledgments
Grant Support: J.E. Talmadge is supported by the NIH under award number P30CA036727.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Vallera DA, Felices M, McElmurry R, McCullar V, Zhou X, Schmohl JE, et al. IL-15 trispecific killer engagers (TriKEs) make natural killer cells specific to CD33+ targets while also inducing persistence, in vivo expansion, and enhanced function. Clin Cancer Res. 2016 Feb 4; doi: 10.1158/1078-0432.CCR-15-2710. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 3.Nunez-Prado N, Compte M, Harwood S, Alvarez-Mendez A, Lykkemark S, Sanz L, et al. The coming of age of engineered multivalent antibodies. Drug Discov Today. 2015;20:588–594. doi: 10.1016/j.drudis.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Waldmann TA. The shared and contrasting roles of IL2 and IL15 in the life and death of normal and neoplastic lymphocytes: implications for cancer therapy. Cancer Immunol Res. 2015;3:219–227. doi: 10.1158/2326-6066.CIR-15-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol. 2009;157:220–233. doi: 10.1111/j.1476-5381.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kontermann RE, Brinkmann U. Bispecific antibodies. Drug Discov Today. 2015;20:838–847. doi: 10.1016/j.drudis.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Gleason MK, Verneris MR, Todhunter DA, Zhang B, McCullar V, Zhou SX, et al. Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol Cancer Ther. 2012;11:2674–2684. doi: 10.1158/1535-7163.MCT-12-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steel JC, Waldmann TA, Morris JC. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol Sci. 2012;33:35–41. doi: 10.1016/j.tips.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munger W, DeJoy SQ, Jeyaseelan R, Sr, Torley LW, Grabstein KH, Eisenmann J, et al. Studies evaluating the antitumor activity and toxicity of interleukin-15, a new T cell growth factor: comparison with interleukin-2. Cell immunol. 1995;165:289–293. doi: 10.1006/cimm.1995.1216. [DOI] [PubMed] [Google Scholar]
- 10.Kiefer JD, Neri D. Immunocytokines and bispecific antibodies: two complementary strategies for the selective activation of immune cells at the tumor site. Immunol Rev. 2016;270:178–192. doi: 10.1111/imr.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halin C, Rondini S, Nilsson F, Berndt A, Kosmehl H, Zardi L, et al. Enhancement of the antitumor activity of interleukin-12 by targeted delivery to neovasculature. Nat Biotechnol. 2002;20:264–269. doi: 10.1038/nbt0302-264. [DOI] [PubMed] [Google Scholar]
- 12.Tzeng A, Kwan BH, Opel CF, Navaratna T, Wittrup KD. Antigen specificity can be irrelevant to immunocytokine efficacy and biodistribution. Proc Natl Acad Sci U S A. 2015;112:3320–3325. doi: 10.1073/pnas.1416159112. [DOI] [PMC free article] [PubMed] [Google Scholar]