Abstract
Salivary protein difference value (SP D-value) is a quantitative measure of salivary protein replenishment, which reportedly relates to individual differences in perceived astringency. This in vitro measure is calculated as the difference in total salivary protein before (S1) and after (S2) stimulation with tannic acid, with a greater absolute value (S2-S1) indicating less protein replenishment. Others report this measure predicts perceived astringency and liking of liquid model systems and beverages containing added polyphenols. Whether this relationship generalizes to astringent compounds other than polyphenols, or to solid foods is unknown. Here, the associations between SP D-values and perceived astringency and overall liking/disliking for alum and tannic acid (experiment 1) as well as solid chocolate-flavored compound coating with added tannic acid or grape seed extract (GSE) (experiment 2) were examined. In both experiments, participants (n=84 and 81, respectively) indicated perceived intensity of astringency, bitterness, sweetness, and sourness, and degree of liking of either aqueous solutions, or solid chocolate-flavored compound coating with added astringents. Data were analyzed via linear regression, and as discrete groups for comparison to prior work. Three discrete groups were formed based on first and third quartile splits of the SP D-value distribution: low (LR), medium (MR), and high responding (HR) individuals. In experiment 1, significantly higher mean astringency ratings were observed for the HR as compared to the LR/MR groups for alum and tannic acid, confirming and extending prior work. In experiment 2, significantly higher mean astringency ratings were also observed for HR as compared to LR groups in solid chocolate-flavored compound containing added tannic acid or GSE. Significant differences in liking were found between HR and LR groups for alum and tannic acid in water, but no significant differences in liking were observed for chocolate-flavored compound samples. A significant linear relationship between SP D-values and perceived astringency was observed for both alum and tannic acid (p’s<0.001), although the variance explained was relatively low (R2 = 0.33 and 0.29, respectively). In the solid chocolate-flavored compound spiked with either tannic acid or GSE, the relationship was not significant (p=0.17 and 0.30; R2=0.03 and 0.02, respectively). Due to the weak associations overall, and the lack of significant differences in perception of astringency between the MR and LR groups, we conclude that SP D-values are not a strong predictor of astringency, especially in solid, high-fat foods. Additional research investigating alternative methods for quantifying individual differences in astringency, as well as exploring the underlying complexities of this percept appears warranted.
Keywords: Astringency, salivary proteins, in vitro methods, individual differences
Introduction
The American Society for Testing and Materials [1] defines astringency as “the complex of sensations due to shrinking, drawing or puckering of the epithelium as a result of exposure to substances such as alums or tannins.” This complex percept has traditionally been associated with negative consumer reactions at high intensities [2]. Common astringent stimuli in the diet are polyphenolic compounds such as those found in red wine, tea, chocolate, and a variety of fruits and nuts. In addition to polyphenols, a number of other classes of compounds can elicit oral astringency, including organic and inorganic acids (e.g. malic or hydrochloric acid), dehydrating agents (e.g. ethanol), and multivalent salts (e.g. ammonium potassium sulfate, or ‘alum’). For more information, see the review by Bajec and Pickering [3],
While it is widely accepted that the prototypical tastes are elicited via G-protein coupled receptors (sweet, bitter, and umami), or ion channels (sour, and salty), the mechanism(s) through which oral astringency is elicited is (are) not fully understood [4–6]. The most commonly cited mechanism for astringent polyphenols – salivary protein precipitation followed by decreased lubricity – is supported by the observation that proline-rich proteins (PRPs) which make up the majority of proteins found in human saliva interact with and are precipitated by tannins [7]. However, recent work by Schöbel et al. [8] proposes an alternative hypothesis involving specific chemosensors for astringency distinct from delubrication.
Sensory responses vary greatly between individuals, which likely influence eating behaviors [9, 10]. Individual differences in saliva characteristics appear to modulate the perception of astringency induced by phenolic stimuli [11–13]. To quantify such individual differences, various in vitro methods have been developed, including approaches based on haze development [11, 12, 14, 15], direct qualitative and quantitative measures of salivary proteins [9, 16–19], colorimetry [20], and voltammetry [21].
Associations between changes in salivary proteins and the perception of oral astringency are well documented, but poorly understood mechanistically. Dinnella and colleagues [17] showed individuals capable of maintaining constant saliva characteristics (i.e. concentration and composition of salivary proteins) are less perceptually responsive than individuals who exhibited significant saliva modifications when challenged with a phenolic astringent. Specifically, they describe an in vitro measure, salivary protein difference value (SP D-value), as the difference in total salivary protein before (S1) and after (S2) stimulation with tannic acid, where a more negative value of S2 minus S1 indicates lesser protein replenishment. That is, individuals with a SP D-value near zero are able to return their saliva to basal conditions relatively quickly after exposure to a model astringent stimulus (tannic acid), while individuals with a negative SP D-value do not. Critically, differences in observed SP D-value were associated with differences in perceived astringency; a relatively stable salivary protein profile (as measured by S2-S1 values near 0) characterizes individuals with lower sensitivity to astringency, while individuals with reduced replenishment of salivary proteins (i.e. more negative SP D-values) report greater oral astringency when asked to rate the intensity of astringent stimuli.
Relationships between SP D-values and real foods were subsequently explored by Dinnella and colleagues [18], who investigated perceptual and hedonic responses to fruit and vegetable juices with added tannic acid. Using first and third quartile splits of the SP D-value distribution, they divided participants into high, medium, and low responding (HR, MR, LR) groups. Generally, added tannic acid induced a greater increase in perceived astringency in the HR group, compared to the MR and LR groups. They also observed a drop in liking for apple juice with added tannic acid in the HR group, with smaller effects in MR and LR groups. These data suggest SP D-values may predict both intensity and hedonic response to astringent stimuli, at least in beverages. However, the extent to which this measure generalizes to astringent compounds other than tannins, and to complex foods, especially solid foods, remains unclear.
According to Peleg [22], “astringency is a complex phenomenon: it elicits a range of sensations, different types of compounds evoke it, and several mechanisms have been suggested to explain it.” As Peleg asserted, astringency is not a simple percept. A diverse set of compounds are capable of eliciting this cluster of sensations, including polyphenols, dehydrating agents, multivalent salts, and organic acids. Alum — a term which collectively refers to both a specific compound (i.e. potassium aluminum sulfate or ammonium aluminum sulfate) as well as any double sulfate salt — is one of the most extensively cited exemplars of an astringent stimulus, and it is widely used for training participants on the sensation of astringency [18, 23–25]. Both alum and tannic acid have previously been shown to precipitate PRPs (in addition to mucins for alum) from saliva [26]. However, a gap in the literature remains, as potential relationships between SP D-values and the astringency from non-phenolic astringents, like alum, have not yet been quantified. Determining the extent to which an in vitro measure like SP D-value generalizes to other classes of astringents may provide insight into whether diverse classes of astringent compounds share a common mechanism.
Previously, the relevance of SP D-values has been explored in beverages like fruit and vegetable juices [18]. While this is a critical step in determining whether SP D-values generalize beyond model systems to real foods, a variety of other foods, including solid foods, also elicit astringency. Solid chocolate is a plant-based food containing a substantial amount of polyphenols embedded in a sugar and fat matrix. Although the consumption of high-cacao-content chocolate has been associated with positive health benefits ascribed to polyphenols (see Visioli [27] for a recent review), these compounds also impart bitterness and astringency when present at the concentrations found in dark chocolate [28]. Thus, potential relationships between SP D-value and the perception of astringency in solid dark chocolate may provide insight into the importance of purported individual differences in astringency perception from polyphenols when they are present in a solid food matrix.
Here, we investigated associations between SP D-values and perceived astringency and liking for aqueous solutions of alum and tannic acid, as well as for solid dark chocolate-flavored compound coating with added astringents (tannic acid and grape seed extract). Collectively, the application of SP D-values to non-polyphenol astringents may help determine the role of salivary protein replenishment in the mechanism(s) underlying oral astringency, and in the context of a solid food, the potential relevance to ingestive behavior and food choice.
Materials and Methods
Overview
We report data from two separate experiments: aqueous tannic acid and alum solutions were presented in experiment 1 (n=84), and solid chocolate compound was presented in experiment 2 (n=81). In each experiment, participants completed an orientation before rating test stimuli in a first session and saliva samples were collected in a second session, which occurred within 1 week. All other procedures were kept consistent. Participants were not eligible to participate in both experiments.
Scaling Methods
Intensity ratings were made on a general labeled magnitude scale (gLMS) [29]. Derived from the labeled magnitude scale [30], the gLMS is a semantically-labeled scale with labels located at 0 (no sensation); 1.4 (barely detectable); 6 (weak); 17 (moderate); 35 (strong); 52 (very strong); and 100 (strongest imaginable sensation of any kind). Affective ratings were made on a generalized bi-polar hedonic scale (e.g. [31]) with −100 (‘strongest disliking of any kind’) on the left, +100 (‘strongest liking of any kind’), on the right, and 0 (‘neither like nor dislike’), at the midpoint. Scales were presented via Compusense five software, v5.2 (Guelph, ONT), and all procedures were approved by the local Institutional Review Board.
Participants
Reportedly healthy individuals were recruited and prescreened for eligibility from the Penn State University campus and surrounding area (State College, PA) via email for their willingness to participate in a taste study. Criteria for eligibility included: between 18–64 years old; not pregnant or breastfeeding; no known defects of smell or taste; no lip, cheek, or tongue piercings; nonsmoker (had not smoked in last 30 days); no food allergies or sensitivities; no history of choking or difficulty swallowing; no history of dry mouth (e.g. Sjögren’s Syndrome); and for experiment 2 only, self-reported consumption of solid chocolate greater than once every two weeks. Participants were asked to refrain from consuming tea or coffee as well as any other polyphenol-containing foods or beverages for at least 8 hours prior to both sessions, as this may alter salivary protein content. All tests were completed at the Sensory Evaluation Center in the Department of Food Science at Penn State University.
Orientation Procedure
At the beginning of session 1, participants were oriented in a common area in our facility before entering isolated sensory testing booths. This orientation consisted of an overview of the gLMS with a warm-up exercise using imagined sensations (e.g. [32] and exemplars of sweet (100 g/L sucrose), astringent (0.9 g/L alum), bitter (0.05 g/L quinine monohydrochloride dihydrate), and sour (0.5 g/L citric acid) stimuli to taste.
Stimuli and presentation for Experiment 1
Aqueous solutions of tannic acid (2.00 g/L) and alum (2.75 g/L) were presented in triplicate in 10 mL aliquots at room temperature. Concentrations were based on a previous study [33]. Two water blanks were also included in the sample set. The sampling instructions were: “Please pour the entire contents of sample [XXX] into your mouth and swish the solution for 10 seconds as if using mouthwash. Spit the sample into the covered spit cup provided after the 10 seconds,” where XXX was a random 3 digit blinding code. Presentation order was counterbalanced using a Williams design. Participants rinsed with room temperature reverse osmosis (RO) water before the first sample and between each sample. A minimum interstimulus interval (ISI) of 2 min was enforced between samples, but participants were encouraged to take as much time between each sample as was required, until they no longer experienced sensations from the previous sample.
Stimuli and presentation for Experiment 2
Solid chocolate-flavored confectioners’ coating (henceforth “compound”) was chosen for ease of sample preparation; unlike chocolate, compound does not require tempering before molding (due to differences in fat sources). For our purposes, it is functionally equivalent to chocolate despite not meeting the standard of identity for chocolate, as it includes plant-based fats besides cocoa butter. Pieces (2 cm × 1.5 cm × 1 cm) of compound (Clasen Quality Coatings, Inc., Madison, WI), without any added astringent (control), or an added astringent — either 1.25% (w/w) tannic acid (Spectrum Chemical Mfg. Corp., New Brunswick, NJ) or 1.25% (w/w) grape seed extract (Tanin VR Grape, Laffort U.S.A, Petaluma, CA) — were prepared in the Dry Pilot Plant in the Department of Food Science, Penn State University. Grape seed extract was used as most polyphenols present in cocoa are condensed tannins, rather than hydrolyzable tannins. Astringents were mixed into melted compound. Mixtures were refined using a horizontal 3-roll refiner to a particle size of approximately 30 μm to ensure the texture of the spiked samples was not markedly different from control. (We did not observe any obvious textural differences in the finished product, although we cannot completely rule out potential effects of texture on liking, as texture was not formally assessed by our participants). Melted compound was poured into rectangular molds (12 cm × 6 cm × 1 cm) and allowed to cool overnight (~12 hours) at room temperature. Bars were broken into smaller individual pieces (2 cm × 1.5 cm × 1 cm; ~24 pieces per bar) for ease of consumption and for consistency of sample size. Samples were presented in duplicate (6 samples total), and the presentation order was counterbalanced using a Williams design. Sampling instructions were: “Place the entire sample in your mouth and chew it five times allowing it to melt/coat your mouth. After swallowing the sample, swirl your tongue on the roof of your mouth five times before making your ratings.” Participants rinsed with room temperature reverse osmosis (RO) water before the first sample, and between each sample; all rinse water was expectorated. A minimum interstimulus interval (ISI) of 2 min was enforced between samples.
Saliva Collection
In the second session of each experiment, two samples of whole mouth saliva were obtained from each participant. Saliva samples were collected as described previously [17] with modifications. Briefly, participants first rinsed their mouths with RO water. They then chewed Parafilm (3 cm × 3 cm) for 10 min to mechanically evoke saliva (first saliva collection, S1). After a 20 min break, participants received an aqueous solution of tannic acid (3.0 g/L). They were instructed to pour the entire contents (15 ml) of the sample into their mouths and rinse with the solution for 10 sec before expectorating. They then rinsed with RO water, before chewing a second piece of Parafilm for an additional 10 mins (second saliva collection, S2). Our protocol differs from that of Dinnella et al. (2009) in the following ways. A 20 minute break was provided between S1 and S2 (versus a 30 minute break) and the duration of saliva collection for both S1 and S2 was 10 minutes (rather than 15 minutes). Our participants did not wear nose clips. Additionally, our participants rated the astringency of test stimuli in session one, rather than immediately after saliva collection, as was done by Dinnella et al. Because participants were asked to abstain from tea and coffee as well as any other polyphenol-containing foods and beverages for 8 h before both sessions, and the sessions occurred within a week of each other, we assume mouth conditions were similar for both, although we did not have a way to confirm this. No attempt was made to control for hydration status across individuals or days. Finally, we did not provide crackers between stimuli; only water was given, as other work suggests this is the most appropriate palette cleanser for astringents [34].
Saliva samples were frozen immediately after collection and stored for ~30 days at −20 °C. For analysis, samples were thawed in a water bath at 37 °C for 10 min, and then centrifuged at 10,000 g for 10 min. The pellet was discarded and the clear upper phase was recovered. Total protein of each sample (S1 and S2) was determined in triplicate using the Biuret Method [35] as reported previously [9, 18]. Bovine Serum Albumin (BSA) was used as the reference for the standard curve.
Data Analyses
The distribution of SP D-values was split using the first and third quartile values to place participants into three groups: low (LR), medium (MR), and high responders (HR) with n’s=21, 42, 21 and 20, 41, 20 for experiments 1 and 2, respectively, for comparison with prior work [17, 18]. Observed SP D-values fell within the range reported previously for these groups [9, 18]. For each attribute within a stimulus, differences in means were compared across groups via analysis of variance (ANOVA); main effects were decomposed using Tukey’s HSD at α=0.05 when the overall F statistic was significant (α of 0.05). As a separate a priori analysis strategy, linear regression was used to model perceptual ratings as a function of the SP D-value. Outliers (defined as residuals greater than 2 standard deviations) were removed, with a maximum of 6 outliers removed from any single model (of > 80 total). Regression was also used to model affective responses as a function of perceived astringency and bitterness. No outliers were removed in these models. All analyses were conducted in DataGraph (Version 3.2, Visual Data Tools, Inc, Chapel Hill, NC) or Minitab (Version 17.1, Minitab Inc., State College, PA).
Results
Experiment 1: alum and tannic acid solutions
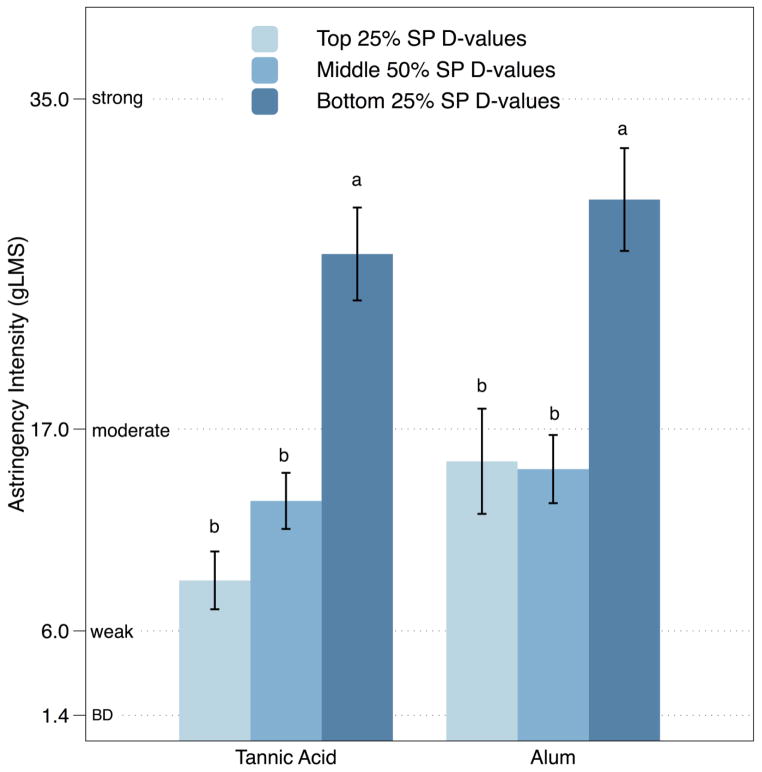
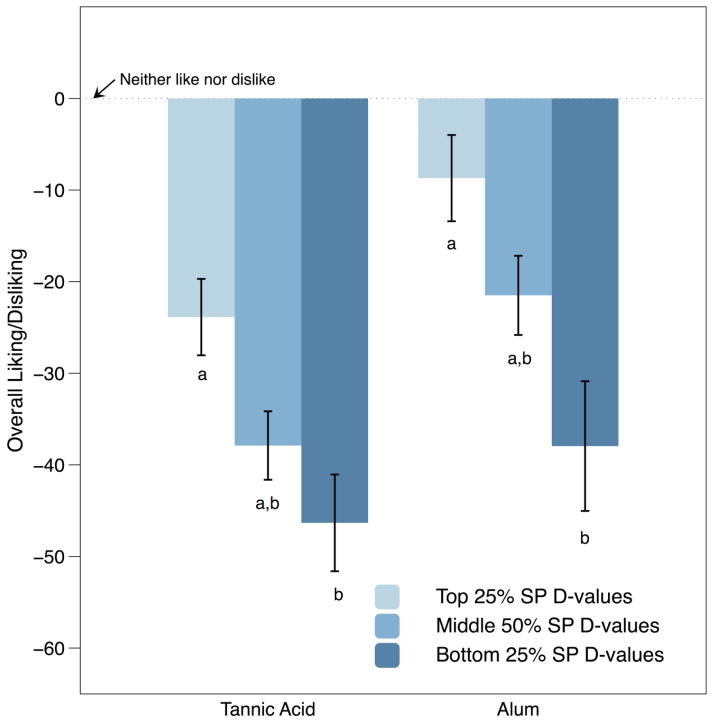
Attribute intensities and hedonic ratings for experiment 1 are shown in Table 1. Ratings for mean perceived astringency from aqueous solutions of tannic acid [F(2,81) = 19.59; p <0.001] and alum [F(2,81) = 10.66; p < 0.001] differed across the SP D-value groups. Mean astringency was significantly higher in the HR group compared to the LR/MR groups for both tannic acid and alum (Fig. 1). Group differences were also observed for hedonic responses (Fig. 2). Liking differed across SP D-value group for both tannic acid [F(2,81) = 5.14; p = 0.008] and alum [F(2,81) = 5.87; p = 0.004]; the HR group had a significantly greater disliking compared to the LR group (Tukey’s HSD p’s< 0.05). None of the other attribute means differed across SP D-value groups.
Table 1.
Mean intensity (gLMS) and hedonic ratings across all participants for experiments 1 (RO water) and 2 (dark chocolate-flavored confectioners’ compound coating). Standard error is shown in parenthesis. Liking ratings were collected with a generalized bipolar hedonic scale ranging from −100 (‘strongest disliking of any kind’) to +100 (‘strongest liking of any kind’); the center point (0) was labeled ‘neither like nor dislike’.
| Stimuli | Conc. | Astringency | Bitterness | Sourness | Sweetness | Liking/Disliking |
|---|---|---|---|---|---|---|
| Experiment 1 | (g/L) | |||||
| Alum | 2.75 | 18.6(1.52) | 5.70(1.34) | 10.6(1.00) | 2.86(0.446) | −22.4(3.20) |
| Tannic Acid | 2.00 | 15.4(1.29) | 14.0(1.35) | 3.45(0.925) | 0.37(0.104) | −36.5(2.64) |
| Water | N/A | 2.43(0.464) | 1.45(0.445) | 0.92(0.223) | 0.55(0.148) | −1.64(2.04) |
|
| ||||||
| Experiment 2 | (w/w) | |||||
| Tannic Acid | 1.25% | 22.0(2.23) | 26.2(2.24) | 8.96(1.45) | 12.0(1.13) | −42.0(3.34) |
| Grape Seed Extract (GSE) | 1.25% | 13.3(1.33) | 14.8(1.47) | 4.48(0.764) | 16.3(1.14) | −8.23(3.55) |
| Control | 0% | 3.27(0.435) | 3.94(0.651) | 1.47(0.266) | 23.7(1.42) | 30.4(2.29) |
Figure 1.
Perceived astringency for aqueous solutions of tannic acid and alum on a general Labeled Magnitude Scale (gLMS), by SP D-value group (see text for details). The columns represent means and bars are standard errors; within a stimulus, columns which do not share a letter are significantly different at α=0.05 (Tukey’s HSD).
Figure 2.
Same as Figure 1, but for overall liking/disliking on a generalized bipolar hedonic scale ranging from −100 (‘strongest disliking of any kind’) to +100 (‘strongest liking of any kind’); the center point (0) was labeled ‘neither like nor dislike’.
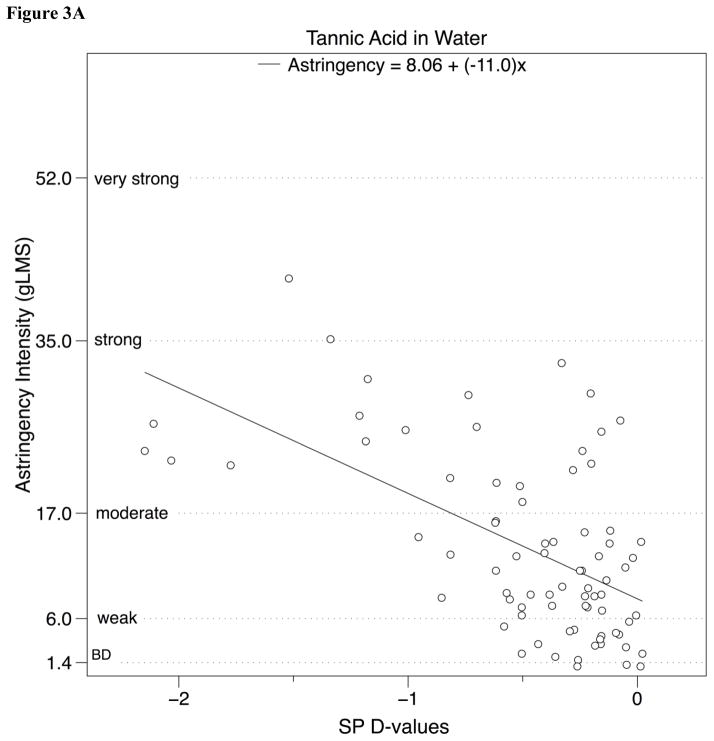
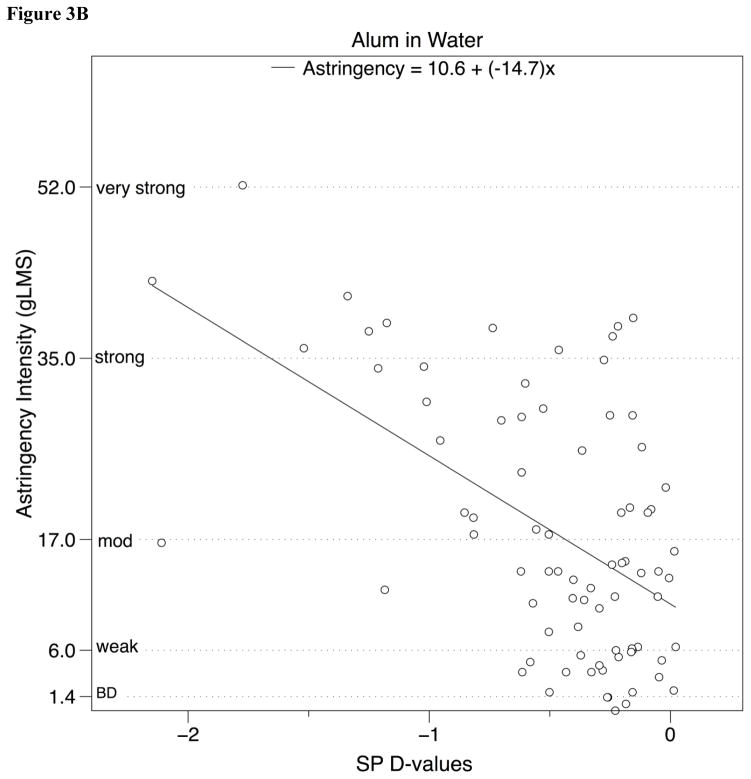
The relationship between SP D-value and mean astringency ratings were also analyzed using linear regression (Fig. 3). Significant relationships between SP D-value and perceived astringency intensity were observed for both alum and tannic acid; a summary of these analyses can be found in Table 2.
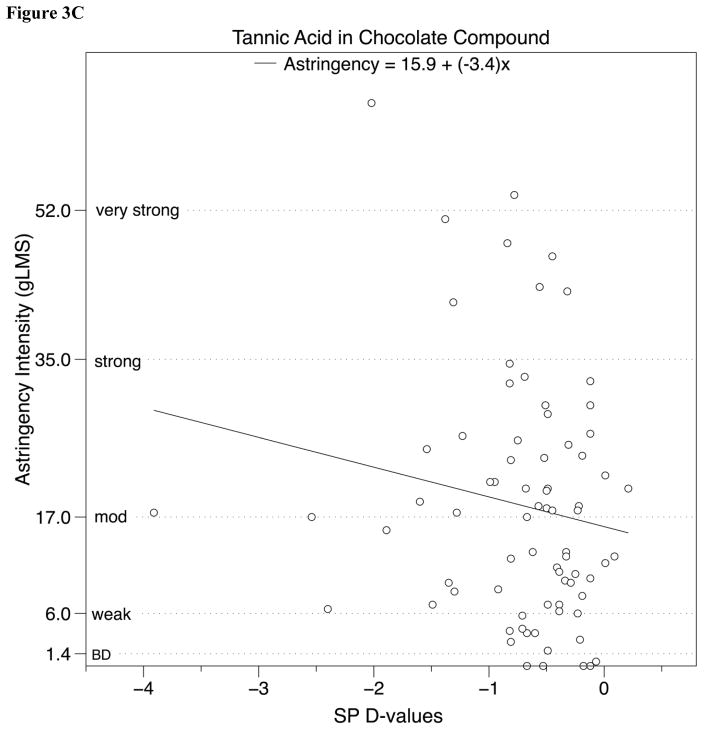
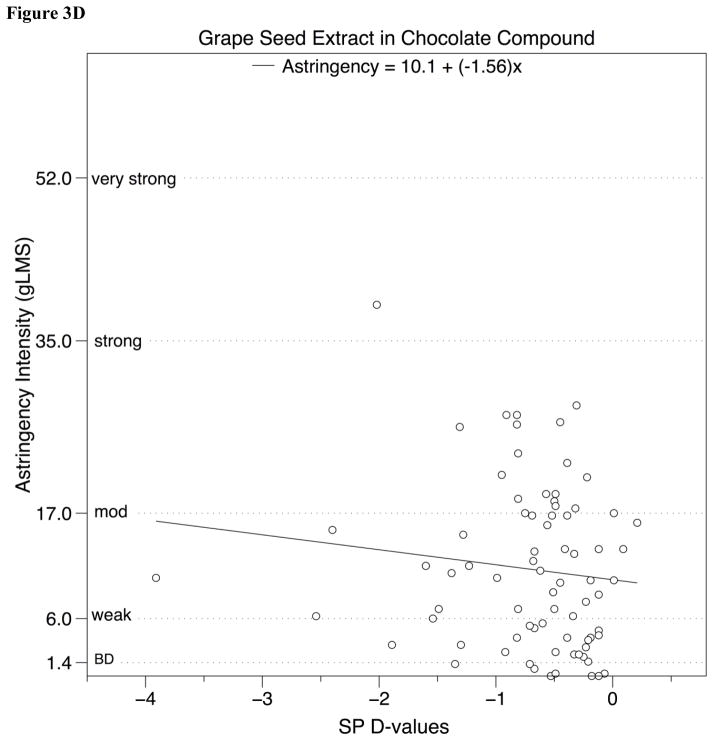
Figure 3.
Perceived astringency for a) tannic acid and b) alum in water (experiment 1) and c) tannic acid and d) grape seed extract in chocolate compound (experiment 2) on a general Labeled Magnitude Scale (gLMS) plotted against SP D-value, after removing outliers (see text for details).
Table 2.
Regression equations showing perceived astringency as a function of SP D-values. Each row represents a simple linear regression model, after removing outliers (see text for details).
| Variable | Slope | Intercept | R2 | F-value | p-value |
|---|---|---|---|---|---|
| Experiment 1 | |||||
| Tannic Acid | −11.0 | 8.06 | 0.33 | 37.46 | <0.001 |
| Alum | −14.7 | 10.6 | 0.29 | 31.19 | <0.001 |
|
| |||||
| Experiment 2 | |||||
| Tannic Acid | −3.40 | 15.9 | 0.03 | 1.94 | 0.168 |
| GSE | −1.56 | 10.1 | 0.02 | 1.11 | 0.295 |
| Control | −0.93 | 2.47 | 0.02 | 1.87 | 0.175 |
Models predicting overall liking/disliking ratings as a function of perceived astringency, bitterness and their cross-product were built using linear regression (Table 3). In water, higher astringency and bitterness ratings were both significant predictors of greater disliking, irrespective of stimulus, explaining 31 and 38% of the variance in hedonic ratings for tannic acid and alum solutions, respectively.
Table 3.
Regression models predicting overall liking/disliking as a function of perceived astringency, bitterness and their cross product. Each row represents a separate multiple regression model. Standardized regression coefficients (β) are shown, with the p-value for that term in parentheses. P-values <0.1 are italicized and <0.05 are bolded.
| Variable | βBitter | βstringent | βcross | Intercept | R2 | F-value | Model p |
|---|---|---|---|---|---|---|---|
| Experiment 1 | |||||||
| Tannic Acid | −1.176(0.001) | −0.899(0.002) | 0.0192(0.061) | −11.60(0.031) | 0.31 | 12.20 | <0.001 |
| Alum | −2.784(0.002) | −0.929(<0.001) | 0.0543(0.035) | 2.00(0.697) | 0.38 | 16.66 | <0.001 |
|
| |||||||
| Experiment 2 | |||||||
| Tannic Acid | −0.519(0.037) | −0.259(0.441) | −0.001 (0.821) | −21.68(0.005) | 0.29 | 10.24 | <0.001 |
| GSE | −1.249(0.001) | −1.192(0.017) | 0.0207(0.118) | 20.16(0.003) | 0.32 | 11.99 | <0.001 |
| Control | −1.204(0.012) | 0.08(0.937) | 0.0262(0.717) | 34.25(<0.001) | 0.09 | 2.55 | 0.062 |
Experiment 2: Solid chocolate
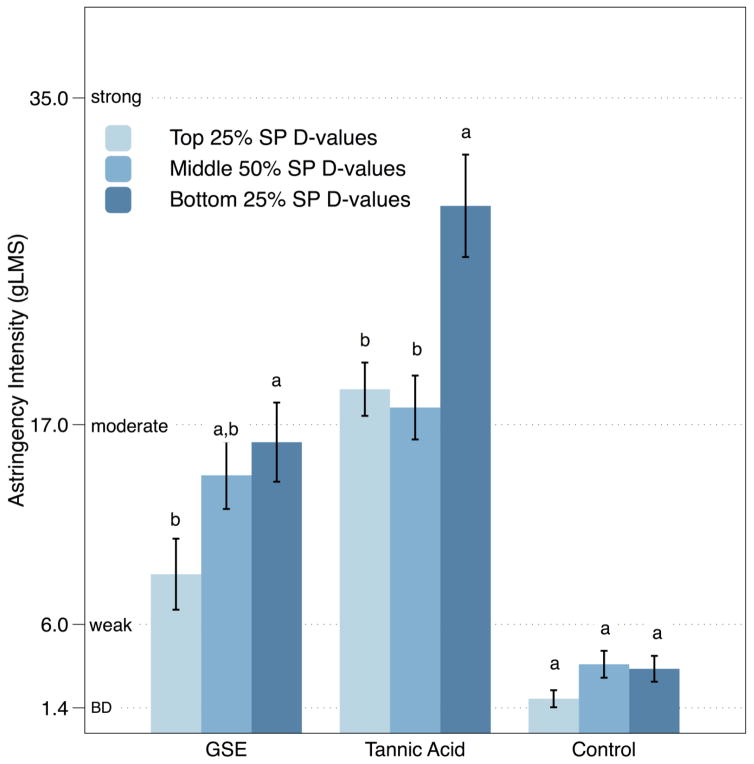
Attribute intensity and hedonic means across all participants are shown in the bottom of Table 1. In separate ANOVA models for each stimulus, the influence of SP D-value group on perceived astringency in chocolate compound fell just below the significance criteria for tannic acid [F(2,78) = 3.24; p = 0.045] and grape seed extract [F(2,78) = 3.12; p = 0.049]; there was no evidence of any differences for the unspiked control [F(2,78) = 1.67; p = 0.194] (Figure 4). Despite the differences in astringency, and in contrast to the aqueous solutions in Experiment 1, there was no evidence that liking differed by SP D-value group for chocolate compound with added tannic acid [F(2,78) = 0.29; p = 0.6], or grape seed extract [F(2,78) = 0.5; p = 0.75]. Consistent with the absence of an effect on astringency in the unspiked control chocolate compound, liking did not differ by SP D-value group [F(2,78) = 0.71; p = 0.50].
Figure 4.
Group differences in perceived astringency for solid chocolate-flavored compound coating with added grape seed extract (GSE), added tannic acid and the unspiked control. The columns represent means and bars are standard errors; within a stimulus, columns which do not share a letter are significantly different at α=0.05 (Tukey’s HSD).
As in experiment 1, we also examined the relationship between SP D-value and astringency using regression (Table 2, and Figure 3, panels C and D), and found no evidence of a relationship between astringency and an individual’s SP D-value for samples with added tannic acid or grape seed extract. Likewise, in the unspiked control, astringency was unrelated to SP D-value. Collectively, these analyses suggest any relationship between SP D-value and perceived astringency in a solid high–fat food like chocolate is likely to be weak at best, at least in a practical sense.
As in experiment 1, we also modeled affective responses as a function of astringency and bitterness via multiple regression (Table 3). In chocolate compound with added grape seed extract, perceived astringency and bitterness were both significant predictors of liking, collectively explaining 32% of the variance in the hedonic scores. Conversely, for the unspiked control and the compound with added tannic acid, bitterness ratings were a significant predictor of overall liking/disliking, while astringency was not. It is also worth noting that the intercept for the tannic acid model is negative, while the intercepts for the grape seed extract and control models are positive. Returning to the overall means in the bottom of Table 1, the compound with added tannic acid tended to be more astringent, more bitter, and less sweet than the other two chocolate compound samples. This suggests that while variation in bitterness and astringency across people influence liking, the overall level of each attribute, irrespective of individual differences, also has a major influence on liking scores.
Discussion
Based on reports that both tannic acid and alum precipitate PRPs present in the saliva [26], we hypothesized that SP D-value would generalize to perceived astringency intensity of alum. Here, mean SP D-values were inside the range reported previously for LR, MR, and HR groups [9, 18]; however, we should also note the SP D-values obtained here also tended to be somewhat lower than previously observed values, which may be attributable to differences in saliva collection protocol with regard to both collection time and resting time between collections (see methods). Despite this minor difference, the HR group here rated the astringency of tannic acid significantly higher than either the LR and MR groups, as was expected from prior work [18]. The same result was observed for alum, thereby extending the work of Dinnella and colleagues to another class of astringent. No significant differences were observed between the MR and LR groups for either stimulus, which may indicate a non-linear relationship between SP D-value and perceived astringency (additional discussion below). The response pattern observed here and by Dinnella and colleagues [18] suggests a minority of individuals (~25% or less) are highly responsive to differences in astringency. Whether this pattern (i.e., HR > MR ≈ LR) is due to underlying genetic variation remains undetermined, although recent data from a twin study suggest there may be a heritable component to the perception and subsequent intake of astringent foods [36].
We observed differences in overall liking/disliking between SP D-value groups for both alum and tannic acid. Although both solutions were disliked, the HR group showed a significantly greater disliking for both as compared to the LR group. A similar relationship was shown previously for apple juices with added tannic acid, with a greater disliking observed for HR relative to the MR and LR groups [18]. This is not surprising, as high levels of astringency are widely cited as a cause for consumers’ rejection of foods [2]. That is, as the HR group experienced greater perceived astringency, it is expected that this group would also exhibit a higher degree of disliking, relative to the LR group. Perceived astringency was a significant predictor of hedonic scores for the alum and tannic acid solutions, even when bitterness was included in the model. This implies the drop in liking was not merely due to increased bitterness from tannic acid. Lea and Arnold [37] characterized bitterness and mouth dryness as ‘twin sensations’ because phenolic astringents like tannic acid are also bitter, and untrained participants may confuse the two sensations. Although we took measures to ensure that participants could differentiate between these sensations, it is possible such confusion may still be present in our data. However, we also note the stimuli were roughly matched for perceived astringency, with alum being slightly more astringent (18.6 versus 15.4 for alum and tannic acid, respectively). This is notable because the tannic acid was substantially more disliked than the alum; this difference is presumably driven by the bitterness of tannic acid (14.0 versus 5.7 for alum) or possibly by the sweetness of the alum (2.86 versus 0.37 for tannic acid). Finally, although individual differences in bitterness perception are well documented [38, 39], the relationship of these differences with respect to astringency is unclear (see Bajec and Pickering [3] for a review). Here, we did not control for 6-n-propylthiouracil (PROP) and phenylthiocarbamide (PTC) phenotypes [40]. However, as no significant differences were observed between SP D-value groups for any other attributes measured, most notably bitterness, it seems unlikely confusion between bitterness and astringency or confounding with bitter phenotype influenced the differences in astringency ratings seen here.
To extend the group-wise scheme used previously (Dinnella et al 2010, 2011), we also examined relationships between SP D-value and astringency via regression. A negative relationship was observed for both tannic acid and alum in water, but the amount of variance explained by SP D-value was modest (33% and 29%, respectively). We are unable to compare our effect sizes directly to prior work, as they do not report R-square values for SP D-values. We explored potential non-linearity in additional models, but no improvement in either R2 values or p-values was observed when quadratic terms were added to the regression model (not shown).
In contrast to the results seen in the aqueous systems, SP D-values showed minimal ability to predict perceived astringency in solid chocolate compound. For the unspiked compound, this could conceivably represent a floor effect, as mean astringency ratings for the control compound did not surpass ‘weak’. Also, while it is possible that SP D-values might have some limited predictive power in very high cocoa polyphenol containing chocolate (e.g. [41], present data from chocolate compound containing elevated levels of condensed tannins (the grape seed extract containing samples) suggest SP D-value is a relatively poor predictor of astringency in a high-fat solid matrix. Notably however, perceived astringency – as opposed to SP D-value – was still a significant predictor of liking for the compound with added grape seed extract, even after controlling for differences in bitterness. While this may seem initially counterintuitive, it is wholly consistent with the idea that astringency occurs via multiple mechanisms, including one that is independent of salivary protein depletion and delubrication.
The chocolate compound containing tannic acid or grape seed extract were both disliked, but the tannic acid sample was disliked more (−42.0 and −8.2; see Table 1). Perceived bitterness had a significant negative influence on liking for both, whereas the influence of astringency on liking was only significant for GSE (Table 3). Although participants were recruited on the basis of their solid chocolate consumption (i.e. self-reported milk or dark solid chocolate consumption greater than once every two weeks), food preferences are extremely complex and can be influenced by a number of factors. These include availability, existing habitual behaviors, socioeconomic status, cultural environment, learning mechanisms, individual expectations, and physiological differences in perceptual abilities [42]. Present results suggest individual differences in salivary protein levels may be relevant for beverage systems containing polyphenols, consistent with [18], but that these differences may be less relevant in solid foods, especially those that are high in fat, as fat presumably provides lubricity. Moreover, the perception of substantial amounts of astringency in tannin spiked chocolate compound despite the high amount of fat in the oral cavity is consistent with the emerging consensus that astringency is not merely a tactile sensation that arises solely from delubrication [8, 43, 44].
Summary and Conclusions
Here, the relationship between SP D-value and perceived astringency was examined for two classes of astringent compounds (i.e., polyphenols and multivalent salts) in water, and two different polyphenols within a complex food (i.e., solid chocolate-flavored confectioners’ compound coating). Previously, SP D-values have been associated with differences in astringency as well as overall liking/disliking of fruit and vegetable juices with added tannic acid [18]. In simple aqueous solutions, we confirmed their findings for polyphenols and extended them to a separate class of astringent stimulus (i.e., the multivalent salt alum). We also used a solid, high fat food matrix, i.e. chocolate-flavored compound to test whether SP D-value would generalize to solid chocolate-flavored compound with added polyphenols — tannic acid (a hydrolyzable tannin) or grape seed extract (a condensed tannin). Unlike the aqueous systems, SP D-values did not predict differences in astringency for solid chocolate.
The utility of SP D-value as an in vitro measure is predicated on the idea that changes in salivary protein levels upon exposure to an astringent are related to individual differences in perceived astringency. Although the tactile nature of astringency is widely supported in the literature, to date, the mechanisms responsible for oral astringency have not been fully elucidated. Emerging evidence suggests astringency may arise via more than one mechanism [8, 43, 44]. Here, we confirm that SP D-value is significantly related to the perceived astringency and liking of astringents in solution, but even in these model systems, it only explains ~30% of the variance in perceived astringency, suggesting it does not capture the mechanistic basis of the percept in its entirety. As currently operationalized, SP D-value only takes into account total change in protein (i.e., the ability of an individual to replenish salivary protein when challenged), but it does not take into account the initial amounts of salivary protein or the types of proteins that are precipitated, which may potentially be a better indication of differential responses to astringent stimuli. However, if astringency arises from multiple mechanisms, including G protein-coupled pathways (e.g. [8], then even more sophisticated methods based on protein replenishment may still have limited utility. Present data also confirm prior work showing an influence of SP D-value on astringency and liking under some conditions, suggesting it may have some utility for wines and other phenolic-containing beverages [18]. Additionally, it should be noted the sub-qualities and side tastes associated with astringent stimuli are complex: whether SP D-value is more predictive of specific sub-qualities like drying, roughing or puckering [45] remains to be determined. Because astringent stimuli come from various classes of chemical compounds, they likely differ in their relative astringent sub-qualities and side tastes, and the mechanisms that give rise to these sensations. Therefore, additional work exploring both the quantitative and qualitative characterization of various types of astringent compounds and how these may contribute to the complexities of this integrated percept is warranted.
Highlights.
Multiple mechanisms have been proposed to explain oral astringency
Depletion of salivary proteins has been associated with differential astringency
Here, protein had a modest relationship with astringency from tannic acid and alum
No relationship between astringency and protein levels were seen in solid chocolate
These data are consistent with multiple mechanisms for astringency
Acknowledgments
The authors would like to thank Rachel Antenucci MS, Rachel Primrose MS, Alissa A. Nolden MS, Demetra Perry, M. Michelle Reyes MS, and Drs. Nadia Byrnes, Alyssa Bakke, and Emma Feeney for assistance in data collection and protocol development, as well as Marlena Sheridan, Dr. Ryan Elias, and Dr. Toral Zaveri for assistance with saliva analysis. We also thank the Pennsylvania State University Statistical Consulting Center for additional guidance, as well as our study participants for their time and participation.
Footnotes
Conflict of interest disclosure
This manuscript was completed in partial fulfillment of the requirements for a Master of Science at the Pennsylvania State University by EEF, who is supported by the Ralph Lee Graduate Fellowship at the Pennsylvania State University; this award is underwritten by PMCA, An International Association of Confectioners. JEH and GRZ have received speaking or consulting fees from corporate clients in the food industry. Additionally, the Sensory Evaluation Center at Penn State routinely conducts taste tests for industrial clients to facilitate experiential learning for students. None of these organizations have had any role in study conception, design or interpretation, or the decision to publish these data. JEH also receives support from a National Institutes of Health grant from the National Institute of Deafness and Communication Disorders [DC010904].
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.ASTM. Standard terminology relating to sensory evaluation of materials and products. Philadelphia, PA: American Society for Testing Materials; pp. 1–3. Annual book of ASTM Standards, 1991. 15.07-end use products (E 253-91a) [Google Scholar]
- 2.Lesschaeve I, Noble AC. Polyphenols: factors influencing their sensory properties and their effects on food and beverage preferences. The American journal of clinical nutrition. 2005;81(1):330S–335S. doi: 10.1093/ajcn/81.1.330S. [DOI] [PubMed] [Google Scholar]
- 3.Bajec MR, Pickering GJ. Astringency: Mechanisms and perception. Critical Reviews in Food Science and Nutrition. 2008;48(9):858–875. doi: 10.1080/10408390701724223. [DOI] [PubMed] [Google Scholar]
- 4.Bate-Smith E. Astringency in foods. Food. 1954;23:124–135. [Google Scholar]
- 5.Bartoshuk LM. History of taste research. Hand book of perception. 1978;6:1. [Google Scholar]
- 6.Brannan G, Setser C, Kemp K. Interaction of astringency and taste characteristics. Journal of sensory studies. 2001;16(2):179–197. [Google Scholar]
- 7.Bennick A. Interaction of plant polyphenols with salivary proteins. Critical Reviews in Oral Biology & Medicine. 2002;13(2):184–196. doi: 10.1177/154411130201300208. [DOI] [PubMed] [Google Scholar]
- 8.Schöbel N, Radtke D, Kyereme J, Wollmann N, Cichy A, Obst K, et al. Astringency Is a Trigeminal Sensation That Involves the Activation of G Protein–Coupled Signaling by Phenolic Compounds. Chemical senses. 2014:bju014. doi: 10.1093/chemse/bju014. [DOI] [PubMed] [Google Scholar]
- 9.Dinnella C, Recchia A, Vincenzi S, Tuorila H, Monteleone E. Temporary modification of salivary protein profile and individual responses to repeated phenolic astringent stimuli. Chemical Senses. 2010;35(1):75–85. doi: 10.1093/chemse/bjp084. [DOI] [PubMed] [Google Scholar]
- 10.Hayes JE, Feeney EL, Allen AL. Do polymorphisms in chemosensory genes matter for human ingestive behavior? Food quality and preference. 2013;30(2):202–216. doi: 10.1016/j.foodqual.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horne J, Hayes J, Lawless HT. Turbidity as a Measure of Salivary Protein Reactions with Astringent Substances. Chemical Senses. 2002;27(7):653–659. doi: 10.1093/chemse/27.7.653. [DOI] [PubMed] [Google Scholar]
- 12.Monteleone E, Condelli N, Dinnella C, Bertuccioli M. Prediction of perceived astringency induced by phenolic compounds. Food Quality and Preference. 2004;15(7):761–769. [Google Scholar]
- 13.Fischer U, Boulton R, Noble A. Physiological factors contributing to the variability of sensory assessments: relationship between salivary flow rate and temporal perception of gustatory stimuli. Food Quality and Preference. 1994;5(1):55–64. [Google Scholar]
- 14.Rodriguez M, Albertengo L, Vitale I, Agullo E. Relationship Between Astringency and Chitosan - Saliva Solutions Turbidity at Different pH. Journal of Food Science. 2003;68(2):665–667. [Google Scholar]
- 15.Fia G, Dinnella C, Bertuccioli M, Monteleone E. Prediction of grape polyphenol astringency by means of a fluorimetric micro-plate assay. Food Chemistry. 2009;113(1):325–330. [Google Scholar]
- 16.Edelmann A, Lendl B. Toward the optical tongue: flow-through sensing of tannin-protein interactions based on FTIR spectroscopy. J Am Chem Soc. 2002;124(49):14741–7. doi: 10.1021/ja026309v. [DOI] [PubMed] [Google Scholar]
- 17.Dinnella C, Recchia A, Fia G, Bertuccioli M, Monteleone E. Saliva Characteristics and Individual Sensitivity to Phenolic Astringent Stimuli. Chemical Senses. 2009;34(4):295–304. doi: 10.1093/chemse/bjp003. [DOI] [PubMed] [Google Scholar]
- 18.Dinnella C, Recchia A, Tuorila H, Monteleone E. Individual astringency responsiveness affects the acceptance of phenol-rich foods. Appetite. 2011;56(3):633–642. doi: 10.1016/j.appet.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 19.Kallithraka S, Bakker J, Clifford MN. Interaction of (+)-catechin, (−)-epicatechin, procyanidin B2 and procyanidin C1 with pooled human saliva in vitro. Journal of the Science of Food and Agriculture. 2001;81(2):261–268. [Google Scholar]
- 20.Guerreiro JRL, Sutherland DS, De Freitas V, Sales MGF. Protein–polyphenol interaction on silica beads for astringency tests based on eye, photography or reflectance detection modes. Analytical Methods. 2013;5(11):2694–2703. [Google Scholar]
- 21.Petrovic SC. Correlation of perceived wine astringency to cyclic voltammetric response. American journal of enology and viticulture. 2009;60(3):373–378. [Google Scholar]
- 22.Peleg H, Bodine KK, Noble AC. The influence of acid on astringency of alum and phenolic compounds. Chemical Senses. 1998;23(3):371–378. doi: 10.1093/chemse/23.3.371. [DOI] [PubMed] [Google Scholar]
- 23.Beecher J, Drake M, Luck P, Foegeding E. Factors regulating astringency of whey protein beverages. Journal of dairy science. 2008;91(7):2553–2560. doi: 10.3168/jds.2008-1083. [DOI] [PubMed] [Google Scholar]
- 24.Yang HH-l, Lawless HT. Descriptive analysis of divalent salts. Journal of Sensory Studies. 2005;20(2):97–113. doi: 10.1111/j.1745-459X.2005.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drobna Z, Wismer WV, Goonewardene LA. Selection of an astringency reference standard for the sensory evaluation of black tea. Journal of sensory studies. 2004;19(2):119–132. [Google Scholar]
- 26.Lee CA, Ismail B, Vickers ZM. The role of salivary proteins in the mechanism of astringency. Journal of food science. 2012;77(4):C381–C387. doi: 10.1111/j.1750-3841.2012.02644.x. [DOI] [PubMed] [Google Scholar]
- 27.Visioli F, Bernardini E, Poli A, Paoletti R. Chocolate and Health. Springer; 2012. Chocolate and Health: A Brief Review of the Evidence; pp. 63–75. [Google Scholar]
- 28.McShea A, Ramiro-Puig E, Munro SB, Casadesus G, Castell M, Smith MA. Clinical benefit and preservation of flavonols in dark chocolate manufacturing. Nutrition reviews. 2008;66(11):630–641. doi: 10.1111/j.1753-4887.2008.00114.x. [DOI] [PubMed] [Google Scholar]
- 29.Bartoshuk LM, V, Duffy B, Fast K, Green BG, Prutkin J, Snyder DJ. Labeled scales (eg, category, Likert, VAS) and invalid across-group comparisons: what we have learned from genetic variation in taste. Food Quality and Preference. 2003;14(2):125–138. [Google Scholar]
- 30.Green BG, Shaffer GS, Gilmore MM. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chemical Senses. 1993;18(6):683–702. [Google Scholar]
- 31.Byrnes NK, Hayes JE. Personality factors predict spicy food liking and intake. Food quality and preference. 2013;28(1):213–221. doi: 10.1016/j.foodqual.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayes JE, Allen AL, Bennett SM. Direct comparison of the generalized visual analog scale (gVAS) and general labeled magnitude scale (gLMS) Food Quality and Preference. 2013;28(1):36–44. doi: 10.1016/j.foodqual.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleming E, Ziegler GR, Hayes JE. Check-All-That-Apply (CATA), Sorting, and Polarized Sensory Positioning (PSP) with Astringent Stimuli. Food quality and preference. 2015 doi: 10.1016/j.foodqual.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee CA, Vickers ZM. Discrimination among astringent samples is affected by choice of palate cleanser. Food Quality and Preference. 2010;21(1):93–99. [Google Scholar]
- 35.Smith P, Krohn RI, Hermanson G, Mallia A, Gartner F, Provenzano M, et al. Measurement of protein using bicinchoninic acid. Analytical biochemistry. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 36.Törnwall O, Dinnella C, Keskitalo-Vuokko K, Silventoinen K, Perola M, Monteleone E, et al. Astringency perception and heritability among young Finnish twins. Chemosensory Perception. 2011;4(4):134–144. [Google Scholar]
- 37.Lea AG, Arnold GM. The phenolics of ciders: bitterness and astringency. Journal of the Science of Food and Agriculture. 1978;29(5):478–483. doi: 10.1002/jsfa.2740290511. [DOI] [PubMed] [Google Scholar]
- 38.Hayes JE, Bartoshuk LM, Kidd JR, Duffy VB. Supertasting and PROP bitterness depends on more than the TAS2R38 gene. Chemical senses. 2008;33(3):255–265. doi: 10.1093/chemse/bjm084. [DOI] [PubMed] [Google Scholar]
- 39.Allen AL, McGeary JE, Hayes JE. Rebaudioside A and Rebaudioside D bitterness do not covary with Acesulfame-K bitterness or polymorphisms in TAS2R9 and TAS2R31. Chemosensory perception. 2013;6(3):109–117. doi: 10.1007/s12078-013-9149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayes JE, Keast RS. Two decades of supertasting: where do we stand? Physiology & behavior. 2011;104(5):1072–1074. doi: 10.1016/j.physbeh.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harwood ML, Ziegler GR, Hayes JE. Tolerance for high flavanol cocoa powder in semisweet chocolate. Nutrients. 2013;5(6):2258–2267. doi: 10.3390/nu5062258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mela DJ. Food choice and intake: the human factor. Proceedings of the Nutrition Society. 1999;58(03):513–521. doi: 10.1017/s0029665199000683. [DOI] [PubMed] [Google Scholar]
- 43.Rossetti D, Bongaerts JHH, Wantling E, Stokes JR, Williamson AM. Astringency of tea catechins: more than an oral lubrication tactile percept. Food Hydrocolloids. 2009;23(7):1984–1992. [Google Scholar]
- 44.Lee CA, Vickers ZM. Astringency of Foods May Not be Directly Related to Salivary Lubricity. Journal of Food Science. 2012;77(9):S302–S306. doi: 10.1111/j.1750-3841.2012.02860.x. [DOI] [PubMed] [Google Scholar]
- 45.Lee CB, Lawless HT. Time-course of astringent sensations. Chemical Senses. 1991;16(3):225–238. [Google Scholar]