Abstract
Introduction and hypothesis
This study aimed to describe a novel strategy to determine the traction forces needed to reproduce physiologic uterine displacement in women with and without prolapse.
Methods
Participants underwent dynamic stress magnetic resonance imaging (MRI) testing as part of a study examining apical uterine support. Physiologic uterine displacement was determined by analyzing uterine location in images taken at rest and at maximal Valsalva. Force-displacement curves were calculated based on intraoperative cervical traction testing. The intraoperative force required to achieve the uterine displacement measured during MRI was then estimated from these curves. Women were categorized into three groups based on pelvic organ support: group 1 (normal apical and vaginal support), group 2 (normal apical support but vaginal prolapse present), and group 3 (apical prolapse).
Results
Data from 19 women were analyzed: five in group 1, five in group 2, and nine in group 3. Groups were similar in terms of age, body mass index (BMI), and parity. Median operating room (OR) force required for uterine displacement measured during MRI was 0.8 N [interquartile range (IQR) 0.62–3.22], and apical ligament stiffness determined using MRI uterine displacement was 0.04 N/mm (IQR 0.02–0.08); differences between groups were nonsignificant. Uterine locations determined at rest and during maximal traction were lower in the OR compared with MRI in all groups.
Conclusions
Using this investigative strategy, we determined that only 0.8 N of traction force in the OR was required to achieve maximal physiologic uterine displacement seen during dynamic (maximal Valsalva) MRI testing, regardless of the presence or absence of prolapse.
Keywords: Apical support, Prolapse, Uterine movement
Introduction
Understanding physiological movements of the uterus in healthy women and how it differs in women with prolapse is an essential part of understanding the etiology of pelvic organ prolapse (POP). Clinically, this information is important for surgical decision making regarding which women with prolapse should have a hysterectomy and which should not. At present, wide variations exist among physicians in their opinions about this issue. One survey of physician opinion on this subject that asked gynecologists whether they would perform a hysterectomy at five different levels of uterine support revealed a 50 % disagreement rate [1]. This difference of opinion exists in part because of an incomplete understanding of how to appropriately assess apical support and a subsequent lack of knowledge regarding the threshold of uterine descent at which hysterectomy should be performed.
The field of biomechanics has a long history of evaluating the functional limits of biological materials. Ideally, measurements should be made that are relevant to the physiological situation encountered during a range of normal activities. At present, much of the available data concerning biomechanical properties of apical suspensory ligaments (cardinal and uterosacral) come from artificial test situations, which may not necessarily reflect how they are loaded in vivo. For example, it is common to perform in vitro uniaxial tension tests on small excised pieces of the uterosacral ligaments and then calculate the tissue stress–strain curves [2, 3]. More recently, in vivo tests performed using cervical traction in the operating room (OR) resulted in force-displacement curves from which the stiffness of the cardinal and uterosacral ligament complexes were calculated [4, 5]. Whether either of these are physiologically relevant measures is unclear.
Dynamic stress magnetic resonance imaging (MRI) provides a means of assessing the relationship between physiological uterine descent and changes in abdominal pressure [6]. However, the critical question concerning how the forces applied along the ligaments with changes in abdominal pressure relate to current measurements of in vitro and in vivo ligament properties remains unanswered. During our work in measuring the in vivo force-displacement behavior of the cardinal and uterosacral ligaments, it became evident that one might be able to estimate the forces placed on the suspensory ligament complex required to reproduce physiologically observed uterine displacement during MRI testing. In other words: How much cervical traction force is required to reproduce the movement seen during a Valsalva maneuver of known pressure?
The aim of our study was to describe a new investigative strategy to address these knowledge gaps, demonstrate the feasibility of using it in women with and without prolapse, and present preliminary data concerning the effect sizes that can be used in planning future large-scale studies. Results of this mechanistic study will be presented in two parts. In Part 1, we compare force displacement behavior in physiologically observed uterine displacement with intraoperative measures. In Part 2, we compare uterus location observed during physiological testing in MRI with intraoperative measurement in the OR.
Methods
This was an analysis of a subset of women who underwent MRI as part of a study at the University of Michigan (Ann Arbor, MI, USA) examining apical uterine support (IRB approval HUM00056743) from September 2012 to September 2013. Women with a full spectrum of pelvic organ support from normal to prolapse were recruited from the gynecology clinics. Inclusion criteria were age ≥18 years planning to have benign gynecologic surgery and willing to undergo MRI. Exclusion criteria were pregnancy (currently or within the past year), prior hysterectomy or surgery for POP, uterine fibroids for >12 weeks or known pelvic inflammatory disease, chronic steroid use, prior pelvic radiation, current treatment for cancer, history of organ transplant, history of vasovagal syncope, neurologic diseases or impairments, mobility issues that would prohibit leg positioning in high lithotomy, metal implants, and claustrophobia or any other contraindications to undergoing MRI.
Women were divided into three groups for analysis according to their pelvic support and cervix location as measured by the Pelvic Organ Quantification (POP-Q) system. We defined apical prolapse as C ≥ −4. and vaginal wall prolapse as Ba and/or Bp ≥ 0. Group 1 comprised women with normal apical and vaginal support, group 2 were women with normal apical support but prolapse of the anterior or posterior vaginal walls, and group 3 comprised women with apical prolapse. Of the nine individuals in group 3, seven also had prolapse of the anterior and posterior vaginal walls. We chose to analyze these groups separately because they may differ in terms of apical ligament properties.
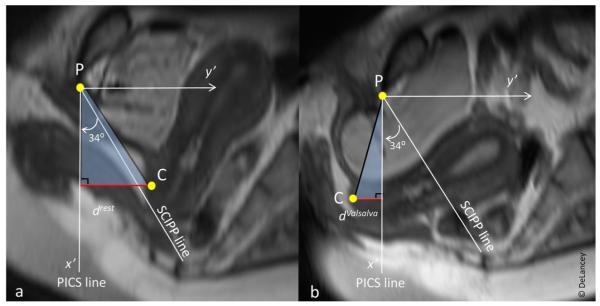
Women underwent dynamic stress MRI in the supine position prior to surgery using a multiple Valsalva technique to achieve maximal prolapse, which has been previously described and validated [7]. MRI was reviewed to confirm that the prolapse was similar in size to that seen during clinical POP-Q examination. Proton-density-weighted fast spin-echo imaging was performed in the axial, sagittal, and coronal planes using a 3-Tesla (3-T) Ingenia MRI scanner (Philips Medical Systems, Best, The Netherlands). Slice thickness was 4 mm with a 1 mm gap. Prior to imaging, a Foley catheter was placed in the bladder and attached to a pressure transducer to monitor abdominal pressure. Twenty milliliters of ultrasound gel were placed in the vagina to delineate the borders. Midsagittal images were taken at rest and during maximal Valsalva. We chose a maximal Valsalva as is done clinically rather than a standardized target pressure for two reasons: First, having a standardized pressure that all individuals are required to achieve requires using a low enough value that all women can generate it, thereby preventing many women from reaching pressures where prolapse is maximally developed. Second, it is difficult for women to adjust their effort during scanning because they are more inhibited, resulting in a suboptimal effort. Once acquired, the images were then used to determine X and Y coordinates for the following points: posterior pubic bone, inferomedial aspect of the fifth sacral vertebra, midpoint of the external cervical os, and the hymenal ring. These coordinates were measured using ImageJ software (Version 1.47, National Institutes of Health, Bethesda, MD, USA) [8] and were verified independently by two experienced researchers for all MRIs. Upon reviewing the images, we observed that with Valsalva, the hymenal ring also moved; therefore, measurements of cervix location relative to the hymenal ring may actually underestimate true uterine movement. In order to more accurately compare uterine movement during MRI to that seen in the OR, we had to calculate the change in cervix location along a plane parallel to the long body axis. The Pelvic Inclination Correction System uses a reference line perpendicular to the long body axis [9]. Therefore, we calculated the vertical distance of the cervix from this reference line at rest (drest) and during maximal Valsalva (dValsalva). Total vertical movement of the cervix was then= ∣dValsalva – drest∣ (Figs. 1 and 2).
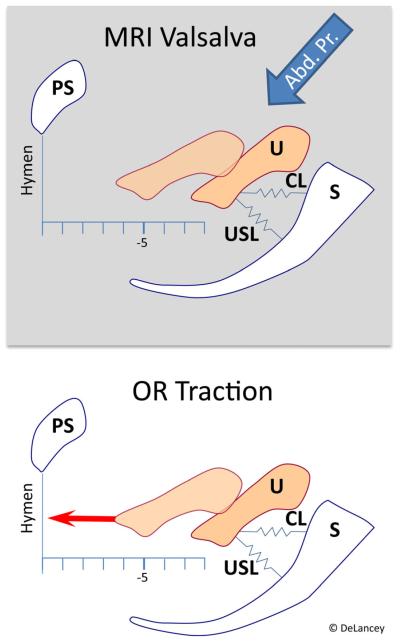
Fig. 1.
Uterine displacement in magnetic resonance imaging vs in the operating room
Fig. 2.
Illustration of how to calculate vertical movement of the cervix using the Pelvic Inclination Correction System (PICS), accounting for pelvic rotation that occurs during Valsalva. P inferior point of the pubic bone; C midpoint of external cervical os; SCIPP sacrococcygeal-inferior pubic point; drest vertical distance of the cervix from the PICS line at rest (negative values above the PICS line, positive values below the PICS line); dValsalva vertical distance of the cervix from the PICS line during maximal Valsalva. Total vertical movement of the cervix = ∣dValsalva – drest∣
The technique for making measurements of cervix location in the OR using a computer-controlled servoactuator device has been previously described [4] and is shown in Fig. 3. To summarize, after induction of anesthesia, location of the anterior lip of the cervix was assessed in the supine position with the legs slightly spread (frog-leg position). The legs were placed in high lithotomy position, and a short-blade Sherback posterior-weight vaginal speculum was placed. A single-tooth tenaculum was then placed across both the anterior and posterior cervical lips and the handle attached to the servoactuator device. Prior to activating the device, resting cervix location was determined by measuring the distance of the lateral cervical edge to the hymenal ring (Rest). The servoactuator then moved at a constant speed to apply a steadily increasing tensile force to the cervix of up to 4 lbs (17.8 N) while simultaneously recording the position of the traction arm. Cervix location at maximal force was determined from these data. Using the force-displacement curves generated during OR testing, we calculated the OR force required to achieve the uterine displacement measured under physiological conditions during MRI. For example, if the uterus descended 2 cm with maximal Valsalva in the MRI, we used the force-displacement curve data measured in the OR to determine the actual force needed for 2 cm of uterine displacement from the resting location for that patient. An estimated stiffness of apical supports of the uterus was calculated by dividing this force by the uterine movement seen in MRI.
Fig. 3.
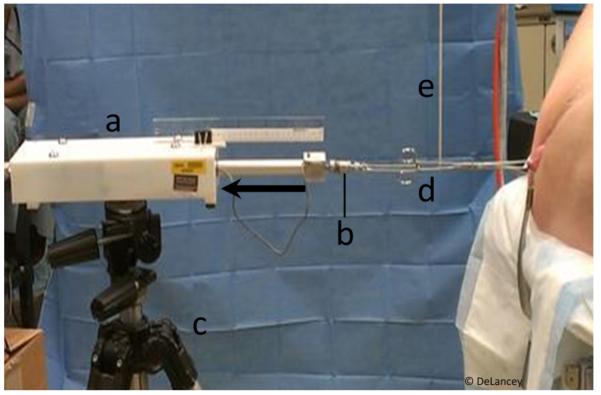
A computer-controlled servoactuator (a) with force transducer (b) mounted on a tripod (c) quantifying force-displacement behavior of the uterine cervix while applying caudally directed tensile force to the handle of a tenaculum (d) supported by a vertical support (e) and attached to the cervix. Blue arrow indicates force vector. Modified from [4]
We performed descriptive statistics on demographic variables of age, parity, and body mass index (BMI, kg/m2), as well as clinic POP-Q points. All data were checked for the assumption of normality. Normally distributed data were analyzed using analysis of variance (ANOVA), while Kruskal–Wallis and Wilcoxon signed-rank tests were used for nonnormally distributed data. Data analyses were performed using SPSS 19 statistical software. Statistical significance was defined at the 5 % significance level.
Results
Twenty women met inclusion criteria; one woman was eventually excluded, as she was unable to effectively perform a Valsalva maneuver during MRI. Therefore, 19 women were included in the final analyses: five with normal pelvic support (group 1), five with normal apical support but vaginal prolapse (group 2), and nine with apical prolapse (group 3). Demographics of the three groups are shown in Table 1. Although there were some differences, groups were statistically similar. By design, women in groups 1 and 2 had similar cervix locations, while those in group 3 had lower cervix location on POP-Q examination.
Table 1.
Demographics of three groups of women with varying degrees of pelvic organ support
| Group 1 Normal support (n = 5) |
Group 2 Normal apex/Vaginal prolapse (n = 5) |
Group 3 Apical prolapse (n = 9) |
P value | |
|---|---|---|---|---|
| Age, years | 46.2 ± 10.4 | 52.2 ± 14.3 | 57.7 ± 10.4 | .23 |
| Parity | 1 (0–3) | 3 (2–3) | 3 (0–4) | .11 |
| BMI kg/m2 | 29.9 ± 6.8 | 28.0 ± 4.6 | 28.0 ± 7.70 | .87 |
| POP-Q point C | −6 (−7 to −6) | −5 (−10 to −5) | −3 (−4 to 5) | .001 |
| Point Ba | −2 (−3 to −1) | 1 (0 to 3) | 1 (−1 to 5) | .006 |
| Point Bp | −3 (−3 to −1) | 0 (−2 to 0) | −1 (−3 to 0) | .11 |
Data reported as mean ± standard deviation (SD) or median (total range). P values determined using analysis of variance (ANOVA) for comparisons of means and Kruskal Wallis test for parity and Pelvic Organ Prolapse Quantification system. All locations measured in centimeters relative to hymenal ring at rest, which is defined as 0 BMI body mass index
Part 1: During MRI, women in group 1 had the smallest amount of total uterine movement (uterine displacement) (Table 2). Compared with group 1, the uterus moved twice as much in group 2 and 2.5 times further in group 3. Abdominal pressure generated during maximal Valsalva in the MRI machine was then compared across groups. Abdominal pressure was highest in women in group 1, but the differences across the three groups did not differ significantly. For all groups, the traction force needed to obtain physiologic uterine movement seen in the MRI was <1 N and was similar across groups. Likewise, estimated stiffness was low and similar across groups.
Table 2.
Uterine displacement in magnetic resonance imaging (MRI)
| All (n = 19) | Group 1 Normal support (n = 5) |
Group 2 Normal apex/Vaginal prolapse (n = 5) |
Group 3 Apical prolapse (n = 9) |
P value | |
|---|---|---|---|---|---|
| OR force required for MRI uterine displacement (N) |
0.80 (0.62 to 3.22) | 0.79 (0.31 to 3.16) | 0.62 (0.52 to 1.39) | 0.88 (0.77 to 8.53) | .06 |
| MRI uterine displacement (cm)a | 3.5 (2.1 to 4.6) | 1.9 (1.1 to 2.5) | 3.9 (2.3 to 4.3) | 4.6 (3.4 to 5.7) | .01 |
| MRI abdominal pressure generated during uterine displacement (mmHg) |
55.0 (46.0 to 71.0) | 71.0 (46.5 to 83.0) | 49.0 (28.0 to 70.0) | 55.0 (47.5 to 64.0) | .38 |
| MRI apical ligament stiffness (N/mm)b | 0.04 (0.02 to 0.08) | 0.04 (0.03 to 0.12) | 0.02 (0.01 to 0.05) | 0.04 (0.02 to 0.14) | .47 |
Data reported as median (interquartile range). P values determined using Kruskal–Wallis. All locations measured in centimeters relative to the hymenal ring at rest, which is defined as 0
Cervix displacement is total movement of the cervix from rest to maximal force in magnetic resonance imaging (MRI)
Estimated stiffness of uterosacral/cardinal ligaments calculated using the operating room (OR) force (N) required to recreate MRI cervix displacement and MRI cervix displacement (mm)
1 N = 0.22 lb
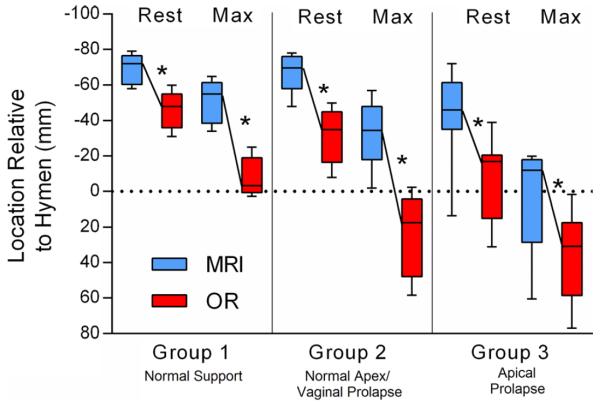
Part 2: Comparison of cervix locations at rest and during maximal traction in MRI vs OR is shown in Fig. 4 and numerical data presented in Supplemental Table S1. In all groups, cervix location at rest was consistently and significantly more caudal in the OR than in MRI. Similar results were found for cervix location at maximal traction. Uterine displacement was significantly greater in the OR vs MRI in women in groups 1 and 2; however, the uterus moved a similar amount in the OR and MRI in the women in group 3. When we analyzed the relationship between uterine displacement and abdominal pressure generated during maximal Valsalva, we found that uterine movement was most highly correlated with abdominal pressure in group 1 (R2 = 0.91, p = .01) vs group 2 (R2 = 0.43, p = .23) and group 3 (R2 = 0.07, p = .48).
Fig. 4.
Uterine location at rest and during maximal traction in magnetic resonance imaging (MRI) vs operating room (OR). All locations shown as millimeters relative to the hymenal ring, which is defined as 0. *Statistical significance, p < .05
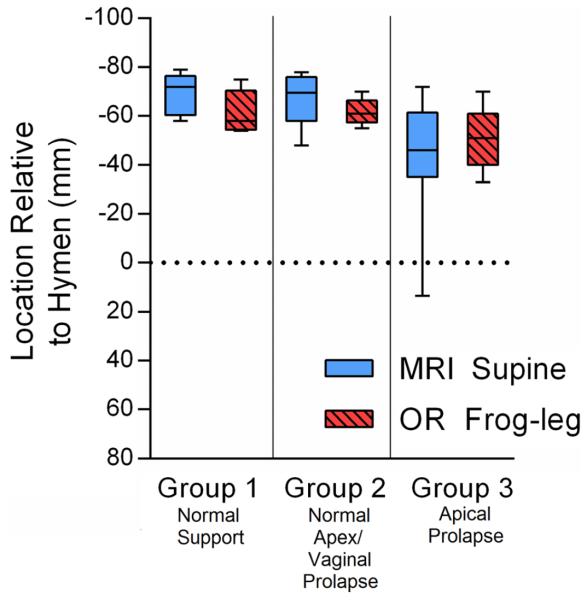
To identify whether a difference in leg positioning (supine in MRI and high lithotomy in the OR) was a possible explanation for why cervix location was lower in the OR, we compared resting cervix location in the frog-leg position to that in the lithotomy position (Fig. 5). In all three groups, we found similar resting cervix locations with MRI and OR frog-leg positioning (data presented in Supplemental Table S2).
Fig. 5.
Uterine Location at Rest: supine in magnetic resonance imaging (MRI) vs Frog-Leg in operating room (OR). All locations shown as millimeters relative to the hymenal ring, which is defined as 0. No significant differences observed between resting locations in all groups
Discussion
We present a novel technique that allows estimation of the amount of tension placed on the apical supports during measured physiological changes in abdominal pressure. A remarkably small traction force, equivalent to <0.2 lb (90 g), was required in the OR to replicate the physiologic uterine movement seen during MRI. To add perspective, a standard metal Grave’s bivalve speculum weighs ~0.4 lb (181 g), which is double the upper limit of the amount of traction force required for maximal uterine movement in the current study. Therefore, the tension placed on the apical ligaments during physiologic events such as Valsalva is small. These findings suggest that the factors involved in determining the location of the uterus are more complex than are simply accounted for by the mechanical properties of the apical supports.
Regarding clinical relevance of our findings: It is common practice for surgeons to apply intraoperative cervical traction in order to assess the degree of uterine descent. A decision regarding whether or not a hysterectomy and/or apical suspension procedure will be performed is often based on the degree of uterine descent seen during this assessment. A study by Foon et al. found that the average traction force applied during this intraoperative assessment for ten gynecologists was 7.9 lb (3.6 kg) [10]. However, our results show that this traction force is 40 times higher than the force required in the OR to achieve maximal physiologic uterine descent during maximal Valsalva in the MRI. Therefore, because supraphysiologic cervical traction may yield a greater degree of uterine descent, this common practice may be biasing surgeons toward performing unnecessary procedures, such as hysterectomy.
Regarding how these findings relate to mechanical properties published in the literature: We calculated apical ligament stiffness using the OR force data and uterine displacement seen during MRI. Apical ligament stiffness was low and did not differ by prolapse group. However, results from in vitro ligament stiffness testing have yielded considerably different results. Using classical techniques for measuring tensile stiffness, Rivaux et al. determined stress–strain curves for excised segments of the uterosacral ligaments of fresh cadavers without POP by performing uniaxial tension tests [2]. Based on their reported data, it would take 26 N, (or 5.8 lb) to stretch the uterosacral ligament by 1 mm (see “Appendix” for calculation). In comparison, we found that the in vivo force required for 1 mm of uterine displacement, and therefore, deformation of the uterosacral and cardinal ligament complexes, was only 0.04 N, or 650 times less. The difference between results of traditional material properties testing and in vivo testing can be understood by recognizing the different testing conditions. Traditional tensile testing evaluates the material behavior of an excised piece of tissue. It is placed in the testing apparatus, and preconditioning is performed that can change the properties of the sample [11]. This involves repetitive cyclic loading prior to data collection to improve test reproducibility. Since the cardinal ligaments have a lattice-like arrangement [12], it seems likely that this preconditioning straightens the fibers so that they are all aligned, making the material properties much stiffer than would be true when loaded in vivo, where the lattice work is open. Results from our physiologic testing suggest that apical ligament stiffness is not the primary factor contributing to uterine support. In a study by Smith et al., in vivo ligament stiffness only explained 19 % of the variation in POP-Q point C [4]. Therefore, when thinking about the etiology of apical support loss, we may presently have a tendency to overestimate the role of apical ligament stiffness and underestimate other important factors, such as ligament length. If so, we must also consider what other factors influence uterine movement.
Although the OR traction force required to achieve maximal uterine displacement seen in MRI was similar in the three groups of women with varying degrees of prolapse, these groups did differ in regards to the magnitude of correlation between abdominal pressure and uterine displacement in MRI. Abdominal pressure accounted for 91 % of the variability seen in uterine displacement in women in group 1. The correlations were low and nonsignificant in women in groups 2 and 3. Therefore, our results show that it takes more intra-abdominal pressure to move the uterus in women with normal pelvic support compared with women with prolapse. As such, we can infer that different factors determine uterine movement in women with prolapse vs those without.
Another observation we made when comparing prolapse groups was the amount of uterine displacement measured in the OR vs MRI. We found that for women in groups 1 and 2, uterine displacement was greater in the OR compared with that during MRI; but for women in group 3, uterine displacement was the same in MRI as it was in the OR. This observation led us to compare the difference in uterine locations seen in the OR vs MRI in Part 2. We found that uterine location at rest and during maximal traction force was universally lower in the OR compared with MRI. In fact, uterine location at rest in the OR was equal to or lower than the maximal Valsalva location seen in MRI for 90 % (17/19) of women. Although this comparison has not been previously reported, Crosby et al. reported that cervix location is, on average, 3.5 cm lower in the OR under traction vs POP-Q exam in clinic during maximal Valsalva [13]. We hypothesized that conditional differences between the OR and MRI environments may help explain some of these findings.
Leg positioning was one factor that differed between the OR and MRI. In the OR, women were in high-lithotomy position, while in MRI they were supine. We know from the literature that leg positioning can affect uterine position. In a study by Barber et al., a higher degree of prolapse was seen in women examined in the upright vs low-lithotomy position [14]. When we compared uterine location at rest during frog-leg position in the OR to supine resting location in MRI, we found similar locations for all prolapse groups. From this finding, we can hypothesize that high-lithotomy leg positioning may indeed result in lower uterine location at rest; however, leg positioning alone does not fully explain the difference in uterine locations we observed between the OR and MRI. The effects of anesthesia on uterine location is another variable that may lead to a lower uterine position in the OR, because general anesthetics could act on the smooth muscle in the uterosacral and cardinal ligaments to cause lengthening, and also on the skeletal muscle of the levator hiatus to cause a widening effect. Furthermore, OR testing was performed with a posterior-weighted speculum in place, which also results in a wider genital hiatus compared with MRI testing.
Although our study is limited by a small sample size, we found significant differences in the small groups, but we cannot make conclusions regarding differences that were not statistically significant, as these may become significant with a larger sample. However, our data can provide effect sizes that may be used in planning subsequent investigations. While it would have been ideal to perform the MRI and servoactuator testing simultaneously, the motor in the servoactuator device is not MRI compatible, and therefore, this was not possible in the current study. Furthermore, Valsalva force generated during clinical POP-Q exam and MRI testing varies by individual and is not standardized; however, our goal is to have the prolapse maximally developed. Finally, we recognize that ultrasound is a more widely accessible imaging tool than MRI. However, being able to make measurements relative to the hymenal ring without artefacts introduced from a transducer pressed on the perineum or a probe in the vagina is essential for the most precise and accurate measurements possible. Strengths of our methods include the use of a custom-designed computer-controlled servoactuator device able to make highly accurate force-displacement measurements, as well as the use of well-developed MRI strategies for quantifying physiological movements. It would be ideal to perform these studies in the standing posture, but at the present time, this has not been feasible, and comparison of upright and supine images of the pelvic floor do not show major differences [15]. Additionally, our technique using dynamic stress MRI to assess physiologic uterine movement allows for precise imaging and measurement of uterine location without instrumentation of the vagina.
In conclusion, we describe a novel investigative strategy to determine traction forces needed to reproduce physiologic uterine displacement in women with and without prolapse. We found that very little traction force in the OR is required to achieve maximal physiologic uterine displacement seen during MRI testing, regardless of the presence or absence of prolapse. Our preliminary data may be useful in designing larger, adequately powered, studies to further assess the various factors contributing to observed differences in physiologic uterine position and uterine displacement to that seen in the OR among women with varying degrees of prolapse.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health Office of Research on Women’s Health grant P50 HD044406 and the National Institute of Child Health and Human Development grant R01 HD038665. Investigator support for CWS was provided by the National Institute of Child Health and Human Development WRHR Career Development Award K12 HD065257.
Appendix
Calculation of the force needed to elongate uterosacral ligament by 1 mm based on in vitro ligament stiffness data [2].
Where ε = strain (m/m)
dL= elongation of the uterosacral ligament (mm)
L = length of the uterosacral ligament≈ 38 mm [16]
σ = stress (N/m2)
F = force (N)
A = cross sectional area of uterosacral ligament ≈ 1 cm2 (based on senior author’s in surgery measurement)
E = Young’s modulus (N/m2) ≈ 10 MPa [2]
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00192-016-2980-1) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflicts of interest None.
References
- 1.Coats E, Agur W, Smith P. When is concomitant vaginal hysterectomy performed during anterior coloporrhaphy? A survey of current practice amongst gynaecologists. Int Urogynecol J. 2010;21(suppl 1):S158–S160. [Google Scholar]
- 2.Rivaux G, Rubod C, Dedet B, Brieu M, Gabriel B, Cosson M. Comparative analysis of pelvic ligaments: a biomechanics study. Int Urogynecol J. 2013;24(1):135–139. doi: 10.1007/s00192-012-1861-5. [DOI] [PubMed] [Google Scholar]
- 3.Chantereau P, Brieu M, Kammal M, Farthmann J, Gabriel B, Cosson M. Mechanical properties of pelvic soft tissue of young women and impact of aging. Int Urogynecol J. 2014;25(11):1547–1553. doi: 10.1007/s00192-014-2439-1. [DOI] [PubMed] [Google Scholar]
- 4.Smith TM, Luo J, Hsu Y, Ashton-Miller J, Delancey JO. A novel technique to measure in vivo uterine suspensory ligament stiffness. Am J Obstet Gynecol. 2013;209(5):484.e1–7. doi: 10.1016/j.ajog.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo J, Smith TM, Ashton-Miller JA, DeLancey JO. In vivo properties of uterine suspensory tissue in pelvic organ prolapse. J Biomech Eng. 2014;136(2):021016. doi: 10.1115/1.4026159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu Y, Chen L, Summers A, Ashton-Miller JA, DeLancey JO. Anterior vaginal wall length and degree of anterior compartment prolapse seen on dynamic MRI. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(1):137–142. doi: 10.1007/s00192-007-0405-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tumbarello JA, Hsu Y, Lewicky-Gaupp C, Rohrer S, DeLancey JO. Do repetitive Valsalva maneuvers change maximum prolapse on dynamic MRI? Int Urogynecol J. 2010;21(10):1247–1251. doi: 10.1007/s00192-010-1178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider CA, Rasband WS, Eliceiri KW. NIH image to image J: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betschart C, Chen L, Ashton-Miller JA, Delancey JO. On pelvic reference lines and the MR evaluation of genital prolapse: a proposal for standardization using the pelvic inclination correction system. Int Urogynecol J. 2013;24(9):1421–1428. doi: 10.1007/s00192-013-2100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foon R, Agur W, Kingsly A, White P, Smith P. Traction on the cervix in theatre before anterior repair: does it tell us when to perform a concomitant hysterectomy? Eur J Obstet Gynecol Reprod Biol. 2012;160(2):205–209. doi: 10.1016/j.ejogrb.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Schatzmann L, Brunner P, Stäubli HU. Effect of cyclic preconditioning on the tensile properties of human quadriceps tendons and patellar ligaments. Knee Surg Sports Traumatol Arthrosc. 1998;6(Suppl 1):S56–S61. doi: 10.1007/s001670050224. [DOI] [PubMed] [Google Scholar]
- 12.Range RL, Woodburned RT. The gross and microscopic anatomy of the transverse cervical ligament. Am J Obstet Gynecol. 1964;15(90):460–467. doi: 10.1016/0002-9378(64)90802-6. [DOI] [PubMed] [Google Scholar]
- 13.Crosby EC, Sharp KM, Gasperut A, Delancey JO, Morgan DM. Apical descent in the office and the operating room: the effect of prolapse size. Female Pelvic Med Reconstr Surg. 2013;19(5):278–281. doi: 10.1097/SPV.0b013e31829c6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barber MD, Lambers A, Visco AG, Bump RC. Effect of patient position on clinical evaluation of pelvic organ prolapse. Obstet Gynecol. 2000;96(1):18–22. doi: 10.1016/s0029-7844(00)00859-0. [DOI] [PubMed] [Google Scholar]
- 15.Fielding JR, Versi E, Mulkern RV, Lerner MH, Griffiths DJ, Jolesz FA. MR imaging of the female pelvic floor in the supine and upright positions. J Magn Reson Imaging. 1996;6(6):961–963. doi: 10.1002/jmri.1880060622. [DOI] [PubMed] [Google Scholar]
- 16.Luo J, Betschart C, Chen L, Ashton-Miller JA, DeLancey JO. Using stress MRI to analyze the 3D changes in apical ligament geometry from rest to maximal Valsalva: a pilot study. Int Urogynecol J. 2014;25(2):197–203. doi: 10.1007/s00192-013-2211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.