Abstract
Purpose
Prognostic markers that identify patients with stage II colon cancers (CC) who are at risk of recurrence are essential to personalize therapy. We evaluated the potential of GIV/Girdin as a predictor of recurrence risk in such patients.
Experimental Design
Expression of full-length GIV was evaluated by immunohistochemistry (IHC) using a newly developed monoclonal antibody together with a mismatch repair (MMR)-specific antibody panel in three stage II CC patient cohorts, ie. a training (n=192), test (n=317), and validation (n=181) cohort, with clinical follow-up data. Recurrence risk stratification models were established in the training cohort of T3, proficient MMR (pMMR) patients without chemotherapy and subsequently validated.
Results
For T3 pMMR tumors, GIV expression and the presence of lymphovascular invasion (LVI) were the only factors predicting recurrence in both training (GIV: HR:2.78, p=0.013; LVI: HR 2.54, p=0.025) and combined test and validation (pooled) cohorts (GIV, HR:1.85, p=0.019; LVI, HR:2.52, p=0.0004). A risk model based on GIV expression and LVI-status classified patients into high- or low-risk groups; 3-year recurrence-free survival was significantly lower in the high-risk versus low-risk group across all cohorts (Training: 52.3% versus 84.8%; HR:3.74, 95%CI: 1.50–9.32; Test: 85.9% versus 97.9%, HR:7.83, 95%CI:1.03–59.54; Validation: 59.4% versus 84.4%, HR:3.71, 95%CI: 1.24–11.12).
Conclusions
GIV expression status predicts recurrence risk in patients with T3 pMMR stage II CC. A risk model combining GIV expression and LVI-status information further enhances prediction of recurrence. Further validation studies are warranted before GIV status can be routinely included in patient management algorithms.
Keywords: GIV, prognostic marker, recurrence, MMR proficient, colon cancer
INTRODUCTION
Stage II colon cancer (CC) represents about 25% of all CC, and surgical resection typically achieves cure in up to 80% of cases (1). For a group of approximately 20% of patients with microsatellite unstable stage II cancers, the prognosis is favourable, and these patients do not benefit from fluoropyrimidine-based adjuvant chemotherapy (2, 3). For the remaining approximately 80% of patients with microsatellite stable stage II cancers, the uncertain benefit from adjuvant treatment (4, 5) poses a management dilemma.
Despite the limited survival impact of adjuvant treatment (6), up to 38% of patients receive adjuvant chemotherapy (7). Consequently, instead of reserving adjuvant chemotherapy for high-risk patients that are likely to derive a greater absolute benefit from treatment, a significant proportion of patients are exposed to potentially toxic treatment only to achieve a modest survival advantage. Conventional high-risk clinico-pathological features include T4 extension, bowel perforation or obstruction, inadequate nodal sampling, poorly differentiated histology, lymphovascular invasion (LVI), peri-neural invasion, and close or positive margins (4, 8, 9). There is currently no compelling evidence that patients with these high-risk features are more likely to benefit from chemotherapy (10). More specifically, it has been demonstrated that the tumor size and lymph node positivity scores (TN system), combined with all clinico-pathologic factors used today, fall short in predicting relapse, particularly for stage II CC (11).
Recently, prediction of recurrence risk in stage II CC through an in-depth molecular analyses of the resected specimen has been attempted. In particular, several gene expression-based prognostic tests are being evaluated and are at varying stages of development (12). Though some of these gene expression signatures have been validated in independent patient cohorts, they are not currently recommended for use in risk assessment and in the determination of adjuvant treatment by clinical guidelines due to the lack of prospective study data to validate their clinical utility (13, 14). Despite a modest association with prognosis [hazard ratios (HR) for patients with high-risk recurrence scores of around 2 to 2.5] and high costs associated with these tests [cost of Oncotype DX assay is US $3200], cost-benefit analysis projected that the routine use of the Oncotype DX assay may reduce adjuvant chemotherapy use by 17% without decreasing quality-adjusted life expectancy, and that it may also decrease direct medical costs by an average of $2971 per patient (15). A less expensive prognostic test, such as an immunohistochemistry-based (IHC) assay, with a similar or higher HR could conceivably further improve the cost-effectiveness and practicality of this risk stratification approach.
Gα-interacting vesicle-associated protein (GIV; also known as Girdin) is a bona fide metastasis-related protein that modulates multiple signaling pathways triggered by diverse classes of receptors [reviewed in (16–19)]. Among the numerous pathways that GIV affects are the prometastatic PI3K/Akt and STAT3 signaling pathways (17, 19, 20). Mechanistically, GIV modulates multi-receptor signaling via its fundamental ability to serve as a guanine exchange factor (GEF) for heterotrimeric G protein, Gαi. GIV couples Gαi proteins to various types of ligand-activated receptors, e.g., growth factor receptor tyrosine kinases (RTKs), integrins, GPCRs, toll-like receptors, etc., many of which are known to engage in tyrosine-based signaling [reviewed in (21, 22)]. By virtue of its ability to link G proteins to multiple receptor classes, GIV facilitates the transactivation of Gαi proteins in response to tyrosine-based signals that are initiated by a variety of external cues.
Consistent with its ability to serve as a central hub for modulation of multi-receptor/pathway signaling, GIV is involved in a wide range of biological processes such as cancer cell migration, tumor angiogenesis, tumor-stroma interaction during cancer progression, cancer invasion, epithelial wound healing, organ fibrosis, neuronal migration, memory formation, macrophage chemotaxis, and vascular repair (17, 19, 23–26). The finding that GIV and its GEF function is essential for signal enhancement (such as PI3K/Akt) and for actin remodeling during cancer cell migration and invasion in vitro (25, 27) led to the discoveries that GIV is essential for tumor invasion and metastasis in murine models (27–32). Depletion of GIV impairs metastasis and inhibits VEGF-mediated neoangiogenesis (27, 32), further supporting GIV’s role in tumor progression. Consistent with its ability to signal at the ‘hub’ of multiple upstream receptors and multiple downstream pathways (17, 19) and its ubiquitous expression pattern (33), GIV is found to be expressed in a wide range of cancers including colorectal, breast, esophageal, gastric, glioblastoma multiforme, lung, and hepatocellular carcinomas [reviewed in (19)]. Its expression at high levels invariably correlates with aggressiveness across the spectrum of tumors (34–38).
In the context of colorectal cancer, full-length GIV has been found to be overexpressed in cells with high metastatic potential and is virtually undetectable in those with poor metastatic potential (29, 39). Furthermore, high GIV expression levels correlate with disease progression (34, 39) and may contribute to the development of chemoresistance (40). Although a previous exploratory study in a small cohort of stage II CC patients indicated that GIV expression may be more prevalent in microsatellite stable tumors and may be associated with a worse prognosis (39), the relevance of those findings in the clinical setting has not been rigorously examined.
In this study, a novel rabbit monoclonal antibody against full length GIV was developed and evaluated by IHC, alongside four other antibodies against mismatch repair (MMR) proteins, in a training cohort of stage II CC patients. Besides the relationship between MMR-status and GIV, the relationship between GIV expression and the gold-standard histopathologic criteria currently used to assess tumor aggressiveness was also evaluated. To eliminate the confounding effects of chemotherapy, recurrence risk stratification and prediction models were developed in this training cohort using the subgroup of patients with MMR proficient (pMMR), T3 tumors, that did not receive adjuvant chemotherapy, who have the greatest clinical need for a prognostic biomarker. The prognostic accuracy of such risk prediction model was further tested on an internal test cohort and subsequently validated using another independent patient cohort.
MATERIALS AND METHODS
Patient Cohorts
For the training and internal testing cohorts, whole tissue sections and data were obtained from 509 consecutive patients who underwent surgical resection of stage II CC at the Royal Melbourne Hospital and Western Hospital, Australia, between 2001 and 2011. Patients were identified from the prospective Australian Comprehensive Cancer Outcomes and Research Database (ACCORD) colorectal cancer database. ACCORD is maintained and managed by BioGrid Australia® and includes prospectively collected multi-disciplinary data relating to diagnosis, histopathological features, patient characteristics, treatment, and outcomes for all patients treated at participating sites. Point of care follow-up data are collected at each clinical visit, including any cancer recurrence. Eligibility criteria for the current study included available archived tumor tissue and follow-up data for at least 24 months. Among the consecutive series of 509 stage II cases, a sub-set of 192 patients was designated as the training cohort. To avoid problems associated with estimating parameters for strata containing no events, the training cohort was purposefully enriched for patients who had recurrence of disease, to bring up the overall recurrence rate to 25% (i.e. 50 patients with recurrence and 150 patients without recurrence). The remaining 317 cases in our consecutive series of 509 stage II cases (with a lower percentage with recurrence) were assigned to the internal testing set.
As an independent validation set, we studied another 181 pMMR stage II CC cases provided as cores within tumor microarrays from the EPICOLON consortium in the independent validation set. The EPICOLON consortium, initiated in 1999 by the Gastrointestinal Oncology Group of the Spanish Gastroenterology Association, is a prospective, multicentre, Spanish study collecting clinical data and biological samples from consecutive, unselected, population-based, colorectal cancer cases without personal or familial history of cancer.
Pathology data were queried including tumor site, number of lymph nodes sampled, tumor differentiation, T stage, and lymphovascular invasion. Although the presence or absence of lymphovascular invasion (LVI) was not centrally reviewed for all cases, these were reported by expert pathologists at major centres that participated in this study. Only tumor tissue samples containing more than 50 viable tumor cells were used for MMR and GIV analysis. We assessed tumor tissue MMR status for the training and internal testing sets and GIV status for all 3 cohorts of patients. The human research ethics committees at each hospital approved this study.
Cell Culture and siRNA Transfection
Cos7 cells were cultured according to American Type Culture Collection guidelines. Silencer negative control scrambled (Scr) siRNA used as control was purchased from Ambion; a previously validated GIV siRNA sequence (31) was custom-ordered from Dharmacon used as described previously (41).
Cell Lysis and Immunoblot Analysis
Whole cell lysates were prepared after washing cells with cold phosphate-buffered saline solution (PBS) prior to resuspending and boiling them in sample buffer. Cell lysates for immunoblotting were run on SDS/PAGE gels and transferred onto PVDF membranes (Millipore, Berford, MA). Membranes were blocked with PBS containing 5% non-fat milk before incubation with primary antibodies. GIV was detected using rabbit anti-GIV-CT (Girdin T-13; Santa Cruz Biotechnologies, Dallas, TX) (1:500) or anti-GIV (SP173; Spring Bioscience, Pleasanton, CA). Goat anti-rabbit and goat anti-mouse Alexa Fluor 680 or IRDye 800 F(ab’)2 used for immunoblotting were from Li-COR Biosciences (Lincoln, NE). Images were acquired using LiCOR Odyssey, processed with ImageJ software (NIH), and assembled as figure panels using Photoshop and Illustrator software (Adobe).
Immunohistochemistry
All IHC assays were developed and performed on the VENTANA BENCHMARK XT automated staining instrument at Ventana Medical Systems, Inc., Tucson, AZ. MMR status was assessed using VENTANA anti-MLH1 (M1) mouse monoclonal, VENTANA anti-MSH2 (G219–1129) mouse monoclonal, CONFIRM anti-MSH6 [clone 44] mouse monoclonal, and VENTANA anti-PMS2 (EPR3947) rabbit monoclonal primary antibodies (Ventana Medical Systems, Inc.).
Formalin-fixed, paraffin-embedded tissue samples (4 µm section) were deparaffinized, pretreated with Cell Conditioning 1 for antigen retrieval (64 min for MLH1, PMS2, and MSH6, and 40 min for MSH2), then treated to inactivate the endogenous peroxidases, followed by incubation with anti-MLH1 primary antibody at room temperature, and with PMS2, MSH2, and MSH6 primary antibodies at 37 °C for 12 min. Antigen-antibody reactions were visualized using Optiview DAB Detection Kit (Ventana Medical Systems, Inc.). To enhance the DAB signal of PMS2 detection, signal amplification (Optiview Amplification Kit, Ventana Medical Systems, Inc.) was utilized for 8 min. After chromogenic detection, all slides were counterstained with Hematoxylin II and Bluing Reagent (Ventana Medical Systems, Inc.) for 4 minutes each, and coverslips were applied.
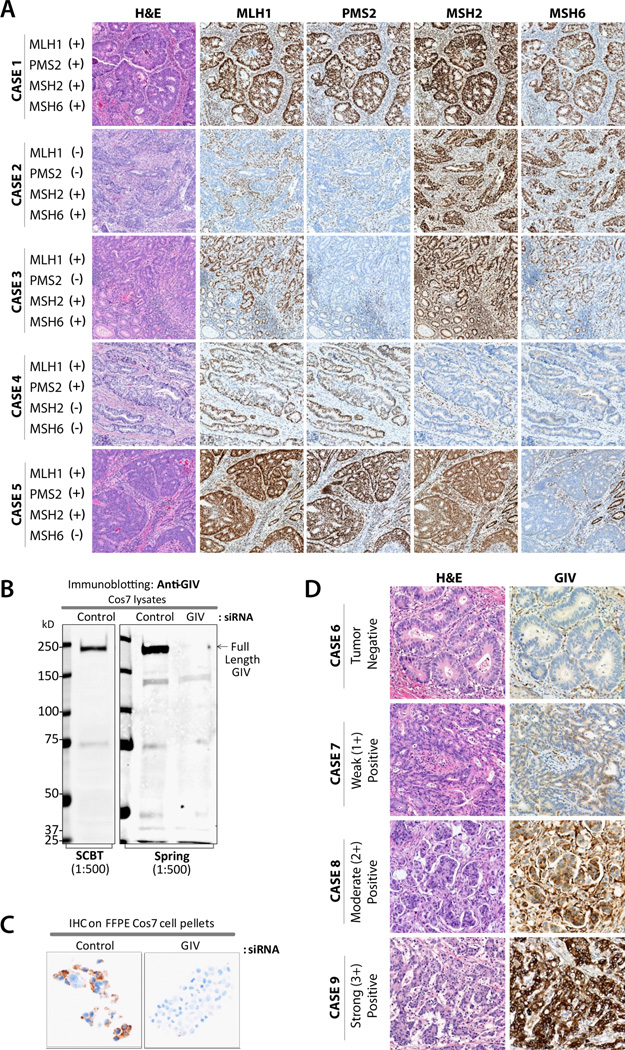
Immunostaining of MMR markers was evaluated for the unequivocal presence or absence of nuclear protein expression in viable tumor cells in the presence of nuclear staining within the internal control cells (lymphocytes, stromal cells, or normal colonic epithelium adjacent to the tumor). Tumor was considered deficient (dMMR) when the tissue lacked staining for one or more MMR proteins; or pMMR when all four MMR proteins were present in malignant cells (Figure 1A).
Figure 1. Immunohistochemical staining of primary colon tumors for mismatch repair (MMR) proteins and GIV.
(A) A proficient MMR case (Case 1) shows intact nuclear staining for all four MMR proteins in tumor and stromal tissue. Deficient MMR cases (Cases 2–5) represent loss of MLH1, PMS2, MSH2, and/or MSH6 proteins, respectively, in tumor cells, while the intact nuclear staining is preserved in stromal cells or normal colonic epithelium. (B) Equal aliquots (~ 75 ug) of whole cell lysates of control or GIV-depleted Cos7 cells were analyzed for GIV expression by immunoblotting with anti-GIV antibodies as indicated. (C) Pellets of control and GIV-depleted Cos7 cells were fixed, embedded in paraffin and analyzed for GIV expression by IHC with anti-GIV antibody. (D) A representative case (Case 6) negative for GIV expression. Cases 7–9 demonstrate weak, moderate, and strong GIV expression, respectively, in tumor cells.
For GIV IHC expression analysis, a rabbit monoclonal, anti-GIV antibody (SP173; Spring BioScience Corp.) was raised against the C-terminus of human GIV. To analyze GIV expression in tumors, the tissue sections were deparaffinized, pretreated in Cell Conditioning 1 for 48 min, treated to inactivate the endogenous peroxidases, and incubated with anti-GIV rabbit monoclonal antibody at 37 °C for 12 min. The presence of GIV protein was detected using Optiview DAB Detection Kit (Ventana Medical Systems, Inc.). Following chromogenic detection, all slides were counterstained with Hematoxylin II and Bluing Reagent (Ventana Medical Systems, Inc.) for 4 min each and coverslips were applied.
GIV staining was scored according to the percentage of viable tumor cell immunoreactivity and staining intensity (0–3: 0 - none; 1- weak; 2 - moderate; 3 - strong) by a subject matter expert pathologist who remained blinded to the clinical outcome (Figure 1C). The scoring algorithm was developed in the training cohort by evaluating several algorithms that incorporated staining intensity and percent staining in terms of their ability to stratify patients into distinguishable survival groups based on the HR in pMMR, chemo-naïve, T3 stage patients. Tumors were considered to be GIV positive when they met one of the two criteria: a) more than 10% of viable tumor cells demonstrated weak to moderate unequivocal cytoplasmic and/or nuclear staining, or b) the presence of any strong (i.e. intensity=3) cytoplasmic and/or nuclear staining in any percentage of viable tumor cells.
Statistical Analysis
Models for identifying high risk patients were developed in a training set of data from pMMR, chemo-naïve, T3 stage patients. The model using clinical variables without GIV status (i.e. clinical model) included age, gender, lateral tumor location, number of lymph nodes sampled, tumor differentiation and LVI status. This clinical model was used as a baseline in order to compare the potential prognostic utility of several reduced models. Such comparative analysis led to the conclusion that this clinical model represented the maximally informative model for these cohorts, based on available clinical data. It should be noted that this is likely an overestimate of the utility of the clinical model, since common clinical practice does not always include consideration of all of these variables (14, 42). Area under the receiver operating characteristic (ROC) curve with 3-year recurrence as the outcome was used in the training set in order to gain insight as to potential for risk stratification in comparison to the clinical model. A 3-year end point was considered appropriate because: a) it reduces risk of bias due to missing data; only four (<5%) patients in the cohort were missing follow-up information at the 3-year time point; b) 3-year and 5-year survival rates are fairly similar, 92% and 90%, respectively, among patients with Stage II CC (11); and c) disease-free survival outcomes after 2- or 3-year median follow-up are excellent predictors of 5-year OS in Stage II CC (43). A Cox Proportional Hazards (CPH) model was used to calculate hazard ratios for risk groups in both univariate and multivariate candidate models in the training data set and the best reduced model (GIV/LVI) was selected. For all analyses where a HR is reported, the recurrence free survival (RFS) at 3 years is also reported to provide additional context. Confidence limits, where reported, are for Clopper-Pearson exact confidence intervals. Risk models that were selected based on the results obtained in the training data set included: 1) a model with all clinical variables; 2) a model combining GIV with all clinical variables; and 3) a reduced model with LVI and GIV only. These risk models were then applied to the internal test and independent validation cohorts, and to pooled data sets (i.e. combined data from the training, test and validation cohorts). Hazard ratios were calculated to assess if the same trends were observed for these risk models, as was seen in the training set. Hazard ratios for univariate association with survival were calculated in test and validation data sets for descriptive purposes only, and not for further model fitting because doing so would have nullified the independence of the validation process. It was assumed that univariate associations likely vary across cohorts, but the clinical utility of the chosen risk model must be robust enough to overcome such cohort-to-cohort variability in order to be considered generalizable. Kaplan-Meier survival curves were plotted and a CPH model was used to calculate comparative HR for risk groups to determine if GIV/LVI could be considered to be as informative as the clinical model in risk stratification. Recurrence-free survival (RFS) was defined as the time from date of surgery to date of disease relapse, or was censored at the date of last follow-up visit for recurrence-free patients.
All risk stratifications were based on the models developed in the training set. Risk stratification was determined in three steps: 1) Build logistic regression model with 3-year recurrence as the response variable; 2) Each patient is given a prediction index based on the linear predictor from this model, which describes the probability of experiencing recurrence within 3 years, given the pattern of the variables included in the model; 3) Find the optimal cut-off. If prediction index is less than the cut-off, the patient is assigned a low risk of recurrence; otherwise, the patient is assigned a high risk. The optimal cut-off was chosen by maximizing the sum of sensitivity and specificity.
The above risk stratification process was used to find optimal cut-offs for 3 models: a) a risk model with all clinical variables; b) a risk model combining GIV with all clinical variables; and c) a risk model combining GIV with LVI status (the GIV/LVI model). These 3 models and the cut-offs that were developed in the training cohort were then applied to an internal validation cohort and used to generate comparative hazard ratios and Kaplan-Meier survival curves. An external validation data set was obtained to further assess the robustness of the GIV/LVI risk model in stratifying risk using hazard ratios. Finally, comparative Kaplan-Meier survival curves were used to assess the value of the GIV/LVI model relative to the model with all clinical variables. Missing clinical information for lymph node yield prohibited clinical model assessment in the independent validation set. All statistical analysis was performed using the R programming environment (44) or SAS Software (Version 9.4 SAS System for Windows, Cary, NC), where a significance level of 0.05 indicated a potentially clinically relevant finding.
As for how the samples size for this retrospective study on a historic cohort was determined, it was primarily based on the availability of tissue samples in the various cohorts. Consequently, our power was low for all but those parameters that had large effects in the training cohort (n=83) with only 26 total events. This was considered an acceptable risk given that a large effect was of primary interest in discovering a model with utility above and beyond current clinical practice.
RESULTS
GIV Expression Analysis by Immunohistochemistry
To validate the potential of GIV protein expression as a biomarker in stage II CC patients, a rabbit monoclonal antibody was developed against the full-length GIV protein. Immunoblot (Figure 1B) and IHC (Figure 1C) analyses of control and GIV-depleted COS-7 cells revealed a high analytical specificity of the Spring Bioscience rabbit monoclonal SP173 clone. An automated GIV IHC assay was developed and applied to three clinical cohorts of stage II CC. GIV IHC showed primarily cytoplasmic staining; in some instances, nuclear staining was also observed. IHC was scored as based on the staining intensity (negative, weak, moderate, or strong positive, see Figure 1D) and the percentage of stained tumor cells (see full scoring algorithm in Materials and Methods).
Patient Characteristics and GIV Status
The clinico-pathological characteristics and their association with GIV status of all 690 patients included in the training, internal testing and independent validation sets are shown in Table 1. Three hundred and seventeen out of 690 patients’ tumors (46%) were GIV positive. Based on the MMR IHC panel testing, the deficient MMR (dMMR) rates were similar between the training (20.8%) and internal testing (19.2%) cohorts; rates are not reported for the independent validation cohort because recruitment included only pMMR cases. A higher proportion of patients in the independent validation cohort received adjuvant chemotherapy (51.9%) than the training (33.3%) and internal testing (16.4%) cohorts. The median follow-up was 53.5 months (interquartile range (IQR): 34–65 months), and 136 of 690 patients (19.7%) experienced recurrent disease during follow-up.
Table 1. Baseline clinico-pathological characteristics as a function of GIV status for patients in individual and all pooled sets prior to sub-setting for model development.
| Characteristics | Training Set (n = 192) |
Internal Testing Set (n=317) |
Independent Validation Set (n = 181) |
Pooled (n=690) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| GIV+ | GIV− | GIV+ | GIV− | GIV+ | GIV− | GIV+ | GIV− | ||
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | ||
| Age (Years) | |||||||||
| <70 | 54 (59.3%) | 55 (54.5%) | 59 (35.8%) | 67 (44.1%) | 24 (39.3%) | 57 (47.5%) | 137 (43.2%) | 179 (48%) | |
| >=70 | 37 (40.7%) | 46 (45.5%) | 106 (64.2%) | 85 (55.9%) | 37 (60.7%) | 63 (52.5%) | 180 (56.8%) | 194 (52%) | |
| Sex | |||||||||
| Female | 47 (51.6%) | 48 (47.5%) | 72 (43.6%) | 63 (41.4%) | 23 (37.7%) | 44 (36.7%) | 142 (44.8%) | 155 (41.6%) | |
| Male | 44 (48.4%) | 53 (52.5%) | 93 (56.4%) | 89 (58.6%) | 38 (62.3%) | 76 (63.3%) | 175 (55.2%) | 218 (58.4%) | |
| Tumor Location | |||||||||
| Left | 44 (48.4%) | 50 (49.5%) | 79 (47.9%) | 79 (52%) | 49 (80.3%) | 88 (73.3%) | 172 (54.3%) | 217 (58.2%) | |
| Right | 47 (51.6%) | 51 (50.5%) | 86 (52.1%) | 73 (48%) | 12 (19.7%) | 32 (26.7%) | 145 (45.7%) | 156 (41.8%) | |
| Tumor Stage | |||||||||
| T3 | 80 (87.9%) | 82 (81.2%) | 142 (86.1%) | 127 (83.6%) | 57 (93.4%) | 109 (90.8%) | 279 (88%) | 318 (85.3%) | |
| T4 | 11 (12.1%) | 19 (18.8%) | 23 (13.9%) | 25 (16.4%) | 4 (6.6%) | 11 (9.2%) | 38 (12%) | 55 (14.7%) | |
| Tumor Differentiation | |||||||||
| Not Reported | 1 (1.1%) | 2 (2%) | 2 (1.2%) | 2 (1.3%) | 1 (1.6%) | 7 (5.8%) | 4 (1.3%) | 11 (2.9%) | |
| Poor | 17 (18.7%) | 25 (24.8%) | 39 (23.6%) | 32 (21.1%) | 3 (4.9%) | 8 (6.7%) | 59 (18.6%) | 65 (17.4%) | |
| Well to Moderate | 73 (80.2%) | 74 (73.3%) | 124 (75.2%) | 118 (77.6%) | 57 (93.4%) | 105 (87.5%) | 254 (80.1%) | 297 (79.6%) | |
| Lymph Node Yield | |||||||||
| <12 | 27 (29.7%) | 23 (22.8%) | 28 (17%) | 34 (22.4%) | NA | NA | 55 (17.4%) | 57 (15.3%) | |
| >=12 | 64 (70.3%) | 78 (77.2%) | 137 (83%) | 118 (77.6%) | NA | NA | 201 (63.4%) | 196 (52.5%) | |
| Lymphovascular Invasion | |||||||||
| Absent | 58 (63.7%) | 67 (66.3%) | 109 (66.1%) | 109 (71.7%) | 30 (49.2%) | 56 (46.7%) | 197 (62.1%) | 232 (62.2%) | |
| Not Reported | 8 (8.8%) | 9 (8.9%) | 11 (6.7%) | 8 (5.3%) | 4 (6.6%) | 12 (10%) | 23 (7.3%) | 29 (7.8%) | |
| Present | 25 (27.5%) | 25 (24.8%) | 45 (27.3%) | 35 (23%) | 27 (44.3%) | 52 (43.3%) | 97 (30.6%) | 112 (30%) | |
| Mismatch Repair Status | |||||||||
| Deficient | 21 (23.1%) | 19 (18.8%) | 35 (21.2%) | 26 (17.1%) | 0 (0%) | 0 (0%) | 56 (17.7%) | 45 (12.1%) | |
| Proficient | 70 (76.9%) | 82 (81.2%) | 130 (78.8%) | 126 (82.9%) | 61 (100%) | 120 (100%) | 261 (82.3%) | 328 (87.9%) | |
| Adjuvant Chemotherapy | |||||||||
| No | 60 (65.9%) | 68 (67.3%) | 140 (84.8%) | 125 (82.2%) | 31 (50.8%) | 56 (46.7%) | 231 (72.9%) | 249 (66.8%) | |
| Yes | 31 (34.1%) | 33 (32.7%) | 25 (15.2%) | 27 (17.8%) | 30 (49.2%) | 64 (53.3%) | 86 (27.1%) | 124 (33.2%) | |
| Progression | |||||||||
| No | 65 (71.4%) | 79 (78.2%) | 144 (87.3%) | 139 (91.4%) | 43 (70.5%) | 84 (70%) | 252 (79.5%) | 302 (81%) | |
| Yes | 26 (28.6%) | 22 (21.8%) | 21 (12.7%) | 13 (8.6%) | 18 (29.5%) | 36 (30%) | 65 (20.5%) | 71 (19%) | |
Note: Only pMMR cases were included in the independent validation set; information on lymph node yield was not available for the independent validation set.
Development of Risk Models Based on GIV expression and Clinico-pathological Factors
Risk stratification models using the data from 83 chemo-naïve patients in the training cohort who had pMMR T3 tumors were developed. The rationale for choosing this subgroup was that among all stage II CC, this is the subgroup that continues to pose the biggest management dilemma for oncologists. Univariate analysis of the training cohort suggested that GIV positivity and the presence of LVI were the only two significant prognostic factors associated with tumor recurrence within pMMR T3 CC patients (Table 2). Both GIV expression and the presence of LVI retained prognostic significance when the data from the training, test and independent validation cohorts were pooled (Table 2, right column).
Table 2. Hazard ratios for probability of recurrence for each univariate model considered and three multivariate models.
Models were developed in T3, pMMR, chemo-naïve cases in the training set then applied to both internal and independent validation sets.
| Variable | Training Set (N = 83) |
Internal Testing Set (N = 175) |
Independent Validation Set (N = 81) |
Pooled (N = 339)** |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95 % CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Univariate Analysis | ||||||||||||
| GIV Status (Positive v. Negative) | 2.78 | [1.24,6.25] | 0.013 | 2.32 | [0.74,7.28] | 0.150 | 1.76 | [0.73,4.25] | 0.210 | 1.85 | [1.10,3.09] | 0.019 |
| Lymphovascular Invasion (Present v. Absent) | 2.54 | [1.12,5.74] | 0.025 | 3.36 | [1.22,9.27] | 0.019 | 1.87 | [0.78,4.50] | 0.163 | 2.52 | [1.51,4.18] | 3.74E-04 |
| Age continuous (per year) | 1.02 | [0.98,1.07] | 0.285 | 1.04 | [0.99,1.10] | 0.115 | 1.00 | [0.96,1.06] | 0.854 | 1.02 | [1.00,1.05] | 0.083 |
| Age (>=70 years v. <70 years) | 1.37 | [0.62,3.02] | 0.439 | 1.27 | [0.43,3.71] | 0.666 | 1.00 | [0.36,2.75] | 0.996 | 1.17 | [0.69,1.98] | 0.553 |
| Sex (Female v. Male) | 1.37 | [0.63,2.98] | 0.422 | 0.49 | [0.14,1.74] | 0.271 | 2.35 | [0.97,5.69] | 0.058 | 1.43 | [0.86,2.36] | 0.166 |
| Tumor Location (Right v. Left) | 1.16 | [0.53,2.50] | 0.712 | 1.19 | [0.43,3.28] | 0.738 | 1.55 | [0.62,3.89] | 0.351 | 1.16 | [0.70,1.92] | 0.572 |
| Lymph Node Yield (<12 v. >=12) | 1.18 | [0.53,2.65] | 0.684 | 0.30 | [0.04,2.26] | 0.241 | N/ A* | 1.05 | [0.51,2.14] | 0.893 | ||
| Tumor Differentiation (Poor v. Well/Moderate) | 1.78 | [0.71,4.46] | 0.221 | 0.85 | [0.19,3.77] | 0.830 | 1.20 | [0.16,9.04] | 0.862 | 1.16 | [0.57,2.35] | 0.688 |
| Multivariate Analysis: All Clinical | ||||||||||||
| Lymphovascular Invasion (Present v. Absent) | 2.71 | [1.11,6.59] | 0.028 | |||||||||
| Age continuous (per year) | 1.03 | [0.98,1.08] | 0.277 | |||||||||
| Sex (Female v. Male) | 1.75 | [0.75,4.08] | 0.196 | |||||||||
| Tumor Location (Right v. Left) | 1.40 | [0.54,3.61] | 0.491 | |||||||||
| Lymph Node Yield (<12 v. >=12) | 1.18 | [0.47,2.96] | 0.725 | |||||||||
| Tumor Differentiation (Poor v. Well/Moderate) | 1.40 | [0.52,3.72] | 0.504 | |||||||||
| All Clinical Model | 2.33 | [1.33,4.09] | 3.18E-03 | 1.79 | [0.87,3.66] | 0.112 | N/A* | 1.96 | [1.27,3.02] | 2.37E-03 | ||
| Multivariate Analysis: GIV + All Clinical | ||||||||||||
| GIV Status (Positive v. Negative) | 2.39 | [0.98,5.85] | 0.057 | |||||||||
| Lymphovascular Invasion (Present v. Absent) | 2.08 | [0.84,5.14] | 0.115 | |||||||||
| Age continuous (per year) | 1.02 | [0.97,1.07] | 0.451 | |||||||||
| Sex (Female v. Male) | 1.39 | [0.58,3.30] | 0.461 | |||||||||
| Tumor Location (Right v. Left) | 1.38 | [0.54,3.51] | 0.496 | |||||||||
| Lymph Node Yield (<12 v. >=12) | 1.02 | [0.40,2.60] | 0.974 | |||||||||
| Tumor Differentiation (Poor v. Well/Moderate) | 1.66 | [0.60,4.59] | 0.328 | |||||||||
| GIV + All Clinical Model | 3.27 | [1.64,6.52] | 7.87E-04 | 1.56 | [0.73,3.33] | 0.251 | N/A* | 2.3 | [1.39,3.81] | 1.20E-03 | ||
| Multivariate Analysis: GIV + LVI | ||||||||||||
| GIV Status (Positive v. Negative) | 2.45 | [1.07,5.60] | 0.034 | |||||||||
| Lymphovascular Invasion (Present v. Absent) | 2.05 | [0.89,4.70] | 0.092 | |||||||||
| GIV/ LVI Risk Model (High v. Low) | 3.74 | [1.50,9.32] | 4.73E-03 | 7.83 | [1.03,59.54] | 0.047 | 3.71 | [1.24,11.12] | 0.019 | 3.44 | [1.79,6.62] | 2.06E-04 |
Models including lymph node yield could not be calculated for the Independent Validation Set; lymph node yield data were not available.
Because clinical models could not be assessed in the independent validation set, hazard ratios for models including lymph node yield in pooled data set do not include cases from the independent validation set; N = 258 for models including lymph node yield in pooled data.
To investigate whether combinations of GIV expression analysis, LVI status, and other clinical variables may provide a synergistic benefit over each factor alone, we developed models with multiple covariates and compared performance of each model in pMMR, T3, chemo-naïve patients from the training set. Models included: clinical variables alone, GIV status combined with clinical variables, and GIV combined with LVI status (Table 2). In the analysis using clinical variables alone, LVI was the only significant prognostic factor for recurrence. In the GIV status combined with clinical variables model, GIV status appeared to be the strongest prognostic factor, followed by LVI status. Based on these results, the reduced model using GIV and LVI status was selected as the best model for further analyses.
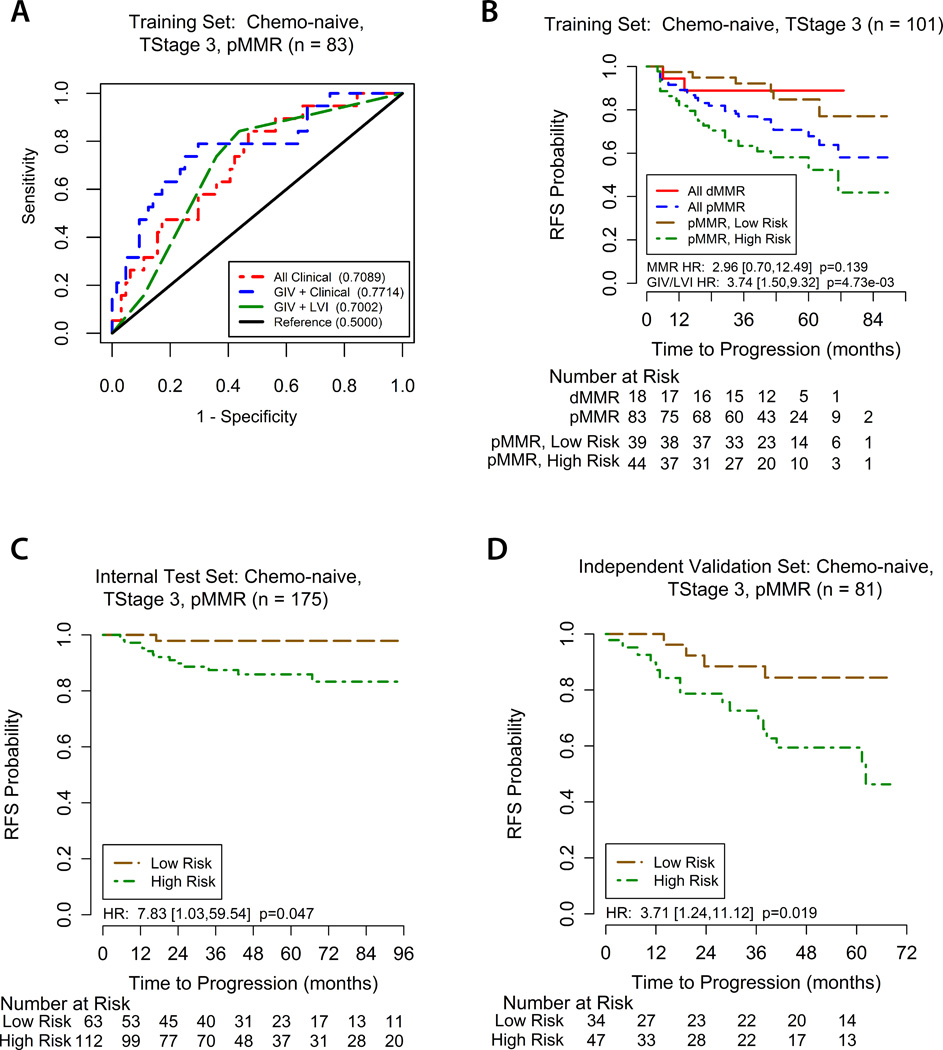
A GIV/LVI risk stratification model was established in which patients with tumors that were positive for either GIV, or LVI, or positive for both are classified as high risk, whereas patients whose tumors were negative for both GIV and LVI were assigned a low risk. Using the training cohort, we assessed the prognostic ability of this GIV/LVI risk model for recurrence at 3 years with ROC curve analysis. This was compared with the ability of the clinical model, and GIV expression status combined with clinical model (Figure 2A). The prognostic ability of the GIV/LVI risk model appeared to be similar to the clinical model.
Figure 2. Assessment of GIV/LVI risk model.
(A) ROC curves comparing the prognostic accuracy of the GIV/LVI risk classifier (high vs low risk) with clinical model alone, or GIV and clinical model combined. Area under the curve (AUC) for recurrence at 3 years (shown in brackets) shows the utility of including GIV analysis in recurrence risk assessment. (B) Kaplan-Meier recurrence-free survival (RFS) based on MMR status and the GIV/LVI risk classifier for T3, surgery-alone patients in the training set. (C, D) Kaplan-Meier RFS based on the GIV/LVI risk classifier for T3, pMMR, surgery-alone patients in the internal testing (C) and independent validation sets (D). ROC = receiver operator characteristics. LVI = lymphovascular invasion. dMMR/pMMR = deficient/proficient mismatch repair.
GIV/LVI Risk Model and Recurrence-Free Survival in pMMR, T3, Surgery-Alone Patients
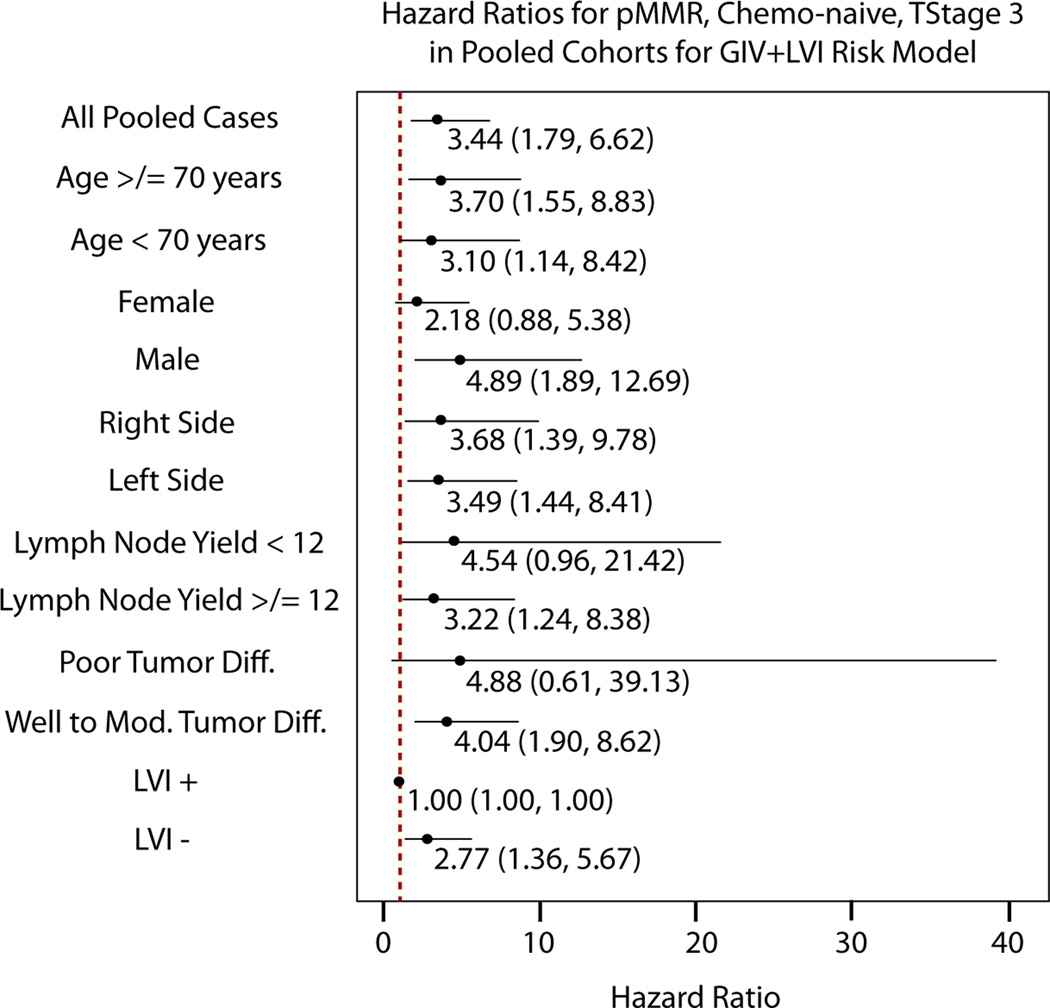
For patients in the training cohort (enriched for recurrence) with pMMR, T3 tumors, treated with surgery alone, 3-year RFS was 63.6% (95%CI 47.8–77.6) in the GIV/LVI high-risk group and 92.3% (95%CI 79.1–98.4) in the low-risk group (HR 3.74, 95% CI 1.50–9.32; P=0.005; Figure 2B). In the internal test cohort, 3-year RFS was once again significantly lower in the high-risk group compared to the low-risk group (89.3% vs 98.4%, HR 7.83, 95%CI 1.03–59.54; P=0.047; Figure 2C). Similar results were seen also in the independent validation cohort; 3-year RFS was again significantly lower in the high-risk group compared to the low-risk group (78.7% vs 91.2%, HR 3.71, 95%CI 1.24–11.12; P=0.019; Figure 2D). It is noteworthy that there was variability in the point estimates of GIV/LVI risk group association with recurrence in the different clinico-pathological subgroups (Figure 3). The association appeared stronger in males than in females, in patients with less than 12 versus 12 or more lymph nodes examined, and in poorly differentiated rather than well-to-moderately differentiated tumors. However, none of these differences were statistically significant, as evidenced by their overlapping confidence intervals.
Figure 3. Risk of recurrence for GIV/LVI high risk versus low risk groups in different clinic-pathological subgroups in all T3, pMMR, chemo-naive, stage II colon cancer patients.
The hazard ratios with 95% confidence intervals represent the difference in expected recurrence free survival between high and low risk groups, as stratified by the GIV/LVI model, in the sub-population listed to the left. Because stratifications are based on the GIV/LVI model and all LVI+ cases were high risk, this prevented any stratification in the LVI+ subpopulation, resulting in a hazard ratio of 1.00. The hazard ratio in the LVI- subpopulation shows the additional contribution of GIV to the ability of LVI to classify risk.
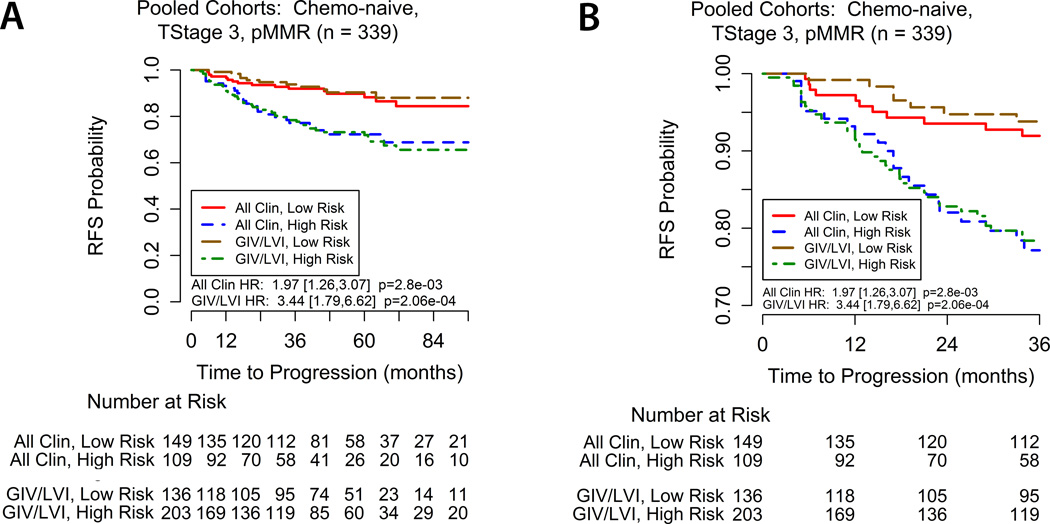
To compare the relative performances of the GIV/LVI risk model and the clinical model, we constructed comparative Kaplan-Meier recurrence-free survival curves and calculated HR for risk groups for the pooled population of pMMR, T3, surgery-alone patients (Figure 4). Patients in the clinical high-risk group had a worse outcome than the clinical low-risk group (3-year RFS 82.44% vs 92.9%, HR 1.96, 95%CI 1.27 – 3.02; P=0.0024). Similarly, the 3-year RFS rate for patients in the GIV/LVI high-risk group was significantly lower than the GIV/LVI low-risk group (81.3% vs 94.9%, HR 3.44, 95%CI 1.79 – 6.62; P=0.0002). These findings indicate that risk stratification by GIV/LVI is at least as informative as the clinical risk model. As noted above, due to missing information for one of the clinic-pathological features, the independent cohort’s data are not included in the pooled assessments of the clinical model.
Figure 4. Kaplan-Meier recurrence-free survival for the pooled population of T3, pMMR, chemo-naive stage II colon cancer patients according to the GIV/LVI risk classifier and clinical (‘All Clin’) model.
(A) Comparative survival stratification for the entire followup perior, and (B) detailed view for the first three years. While the GIV/LVI stratification is shown for all cohorts pooled, the clinical model does not include cases from the independent validation set due to missing lymph node yield data.
DISCUSSION
The major finding in this work is the development and validation of a novel prognostic algorithm based on GIV expression and LVI status to predict recurrence risk in patients with stage II CC. First, we found that patients with T3, pMMR, stage II CC, the subgroup of patients who create the biggest dilemma for adjuvant treatment decision making, can be stratified into low- and high-risk groups using the GIV/LVI risk prediction model with a significant difference observed in 3-year RFS. Second, we found that this GIV/LVI risk stratification model is as good as an ‘all-clinical’ test model in its ability to predict the 3-year recurrence risk; the latter accounts for all clinico-pathological risk factors. It is noteworthy that the ‘all clinical’ model we used here does not represent any gold standard clinical practice, but was used as an internal reference only because we had access to detailed clinico-pathological information in our test cohort. Such a ‘all clinical’ model suffers from subjective reporting of pathological features and inter-observer variability, and is impractical in the community setting because of missing information. Thus, the GIV/LVI risk model offers both objectivity [GIV staining by IHC] and robustness; it is less ambiguous and more practical. Consequently, the staining and scoring methodology we define here and the diagnostic antibody we validate in this work has the potential to impact patient care in the broader community.
The decision to treat or not to treat a patient with stage II CC remains one of the most challenging areas in colorectal oncology. Whilst the benefit of adjuvant chemotherapy in stage III CC is well established, the benefit of adjuvant 5-fluorouracil based chemotherapy in stage II CC has not been clearly demonstrated. Several large pooled analyses have failed to detect a significant overall survival benefit of adjuvant chemotherapy in stage II CC (4, 5). The QUASAR study, the largest randomised study to assess the benefit of adjuvant 5-fluorouracil chemotherapy in patients at low risk of recurrence, included a rate of 91% of patients with stage II CC (6). Although this trial demonstrated an overall small, but significant survival benefit of 3.6% in favor of 5-fluorouracil chemotherapy, the benefit was not significant in the stage II CC subset alone (excluding patients with stage III and rectal cancer). The addition of oxaliplatin to 5-fluorouracil has not been shown to provide an overall survival benefit in stage II patients, despite a trend toward a greater benefit in high risk patients (45).
Notwithstanding the modest potential overall survival benefit from adjuvant therapy in the average risk stage II CC patients, most trials have demonstrated at least some RFS benefit (4, 6, 8). This has prompted approaches to utilize clinico-pathological and molecular features to select patient subgroups with a higher risk of recurrence who might, due to the elevated baseline risk, derive a greater benefit from adjuvant chemotherapy. Most of the traditional clinico-pathological risk factors suffer from lack of standardization and prospective validation of their clinical utility, resulting in clinical guidelines using varying definitions of “high risk” stage II disease. There is also little evidence in the literature to suggest that patients with any poor prognostic features are more likely to benefit from chemotherapy (10). For example, in a recent population-based study in 1,697 stage II CC patient, a recurrence-free and overall survival benefit of adjuvant chemotherapy was observed in patients with T4 primary cancers, but not other high risk clinical features (46).
Amongst the many molecular markers and gene expression signatures examined to date, MMR status is the most robust prognostic and predictive marker in stage II CC and is the only marker recommended for clinical use at this time. Not only are dMMR tumors associated with a better prognosis than pMMR tumors, MMR deficient status is associated with a lack of benefit, or even a detrimental effect, from adjuvant 5-fluoropyrimidine chemotherapy (2, 3, 47). Consequently, T4 invasion and MMR deficiency are two prognostic factors commonly used clinically to argue in favor or against the use of adjuvant therapy. For the remaining stage II patients with T3, pMMR tumors, there is a strong need for a risk prediction model to guide treatment decisions.
We developed and validated a simple IHC-based approach to stratify recurrence risk using GIV staining and LVI status in T3, pMMR tumors. In our study, many of the conventional features appear to have little prognostic impact within the T3, pMMR population. This is consistent with the finding from a recent study in 416 patients comparing the ColoPrint signature and the National Comprehensive Cancer Network (NCCN) clinical risk factors, where additional clinical features in the NCCN guidelines did not correlate with outcome in the T3, pMMR subgroup (HR 1.01, P = 0.9) (48). It is notable that GIV and LVI status were the only two significant predictors of recurrence in our study. Within the pooled T3, pMMR subgroup, the GIV/LVI risk model identified 40% of patients with low risk of recurrence, where the 3-year RFS of 94.9% (Figure 4A) is similar to that of patients with dMMR tumors (2, 47). These findings indicate that the GIV/LVI model consistently performs well in stratifying patients into high- and low-risk groups and effectively prognosticates recurrence risk. The 3.44-fold hazard of recurrence for the GIV/LVI risk model in our study for pMMR, T3 tumors is comparable to or greater than the HR reported for gene expression assays. The advantages of an IHC-based approach compared to gene expression microarray- or quantitative PCR-based analysis are the simplicity of the test, the ability to use readily available FFPE samples, and the relatively low cost.
This study has several limitations. First, we did not examine the predictive effect to adjuvant chemotherapy with the GIV/LVI risk model. The retrospective nature of our study and the population-based cohorts impaired our ability to draw reliable conclusions about the ability of this risk model to predict which patients are more or less likely to benefit from adjuvant chemotherapy. This question is best addressed with samples and data from a randomised clinical trial. Second, although we used three cohorts (training, test and validation), the size of our training cohort was small. Consequently, the power was low for all but large effects in the training cohort; only a large effect would likely have utility comparable to or better than current clinical practice and be robust to the noise and potential biases of retrospective analysis. An additional limitation of this study is the lack of lymph node yield data from the validation cohort.
In conclusion, this study showed that the combination of GIV and LVI status can reproducibly and effectively stratify patients with T3, pMMR, stage II CC into groups with a low and high risk of recurrence. Further studies have been planned to validate these findings in a randomised clinical trial cohort, with the additional goal of examining the value of the GIV/LVI model in predicting benefit from adjuvant chemotherapy.
Translational relevance.
This study investigated the prognostic impact of GIV (also known as Girdin) expression assessed by immunohistochemistry and lymphovascular invasion (LVI) in stage II colon cancer. GIV is a multidomain signal transducer which enhances cellular phenotypes that fuel metastatic progression of cancers, e.g., invasiveness, stem-ness, survival and drug resistance. The key finding here was that patients with T3, pMMR, stage II CC, the subgroup of patients who create the biggest dilemma for adjuvant treatment decision making, could be stratified into low- and high-risk groups using the GIV/LVI risk prediction model with a significant difference observed in 3-year recurrence-free survival. Given the modest benefit of adjuvant chemotherapy in stage II colon cancer, the ability to identify patients with a low risk of recurrence is clinically useful to define a subgroup of patients who do not require adjuvant chemotherapy.
Acknowledgments
Funding Support: This work was supported by Ventana Medical Systems, Inc., the NIH (R01CA160911 and R01 CA100768 to P.G; R01 CA72851, CA 181572, and U01 CA187956 to A.G), and funds from the Moores Cancer Center to P.G and the Baylor Research Institute to A.G.
We thank Antonia Miller for her technical contributions, Chang Xu for statistical analysis, and Kassie Smith and Marissa Blackburn for project coordination efforts.
Footnotes
Conflicts of interest: None to declare
REFERENCES
- 1.Edge M, Byrd D, Compton C, Fritz A, Greene F, Trotti A. AJCC cancer staging manual. 7th. New York, NY: Springer; 2010. [Google Scholar]
- 2.Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:3219–3226. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. The New England journal of medicine. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Figueredo A, Charette ML, Maroun J, Brouwers MC, Zuraw L. Adjuvant therapy for stage II colon cancer: a systematic review from the Cancer Care Ontario Program in evidence-based care’s gastrointestinal cancer disease site group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:3395–3407. doi: 10.1200/JCO.2004.03.087. [DOI] [PubMed] [Google Scholar]
- 5.Mamounas E, Wieand S, Wolmark N, Bear HD, Atkins JN, Song K, et al. Comparative efficacy of adjuvant chemotherapy in patients with Dukes’ B versus Dukes’ C colon cancer: results from four National Surgical Adjuvant Breast and Bowel Project adjuvant studies (C-01, C-02, C-03, and C-04) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17:1349–1355. doi: 10.1200/JCO.1999.17.5.1349. [DOI] [PubMed] [Google Scholar]
- 6.Quasar Collaborative G, Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370:2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 7.Wirtzfeld DA, Mikula L, Gryfe R, Ravani P, Dicks EL, Parfrey P, et al. Concordance with clinical practice guidelines for adjuvant chemotherapy in patients with stage I-III colon cancer: experience in 2 Canadian provinces. Canadian journal of surgery Journal canadien de chirurgie. 2009;52:92–97. [PMC free article] [PubMed] [Google Scholar]
- 8.Gill S, Loprinzi CL, Sargent DJ, Thome SD, Alberts SR, Haller DG, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:1797–1806. doi: 10.1200/JCO.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 9.Benson AB, 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:3408–3419. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor ES, Greenblatt DY, LoConte NK, Gangnon RE, Liou JI, Heise CP, et al. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:3381–3388. doi: 10.1200/JCO.2010.34.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsikitis VL, Larson DW, Huebner M, Lohse CM, Thompson PA. Predictors of recurrence free survival for patients with stage II and III colon cancer. BMC Cancer. 2014;14:336. doi: 10.1186/1471-2407-14-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sveen A, Nesbakken A, Agesen TH, Guren MG, Tveit KM, Skotheim RI, et al. Anticipating the clinical use of prognostic gene expression-based tests for colon cancer stage II and III: is Godot finally arriving? Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:6669–6677. doi: 10.1158/1078-0432.CCR-13-1769. [DOI] [PubMed] [Google Scholar]
- 13.Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23:2479–2516. doi: 10.1093/annonc/mds236. [DOI] [PubMed] [Google Scholar]
- 14.Ponz de Leon M, Sant M, Micheli A, Sacchetti C, Di Gregorio C, Fante R, et al. Clinical and pathologic prognostic indicators in colorectal cancer. A population-based study. Cancer. 1992;69:626–635. doi: 10.1002/1097-0142(19920201)69:3<626::aid-cncr2820690305>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Hornberger J, Lyman GH, Chien R, Meropol NJ. A multigene prognostic assay for selection of adjuvant chemotherapy in patients with T3, stage II colon cancer: impact on quality-adjusted life expectancy and costs. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2012;15:1014–1021. doi: 10.1016/j.jval.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Marcos M, Ghosh P, Farquhar MG. GIV/Girdin transmits signals from multiple receptors by triggering trimeric G protein activation. J Biol Chem. 2015;290:6697–6704. doi: 10.1074/jbc.R114.613414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh P. G protein coupled growth factor receptor tyrosine kinase: no longer an oxymoron. Cell Cycle. 2015:1–5. doi: 10.1080/15384101.2015.1066538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh P, Garcia-Marcos M, Farquhar MG. GIV/Girdin is a rheostat that fine-tunes growth factor signals during tumor progression. Cell Adh Migr. 2011;5:237–248. doi: 10.4161/cam.5.3.15909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh P. Heterotrimeric G proteins as emerging targets for network based therapy in cancer: End of a long futile campaign striking heads of a Hydra. Aging. 2015;7:469–474. doi: 10.18632/aging.100781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin C, Ear J, Pavlova Y, Mittal Y, Kufareva I, Ghassemian M, et al. Tyrosine phosphorylation of the Galpha-interacting protein GIV promotes activation of phosphoinositide 3-kinase during cell migration. Sci Signal. 2011;4:ra64. doi: 10.1126/scisignal.2002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aznar N, Kalogriopoulos N, Midde K, Lo IC, Ghosh P. Heterotrimeric G Protein Signaling via GIV/Girdin: Breaking the rules of engagement, space and time. BioEssays. 2016 doi: 10.1002/bies.201500133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghosh P. The Untapped Potential of Tyrosine-based G protein Signaling. Pharmacol Res. 2016 doi: 10.1016/j.phrs.2016.01.017. Accepted, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weng L, Enomoto A, Ishida-Takagishi M, Asai N, Takahashi M. Girding for migratory cues: roles of the Akt substrate Girdin in cancer progression and angiogenesis. Cancer science. 2010;101:836–842. doi: 10.1111/j.1349-7006.2009.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunkel Y, Ong A, Notani D, Mittal Y, Lam M, Mi X, et al. STAT3 protein up-regulates Galpha-interacting vesicle-associated protein (GIV)/Girdin expression, and GIV enhances STAT3 activation in a positive feedback loop during wound healing and tumor invasion/metastasis. J Biol Chem. 2012;287:41667–41683. doi: 10.1074/jbc.M112.390781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma GS, Aznar N, Kalogriopoulos N, Midde KK, Lopez-Sanchez I, Sato E, et al. Therapeutic effects of cell-permeant peptides that activate G proteins downstream of growth factors. Proc Natl Acad Sci U S A. 2015;112:E2602–E2610. doi: 10.1073/pnas.1505543112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez-Sanchez I, Dunkel Y, Roh YS, Mittal Y, De Minicis S, Muranyi A, et al. GIV/Girdin is a central hub for profibrogenic signalling networks during liver fibrosis. Nat Commun. 2014;5:4451. doi: 10.1038/ncomms5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitamura T, Asai N, Enomoto A, Maeda K, Kato T, Ishida M, et al. Regulation of VEGF-mediated angiogenesis by the Akt/PKB substrate Girdin. Nature cell biology. 2008;10:329–337. doi: 10.1038/ncb1695. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Marcos M, Ghosh P, Farquhar MG. GIV is a nonreceptor GEF for G alpha i with a unique motif that regulates Akt signaling. Proc Natl Acad Sci U S A. 2009;106:3178–3183. doi: 10.1073/pnas.0900294106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosh P, Garcia-Marcos M, Bornheimer SJ, Farquhar MG. Activation of Galphai3 triggers cell migration via regulation of GIV. The Journal of cell biology. 2008;182:381–393. doi: 10.1083/jcb.200712066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamura Y, Asai N, Enomoto A, Kato T, Mii S, Kondo Y, et al. Akt-Girdin signaling in cancer-associated fibroblasts contributes to tumor progression. Cancer Res. 2015;75:813–823. doi: 10.1158/0008-5472.CAN-14-1317. [DOI] [PubMed] [Google Scholar]
- 31.Enomoto A, Murakami H, Asai N, Morone N, Watanabe T, Kawai K, et al. Akt/PKB regulates actin organization and cell motility via Girdin/APE. Developmental cell. 2005;9:389–402. doi: 10.1016/j.devcel.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Jiang P, Enomoto A, Jijiwa M, Kato T, Hasegawa T, Ishida M, et al. An actin-binding protein Girdin regulates the motility of breast cancer cells. Cancer Res. 2008;68:1310–1318. doi: 10.1158/0008-5472.CAN-07-5111. [DOI] [PubMed] [Google Scholar]
- 33.Le-Niculescu H, Niesman I, Fischer T, DeVries L, Farquhar MG. Identification and characterization of GIV, a novel Galpha i/s-interacting protein found on COPI, endoplasmic reticulum-Golgi transport vesicles. J Biol Chem. 2005;280:22012–22020. doi: 10.1074/jbc.M501833200. [DOI] [PubMed] [Google Scholar]
- 34.Jun BY, Kim SW, Jung CK, Cho YK, Lee IS, Choi MG, et al. Expression of girdin in human colorectal cancer and its association with tumor progression. Diseases of the colon and rectum. 2013;56:51–57. doi: 10.1097/DCR.0b013e31826b9b7e. [DOI] [PubMed] [Google Scholar]
- 35.Jin F, Liu C, Guo Y, Chen H, Wu Y. Clinical implications of Girdin and PI3K protein expression in breast cancer. Oncology letters. 2013;5:1549–1553. doi: 10.3892/ol.2013.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song JY, Jiang P, Li N, Wang FH, Luo J. Clinical significance of Girdin expression detected by immunohistochemistry in non-small cell lung cancer. Oncology letters. 2014;7:337–341. doi: 10.3892/ol.2013.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao K, Lu C, Han S, Zou Q, Li J, Xie D, et al. Expression of Girdin in primary hepatocellular carcinoma and its effect on cell proliferation and invasion. International journal of clinical and experimental pathology. 2015;8:551–559. [PMC free article] [PubMed] [Google Scholar]
- 38.Wang C, Lin J, Li L, Wang Y. Expression and clinical significance of girdin in gastric cancer. Molecular and clinical oncology. 2014;2:425–428. doi: 10.3892/mco.2014.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia-Marcos M, Jung BH, Ear J, Cabrera B, Carethers JM, Ghosh P. Expression of GIV/Girdin, a metastasis-related protein, predicts patient survival in colon cancer. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:590–599. doi: 10.1096/fj.10-167304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang YJ, Li AJ, Han Y, Yin L, Lin MB. Inhibition of Girdin enhances chemosensitivity of colorectal cancer cells to oxaliplatin. World J Gastroenterol. 2014;20:8229–8236. doi: 10.3748/wjg.v20.i25.8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghosh P, Beas AO, Bornheimer SJ, Garcia-Marcos M, Forry EP, Johannson C, et al. A G{alpha}i-GIV molecular complex binds epidermal growth factor receptor and determines whether cells migrate or proliferate. Molecular biology of the cell. 2010;21:2338–2354. doi: 10.1091/mbc.E10-01-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quah HM, Chou JF, Gonen M, Shia J, Schrag D, Landmann RG, et al. Identification of patients with high-risk stage II colon cancer for adjuvant therapy. Diseases of the colon and rectum. 2008;51:503–507. doi: 10.1007/s10350-008-9246-z. [DOI] [PubMed] [Google Scholar]
- 43.Sargent DJ, Patiyil S, Yothers G, Haller DG, Gray R, Benedetti J, et al. End points for colon cancer adjuvant trials: observations and recommendations based on individual patient data from 20,898 patients enrolled onto 18 randomized trials from the ACCENT Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:4569–4574. doi: 10.1200/JCO.2006.10.4323. [DOI] [PubMed] [Google Scholar]
- 44.Team RC. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 45.Tournigand C, Andre T, Bonnetain F, Chibaudel B, Lledo G, Hickish T, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:3353–3360. doi: 10.1200/JCO.2012.42.5645. [DOI] [PubMed] [Google Scholar]
- 46.Kumar A, Kennecke HF, Renouf DJ, Lim HJ, Gill S, Woods R, et al. Adjuvant chemotherapy use and outcomes of patients with high-risk versus low-risk stage II colon cancer. Cancer. 2015;121:527–534. doi: 10.1002/cncr.29072. [DOI] [PubMed] [Google Scholar]
- 47.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 48.Kopetz S, Tabernero J, Rosenberg R, Jiang ZQ, Moreno V, Bachleitner-Hofmann T, et al. Genomic classifier ColoPrint predicts recurrence in stage II colorectal cancer patients more accurately than clinical factors. The oncologist. 2015;20:127–133. doi: 10.1634/theoncologist.2014-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]