Abstract
B7-H3 (CD276) is an important immune checkpoint member of the B7 and CD28 families. Induced on antigen presenting cells, B7-H3 plays an important role in the inhibition of T cell function. Importantly, B7-H3 is highly overexpressed on a wide range of human solid cancers and often correlates with both negative prognosis and poor clinical outcome in patients. Challenges remain to identify the receptor(s) of B7-H3 and thus better elucidate the role of the B7-H3 pathway in immune responses and tumor evasion. With a preferential expression on tumor cells, B7-H3 is an attractive target for cancer immunotherapy. Based on the clinical success of inhibitory immune checkpoint blockade (CTLA-4, PD-1 and PD-L1), developing monoclonal antibodies (mAbs) against B7-H3 appears promising therapeutic strategies. An unconventional mAb against B7-H3 with antibody-dependent cell-mediated cytotoxicity is currently being evaluated in a Phase I clinical trial and has shown encouraging preliminary results. Additional therapeutic approaches in targeting B7-H3, such as blocking mAbs, bispecific mAbs, chimeric antigen receptor T cells, small molecules inhibitors, and combination therapies should be evaluated as these technologies have already shown positive results in various cancer settings. A better understanding of the B7-H3 pathway in humans will surely help to further optimize associated cancer immunotherapies.
Background
During an immune response, naive T cells engage their T cell receptor (TCR) to interact with a complex of major-histocompatibility complex (MHC) and peptide expressed by antigen presenting cells (APCs). This first signal is not sufficient to trigger full T cell activation. A second signal is provided by the interaction of costimulatory molecules (most importantly B7-1/2 and CD28) leading to full T cell activation. Following activation, coinhibitory molecules such as cytotoxic T-lymphocyte associated protein 4 (CTLA-4) function to restrain T cell responses, resulting in T cell exhaustion and tolerance. Interactions between members of the B7 ligand family and the CD28 receptor family provide T cell co-stimulation and co-inhibition, regulating T cell activation and tolerance, exhaustion and effector function, differentiation and memory generation. B7-H3, also known as CD276, is an immune checkpoint molecule belonging to the B7-CD28 pathways.
Structure and functional significance of the B7-H3 pathway
B7-H3 is a type I transmembrane protein encoded by chromosome 9 in mice and 15 in humans. The extracellular domain is composed of a single pair of immunoglobulin variable domain and immunoglobulin constant domain in mice (2IgB7-H3 isoform) and two identical pairs in human (4IgB7-H3 isoform) due to exon duplication (1,2). The intracellular tail of B7-H3 is short and has no known signaling motif. B7-H3 was first described in humans (3) and then in mice (2), but is universally expressed among species (4). A soluble form, cleaved from the surface by a matrix metallopeptidase MMP (5) or produced through alternative splicing of the intron (6), is also detectable in human sera.
B7-H3 is expressed on many tissues and cell types. At the mRNA level, it is ubiquitously found in non-lymphoid and lymphoid organs as liver, heart, prostate, spleen and thymus. Despite broad mRNA expression, protein expression is limited at steady state, suggesting the presence of an important post-transcriptional control mechanism. B7-H3 is constitutively found on non-immune resting fibroblasts, endothelial cells (EC), osteoblasts, and amniotic fluid stem cells. Moreover, B7-H3 expression is induced on immune cells, specifically antigen-presenting cells. In particular, coculture with regulatory T cells (Treg)(7), IFN-γ, lipopolysaccharide (LPS) or anti-CD40 in vitro stimulation (8) all induce the expression of B7-H3 on dendritic cells (DCs). Monocytes and monocytes-derived DCs upregulate B7-H3 after LPS stimulation or cytokine-induced differentiation respectively (9). Additionally, B7-H3 is also detected on natural killer (NK) cells, B cells, and a minor population of T cells following PMA/ionomycin stimulation (1).
The B7-H3 pathway has a dual role in contributing to the regulation of innate immune responses. One study found that neuroblastoma cells express B7-H3 on their cell surface, which protect them from NK cell-mediated lysis (10). Another group argues that B7-H3 costimulates innate immunity by augmenting proinflammatory cytokines release from LPS-stimulated monocytes/macrophages, in both a Toll like receptor 4- and 2-dependent manner (11). The role of B7-H3 in controlling the innate immunity is clearly complex and requires more elucidation.
A larger body of literature suggests that B7-H3 plays an important role in T cell-mediated adaptive immunity, although the nature of its signalling remains controversial (12). A co-stimulatory role of B7-H3 on human T cells was initially reported in vitro (3). Murine studies showing B7-H3 worsens experimental autoimmune encephalomyelitis (EAE), arthritis, bacterial meningitis and chronic allograft rejection (13–15) supported this claim. However, subsequent studies have mostly shown that B7-H3 acts as a T cell co-inhibitor. B7-H3 inhibits polyclonal or allogeneic CD4 and CD8 T cell activation, proliferation and effector cytokine production (IFN-γ and IL-2) in mice and humans. This negative regulation of T cells is associated with diminished NFAT, NF-kB and AP-1 transcriptional factor activity (16). Independent studies utilizing either protein blockade or gene-knockout mice have reported that B7-H3 ameliorates graft-versus-host-disease, prolongs cardiac allograft survival, reduces airway hypersensitivity, and delays EAE onset, especially by down-regulating Th1 responses (8,17,18). These examples lend more credence to the co-inhibitory nature of B7-H3.
The receptor(s) for B7-H3 has yet to be discovered (19,20). Nevertheless, the crystal structure of mouse B7-H3 reveals that its receptor engagement on T cells involves the particular segment connecting F and G strands (the FG loop) of the immunoglobulin variable domain of B7-H3 (19). Moreover, B7-H3 crystallizes as a glycosylated monomer but also undergoes an unusual dimerization in vitro. Altogether, the nature of the receptor(s), differences in cellular context, and various disease models certainly account for the discrepancies in the function of the B7-H3 pathway in regulating both innate and adaptive immunity during homeostasis and inflammation.
Beyond the immune system, the B7-H3 pathway has a non-immunological role in promoting osteoblastic differentiation and bone mineralization in mice, ensuring normal bone formation (21). Indeed, B7-H3 knockout mice had reduced bone mineral density and were more susceptible to bone fractures compared to wild-type mice. Furthermore, similar to other immune checkpoints of the B7-CD28 pathways, B7-H3 is also expressed in human cancers and participates in tumorigenesis through modulation of both immune and non-immune related pathways.
B7-H3 in the tumor microenvironment and immune evasion
Numerous studies have described B7-H3 overexpression in human malignancies, including melanoma (22), leukemia (23), breast (24), prostate (25), ovarian (26), pancreatic (27) colorectal (28) and other cancers. As detected by immunochemistry technique, over 60% and up to 93% of patient tumor tissues display aberrant expression of B7-H3 in the vast majority of cancer types (Table 1), while limited expression is seen on normal healthy tissues. Within positively stained samples, B7-H3 is found either on the membrane, in the cytoplasm or within the nucleus of cancer cells but also on the tumor-associated vasculature. When studying over 700 colorectal cancer patients, cytoplasmic/membrane and stromal expression were respectively seen in 86% and 77% of the samples while nuclear expression of B7-H3 in cancer cells was present in 27% of the samples (29). In most studies, the intensity of the B7-H3 staining was further quantified and ranged from low to high expression. Finally, association studies investigated potential clinical correlation between tumor-associated B7-H3 and disease severity. Various clinicopathological parameters were assessed, including tumor size, metastasis, cancer stage, survival, and recurrence rate. In most cases, a high expression of B7-H3 correlated with bad prognosis and poor clinical outcome. One study with over 800 prostate cancer patients revealed that patients with strong B7-H3 expression on tumor cells had a significantly increased risk of disease spread at time of surgery, clinical cancer recurrence and cancer-specific death (25). B7-H3 expression on lung cancer was associated with a lower number of tumor infiltrating lymphocytes (TILs) and with lymph node metastasis, suggesting a role for B7-H3 in immune evasion and tumorigenesis (30). Importantly, B7-H3 protein expression in tumors is known to be modulated by miR-29 (31), up-regulated upon IFN-γ stimulation (32) and potentially increased by Immunoglobulin-like transcript 4 signalling (33).
Table 1.
B7-H3 aberrant expression in human cancers and association with clinical-pathological characteristics
| Cancer type | B7-H3 expressing tumor tissues | Clinical correlation | Refs |
|---|---|---|---|
| Hepatocellular carcinoma | 93.8 % | Poorer survival, increased recurrence | (32) |
| Pancreas | 93.7% | Lymph node metastasis, lower differentiation grade | (27) |
| Prostate | - 93% | - Disease spread, increased risk of clinical cancer recurrence and cancer-specific death | (25) |
| - 100% | - larger tumor volume, extraprostatic extension, higher Gleason score, seminal vesicle involvement, positive surgical margins, >4-fold increased risk of cancer progression after surgery | (62) | |
| Osteosarcoma | 91.8% | Shorter survival and recurrence time, lower CD8 TIL | (63) |
| Breast | - 90.60% | - Lymph nodes metastasis, advanced disease, IL-10 in tumor cells | (64) |
| - 80.55% | - Negative relation with VEGF, microvascular density for CD34, and tumor size | (24) | |
| Colorectal cancer | - Cytoplasmic/membrane 86% Stroma 77% Nuclear 27% |
- reduced recurrence-free survival in TNM stage 1 | (28) |
| - Cytoplasm 62% Membrane 46% Nuclear 30% |
- reduced metastasis-free, disease-specific and overall survival | (29) | |
| Ovarian carcinoma | Cytoplasm/membrane 83% Tumor endothelium 44% |
High-grade serous histological subtype, increased recurrence and reduced survival | (26) |
| Endometrial cancer | 75.7% | TIL infiltration, shortened overall survival | (65) |
| Oral squamous cell carcinoma | 74.75% | Larger tumor size, advanced clinical stage, low survival rate | (34) |
| Cervical | 72.22% | Tumor size, positive correlation with FoxP3, negative correlation with IL-2 | (66) |
| Non-small lung cancer | - 69.5% | - Lymph nodes metastasis, TNM stage | (67) |
| - 37.1% | - Lower TILs, lymph nodes metastasis | (30) | |
| Bladder | 58.6% | No association | (68) |
| Clear cell renal cell carcinoma | Cancer cell 19% Tumor vasculature 18% |
large tumor size, advanced TNM stage, high nuclear grade, coagulative tumor necrosis, and capsular invasion | (69) |
| Glioma | Not specified | Malignancy grade | (70) |
| Melanoma | Not specified | Stage of melanoma, melanoma specific survival in stage III and IV | (22) |
TIL, tumor infiltrating lymphocyte; VEGF, vascular endothelial growth factor; TNM, TNM Classification of Malignant Tumors.
*Not all clinical studies were included in this table due to the space limitation
Up to date, the molecular mechanisms by which B7-H3 participates in tumor growth and immune evasion still remain elusive and need further investigation. Interestingly, aberrant glycosylation of B7-H3 was described in oral cancer. Its’ glycans, more diverse and with higher fucosylation, seem to interact better with DC-SIGN [DC-specific intercellular adhesion molecule-3 (ICAM-3)-grabbing non-integrin] and Langherin (34), proteins expressed on the membrane of DCs, suggesting a possible engagement and tolerization of DCs. Moreover, the crosstalk between lung cancer cells and tumor associated macrophages, partially through IL-10, induces B7-H3 membrane expression and inhibits T cell anti-tumor immunity in mice (35). Besides its role in modulating tumor immunity, B7-H3 also has a non-immunological function in regulating tumor aggressiveness. It was shown to modulate migration, invasion and adhesion to fibronectin of various cancer cells (36) through the Jak2/Stat3/MMP-9 signaling pathway (37). Additionally, overexpression of B7-H3 in colorectal and breast cancer cells augments resistance to apoptosis by activating the Jak2/STAT3/survivin signaling pathway. This in turn weakens tumor cell sensitivity to the chemotherapeutic drug Paclitaxel (38,39). Furthermore, B7-H3 was shown to modulate the metastasis-associated proteins MMP-2, TIMP-1, TIMP-2, STAT3 and IL-8 in melanoma cells (40). In hepatoma cells, B7-H3 targeted the epithelial-to-mesenchymal transition via the Jak2/STAT3/Slug signaling pathway (41). Lastly, a recent study showed that decreased expression of B7-H3 reduces the glycolytic capacity and sensitizes breast cancer cells to AKT/mTOR inhibitors, unveiling a previously unknown link between B7-H3 and metabolism (42). Altogether, these mechanisms promote aggression and invasion of the tumor.
Clinical-Translational Advances
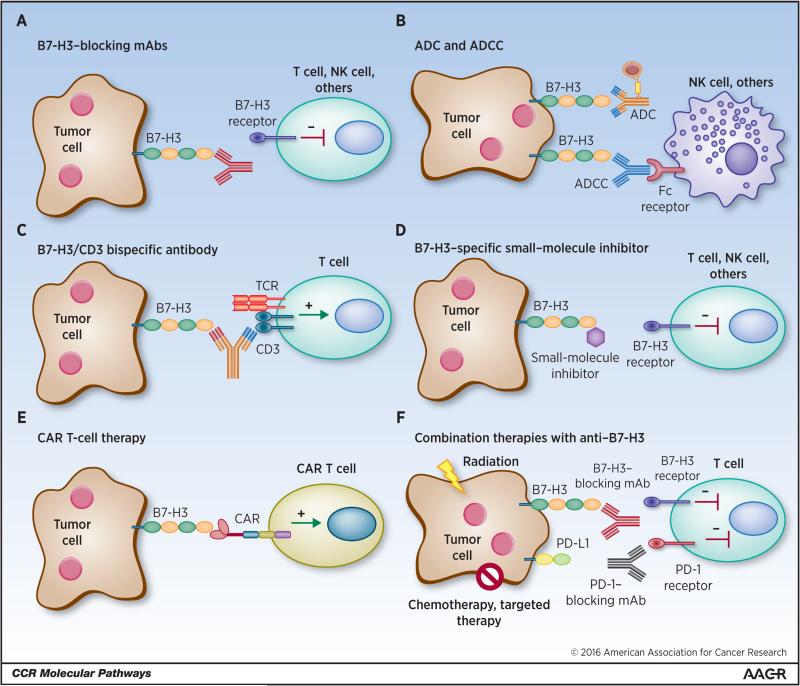
The precise role of B7-H3 in regulating the function of tumor-infiltrating immune cells and its’ activity in cancer cells has yet to be fully elucidated. This is due in large part to the conflicting studies that have demonstrated B7-H3 to be either co-stimulatory or co-inhibitory in several disease models. Additionally, the receptor(s) that interact with B7-H3 has yet to be identified, magnifying the scrutiny. However, there is no doubt that aberrant expression of B7-H3 consists of a possible biomarker and a promising immune checkpoint target for multiple cancer immunotherapy approaches (Figure 1), as anticipated almost 10 years ago (43). The scientific community is beginning to explore its therapeutic role in cancer in a variety of ways.
Figure 1. Human cancer immunotherapy strategies targeting B7-H3.
(A) Blockade of B7-H3 with blocking monoclonal antibodies (mAbs) neutralize inhibitory signaling in its’ unidentified receptor(s) in T cells, NK cells and other immune cells enabling effector function. (B) B7-H3 specific antibody-dependent cell-mediated cytotoxicity (ADCC) initiated by Fc receptor engagement of NK cells and other immune cells induces death of tumor cells. Antibody drug conjugates (ADC) bind to B7-H3 expressed by tumor cells and are internalized and generate cytotoxicy to tumor cells. (C) CD3/B7-H3 bispecific antibodies bind to tumor-expressed B7-H3 and crosslink the CD3 portion of the TCR complex, activating T cells in the tumor microenvironment for tumor cell death. (D) Small molecule inhibitors may bind to specific regions of B7-H3, such as the FG loop of the IgV domain, inhibiting the ligand-receptor interaction between tumor cells and immune cells, thus blocking receptor signaling and restoring effector function of immune cells. (E) Engineered chimeric antigen receptor (CAR) T cells recognize membrane B7-H3 and directly kill tumor cells. (F) Blocking mAbs against B7-H3 in combination with radiation, chemotherapy, targeted therapy, or other immune checkpoint inhibitors blockade synergize to generate more effective anti-tumor immune responses.
Blocking monoclonal antibodies
The B7 ligand and CD28 receptor families have become attractive targets for cancer immunotherapy, with specific emphasis placed on the development of monoclonal antibodies (mAb) blocking B7-CD28 pathways. Blocking mAbs against the immune checkpoints CTLA-4, programmed cell death protein 1 (PD-1), and PD-1 ligand 1 (PD-L1) have shown significant clinical success in patients with a variety of cancers (44–46). This same logic and success can be extended to B7-H3 as well (Figure 1A). Blocking mAbs are effective because they either partially or completely neutralize inhibitory ligand to receptor interactions, thus allowing effector functions. Despite the fact that the B7-H3 binding partner(s) remain unknown and that mAbs generated against B7-H3 are specific to the protein, the ability of these mAbs to neutralize B7-H3 interactions and the signaling pathway remain unknown. Thus, currently, no blocking mAb against B7-H3 is available. Until this receptor(s) is found, additional strategies in screening antibodies for neutralization capacity need to be developed.
Targeting B7-H3 through antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody drug conjugate (ADC) therapies
The difficulties that have been encountered in creating blocking mAbs against B7-H3, have led to the optimization of Abs against B7-H3 for therapy through alternative means (Figure 1B). Enoblituzumab (MGA271), a mAb reactive to cancer-associated B7-H3 demonstrated enhanced anti-tumor function through potent ADCC against a broad range of tumor cell types. In mice, weekly doses of MGA271 in both renal and bladder carcinoma xenografts resulted in sustained tumor growth inhibition, effects that were Fc mediated (47). Currently, an ongoing Phase I study of Enoblituzumab is being conducted in patients with refractory B7-H3-expressing tumors or B7-H3-expressing vasculature (trial NCT01391143). Preliminary results of the dose escalation study indicate that as a monotherapy, the Fc-enhanced mAb Enoblitzumab, demonstrates anti-tumor activity in several tumor types and modulates T cells by increasing the T cell repertoire clonality in the peripheral blood of patients following treatment (48). Though Enoblituzumab is not a blocking mAb and its’ success largely depends on ADCC, the results are encouraging and open the door for more clinical trials targeting this protein by way of mAbs.
Alternately, mAbs can be stably conjugated to a biologically active cytotoxic drug or compound that induces cell death. Once the mAb binds the cell surface antigen, the complex is internalized, releasing the cytotoxic substance and killing cancer cells. 8H9 is a mAb specific to B7-H3 that demonstrated clinical success as an ADC following it being radiolabeled to iodine-131 (131I) and administrated to patients with metastatic central nervous system neuroblastoma (49). 8H9 also distinguishes itself from other B7-H3 specific antibodies in that it binds to the FG loop of B7-H3, a region critical to its immunological function (50). Recently, 8H9 was humanized and affinity matured and maintained its ability to kill B7-H3 positive neuroblastoma cells in vitro. Two-fold and five-fold enhancements in killing were observed by the affinity matured and humanized 8H9 compared to the non-matured and chimeric generations, respectively. Furthermore, the mAb was labeled with 131I and injected into athymic nude mice xenografted with human neuroblastoma and showed successful biodistribution to the tumor (50). Currently, clinical trials with radiolabeled 8H9 are ongoing in patients with peritoneal cancers, gliomas, and advanced central nervous system cancers (NCT01099644, NCT01502917, and NCT00089245).
Bispecific antibodies
Bispecific antibodies are another suitable option beginning to pick up steam in the area of tumor immunotherapy. Bispecific antibodies are artificially generated antibodies composed of fragments of two distinct mAbs, thus combining two specificities. One arm can bind to the CD3 component of the TCR complex on T cells while the other arm recognizes a tumor specific antigen, for instance B7-H3, overexpressed on cancer cells (Figure 1C). That way, T cells are recruited to the tumor site and activated to kill cancer cells (51). Given the up-regulated expression of B7-H3 on multiple cancers, it seems like a promising option that should be pursued. Side-effects of bispecific antibody treatment include an excessive inflammatory reaction due to cytokines produced by over-activated T cells, but can be limited by corticosteroids administration.
Targeting B7-H3 with small molecule inhibitors
With no current information known about the receptor(s) of B7-H3, the only viable target for disruption of this pathway is tumor-expressed B7-H3. In addition to conventional therapeutic mAbs, the roles of small molecule inhibitors have also began to gain interest in the immune-oncology field (52). Small molecule inhibitors are low molecular weight organic compounds (dinucleotides, peptides, monosaccharides etc.) that bind specific biological targets. They are readily used because of the advantages they offer in cheaper manufacturing costs, ease of delivery due to oral administration, greater tissue distribution due to size, and shorter half-life when compared to antibodies. Knowing that the receptor(s) of B7-H3 on activated T cells engages the FG loop of the IgV domain of B7-H3 (19), a small molecule inhibitor could be designed to disturb this specific ligation area (Figure 1D). Although often unpredictable, off-target effects can arise and should be assessed as thoroughly as possible to limit detrimental consequences.
Targeting B7-H3 with chimeric antigen receptor (CAR) T cells
Another interesting way to target B7-H3 for immunotherapy is with CAR T cell technology (Figure 1E). This therapy recently demonstrated outstanding results in treating human refractive acute lymphoblastic leukemia (53,54). Autologous T cells are engineered with a CAR targeting a tumor antigen and adoptively transferred to patients to kill cancer cells. So far, this technology has been successfully applied to hematological cancers only. Although challenging, efforts are being made to translate CAR T cell therapy to the treatment of solid tumors. Importantly, the target must be highly overexpressed by the tumor and low or absent in normal peripheral tissues, as B7-H3, in order to avoid off-tumor effects. Engineered T cells would have to reach the tumor site and penetrate the stroma to specifically kill the targeted tumor cells. Moreover, CAR T cells would be exposed to the immunosuppressive tumor microenvironment, which could alter their function. Some optimizations of CAR T cells are currently being made and will hopefully help, either alone or in a combination therapy, to treat solid cancers (55). One clinical trial evaluated the safety and antitumor activity of CAR T cells in patients with chemotherapy refractory metastatic pancreatic cancer, with prelimimary evidence of good tolerance and antitumor efficacy (56). Of note, a cytokine release syndrome has been described in some patients and must be addressed to fully ensure the safety of this technique.
Synergistic options with anti-B7-H3 therapy: chemotherapy or targeted therapy, immune checkpoint inhibitors, and radiation
The clinical successes of mAbs blocking immune checkpoints such as CTLA-4, PD-1, and PD-L1 has led to the rationale of combining these modalities with conventional therapeutics or additional checkpoint inhibitors, with the goal of synergizing their actions and improving patient survival. The most traditional therapeutic regimen for treating cancers has been with chemotherapy. Recent studies have shown that the combination of a variety of chemotherapeutics with checkpoint inhibitors display great synergistic effects that enhanced the prospects of its full utilization in standard clinical practice. The combination of an anti-CTLA-4 mAb (Ipilimumab) and the chemotherapeutic drug Dacarbazine, when compared to Dacarbazine plus placebo, led to the improved overall survival of patients with metastatic melanoma (57). Based on a few preclinical animal studies, the combination of B7-H3 blockade and chemotherapy looks promising (Figure 1F). Indeed, the silencing of B7-H3 through shRNA in an histiocytic lymphoma derived human cell line, U937, in combination with the anti-neoplastic drug Ara-C led to 80% tumor reduction compared to the 40% inhibition observed in wild-type U937 cells combined with Ara-C in a mouse xenograft model (58). Similarly, shRNA silencing of B7-H3 in a murine model of breast cancer, combined with the chemotherapeutic Paclitaxel, showed approximately 80% reduction in tumor growth compared to the untreated wild-type cells (38). In both studies, silencing B7-H3 significantly enhanced tumor cells chemosensitivity and drug-induced apoptosis. Moreover, exploiting the differences between normal cell and cancer cells through targeted therapy as opposed to conventional chemotherapy may also deliver exciting results as a combination strategy. Taken together these studies provide rationale for the potential synergistic effects between B7-H3 blockade and chemotherapy or targeted therapy for patients with a variety of cancers.
The combination of multiple of immune checkpoint inhibitors as a means for treating cancers has also been emerging quite rapidly. A recent study has shown that the combination of anti-PD-1 mAb (Nivolumab) and Ipilimumab in patients with previously untreated melanoma resulted in significantly longer progression-free survival than Ipililumab alone (59). Furthermore, the combination of PD-1 and CTLA-4 blockade was able to demonstrate efficacy in patients with PD-L1 negative tumors compared to either agent alone. The expression pattern of B7-H3 contrasts greatly with the other checkpoint inhibitors in that the majority of B7-H3 can be found on tumor and tumor associated tissue, while the others are expressed on immune cells, normal tissue, and cancerous cells. This difference in expression can be highly advantageous for generating not only local responses through the tumor-specific targeting of B7-H3, but also systemic activation of immune cells through additional checkpoint blockade, altogether potentially further enhancing anti-tumor immunity (Figure 1F). Despite no available studies in preclinical models, Phase I clinical trials are underway to explore the safety of Enoblituzumab in combination with either Ipilimumab or anti-PD-1 (Pembrolizumab) in patients with refractory cancer (NCT02381314 and NCT02475213).
Radiation is an additional avenue that can be looked at in combination with B7-H3 targeting to be utilized in a future clinical setting (Figure 1F). An anecdotal clinical report suggests that Ipilimumab plus radiation cooperates to limit melanoma growth (60). Further studies confirmed these results in a small subset of melanoma patients treated with Ipilimumab and radiation (61). Of note, resistance was commonly seen and explained by PD-L1 up-regulation on the melanoma cells, causing T cell exhaustion, and highlighting the need for a triple combination therapy. Similarly, one should explore the potential synergistic effects of B7-H3 blockade and radiotherapy and its underlying mechanisms for future development of novel cancer immunotherapies (Figure 1F).
Concluding Remarks
B7-H3 has both immunological and non-immunological functions. Largely overexpressed in human tumor tissues, B7-H3 positively correlates with cancer severity and poor outcome. Compared to other immune checkpoints, B7-H3 pathway not only regulates innate and adaptive immunity but also promotes cancer cells aggressiveness through various non-immunological functions. Therefore, B7-H3 consists of a unique and interesting target for future cancer immunotherapies. One of the most promising therapeutic strategies may be the use of blocking mAbs against the B7-H3 pathway. Rather than administered alone, blocking mAbs are more likely to achieve synergistic anti-tumor effects if combined with a chemotherapeutic regimen or other checkpoint inhibitors. In parallel, finding its receptor(s) and better elucidating the involvement of B7-H3 pathway in immune responses and cancer development is crucial, as it would help for the design of more effective therapeutic agents, with the ultimate goal of complete and durable treatment of human cancers.
Acknowledgments
Grant Support
Research in the Zang lab is supported by the NIH under award numbers R01CA175495 and R01DK100525, the U.S. Department of Defense Established Investigator Idea Development Award (PC131008), Pfizer CTI, Jiangsu Hengrui Medicine Co., and Irma T. Hirschl/Monique Weill-Caulier Trusts (to X. Zang).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Steinberger P, Majdic O, Derdak SV, Pfistershammer K, Kirchberger S, Klauser C, et al. Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J Immunol. 2004;172:2352–9. doi: 10.4049/jimmunol.172.4.2352. [DOI] [PubMed] [Google Scholar]
- 2.Sun M, Richards S, Prasad DVR, Mai XM, Rudensky A, Dong C. Characterization of mouse and human B7-H3 genes. J Immunol. 2002;168:6294–7. doi: 10.4049/jimmunol.168.12.6294. [DOI] [PubMed] [Google Scholar]
- 3.Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, et al. B7-H3: A costimulatory molecule for T cell activation and IFN-γ production. Nat Immunol. 2001;2:269–74. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 4.Sun J, Fu F, Gu W, Yan R Zhang G, Shen Z, et al. Origination of new immunological functions in the costimulatory molecule B7-H3: the role of exon duplication in evolution of the immune system. PLoS One. 2011;6:e24751. doi: 10.1371/journal.pone.0024751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang G, Hou J, Shi J, Yu G, Lu B, Zhang X. Soluble CD276 (B7-H3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum. Immunology. 2008;123:538–46. doi: 10.1111/j.1365-2567.2007.02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W, Liu P, Wang Y, Nie W, Li Z, Xu W, et al. Characterization of a soluble B7-H3 (sB7-H3) spliced from the intron and analysis of sB7-H3 in the sera of patients with hepatocellular carcinoma. PloS One. 2013;8:e76965. doi: 10.1371/journal.pone.0076965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahnke K, Ring S, Johnson TS, Schallenberg S, Schönfeld K, Storn V, et al. Induction of immunosuppressive functions of dendritic cells in vivo by CD4+CD25+ regulatory T cells: Role of B7-H3 expression and antigen presentation. Eur J Immunol. 2007;37:2117–26. doi: 10.1002/eji.200636841. [DOI] [PubMed] [Google Scholar]
- 8.Suh W-K, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1–mediated immune responses. Nat Immunol. 2003;4:899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- 9.Zhang G, Dong Q, Xu Y, Yu G, Zhang X. B7-H3: another molecule marker for Mo-DCs? Cell Mol Immunol. 2005;2:307–11. [PubMed] [Google Scholar]
- 10.Castriconi R, Dondero A, Augugliaro R, Cantoni C, Carnemolla B, Sementa AR, et al. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci U S A. 2004;101:12640–5. doi: 10.1073/pnas.0405025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang G, Wang J, Kelly J, Gu G, Hou J, Zhou Y, et al. B7-H3 augments the inflammatory response and is associated with human sepsis. J Immunol. 2010;185:3677–84. doi: 10.4049/jimmunol.0904020. [DOI] [PubMed] [Google Scholar]
- 12.Hofmeyer KA, Ray A, Zang X. The contrasting role of B7-H3. Proc Natl Acad Sci U S A. 2008;105:10277–8. doi: 10.1073/pnas.0805458105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo L, Zhu G, Xu H, Yao S, Zhou G, Zhu Y, et al. B7-H3 promotes pathogenesis of autoimmune disease and inflammation by regulating the activity of different T cell subsets. PloS One. 2015;10:e0130126. doi: 10.1371/journal.pone.0130126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Fraser CC, Kikly K, Wells AD, Han R, Coyle AJ, et al. B7-H3 promotes acute and chronic allograft rejection. Eur J Immunol. 2005;35:428–38. doi: 10.1002/eji.200425518. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Quinn EM, Ni H, Wang J, Blankson S, Redmond HP, et al. B7-H3 participates in the development of experimental pneumococcal meningitis by augmentation of the inflammatory response via a TLR2-dependent mechanism. J Immunol. 2012;189:347–55. doi: 10.4049/jimmunol.1103715. [DOI] [PubMed] [Google Scholar]
- 16.Prasad DVR, Nguyen T, Li Z, Yang Y, Duong J, Wang Y, et al. Murine B7-H3 is a negative regulator of T cells. J Immunol. 2004;173:2500–6. doi: 10.4049/jimmunol.173.4.2500. [DOI] [PubMed] [Google Scholar]
- 17.Ueno T, Yeung MY, McGrath M, Yang S, Zaman N, Snawder B, et al. Intact B7-H3 signaling promotes allograft prolongation through preferential suppression of Th1 effector responses. Eur J Immunol. 2012;42:2343–53. doi: 10.1002/eji.201242501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veenstra RG, Flynn R, Kreymborg K, McDonald-Hyman C, Saha A, Taylor PA, et al. B7-H3 expression in donor T cells and host cells negatively regulates acute graft-versus-host disease lethality. Blood. 2015;125:3335–46. doi: 10.1182/blood-2014-09-603357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vigdorovich V, Ramagopal UA, Lázár-Molnár E, Sylvestre E, Lee JS, Hofmeyer KA, et al. Structure and T cell inhibition properties of B7 family member, B7-H3. Structure. 2013;21:707–17. doi: 10.1016/j.str.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leitner J, Klauser C, Pickl WF, Stöckl J, Majdic O, Bardet AF, et al. B7-H3 is a potent inhibitor of human T cell activation: No evidence for B7-H3 and TREML2 interaction. Eur J Immunol. 2009;39:1754–64. doi: 10.1002/eji.200839028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suh W-K, Wang SX, Jheon AH, Moreno L, Yoshinaga SK, Ganss B, et al. The immune regulatory protein B7-H3 promotes osteoblast differentiation and bone mineralization. Proc Natl Acad Sci U S A. 2004;101:12969–73. doi: 10.1073/pnas.0405259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Chong KK, Nakamura Y, Nguyen L, Huang SK, Kuo C, et al. B7-H3 associated with tumor progression and epigenetic regulatory activity in cutaneous melanoma. J Invest Dermatol. 2013;133:2050–8. doi: 10.1038/jid.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu Y, Lv X, Wu Y, Xu J, Wang L, Chen W, et al. Expression of costimulatory molecule B7-H3 and its prognostic implications in human acute leukemia. Hematology. 2015;20:187–95. doi: 10.1179/1607845414Y.0000000186. [DOI] [PubMed] [Google Scholar]
- 24.Sun J, Guo Y-D, Li X-N, Zhang Y-Q, Gu L, Wu P-P, et al. B7-H3 expression in breast cancer and upregulation of VEGF through gene silence. OncoTargets Ther. 2014;7:1979–86. doi: 10.2147/OTT.S63424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, et al. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci U S A. 2007;104:19458–63. doi: 10.1073/pnas.0709802104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zang X, Sullivan PS, Soslow RA, Waitz R, Reuter VE, Wilton A, et al. Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Mod Pathol. 2010;23:1104–12. doi: 10.1038/modpathol.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Sun J, Zhao H, Zhu D, Zhi Q, Song S, et al. The coexpression and clinical significance of costimulatory molecules B7-H1, B7-H3, and B7-H4 in human pancreatic cancer. OncoTargets Ther. 2014;7:1465–72. doi: 10.2147/OTT.S66809. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Ingebrigtsen VA, Boye K, Nesland JM, Nesbakken A, Flatmark K, Fodstad Ø . B7-H3 expression in colorectal cancer: associations with clinicopathological parameters and patient outcome. BMC Cancer. 2014;14:602. doi: 10.1186/1471-2407-14-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ingebrigtsen VA, Boye K, Tekle C, Nesland JM, Flatmark K, Fodstad O. B7-H3 expression in colorectal cancer: nuclear localization strongly predicts poor outcome in colon cancer. Int J Cancer. 2012;131:2528–36. doi: 10.1002/ijc.27566. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Wang Y, Zhao J, Gu M, Giscombe R, Lefvert AK, et al. B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung Cancer. 2006;53:143–51. doi: 10.1016/j.lungcan.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Xu H, Cheung IY, Guo H-F, Cheung N-KV. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: potential implications for immune based therapy of human solid tumors. Cancer Res. 2009;69:6275–81. doi: 10.1158/0008-5472.CAN-08-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun T-W, Gao Q, Qiu S-J, Zhou J, Wang X-Y, Yi Y, et al. B7-H3 is expressed in human hepatocellular carcinoma and is associated with tumor aggressiveness and postoperative recurrence. Cancer Immunol Immunother. 2012;61:2171–82. doi: 10.1007/s00262-012-1278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang P, Yu S, Li H, Liu C, Li J, Lin W, et al. ILT4 drives B7-H3 expression via PI3K/AKT/mTOR signalling and ILT4/B7-H3 co-expression correlates with poor prognosis in non-small cell lung cancer. FEBS Lett. 2015;589:2248–56. doi: 10.1016/j.febslet.2015.06.037. [DOI] [PubMed] [Google Scholar]
- 34.Chen J-T, Chen C-H, Ku K-L, Hsiao M, Chiang C-P, Hsu T-L, et al. Glycoprotein B7-H3 overexpression and aberrant glycosylation in oral cancer and immune response. Proc Natl Acad Sci U S A. 2015;112:13057–62. doi: 10.1073/pnas.1516991112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen C, Shen Y, Qu Q-X, Chen X-Q, Zhang X-G, Huang J-A. Induced expression of B7-H3 on the lung cancer cells and macrophages suppresses T-cell mediating anti-tumor immune response. Exp Cell Res. 2013;319:96–102. doi: 10.1016/j.yexcr.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y-W, Tekle C, Fodstad O. The immunoregulatory protein human B7H3 is a tumor- associated antigen that regulates tumor cell migration and invasion. Curr Cancer Drug Targets. 2008;8:404–13. doi: 10.2174/156800908785133141. [DOI] [PubMed] [Google Scholar]
- 37.Liu F, Zhang T, Zou S, Jiang B, Hua D. B7-H3 promotes cell migration and invasion through the Jak2/Stat3/MMP9 signaling pathway in colorectal cancer. Mol Med Rep. 2015;12:5455–60. doi: 10.3892/mmr.2015.4050. [DOI] [PubMed] [Google Scholar]
- 38.Liu H, Tekle C, Chen Y-W, Kristian A, Zhao Y, Zhou M, et al. B7-H3 silencing increases paclitaxel sensitivity by abrogating Jak2/Stat3 phosphorylation. Mol Cancer Ther. 2011;10:960–71. doi: 10.1158/1535-7163.MCT-11-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang T, Jiang B, Zou S-T, Liu F, Hua D. Overexpression of B7-H3 augments anti-apoptosis of colorectal cancer cells by Jak2-STAT3. World J Gastroenterol. 2015;21:1804–13. doi: 10.3748/wjg.v21.i6.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tekle C, Nygren MK, Chen Y-W, Dybsjord I, Nesland JM, Maelandsmo GM, et al. B7-H3 contributes to the metastatic capacity of melanoma cells by modulation of known metastasis-associated genes. Int J Cancer. 2012;130:2282–90. doi: 10.1002/ijc.26238. [DOI] [PubMed] [Google Scholar]
- 41.Kang F-B, Wang L, Jia H-C, Li D, Li H-J, Zhang Y-G, et al. B7-H3 promotes aggression and invasion of hepatocellular carcinoma by targeting epithelial-to-mesenchymal transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int. 2015;15:45. doi: 10.1186/s12935-015-0195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nunes-Xavier CE, Karlsen KF, Tekle C, Pedersen C, Øyjord T, Hongisto V, et al. Decreased expression of B7-H3 reduces the glycolytic capacity and sensitizes breast cancer cells to AKT/mTOR inhibitors. Oncotarget. 2016;7:6891–901. doi: 10.18632/oncotarget.6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zang X, Allison JP. The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res. 2007;13:5271–9. doi: 10.1158/1078-0432.CCR-07-1030. [DOI] [PubMed] [Google Scholar]
- 44.Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with Ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loo D, Alderson RF, Chen FZ, Huang L, Zhang W, Gorlatov S, et al. Development of an Fc- enhanced anti–B7-H3 monoclonal antibody with potent antitumor activity. Clin Cancer Res. 2012;18:3834–45. doi: 10.1158/1078-0432.CCR-12-0715. [DOI] [PubMed] [Google Scholar]
- 48.Enoblituzumab (anti-B7-H3) [about 2 screens] [cited 2016 Feb 24] Available from: http://www.macrogenics.com/enoblituzumab-anti-b7-h3/
- 49.Kramer K, Kushner BH, Modak S, Pandit-Taskar N, Smith-Jones P, Zanzonico P, et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J Neurooncol. 2010;97:409–18. doi: 10.1007/s11060-009-0038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmed M, Cheng M, Zhao Q, Goldgur Y, Cheal SM, Guo H-F, et al. Humanized affinity-matured monoclonal antibody 8H9 has potent antitumor activity and binds to FG loop of tumor antigen B7-H3. J Biol Chem. 2015;290:30018–29. doi: 10.1074/jbc.M115.679852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weidle UH, Kontermann RE, Brinkmann U. Tumor-antigen–binding bispecific antibodies for cancer treatment. Semin Oncol. 2014;41:653–60. doi: 10.1053/j.seminoncol.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 52.Adams JL, Smothers J, Srinivasan R, Hoos A. Big opportunities for small molecules in immuno oncology. Nat Rev Drug Discov. 2015;14:603–22. doi: 10.1038/nrd4596. [DOI] [PubMed] [Google Scholar]
- 53.Gill S, June CH. Going viral: chimeric antigen receptor T-cell therapy for hematological malignancies. Immunol Rev. 2015;263:68–89. doi: 10.1111/imr.12243. [DOI] [PubMed] [Google Scholar]
- 54.Sadelain M. CAR therapy: the CD19 paradigm. J Clin Invest. 2015;125:3392–400. doi: 10.1172/JCI80010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kakarla S, Gottschalk S. CAR T cells for solid tumors: armed and ready to go? Cancer J. 2014;20:151–5. doi: 10.1097/PPO.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beatty GL, O'Hara MH, Nelson AM, McGarvey M, Torigian DA, Lacey SF, et al. Safety and antitumor activity of chimeric antigen receptor modified T cells in patients with chemotherapy refractory metastatic pancreatic cancer. J Clin Oncol. 2015;33(suppl) abstr 3007. [Google Scholar]
- 57.Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 58.Zhang W, Wang J, Wang Y, Dong F, Zhu M, Wan W, et al. B7-H3 silencing by RNAi inhibits tumor progression and enhances chemosensitivity in U937 cells. OncoTargets Ther. 2015;8:1721–33. doi: 10.2147/OTT.S85272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Larkin J, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:1270–1. doi: 10.1056/NEJMc1509660. [DOI] [PubMed] [Google Scholar]
- 60.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–7. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roth TJ, Sheinin Y, Lohse CM, Kuntz SM, Frigola X, Inman BA, et al. B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 2007;67:7893–900. doi: 10.1158/0008-5472.CAN-07-1068. [DOI] [PubMed] [Google Scholar]
- 63.Wang L, Zhang Q, Chen W, Shan B, Ding Y, Zhang G, et al. B7-H3 is overexpressed in patients suffering osteosarcoma and associated with tumor aggressiveness and metastasis. PloS One. 2013;8:e70689. doi: 10.1371/journal.pone.0070689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu C, Liu J, Wang J, Liu Y, Zhang F, Lin W, et al. B7-H3 expression in ductal and lobular breast cancer and its association with IL-10. Mol Med Rep. 2013;7:134–8. doi: 10.3892/mmr.2012.1158. [DOI] [PubMed] [Google Scholar]
- 65.Brunner A, Hinterholzer S, Riss P, Heinze G, Brustmann H. Immunoexpression of B7-H3 in endometrial cancer: relation to tumor T-cell infiltration and prognosis. Gynecol Oncol. 2012;124:105–11. doi: 10.1016/j.ygyno.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 66.Huang C, Zhou L, Chang X, Pang X, Zhang H, Zhang S. B7-H3, B7-H4, Foxp3 and IL-2 expression in cervical cancer: Associations with patient outcome and clinical significance. Oncol Rep. 2016;35:2183–90. doi: 10.3892/or.2016.4607. [DOI] [PubMed] [Google Scholar]
- 67.Mao Y, Li W, Chen K, Xie Y, Liu Q, Yao M, et al. B7-H1 and B7-H3 are independent predictors of poor prognosis in patients with non-small cell lung cancer. Oncotarget. 2015;6:3452–61. doi: 10.18632/oncotarget.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xylinas E, Robinson BD, Kluth LA, Volkmer BG, Hautmann R, Küfer R, et al. Association of T- cell co-regulatory protein expression with clinical outcomes following radical cystectomy for urothelial carcinoma of the bladder. Eur J Surg Oncol. 2014;40:121–7. doi: 10.1016/j.ejso.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 69.Qin X, Zhang H, Ye D, Dai B, Zhu Y, Shi G. B7-H3 is a new cancer-specific endothelial marker in clear cell renal cell carcinoma. OncoTargets Ther. 2013;6:1667–73. doi: 10.2147/OTT.S53565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baral A, Ye HX, Jiang PC, Yao Y, Mao Y. B7-H3 and B7-H1 expression in cerebral spinal fluid and tumor tissue correlates with the malignancy grade of glioma patients. Oncol Lett. 2014;8:1195–201. doi: 10.3892/ol.2014.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]