Abstract
The pro-inflammatory cytokine tumor necrosis factor (TNF)-α has an important role in control of experimental Leishmania donovani infection. Less is known about the role of TNF-α in human visceral leishmaniasis (VL). Evidence for a protective role is primarily based on case reports of VL development in individuals treated with TNF-α neutralizing antibody. In this study, we have evaluated how TNF-α neutralization affects parasite replication and cytokine production in ex vivo splenic aspirates (SA) from active VL patients. The effect of TNF-α neutralization on cell mediated antigen specific responses were also evaluated using whole blood cultures. Neutralization of TNF-α did not affect parasite numbers in SA cultures. Interferon (IFN)-γ levels were significantly reduced, but interleukin (IL)-10 levels were unchanged in these cultures. Leishmania antigen stimulated SA produced significant TNF-α which suggests that TNF-α is actively produced in VL spleen. Further it stimulates IFN-γ production, but no direct effect on parasite replication.
Keywords: Visceral leishmaniasis, TNF-α, Enbrel, IL-10, splenic aspirate, whole blood assay
1. Introduction
Visceral Leishmaniasis (VL) remains an important neglected tropical disease, which still accounts for upto 50,000 deaths annually [1]. Drugs against VL are costly and are associated with severe side effects. Modulation of immune responses to facilitate cure of leishmaniasis has been suggested as a strategy to improve VL therapy [2]. However, the immune events mediating progression or control of Leishmania donovani during VL remain unclear in humans.
TNF-α is a multipotent, pro-inflammatory cytokine implicated in wide array of immune responses. TNF-α is important in the defence against many infections, being involved in macrophage activation, granuloma formation and maintenance [3], and upregulation of chemokine production and expression of cell adhesion molecules required for cellular recruitment [4; 5]. Reactivation of latent disease following anti-TNF-α treatment is a concern and a known risk factor for infections such as tuberculosis (TB) [6; 7]. Since Leishmania and mycobacterium have the same host cells and are controlled by similar immune responses, neutralization of TNF-α can be envisaged to increase susceptibility to Leishmania parasites. In experimental cutaneous leishmaniasis, treatment with TNF-α resulted in decreased parasite burden and lesion size [8; 9]. Consistent with a TNF requirement for protection, neutralization with anti-TNF causes transient aggravation of cutaneous disease [8; 10]. TNF receptor I (TNF-RI) and -II (TNF-RII) deficient mice were able to clear parasites but they exhibited continued swelling at the site of infection [11; 12]. In a visceral model, TNF-α was found to be critical for resistance to Leishmania donovani infection and resolution of disease [3; 9; 13]. TNF-α is believed to act in concert with IFN-γ through induction of nitric oxide (NO) production by activated macrophages to kill intracellular Leishmania parasites [14]. As a measure to control the inflammatory and potentially dangerous effects of excess TNF-α, production of the regulatory cytokine interleukin (IL)-10 is required [15]. Elevated co-expression of TNF-α and IL-10 has been a consistent finding in VL [16], and while the up-regulation of IL-10 is important to limit tissue pathology [17], it also allows parasites to persist and can facilitate their replication in macrophages [18; 19].
However, the protective role of TNF-α in human VL is not necessarily clear. VL development and/or reactivation of leishmaniasis has been observed following TNF-α neutralizing therapy [20; 21; 22; 23; 24; 25; 26; 27]. Higher levels of TNF-α may exacerbate disease, and polymorphism in an allele associated with higher production of serum TNF-α has been linked to VL [28]. Therefore, a better understanding about TNF-α activity in humans and a determination about whether this cytokine is a suitable target for immune modulation in VL is needed. Here, we examined the expression of TNF-α, TNF-RI and TNF-RII, as well as the activity of endogenous TNF-α during human VL by testing the effect of TNF-α neutralization on parasite load and cytokine production in splenic cells and on antigen specific cytokine production in peripheral blood from patients.
2. Materials and Methods
2.1 Study Subjects
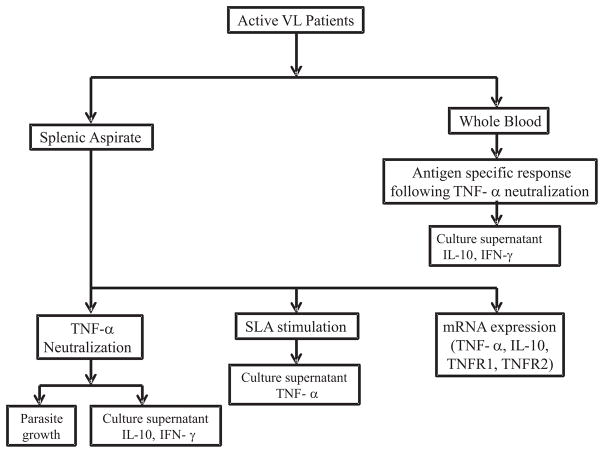
All the patients were presented with symptoms of VL at the Kala-azar Medical Research Center (KAMRC), Muzaffarpur, Bihar, India. In total, 45 VL cases, confirmed by detection of amastigotes in splenic aspirate smears and/or by a positive result in the rK39 diagnostic test, were recruited to the study with their prior consent and ethical clearance from the institutional ethics committee of Banaras Hindu University (IRB No. Dean/2008–09/314, Dean/2012–2013/89). Patients positive for HIV, tuberculosis, hepatitis or children <14 years were not included in the study. The clinical data of all the enrolled subjects are shown in Table 1. The analysis plan for various experimental assays performed is represented in Figure 1.
Table 1.
Clinical characteristics of the subjects enrolled in the study
| N | 45 |
|---|---|
| Sex (M/F) | 31/14 |
| Age (years) | 35.73 ± 15.79 (35)a |
| Duration of illeness (days) | 47.75 ± 43.11 (30) |
| WBC counts (pre treatment) | 3770 ± 2215 (3300) |
| WBC counts (post treatment)b | 7433 ± 2813 (7150) |
| Spleen size (cm), (pre treatment) | 4.78 ± 2.81 (4) |
| Spleen size (cm), (post treatment) | 0.59 ± 1.21 (0) |
| Spleen scorec# | 2.47 ± 1.2 (2) |
Value in parentheses represents median values.
Post treatment values are from day 15 or day 30 post treatment.
Parasite scoring is on logarithmic scale from 0 to 6, where 0 is no parasites per 1000 microscopic fields (1000X), 1 is 1–10 parasites per 1000 fields, and 6 is >100 parasites per field.
Splenic scores presented are only based on patients underwent biopsy (some patients are treated based on rk39 strip test)
Figure 1.
Schematic representation of various assays performed on VL patients.
2.2 Collection of spleen tissue and venous blood
Needle aspiration from spleen (SA) tissue was performed for diagnostic purposes (n=34). The remaining SA (50–150μl) material following the preparation of tissue smears was collected in 0.9ml heparinized RPMI 1640 medium (Gibco, U.S.A.), supplemented with 10% heat inactivated (HI) FBS (Gibco), 50 Units/ml Penicillin, 50μg/ml Streptomycin (PenStrep, Gibco), 25mM HEPES (Gibco).
In a separate set of experiments, 5ml venous blood was collected in heparinized syringes from VL patients (n=11) for whole blood cell culture. Cell culture was performed at KAMRC immediately after collection of blood, while splenic samples were transported at 4–8°C to the central lab at Banaras Hindu University and processed immediately upon arrival.
2.3 Spleen Cell Culture
2.3.1 Determination of numbers of viable Leishmania parasites in splenic aspirates
The number of Leishmania parasites in SA were quantified by a serial dilution method as described previously [29; 30]. Briefly, parasites from SA were grown out on NNN- blood agar plates, overlaid with M199+20% FBS. Each sample was diluted 3-fold in 96-well microtiter plates over 12 or more wells. The blood agar culture plates were routinely monitored for parasite growth and the number of viable parasites was determined by the last well of growth or highest dilution in which promastigotes could be detected following 7–10 days of incubation at 25°C in a BOD incubator.
2.3.2 Neutralization of cytokines in SA cultures
The SA suspension was divided into three equal parts in U-bottom 96-well, polypropylene culture plates (Nunc) and treated with purified mouse monoclonal antibodies against TNF-α (clone 1825, R&D Systems, U.S.A.) or Enbrel (Etanercept, Pfizer, USA). Control SA was incubated with purified mouse monoclonal IgG or IgG1 isotype antibody (clone 20116, R&D Systems). We previously reported that IL-10 neutralization promotes parasite clearance in SA cells from VL patients [31]. As a control, and to confirm that our assay system was working properly, we also cultured SA cells in presence of anti-IL-10 (clone 25209, R&D systems) or control IgG2b antibodies (clone 20116, R&D systems). All antibodies were used at a final concentration of 20μg/ml. SA was incubated for 3 days at 37°C in 5% CO2. SA culture supernatants were removed and stored at −80°C until processed for cytokine measurements. The removed volume was replaced by promastigote growth medium M199, containing 20% FCS, 100U/ml penicillin, 100μg/ml streptomycin, 2mM L-glutamine, 40mM HEPES, 0.1mM adenine (in 50mM HEPES). The SA was then cultured in serial dilutions, as described above, to determine the number of viable parasites in the culture.
2.4 Splenic aspirate and whole blood culture
The SA suspension was divided into two equal parts and either stimulated with SLA (10μg/ml) or media for 24 hours at 37°C in 5% CO2. Culture supernatant was collected following incubation for cytokine estimation by ELISA. Whole blood cell cultures were performed as described previously [32; 33]. In brief, heparinised blood was collected from VL patients and centrifuged at 450g. To remove the background levels of cytokines, plasma was removed, and the remaining blood cells were washed with PBS and an equal volume of heat inactivated FBS was added. One ml of whole blood was dispensed into polypropylene tubes and stimulated with soluble leishmania antigen (SLA; 10μg/μl) in the presence or absence of anti-TNF-α mAb or an isotype control mAb. PBS and PHA (10μg/μl) were used as a control in this assay. Whole blood was incubated at 37°C in 5% CO2 for 24hours, prior to removing cell culture supernatant and storing at −80°C until used for cytokine measurements.
2.5 Cytokines measurements
Commercial ELISA kits (Biolegend, U.S.A) were used to measure levels of IL-10, IFN-γ and TNF-α in cell culture supernatants, according to the manufacturer’s instructions.
2.6 RNA isolation, cDNA synthesis and Real time PCR
RNA was isolated from splenic tissues stored in RLT buffer, using RNAeasy minikit (Qiagen, Germany), as per manufacturer’s instruction. cDNA synthesis was performed on RNA samples using High Capacity cDNA Reverse transcription kits (Applied Biosystems, USA) as per their instructions in 20μl reaction volume. Real time PCR was performed by using ABI-Prism 7500 (Applied Biosystems). cDNA specific for FAM-MGB labelled probe for TNF-α, TNF-RI, TNF-RII, IL-10 and VIC-MGB labelled 18s mRNA were used to determine normalised expression of gene of interest over 18s (all reagents from Applied Biosystems).
2.7 Statistical Analyses
Statistical analysis was performed using PRISM5 (GraphPad Software, La Jolla, CA, USA) and employing either Mann Whitney U Test or Wilcoxon matched pair test. The effect of treatment was considered significant when p<0.05.
3. Results
3.1 IL-10 neutralization reduces the parasite burden in SA cultures
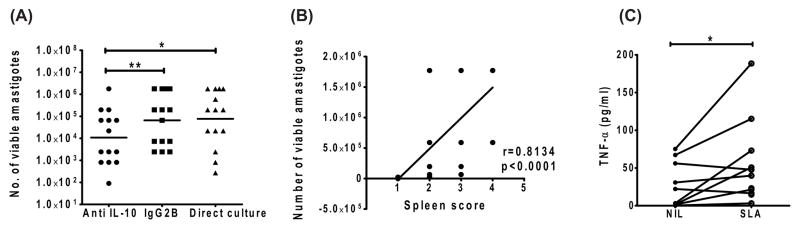
We previously reported the effect of IL-10 neutralization on parasite clearance in SA cells from VL patients [34]. As a positive control for our cell culture system, we first confirmed the effect of IL-10 neutralization on parasite growth. In line with our previous findings, we found a significant reduction in the number of viable parasites in anti-IL-10 mAb-treated SA cell cultures in 86% of the samples tested, compared to cell cultures treated with isotype control mAb (Figure 2A). In addition, we did not observe any significant changes in the number of viable amastigotes in splenic cultures plated directly over the same cultures which were plated following three days of incubation (Figure 2A). However, we observed a positive correlation (Spearman r=0.81, p<0.0001) between the number of viable parasites, as determined by limiting dilution culture, and the measurement of parasite burden as spleen score, determined by microscopy (Figure 2B). Furthermore, SLA stimulation of splenic aspirate cells showed significant production of TNF-α in VL spleen (Figure 2C). Thus, we found parasite growth in SA cultures, despite the presence of TNF-α.
Figure 2. Effect of IL-10 neutralization on parasite growth and relationship between parasite growth and spleen score.
A) Spleen cells were cultured in blood agar in the presence of anti IL-10 or control IgG2B for 3 days, as indicated, before counting the number of viable amastigotes by limiting dilution in blood agar plate (n=13). Base line quantification of viable parasite using direct culture was also included for comparison. B) Correlation between number of viable parasites as measured by base line quantification using direct plating and spleen score determined by microscopy. Statistical differences of p<0.05 (*), p < 0.01 (**) and p < 0.001 (***) are indicated. C) TNF-α production by splenic cells after stimulation with SLA in 24hour culture.
3.2 Leishmania parasite load in SA cultures was not affected by TNF-α neutralization
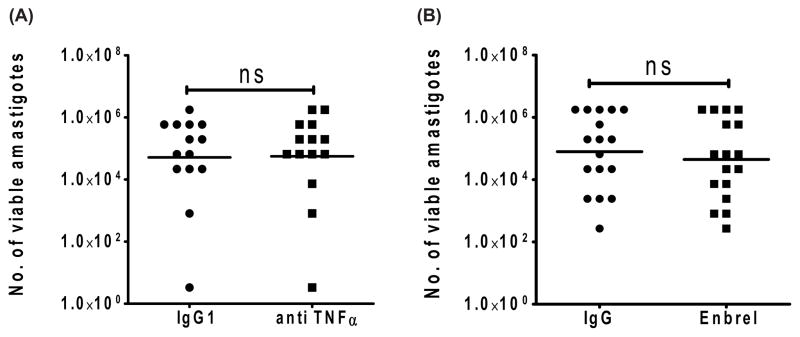
To investigate the role of endogenous TNF-α on parasite growth, we cultured SA tissue from VL patients in the presence or absence of two different TNF-α neutralizing monoclonal antibodies, including Enbrel, a clinically approved TNF-α neutralizing soluble receptor routinely used in several clinical settings to reduce inflammation. Somewhat surprisingly, we found no significant changes in parasite burden following TNF-α blockade, regardless of the neutralizing mAb used (Figure 3A & 3B).
Figure 3. TNF-α neutralization using mouse anti-TNF-α or Enbrel (Humanized anti TNF-α Ab) had no effect on parasite replication in ex vivo SA culture.
Splenic aspirate cells were cultured at 37°C, 5% CO in the presence of either A) mouse anti human TNF-α Ab (20μg/ml) and Control IgG1 (20μg/ml) or B) Enbrel (20μg/ml) and isotype control human IgG (20 μg/ml). Values shown are the number of viable amastigotes present after 3 days, as estimated by microtitration culture in blood agar plates. Data represent the geometric mean of number of parasites. Comparison was made using Wilcoxon signed rank test for paired samples. Samples where growth was detected in the last well of titration in both control IgG and anti-TNF treated were excluded from analysis.
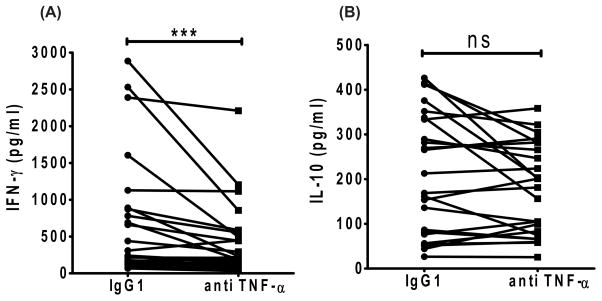
3.3 Blockade of TNF-α reduces IFN-γ levels in SA cell cultures
Next, we tested if TNF-α influenced endogenous IFN-γ and IL-10 production during Leishmania infection by assessing how TNF-α blockade affected the levels of these cytokines in SA cell culture supernatants. Neutralization of TNF-α resulted in a significant decrease in IFN-γ levels in 84% of samples, compared to control treated samples (Figure 4A). However, there was no change in IL-10 levels in these same SA cell cultures (Figure 4B).
Figure 4. TNF-α Blockade significantly reduces the IFN-γ secretion but not IL-10 in 3 days spleen culture.
Reduction of IFN-γ but not IL-10 in 3-day splenic culture supernatant of VL patients after TNF-α neutralization. A) IFN-γ and B) IL-10 level in SA culture supernatants. Statistical differences of p < 0.001 (***) are indicated. Each dot represents one sample. Lines indicate paired samples, treated and controls.
3.4 Expression of TNF-α, TNF-RI and TNF-RII mRNA in the spleen was not significantly altered by drug treatment
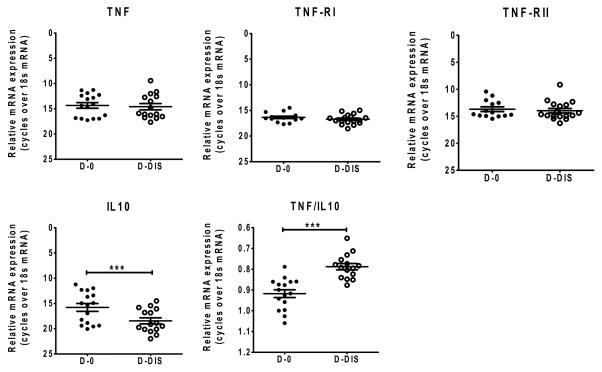
Ex-vivo gene expression analysis in whole splenic aspirate cells using real time PCR showed no significant changes in expression of TNF-α, TNF-RI or TNF-RII mRNA in active VL, when compared with cells from the same patients after drug treatment. However, we found decreased TNF/IL-10 expression index in active VL (Figure 5), which was likely caused by the significantly higher expression of IL-10 in active VL.
Figure 5. mRNA expression analysis in VL pre and post treated splenic aspirates.
TNF-α, TNF-RI, TNF-RII, IL-10 mRNA and TNF-α:IL-10 expression index in splenic aspirate from VL patients before drug treatment (D-0) and after drug treatment D(-Dis) were measured by qPCR. The expression level shown are relative to the 18S control gene (n=14 subjects per group).
3.5 Neutralization of TNF-α does not alter antigen specific IFN-γ and IL-10 production in whole blood cultures
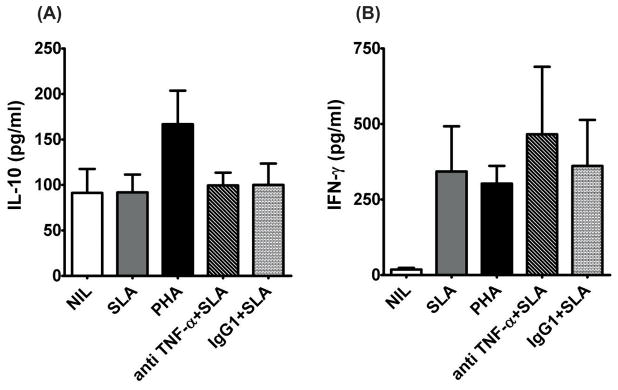
To further evaluate the role of TNF-α during VL, we next investigated how TNF-α neutralization affected antigen-specific responses in whole blood stimulated with SLA from VL patients (n=11) [33; 35]. In this short term assay, we found no effect of TNF-α blockade on the IL-10 or IFN-γ responses to SLA (Figure 6A & 6B). This latter result suggested differences in the relationship between TNF-α and IFN-γ production in the spleen and blood.
Figure 6. TNF-α neutralization does not affects SLA induced IFN-γ or IL-10 production in peripheral blood of VL patients.
Cytokine levels in SLA stimulated whole blood culture of VL patients. Whole blood (WB) samples of VL patients were stimulated with SLA (10μg/ml) in presence or absence of anti TNF-α antibody or control IgG1 (20μg/ml) and cultured for 24 h in 5% CO2 at 37°C. A) IL-10 and B) IFN-γ in WB culture supernatant. Values shown are mean +1 SD, of 11 patient samples assessed.
4. Discussion
Blockade of TNF-α can result in increased susceptibility to infections such as TB and leishmaniasis, or reactivation of disease following therapeutic cure. This indicates that TNF-α plays an important role in the control of intracellular pathogens, especially those that infect macrophages. In mice, TNF-α was found to be required for the control of L. donovani infection and for the formation of granulomas [13; 36]. We have previously shown that IL-10 neutralization increases production of TNF-α by SA cells and suggested that the increased TNF-α could contribute to parasite killing [34]. To better understand whether TNF-α has a direct role in control of parasite growth in human L. donovani infection, we tested the effect of TNF-α blockade on SA cell cultures. Culture of SA cells has previously been used to examine the role of cells and cytokines in tissue from target site of infection in VL patients [34]. In contrast to expectations, we found no direct effect of TNF-α neutralization on parasite burden in ex vivo SA cultures. However, there was significant decrease in IFN-γ levels, but little effect on IL-10 secretion. Using the same short term culture assay involving splenic aspirate cells, we have previously shown that both IFN-γ and IL-10 plays an important role in regulating parasite replication in spleen tissue from VL patients [31; 33]. Hence, it is surprising that the reduced IFN-γ levels observed after TNF-α blockade did not cause increased parasite growth. One possible explanation could be that other anti-parasitic pro-inflammatory cytokines may have compensated for reduced IFN-γ levels in this system. Both IL-17 and IL-22 have been implicated in better control of L. donovani growth in African VL patients [37]. It can also be reasonably expected that our short term SA assay may only allow us to observe very direct effects on parasite replication. Manipulations that act though up or down regulation of another cytokines targeting parasite replication may thus not be readily detectable. More importantly, in L. major infection, mice can eliminate parasites without signalling via TNF receptors, and this was found to be associated with macrophage activation and NO production, which suggest that TNF-α is not absolutely required for parasite containment [12]. In the absence of TNF-α, T cells can activate macrophages for NO production and hence Leishmania parasite killing [38]. Such TNF independent parasite killing mechanisms initiated by T cells may explain the absence of any effect on parasite growth in our blockade system.
TNF-α was found to be critical for Th1 cell-dependent granuloma formation in an experimental model of VL [39]. Although we did not show a direct role for TNF-α in killing of parasites, our results indicate immune modulatory effects of TNF-α during VL, especially in the spleen. Leishmania infected macrophages produces large amount of TNF-α [40] and this TNF-α can contribute to IFN-γ production by T cells and NK cells [41], so TNF-α blockade could interfere with IFN-γ secretion, as supported by our data. Neutralization of TNF-α has previously been reported to inhibit IFN-γ production by CD8+ T cells [42; 43], and we have also shown that CD8+ T cells contribute to endogenous IFN-γ production in SA cell cultures [44]. This suggest that TNF-α can induce exaggerated production of IFN-γ which induces tissue damage and pathology as seen in disease severity. The spleen is a site of chronic infection, associated with splenomegaly and tissue pathology [45; 46]. Under these conditions, immune regulation is likely to be compromised. Therefore, although reactivation of VL has been observed following TNF-α blockade [24; 47], and it has been shown to play critical roles in controlling parasite growth in experimental models [3; 36], the requirement for TNF-α in chronic VL may be different.
TNF and its receptors show no changes in expression following therapeutic cure in VL. The fact that TNF-α neutralization did not affect antigen specific IFN-γ or IL-10 production in 24-hour whole blood cell cultures suggests that TNF-α has minimal effects on Leishmania specific CD4+ T cell responses in peripheral blood, unlike what we find for IFN-γ production by spleen cells.
5. Conclusions
Together, our results indicate that TNF-α is actively produced in the spleen during human VL but has no direct impact on parasite replication despite promoting production of IFN-γ. Importantly, TNF-α may have deleterious roles during VL by causing tissue damage, and targeting this cytokine in a way that minimises its impact on IFN-γ production may have therapeutic advantages.
Acknowledgments
Funding
This work was supported by the Extramural Research Program of the National Institute of Allergy (NIAID), National Institutes of Health (NIH) Tropical Medicine Research Centers [TMRC Grant No IP50 AI074321]. Neetu Singh is funded by UGC-CSIR, New Delhi, India.
We thank hospital staffs at KAMRC for their assistance in the collection of patient samples. The care of patients was supported by the Sitaram Memorial Trust, KAMRC, Muzaffarpur, Bihar, India.
Abbreviations
- VL
Visceral leishmaniasis
- SA
splenic aspirate
- TB
Tuberculosis
- SLA
soluble leishmania antigen
- PHA
Phytohemagglutinin
- TNF-α
tumor necrosis factor-α
- IFN-γ
Interferon-γ
- IL-10
interleukin-10
- TNF-RI
Tumor necrosis factor receptor type I
- TNF-RII
Tumor necrosis factor receptor type II
- NO
Nitric oxide
Footnotes
Conflict of interest
All authors reported no potential financial conflict of interest related to the content of this manuscript.
References
- 1.Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nylen S, Sacks D. Interleukin-10 and the pathogenesis of human visceral leishmaniasis. Trends Immunol. 2007;28:378–84. doi: 10.1016/j.it.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Murray HW, Jungbluth A, Ritter E, Montelibano C, Marino MW. Visceral leishmaniasis in mice devoid of tumor necrosis factor and response to treatment. Infect Immun. 2000;68:6289–93. doi: 10.1128/iai.68.11.6289-6293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sedgwick JD, Riminton DS, Cyster JG, Korner H. Tumor necrosis factor: a master-regulator of leukocyte movement. Immunol Today. 2000;21:110–3. doi: 10.1016/s0167-5699(99)01573-x. [DOI] [PubMed] [Google Scholar]
- 5.Roach DR, Bean AG, Demangel C, France MP, Briscoe H, Britton WJ. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol. 2002;168:4620–7. doi: 10.4049/jimmunol.168.9.4620. [DOI] [PubMed] [Google Scholar]
- 6.Ehlers S. Role of tumour necrosis factor (TNF) in host defence against tuberculosis: implications for immunotherapies targeting TNF. Ann Rheum Dis. 2003;62(Suppl 2):ii37–42. doi: 10.1136/ard.62.suppl_2.ii37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winthrop KL. Risk and prevention of tuberculosis and other serious opportunistic infections associated with the inhibition of tumor necrosis factor. Nat Clin Pract Rheumatol. 2006;2:602–10. doi: 10.1038/ncprheum0336. [DOI] [PubMed] [Google Scholar]
- 8.Titus RG, Sherry B, Cerami A. Tumor necrosis factor plays a protective role in experimental murine cutaneous leishmaniasis. J Exp Med. 1989;170:2097–104. doi: 10.1084/jem.170.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilhelm P, Ritter U, Labbow S, Donhauser N, Rollinghoff M, Bogdan C, Korner H. Rapidly fatal leishmaniasis in resistant C57BL/6 mice lacking TNF. J Immunol. 2001;166:4012–9. doi: 10.4049/jimmunol.166.6.4012. [DOI] [PubMed] [Google Scholar]
- 10.Theodos CM, Povinelli L, Molina R, Sherry B, Titus RG. Role of tumor necrosis factor in macrophage leishmanicidal activity in vitro and resistance to cutaneous leishmaniasis in vivo. Infect Immun. 1991;59:2839–42. doi: 10.1128/iai.59.8.2839-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vieira LQ, Goldschmidt M, Nashleanas M, Pfeffer K, Mak T, Scott P. Mice lacking the TNF receptor p55 fail to resolve lesions caused by infection with Leishmania major, but control parasite replication. J Immunol. 1996;157:827–35. [PubMed] [Google Scholar]
- 12.Nashleanas M, Kanaly S, Scott P. Control of Leishmania major infection in mice lacking TNF receptors. J Immunol. 1998;160:5506–13. [PubMed] [Google Scholar]
- 13.Tumang MC, Keogh C, Moldawer LL, Helfgott DC, Teitelbaum R, Hariprashad J, Murray HW. Role and effect of TNF-alpha in experimental visceral leishmaniasis. J Immunol. 1994;153:768–75. [PubMed] [Google Scholar]
- 14.Liew FY, Li Y, Millott S. Tumor necrosis factor-alpha synergizes with IFN-gamma in mediating killing of Leishmania major through the induction of nitric oxide. J Immunol. 1990;145:4306–10. [PubMed] [Google Scholar]
- 15.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy--review of a new approach. Pharmacol Rev. 2003;55:241–69. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 16.Nylen S, Maurya R, Eidsmo L, Manandhar KD, Sundar S, Sacks D. Splenic accumulation of IL-10 mRNA in T cells distinct from CD4+CD25+ (Foxp3) regulatory T cells in human visceral leishmaniasis. J Exp Med. 2007;204:805–17. doi: 10.1084/jem.20061141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mege JL, Meghari S, Honstettre A, Capo C, Raoult D. The two faces of interleukin 10 in human infectious diseases. Lancet Infect Dis. 2006;6:557–69. doi: 10.1016/S1473-3099(06)70577-1. [DOI] [PubMed] [Google Scholar]
- 18.Vouldoukis I, Becherel PA, Riveros-Moreno V, Arock M, da Silva O, Debre P, Mazier D, Mossalayi MD. Interleukin-10 and interleukin-4 inhibit intracellular killing of Leishmania infantum and Leishmania major by human macrophages by decreasing nitric oxide generation. Eur J Immunol. 1997;27:860–5. doi: 10.1002/eji.1830270409. [DOI] [PubMed] [Google Scholar]
- 19.Murphy ML, Wille U, Villegas EN, Hunter CA, Farrell JP. IL-10 mediates susceptibility to Leishmania donovani infection. Eur J Immunol. 2001;31:2848–56. doi: 10.1002/1521-4141(2001010)31:10<2848::aid-immu2848>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 20.Zanger P, Kotter I, Kremsner PG, Gabrysch S. Tumor necrosis factor alpha antagonist drugs and leishmaniasis in Europe. Clin Microbiol Infect. 2012;18:670–6. doi: 10.1111/j.1469-0691.2011.03674.x. [DOI] [PubMed] [Google Scholar]
- 21.Xynos ID, Tektonidou MG, Pikazis D, Sipsas NV. Leishmaniasis, autoimmune rheumatic disease, and anti-tumor necrosis factor therapy. Europe Emerg Infect Dis. 2009;15:956–9. doi: 10.3201/eid1506.090101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeziorski E, Dereure J, Mac Bullen G, Blanchet C, Ludwig C, Costes V, Rodiere M. Mucosal relapse of visceral leishmaniasis in a child treated with anti-TNFalpha. Int J Infect Dis. 2015;33:135–6. doi: 10.1016/j.ijid.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 23.Guedes-Barbosa LS, Pereira da Costa I, Fernandes V, Henrique da Mota LM, de Menezes I, Aaron Scheinberg M. Leishmaniasis during anti-tumor necrosis factor therapy: report of 4 cases and review of the literature (additional 28 cases) Semin Arthritis Rheum. 2013;43:152–7. doi: 10.1016/j.semarthrit.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 24.De Leonardis F, Govoni M, Lo Monaco A, Trotta F. Visceral leishmaniasis and anti-TNF-alpha therapy: case report and review of the literature. Clin Exp Rheumatol. 2009;27:503–6. [PubMed] [Google Scholar]
- 25.Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis. 2004;38:1261–5. doi: 10.1086/383317. [DOI] [PubMed] [Google Scholar]
- 26.Bagalas V, Kioumis I, Argyropoulou P, Patakas D. Visceral leishmaniasis infection in a patient with rheumatoid arthritis treated with etanercept. Clin Rheumatol. 2007;26:1344–5. doi: 10.1007/s10067-006-0356-5. [DOI] [PubMed] [Google Scholar]
- 27.Catala A, Roe E, Dalmau J, Pomar V, Munoz C, Yelamos O, Puig L. Anti-tumour necrosis factor-induced visceral and cutaneous leishmaniasis: case report and review of the literature. Dermatology. 2015;230:204–7. doi: 10.1159/000370238. [DOI] [PubMed] [Google Scholar]
- 28.Karplus TM, Jeronimo SM, Chang H, Helms BK, Burns TL, Murray JC, Mitchell AA, Pugh EW, Braz RF, Bezerra FL, Wilson ME. Association between the tumor necrosis factor locus and the clinical outcome of Leishmania chagasi infection. Infect Immun. 2002;70:6919–25. doi: 10.1128/IAI.70.12.6919-6925.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sacks DL, Melby PC. Animal models for the analysis of immune responses to leishmaniasis. Curr Protoc Immunol Chapter. 2001;19(Unit 19.2) doi: 10.1002/0471142735.im1902s28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maurya R, Mehrotra S, Prajapati VK, Nylen S, Sacks D, Sundar S. Evaluation of blood agar microtiter plates for culturing leishmania parasites to titrate parasite burden in spleen and peripheral blood of patients with visceral leishmaniasis. J Clin Microbiol. 2010;48:1932–4. doi: 10.1128/JCM.01733-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gautam S, Kumar R, Maurya R, Nylen S, Ansari N, Rai M, Sundar S, Sacks D. IL-10 neutralization promotes parasite clearance in splenic aspirate cells from patients with visceral leishmaniasis. J Infect Dis. 2011;204:1134–7. doi: 10.1093/infdis/jir461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ansari NA, Kumar R, Gautam S, Nylen S, Singh OP, Sundar S, Sacks D. IL-27 and IL-21 are associated with T cell IL-10 responses in human visceral leishmaniasis. J Immunol. 2011;186:3977–85. doi: 10.4049/jimmunol.1003588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar R, Singh N, Gautam S, Singh OP, Gidwani K, Rai M, Sacks D, Sundar S, Nylen S. Leishmania specific CD4 T cells release IFNgamma that limits parasite replication in patients with visceral leishmaniasis. PLoS Negl Trop Dis. 2014;8:e3198. doi: 10.1371/journal.pntd.0003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gautam S, Kumar R, Maurya R, Nylen S, Ansari N, Rai M, Sundar S, Sacks D. IL-10 neutralization promotes parasite clearance in splenic aspirate cells from patients with visceral leishmaniasis. J Infect Dis. 2011;204:1134–7. doi: 10.1093/infdis/jir461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh OP, Gidwani K, Kumar R, Nylen S, Jones SL, Boelaert M, Sacks D, Sundar S. Reassessment of immune correlates in human visceral leishmaniasis as defined by cytokine release in whole blood. Clin Vaccine Immunol. 2012;19:961–6. doi: 10.1128/CVI.00143-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engwerda CR, Ato M, Stager S, Alexander CE, Stanley AC, Kaye PM. Distinct roles for lymphotoxin-alpha and tumor necrosis factor in the control of Leishmania donovani infection. Am J Pathol. 2004;165:2123–33. doi: 10.1016/s0002-9440(10)63262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pitta MG, Romano A, Cabantous S, Henri S, Hammad A, Kouriba B, Argiro L, el Kheir M, Bucheton B, Mary C, El-Safi SH, Dessein A. IL-17 and IL-22 are associated with protection against human kala azar caused by Leishmania donovani. J Clin Invest. 2009;119:2379–87. doi: 10.1172/JCI38813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nashleanas M, Scott P. Activated T cells induce macrophages to produce NO and control Leishmania major in the absence of tumor necrosis factor receptor p55. Infect Immun. 2000;68:1428–34. doi: 10.1128/iai.68.3.1428-1434.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stanley AC, Engwerda CR. Balancing immunity and pathology in visceral leishmaniasis. Immunol Cell Biol. 2007;85:138–47. doi: 10.1038/sj.icb7100011. [DOI] [PubMed] [Google Scholar]
- 40.Giudice A, Vendrame C, Bezerra C, Carvalho LP, Delavechia T, Carvalho EM, Bacellar O. Macrophages participate in host protection and the disease pathology associated with Leishmania braziliensis infection. BMC Infect Dis. 2012;12:75. doi: 10.1186/1471-2334-12-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wherry JC, Schreiber RD, Unanue ER. Regulation of gamma interferon production by natural killer cells in scid mice: roles of tumor necrosis factor and bacterial stimuli. Infect Immun. 1991;59:1709–15. doi: 10.1128/iai.59.5.1709-1715.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Via CS, Shustov A, Rus V, Lang T, Nguyen P, Finkelman FD. In vivo neutralization of TNF-alpha promotes humoral autoimmunity by preventing the induction of CTL. J Immunol. 2001;167:6821–6. doi: 10.4049/jimmunol.167.12.6821. [DOI] [PubMed] [Google Scholar]
- 43.Bruns H, Meinken C, Schauenberg P, Harter G, Kern P, Modlin RL, Antoni C, Stenger S. Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J Clin Invest. 2009;119:1167–77. doi: 10.1172/JCI38482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gautam S, Kumar R, Singh N, Singh AK, Rai M, Sacks D, Sundar S, Nylen S. CD8 T cell exhaustion in human visceral leishmaniasis. J Infect Dis. 2014;209:290–9. doi: 10.1093/infdis/jit401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smelt SC, Engwerda CR, McCrossen M, Kaye PM. Destruction of follicular dendritic cells during chronic visceral leishmaniasis. J Immunol. 1997;158:3813–21. [PubMed] [Google Scholar]
- 46.Sundar S, Rai M. Laboratory diagnosis of visceral leishmaniasis. Clin Diagn Lab Immunol. 2002;9:951–8. doi: 10.1128/CDLI.9.5.951-958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franklin G, Greenspan J, Chen S. Anti-tumor necrosis factor-alpha therapy provokes latent leishmaniasis in a patient with rheumatoid arthritis. Ann Clin Lab Sci. 2009;39:192–5. [PubMed] [Google Scholar]