Abstract
Recent few years have seen great progress in understanding tumor biology and surrounding microenvironment. Solid tumors create regions with low oxygen levels, generally termed as hypoxic regions. These hypoxic areas offer a tremendous opportunity to develop targeted therapies. Hypoxia is not a random by-product of cellular milieu due to uncontrolled tumor growth; rather it is a constantly evolving participant in overall tumor growth and fate. This article reviews current trends and recent advances in drug therapies and delivery systems targeting hypoxia in the tumor microenvironment. In the first part, we give an account of important physicochemical changes and signaling pathways activated in the hypoxic microenvironment. This is then followed by various treatment strategies including hypoxia-sensitive signaling pathways and approaches to develop hypoxia-targeted drug delivery systems.
Keywords: Hypoxia, targeted therapy, HIF-1, pH targeted, solid tumor microenvironment, physicochemical change
1. Introduction
Oxygen is essential for energy metabolism, which drives cellular bioenergetics. Precisely distributed vasculature aids in delivering oxygenated blood to the body tissues. Regions of low oxygen levels are generally termed as hypoxic regions. Hypoxia is profound in various solid tumors, myocardial infraction, some chronic renal diseases and gliomas (Brown and Giaccia, 1998, Harris, 2002, Kaur et al., 2005). The clinical significance of hypoxia in cancer has been reviewed extensively (Dhani et al., 2015, Vaupel and Mayer, 2007). Rapid proliferation of tumor mass prevents vascularization in time, leading to hypoxia in solid tumors (Bertout et al., 2008, Gleadle and Ratcliffe, 2001). Although the growing tumor is capable of stimulating growth of new vasculature, such tumor-induced angiogenesis results in disorganized growth of vasculature. Spatial distortion of tumor vasculature resulting from lasting hypoxic conditions may lead to increase in intercapillary distance, which are beyond the diffusion capacity of oxygen. Such a condition is termed as chronic hypoxia or diffusion-limited hypoxia where tumor cells within 180 µm periphery of blood vessels are subjected to necrose (Harris, 2002, Thomlinson and Gray, 1955, Wilson and Hay, 2011). The other type of hypoxia, called acute hypoxia, perfusion-limited hypoxia or intermittent hypoxia can occur due to temporary blockade or variable blood flow in tumor blood vessels. Closed vessels can reopen causing re-oxygenation injury, an increase in free radicals, activation of stress response genes and tissue damage (Prabhakar, 2001). This also makes diffusion of drugs to the tumor sites difficult. Studies have shown that current anticancer strategies can only target tumor cells around blood vessels, but not those in poorly perfused areas (Carlisle and Coussios, 2013, Loges et al., 2009, Minchinton and Tannock, 2006). Hypoxic condition forces tumors to undergo adaptive genetic changes that resist hypoxia-induced cell death and tissue necrosis. In fact, the hypoxic tumor region is associated with the higher expression of MDR1 (multidrug resistance gene) and P-glycoprotein genes responsible for development of multidrug resistance to anticancer drugs (Brown and Giaccia, 1998). Low oxygen tension in tumors is found to be responsible for increased metastasis, poor patient survival as well as drug resistance in cases of squamous tumors of the head and neck, cervical or breast cancers (Höckel et al., 1999, Höckel and Vaupel, 2001). Moreover, slowly dividing cells in the hypoxic region escape from the action of most of the cytotoxic drugs that target rapidly dividing cells. Poorly perfused hypoxic areas may also harbor cancer stem cells that are capable of undergoing epithelial to mesenchymal transition (Carlisle and Coussios, 2013). Moreover, hypoxia also suppresses immune response and promotes epithelial to mesenchymal transition, invasiveness and metastasis (Birner et al., 2000). The effect of the hypoxic tumor microenvironment on metastatic progression is reviewed by Subarsky and Hill (Subarsky and Hill, 2003). Increasing evidence suggests hypoxia to be responsible not only for angiogenesis but also for changes in cellular metabolism. Exploiting such changes for selectively targeting hypoxic areas in tumors or other diseased conditions is an attractive strategy. Targeting hypoxia is one of the important strategies for treating solid tumors with fewer chances of recurrence (Dhani, Fyles, 2015). Drug delivery research has been moving to innovative strategies for various applications including engineered nanoparticle based drug/gene delivery systems (Goenka et al., 2014, Mason et al., 2015, Sant et al., 2005, Sant et al., 2008a, Sant et al., 2008b), with efforts for more accurately targeting strategies at its peak.
In this review, we briefly discuss various cellular signaling responses and other physicochemical changes triggered in the hypoxic microenvironment. In the later part, several established and experimental approaches to exploit hypoxia are reviewed briefly with a special focus on smart drug delivery systems selectively targeting the hypoxic regions. Finally, the review concludes with a brief overview of important clinical studies exploiting hypoxia as a viable approach for cancer treatment via direct or indirect strategies.
2. Hypoxia and tumor microenvironment
2.1. Physicochemical changes in hypoxic environment
Hypoxia leads to changes in the interstitial fluid pressure, reduced pH and increased generation of reactive oxygen species (ROS). Hypoxic areas exhibit high interstitial fluid pressure as a result of leaky vasculature as well as abnormal lymphatic drainage in the tumor (Nelson et al., 2004). In addition, hypoxic microenvironment leads to overproduction of lactic acid and carbonic acid (HCO3−) through the activation of glycolysis, which results in acidic pH. Precisely, carbonic anhydrase IX or XII, which are activated by hypoxia inducible factor (HIF), causes the reversible conversion of carbon dioxide and water into HCO3−, which diffuses out of the cell membrane resulting in excess HCO3− in the tumor microenvironment. Moreover, the pH of endosomal and lysosomal vesicles inside the tumor cells tend to be more acidic than the cytosolic pH (Harris, 2002). Such reduced intracellular pH may aid in metastasis by activating proteases.
The hypoxic region also exhibits a high redox potential difference between intracellular space (reducing) and extracellular space (oxidizing). Such redox potential difference offers the opportunity for devising bioconjugation strategies for selective and controlled delivery of active ingredient (Saito et al., 2003). Chemical classes such as nitro, quinones, aromatic N-oxides, aliphatic N-oxides and transition metals can be metabolized selectively under hypoxic microenvironment by enzymatic reduction (Wilson and Hay, 2011). Such oxygen-dependent and reversible reductions of prodrug complexes into their active form in the hypoxic region has been exploited by designing trigger-linker systems (Naughton, 2001).
Hypoxia also generates ROS, which alter genomic stability and impair DNA repair pathways (Guzy et al., 2005). Enzymatic reduction of specific chemical classes in hypoxic regions due to presence of ROS provides the basis for the design of bioreductive prodrugs (Wilson and Hay, 2011) as described in section 4.
2.2. Hypoxia activated signaling pathways
2.2.1. HIF-1 pathway
Hypoxic condition tends to promote molecular changes in both normal and neoplastic sites eventually leading to activation of HIF (Loges, Mazzone, 2009, Minchinton and Tannock, 2006). HIF-1 is a heterodimeric transcription factor consisting of HIF-1α and HIF-1β (also known as aryl hydrocarbon receptor nuclear translocator (ARNT)) subunits, both being expressed constitutively, with precise regulation of the α subunit (Jiang et al., 1996). HIF-1 leads to transcription of critical genes responsible for angiogenesis, glucose metabolism, invasion and cell fate (Krishnamachary et al., 2003, Pennacchietti et al., 2003). Expression of a transcription factor can be determined by the rates of protein synthesis and protein degradation. For HIF-1α, synthesis is regulated by oxygen-independent mechanisms whereas degradation is regulated by oxygen-dependent mechanisms. Oxygen availability promotes binding of HIF-1α to the tumor suppressor Von Hippel-Lindau (VHL) protein, which in turn leads to ubiquitinylation of HIF-1α. Subsequently, the HIF-1α subunit is hydroxylated by HIF-1 prolyl hydroxylase enzyme (HPH) facilitating its degradation in an oxygen-dependent manner (Bruick and McKnight, 2001, Epstein et al., 2001). However, in hypoxic condition, limited oxygen availability inhibits HPH and HIF-1 degradation (Lee et al., 2009). Accumulated HIF-1 then binds to several hypoxia response elements (HREs) resulting in expression of hypoxia response genes, also known as HIF target genes such as lactate dehydrogenase, carbonic anhydrase, vascular endothelial growth factor (VEGF) etc. (Table 1). Although HIF-1 can be activated by hypoxia in all cell types, cell type-specific expression of HIF-1 target genes can be explained by functional interaction of HIF-1 with other transcription factors modulated in those specific cell types. Therefore, HIF-1 can just be viewed as a relay race runner, which hands the activating baton to other transcription factors harbored in specific cell type in response to hypoxia (Semenza, 2003). However, one of the exceptions to this is VEGF, a HIF target gene induced in almost all cell types in response to hypoxia.
Table 1.
Major classes of HIF-1 target genes (Belozerov and Van Meir, 2005)
| Short term adaptation | Long term survival and proliferation | ||
|---|---|---|---|
| Function | Gene | Function | Gene |
| Glucose transport Glucose metabolism | Glucose transporter 1 (Glut-1) | Angiogenesis | VEGF |
| Glyceraldehyde 3- phosphate dehydrogenase (GAPDH) | Transforming growth factor beta 3 (TGF- β3) | ||
| Enolase 1 | Cell survival | Erythropoietin (EPO) | |
| Hexokinase 1 | Adrenomedullin (ADM) | ||
| Lactate dehydrogenase A (LDHA) | Cell proliferation | TGF-α | |
| 6-phosphofructo-2- kinase/fructose-2,6- biphosphatase 3 (PFKFB3) | Insulin-like growth factor 2 (IGF2) | ||
| Amino acid metabolism | Transgluaminase 2 | Drug resistance | Multidrug resistance (MDR1) |
| Iron homeostasis | Transferrin | P-glycoprotein | |
| Nucleotide metabolism | Adenylate kinase 3 | Invasion | Matrix metalloproteinase-2 (MMP2) |
| pH regulation | Carbonic anhydrase IX | Urokinase receptor (UPAR) | |
2.2.2. mTOR kinase and UPR pathways
In addition to HIF, two other oxygen sensitive signaling pathways are activated independently, namely Mammalian (or mechanistic) target of rapamycin (mTOR) and Unfolded Protein Response (UPR). Major function of mTOR is to integrate upstream metabolic signals that are essential for basic cellular functions. On the other hand, UPR is activated in response to endoplasmic reticulum (ER) stress and homeostatic imbalance. ER stress promotes the accumulation of unfolded or misfolded proteins triggering UPR activation, thus affecting downstream signaling such as protein production, maturation, degradation, cell metabolism and cell death (Wouters and Koritzinsky, 2008).
Several elements of both of these pathways are altered by hypoxia. The severity and/or duration of hypoxic condition determine influence of hypoxia on these pathways. Hypoxia inhibits the downstream signaling of mTOR kinase and initiation of mRNA translation via independent mechanisms. Alteration of the mTOR kinase signaling pathway changes tumor progression and hypoxic tolerance in advanced stages of the tumor. UPR is triggered in the case of severe hypoxic condition promoting hypoxic tolerance and aiding in cell adaptation and survival. Alternatively, through regulation of apoptotic pathways, UPR also triggers cell death. Several downstream effector pathways execute this tolerance effect (Guertin and Sabatini, 2007, Wouters and Koritzinsky, 2008). Targeting these pathways can prevent significant changes in mRNA translation, which can control gene expression in hypoxic cells ultimately controlling tumor growth.
Although HIF, mTOR and UPR get activated independently, increasing evidence points to a yet to be discovered, upstream transcription factor resulting in integrated response to hypoxia; however precise understanding of their influence on each other remains to be elucidated. A clear link between these interrelated hypoxia responsive pathways may provide clues making these pathways easier molecular targets for therapeutic benefit.
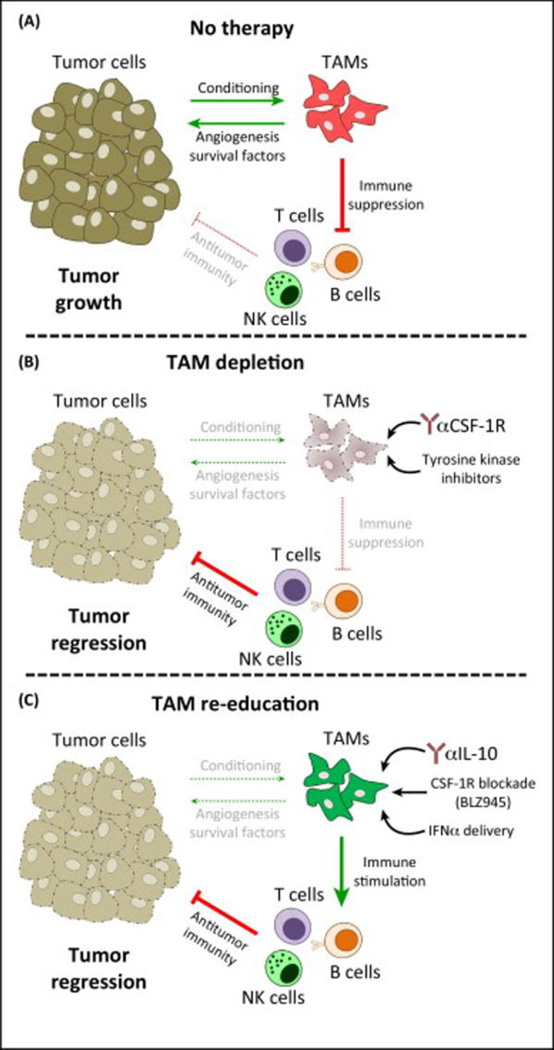
2.3 Hypoxia-induced changes in Tumor Associated Macrophages (TAMs)
Tumor microenvironment can be viewed as a supportive cellular ecology to support the malignant transition. During such a transition, tumor associated macrophages (TAMs) have been found to be recruited in a large population in breast cancer. They are attracted by dead cells and other debris in tumors and remain in necrotic areas of tumor without further migration (Murdoch et al., 2004, Turner et al., 1999). TAMs are also found to be involved in cancer-initiating inflammatory responses mostly by suppressing the anti-cancer immune response (Figure 2A). This is made possible by reducing the production of pro-inflammatory cytokine-interleukin (IL)-12 and cytotoxic type 1 T helper (TH1) CD4+ T cells. This, along with the production of immunosuppressive factors (such as IL-10, TGFβ, prostaglandin E2), primes the tumor microenvironment to suppress the anticancer immune response (Biswas et al., 2006, Ojalvo et al., 2009, Torroella-Kouri et al., 2009). Moreover, accumulation of TAMs in hypoxic regions is found to be associated with aggressive tumor behavior in breast cancer by expressing VEGF in poorly vascularized areas together with existing immunosuppressive microenvironment (Doedens et al., 2010, Leek et al., 1996, Lewis et al., 2000, Murata et al., 2002). In addition, hypoxic macrophages release macrophage migratory inhibitory factor (MIF), a gene product increased by HIF-1α upregulation. MIF stabilizes HIF-1α protein, eventually promoting the degradation of basement membranes in tumors and providing an easy escape for tumor cells (Oda et al., 2008). Obeid et al has recently reviewed the role of TAMs in the breast cancer progression discussing factors affecting TAMs other than hypoxia such as angiogenesis, tumor matrix and toll-like receptors, all of which need to be accounted for when estimating overall effect of TAMs on tumor invasiveness (Obeid et al., 2013). Comprehensive reviews focused on complex mechanisms of the influence of TAMs and therapeutic targeting opportunities are suggested for further reading (Chanmee et al., 2014, Noy and Pollard, 2014, Ostuni et al., 2015).
Figure 2.
Therapeutic targeting of tumor associated macrophages (TAMs). (A) During cancer progression, tumor-derived signals condition TAMs to directly promote tumor growth via neovascularization and the production of growth/survival factors. In addition, TAMs operate a range of immunosuppressive mechanisms that restrain the antitumor activity of infiltrating immune cells. (B) Therapies with anti-CSF-1R antibodies or (quasi-) specific inhibitors of CSF-1R tyrosine kinase activity effectively deplete TAMs, thus ablating their direct and indirect tumor-promoting actions. In turn, this results in tumor regression (or growth inhibition) via repression of cytotoxic immune responses. (C) Inhibition of tumor growth can also be achieved by functionally re-educating TAMs, rather than by killing them. This approach may be the most efficient because blockade of the tumor-promoting functions of TAMs may be coupled with enhancement of their immunostimulatory properties. Recent examples include IL-10 blockade, CSF-1R blockade in glioblastoma, or exogenous administration of pro-inflammatory cytokines. Abbreviations: NKs, natural killer cells; TAMs, tumor-associated macrophages. (Adopted in its original form from (Ostuni, Kratochvill, 2015))
Hypoxia-induced changes in tumor microenvironment are summarized in Table 2.
Table 2.
Hypoxia inducible changes in tumor microenvironment
| Physicochemical characteristics | Acidic pH (due to activation of glycolysis which results in overproduction of lactic acid and carbonic acid) |
| Changes in interstitial fluid pressure | |
| Relatively higher redox potential difference between intracellular space (reducing) and extracellular space (oxidizing). | |
| Generation of reactive oxygen species | |
| Signaling pathways | Activation of hypoxia inducible factor 1 (HIF-1) which leads to transcription of critical genes responsible for angiogenesis, glucose metabolism, invasion and cell fate. |
| Alteration of the mTOR kinase signaling pathway changes tumor progression and hypoxic tolerance in advanced stages of the tumor. UPR is triggered in the case of severe hypoxic condition promoting hypoxic tolerance and aiding in cell adaptation and survival. | |
| Tumor associated macrophages (TAMs) | Recruitment of TAMs in a large number in the tumor area and they remain there without further migration. |
3. Therapeutic approaches to exploit hypoxic tumor microenvironment
Hypoxic regions pose challenges for delivering sufficient amounts of drug to the tumor cells as they are remote to the blood vessels. Changes in physicochemical and biological properties of tumor vasculature and strategies to normalize it using anti-angiogenic factors have been reviewed by Fukumura et al (Fukumura and Jain, 2007). Leaky vasculature and a negative charge on the vessel luminal face can also be exploited by controlling the molecular weight of drug carrier polymers and using cationic molecules (Campbell et al., 2002, Fang et al., 2011). A device with a depot of growth factors to promote angiogenesis locally may help make systemic delivery of chemotherapeutic agents more effective to hypoxic regions of solid tumors. Such a device incorporating angiogenic growth factors in a collagen matrix has been shown to be induced by hypoxia (Hadjipanayi et al., 2011). However, the study did not report its applicability in hypoxic tumor and capability of normalizing the vessels and improving the drug delivery.
Drug distribution mechanisms in tumors and methods of modifying them have been reviewed in details by Paolo et al (Di Paolo and Bocci, 2007). Drug diffusion using detailed knowledge of physicochemical factors of tumor region and structural or pharmaceutical modifications of drug have been emphasized in this review. Gene therapies can take advantage of the HIF-1 active microenvironment to improve drug penetration and targeting of hypoxic areas. Unique approaches based on such principle have also been reviewed by Kizaka-Kondoh et al (Kizaka-Kondoh et al., 2009).
The following section gives brief accounts of currently used approaches exploiting hypoxia, which can be broadly classified as HIF inhibition by small molecules, bioreductive prodrugs and hypoxia-targeted delivery systems. Details of various prodrug strategies including the ones that exploit hypoxia and rationale for hypoxia-activated prodrug design are reviewed by Denny (Denny, 2001). A summary of therapeutic approaches targeting hypoxia can be found in Table 3.
Table 3.
Summary of therapeutic approaches exploiting hypoxic tumor microenvironment
| Therapeutic strategy |
Drug class /Drug name/ Drug delivery system |
Mechanism of action | Reference(s) |
|---|---|---|---|
| Hypoxia sensitive bioreductive prodrugs (releases the drug from the complex once activated) | Quinone lactonization | The quinone propionic-based carrier skeleton is used in this delivery system; the propionic moiety links the trigger (benzoquinone) to the active drug and lactonization is made possible by providing steric hindrance by three methyl groups on benzoquinone. The benzoquinone trigger gets reduced leading to intramolecular cyclization between benzoquinone and propionic moieties, the process by which active drug is released from the delivery system. | (Amsberry and Borchardt, 1991, Gharat, Taneja, 2001, Levine and Raines, 2012) |
| Self-alkylating bioreductive agents | They avoid DNA adduct formation by accommodating a nucleophile within bioreductive skeleton, which reacts with the activated remnant (capable of forming cytotoxic conjugate) after the drug is released. This nucleophile inactivates the cytotoxic residue by facilitating the nucleophile in the moiety itself. This class of agents are more suitable for diseases where cytotoxicity is not required. | (Blake, Naughton, 1998, Freeman, Jaffor, 2005, Jaffar, Williams, 2001) | |
| Vitamin E analogues (Trolox (6- hydroxy- 2,5,7,8- tetramethylchr oman-2- carboxylic acid)) | An oxidized vitamin E moiety (tocopheryl quinone, TQ) is used for drug release via intramolecular cyclization in a redox-driven manner. As TQ gets reduced in the hypoxic environment, it cyclizes and results in the release of a hydroxyl group. | (Liebler, 1993, Naughton, 2001) | |
| Selected examples of hypoxia sensitive bioactive prodrugs (get activated as a whole) | Aromatic N- oxides (tirapazamine) | In hypoxic condition, intracellular reduction of TPZ leads to the formation of its radical causing extensive DNA damage in the form of base pair damage, single strand breaks and double strand breaks via tumor-specific poisoning of topoisomerase II. | (Chowdhury, Junnotula, 2007, Peters and Brown, 2002, Shinde, Hay, 2009, von Pawel, von Roemeling, 2000) |
| Aliphatic N- oxides | Oxygen-dependent inhibition of two- electron reductases and thus, produce selective cytotoxicity. | ||
| Transition metals (Cobalt III and Copper II) | It gets activated via one-electron reductions, to unstable complexes, which later dissociate to release an active ligand. | (Ahn, Botting, 2006, Parker, Lacy, 2004, Ware, Palmer, 1993) | |
| Selected examples of pH sensitive/ responsive drug delivery systems | Layer by layer, pH-responsive sheddable nanoparticle shell | The shell consists of multiple layers, of which neutral ones shed in response to the acidic environment, finally exposing the charged nanoparticle surface that could be easily taken up by the tumor cells. These nanoparticles use a trilayer skeleton of poly-L-lysine (PLL) modified with iminobiotin, followed by the linker protein neutravidin and biotin end-functionalized poly(ethylene glycol) (PEG). | (Poon, Chang, 2011) |
| pH responsive nanovalves | Porous yet rigid mesoporous silica nanospheres/nanoparticles (MSNP) were loaded with drug particles and later, the pores were plugged with β- cyclodextrin (β-CD)-capped valves. A change in pH from 7.4 to low/ mild acidifying endosomal conditions results in the protonation of aromatic amines, releasing the β-CD cap and eventually facilitating diffusion of the drug from nanopores. | (Meng, Xue, 2010) |
3.1. Prodrugs activated by reduction
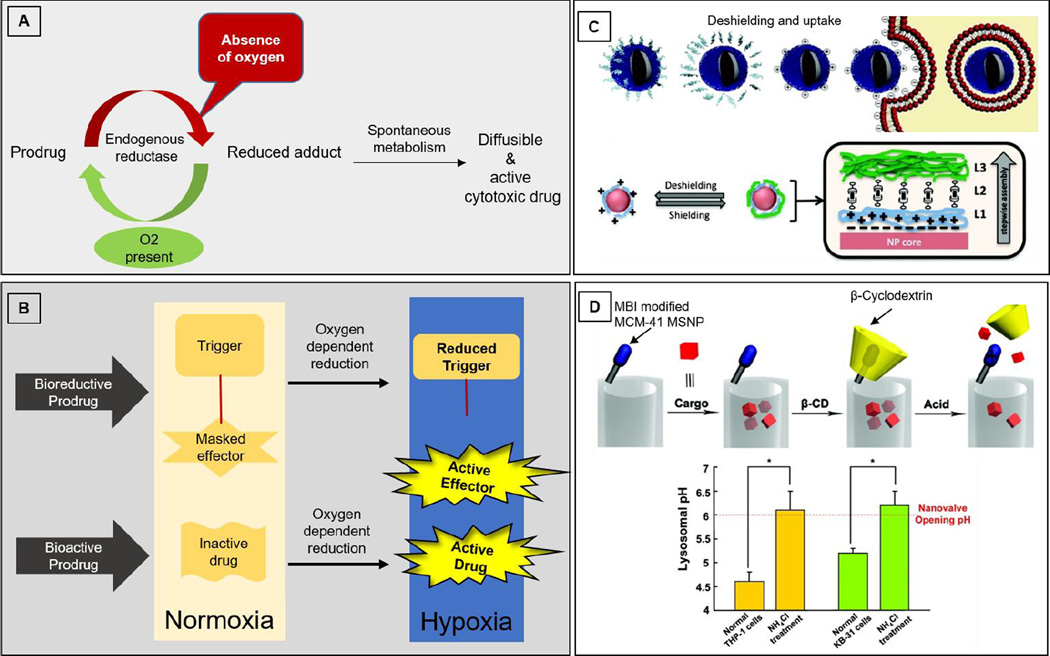
Hypoxic regions exhibit differences in redox potential between extracellular and intracellular regions. Drugs can be delivered specifically to hypoxic regions by exploiting such differences in reduction potential in normoxic and hypoxic tissues. Basic common activation mechanism of prodrugs activated by reduction is depicted in Figure 1A. To be successful, it is important that the bioreductive moiety does not get reduced and/or re-oxidized easily in normoxic conditions; however, it only responds to the range/window of redox potential change in the hypoxic region. Moreover, the most cytotoxic bioreductive systems easily form adducts with DNA via nucleophile attack, resulting in cytotoxicity. Such a cytotoxic effect may be desirable in treating tumors but may be adverse for treating other hypoxic diseases.
Figure 1.
A) Common mechanisms for oxygen-dependent activation of prodrugs; B) Bioreductive prodrugs and bioactive prodrugs; C) Concept of de-shielding and cell uptake (Top schematic); schematic illustration of the design and concept for achieving tumor specificity with layer-by-layer nanoparticles. This design takes advantage of a lower pH in hypoxic tissues to de-shield the terminal, polyethylene glycol) (PEG) layer, exposing the underlying positively charged poly-L-lysine (PLL) layer for cell targeting. L1: PLL modified with iminobiotin; L2: neutravidin; L3: biotin end-functionalized PEG (Bottom schematic); Reproduced from Poon et al (Poon, Chang, 2011); D) A graphical representation of the pH-responsive MSNP (Mesoporous silica nanoparticles) nanovalve. Schematic shows the working principle of nanovalves via protonation of the stalk and release of the β-cyclodextrin. (Top schematic); Measurement of lysosomal pH in THP-1 and KB-31 cells prior to and after NH4Cl treatment (*p < 0.05). The dashed line indicates that a threshold pH (6.0) is required for nanovalve opening. Since NH4Cl treatment elevates the pH to above this threshold, it eliminates the microenvironment that is required for cargo release. (Bottom graph); Reproduced from Meng et al (Meng, Xue, 2010).
Prodrug is a biologically inactive compound which can be transformed into its active form in the body to give rise to an active drug compound. Classes of such prodrugs that exploit the principle of bioreduction include (1) bioreductive prodrug and (2) bioactive prodrugs. Bioreductive prodrugs undergo intramolecular cyclization to “release” a drug whereas bioactive prodrug moieties undergo reduction to form an active radical moiety for activity. Both major types of prodrugs, which require oxygen sensitive reduction for activation, are depicted in Figure 1B.
3.1.1. Bioreductive prodrugs
Hypoxic tissue can reduce the prodrug into an active compound, which is reversible by oxygen availability. Naughten reviewed three major bioreductive delivery systems (Naughton, 2001). A common mechanism for bioreductive prodrugs is depicted in the top panel of Figure 1B, in which oxygen-dependent reduction cleaves the linker and releases the effector in its active form. A quinone lactonization system consists of three major components, namely, trigger, linker and steric hindrance groups. The quinone propionic-based carrier skeleton is used in this delivery system; the propionic moiety links the trigger (benzoquinone) to the active drug and lactonization is made possible by providing steric hindrance by three methyl groups on benzoquinone. The benzoquinone trigger gets reduced leading to intramolecular cyclization between benzoquinone and propionic moieties, the process by which active drug is released from the delivery system (Amsberry and Borchardt, 1991, Levine and Raines, 2012). Another similar study also confirmed that an alkylating agent called melphalan is released as a result of lactonization on bioreduction in a human adenocarcinoma cell line (Gharat et al., 2001).
The second delivery system is aimed to treat diseases such as rheumatoid arthritis, stroke and diabetes in which cytotoxicity is not required at the site of action. Such agents are often called self-alkylating bioreductive agents. Blake et al claimed a patent (Blake et al., 1998) for avoiding DNA adduct formation by accommodating a nucleophile within bioreductive skeleton, which reacts with the activated remnant (capable of forming cytotoxic conjugate) after the drug is released. This nucleophile inactivates the cytotoxic residue by facilitating the nucleophile in the moiety itself. Another patent also claims innovation in a similar approach (Freeman et al., 2005). Jaffer et al (Jaffar et al., 2001) also reviewed bioreductive prodrugs emphasizing three main classes: the N-oxides, the nitroaromatics/ heterocyclics and quinones. Nitro- functionality of nitroaromatics/ heterocyclics can be reduced under hypoxic condition, which produces reactive intermediates (e.g. nitroquinoline prodrug of cyclophosphoramide).
The third approach utilizes an oxidized vitamin E moiety (tocopheryl quinone, TQ) for drug release via intramolecular cyclization in a redox-driven manner (Liebler, 1993). As TQ gets reduced in the hypoxic environment, it cyclizes and results in the release of a hydroxyl group. Based on this concept, Naughten suggested a strategy to conjugate a drug to TQ via the hydroxyl group, whereby active drug can be released selectively upon reduction of TQ (Naughton, 2001).
3.1.2. Bioactive prodrugs
Bioactive prodrugs are activated as a whole rather than ‘delivery’ or ‘release’ of active therapeutic upon activation. Aromatic N-oxide drug, tirapazamine (TPZ), upon activation under hypoxic condition inhibits DNA and works synergistically with chemotherapy and radiation therapy (von Pawel et al., 2000). In hypoxic condition, intracellular reduction of TPZ leads to the formation of its radical causing extensive DNA damage in the form of base pair damage, single strand breaks and double strand breaks. Later studies demonstrated that it is not the TPZ radical itself but its downstream radicals (e.g. oxidizing hydroxyl or benzotriazinyl), which are responsible for cytotoxicity (Chowdhury et al., 2007, Shinde et al., 2009). Double strand breaks are responsible for cytotoxicity exerted by TPZ which to an extent is due to tumor-specific poisoning of topoisomerase II (Peters and Brown, 2002). Aliphatic N-oxides show oxygen-dependent inhibition of two-electron reductases and thus, produce selective cytotoxicity. Transition metals (Cobalt III and Copper II) can also be activated through one-electron reductions, to unstable complexes, which later dissociate to release an active ligand (Ahn et al., 2006, Parker et al., 2004, Ware et al., 1993). Different strategies utilizing this bioreductive principle for both the prodrug skeleton releasing active drug and the drug molecule itself getting activated by bioreduction, has been reviewed earlier (Denny, 2001). A recent report on in vivo efficacy of violacein by Hashimi et al also suggests its hypoxia synergized effect (Hashimi et al., 2015). Choi et al demonstrated enhancement of antitumor and anti-angiogenic effects of axitinib under hypoxic conditions by designing a novel drug delivery system (Choi et al., 2015).
Significant amount of efforts has been put together to exploit and target accurately reproducible behavior of hypoxic cells. As pointed out in a short review by Denny, even though there hasn’t been a registered clinical success in the clinical trials yet, these efforts have provided a wealth of information about drug diffusion, appropriate cytotoxic mechanisms, generation of potent active species, bystander effects of the generated active species (Denny, 2010). It is difficult to compare various classes of such drugs for their clinical efficacy as the precise understanding of the effect of physicochemical properties of drugs on rate of their extravascular diffusion is missing. Moreover, early results of some relevant clinical trials employing prodrugs activated by hypoxia also suggest for improvement in preclinical xenograft models so as to closely match clinical situation with respect to cytotoxic or cytostatic activity of the drug and extent of hypoxia.
3.2. pH-sensitive drug delivery systems
pH around the hypoxic region in tumors is generally more acidic than in other regions of the body due to overproduction of lactic acid and carbonic acid. This happens because the hypoxic environment enhances the action of some isoforms of hydrogen ions or lactate monocarboxylate transporters causing lactate to eventually be pumped out of the cells. The decreased pH in the hypoxic tumor microenvironment facilitates tumor invasion and slows down the uptake of anticancer drugs like doxorubicin (Tian and Bae, 2012). This pH sensitivity in the hypoxic tumor region has been exploited to design a layer by layer, pH-responsive sheddable nanoparticle shell (Poon et al., 2011). As shown in Figure 1C, such a shell is designed by incorporating multilayer polyelectrolyte assemblies on nanoparticles. The shell consists of multiple layers, of which neutral ones shed in response to the acidic environment, finally exposing the charged nanoparticle surface that could be easily taken up by the tumor cells. Research by Poon et al provides proof of concept for layer by layer nanoparticles which can expose the charged layer to facilitate their cellular uptake in an acidic microenvironment and inhibit uptake in a non-tumor region (Poon, Chang, 2011). These nanoparticles use a trilayer skeleton of poly-L-lysine (PLL) modified with iminobiotin, followed by the linker protein neutravidin and biotin end-functionalized poly(ethylene glycol) (PEG). The PLL layer aids in cellular uptake and the neutravidin layer links PLL and PEG by a neutravidin-iminobiotin bond. These neutravidin-iminobiotin bonds are pH-sensitive bonds similar to biotin-avidin bonds. They are stable at pH 8–12 but vulnerable to cleavage at pH 4–6 because protonated iminobiotin has a low affinity for neutravidin. The outer PEG helps to bypass rapid reticuloendothelial system (RES) clearance and also promotes their accumulation in the tumor site due to leaky vasculature and poor lymphatic drainage, exerting enhanced permeability and retention (EPR) effect. Due to EPR effect, polymer/liposome drug complexes have achieved a 10–100 fold increase in drug concentration in the tumor tissue and exerted a potent drug effect on tumors compared to free drug molecules (Maeda et al., 2003, Milane et al., 2011, van Vlerken et al., 2007).
In another study, pH-responsive nanovalves have been successfully fabricated (Meng et al., 2010). Figure 1D depicts the concept behind the design of such nanovalves, its working mechanism and pH threshold as compared to a control and ammonium chloride (NH4Cl) treated cells. Porous yet rigid mesoporous silica nanospheres/nanoparticles (MSNP) were loaded with drug particles and later, the pores were plugged with β-cyclodextrin (β-CD)-capped valves. The approximate diameter of MSNP was 100nm and the hexagonal arrays of tubular pores were approx. 2nm in diameter. A series of aromatic amines were chosen as stalks and β-CD was chosen as a cap that is capable of blocking the nanopore openings reversibly. Stalks were designed to attach to the nanopore openings and to a capping agent via covalent bonds. The interaction between both β-cyclodextrin and stalk is a function of the binding constant, which depends on pH. A change in pH from 7.4 to low/ mild acidifying endosomal conditions results in the protonation of aromatic amines, releasing the β-CD cap and eventually facilitating diffusion of the drug from nanopores (Meng, Xue, 2010). Dong et al (Dong et al., 2013) also successfully utilized two different surface coating strategies for pH-responsive nanovalves for on-demand release of cargo. The first model utilized 120nm MSNs coated with polyethylene imine (PEI) leading to acid-responsive nanovalves that open in acidic pH whereas the second model employed 50nm MSNs coated with PEI-PEG copolymers leading to nanovalves that open at pH 5. With the advantage of EPR effect due to PEG coating, the latter system proved to be advantageous with improved in vivo accumulation but overall, both the coatings improved biodistribution of silica particles (Dong, Xue, 2013). Several other researchers have also explored this approach recently (Koçer et al., 2006, Lee et al., 2013, Nguyen et al., 2006, Xue and Findenegg, 2012, Xue et al., 2011).
The pH sensitive delivery systems are relatively new to the hypoxic tumor targeting area as compared to prodrugs activated by hypoxia, therefore, even though preliminary studies seem promising, results from clinical trials are awaited before their complete assessment.
3.3. Hypoxia-sensitive gene therapy
A major limitation of current gene therapy approaches is less selectivity to tumor cells. Direct injection into the tumor tissue to deliver genes to the site is one way to achieve a desired selectivity but the risk of migration of the delivered gene to the other sites still exists. Selective gene therapy can be achieved by designing a therapeutic gene, which can only be transcribed or translated in response to binding of HIF-1 to HREs. Upon translation, the therapeutic gene can produce an enzyme, which will convert a prodrug into an active cytotoxic drug. HIF-1 is not expressed in normoxic tissues, making the activation of prodrug selective to hypoxic tissues (Binley et al., 2003, Patterson et al., 2002, Shibata et al., 2002). Increased selectivity can be achieved by expressing enzymes that are not generally expressed in humans (e.g. cytosine deaminase from E. coli). Shibata et al (Shibata, Giaccia, 2002) successfully demonstrated this concept using vectors containing bacterial nitroreductase gene to activate anticancer prodrug CB1954. The vectors were prepared with HREs derived from human VEGF (Shibata, Giaccia, 2002). In another approach, Patterson et al (Patterson, Williams, 2002) combined selective gene activation with the bioreductive approach where the HRE vector for human flavoprotein cytochrome C P450 reductase was used for transfecting the human fibrosarcoma cell line, HT1080. The prodrug, RSU1069, showed greater than a 30 fold increase in the normoxic to hypoxic cytotoxicity ratio compared to negative controls in vitro and also preferential cytotoxicity to hypoxic cells in vivo (Patterson, Williams, 2002). In another study, hypoxia-driven triple suicide genes TK/CD/UPRT were expressed to increase the cytotoxicity of ganciclovir (GCV) and 5-fluorocytosine (5-FC) as well as increase sensitivity of human colorectal cancer towards radiation both in vitro and in vivo (Hsiao et al., 2014). Alternative gene therapy strategies employ antisense or small interfering RNA (siRNA) delivery to hypoxic tumors for inducing tumor apoptosis (Mizuno et al., 2005, Sun et al., 2010).
Viral gene delivery is the most efficacious strategy for targeting, however, it suffers from safety issues. Non-viral gene delivery systems overcome the safety issue but they miss out on the efficacy when evaluated for transfection. Moreover, as pointed out in another recent review (Rhim et al., 2013), hypoxia is not a suitable target for systemic gene delivery of hypoxia-inducible gene expression systems as it can easily be localized in organs with low physiological oxygen levels resulting in unwanted side effects. Therefore, dual specific expression systems should be considered for gene delivery approaches. Indeed, such dual specific expression systems have exhibited high tumor specificity as demonstrated by a combination of cancer specific survivin promoter with HREs for enhanced specificity (Farokhimanesh et al., 2010). Additional priming of delivery carrier to hypoxic microenvironment can further improve the efficacy and safety.
3.4 Tumor associated macrophages (TAMs) as a targeting tool
As discussed in section 2.3, role of TAMs in metastasis and promotion of angiogenesis is well evident. Manipulation of TAMs can be useful to control the tumor microenvironment either alone or along with other chemotherapeutic agents. Indeed, colony stimulating factor 1 receptor (CSF-1R) blocker increased the efficacy of paclitaxel in a breast cancer mouse model by eliminating immunosuppressive TAMs because macrophages rely on colony stimulating factor 1 (CSF-1) for their differentiation and survival. (DeNardo et al., 2011). As depicted in figure 2B, popular strategies involve depletion of TAMs by the inhibition of the key survival pathway for macrophages (CSF-1/CSF-1R signaling) (Ostuni, Kratochvill, 2015). Emactuzumab (RG7155) is one such CSF-1R blocker currently in phase I clinical trial (NCT01494688) in patients with diffuse-type giant cell tumors, which is scheduled to be over in 2018, has shown to be promising (Cassier et al., 2015).
Passive strategies to deplete TAMs rely on blocking the recruitment of monocytes to the tumor site. This can be effectively achieved by interfering with CCL2-CCR2 signaling. Indeed, antibody mediated inhibition of CCL2 prevented monocyte recruitment and improved the therapeutic outcome as evident by paradoxical effect of CCL2 in four syngeneic mouse models of metastatic breast cancer (Bonapace et al., 2014, Qian et al., 2011). However, it led to rapid rebound of monocyte infiltration in tumors due to adaptive response from tumor cells as a result of anti-CCL2 therapy.
Another approach involves re-education/reprogramming of TAMs to stimulate, rather than suppress, the immune response, thereby weakening the antitumor immunity as conceptualized in figure 2C (Ostuni, Kratochvill, 2015, Ruffell et al., 2014). Moreover, the ability of TAMs to localize in the hypoxic area can be exploited for selective targeting. For example, patient-derived macrophages can be activated ex-vivo with IFN-γ and exploited for tumoricidal effect (Hennemann et al., 1998). However, efficacy of this clinical therapy was reported to be low. In a more recent approach, gene-dependent enzyme prodrug therapy (GDEPT) was successfully tried in tumor spheroids (Griffiths et al., 2000). Increased expression of scavenger receptors, such as mannose receptors, on TAMs and biochemical changes in their surrounding microenvironment have been exploited for delivering DNA, drugs, imaging agents as well as vaccines (Locke et al., 2012, Yu et al., 2010). In addition, acid-sensitive PEG shielding of nanoparticles has been used to selectively spare normal macrophages and to target TAMs using PEG-sheddable, mannose-modified nanoparticles (Zhu et al., 2013).
As mentioned earlier, blockade of monocyte recruitment is a tricky strategy due to the adaptive response from tumor cells resulting in a complete relapse. Moreover, re-education or reprogramming was found to be less efficacious. By far, CSF-1R inhibitory treatments are shown to be the most promising approach for TAM targeting approaches. However, CSF-1 is essential for the survival of non-tumor macrophages as well, therefore safety concerns remain to be assessed with the ongoing trials with various CSF-1R inhibitor peptides and antibodies.
3.5. Small molecule inhibitors of HIF-1
HIF-1 is one of the important factors for tumor growth, therefore, it is imperative to devise strategies to target inhibition of HIF-1 pathways in hypoxic cells in order to control the tumor progression. Although some cell-based screening methods have identified certain pathways that inhibit HIF-1 transcription activity, the mechanism of action is still not clear. Specifically, targeting HIF-1α mediating functions like DNA binding (bHLH), protein-protein interaction and transcriptional activity (COOH-terminal transactivation domain or C-TAD) has led to the creation of more selective inhibitors; however, they result in poor HIF inhibition (Belozerov and Van Meir, 2005). Ironically, these inhibitors cannot interrupt HIF-1α pathway as their exclusive target, demanding the design of more efficient targeting agents. Designing the screening method to verify specificity of novel compound is as challenging as designing a selective inhibitor itself (Xia et al., 2012). Readers are encouraged to read more on pharmacological targeting of HIF-1 reviewed by Belozerov et al (Belozerov and Van Meir, 2005).
4. Brief review of clinical status of drugs exploiting tumor hypoxia
Promising results in preclinical studies encouraged clinical trials testing the validity of therapies targeted to the hypoxic microenvironment. Several clinical trials have been conducted to test the benefit of bioactive and bioreductive prodrug drugs in humans. Most of the related clinical trials involving hypoxia-targeted therapies to tumors are in combination with current standard-of-care therapies (e.g. chemotherapy or radiotherapy). One of such promising drugs is TPZ, which has been studied in several solid cancer types in combination with the other chemo-or radiotherapy (Le et al., 2006, Rischin et al., 2005, Rischin et al., 2001). In two separate clinical trials, TPZ showed contradicting evidence for its efficacy when combined with other cytotoxic drugs, such as cisplatin, or radiation therapy. For instance, in a phase III trial in advanced non-small cell lung tumor patients, TPZ enhanced the activity of cisplatin, with improved median survival and response rate (von Pawel, von Roemeling, 2000). While in another clinical trial in advanced non-small cell lung tumor patients, the addition of TPZ to paclitaxel and carboplatin did not show any significant improvement in patient survival, or response rate, and instead resulted in increased toxicity (Mack et al., 2008, von Pawel, von Roemeling, 2000, Williamson et al., 2005). Although TPZ is the only clinically most advanced hypoxia-activated cytotoxin, it suffers from poor tumor penetration and higher toxicity at effective concentrations. Hence, current research efforts are also directed in synthesizing new prodrug strategies and studying structure activity relationships (Duan et al., 2008). One such bioactive prodrug activated in hypoxic regions, PR104, exhibited promising activity in preclinical xenograft tumor models when combined with radiotherapy or chemotherapy (gemcitabine in pancreatic tumors and docetaxel in prostate tumors) (Patterson et al., 2007). However, in Phase I clinical trials in advanced solid tumor patients, its combination with docetaxel or gemcitabine resulted in dose-limiting myelotoxicity. Similarly, many phase II clinical trials with PR104 were terminated due to safety and toxicity issues (e.g. PR104 with sorafenib in advanced hepatocellular carcinoma patients, clinical trial identifier: NCT00862082). Hypoxia-activated prodrug TH-302 is currently being tested in many Phase I and Phase II trials for kidney and liver cancers (NCT01497444), non-small cell lung cancers (NCT02093962), multiple myeloma (NCT01522872) and others. TH-302 has also been tested in combination with Doxorubicin and compared with Doxorubicin alone in a phase III clinical trial (NCT01440088), for which results are awaited. Banoxantrone (AQ4N), an aliphatic N-oxide, is also being tested in phase I/ phase II clinical trials (NCT00394628, NCT00109356, NCT00090727), either alone or in combination with chemo- or radiotherapy. It was found to be safe and the concentration was found to be more than 1 µg/g in majority of tumor samples, at lower levels in adjacent tissues and at very low levels (0.037 µg/g) in distant skin samples. This reiterated its selective activation in hypoxic regions in human solid tumors. Detailed information on these trials can be found on clinicaltrials.gov. With initial promising preclinical results shown by pH-responsive and hypoxia-targeted drug delivery systems, clinical trials with these systems are expected to be initiated. Relevant clinical trials and their current status is summarized in Table 4.
Table 4.
Selected examples of relevant clinical trials and their current status
| Drug /Drug delivery system/ therapeutic approach |
Current status in clinical trial | Reference(s)/ clincical trial identifier |
|---|---|---|
| Tirapazamine (TPZ) (Arom N-oxide) |
It has been studied in several solid cancer types in combination with the other chemo- or radiotherapy. In two separate clinical trials, TPZ showed contradicting evidence for its efficacy when combined with other cytotoxic drugs, such as cisplatin, or radiation therapy. For instance, in a phase III trial in advanced non-small cell lung tumor patients, TPZ enhanced the activity of cisplatin, with improved median survival and response rate. While in another clinical trial in advanced non-small cell lung tumor patients, the addition of TPZ to paclitaxel and carboplatin did not show any significant improvement in patient survival, or response rate, and instead resulted in increased toxicity. |
NCT00005078 NCT00262821 NCT00066742 (Mack, Redman, 2008, von Pawel, von Roemeling, 2000, Williamson, Crowley, 2005) |
| PR104 (Nitro compound) |
Phase I clinical trial in advanced solid tumor patients using PR104 in combination with docetaxel or gemcitabine resulted in dose- limiting myelotoxicity. Similarly, many phase II clinical trials with PR104 were terminated due to safety and toxicity issues. |
NCT00862082 NCT01358227 NCT00262821 |
| TH-302 (Nitro compound) |
It is being tested in many Phase I and Phase II trials for kidney and liver cancers, non- small cell lung cancers, multiple myeloma. TH-302 has also been tested in combination with Doxorubicin versus Doxorubicin alone in a phase III clinical trial expected to be concluded recently phase III clinical trial, for which results are not posted yet. |
NCT01497444 NCT01440088 NCT02093962 NCT01522872 |
| Banoxantrone (AQ4N) (Aliphatic N-oxide) |
Phase I/ phase II clinical trials, either alone or in combination with chemo- or radiotherapy, have been conducted. Phase I studies showed promising safety results. |
NCT00394628 NCT00109356 NCT00090727 |
5. Conclusion
Significant progress has been made to characterize hypoxic tumor microenvironment. However, there is still scope for deciphering some ambiguity in the molecular mechanisms of hypoxic tumors. Nonetheless, an in-depth understanding of physico-chemical changes in the tumor microenvironment due to hypoxia has offered great opportunity to design drug delivery systems that release drugs selectively in such conditions. Except for hypoxia-activated prodrugs with bioreductive ‘trigger’ chemistries, other approaches haven not made it to the clinical trials yet. Results of clinical trials so far with bioreductive prodrugs have been somewhat disappointing, suggesting need for improvement in currently employed hypoxic xenograft models. Other approaches such as pH-sensitive, gene therapy or macrophage-targeted approaches have shown convincing proof-of-principle results. The fact that hypoxic tumor microenvironment is a constantly evolving system, makes it challenging for therapeutic interventions to avoid resistance issues. However, as discussed throughout this review, tumors exhibit more than one such property in the hypoxic tissue. This is a great opportunity for designing a highly specific drug delivery system that is capable of exploiting more than one such property to target hypoxic areas. Indeed, in a recent study, a pH sensitive and bioreductible polymer (PPCBA)- coated oncolytic adenovirus was used to demonstrate a combinatorial approach exploiting hypoxic microenvironmental features in vitro (for its uptake) as well as in a mouse xenograft model (Moon et al., 2015). Such combinatorial strategies can deliver safe and resistance-free treatment outcomes. Further collaborative research in molecular pharmacology, materials science, and pharmaceutics is needed to understand and eventually exploit the molecular/physicochemical changes in hypoxic regions for developing better hypoxia targeted drug delivery systems.
Highlights.
Hypoxia is not a random by-product of cellular milieu due to uncontrolled tumor growth; rather it is a constantly evolving participant in overall tumor growth and fate.
Hypoxic microenvironment changes physicochemical features of tumor microenvironment, primes macrophages and gene expression towards the survival of the tumor. These changes can be exploited for designing targeted delivery systems.
Hypoxia targeting therapeutic approaches utilizing single targeting strategy have shown some promise, with clinical trials showing lesser prospects than expected so far. Combination of multiple targeting strategies in a single treatment regime holds a greater promise for safe, effective and resistance-free treatment outcomes.
Acknowledgments
We thank Dr. Vinayak Sant and Ms. Tatyana Yatsenko for critical reading of the manuscript. We also thank Ms. Jr-Jiun Jean Liou for help with the literature search on clinical trials. Dr. Shilpa Sant acknowledges NIH funding (EB018575) and the start-up funds from the Department of Pharmaceutical Sciences, School of Pharmacy, University of Pittsburgh.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn G-O, Botting KJ, Patterson AV, Ware DC, Tercel M, Wilson WR. Radiolytic and cellular reduction of a novel hypoxia-activated cobalt(III) prodrug of a chloromethylbenzindoline DNA minor groove alkylator. Biochem Pharmacol. 2006;71:1683–1694. doi: 10.1016/j.bcp.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Amsberry K, Borchardt R. Amine Prodrugs Which Utilize Hydroxy Amide Lactonization I. A Potential Redox-Sensitive Amide Prodrug. Pharm Res. 1991;8:323–330. doi: 10.1023/a:1015885213625. [DOI] [PubMed] [Google Scholar]
- Belozerov VE, Van Meir EG. Hypoxia inducible factor-1: a novel target for cancer therapy. Anticancer Drugs. 2005;16:901–909. doi: 10.1097/01.cad.0000180116.85912.69. [DOI] [PubMed] [Google Scholar]
- Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley K, Askham Z, Martin L, Spearman H, Day D, Kingsman S, et al. Hypoxia-mediated tumour targeting. Gene Ther. 2003;10:540–549. doi: 10.1038/sj.gt.3301944. [DOI] [PubMed] [Google Scholar]
- Birner P, Schindl M, Obermair A, Plank C, Breitenecker G, Oberhuber G. Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res. 2000;60:4693–4696. [PubMed] [Google Scholar]
- Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-κB and enhanced IRF-3/STAT1 activation) Blood. 2006;107:2112–2122. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- Blake D, Naughton D, Adams G, Stratford I, Morris C, Jaffar M, et al. Bioreductive conjugate for targeting a therapeutic agent. Google Patents. 1998 [Google Scholar]
- Bonapace L, Coissieux M-M, Wyckoff J, Mertz KD, Varga Z, Junt T, et al. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. 2014;515:130–133. doi: 10.1038/nature13862. [DOI] [PubMed] [Google Scholar]
- Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–1416. [PubMed] [Google Scholar]
- Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- Campbell RB, Fukumura D, Brown EB, Mazzola LM, Izumi Y, Jain RK, et al. Cationic charge determines the distribution of liposomes between the vascular and extravascular compartments of tumors. Cancer Res. 2002;62:6831–6836. [PubMed] [Google Scholar]
- Carlisle R, Coussios C-C. Mechanical approaches to oncological drug delivery. Ther Deliv. 2013;4:1213–1215. doi: 10.4155/tde.13.94. [DOI] [PubMed] [Google Scholar]
- Cassier PA, Italiano A, Gomez-Roca CA, Le Tourneau C, Toulmonde M, Cannarile MA, et al. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: a dose-escalation and dose-expansion phase 1 study. The Lancet Oncology. 2015;16:949–956. doi: 10.1016/S1470-2045(15)00132-1. [DOI] [PubMed] [Google Scholar]
- Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6:1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JY, Ramasamy T, Tuan Hiep T, Ku SK, Shin BS, Choi H-G, et al. Systemic delivery of axitinib with nanohybrid liposomal nanoparticles inhibits hypoxic tumor growth. Journal of Materials Chemistry B. 2015;3:408–416. doi: 10.1039/c4tb01442a. [DOI] [PubMed] [Google Scholar]
- Chowdhury G, Junnotula V, Daniels JS, Greenberg MM, Gates KS. DNA strand damage product analysis provides evidence that the tumor cell-specific cytotoxin tirapazamine produces hydroxyl radical and acts as a surrogate for O(2) J Am Chem Soc. 2007;129:12870–12877. doi: 10.1021/ja074432m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte Complexity Predicts Breast Cancer Survival and Functionally Regulates Response to Chemotherapy. Cancer Discovery. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny WA. Prodrug strategies in cancer therapy. European Journal of Medicinal Chemistry. 2001;36:577–595. doi: 10.1016/s0223-5234(01)01253-3. [DOI] [PubMed] [Google Scholar]
- Denny WA. Hypoxia-activated prodrugs in cancer therapy: progress to the clinic. Future Oncology. 2010;6:419–428. doi: 10.2217/fon.10.1. [DOI] [PubMed] [Google Scholar]
- Dhani N, Fyles A, Hedley D, Milosevic M. The Clinical Significance of Hypoxia in Human Cancers. Seminars in Nuclear Medicine. 2015;45:110–121. doi: 10.1053/j.semnuclmed.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Di Paolo A, Bocci G. Drug distribution in tumors: mechanisms, role in drug resistance, and methods for modification. Curr Oncol Rep. 2007;9:109–114. doi: 10.1007/s11912-007-0006-3. [DOI] [PubMed] [Google Scholar]
- Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70:7465–7475. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Xue M, Zink JI. Functioning of nanovalves on polymer coated mesoporous silica Nanoparticles. Nanoscale. 2013;5:10300–10306. doi: 10.1039/c3nr03442a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J-X, Jiao H, Kaizerman J, Stanton T, Evans JW, Lan L, et al. Potent and highly selective hypoxia-activated achiral phosphoramidate mustards as anticancer drugs. J Med Chem. 2008;51:2412–2420. doi: 10.1021/jm701028q. [DOI] [PubMed] [Google Scholar]
- Epstein ACR, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, et al. C. elegans EGL-9 and Mammalian Homologs Define a Family of Dioxygenases that Regulate HIF by Prolyl Hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Fang P-A, Margolis HC, Conway JF, Simmer JP, Dickinson GH, Beniash E. Cryogenic transmission electron microscopy study of amelogenin self-assembly at different pH. Cells Tissues Organs. 2011;194:166–170. doi: 10.1159/000324250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farokhimanesh S, Rahbarizadeh F, Rasaee MJ, Kamali A, Mashkani B. Hybrid promoters directed tBid gene expression to breast cancer cells by transcriptional targeting. Biotechnology progress. 2010;26:505–511. doi: 10.1002/btpr.353. [DOI] [PubMed] [Google Scholar]
- Freeman S, Jaffor M, Stratford I. Drug targeting. Google Patents. 2005 [Google Scholar]
- Fukumura D, Jain RK. Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. J Cell Biochem. 2007;101:937–949. doi: 10.1002/jcb.21187. [DOI] [PubMed] [Google Scholar]
- Gharat L, Taneja R, Weerapreeyakul N, Rege B, Polli J, Chikhale PJ. Targeted drug delivery systems 6: Intracellular bioreductive activation, uptake and transport of an anticancer drug delivery system across intestinal Caco-2 cell monolayers. Int J Pharm. 2001;219:1–10. doi: 10.1016/s0378-5173(01)00599-3. [DOI] [PubMed] [Google Scholar]
- Gleadle J, Ratcliffe P. Hypoxia. eLS: John Wiley & Sons, Ltd; 2001. [Google Scholar]
- Goenka S, Sant V, Sant S. Graphene-based nanomaterials for drug delivery and tissue engineering. J Control Release. 2014;173:75–88. doi: 10.1016/j.jconrel.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Griffiths L, Binley K, Iqball S, Kan O, Maxwell P, Ratcliffe P, et al. The macrophage - a novel system to deliver gene therapy to pathological hypoxia. Gene Ther. 2000;7:255–262. doi: 10.1038/sj.gt.3301058. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, et al. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Hadjipanayi E, Cheema U, Mudera V, Deng D, Liu W, Brown RA. First implantable device for hypoxia-mediated angiogenic induction. J Control Release. 2011;153:217–224. doi: 10.1016/j.jconrel.2011.03.029. [DOI] [PubMed] [Google Scholar]
- Harris AL. Hypoxia [mdash] a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- Hashimi SM, Xu T, Wei MQ. Violacein anticancer activity is enhanced under hypoxia. Oncology Reports. 2015;33:1731–1736. doi: 10.3892/or.2015.3781. [DOI] [PubMed] [Google Scholar]
- Hennemann B, Beckmann G, Eichelmann A, Rehm A, Andreesen R. Phase I trial of adoptive immunotherapy of cancer patients using monocyte-derived macrophages activated with interferon gamma and lipopolysaccharide. Cancer Immunol Immunother. 1998;45:250–256. doi: 10.1007/PL00006671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höckel M, Schlenger K, Höckel S, Vaupel P. Hypoxic Cervical Cancers with Low Apoptotic Index Are Highly Aggressive. Cancer Research. 1999;59:4525–4528. [PubMed] [Google Scholar]
- Höckel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- Hsiao HT, Xing L, Deng X, Sun X, Ling CC, Li GC. Hypoxia-targeted triple suicide gene therapy radiosensitizes human colorectal cancer cells. Oncol Rep. 2014;32:723–729. doi: 10.3892/or.2014.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffar M, Williams KJ, Stratford IJ. Bioreductive and gene therapy approaches to hypoxic diseases. Adv Drug Deliv Rev. 2001;53:217–228. doi: 10.1016/s0169-409x(01)00228-9. [DOI] [PubMed] [Google Scholar]
- Jiang BH, Rue E, Wang GL, Roe R, Semenza GL. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J Biol Chem. 1996;271:17771–17778. doi: 10.1074/jbc.271.30.17771. [DOI] [PubMed] [Google Scholar]
- Kaur B, Khwaja FW, Severson EA, Matheny SL, Brat DJ, Van Meir EG. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro Oncol. 2005;7:134–153. doi: 10.1215/S1152851704001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizaka-Kondoh S, Tanaka S, Harada H, Hiraoka M. The HIF-1-active microenvironment: an environmental target for cancer therapy. Adv Drug Deliv Rev. 2009;61:623–632. doi: 10.1016/j.addr.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Koçer A, Walko M, Bulten E, Halza E, Feringa BL, Meijberg W. Rationally Designed Chemical Modulators Convert a Bacterial Channel Protein into a pH-Sensory Valve. Angewandte Chemie International Edition. 2006;45:3126–3130. doi: 10.1002/anie.200503403. [DOI] [PubMed] [Google Scholar]
- Krishnamachary B, Berg-Dixon S, Kelly B, Agani F, Feldser D, Ferreira G, et al. Regulation of Colon Carcinoma Cell Invasion by Hypoxia-Inducible Factor 1. Cancer Research. 2003;63:1138–1143. [PubMed] [Google Scholar]
- Le Q-T, Taira A, Budenz S, Jo Dorie M, Goffinet DR, Fee WE, et al. Mature results from a randomized Phase II trial of cisplatin plus 5-fluorouracil and radiotherapy with or without tirapazamine in patients with resectable Stage IV head and neck squamous cell carcinomas. Cancer. 2006;106:1940–1949. doi: 10.1002/cncr.21785. [DOI] [PubMed] [Google Scholar]
- Lee K, Zhang H, Qian DZ, Rey S, Liu JO, Semenza GL. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proceedings of the National Academy of Sciences. 2009;106:17910–17915. doi: 10.1073/pnas.0909353106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lee S-F, Zhu X-M, Wang Y-XJ, Xuan S-H, You Q, Chan W-H, et al. Ultrasound, pH, and magnetically responsive crown-ether-coated core/shell nanoparticles as drug encapsulation and release systems. ACS Appl Mater Interfaces. 2013;5:1566–1574. doi: 10.1021/am4004705. [DOI] [PubMed] [Google Scholar]
- Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–4629. [PubMed] [Google Scholar]
- Levine MN, Raines RT. Trimethyl lock: a trigger for molecular release in chemistry, biology, and pharmacology. Chemical Science. 2012;3:2412–2420. doi: 10.1039/C2SC20536J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JS, Landers RJ, Underwood JCE, Harris AL, Lewis CE. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. The Journal of Pathology. 2000;192:150–158. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH687>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Liebler DC. The role of metabolism in the antioxidant function of vitamin E. Crit Rev Toxicol. 1993;23:147–169. doi: 10.3109/10408449309117115. [DOI] [PubMed] [Google Scholar]
- Locke LW, Mayo MW, Yoo AD, Williams MB, Berr SS. PET imaging of tumor associated macrophages using mannose coated 64Cu liposomes. Biomaterials. 2012;33:7785–7793. doi: 10.1016/j.biomaterials.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell. 2009;15:167–170. doi: 10.1016/j.ccr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Mack PC, Redman MW, Chansky K, Williamson SK, Farneth NC, Lara PN, et al. Lower osteopontin plasma levels are associated with superior outcomes in advanced non-small-cell lung cancer patients receiving platinum-based chemotherapy: SWOG Study S0003. J Clin Oncol. 2008;26:4771–4776. doi: 10.1200/JCO.2008.17.0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H, Fang J, Inutsuka T, Kitamoto Y. Vascular permeability enhancement in solid tumor: various factors, mechanisms involved and its implications. Int Immunopharmacol. 2003;3:319–328. doi: 10.1016/S1567-5769(02)00271-0. [DOI] [PubMed] [Google Scholar]
- Mason D, Chen Y-Z, Krishnan HV, Sant S. Cardiac gene therapy: Recent advances and future directions. Journal of Controlled Release. 2015;215:101–111. doi: 10.1016/j.jconrel.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Meng H, Xue M, Xia T, Zhao Y-L, Tamanoi F, Stoddart JF, et al. Autonomous in Vitro Anticancer Drug Release from Mesoporous Silica Nanoparticles by pH-Sensitive Nanovalves. Journal of the American Chemical Society. 2010;132:12690–12697. doi: 10.1021/ja104501a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milane L, Ganesh S, Shah S, Duan ZF, Amiji M. Multi-modal strategies for overcoming tumor drug resistance: hypoxia, the Warburg effect, stem cells, and multifunctional nanotechnology. Journal of controlled release : official journal of the Controlled Release Society. 2011;155:237–247. doi: 10.1016/j.jconrel.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Nagao M, Yamada Y, Narikiyo M, Ueno M, Miyagishi M, et al. Small interfering RNA expression vector targeting hypoxia-inducible factor 1 alpha inhibits tumor growth in hepatobiliary and pancreatic cancers. Cancer Gene Ther. 2005;13:131–140. doi: 10.1038/sj.cgt.7700871. [DOI] [PubMed] [Google Scholar]
- Moon CY, Choi J-W, Kasala D, Jung S-J, Kim SW, Yun C-O. Dual tumor targeting with pH-sensitive and bioreducible polymer-complexed oncolytic adenovirus. Biomaterials. 2015;41:53–68. doi: 10.1016/j.biomaterials.2014.11.021. [DOI] [PubMed] [Google Scholar]
- Murata Y, Ohteki T, Koyasu S, Hamuro J. IFN-gamma and pro-inflammatory cytokine production by antigen-presenting cells is dictated by intracellular thiol redox status regulated by oxygen tension. Eur J Immunol. 2002;32:2866–2873. doi: 10.1002/1521-4141(2002010)32:10<2866::AID-IMMU2866>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2224–2234. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- Naughton DP. Drug targeting to hypoxic tissue using self-inactivating bioreductive delivery systems. Adv Drug Deliv Rev. 2001;53:229–233. doi: 10.1016/s0169-409x(01)00229-0. [DOI] [PubMed] [Google Scholar]
- Nelson DA, Tan T-T, Rabson AB, Anderson D, Degenhardt K, White E. Hypoxia and defective apoptosis drive genomic instability and tumorigenesis. Genes Dev. 2004;18:2095–2107. doi: 10.1101/gad.1204904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TD, Leung KC-F, Liong M, Pentecost CD, Stoddart JF, Zink JI. Construction of a pH-driven supramolecular nanovalve. Org Lett. 2006;8:3363–3366. doi: 10.1021/ol0612509. [DOI] [PubMed] [Google Scholar]
- Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeid E, Nanda R, Fu Y-X, Olopade OI. The role of tumor-associated macrophages in breast cancer progression (review) Int J Oncol. 2013;43:5–12. doi: 10.3892/ijo.2013.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda S, Oda T, Nishi K, Takabuchi S, Wakamatsu T, Tanaka T, et al. Macrophage migration inhibitory factor activates hypoxia-inducible factor in a p53-dependent manner. PLoS One. 2008;3:e2215. doi: 10.1371/journal.pone.0002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojalvo LS, King W, Cox D, Pollard JW. High-Density Gene Expression Analysis of Tumor-Associated Macrophages from Mouse Mammary Tumors. The American Journal of Pathology. 2009;174:1048–1064. doi: 10.2353/ajpath.2009.080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostuni R, Kratochvill F, Murray PJ, Natoli G. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol. 2015;36:229–239. doi: 10.1016/j.it.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Parker LL, Lacy SM, Farrugia LJ, Evans C, Robins DJ, O'Hare CC, et al. A novel design strategy for stable metal complexes of nitrogen mustards as bioreductive prodrugs. J Med Chem. 2004;47:5683–5689. doi: 10.1021/jm049866w. [DOI] [PubMed] [Google Scholar]
- Patterson AV, Ferry DM, Edmunds SJ, Gu Y, Singleton RS, Patel K, et al. Mechanism of action and preclinical antitumor activity of the novel hypoxia-activated DNA cross-linking agent PR-104. Clin Cancer Res. 2007;13:3922–3932. doi: 10.1158/1078-0432.CCR-07-0478. [DOI] [PubMed] [Google Scholar]
- Patterson AV, Williams KJ, Cowen RL, Jaffar M, Telfer BA, Saunders M, et al. Oxygen-sensitive enzyme-prodrug gene therapy for the eradication of radiation-resistant solid tumours. Gene Ther. 2002;9:946–954. doi: 10.1038/sj.gt.3301702. [DOI] [PubMed] [Google Scholar]
- Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–361. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- Peters KB, Brown JM. Tirapazamine: A Hypoxia-activated Topoisomerase II Poison. Cancer Research. 2002;62:5248–5253. [PubMed] [Google Scholar]
- Poon Z, Chang D, Zhao X, Hammond PT. Layer-by-layer nanoparticles with a pH-sheddable layer for in vivo targeting of tumor hypoxia. ACS Nano. 2011;5:4284–4292. doi: 10.1021/nn200876f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR. Oxygen sensing during intermittent hypoxia: cellular and molecular mechanisms. J Appl Physiol (1985) 2001;90:1986–1994. doi: 10.1152/jappl.2001.90.5.1986. [DOI] [PubMed] [Google Scholar]
- Qian B-Z, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim T, Lee DY, Lee M. Hypoxia as a target for tissue specific gene therapy. Journal of Controlled Release. 2013;172:484–494. doi: 10.1016/j.jconrel.2013.05.021. [DOI] [PubMed] [Google Scholar]
- Rischin D, Peters L, Fisher R, Macann A, Denham J, Poulsen M, et al. Tirapazamine, Cisplatin, and Radiation versus Fluorouracil, Cisplatin, and Radiation in patients with locally advanced head and neck cancer: a randomized phase II trial of the Trans-Tasman Radiation Oncology Group (TROG 98.02) J Clin Oncol. 2005;23:79–87. doi: 10.1200/JCO.2005.01.072. [DOI] [PubMed] [Google Scholar]
- Rischin D, Peters L, Hicks R, Hughes P, Fisher R, Hart R, et al. Phase I trial of concurrent tirapazamine, cisplatin, and radiotherapy in patients with advanced head and neck cancer. J Clin Oncol. 2001;19:535–542. doi: 10.1200/JCO.2001.19.2.535. [DOI] [PubMed] [Google Scholar]
- Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho Christine MT, Pryer N, et al. Macrophage IL-10 Blocks CD8+ T Cell-Dependent Responses to Chemotherapy by Suppressing IL-12 Expression in Intratumoral Dendritic Cells. Cancer Cell. 2014;26:623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito G, Swanson JA, Lee K-D. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: role and site of cellular reducing activities. Adv Drug Deliv Rev. 2003;55:199–215. doi: 10.1016/s0169-409x(02)00179-5. [DOI] [PubMed] [Google Scholar]
- Sant S, Nadeau W, Hildgen P. Effect of porosity on the release kinetics of propafenone-loaded PEG-g-PLA nanoparticles. Journal of Controlled Release. 2005;107:203–214. doi: 10.1016/j.jconrel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Sant S, Poulin S, Hildgen P. Effect of polymer architecture on surface properties, plasma protein adsorption, and cellular interactions of pegylated nanoparticles. J Biomed Mater Res A. 2008a;87A:885–895. doi: 10.1002/jbm.a.31800. [DOI] [PubMed] [Google Scholar]
- Sant S, Thommes M, Hildgen P. Microporous structure and drug release kinetics of polymeric nanoparticles. Langmuir. 2008b;24:280–287. doi: 10.1021/la702244w. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Shibata T, Giaccia AJ, Brown JM. Hypoxia-inducible regulation of a prodrug-activating enzyme for tumor-specific gene therapy. Neoplasia. 2002;4:40–48. doi: 10.1038/sj.neo.7900189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde SS, Hay MP, Patterson AV, Denny WA, Anderson RF. Spin trapping of radicals other than the *OH radical upon reduction of the anticancer agent tirapazamine by cytochrome P450 reductase. J Am Chem Soc. 2009;131:14220–14221. doi: 10.1021/ja906860a. [DOI] [PubMed] [Google Scholar]
- Subarsky P, Hill RP. The hypoxic tumour microenvironment and metastatic progression. Clinical & Experimental Metastasis. 2003;20:237–250. doi: 10.1023/a:1022939318102. [DOI] [PubMed] [Google Scholar]
- Sun X, Vale M, Jiang X, Gupta R, Krissansen GW. Antisense HIF-1[alpha] prevents acquired tumor resistance to angiostatin gene therapy. Cancer Gene Ther. 2010;17:532–540. doi: 10.1038/cgt.2010.7. [DOI] [PubMed] [Google Scholar]
- Thomlinson RH, Gray LH. The Histological Structure of Some Human Lung Cancers and the Possible Implications for Radiotherapy. Br J Cancer. 1955;9:539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Bae YH. Cancer nanomedicines targeting tumor extracellular pH. Colloids Surf B Biointerfaces. 2012;99:116–126. doi: 10.1016/j.colsurfb.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torroella-Kouri M, Silvera R, Rodriguez D, Caso R, Shatry A, Opiela S, et al. Identification of a Subpopulation of Macrophages in Mammary Tumor-Bearing Mice That Are Neither M1 nor M2 and Are Less Differentiated. Cancer Research. 2009;69:4800–4809. doi: 10.1158/0008-5472.CAN-08-3427. [DOI] [PubMed] [Google Scholar]
- Turner L, Scotton C, Negus R, Balkwill F. Hypoxia inhibits macrophage migration. Eur J Immunol. 1999;29:2280–2287. doi: 10.1002/(SICI)1521-4141(199907)29:07<2280::AID-IMMU2280>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- van Vlerken LE, Duan Z, Seiden MV, Amiji MM. Modulation of intracellular ceramide using polymeric nanoparticles to overcome multidrug resistance in cancer. Cancer Res. 2007;67:4843–4850. doi: 10.1158/0008-5472.CAN-06-1648. [DOI] [PubMed] [Google Scholar]
- Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer and Metastasis Reviews. 2007;26:225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- von Pawel J, von Roemeling R, Gatzemeier U, Boyer M, Elisson LO, Clark P, et al. Tirapazamine plus cisplatin versus cisplatin in advanced non-small-cell lung cancer: A report of the international CATAPULT I study group. Cisplatin and Tirapazamine in Subjects with Advanced Previously Untreated Non-Small-Cell Lung Tumors. J Clin Oncol. 2000;18:1351–1359. doi: 10.1200/JCO.2000.18.6.1351. [DOI] [PubMed] [Google Scholar]
- Ware DC, Palmer BD, Wilson WR, Denny WA. Hypoxia-selective antitumor agents. 7. Metal complexes of aliphatic mustards as a new class of hypoxia-selective cytotoxins Synthesis and evaluation of cobalt(III) complexes of bidentate mustards. J Med Chem. 1993;36:1839–1846. doi: 10.1021/jm00065a006. [DOI] [PubMed] [Google Scholar]
- Williamson SK, Crowley JJ, Lara PN, McCoy J, Lau DHM, Tucker RW, et al. Phase III Trial of Paclitaxel Plus Carboplatin With or Without Tirapazamine in Advanced Non-Small-Cell Lung Cancer: Southwest Oncology Group Trial S0003. Journal of Clinical Oncology. 2005;23:9097–9104. doi: 10.1200/JCO.2005.01.3771. [DOI] [PubMed] [Google Scholar]
- Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer. 2008;8:851–864. doi: 10.1038/nrc2501. [DOI] [PubMed] [Google Scholar]
- Xia Y, Choi H-K, Lee K. Recent advances in hypoxia-inducible factor (HIF)-1 inhibitors. European Journal of Medicinal Chemistry. 2012;49:24–40. doi: 10.1016/j.ejmech.2012.01.033. [DOI] [PubMed] [Google Scholar]
- Xue M, Findenegg GH. Lysozyme as a pH-responsive valve for the controlled release of guest molecules from mesoporous silica. Langmuir. 2012;28:17578–17584. doi: 10.1021/la304152j. [DOI] [PubMed] [Google Scholar]
- Xue M, Zhong X, Shaposhnik Z, Qu Y, Tamanoi F, Duan X, et al. pH-Operated mechanized porous silicon nanoparticles. J Am Chem Soc. 2011;133:8798–8801. doi: 10.1021/ja201252e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Liu C, Liu Y, Zhang N, Xu W. Mannan-modified solid lipid nanoparticles for targeted gene delivery to alveolar macrophages. Pharm Res. 2010;27:1584–1596. doi: 10.1007/s11095-010-0149-z. [DOI] [PubMed] [Google Scholar]
- Zhu S, Niu M, O’Mary H, Cui Z. Targeting of Tumor-Associated Macrophages Made Possible by PEG-Sheddable, Mannose-Modified Nanoparticles. Molecular Pharmaceutics. 2013;10:3525–3530. doi: 10.1021/mp400216r. [DOI] [PMC free article] [PubMed] [Google Scholar]