Abstract
Purpose
The effectiveness of NK cell infusions to induce leukemic remission is limited by lack of both antigen specificity and in vivo expansion. To address the first issue we previously generated a bispecific killer engager (BiKE) containing single chain scFV against CD16 and CD33 to create an immunologic synapse between NK cells and CD33+ myeloid targets. We have now incorporated a novel modified human IL-15 crosslinker, producing a 161533 trispecific killer engager (TriKE) to induce expansion, priming, and survival, which we hypothesize will enhance clinical efficacy.
Methods
Reagents were tested in proliferation and functional assays and in an in vivo xenograft model of AML.
Results
When compared to the 1633 BiKE, the 161533 TriKE induced superior NK cell cytotoxicity, degranulation, and cytokine production against CD33+ HL-60 targets and increased NK survival and proliferation. Specificity was shown by the ability of a 1615EpCAM TriKE to kill CD33-EpCAM+ targets. Using NK cells from patients after allogeneic stem cell transplantation when NK cell function is defective, the 161533 TriKE restored potent NK function against primary AML targets and induced specific NK cell proliferation. These results were confirmed in an immunodeficient mouse HL-60-Luc tumor model where the 161533 TriKE exhibited superior anti-tumor activity and induced in vivo persistence and survival of human NK cells for at least 3 weeks.
Conclusions
Off-the-shelf 161533 TriKE imparts antigen specificity and promotes in vivo persistence, activation, and survival of NK cells. These qualities are ideal for NK cell therapy of myeloid malignancies or targeting antigens of solid tumors.
Keywords: NK cell, ADCC, IL-15, bispecific antibodies, carcinoma
Introduction
Natural killer (NK) cells are cytotoxic lymphocytes of the innate immune system capable of immune surveillance. Like cytotoxic T cells, upon activation NK cells deliver a store of membrane penetrating and apoptosis-inducing molecules, including granulysin, granzyme and perforin (1). Unlike T cells, NK cells do not require antigen priming and recognize targets by engaging activating receptors in the absence of self-MHC recognition by inhibitory receptors. We have shown that adoptive transfer of haploidentical NK cells after lymphodepleting chemotherapy can induce complete remissions in 30–50% of patients with refractory acute myeloid leukemia (AML) when given with IL-2 to stimulate in vivo donor NK cell expansion (2,3). However, this approach is limited by lack of antigen specificity and by IL-2 mediated induction of regulatory T (Treg) cells that suppress NK cell proliferation and function (3,4). Thus we have developed a reagent that targets NK cells to specific tumor antigens and drives NK expansion and persistence, while bypassing the negative effects of Tregs and the morbidity of IL-2.
NK cells mediate antibody directed cellular cytotoxicity (ADCC) through the highly potent CD16 (FcγRIII) activating receptor. Signaling through CD16 induces a calcium flux and phosphorylation of ITAMs triggering the release of lytic granules and cytokines such as interferon (IFN-γ) and tumor necrosis factor (TNF-α) (5–7). To better target NK cells to malignant targets, we created and tested bispecific or trispecific killer engagers (BiKEs and TriKEs respectively) (8–11) each incorporating an anti-human anti-CD16 scFv derived from a human phage display library (12) with other scFvs directed against epitopes on malignant cells. These agents form an immunologic synapse between the highly activating CD16 receptor on NK cells and specific tumor antigens and markedly enhance cytotoxic killing of various human cancers (8–11). Our 1633 BiKE enhances NK cell responses to CD33+ AML (9) and myelodyplastic syndrome (MDS) targets (8).
IL-15 plays key role in NK cell development homeostasis, proliferation, survival, and activation (13). IL-15 and IL-2 share several signaling components including the IL-2/IL-15Rβ (CD122) and the common gamma chain (CD132). However, unlike IL-2, IL-15 does not stimulate CD25+ Tregs, allowing for NK cell activation without inducing concurrent Treg-mediated immune inhibition (14). IL-15 also activates NK cells, and can restore functional defects in engrafting NK cells after hematopoietic stem cell transplantation (HSCT) (15). IL-15 also stimulates CD8+ cytotoxic T cells, which further enhances its immunotherapeutic potential. Importantly, based on pre-clinical animal studies, the toxicity profile of low dose IL-15 may be more favorable than that of IL-2 (14,16). This report describes the generation of a 161533 TriKE that utilizes IL-15 as an intramolecular linker between CD16 and CD33 scFvs to direct NK cell mediated killing of CD33+ tumors while simultaneously generating an NK cell self-sustaining proliferation/survival signal through IL-15.
Materials and Methods
Cell Isolation, Patients and Samples
Peripheral blood mononuclear cells (PBMCs) were isolated from normal adult donor blood obtained from Memorial Blood Center (Minneapolis, MN) by centrifugation using a Histopaque gradient (Sigma-Aldrich, St. Louis, MO) and cryopreserved. Where noted, NK cells were enriched with magnetic beads using Stemcell EasySep Human NK cell enrichment kit while CD3/CD19 depleted products were generated with Miltenyi’s CliniMACS beads. Post-HSCT PBMCs from patients undergoing HLA-matched sibling transplants were obtained from an immune reconstitution biobank protocol. Recipient blood was collected and cryopreserved early (day 20–100) [n = 10] after HSCT. All samples were obtained after informed consent, using guidelines approved by the Committee on the Use of Human Subjects in Research at the University of Minnesota in accordance with the Declaration of Helsinki.
Cell Lines
Two cell lines were used and authenticated in these studies. HL-60, a CD33+ human acute promyelocytic leukemia cell line, was obtained in 2/2013 from ATCC, Manassas, VA 2013, and authenticated by flow cytometry (FC) against a panel of FITC labeled anti-CD33 and anti-CD45 as positive markers and anti-CD19, anti-CD22, anti-CD3, and anti-EpCAM as negative markers (BioLegend, San Diego, CA). The line was cultured in Iscove’s medium (Invitrogen, Carlsbad, CA) supplemented with 20% FBS (Gibco-Invitrogen) and 100U/mL penicillin and 100U/mL streptomycin (Invitrogen) at 37°C and 5% CO2. The control, murine colorectal carcinoma cell line HT-29 was also obtained from ATCC in 2013 and authenticated against a panel of BV7 anti-Integrin beta 1 antibody, MOC-31 epithelial glycoprotein 2, and anti-EpCAM as positive markers and anti-CD19, anti-CD22, and anti-CD45 as negative markers. The line was cultured at 37°C with 5% CO2 in Dulbecco’s Modified Eagle’s Medium (DMEM), high glucose (Invitrogen) supplemented with 10% FBS and 100U/mL penicillin and 100U/mL streptomycin and last tested in 2015.
Construction, Expression and Purification of BiKEs and TriKEs
The hybrid gene encoding 161533 was synthesized using DNA shuffling and DNA ligation techniques (10, 17). The VL and VH genes of each sFv were linked by a fragment encoding G4S linker. In its final configuration, the 161533 NcoI/XhoI gene fragment encoded a start codon followed first by anti-human CD16 scFv (12), a 20 amino acid flanking sequence PSGQAGAAASESLFVSNHAY, human IL-15, and then a 7 amino acid flanking sequence EASGGPE, and then the anti-CD33 scFv. The final target gene was spliced into the pET21d expression vector and inclusion bodies expressed. DNA-sequencing analysis (Biomedical Genomics Center, University of Minnesota) was used to verify that the gene was correct in sequence and cloned in frame.
Plasmid was transformed into the Escherichia coli strain BL21(DE3)(EMD, Madison WI). Bacteria were grown in 600 ml Luria Broth supplemented with 100 ug/ml carbenicillin in a 2L flask at 37°C with shaking. Expression of the hybrid gene was induced by the addition of isopropyl-b-D-thiogalactopyranoside (IPTG, FisherBiotech Fair Lawn, NJ). Two hours after induction, the bacteria were harvested by centrifugation. The cell pellets were suspended and homogenized using a polytron homogenizer. After sonication and centrifugation, the pellets were extracted with 0.3% sodium Deoxycholate, 5% Triton X-100, 10% Glycerin, 50 mM Tris, 50 mM NaCl, 5 mM EDTA, pH8.0 and inclusion bodies extensively washed to remove endotoxin.
The proteins were refolded using a sodium N-lauroyl-sarcosine (SLS) air oxidation method modified from a previously reported procedure for isolating scFv (18). Refolded 161533 was purified by FPLC ion exchange chromatography (Q Sepharose Fast Flow, Sigma, St. Louis, MO) using a stepwise gradient from 0.2 M to 0.5 M NaCl in 20 mM Tris-HCl, pH 9.0 over 4 column volumes.
Flow Cytometry
Cells were immunophenotyped with the following fluorescent-labeled monoclonal antibodies (mAb) against: PE-Cy7-conjugated CD56 (HCD56; BioLegend, San Diego, CA), ECD/PE-CF594-conjugated CD3 (UCHT1; Beckman Coulter, Brea, CA), APC-Cy7-conjugated CD16 (3G8; BioLegend), Pacific Blue-conjugated CD45 (HI30; BioLegend), PerCP-Cy5.5/FITC-conjugated anti-human CD107a (LAMP-1) (H4A3; BioLegend), Pacific Blue/BV421-conjugated anti-human IFN-γ (4S.B3; BioLegend), FITC/Alexa Fluor 647-conjugated TNF-α (MAb11; BioLegend), FITC/PE-conjugated CD33 (P67.6; BD Biosciences), and APC-conjugated CD45 (HI30; BioLegend), FITC-conjugated EpCAM; (BioLegend). Phenotypic acquisition of cells was performed on the LSRII (BD Biosciences) and analyzed with FlowJo software (Tree Star Inc., Ashland, OR).
CD107a and IFNγ/TNFα Functional Flow Assay
Post-HSCT recipient PBMCs and primary AML blasts were thawed and placed in RPMI-10 overnight. After 16–24 hrs, the PBMCs plus 50 nM 1633 BiKE or 161533 TriKE was added to the cultures. The following morning cells were washed and another round of 50 nM 1633 BiKE or 161533 TriKE was added. HL-60 Targets or primary AML blasts were added immediately after to generate a 5:1 effector to target ratio. PBMCs, HL-60 targets or primary AML blasts, and BiKE or TriKE molecules were co-cultured for 4 hours and CD107a expression and intracellular IFN-γ and TNF-α production were evaluated as previously described (10).
Proliferation Assay
PBMCs from post-transplant patients (day 22–44 or day100) were labeled with CellTrace Violet Cell Proliferation Dye (Invitrogen), per manufacturer’s protocol, placed in culture medium with HL-60 target cells at 5:1 (E:T) ratio, and treated with 50 nM 1633 BiKE or 161533 TriKE. Cells were then harvested 7 days later and analyzed for viability, through Live/Dead staining, and proliferation, through dilution of CellTrace, in the NK cell (CD56+CD3−) population.
51Chromium Release Cytotoxicity Assay
Cytotoxicity was evaluated by 4-hour 51Cr-release assays. Briefly, resting PBMC from normal donors treated with 161533 TriKE or 1633 BiKE (10μg/mL), scFvCD16 control reagent (10μg/mL) or no reagent were co-cultured for 4-hours with 51Cr-labeled or HL-60 targets at varying E:T ratios. For post-transplant study PBMCs cells were with HL-60 targets at a 20:1 (E:T) ratio in the presence of 50 nM 1633 BiKE or 50 nM 161533 TriKE. 51Cr release was measured by a gamma scintillation counter (Perkin Elmer, Walthman, MA) and specific target lysis was determined (10).
In vivo mouse study and Imaging
HL-60-luc cells were incorporated into a previously described NK cell xenogeneic mouse model system (19). NSG mice (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ, n = 5/group) were conditioned with 275 cGy and injected IV with 0.75 × 105 HL-60-luc S4 subcultured for tumor invasiveness. The control group did receive NK cells while the 1633 BiKE and the 161533 TriKE groups received 1 × 106 NK cells, calculated from a CD3/CD19 magnetically depleted product, on day 3. BiKE and TriKE treatment was also initiated after injection of the NK cells on day 3. A single course of treatment consisted of an IP injection of 50ug of drug given every day of the week (MTWThF) and mice were treated for two weeks. The HL-60-luc cells are a subline of HL-60 and have been transfected with a luciferase reporter gene, allowing for imaging of the mice each week to determine their bioluminescent activity and to monitor leukemia progression as described previously (20). Briefly, mice were injected with 100ul of 30mg/ml luciferin substrate 10 minutes prior to imaging and then anesthetized via inhalation of isoflurane gas. The mice were then imaged using the Xenogen Ivis 100 imaging system and analyzed with Living Image 2.5 software (Xenogen Corporation, Hopkington MA). On day 20, all the animals were bled and two-minute exposures were made and units for the regions of interest (ROI) were expressed as counts. The blood was analyzed by flow cytometry for presence of human CD45+CD56+CD3− NK cells. A second experiment was performed in order to verify reproducibility of data.
Cytokine Measurement by Luminex
For analysis of cytokines/chemokines, 500,000 NK cells co-cultured with 250,000 HL-60 cells were treated with the BiKE, TriKE, or no reagent for 24 hours at 37°C, 5%CO2. Supernatant levels were determined using a human-specific panel for IFN-γ, TNF-α, GM-CSF, or MIP-1α (EMD Millipore, Billerica, MA) and the Luminex system (Luminex, Austin, TX). Final pg/mL values were interpolated from standard curves of the corresponding recombinant human proteins with Bioplex software v.5.1 (BioRad, Hercules, CA).
Statistical Analysis
Grouped data were expressed as mean ± standard error mean (SEM). Differences between two groups were analyzed by Student’s t test. Multiple comparisons were analyzed by paired one-way ANOVA with Tukey correction. Analysis was carried out in Graphpad Prism software. On figures statistical significance is indicated as * = P≤0.05; ** = P<0.01; *** = P<0.001.
Results
Synthesis and purity of a 161533 TriKE
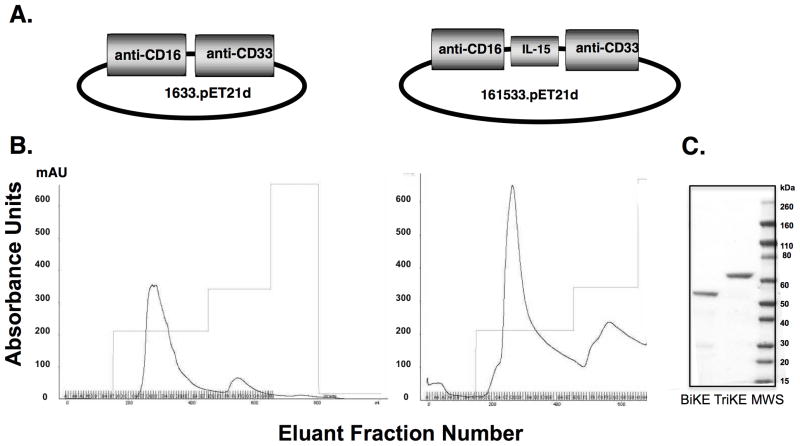
We introduced a human modified IL-15 cross-linker into our 1633 BiKE to create a 161533 TriKE designed to mediate antigen specificity and to deliver a proliferation and survival signal to NK cells (Figure 1A). The bacterial expression system, which requires refolding, delivered high yield 1633 BiKE and the 161533 TriKE products as shown by fast protein liquid chromatography (FPLC) profiles (Figure 1B). The addition of IL-15 to the molecule reduced its isoelectric point by two pH units, creating more favorable conditions for purification resulting in nearly double the yield compared to 1633 BiKE after purifications that began with identical amounts of inclusion bodies. Products were >95% pure by SDS-PAGE gel analysis and Coomasie Blue Staining (Figure 1C). To verify that binding and specificity remained intact in the new TriKE molecule, selectivity was measured by direct binding and competition flow cytometry assays against CD33+ HL-60 cells and CD33− HT-29 cells (Supplementary Tables 1 and 2). The IL-15 flanking sequences were important for optimal TriKE activity.
Figure 1. 161533 TriKE elicits superior purification properties over 1633 BiKE.
A) Schematic of gene placement of the BiKE (left) and TriKE (right) moieties in the pET expression vector. B) Absorbance tracings for 1633 BiKE (left) and 161533 TriKE (right), eluted from the ion exchange column as the first phase in drug purification using a 3-step elution protocol. For both agents a similar quantity of inclusion bodies was refolded and purified. The first peak eluted from the column represents the product. C) SDS-PAGE gel and Coomasie Blue staining of the BiKE and TriKE agents after a second step purification over a size exclusion column.
161533 TriKE increases NK cell function
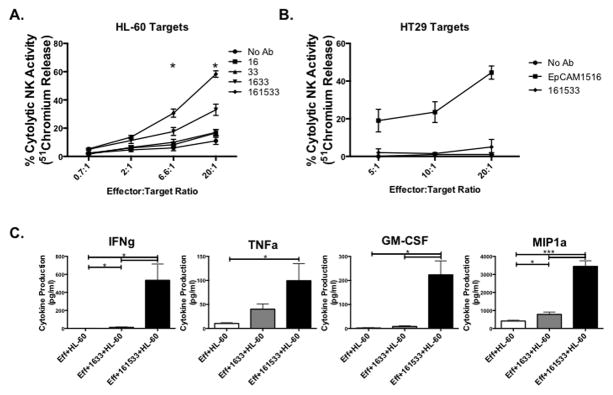
To test whether the 161533 TriKE retained the ability to mediate ADCC, as demonstrated with the 1633 BiKE (8,9), killing of CD33+ HL-60 targets by normal donor PBMCs was measured with both agents in a 4-hour chromium release assay (Figure 2A). The 161533 TriKE induced higher NK cell mediated killing than the 1633 BiKE, particularly at the 20:1 ratio (58.3 ± 2.3% vs. 33 ± 4%, P = 0.02). Control conditions with anti-CD16 and anti-CD33 scFv alone did not increase killing compared to untreated PBMC controls, demonstrating that these components alone have minimal activity. To address the input of the IL-15 moiety on NK cell cytotoxicity we compared the 161533 TriKE to equivalent molar concentrations (30 nM) of IL-15 or IL-15 plus 1633 BiKE (Supplementary Figure 1A). Results indicate that combining 1633 BiKE and IL-15 yields similar NK cell mediated cytotoxicity when compared to utilization of the 161533 TriKE, as expected. Although a trend of enhanced cytotoxicity can be seen with addition of IL-15 alone, likely due to enhanced priming of natural cytotoxicity, the 1633 specificity elicited by the scFvs in the TriKE or BiKE + IL-15 clearly enhance killing of targets indicating that combination of these molecules are paramount to enhancing NK cell function. The specificity of 161533 TriKE was further demonstrated by its inability to mediate killing of CD33− HT-29 target cells after co-incubation with PBMCs (Figure 2B). A novel IL-15 TriKE made with an anti-EpCAM scFv in place of anti-CD33 was used as a positive control to confirm that the HT-29 target cells were not inherently resistant to NK-mediated killing. The robust killing of CD33−EpCAM+ HT-29 cells mediated by the anti-EpCAM TriKE highlights the versatility of the IL-15 linker TriKE platform to induce antigen specificity against both hematologic and solid tumor malignancies.
Figure 2. 161533 TriKE elicits superior NK cell function against CD33+ targets.
A) Freshly isolated PBMCs were cultured with chromium loaded HL-60 cells for 4 hours at E:T ratios of 20:1, 6.6:1, and 2:1. The listed reagents were added at the beginning of co-culture at a 20 nM concentration. Data are displayed as percent NK cell cytolytic activity. Statistical significance only shown for 161533 TriKE and 1633 BiKE comparison (n=3). B) To evaluate the specificity of 161533 TriKE, a chromium release assay was performed against CD33−EpCAM+ HT29 targets. EpCAM1533 TriKE was used as a positive control (n = 2). Data are displayed as percent NK cell cytolytic activity. C) NK cells were enriched from normal donor PBMCs utilizing magnetic beads and placed in culture with HL-60 targets at an E:T ratio of 2:1 alone, in the presence of 1633 BiKE or in the presence of 161533 TriKE for 24 hours. Supernatants collected and frozen at the end of the incubation were later assessed for levels of of secreted IFNγ, TNFα, GM-CSF, and MIP1a using a Luminex-based multiplex assay (n=5). Points and bars represent mean ± SEM.
To directly investigate the role of the 1633 BiKE and 161533 TriKE on NK cell activation, PBMCs were incubated alone, with HL-60 cells, or with HL-60 cells plus 1633 BiKE or 161533 TriKE for 24 hrs and activation markers were evaluated on the NK cells (Supplementary Figure 1B). A clear hierarchy of activation can be noted through induction of CD69 expression, which is highest on NK cells incubated with 161533 and targets, followed by NK cells incubated with 1633 and targets, NK cells incubated with targets alone, and NK cells that were left untreated. CD25 expression was also highest on the NK cells incubated with 161533 and HL-60s, but the magnitude of the increase is likely also contributed by the effect of IL-15 signaling on CD25 induction. A trend for an increase in HLA-DR expression, which has slower induction dynamics, can also be noted in the NK cells treated with 1633 and 161533 and targets. Taken together these data indicate potent NK cell activation through the 161533 TriKE.
161533 TriKE affects cytokine production
In addition to cytotoxicity, another key anti-cancer function of NK cells is the production of cytokines and chemokines upon target cell recognition. Cytokine production was measured by incubating enriched NK cells and HL-60 targets alone, with 1633 BiKE, or with 161533 TriKE. Inflammatory cytokines and chemokines were measured in supernatants collected after 24 hours of incubation (Figure 2C). 161533 TriKE induced significantly more IFNγ and TNFα secretion compared to 1633 BiKE (left panels). No significant differences were noted between levels of IL-6, IL-10, or IL-8 (data not shown). GM-CSF and MIP-1α (right panels) were also induced by the 161533 TriKE while RANTES remained unchanged from baseline (data not shown). These data indicate that incorporation of the IL-15 molecule into the TriKE enhances the production of pro-inflammatory cytokines and chemokines by NK cells, augmenting their anti-tumor activity.
161533 TriKE induces survival and proliferation of post-transplant NK cells
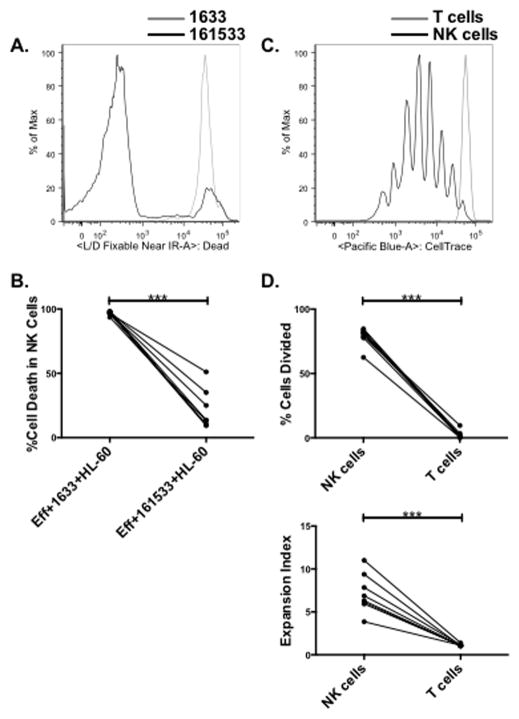
The ability of the IL-15 linker in our 161533 TriKE to deliver homeostatic and expansion signals to NK cells was tested in a physiologically relevant context using early post-HSCT recipient samples. This time point is of particular interest because we have shown major defects in NK cell mediated target cell induced cytokine production at this time, when engrafting NK cells are needed to mediate graft versus leukemia (GvL) responses (15). The proliferation induced by 1633 BiKE or 161533 TriKE in post-HSCT recipient PBMCs (day 20–100; n = 8) was measured after labeling with CellTrace dye and 7 days of incubation with HL-60 targets. Cell survival at 7 days, measured with Live/Dead dye, was significantly greater in the 161533 TriKE condition compared to the 1633 BiKE condition (Figures 3A and 3B; 96.9 ± 0.5% vs. 21 ± 5.4%; P < 0.0001). The proliferation induced by the IL-15 moiety in the 161533 TriKE was measured by CellTrace dye dilution in the viable NK and T cell populations. Unexpectedly, the 161533 TriKE induced robust and specific NK cell proliferation, with minimal T cell proliferation in the same samples (Figures 3C and 3D, top; cell division in 79.1 ± 2.5% of NK cells vs. 2.3 ± 1.1% of T cells, P < 0.0001). Compared to T cells, the expansion index or total fold expansion of the NK cells was significantly higher in the post-transplant setting (Figure 3D, bottom: 7.2 ± 0.8% vs. 1.1 ± 0.1%, P < 0.0001). However, in normal donors the 161533 TriKE was capable of expanding T cells (Supplementary Figure 2A), albeit to a much lower extent than NK cells, indicating that the reason there is no expansion of T cells in the post-transplant setting might be that at early time points after HSCT T cells capable of responding to the IL-15 moiety are diminished or their activity is blunted by concomitant pharmacologic agents given for GVHD prophylaxis.
Figure 3. The 161533 TriKE mediates NK cell survival and proliferation.
Post-HSCT recipient PBMCs were loaded with CellTrace proliferation dye and co-cultured with HL-60 Targets at a 5:1 (E:T) ratio for 7 days in the presence of 50 nM 1633 BiKE or 161533 TriKE. At the end of the incubation CD56+CD3− NK cells were assessed for viability through Live/Dead Near IR staining. A) An individual histogram and B) pooled analyses of viability in the NK cell population treated with the 1633 BiKE (gray) or 161533 TriKE (black) are shown. Individual dots represent separate post-HSCT samples treated with noted reagents (n=8). C) After 7 days, significantly fewer live cells remained in the 1633 BiKE condition precluding proliferation analysis. Proliferation in the 161533 TriKE condition was assessed by CellTrace dilution on live CD56+CD3− NK cells (black) and CD56−CD3+ T cells (gray). D) Pooled analyses depict the percent of dividing cells (top) and expansion index (bottom). The expansion index is calculated based on fold expansion within the population for a given amount of CellTrace dilution. Individual dots represent separate post-HSCT samples for noted populations (n=8).
We compared the biologic activity of the IL-15 moiety in the 161533 TriKE molecule to that of the clinical grade recombinant human IL-15 produced by the NCI. Enriched NK cells from normal donors were labeled with CellTrace and incubated for 7 days with no reagent, NCI IL-15, 1633 BiKE or 161533 TriKE. As expected, NK cell proliferation required IL-15, and was maintained by either NCI IL-15 or the 161533 TriKE (Supplementary Figures 2B and 2C). In marked contrast, the NK cells in the no reagent or 1633 BiKE groups failed to proliferate. These data indicate that the IL-15 linker incorporated into the 161533 TriKE is functionally active and capable of delivering survival and proliferation signals to normal donor or post-HSCT recipient NK cells.
161533 TriKE potently restores function in defective post-HSCT recipient NK cells
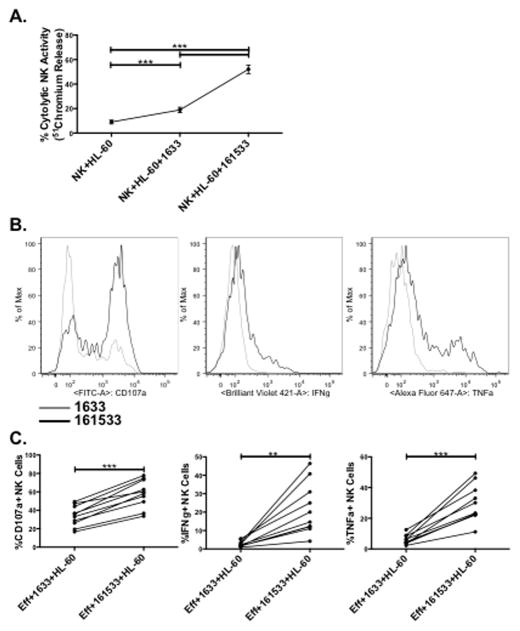
Given the potential clinical development of 1633 BiKE and 161533 TriKE as post-HSCT immunotherapy, the ability of each molecule to restore defective NK cell function was tested using post-HSCT recipient PBMCs (Figure 4). Thawed and rested PBMC were incubated with 1633 BiKE or 161533 TriKE overnight, washed, and reincubated with the same agent immediately prior to addition of targets. Responses after a 4 hour incubation with HL-60 targets were measured by chromium release and flow cytometry. While the 1633 BiKE promoted double the cytotoxicity against HL-60 targets compared to PBMCs alone (18.9 ± 2.1% versus 9 ± 1.5%, P < 0.0001), incubation with the 161533 TriKE further enhanced NK cell-mediated cytotoxic function (52.1 ± 3.5%) (Figure 4A). The increased cytotoxicity mediated by 161533 TriKE correlated with increased NK cell degranulation measured by CD107a expression (Figures 4B and 4C left panel). Defective target cell-induced production of IFNγ by NK cells was not restored by 1633 BiKE, whereas 161533 TriKE promoted a potent response (2.6 ± 0.5% vs. 21.7 ± 4.4%, respectively; P = 0.001, Figures 4B and 4C, center panels). A similar rescue of TNFα production by 161533 TriKE, but not 1633 BiKE was observed (29.9 ± 3.8% vs. 6.1 ± 1%, respectively; P < 0.0001, Figures 4B and 4C, right panels). The magnitude of post-HSCT recipient NK cell responses to CD33+ targets mediated by 161533 TriKE clearly demonstrate the immunotherapeutic potential of the this agent.
Figure 4. 161533 TriKE potently restores function in defective post-HSCT recipient NK cells.
The NK cell function induced by 161533 TriKE was compared to1633 BiKE using post-HSCT recipient PBMCs that were thawed and rested overnight with no cytokines. The next night they were incubated with no drug, 1633 BiKE (50 nM), or 161533 TriKE (50 nM). The next morning they were washed and reincubated with the specified agent immediately prior to addition of targets. A) Cytotoxicity against chromium loaded HL-60 targets was measured after 4 hours and the percent cytolytic activity was calculated. Dots denote mean ± SEM (n=9). B) Target cell-induced function is shown in representative histograms and C) pooled data of CD56+CD3− NK cell expression of CD107a (left panels), IFNγ (center panels), and TNFα (right panels) after 4 hour incubation with HL-60 targets. Dots denote individual patient samples treated with noted reagents (n=10).
161533 TriKE increases NK cell function against primary AML blasts
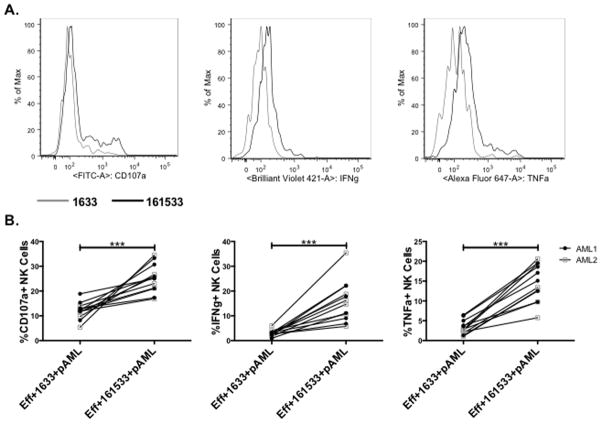
We compared the function of post-HSCT recipient NK cells incubated with 1633 BiKE or 161533 TriKE against primary AML blasts from two different patients (AML1 and AML2). While the overall production of CD107a, IFNγ and TNFα by post-HSCT NK cells exposed to primary blasts was reduced when compared to HL-60 targets, the 161533 TriKE again mediated significantly more degranulation and cytokine production compared to the1633 BiKE (Figures 5A and 5B). Reduced expression of CD33 on the primary AML blasts compared to HL-60 (Supplementary Figure 2D) could explain the decreased NK cell response to primary AML blasts. Importantly, the post-HSCT recipient NK cells exhibited similar 161533 TriKE-mediated responses to activation by AML1 and AML2. Taken together, these in vitro data indicate that the 161533 TriKE induces antigen specific NK cell cytotoxic and cytokine producing responses against primary CD33+ tumor cells, and that the costimulation provided by the IL-15 linker increases NK responses compared to those seen with the 1633 BiKE agent.
Figure 5. 161533 TriKE induces enhanced NK cell function against primary AML blasts.
Post-transplant patient PBMCs were thawed and rested overnight. The next night they were incubated with 1633 BiKE (50 nM), or 161533 TriKE (50 nM). The next morning they were washed and reincubated with the specified agent immediately prior to addition of targets. Primary AML blasts from apheresis products of two separate patients were thawed and rested overnight. Treated post-transplant patient PBMCs (n = 6) were incubated with the two different primary AML blasts (n = 12 total) for four hours and NK cell function was assessed by flow cytometry. A) Representative histograms denoting CD107a (left), IFNγ (center), and TNFα (right) expression on post-transplant patient NK cells treated with 1633 BiKE (gray) or 161533 TriKE (black) after 4 hour incubation with primary AML blasts. C) Pooled data for CD107a (left), IFNγ (center), and TNFα (right) expression on post-transplant patient NK cells treated with 1633 BiKE and 161533 TriKE and incubated with primary AML blasts. Each box represents a separate post-transplant patient sample incubated against two separate patient AML blast targets, denoted by filled and open boxes (n=12 total).
161533 TriKE induces enhanced in vivo survival of NK cells with anti-tumor function
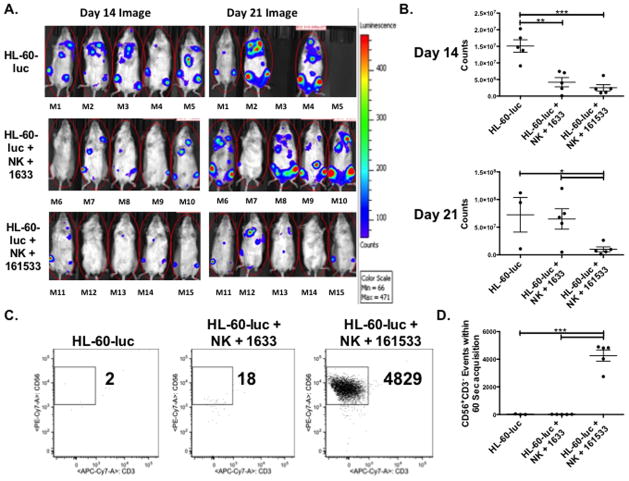
We developed a murine xenograft model incorporating human NK cells and CD33+ tumor cells to compare the activity of the 1633 BiKE and the 161533 TriKE in vivo. NSG mice were conditioned and injected intravenously with HL-60 cells containing a luciferase reporter gene (7.5 × 105 cells/mouse), followed 3 days later by intravenous infusion of 1 × 106 normal human donor NK cells activated overnight with IL-15. Mice then were treated with no drug (and no NK cells in the control group), 1633 BiKE or 161533 TriKE (20 ug) administered intraperitoneally MTWThF through the first two weeks of the study (10 doses total). Mice were imaged on day 14 and day 21 to measure tumor burden (Figures 6A and 6B). At day 14 the 1633 BiKE and 161533 TriKE groups showed similar control of tumor burden, both of which were significantly better than the control group. However on day 21, by which time 2 mice from the no-drug control group had died, only the 161533 TriKE group showed significantly reduced tumor burden, indicating superior sustained control of the HL-60 tumor burden with 161533 TriKE therapy.
Figure 6. 161533 TriKE limits HL-60 tumor growth in vivo better than 1633 BiKE through enhanced maintenance of NK cells.
An in vivo tumor model was established by conditioning NSG mice (275 cGy) and then injecting HL-60-luc cells intravenously (7.5 × 105 cells/mouse). Three days later 1×106 normal human donor NK cells (calculated from a magnetically depleted CD3/CD19 product) activated overnight with IL-15 were infused. 1633 BiKE and 161533 TriKE (50 ug) was administered MTWThF through the next two weeks of the study (10 doses total), and a control group only received HL-60-luc cells. A) Individual mouse photoluminscence after 2 minute exposures on day 14 (left) and day 21 (right) after NK infusion are shown (n=5 per treatment group,). B) Quantification of luminescence in mice from the three treatment groups at day 14 (top) and day 21 (bottom). Each dot represents a different mouse and bars denote mean ± SEM (n=5 per treatment group, representative of two separate experiments). C) Blood was collected on day 20 from the mice in each of the experiential treatment groups. Circulating CD45+CD56+CD3− human NK cells were quantified by flow cytometry. Events were collected over 60 seconds and the number of human NK cell events was calculated. Representative dot plots are shown denoting the number of NK (CD56+CD3−) cell events within the CD45+ gate. D) Aggregate data demonstrating the number of human NK cell events in each treatment group at day 20. Individual dots represent different mice and bars denote mean ± SEM (n=3 for HL-60-luc group [two mice died], n=5 for the 1633 BiKE and 161533 TriKE groups).
To evaluate the effect of the 1633 BiKE and the 161533 TriKE on in vivo NK cell maintenance, accounting for NK cell survival and proliferation, we bled the mice (20 μL) at day 20 and measured the number of human NK cell (CD45+CD56+CD3−) events in 60 seconds of acquisition by flow cytometry. Essentially no circulating human NK cells were detected in the no-drug control or the 1633 BiKE treated animals (17.3 ± 14.3 and 11.6 ± 2.3 events respectively; Figures 6C and 6D). In marked contrast, all of the 161533 TriKE treated animals had a substantial number circulating human NK cells (4261 ± 410.6 events) during the same acquisition period. Thus, mice treated with 161533 TriKE had over 350-fold more human NK cells circulating in the peripheral blood than did BiKE treated mice, indicating that the IL-15 linker within the TriKE molecule delivers robust proliferation and survival signals.
A second experiment was performed using this same murine xenograft model (n=4–5/group) to reproduce this data. Only in the second experiment, a higher number (106) of highly HL-60 cells were administered. Fifty percent of the TriKE treated mice were complete responders to therapy. Again, the BiKE treated mice showed only minimal effects on day 21 (data not shown). Also, a separate group of animals were given high dose IL-15 alone without BiKE or TriKE (5 ug MWF). These showed no response indicating that IL-15 itself is not sufficient to mediate an NK cell mediated anti-tumor effect.
Discussion
The original contribution of this work is a self-sustaining 161533 TriKE using anti-CD16 and anti-CD33 scFvs with a modified IL-15 linker capable of directing antigen specific ADCC while inducing NK cell proliferation and survival. We previously reported that our 1633 BiKE molecule successfully drives antigen-specific ADCC against primary AML and MDS (8,9), and we have substantially improved this platform by introducing an IL-15 signal that costimulates the NK cell response to CD33+ targets, including primary AML blasts, with significantly enhanced cytotoxic and cytokine production compared to the 1633 BiKE. The 161533 TriKE induces potent responses by NK cells from normal donor samples as well as from post-HSCT recipient NK cells. Most importantly, using our novel xenogeneic tumor model we demonstrate that the 161533 TriKE is capable of driving human NK cells to proliferate and survive in vivo while mediating an antigen specific sustained anti-cancer response. Neither 1633 BiKE or IL-15 alone had all these properties.
Combining cytokines with antibodies is not a new concept and early efforts focused on IL-2. IL-2 cytobodies were generated in the early 2000s (21–24), but unfortunately clinical responses were marginal and IL-2 toxicities were dose limiting. The effectiveness of IL-2 as a cancer immunotherapy is also limited by its potent induction of CD25+ Tregs. IL-15 plays a critical role in NK cell development, homeostasis, proliferation, activation and survival (13, 25–30). IL-15 also may be less toxic than IL-2, as has been demonstrated by Munger et al who used a vascular leak mouse model that measures the extravasation of 125I-labeled albumin. They showed that high dose IL-2 caused vascular leak syndrome (VLS) in mice, but high dose IL-15 did not (16). Thus we chose to incorporate a modified IL-15 molecule into our 161533 TriKE to simultaneously function as an intramolecular linker and as a stimulator of NK function. Although IL-15 is considered less toxic than IL-2 based on its reduced propensity to induce VLS, it does trigger a cytokine cascade including TNF-α, IL-1, IL-6, IL-8, MCP-1, GM-CSF, and pro-inflammatory chemokines (31,32). The most common severe side effects are fever, fatigue, nausea, vomiting, weight loss, severe neutropenia, and a generalized skin rash associated with cytokine inflammatory syndrome. In mice, weight loss precedes death, so weight loss can be used as a measure of toxicity. We did not observe weight loss in NSG mice that received NK cells and NCI rH IL-15 or IL-15 in the form of the 161533 TriKE (10 injections of 20ug; data not shown). These studies do not demonstrate toxicity, either from the 161533 TriKE or from the activated NK cells. Although further investigation is warranted to assess whether IL-15-activated NK cells can induce toxicity indirectly, our pre-clinical data indicate that IL-15 activation of NK cells by the 161533 TriKE agent may result in decreased toxicity by more localized delivery.
Other potential toxicities associated with exogenous IL-15 administration should be considered. In certain settings, IL-15 has been found to participate in the development of leukemias and solid tumors (33–40), to inhibit apoptosis of tumor cells (41), and to promote their proliferation (38,42), migration (43), epithelial-to-mesenchymal transition (35), invasion (37), metastasis and tumor angiogenesis (36). Therefore, we believe that selectively delivering IL-15 signals to NK cells in the context of the TriKE molecule will facilitate more direct interactions with the immune synapse formed between NK effector cells and tumor targets and will potentially decrease systemic effects.
We have demonstrated that unlike the 1633 BiKE, the 161533 TriKE is capable of inducing proliferation and increasing survival in normal donor and post-HSCT recipient NK cells. As shown in our xenogeneic tumor model, the increased number of live NK cells will likely translate to increased anti-tumor activity. Importantly, our in vitro assays showed that the TriKE induced proliferation of NK but not T cells in post-HSCT samples, which is important in the post-HSCT setting where T cells mediate graft versus disease. We have previously described functional defects in recipient NK cells early post-HSCT (15,44), which may account for early relapses (45). Compared to the 1633 BiKE, our 161533 TriKE restored robust NK cell function in post-HSCT recipient NK cells. Thus, there may be great benefit of this agent in the HSCT arena.
We and others have previously reported BiKEs (8–11,45,46). Our preclinical data show that 161533 TriKE holds more promise for clinical testing to treat AML and MDS. Allogeneic HSCT is standard therapy for MDS and AML, but older patients transplanted with reduced intensity conditioning have an unacceptably high relapse rate of 30%. Thus, new therapeutic strategies are urgently needed to reduce relapse of MDS and AML after HSCT and to treat patients who are not transplant candidates. Our 161533 TriKE adds a critical component to the BiKE scaffold by inducing maintenance and expansion of the effector NK cells mediating tumor killing. This is particularly important since resting NK cell function is diminished in MDS and AML patients from the disease itself or its therapy. A unique advantage of using the CD33 scFv in our TriKE is that the molecule may also direct NK cells to eliminate CD33+ myeloid derived suppressor cells (MDSC). Thus the agent may directly target tumor as well as reducing tumor-induced immune suppression.
The 161533 TriKE molecules could also be used to enhance endogenous NK cell function against NK sensitive malignancies in the post-transplant setting, as demonstrated by our in vitro studies, particularly in refractory disease or in patients undergoing reduced intensity conditioning. Although studies shown here target the CD33 antigen in myeloid tumors, the IL-15 TriKE platform can easily be adapted to target other tumors by switching the anti-CD33 scFv portion for an scFv targeting a different tumor antigen such as EpCAM present on solid tumor targets, making this a versatile platform for self-sustained NK cell based immunotherapies.
Supplementary Material
Statement of Translational Relevance.
IL-15 is a signal for NK cell development, proliferation, survival, and activation. A major advantage of IL-15 therapy over IL-2 therapy is that does not engage Treg suppressor cells and may minimize toxicities associated with IL-2 therapy. In this paper, we report for the first time that we have stably integrated IL-15 into a bispecific antibody platform recognizing CD16 on NK cells and CD33 on myeloid cancer cells. This trispecific 16 × 15 × 33 recombinant molecule successfully boosted antibody dependent cell mediated toxicity (ADCC) in vitro and in vivo compared to the bispecific antibody without IL-15. Importantly, the new molecule had the ability to expand and sustain human NK cells in vivo in a xenograft model. This off the shelf drug is cost effective and easy to use, desirable qualities for enhancing the selectivity of NK cell therapy of myeloid malignancies or targeting antigens of solid tumors.
Acknowledgments
Financial Support: This work was supported in part by the US Public Health Service Grant R01-CA36725, R01-CA72669, P01-CA65493, P01-CA111412 and R35 CA197292 awarded by the NCI and the NIAID, DHHS. It was also supported by an NIH Research Evaluation and Commercialization Hub (REACH) Award (U01), the Mayo Partnership Award, the Lion Fund, William Lawrence and Blanche Hughes Fund, the Randy Shaver Foundation, the Atwater Cancer Drug Development Award, the Deutsche Krebshilfe (D.S.) and a CETI translational award from the University of Minnesota Masonic Cancer Center.
The authors acknowledge cell-processing services from the Translational Therapy and FACS Cores of the Masonic Cancer Center (P30 CA77598). We acknowledge the excellent technical assistance of Elizabeth Taras, Deborah Todhunter, Andy Sicheneder, Sami Chu, and Seunguk Oh.
Footnotes
Conflict-of-interest disclosure: Drs. Vallera and Miller are members of the Oxis Biotech Scientific Advisory Board and hold equity in the company. This relationship has been reviewed and managed by the University of Minnesota in accordance with its conflict of interest policies.
References
- 1.Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 2.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 3.Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lewis D, Hippen K, McGlave P, Weisdorf DJ, Blazar BR, Miller JS. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123:3855–63. doi: 10.1182/blood-2013-10-532531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell CJ, Sun Y, Nowak UM, Clark J, Howlett S, Pekalski ML, Yang X, Ast O, Waldhauer I, Freimoser-Grundschober A, Moessner E, Umana P, Klein C, Hosse RJ, Wicker LS, Peterson LB. Sustained in vivo signaling by long-lived IL-2 induces prolonged increases of regulatory T cells. J Autoimmun. 2015;56:66–80. doi: 10.1016/j.jaut.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanier LL, Ruitenberg JJ, Phillips JH. Functional and biochemical analysis of CD16 antigen on natural killer cells and granulocytes. J Immunol. 1988;141:3478–85. [PubMed] [Google Scholar]
- 6.Yokoyama WM, Plougastel BF. Immune functions encoded by the natural killer gene complex. Nat Rev Immunol. 2003;3:304–16. doi: 10.1038/nri1055. [DOI] [PubMed] [Google Scholar]
- 7.Lanier LL. Natural killer cell receptor signaling. Curr Opin Immunol. 2003;15:308–14. doi: 10.1016/s0952-7915(03)00039-6. [DOI] [PubMed] [Google Scholar]
- 8.Gleason MK, Ross JA, Warlick ED, Lund TC, Verneris MR, Wiernik A, Spellman S, Haagenson MD, Lenvik AJ, Litzow MR, Epling-Burnette PK, Blazar BR, Weiner LM, Weisdorf DJ, Vallera DA, Miller JS. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood. 2014;123:3016–26. doi: 10.1182/blood-2013-10-533398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiernik A, Foley B, Zhang B, Verneris MR, Warlick E, Gleason MK, Ross JA, Luo X, Weisdorf DJ, Walcheck B, Vallera DA, Miller JS. Targeting natural killer cells to acute myeloid leukemia in vitro with a CD16 x 33 bispecific killer cell engager and ADAM17 inhibition. Clin Cancer Res. 2013;19:3844–55. doi: 10.1158/1078-0432.CCR-13-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vallera DA, Zhang B, Gleason MK, Oh S, Weiner LM, Kaufman DS, McCullar V, Miller JS, Verneris MR. Heterodimeric bispecific single-chain variable-fragment antibodies against EpCAM and CD16 induce effective antibody-dependent cellular cytotoxicity against human carcinoma cells. Cancer Biother Radiopharm. 2013;28:274–82. doi: 10.1089/cbr.2012.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gleason MK, Verneris MR, Todhunter DA, Zhang B, McCullar V, Zhou SX, Panoskaltsis-Mortari A, Weiner LM, Vallera DA, Miller JS. Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol Cancer Ther. 2012;11:2674–84. doi: 10.1158/1535-7163.MCT-12-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCall AM, Adams GP, Amoroso AR, et al. Isolation and characterization of an anti-CD16 single-chain Fv fragment and construction of an anti-HER2/neu/anti-CD16 bispecific scFv that triggers CD16-dependent tumor cytolysis. Mol Immunol. 1999;36:433–445. doi: 10.1016/s0161-5890(99)00057-7. [DOI] [PubMed] [Google Scholar]
- 13.Steel JC, Waldmann TA, Morris JC. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol Sci. 2012;33:35–41. doi: 10.1016/j.tips.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger C, et al. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood. 2009;114:2417–2426. doi: 10.1182/blood-2008-12-189266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, Weisdorf DJ, Miller JS. NK cell education after allogeneic transplantation: dissociation between recovery of cytokine-producing and cytotoxic functions. Blood. 2011;118:2784–92. doi: 10.1182/blood-2011-04-347070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munger W, et al. Studies evaluating the antitumor activity and toxicity of interleukin-15, a new T cell growth factor: comparison with interleukin-2. Cell Immunol. 1995;165:289–293. doi: 10.1006/cimm.1995.1216. [DOI] [PubMed] [Google Scholar]
- 17.Vallera DA, Chen H, Sicheneder AR, Panoskaltsis-Mortari A, Taras EP. Genetic alteration of a bispecific ligand-directed toxin targeting human CD19 and CD22 receptors resulting in improved efficacy against systemic B cell malignancy. Leuk Res. 2009;33:1233–42. doi: 10.1016/j.leukres.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vallera DA, Todhunter D, Kuroki DW, Shu Y, Sicheneder A, Panoskaltsis-Mortari A, Vallera VD, Chen H. Molecular modification of a recombinant, bivalent anti-human CD3 immunotoxin (Bic3) results in reduced in vivo toxicity in mice. Leuk Res. 2005;29:331–41. doi: 10.1016/j.leukres.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Miller JS, Rooney CM, Curtsinger J, McElmurry R, McCullar V, Verneris MR, Lapteva N, McKenna D, Wagner JE, Blazar BR, Tolar J. Expansion and homing of adoptively transferred human natural killer cells in immunodeficient mice varies with product preparation and in vivo cytokine administration: implications for clinical therapy. Biol Blood Marrow Transplant. 2014;20:1252–7. doi: 10.1016/j.bbmt.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waldron NN, Kaufman DS, Oh S, Inde Z, Hexum MK, Ohlfest JR, Vallera DA. Targeting tumor-initiating cancer cells with dCD133KDEL shows impressive tumor reductions in a xenotransplant model of human head and neck cancer. Mol Cancer Ther. 2011;10:1829–38. doi: 10.1158/1535-7163.MCT-11-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osenga KL, Hank JA, Albertini MR, Gan J, Sternberg AG, Eickhoff J, Seeger RC, Matthay KK, Reynolds CP, Twist C, Krailo M, Adamson PC, Reisfeld RA, Gillies SD, Sondel PM. A phase I clinical trial of the hu14. 18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: A study of the Children’s Oncology Group. Clin Cancer Res. 2006;12:1750–1759. doi: 10.1158/1078-0432.CCR-05-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King DM, Albertini MR, Schalch H, Hank JA, Gan J, Surfus J, Mahvi D, Schiller JH, Warner T, Kim K, Eickhoff J, Kendra K, Reisfeld R, Gillies SD, Sondel P. Phase I clinical trial of the immunocytokine EMD 273063 in melanoma patients. J Clin Oncol. 2004;22:4463–4473. doi: 10.1200/JCO.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko YJ, Bubley GJ, Weber R, Redfern C, Gold DP, Finke L, Kovar A, Dahl T, Gillies SD. Safety, pharmacokinetics, and biological pharmacodynamics of the immunocytokine EMD 273066 (huKS-IL2): Results of a phase I trial in patients with prostate cancer. J Immunother. 2004;27:232–239. doi: 10.1097/00002371-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Connor JP, Felder M, Hank J, Harter J, Gan J, Gillies SD, Sondel P. Ex vivo evaluation of anti-EpCAM immunocytokine huKS-IL2 in ovarian cancer. J Immunother. 2004;27:211–219. doi: 10.1097/00002371-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Waldmann TA. Interleukin-15 in the treatment of cancer. Expert Rev Clin Immunol. 2014;10:1689–701. doi: 10.1586/1744666X.2014.973856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mishra A, Sullivan L, Caliguri MA. Molecular pathways: Interleukin-15 signaling in health and cancer. Clin Cancer Res. 2014;20:2044–50. doi: 10.1158/1078-0432.CCR-12-3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ochoa MC, Mazzolini G, Hervas-Stubbs S, de Sanmamed MF, Berraondo P, Melero I. Interleukin-15 in gene therapy of cancer. Curr Gene Ther. 2013;13:15–30. doi: 10.2174/156652313804806561. [DOI] [PubMed] [Google Scholar]
- 28.Jakobisiak M, Golab J, Lasek W. Interleukin 15 as a promising candidate for tumor immunotherapy. Cytokine Growth Factor Rev. 2011;22:99–108. doi: 10.1016/j.cytogfr.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Shanmugham LN, Petrarca C, Frydas S, Donelan J, Castellani ML, Boucher W, Madhappan B, Tete S, Falasca K, Conti P, Vecchiet J. IL-15 an immunoregulatory and anti-cancer cytokine. Recent advances. J Exp Clin Cancer Res. 2006;25:529–36. [PubMed] [Google Scholar]
- 30.Becknell B, Caligiuri MA. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol. 2005;86:209–39. doi: 10.1016/S0065-2776(04)86006-1. [DOI] [PubMed] [Google Scholar]
- 31.Lin JX, Migone TS, Tsang M, Friedmann M, Weatherbee JA, Zhou L, et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–9. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 32.Badolato R, Ponzi AN, Millesimo M, Notarangelo LD, Musso T. Interleukin-15 (IL-15) induces IL-8 and monocyte chemotactic protein 1 production in human monocytes. Blood. 1997;90:2804–9. [PubMed] [Google Scholar]
- 33.Hodge DL, Yang J, Buschman MD, Schaughency PM, Dang H, Bere W, et al. Interleukin-15 enhances proteasomal degradation of bid in normal lymphocytes: implications for large granular lymphocyte leukemias. Cancer Res. 2009;69:3986–94. doi: 10.1158/0008-5472.CAN-08-3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fehniger TA, Suzuki K, Ponnappan A, VanDeusen JB, Cooper MA, Florea SM, et al. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. J Exp Med. 2001;193:219–31. doi: 10.1084/jem.193.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khawam K, Giron-Michel J, Gu Y, Perier A, Giuliani M, Caignard A, et al. Human renal cancer cells express a novel membrane-bound interleukin-15 that induces, in response to the soluble interleukin-15 receptor alpha chain, epithelial-to-mesenchymal transition. Cancer Res. 2009;69:1561–9. doi: 10.1158/0008-5472.CAN-08-3198. [DOI] [PubMed] [Google Scholar]
- 36.Kuniyasu H, Ohmori H, Sasaki T, Sasahira T, Yoshida K, Kitadai Y, et al. Production of interleukin 15 by human colon cancer cells is associated with induction of mucosal hyperplasia, angiogenesis, and metastasis. Clin Cancer Res. 2003;9:4802–10. [PubMed] [Google Scholar]
- 37.Kuniyasu H, Oue N, Nakae D, Tsutsumi M, Denda A, Tsujiuchi T, et al. Interleukin-15 expression is associated with malignant potential in colon cancer cells. Pathobiology. 2001;69:86–95. doi: 10.1159/000048761. [DOI] [PubMed] [Google Scholar]
- 38.Trentin L, Cerutti A, Zambello R, Sancretta R, Tassinari C, Facco M, et al. Interleukin-15 promotes the growth of leukemic cells of patients with B-cell chronic lymphoproliferative disorders. Blood. 1996;87:3327–35. [PubMed] [Google Scholar]
- 39.Wu S, Fischer L, Gokbuget N, Schwartz S, Burmeister T, Notter M, et al. Expression of interleukin 15 in primary adult acute lymphoblastic leukemia. Cancer. 2010;116:387–92. doi: 10.1002/cncr.24729. [DOI] [PubMed] [Google Scholar]
- 40.Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006;17:259–80. doi: 10.1016/j.cytogfr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Zambello R, Facco M, Trentin L, Sancetta R, Tassinari C, Perin A, et al. Interleukin-15 triggers the proliferation and cytotoxicity of granular lymphocytes in patients with lymphoproliferative disease of granular lymphocytes. Blood. 1997;89:201–11. [PubMed] [Google Scholar]
- 42.Budagian V, Bulanova E, Orinska Z, Pohl T, Borden EC, Silverman R, et al. Reverse signaling through membrane-bound interleukin-15. J Biol Chem. 2004;279:42192–2. doi: 10.1074/jbc.M403182200. [DOI] [PubMed] [Google Scholar]
- 43.Foley B, Felices M, Cichocki F, Cooley S, Verneris MR, Miller JS. The biology of NK cells and their receptors affects clinical outcomes after hematopoietic cell transplantation (HCT) Immunol Rev. 2014;258:45–63. doi: 10.1111/imr.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verneris MR, Lee SJ, Ahn KW, Wang HL, Battiwalla M, Inamoto Y, Fernandez-Vina MA, Gajewski J, Pidala J, Munker R, Aljurf M, Saber W, Spellman S, Koreth J. HLA Mismatch Is Associated with Worse Outcomes after Unrelated Donor Reduced-Intensity Conditioning Hematopoietic Cell Transplantation: An Analysis from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2015;21:1783–9. doi: 10.1016/j.bbmt.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stein C, Kellner C, Kügler M, Reiff N, Mentz K, Schwenkert M, Stockmeyer B, Mackensen A, Fey GH. Novel conjugates of single-chain Fv antibody fragments specific for stem cell antigen CD123 mediate potent death of acute myeloid leukaemia cells. Br J Haematol. 2010;148:879–885. doi: 10.1111/j.1365-2141.2009.08033.x. [DOI] [PubMed] [Google Scholar]
- 46.Singer H, Kellner C, Lanig H, Aigner M, Stockmeyer B, Oduncu F, Schwemmlein M, Stein C, Mentz K, Mackensen A, Fey GH. Effective elimination of acute myeloid leukemic cells by recombinant bispecific antibody derivatives directed against CD33 and CD16. J Immunother. 2010;33:599–610. doi: 10.1097/CJI.0b013e3181dda225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.