Abstract
Listeria monocytogenes, the causative organism of the serious food-borne disease listeriosis, has a membrane abundant in branched-chain fatty acids (BCFAs). BCFAs are normally biosynthesized from branched-chain amino acids via the activity of branched chain α-keto acid dehydrogenase (Bkd), and disruption of this pathway results in reduced BCFA content in the membrane. Short branched-chain carboxylic acids (BCCAs) added as media supplements result in incorporation of BCFAs arising from the supplemented BCCAs in the membrane of L. monocytogenes bkd mutant MOR401. High concentrations of the supplements also effect similar changes in the membrane of the wild type organism with intact bkd. Such carboxylic acids clearly act as fatty acid precursors, and there must be an alternative pathway resulting in the formation of their CoA thioester derivatives. Candidates for this are the enzymes phosphotransbutyrylase (Ptb) and butyrate kinase (Buk), the products of the first two genes of the bkd operon. Ptb from L. monocytogenes exhibited broad substrate specificity, a strong preference for branched-chain substrates, a lack of activity with acetyl CoA and hexanoyl CoA, and strict chain length preference (C3–C5). Ptb catalysis involved ternary complex formation. Additionally, Ptb could utilize unnatural branched-chain substrates such as 2-ethylbutyryl CoA, albeit with lower efficiency, consistent with a potential involvement of this enzyme in the conversion of the carboxylic acid additives into CoA primers for BCFA biosynthesis.
1. Introduction
Listeria monocytogenes, the dangerous foodborne pathogen, grows actively at low temperatures and is a cause for concern due to the widespread use of refrigeration as a food preservation method [1]. Listeria outbreaks remain a continuing problem (http://www.cdc.gov/listeria/outbreaks). The economic costs of a 2008 L. monocytogenes outbreak in Canada linked to contaminated delicatessen meat from one processing plant, which resulted in 57 cases of listeriosis and 24 deaths, were estimated to be $242 million Canadian dollars [2].
A fluid membrane ensured by a high content of branched-chain fatty acids (BCFAs), which have low phase transition temperatures, supports the growth of L. monocytogenes at low temperatures [3–5]. Branched-chain amino acids, namely isoleucine, leucine and valine, through the activity of branched-chain amino transferase (Bcat) and branched-chain α-keto acid dehydrogenase complex (Bkd) result in the formation of the CoA derivatives of 2-methylbutyrate, isobutyrate and isovalerate respectively, which are then elongated by the wellcharacterized dissociated fatty acid biosynthesis (FAS II) system to form membrane BCFAs [6]. Mutants of L. monocytogenes (cld-2/MOR401) lacking functional Bkd have low membrane BCFA content and demonstrate diminished growth at refrigeration temperatures, reduced tolerance to adverse environmental conditions such as oxidative stress, and poor survival in macrophages [7,8]. Growth of bkd mutants in Bacillus subtilis, L. monocytogenes and Staphylococcus aureus could be rescued by the addition of branched-chain carboxylic acids (BCCAs) to the medium [9–11].
Extensive studies on medium supplementation by Kaneda [12] in B. subtilis and by Sen et al. [13] in L. monocytogenes show that addition of a specific BCCA results in an increase in membrane fatty acids biosynthesized from that particular BCCA. The carboxylic acids that are capable of causing an alteration of membrane fatty acid composition are not restricted to the normal products of BCAA metabolism but also include unnatural C6 BCCAs that have a similar structure such as 2-ethylbutyrate, 2-methylpentanoate, and straight-chain carboxylic acids (SCCAs) such as butyrate and propionate [12,13]. Incorporation of novel fatty acids into the membrane as a result of elongation of unnatural C6 BCCAs is indicative of the entry of the supplements into the FAS II pathway as their respective acyl CoA derivatives, since the only known source of membrane BCFAs is via biosynthesis [6]. Formation of acyl CoA derivatives despite the absence of functional Bkd confirms the presence of an alternate pathway which catalyzes the conversion of supplemented carboxylic acids into their respective acyl CoA end products [10,13]. Willecke and Pardee [11] first suggested the involvement of an alternate system in the conversion of BCCAs into precursors of fatty acids in a bkd mutant of B. subtilis. Integration of the products of such a wide range of substrates into the membrane implies that the pathway involved in their activation must be remarkably flexible in its substrate specificity [10,13]. This pathway has not been characterized in L. monocytogenes thus far despite ample evidence of its existence.
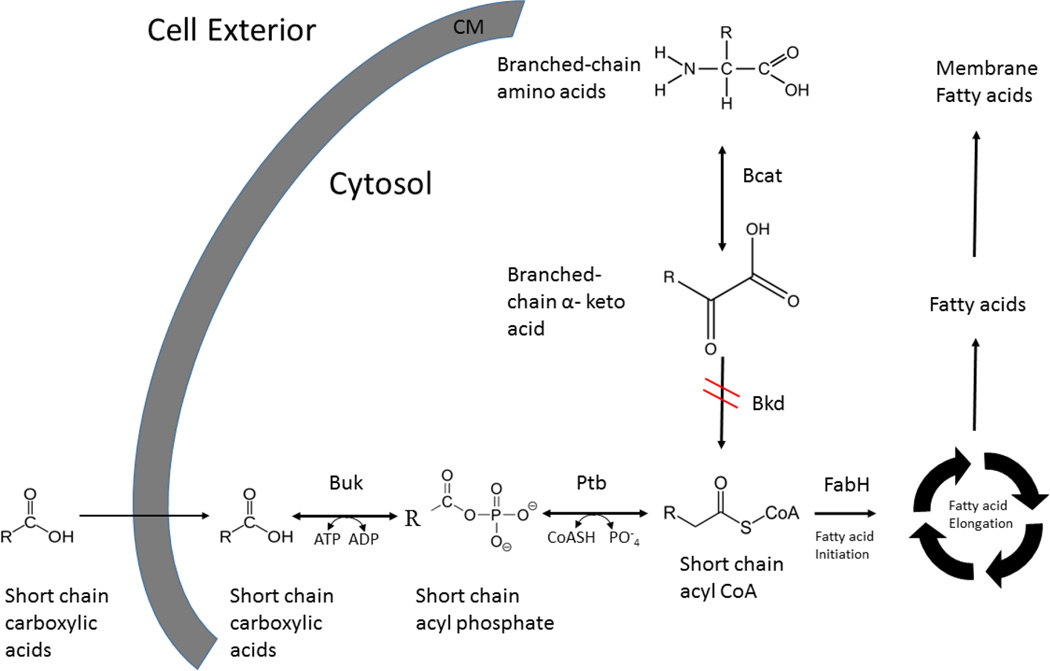
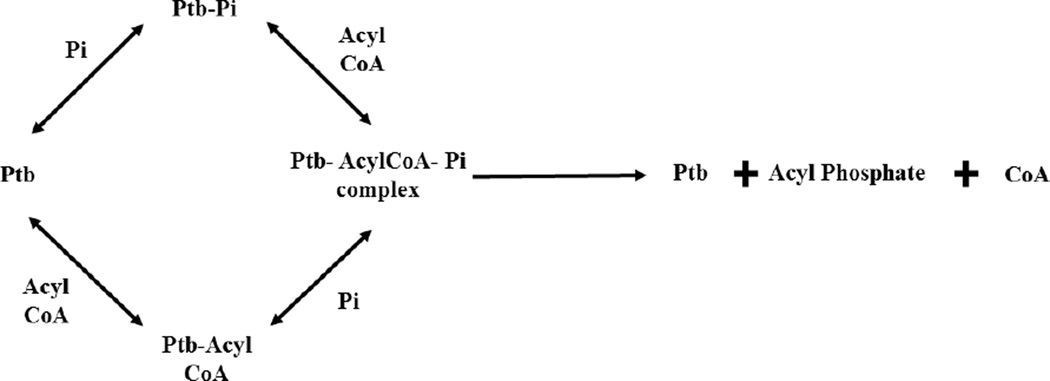
Two genes, namely phosphotransbutyrylase (ptb) and butyrate kinase (buk), have been identified upstream of the genes encoding the Bkd complex in the bkd operon of L. monocytogenes based on sequence similarity to the bkd operon in B. subtilis and Enterococcus faecalis [7,14–16]. ptb and buk are followed by the genes encoding the polypeptides of the Bkd complex in the bkd operon in L. monocytogenes [7]. The molecular organization of these two genes in L. monocytogenes is markedly different from Clostridium acetobutylicum in which ptb and buk form a separate operon expressed from a single promoter [17]. Buk (EC 2.7.2.7) is known to phosphorylate short chain carboxylic acids in the fermentative organism C. acetobutylicum and a marine spirochete MA-2 [18,19]. Ptb (EC 2.3.1.19) from C. acetobutylicum is an enzyme catalyzing the reversible conversion of acyl phosphates to acyl CoAs [20] (Fig. 1). We propose that these two enzymes, given their functions and location in the bkd operon, reverse their physiological direction in the presence of exogenous carboxylic acids and catalyze the conversion of these substrates into their corresponding acyl CoA thioesters (Fig. 1).
Fig. 1. Proposed alternative pathway for provision of acyl CoA precursors for fatty acid biosynthesis.
Schematic showing the proposed sequential conversion of exogenous carboxylic acids into their acyl CoA derivatives by the activity of Buk and Ptb and their subsequent incorporation into membrane fatty acids. R-indicates SCCAs or BCCAs. Absence of functional Bkd allows greater incorporation of the products of the alternate pathway into the membrane fatty acids.
Ptb has been well studied in C. acetobutylicum, an important producer of industrial solvents, and it plays a crucial role in the energy metabolism in the acidogenesis phase leading to the production of ATP by substrate phosphorylation [20–22]. Similar studies in E. faecalis demonstrate the involvement of Ptb in branched-chain amino acid catabolism resulting in energy production and secretion of catabolic end products such as 2-methylbutyrate and isovalerate [16]. These studies on Ptb were focused on its involvement in energy metabolism and have been limited in scope.
Ptb from L. monocytogenes is a putative 288 amino acid protein with 38.5% identity to the enzyme from C. acetobutylicum and 41% identity to Ptb from E. faecalis. In this work we describe the purification of Ptb from L. monocytogenes expressed in E. coli and investigation of its substrate preferences and enzyme kinetic constants (KM and kcat). The results indicate that Ptb demonstrates broad substrate specificity, which is consistent with our hypothesis of its role in the incorporation of exogenous carboxylic acids into the membrane fatty acids. SCCAs such as propionate and butyrate, which are used as food preservatives, additionally may contribute to the control of L. monocytogenes by resulting in the biosynthesis of straight-chain fatty acids that rigidify the membrane [10,13]. The substantial activity with these substrates suggests that Ptb may play a role in this process.
2. Materials and methods
2.1 Materials
Pentanoyl CoA was purchased from Crystalchem (Downers Grove, IL). All other materials used in this work including the chemicals utilized for synthesis of 2-methylbutyryl CoA and 2-ethylbutyryl CoA were purchased from Sigma Aldrich (St. Louis, MO).
2.2 Strains and plasmids
L. monocytogenes 10403S was grown in Brain Heart Infusion (BHI) broth (Becton Dickinson, Sparks, MD) and used for isolation of genomic DNA using a Masterpure genomic DNA purification kit according to the manufacturer’s instructions (Epicenter, Madison, WI). Primers (forward 5’ GGGGAGGTCGACAAATGACAAAAAGCAGATTTTTTTCA and reverse 5’GGGGAGCTCGAGTTTCTCAACTAGTCTTACAG) were designed with restriction sites for cloning and used for amplification of ptb from L. monocytogenes 10403S genomic DNA. The amplified gene (864 bp) was ligated into the expression vector pET28a (Novagen, Madison, WI) using T4 DNA ligase (Fermentas, Waltham, MA) to generate pET28a-ptb and transformed into competent Escherichia coli BL21 (DE3) cells. Kanamycin (50 µg/ml) was used as the selection agent for growth of pET28a and pET28a-ptb carrying E. coli cells in Luria broth (Becton Dickinson, Sparks, MD). Purification of DNA, ligation and transformation were performed according to the manufacturer’s instructions (Qiagen, Valencia, CA).
2.3 Purification of Ptb (EC 2.3.1.19)
An overnight culture of E. coli BL21 DE3 cells carrying pET28a-ptb was used to inoculate 500 ml Luria broth (2% inoculum) which was incubated at 37 °C until the OD600 reached 0.6. Overexpression of Ptb was induced by the addition of isopropyl β-D-thiogalactopyranoside (IPTG) to a final concentration of 1 mM. The cells were harvested by centrifugation at 4°C at 3,000 g and the pellets were stored at −80 °C. To purify Ptb, the cell pellet was resuspended in binding buffer (1.5 M sodium chloride-NaCl, 25 mM N-(2-Hydroxyethyl) piperazine-N′-(2-ethanesulfonic acid) [HEPES], 1 mM MgCl2, 5 mM imidazole, 5% glycerol and 2 mM β-mercapto ethanol pH 7.5) and the cells were broken using a French press at 16,000 psi with two passes through the machine. Debris was removed by centrifugation at 20,000 g for 30 min and the cell-free extract was allowed to bind for one hour with nickel-chelated nitrilotriacetic acid (Ni2+-NTA) resin (Thermo Scientific, Waltham, MA), which was pretreated with binding buffer. The resin was then packed into a column and washed with 5 column volumes of wash buffer (1.5 M NaCl, 25 mM HEPES, 1 mM MgCl2, 10 mM imidazole, 5% glycerol and 2 mM β-mercaptoethanol, pH 7.5). The bound hexa histidine-tagged Ptb was then eluted with 4 ml of elution buffer (1.5 M NaCl, 25 mM HEPES, 1 mM MgCl2, 250 mM imidazole, 25% glycerol and 2 mM β-mercaptoethanol, pH 7.5). The concentration of protein was determined by the Bradford assay using bovine serum albumin as the standard (Biorad, Hercules, CA).
2.4 Synthesis of acyl CoA substrates
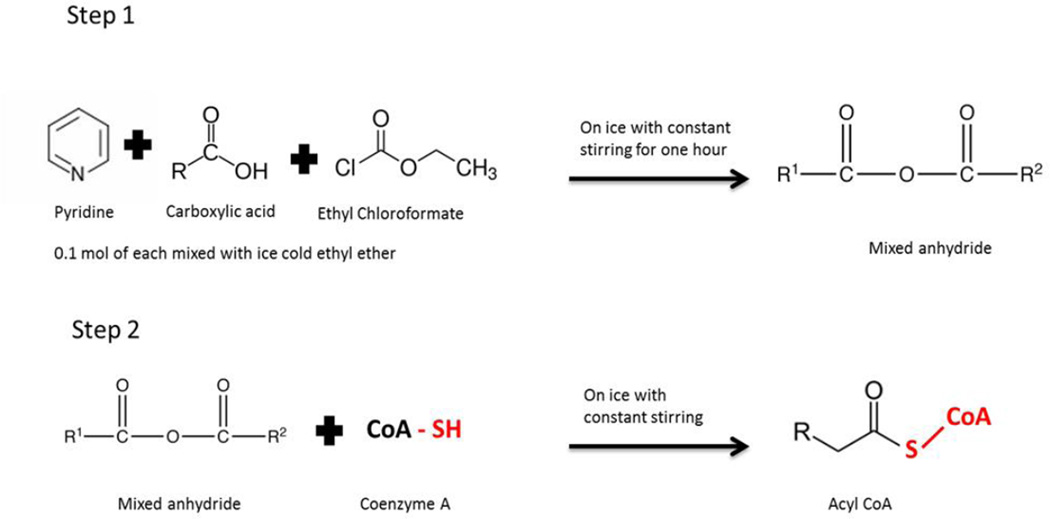
The acyl CoA substrates 2-methylbutyryl CoA and 2-ethylbutyryl CoA were prepared (Fig. 2) according to the protocol outlined by Stadtman [23]. Briefly, the mixed anhydride was prepared by the addition of 0.1 mol of the organic acid and anhydrous pyridine each to 10 ml icecold ethyl ether drop wise. This was followed by the addition of 0.1 mol of ethyl chloroformate drop wise to the mixture. The mixture was stirred for one hour in an ice bath. The insoluble pyridine hydrochloride that formed as a precipitate was removed by filtration and discarded. Next an aqueous solution of coenzyme A was prepared in 0.2 M KH2CO3 at pH 7.5. To this solution an equimolar amount of the mixed anhydride was added dropwise with constant stirring on ice for five minutes. The solution was tested for the presence of free CoA by the nitroprusside test as described by Toennies and Kolb [24]. Additional mixed anhydride was added until the nitroprusside test no longer revealed free CoA. The pH was then adjusted to 6.0 by the addition of 1 M HCl and the aqueous layer was extracted with an equal volume of ethyl ether three times to remove unreacted mixed anhydride. The remaining traces of ethyl ether were removed by bubbling nitrogen through the solution. The prepared acyl CoA substrate was quantitated by the hydroxylamine method [25].
Fig. 2. Preparation of acyl CoA substrates.
Step 1 shows the synthesis of mixed acyl anhydrides from the different carboxylic acids (R group-2-methylbutyrate and 2-ethylbutyrate). Step 2 shows the reaction of the mixed anhydride with an aqueous solution of CoA resulting in the formation of acyl CoA substrates according to the method outlined by Stadtman [23].
2.5 Ptb assay
All kinetic studies were performed at room temperature (23–25 °C) in a reaction volume of 200 µl in microcuvettes (path length = 1 cm). Ptb was assayed in the acyl phosphate forming direction according to the method of Klotsch [26] as described by Weisenborn et al., [20]. This assay was chosen for analysis of Ptb due to the commercial availability of acyl CoA derivatives varying in the carbon skeleton and also due to the lack of available corresponding acyl phosphate derivatives. Ptb catalyzes the nucleophilic attack from inorganic phosphate on an existing acyl CoA derivative to produce the corresponding acyl phosphate derivative and concomitantly liberates coenzyme-A. The free sulfhydryl group on coenzyme-A was then quantified by reacting it with Ellman’s reagent [5,5’-dithiobis-(2-nitrobenzoic acid), (DTNB)] [27]. Free thiols react with the disulfide bond in DTNB and liberate 5-thio-2-nitrobenzoic acid (TNB), which at neutral and alkaline pH ionizes to produce a yellow color which we measured spectrophotometrically (412 nm, Nanodrop 2000c UV-Vis spectrophotometer, Wilmington, DE). The production of TNB is stoichiometric with the amount of thiol present and thus was used calculate the initial velocity of Ptb production of each acyl phosphate derivative.
2.6 Steady state kinetic analysis
The assay for Ptb activity was standardized by using butyryl CoA as the substrate with a saturating concentration of inorganic phosphate (0.1 M) in the assay mixture. Enzyme-dependent rate of product formation in terms of the change in absorbance at 412 nm was studied and it was decided that a final concentration of Ptb in the assay of 5–10 nM would be used to obtain reaction velocities. Assay medium contained: 50 mM Tris (pH 7.5), 4 mM MgCl2, 0.08 mM DTNB, 0.1 M KH2PO4 and varying amounts of acyl CoA (10 µM to 500 µM). Stock solutions of the acyl CoA substrates (10 mM) were prepared using 50 mM Tris-HCl (pH 4.0) and the concentrations were calculated based on the purity of the substrate as reported by the manufacturer. Tris buffer at low pH was chosen for preparing stock solutions in order to rapidly bring up the pH of the working solution prior to use. Each substrate was diluted to 1 mM concentration prior to use with 50 mM Tris-HCl (pH 7.5). Reactions were initiated by the addition of Ptb and absorbance (412 nm) was recorded at two second intervals for the duration of the reaction (60 sec). Initial rates were calculated from the linear portion of the reaction curve using Microsoft Excel and plotted against the substrate concentration, and apparent kinetic parameters were determined using KaleidaGraph software by fitting the data to the Michaelis-Menten equation, v = Vmax * [S] / (KM +[S]) where Vmax is the maximum velocity and KM is the substrate concentration that produces half maximal velocity and [S] is the substrate concentration (Kaleidagraph, Synergy software, Reading, PA). At least three replicates of each reaction were performed and the results were presented as the mean ± SEM. Coenzyme-A (25–125 µM) (Sigma-Aldrich, St. Louis, MO) was used to establish a standard curve. Assays were conducted in the absence of enzyme to determine background degradation of the substrate, if any, and also in the absence of the substrate to rule out interference of β-mercaptoethanol. The apparent KM and kcat values were determined for a variety of straight-chain and branched-chain acyl CoA compounds listed in Table 1. Ptb was assayed in the presence of excess isovaleryl CoA (200 µM) and varying inorganic phosphate concentrations (50 µM – 25 mM) in order to determine the kinetic constants with respect to inorganic phosphate.
Table 1.
Steady state kinetic analysis of Ptb from L. monocytogenes
| Substrate | kcat (sec−1) | KM (µM) | kcat/KM (µM−1sec−1) |
|---|---|---|---|
| Straight-chain | |||
| Acetyl CoA | 302.4 ±103.9 | 992.1 ± 471.9 | 0.3 |
| Propionyl CoA | 518.8 ± 25.8 | 190.6 ± 20.9 | 2.72 |
| Butyryl CoA | 673.9 ± 25.2 | 79.9 ± 8.8 | 8.43 |
| Pentanoyl CoA | 676.1 ± 19 | 160.8 ± 10.5 | 4.2 |
| Hexanoyl CoA | 47 ± 2.6 | 31.5 ± 8.4 | 1.7 |
| Branched-chain | |||
| Iso butyryl CoA | 1263.4 ± 31.7 | 57.9 ± 4.8 | 21.4 |
| Isovaleryl CoA | 1043.4 ± 12.2 | 53.2 ± 2.1 | 19.6 |
| 2-methylbutyryl CoA | 849.1 ±18 | 48.1 ± 3.4 | 17.7 |
| 2-ethyl butyryl CoA | 100.9 ± 11 | 43.8 ± 20.3 | 2.3 |
Initial velocities were measured at 25 °C in the presence of 100 mM phosphate at pH 7.5. The kcat and KM were calculated from the least squares fit of the experimental data from the different substrates to the Michaelis-Menten equation. The values indicated are the mean of experiments performed at least in triplicate ± SEM.
Ptb assay in the acyl CoA forming direction was performed via colorimetric detection of released inorganic phosphate according to the method described by Helms et al. [28] based on the method of Brotherus and co-workers [29]. Briefly, the assay solution (200 µl) contained 100 mM Tris (pH 7.5), 500 µM CoA and varying concentrations of butyryl phosphate (200 µM - 3 mM). The reaction was initiated by the addition of Ptb enzyme (5–10 nM final concentration). The reaction was incubated at 25 °C for 10 minutes and 50 µl of the assay solution was plunged into a microtiter plate well containing 80 µL of “ice-cold” stopping solution (0.5 M HCl, 0.129 g ascorbic acid and 0.2 ml of freshly prepared 10% ammonium molybdate). After incubation on ice for 10 minutes, 120 µl of ACG (Arsenite, Citrate, Glacial acetic acid) solution (150 mM sodium m-arsenite, 70 mM sodium citrate, 350 mM acetic acid) was added and incubated for 5 minutes at 37 °C. The resultant blue color was quantified via absorbance at 800 nm. Inorganic phosphate (100 – 500 µM) was utilized to build a standard curve. Data were plotted as a function of butyryl phosphate concentration and fitted to the Michaelis-Menten equation to determine kinetic constants.
3 Results
3.1 Purification of Ptb
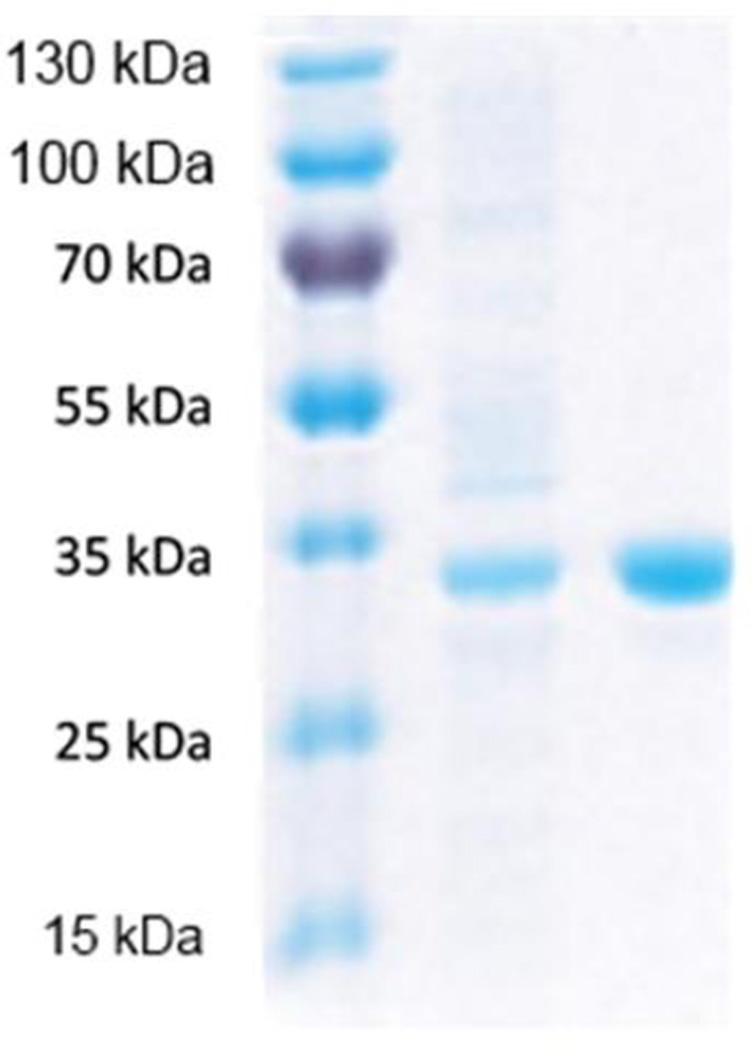
Ptb was purified by its N-terminal His-tag through Ni2+- NTA affinity purification as described in Methods. Loss of catalytic activity due to precipitation of purified Ptb was observed at concentrations ranging from 1–6 mg/ml in 50 mM Tris (pH 7.5) and in 25 mM HEPES (pH 7.5) buffers. Similar problems with stabilization of purified Ptb from Clostridium beijerinckii have been reported by Thompson and Chen [30], which were resolved by storage in high NaCl concentrations (1.3–3M). Ptb from L. monocytogenes could be stored for two weeks without detectable loss of activity by dilution of eluted protein to < 0.5 mg/ml in HEPES buffer with 1.5 M NaCl. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis confirmed the calculated molecular mass of Ptb (~34 kDa) and its purity (Fig. 3).
Fig. 3. SDS-PAGE analysis of purified preparation of Ptb.
Samples were resolved on 10% (w/v) acrylamide gel and stained with Coomassie Blue. Lane 1 protein molecular weight markers, Lane 2 Cell-free lysate showing overexpression of Ptb, Lane 3 Recombinant Ptb after purification by Ni2+- affinity chromatography.
3.2 Kinetic analysis of Ptb
3.2.1 Straight chain acyl CoA substrates
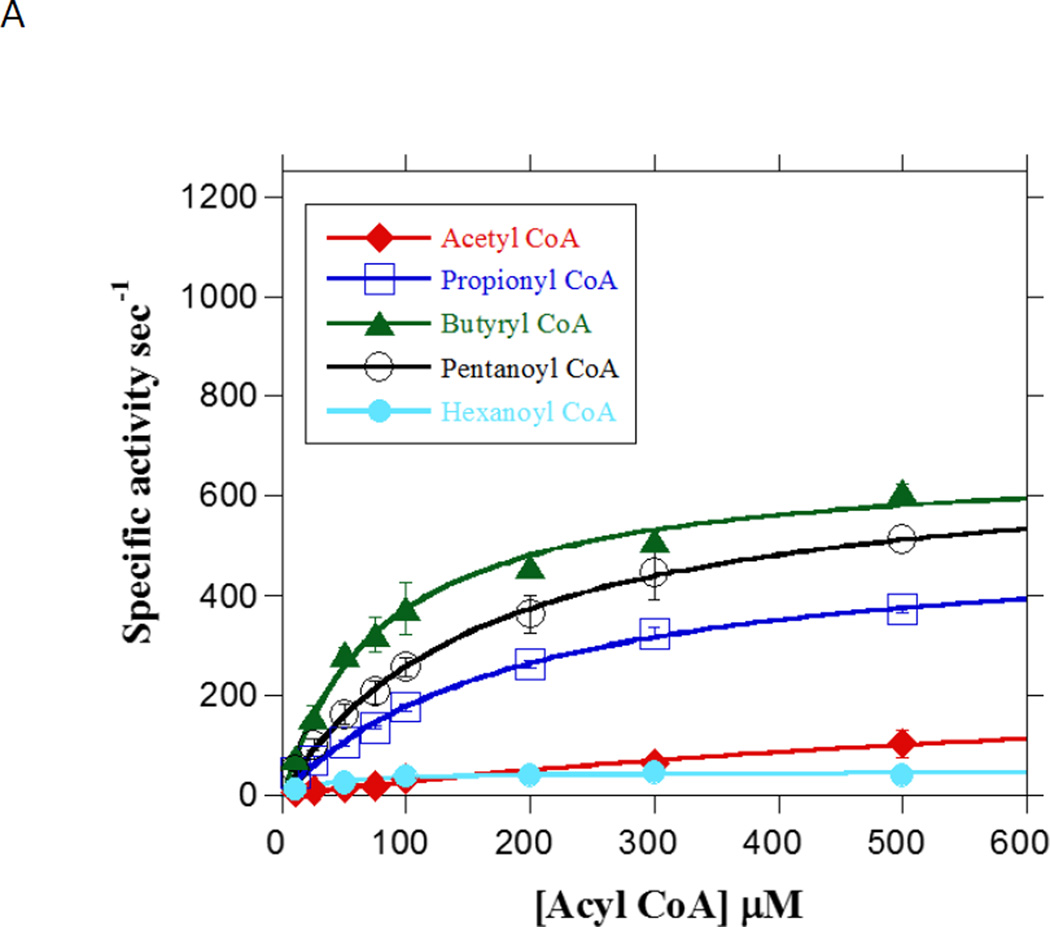
It was of interest to understand the substrate specificity of Ptb in order to determine if it could fulfill the role of activation of different carboxylic acids. To this end Ptb was assayed systematically with acyl CoA substrates differing in carbon chain length by one carbon (C2–C6). The data were fitted to the Michaelis –Menten equation (Fig. 4A) and the kinetic constants presented in Table 1. As shown in Table 1, the KM of butyrate was 79.9 µM and was the lowest of all the SCCAs tested. This KM value was similar to those previously reported for Ptb from other organisms [20,31]. The shortest and longest substrates tested, acetyl CoA (C2) and hexanoyl CoA (C6), proved to be poor substrates for Ptb judging by their poor fit to the Michaelis-Menten equation and low catalytic efficiency (kcat / KM) suggesting that the carbon chain length was suboptimal for efficient binding to the active site. The overall catalytic efficiency and the turnover number (kcat) for this series of substrates suggested that increase/decrease in chain length from the optimal size of C4 (butyryl CoA-8.43 µM−1 sec−1) resulted in a decrease in catalytic efficiency with a sharp drop with an increase/decrease of two carbons (C2–0.3 µM−1 sec−1and C6- 1.7 µM−1 sec−1) (Table 1). From these data, we concluded that the ideal chain length of substrates for Ptb was C3–C5.
Fig. 4. Substrate preference of LmPtb.
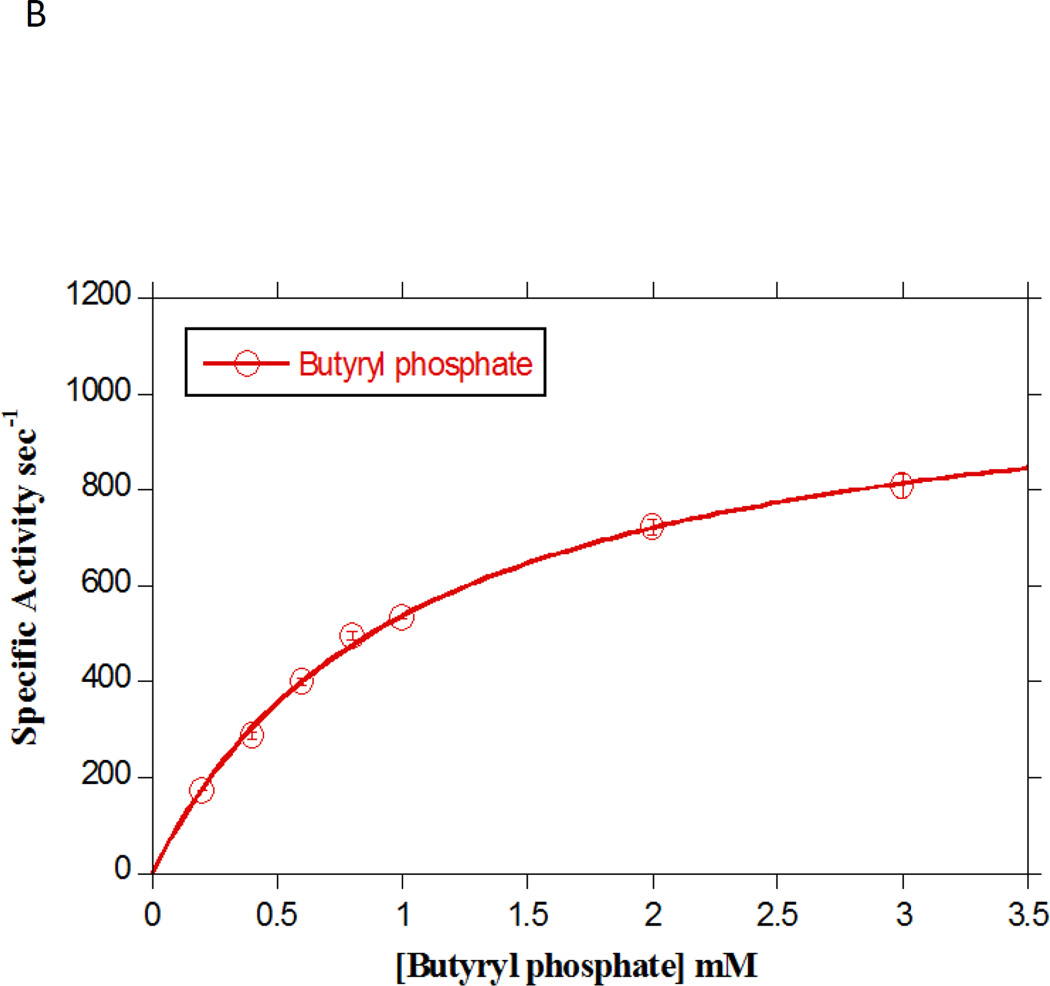
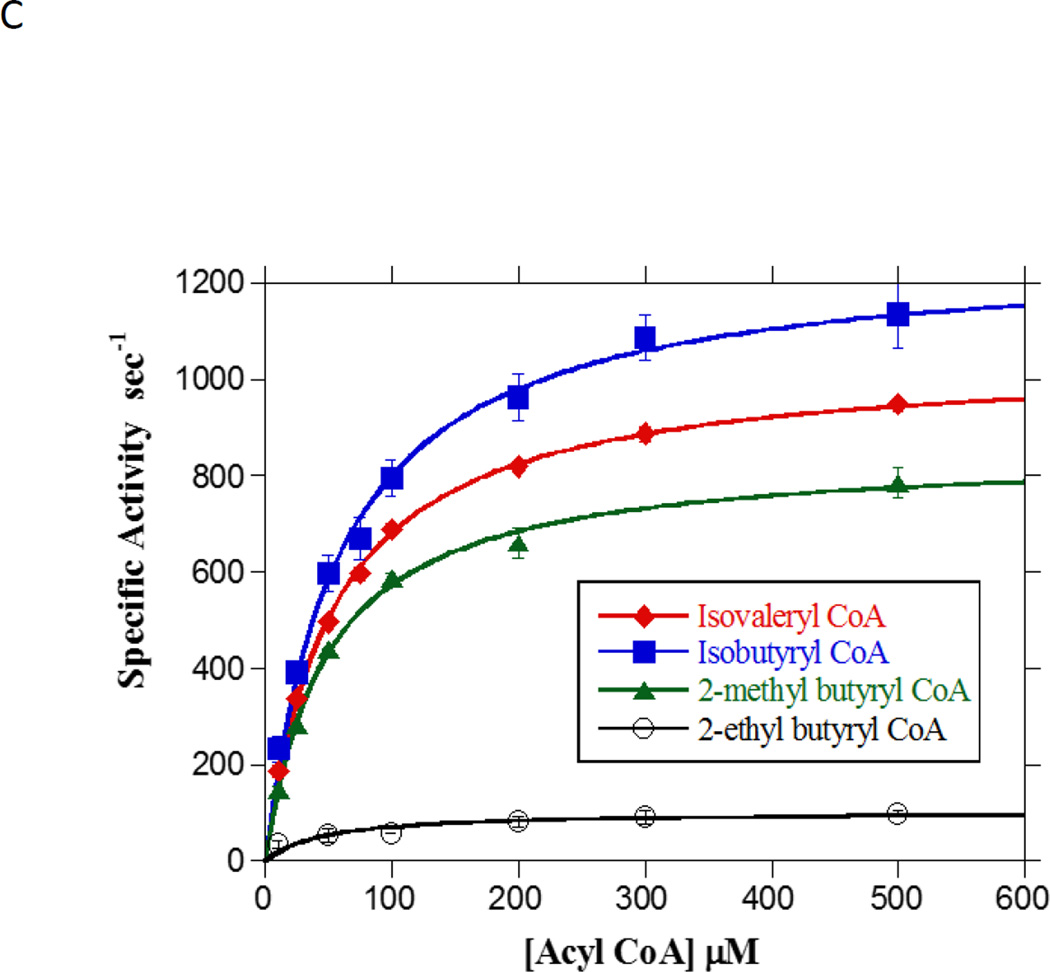
A. Concentration-dependence of straight-chain acyl CoA substrates on Ptb activity assayed in the in the acyl phosphate forming direction. Initial rates for Ptb utilization of the substrates acetyl CoA (closed diamonds), propionyl CoA (open squares), butyryl CoA (closed triangles) and pentanoyl CoA (open circles) and hexanoyl CoA (closed circles) were determined at 25 °C in the presence of 100 mM inorganic phosphate under standard assay conditions (see Materials and Methods). Data are plotted as a function of substrate concentration and KM and kcat were determined from fitting the data to the Michaelis-Menten equation using Kaleidagraph software. Data are means ± SEM of at least three experiments. B. Concentration-dependence of butyryl phosphate on Ptb activity assayed in the acyl CoA forming direction. Initial rates for Ptb utilization of butyryl phosphate (open circles) were determined at 25 oC in the presence of 100 mM Tris and 500 µM CoA (see Materials and Methods). Data were plotted as a function of substrate concentration and KM and kcat were determined from the fit of the data to the Michaelis-Menten equation using Kaleidagraph software. Data plotted are means ± SEM of three experiments. C. Concentration-dependence of branched-chain acyl CoA substrates on Ptb activity assayed in the in the acyl phosphate forming direction. Initial rates for Ptb utilization of the substrates isovaleryl CoA (closed diamonds), isobutyryl CoA (closed squares), 2-methyl butyryl CoA (closed triangles) and 2-ethyl butyryl CoA (open circles) were determined at 25 oC in the presence of 100 mM inorganic phosphate (see Materials and Methods). Data were plotted as a function of substrate concentration and KM and kcat were determined from the fit of the data to the Michaelis-Menten equation using Kaleidagraph software. Data plotted are means ± SEM of at least three experiments.
Ptb was also assayed in the acyl CoA forming direction using butyryl phosphate. The KM for butyryl phosphate was 1.04 mM, which is higher than the previously reported values [20], with a corresponding catalytic efficiency (kcat / KM) of 1.05 (Fig. 4B).
3.2.2 Branched-chain acyl CoA substrates
In order to understand the substrate preferences of Ptb in detail we also examined its activity with a variety of branched-chain substrates. Our measurements were limited by the commercial availability of branched-chain substrates (isobutyryl CoA and isovaleryl CoA) and the substrates that we could prepare (2-methylbutyryl CoA and 2-ethylbutyryl CoA). Nonetheless, our studies utilized enough substrate structural diversity to deduce critical features of the Ptb active site (Fig. 4C). Presence of an alkyl side chain improved the binding affinity of the substrate to Ptb as evidenced by the KM values (Table 1). Additionally, the KM values of the different branched-chain substrates were similar suggesting that the presence of the alkyl side chain likely produces critical contacts within the active site resulting in improved binding. Perhaps the presence of the side chain restricted freedom of movement of the substrate in the active site, and thus resulted in increased turnover numbers (kcat) of the branched-chain substrates in comparison to straight-chain substrates of the same chain length (e.g. butyryl-CoA-673.9 sec−1, isovaleryl-CoA 1043.4 sec−1, 2-methylbutyryl-CoA-849.1 sec−1) (Table 1). Furthermore, binding affinity did not appear to be influenced by the branch position nor by the length of the side chain as demonstrated by the similar KM values for isovaleryl-CoA (β-position) and 2-methylbutyryl-CoA (α-position) and 2-ethylbutyryl-CoA (ethyl branch in the α-position) (Table 1). However, the increase in side chain length was found to be associated with lower kcat values suggesting a decrease in product formation. Analysis of our experimental data (Table 1 & 2) indicated a strong preference for substrates with an alkyl side chain.
Table 2.
Relative activity of Ptb.
| Substrate | Relative activity % |
|---|---|
| Isobutyryl CoA | 100.0 |
| Isovaleryl CoA | 86.7 |
| 2-methyl butyryl CoA | 73.8 |
| Butyryl CoA | 47.1 |
| Pentanoyl CoA | 32.6 |
| Propionyl CoA | 22.3 |
| 2-ethyl butyryl CoA | 7.1 |
| Hexanoyl CoA | 4.8 |
| Acetyl CoA | 4.2 |
Relative activity was calculated from the experimental data obtained at 100 µM acyl CoA concentration and 100 mM phosphate measured at 25 °C under the same buffer conditions as stated in “Materials and Methods”. The activity of isobutyryl CoA (kcat) was designated as 100% and the relative activity of the other substrates were calculated in comparison to isobutyryl CoA.
3.2.3. Inorganic phosphate
The kinetic constants of Ptb with respect to inorganic phosphate were determined at excess isobutyryl CoA, isovaleryl-CoA and propionyl CoA concentrations (200 µM) based on curve fitting to the Hill equation (Fig. 5A and 5B). Others have reported that high concentrations of inorganic phosphate can inhibit Ptb from some other bacteria [30]. It has also been reported that KM values for phosphate can differ with various acyl-CoA substrates [30]. Consequently, we examined the phosphate parameters more closely in L. monocytogenes Ptb. We did not observe any inhibition by increasing concentrations of inorganic phosphate in our experiments.
Fig. 5. Concentration dependence of phosphate utilization by LmPtb.
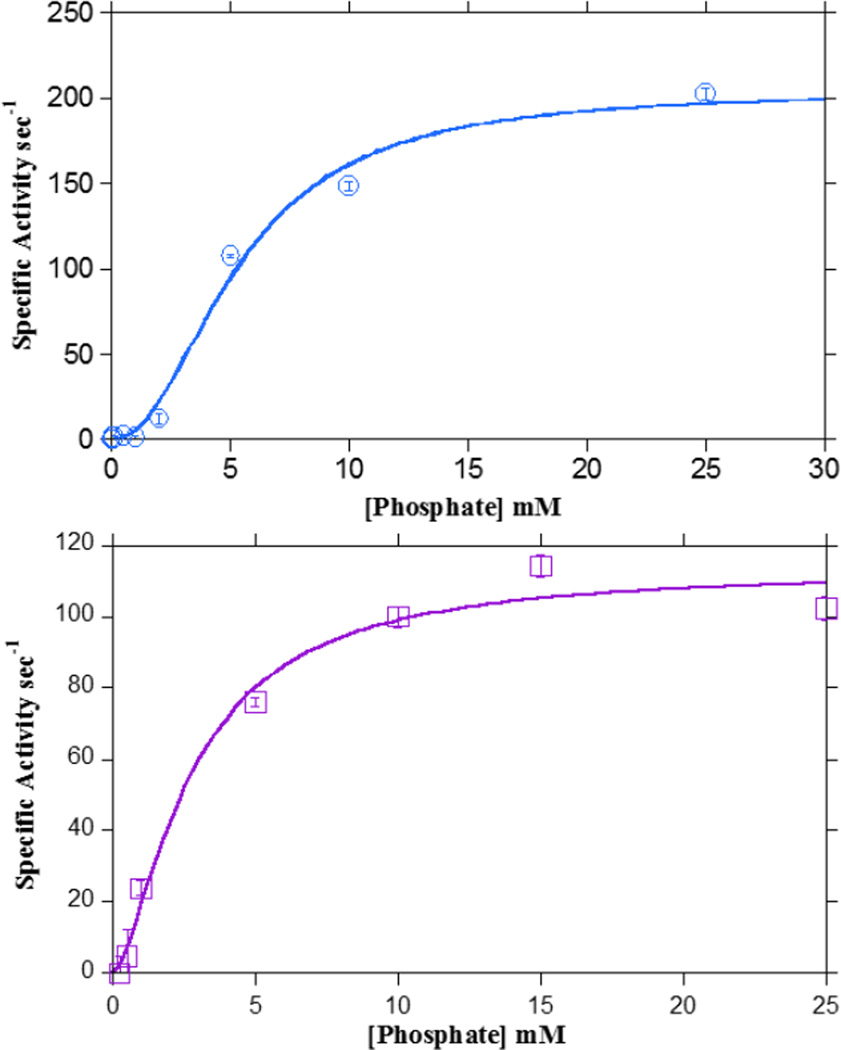
Ptb activity was determined at 25oC in the presence of varying phosphate concentrations (10 µM – 25 mM) and excess A) isobutyryl CoA (200 µM) (open circles) or B) isovaleryl CoA (200 µM) (open squares). Data were fitted to the Hill equation using Kaleidagraph software providing values for KM and kcat. Means of data from at least three experiments ± SEM were plotted as reaction rate versus phosphate concentration.
When the specific activity of Ptb was plotted as a function of phosphate concentration (Fig. 5A. and 5B), product formation was minimal at low phosphate concentrations and an increase in activity was observed with concurrent increase in phosphate concentration (>1mM) until a maximum was obtained at about 15–20 mM of phosphate. The sigmoidal nature of the curve was readily apparent only at low concentrations of phosphate, thus confirming positive cooperativity, and curve fitting of the data to the Hill equation (v = Vmax. [S]n/ K+[S]n, where n is the Hill coefficient) allowed the estimation of the parameters for binding of phosphate to Ptb (Fig. 5A. and 5B). The apparent KM for phosphate when isobutyryl CoA was utilized as the cosubstrate was found to be 5.35 ± 0.55 mM with a Hill coefficient of 2.11± 0.37 and comparable parameters were obtained with isovaleryl CoA (KM - 2.78 mM) as the second substrate. However, the apparent KM was found to be higher when the second substrate utilized was not a preferred substrate, as in the case of propionyl CoA (KM =16.4 mM), a C3 compound which lacks a methyl branch unlike isobutyryl CoA and isovaleryl CoA.
3.3 Mechanism of action of Ptb
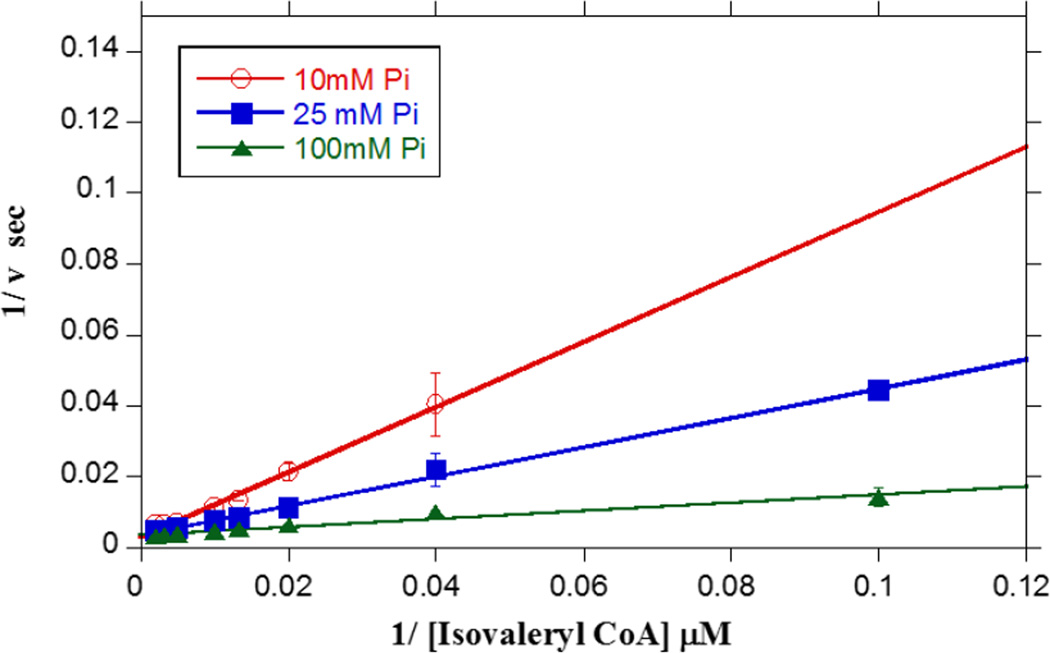
The initial rates of Ptb were further analyzed at varying concentrations of isovaleryl CoA in the presence of different fixed concentrations of inorganic phosphate (10, 25 and 100 mM). These data were used to determine the binding mechanism of Ptb which utilized the two substrates, acyl CoA and inorganic phosphate, with the formation of two products, CoA and acyl phosphate. Experiments were conducted to determine whether Ptb catalysis followed the pingpong model or required ternary complex formation. Data were normalized to the kcat of isovaleryl CoA at 100 mM inorganic phosphate and fitted to a double reciprocal plot (Fig. 6). The double reciprocal fit of the data yielded lines intersecting at a single point on the Y-axis and was consistent with ternary complex formation rather than a ping-pong mechanism. The intersection of the lines at a single point on the Y-axis confirmed the mechanism to be that of rapid equilibrium ordered mechanism as outlined schematically in Fig. 7 [32]. Furthermore, Scheme 1 describing a simple ternary complex mechanism of action was mathematically simplified to the expression in equation 1 where x is the concentration of the varying substrate (isobutyryl CoA) and S is the concentration of the fixed substrate (inorganic phosphate). The initial rate data were fitted globally to equation 1 using the graphing software OriginPro (OriginLab, Northampton, MA) which confirmed the mechanism of action to involve ternary complex formation.
| Equation 1 |
Fig. 6. Ptb activity follows a sequential kinetic model.
The concentration dependence of isovaleryl-CoA on Ptb activity was determined in the presence of varying phosphate concentrations, i.e. 10 mM (open circles), 25 mM (closed squares), and 100 mM (closed triangles) under standard assay conditions at 25 °C. Double reciprocal (Lineweaver-Burk) plots 33 of means ± SEM from at least three experiments at each phosphate concentration were plotted. The three lines clearly intersect, which is indicative of a sequential kinetic mechanism (pingpong kinetics is characterized by parallel lines).
Fig. 7. Mechanism of action of Ptb.
Schematic showing the proposed mechanism of action of Ptb involving the sequential binding of the substrates to Ptb to form the ternary complex followed by catalysis and subsequent release of products.
Scheme 1.
4. Discussion
4.1 Substrate specificity of Ptb
The high membrane BCFA content of L. monocytogenes 10403S is ensured due to the strong affinity of its FabH enzyme towards the precursors of BCFAs [33]. Although the membrane fatty acid composition of the wild-type organism is affected only by high external concentrations of propionate, butyrate and certain unnatural BCCAs, the bkd mutant MOR401(cld-2) is more susceptible to manipulation by supplementation of the media by various carboxylic acids [10,13]. Sen et al. [13] showed that in addition to the natural products of amino acid catabolism, certain BCCAs with similar structural characteristics such as 2-ethylbutyrate and 2-methylpentanoate also cause significant alteration of the membrane fatty acid composition to reflect the incorporation of the fatty acids resulting from the elongation of the added BCCAs. These studies strongly indicate the presence of a bypass pathway forming CoA thioesters of the BCCAs and SCCAs for entry into the bacterial FAS II system. Ptb and Buk, whose activities resemble that of phosphotransacetylase (pta) and acetate kinase (ack) respectively, were concluded to be the most likely candidates due to their reported ability to utilize multiple substrates in other organisms, reversibility of their catalytic activity, and their location in the bkd operon [18,20,34,35].
Our investigation into the kinetic characteristics of Ptb revealed its substantial activity with a wide range of acyl CoA substrates thus supporting our hypothesis. The substrates that were studied differed in their chain length and also in the presence and location of alkyl side chains. The broad substrate specificity of the enzyme is in agreement with the available data on Ptb from C. acetobutylicum and Ptb from E. faecalis [16,20]. Although Ptb has been studied in these organisms, the scope of these investigations has been limited to exploration of its activity with respect to the secreted products, such as butyrate in case of C. acetobutylicum, and the end products of BCAA catabolism in E. faecalis, with the inclusion of a few closely related compounds [16,20,22]. L. monocytogenes does not secrete these products under either aerobic or anaerobic growth conditions in defined medium according to a report by Romick et al. [36]. Additionally, a relationship between the activities of the bkd operon and membrane fatty acid composition could not be established in E. faecalis although 21% of the membrane fatty acids were BCFAs [16]. In contrast, Ptb from L. monocytogenes demonstrates considerable activity with the various substrates which have been previously demonstrated to influence the membrane fatty acid composition thus making it a likely candidate in the activation of exogenous carboxylic acids. Such a relationship between the activity of Ptb and the membrane composition has not been reported thus far.
4.2. Ptb exhibits chain length specificity
Although Ptb demonstrated broad substrate specificity, the activity is limited to substrates having a carbon chain length of C3–C5. Negligible activity with acetyl CoA as the substrate indicates that Ptb is distinct from Pta and that it is not involved in acetyl CoA metabolism. These data agree with previously published reports on Ptb from C. acetobutylicum, which likewise demonstrated no activity with acetyl CoA as the substrate, and the fact that addition of acetate into the medium does not cause any change in the membrane fatty acid composition [10,20,22]. Long chain fatty acids such as C14:0, C16:0 and C18:1 and addition of monolaurin and bile salts to the medium cause alteration of the membrane fatty acids to reflect the added substrates in L. monocytogenes [37–39]. While such fatty acids are incorporated into the membrane, their activation by Ptb is unlikely since a drastic drop in catalytic efficiency was exhibited by Ptb with hexanoyl CoA (C6) as its substrate when compared with its activity with pentanoyl CoA (C5) (Fig. 4A, Table 1 & 2). Recent reports by Parsons et al. [40,41] revealed a novel pathway involving the activation of long chain fatty acids by means of phosphorylation by a fatty acid kinase (Fak) thereby resulting in the production of fatty acyl phosphate. This pathway appears to be a more likely mechanism for activation of long chain fatty acids.
It is noteworthy that Ptb exhibited the highest affinity towards butyryl CoA among the straight chain substrates, and also exhibits significant product formation in the butyryl CoA forming direction (Fig. 4B). SCCAs, mainly acetate, propionate and butyrate, make up the majority of the byproducts of anaerobic metabolism by the mammalian gut commensal bacteria and L. monocytogenes is exposed to these carboxylic acids during infection [42]. Exposure to butyrate results in reduced expression of virulence genes in L. monocytogenes and Salmonella enterica Enteriditis thus indicating its role of a signal molecule [43,44]. The role of butyrate as a signal molecule is also apparent in Enterohemorrhagic E. coli (EHEC) in which it causes an upregulation of the virulence genes and increased motility [45]. Moreover, increased expression of solvent producing and stationary phase genes coinciding with a butyryl phosphate peak has been reported in C. acetobutylicum. Butyryl phosphate is hypothesized to behave as a small molecule phosphate donor similar to acetyl phosphate in E. coli in its role as a global gene regulator in C. acetobutylicum [46]. Additionally, high concentrations of butyrate result in growth inhibition due to incorporation of SCFAs in the membrane of L. monocytogenes, a property that could be used to aid in control of the organism [10,13]. Butyrate has been used as a feed additive to aid in the control S. enterica Enteriditis in turkeys and chicken [47,48].
4.3. Ptb demonstrates preference for branched-chain substrates
Ptb from L. monocytogenes preferred branched-chain CoA substrates, especially the natural degradation products of the branched-chain amino acids. The order of catalytic efficiency of Ptb in decreasing order was isobutyryl CoA > isovaleryl CoA > 2-methylbutyryl CoA > 2-ethylbutyryl CoA (Table 1). Such distinct preference of Ptb towards branched-chain substrates has not been established in any organism thus far. Ward et al. [16] showed that Ptb was involved in the catabolism of branched-chain amino acids by determining its activity with various substrates compared to that of butyrate. However, the kinetic constants were not determined for the other related acyl CoA substrates. FabH has been reported to prefer BCFA substrates in the order 2-methylbutyryl CoA > isovaleryl CoA > isobutyryl CoA at 30 °C in L. monocytogenes [33]. The substrate specificity of Ptb is unlike that of Bkd, the normal source of the intracellular acyl CoA pool, whose preference is α-keto methylvalerate > α-keto isovalerate > α-keto isocaproate and results in the formation of 2-methylbutyryl CoA, isobutyryl CoA and isovaleryl CoA respectively in B. subtilis [6]. This variation in the substrate specificities of the two enzymes may help explain the lack of restoration of the membrane fatty acid composition in the bkd mutant in L. monocytogenes (cld-2), to that of the wild type organism, despite the presence of equal amounts of the three BCCAs [7]. In other words, the significant branched-chain acyl CoA product formation by Ptb due to exogenous supplementation could outcompete the endogenous substrates for FabH and the substrate preference of Ptb could be reflected in the membrane fatty acid composition.
4.4. Ptb activity exhibits positive cooperativity with phosphate
Ptb from L. monocytogenes displays positive cooperativity with respect to phosphate in the absence of limiting conditions of acyl CoA substrate and is also not inhibited by increasing concentrations of phosphate. Wiesenborn et al. [20] investigated the binding of phosphate by Ptb from C. acetobutylicum and reported non-competitive inhibition at high concentrations of phosphate in the presence of excess butyryl CoA. Similar studies by Thompson and Chen [30] on Ptb from C. beijerinckii showed higher KM values for phosphate when the cosubstrate was changed from butyryl CoA to a weaker substrate namely acetoacetyl CoA. Both the studies utilized a minimum concentration of 5 mM for phosphate for their kinetic investigations, which precluded a demonstration of positive cooperativity by the enzyme. We investigated Ptb from L. monocytogenes using a minimum concentration of 50 µM to a maximum of 25 mM which allowed the observation of sigmoidal behavior by the enzyme, a confirmation of positive cooperativity. Although binding of phosphate molecules at more than one site was offered as an explanation for the nonlinearity of the double reciprocal plots by Wiesenborn et al. [20], our studies suggest that binding of the acyl CoA molecule likely influences the binding of phosphate within the active site. Thus binding of an optimal substrate, such as isobutyryl CoA, was accompanied by a lower KM for phosphate, indicating higher affinity, whereas binding of a substrate that is suboptimal, such as propionyl CoA, resulted in a higher KM for phosphate.
4.5. Ptb activity involves ternary complex formation
The mechanism of action of Ptb was confirmed to be that of rapid equilibrium ordered mechanism (Fig. 7) by the global fit of the initial rate data to the mathematical expression corresponding to the ternary complex formation or random Bi Bi binding scheme (Equation 1). This was similar to the mechanism determined for Ptb in C. acetobutylicum in which the data fit to a family of parabolic curves but was still not consistent with the fit for the ping pong mechanism. Formation of the ternary complex in phosphate acetyltransferase (pta) has been demonstrated in Clostridium kluyveri and also in the thermophilic archaeon Methanosarcina thermophila [34,49,50]. However, the double reciprocal plot of the data in M. thermophila shows lines intersecting to the left of the Y-axis and is unlike that of Ptb which shows the lines intersecting on a single point on the Y-axis [49]. Convergence of the lines to a point on the Y axis suggests the mechanism of action to be that of random Bi Bi and rapid equilibrium ordered mechanism wherein the enzyme forms a complex with both the substrates before the formation of products by chemical alteration [32]. This is the first report of the mechanism of action of Ptb from L. monocytogenes.
4.5. Conclusions
Analysis of Ptb from L. monocytogenes reveals that it is active with a large number of substrates and prefers substrates with a carbon chain length of C3-C5 and the presence of an alkyl side chain. Activity with natural SCCAs and BCCAs and also with unnatural substrates such as 2-ethyl butyrate, that are capable of altering the membrane fatty acid composition, strongly indicates that Ptb is the probable candidate for the alternate pathway for primer production for fatty acid biosynthesis. Butyrate is likely involved in regulation of gene expression since exposure to butyrate leads to decreased expression of virulence genes in L. monocytogenes. Additionally, high concentrations of butyrate also lead to diminished growth of L. monocytogenes suggesting a possible control strategy for the organism. The high activity of Ptb with butyryl CoA indicates that it could be involved in signal processing upon exposure of L. monocytogenes to butyrate.
Highlights.
Ptb from L. monocytogenes exhibited broad substrate specificity
Ptb preferred substrates with a chain length of 3 to 5 carbons
An alkyl side chain was required for higher catalytic efficiency
Ptb could utilize unnatural substrates such as 2-ethyl butyryl CoA
Ptb catalysis involved ternary complex formation
Acknowledgments
This work was supported by grant 1 R15 AI099977-01 from the National Institutes of Health to Brian J Wilkinson and Craig Gatto and 1 R15 GM61583 to Craig Gatto. This work was also supported by the R. D. Weigel grant from the Beta Lambda Chapter of the Phi Sigma Biological Honor Society at Illinois State University. The funding sources had no role in study design, collection, analysis and interpretation of data, writing of this manuscript, or the decision to submit it for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walker SJ, Archer P, Banks JG. Growth of Listeria monocytogenes at refrigeration temperatures. J. Appl. Bacteriol. 1990;68:157–162. doi: 10.1111/j.1365-2672.1990.tb02561.x. [DOI] [PubMed] [Google Scholar]
- 2.Thomas MK, Vriezen R, Farber JM, Currie A, Schlech W, Fazil A. Economic cost of a Listeria monocytogenes outbreak in Canada, 2008. Foodborne Pathog. Dis. 2015;12 doi: 10.1089/fpd.2015.1965. fpd.2015.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Annous BA, Becker LA, Bayles DO, Labeda DP, Wilkinson BJ. Critical role of anteiso-C15:0 fatty acid in the growth of Listeria monocytogenes at low temperatures. Appl. Environ. Microbiol. 1997;63:3887–3894. doi: 10.1128/aem.63.10.3887-3894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mastronicolis SK, Arvanitis N, Karaliota A, Magiatis P, Heropoulos G, Litos C, Moustaka H, Tsakirakis A, Paramera E, Papastavrou P. Coordinated regulation of coldinduced changes in fatty acids with cardiolipin and phosphatidylglycerol composition among phospholipid species for the food pathogen Listeria monocytogenes. Appl. Environ. Microbiol. 2008;74:4543–4549. doi: 10.1128/AEM.02041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu K, Ding X, Julotok M, Wilkinson BJ. Exogenous isoleucine and fatty acid shortening ensure the high content of anteiso-C15:0 fatty acid required for low-temperature growth of Listeria monocytogenes. Appl. Environ. Microbiol. 2005;71:8002–8007. doi: 10.1128/AEM.71.12.8002-8007.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oku H, Kaneda T. Biosynthesis of branched-chain fatty acids in Bacillus subtilis: A decarboxylase is essential for branched-chain fatty acid synthetase. J. Biol. Chem. 1988;263:18386–18396. [PubMed] [Google Scholar]
- 7.Zhu K, Bayles DO, Xiong A, Jayaswal RK, Wilkinson BJ. Precursor and temperature modulation of fatty acid composition and growth of Listeria monocytogenes cold-sensitive mutants with transposon-interrupted branched-chain alpha-keto acid dehydrogenase. Microbiology. 2005;151:615–623. doi: 10.1099/mic.0.27634-0. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y, O’Riordan MXD. Branched-chain fatty acids promote Listeria monocytogenes intracellular infection and virulence. Infect. Immun. 2010;78:4667–4673. doi: 10.1128/IAI.00546-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh VK, Hattangady DS, Giotis ES, Singh AK, Chamberlain NR, Stuart MK, Wilkinson BJ. Insertional inactivation of branched-chain alpha-keto acid dehydrogenase in Staphylococcus aureus leads to decreased branched-chain membrane fatty acid content and increased susceptibility to certain stresses. Appl. Environ. Microbiol. 2008;74:5882–5890. doi: 10.1128/AEM.00882-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Julotok M, Singh AK, Gatto C, Wilkinson BJ. Influence of fatty acid precursors, including food preservatives, on the growth and fatty acid composition of Listeria monocytogenes at 37 and 10 °C. Appl. Environ. Microbiol. 2010;76:1423–1432. doi: 10.1128/AEM.01592-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willecke K, Pardee AB. Fatty acid-requiring mutant of Bacillus subtilis defective in branched chain alpha-keto acid dehydrogenase. J. Biol. Chem. 1971;246:5264–5272. [PubMed] [Google Scholar]
- 12.Kaneda T. Incorporation of branched-chain C6 -fatty acid isomers into the related long-chain fatty acids by growing cells of Bacillus subtilis. Biochemistry. 1970;10:340–347. doi: 10.1021/bi00778a022. [DOI] [PubMed] [Google Scholar]
- 13.Sen S, Sirobhushanam S, Hantak MP, Lawrence P, Thomas Brenna J, Gatto C, Wilkinson BJ. Short branched-chain C6 carboxylic acids result in increased growth, novel “unnatural” fatty acids and increased membrane fluidity in a Listeria monocytogenes branched-chain fatty acid-deficient mutant. Biochim. Biophys. Acta. 2015;1851:1406–1415. doi: 10.1016/j.bbalip.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debarbouille M, Gardan R, Arnaud M, Rapoport G. Role of bkdR, a transcriptional activator of the sigL-dependent isoleucine and valine degradation pathway in Bacillus subtilis. J. Bacteriol. 1999;181:2059–2066. doi: 10.1128/jb.181.7.2059-2066.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson KE, Fouts DE, Mongodin EF, Ravel J, DeBoy RT, Kolonay JF, Rasko DA, Angiuoli SV, Gill SR, Paulsen IT, Peterson J, White O, Nelson WC, Nierman W, Beanan MJ, Brinkac LM, Daugherty SC, Dodson RJ, Durkin AS, Madupu R, et al. Whole genome comparisons of serotype 4b and 1/2a strains of the food-borne pathogen Listeria monocytogenes reveal new insights into the core genome components of this species. Nucleic Acids Res. 2004;32:2386–2395. doi: 10.1093/nar/gkh562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward DE, Ross RP, van der Weijden CC, Snoep JL, Claiborne A. Catabolism of branched-chain alpha-keto acids in Enterococcus faecalis: the bkd gene cluster, enzymes, and metabolic route. J. Bacteriol. 1999;181:5433–5442. doi: 10.1128/jb.181.17.5433-5442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walter KA, Nair RV, Cary JW, Bennett GN, Papoutsakis ET. Sequence and arrangement of two genes of the butyrate-synthesis pathway of Clostridium acetobutylicum ATCC 824. Gene. 1993;134:107–111. doi: 10.1016/0378-1119(93)90182-3. [DOI] [PubMed] [Google Scholar]
- 18.Hartmanis MGN. Butyrate kinase from Clostridium acetobutylicum. J. Biol. Chem. 1987;262:617–621. [PubMed] [Google Scholar]
- 19.Harwood CS, Canale-Parola E. Properties of acetate kinase isozymes and a branchedchain fatty acid kinase from a Spirochete. J. Bacteriol. 1982;152:246–254. doi: 10.1128/jb.152.1.246-254.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiesenborn DP, Rudolph FB, Papoutsakis ET. Phosphotransbutyrylase from Clostridium acetobutylicum ATCC 824 and its role in acidogenesis. Appl. Environ. Microbiol. 1989;55:317–322. doi: 10.1128/aem.55.2.317-322.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cary JW, Petersen DJ, Papoutsakis ET, Bennett GN. Cloning and expression of Clostridium acetobutylicum phosphotransbutyrylase and butyrate kinase genes in Escherichia coli. J. Bacteriol. 1988;170:4613–4618. doi: 10.1128/jb.170.10.4613-4618.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartmanis MGN, Gatenbeck S. Intermediary metabolism in Clostridium acetobutylicum : Levels of enzymes involved in the formation of acetate and butyrate. Appl. Environ. Microbiol. 1984;47:1277–1283. doi: 10.1128/aem.47.6.1277-1283.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stadtman ER. Preparation and assay of acyl coenzyme A and other thiol esters; use of hydroxylamine. Methods Enzymol. 1957;3:931–941. [Google Scholar]
- 24.Toennies G, Kolb JJ. Techniques and reagents for paper chromatography. Anal. Chem. 1951;23:823–826. [Google Scholar]
- 25.Lipmann F, Tuttle LC. A specific micromethod for the determination of acyl phosphates. J. Biol. Chem. 1945;159:21–28. [Google Scholar]
- 26.Klotzsch HR. Phosphotransacetylase from Clostridium kluyveri. Methods Enzymol. 1969;13:381–386. [Google Scholar]
- 27.Ellman GL. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 28.Helms JB, Saunders LP, Meyer J, Costa CJ, Plowman E, Williford N, Corbitt M, Holden JP, Gatto C. Kinetic characterization of Na, K-ATPase inhibition by the acetaminophen metabolite. Open J. Mol. Integr. Physiol. 2015;5:1–17. [Google Scholar]
- 29.Brotherus JR, Moller JV, Jorgensen PL. Soluble and active renal Na,K-ATPase with maximum protein molecular mass 170,000 + 9,000 daltons; Formation of larger units by secondary aggregation. Biochem. Biophys. Res. Commun. 1981;100:146–154. doi: 10.1016/s0006-291x(81)80075-7. [DOI] [PubMed] [Google Scholar]
- 30.Thompson DK, Chen JS. Purification and properties of an acetoacetyl coenzyme Areacting phosphotransbutyrylase from Clostridium beijerinckii (“Clostridium butylicum”) NRRL B593. Appl. Environ. Microbiol. 1990;56:607–613. doi: 10.1128/aem.56.3.607-613.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aboulnaga E-HH, Pinkenburg O, Schiffels J, El-Refai A, Buckel W, Selmer T. Effect of an oxygen-tolerant bifurcating butyryl coenzyme A dehydrogenase/electron-transferring flavoprotein complex from Clostridium difficile on butyrate production in Escherichia coli. J. Bacteriol. 2013;195:3704–3713. doi: 10.1128/JB.00321-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segel IH. Enzyme kinetics: behavior and analysis of rapid equilibrium and steady-state enzyme systems. New York: John Wiley Sons; 1993. [Google Scholar]
- 33.Singh AK, Zhang Y, Zhu K, Subramanian C, Li Z, Jayaswal K, Gatto C, Rock CO, Wilkinson BJ. FabH selectivity for anteiso branched-chain fatty acid precursors in low temperature adaptation in Listeria monocytogenes. FEMS Microbiol. Lett. 2009;301:1–8. doi: 10.1111/j.1574-6968.2009.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergmeyer HU, Holz G, Klotzch H, Lang G. Phosphotransacetylase from Clostridium kluyveri. Culture of the bacterium, isolation, crystallization and properties of the enzyme. Biochem. Z. 1963;338:114–21. [PubMed] [Google Scholar]
- 35.Bock A, Glasemacher J, Schmidt R, Schönheit P, Glasemacher R. Purification and characterization of two extremely thermostable enzymes, phosphate acetyltransferase and acetate kinase , from the hyperthermophilic eubacterium Thermotoga maritima. J. Bacteriol. 1999;181:1861–1867. doi: 10.1128/jb.181.6.1861-1867.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romick TL, Fleming HP, McFeeters RF. Aerobic and anaerobic metabolism of Listeria monocytogenes in defined glucose medium. Appl. Environ. Microbiol. 1996;62:304–307. doi: 10.1128/aem.62.1.304-307.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juneja VK, Davidson PM. Influence of altered fatty acid composition on resistance of Listeria monocytogenes to antimicrobials. J. Food Prot. 1993;56:302–305. doi: 10.4315/0362-028X-56.4.302. [DOI] [PubMed] [Google Scholar]
- 38.Tokarskyy O, Marshall DL. Mechanism of synergistic inhibition of Listeria monocytogenes growth by lactic acid, monolaurin, and nisin. Appl. Environ. Microbiol. 2008;74:7126–7129. doi: 10.1128/AEM.01292-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gahan CGM, Hill C. Listeria monocytogenes: survival and adaptation in the gastrointestinal tract. Front. Cell. Infect. Microbiol. 2014;4:9. doi: 10.3389/fcimb.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parsons JB, Broussard TC, Bose JL, Rosch JW, Jackson P, Subramanian C, Rock CO. Identification of a two-component fatty acid kinase responsible for host fatty acid incorporation by Staphylococcus aureus. Proc. Natl. Acad. Sci. 2014;111:10532–10537. doi: 10.1073/pnas.1408797111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parsons JB, Frank MW, Jackson P, Subramanian C, Rock CO. Incorporation of extracellular fatty acids by a fatty acid kinase-dependent pathway in Staphylococcus aureus. Mol. Microbiol. 2014;92:234–245. doi: 10.1111/mmi.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun Y, Wilkinson BJ, Standiford TJ, Akinbi HT, O’Riordan MXD. Fatty acids regulate stress resistance and virulence factor production for Listeria monocytogenes. J. Bacteriol. 2012;194:5274–5284. doi: 10.1128/JB.00045-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gantois I, Ducatelle R, Pasmans F, Haesebrouck F, Thompson A, Hinton JC, Van Immerseel F, Hautefort I. Butyrate specifically down-regulates Salmonella pathogenicity island 1 gene expression. Appl. Environ. Microbiol. 2006;72:946–949. doi: 10.1128/AEM.72.1.946-949.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tobe T, Nakanishi N, Sugimoto N. Activation of motility by sensing short-chain fatty acids via two steps in a flagellar gene regulatory cascade in enterohemorrhagic Escherichia coli. Infect. Immun. 2011;79:1016–1024. doi: 10.1128/IAI.00927-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Y, Tomas CA, Rudolph FB, Papoutsakis ET, Bennett GN. Intracellular butyryl phosphate and acetyl phosphate concentrations in Clostridium acetobutylicum and their implications for solvent formation. Appl. Environ. Microbiol. 2005;71:530–537. doi: 10.1128/AEM.71.1.530-537.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milbradt EL, Zamae JR, Araujo Junior JP, Mazza P, Padovani CR, Carvalho VR, Sanfelice C, Rodrigues DM, Okamoto AS, Andreatti Filho RL. Control of Salmonella enteritidis in turkeys using organic acids and competitive exclusion product. J. Appl. Microbiol. 2014;117:554–563. doi: 10.1111/jam.12537. [DOI] [PubMed] [Google Scholar]
- 48.Fernández-Rubio C, Ordóñez C, Abad-Gonzalez J, Garcia-Gallego A, Honrubia MP, Mallo JJ, Balaña-Fouce R, Fernandez-Rubio C, Ordonez C, Balana-Fouce R. Butyric acid-based feed additives help protect broiler chickens from Salmonella enteritidis infection. Poult. Sci. 2009;88:943–948. doi: 10.3382/ps.2008-00484. [DOI] [PubMed] [Google Scholar]
- 49.Lawrence SH, Ferry JG. Steady-state kinetic analysis of phosphotransacetylase from Methanosarcina thermophila. 2006;188:1155–1158. doi: 10.1128/JB.188.3.1155-1158.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henkin J, Abeles RH. Evidence against an acyl-enzyme intermediate in the reaction catalyzed by Clostridial phosphotransacetylase. Biochemistry. 1976;15:3472–3479. doi: 10.1021/bi00661a012. [DOI] [PubMed] [Google Scholar]